Abstract
The role of microRNA-132 in human pancreatic ductal adenocarcinomas is still ambiguous. We explored the association between microRNA-132 and pancreatic ductal adenocarcinoma prognosis. The expression of microRNA-132 in 50 pancreatic ductal adenocarcinoma tissue samples and pancreatic ductal adenocarcinoma cell lines was examined, and the association between its expression and pancreatic ductal adenocarcinoma prognosis was assessed. Functional analysis and factors downstream of microRNA-132 were investigated. Kaplan-Meier survival curves showed that high expression of microRNA-132 was a significant prognostic factor for 1-year survival of patients with pancreatic ductal adenocarcinoma (P = .028). Multivariate analysis for overall survival indicated that high expression of microRNA-132 was an independent prognostic factor for patients with pancreatic ductal adenocarcinoma (P = .044). Low expression of microRNA-132 was associated with poor prognosis in pancreatic ductal adenocarcinoma. Ectopic expression of microRNA-132 significantly inhibited proliferation and promoted apoptosis of 2 pancreatic ductal adenocarcinoma cell lines. Bioinformatic analysis revealed that microRNA-132 may exert its effects on pancreatic ductal adenocarcinoma through downregulating mitogen-activated protein kinase 3 and nuclear transcription factor Y subunit α. The results of this study further our understanding of the relationship between microRNA-132 and pancreatic ductal adenocarcinoma by showing that microRNA-132 might inhibit the progression of pancreatic ductal adenocarcinoma by regulating mitogen-activated protein kinase and nuclear transcription factor Y subunit alpha.
Keywords: miR-132, PDAC, tumor suppressor, prognostic, biomarker
Introduction
In the United States, pancreatic ductal adenocarcinoma (PDAC) is a highly lethal malignancy and the third leading cause of cancer-related death.1 Despite much research and progress on this disease over the past several decades, there are no effective treatment regimens. Chemotherapy and radiation therapy remain mostly ineffective.2,3 Surgical resection is the only attempt at a cure, but only 15% to 20% of patients with pancreatic cancer are eligible for surgery at the time of presentation. Furthermore, those who undergo successful surgical resection have a dismal 5-year survival rate of 15% to 23%.4 Novel treatment strategies are needed to combat this disease. However, a better understanding of the molecular pathogenesis of PDAC must be achieved before new therapies can be developed.
Emerging evidence indicates that the deregulation or dysfunction of microRNAs (miRNAs) contribute to human carcinogenesis and cancer progression.5 The miRNAs can either promote or inhibit tumor development and growth according to the function of the target gene. The miR-132, a member of the miR-132/212 family, is involved in the development of a variety of cancers.6 The miR-132/212 cluster has the potential to diagnose and predict the prognosis of hepatocellular carcinoma as a noninvasive serum biomarker.7 The miR-132/212 can augment glucose and glucagon-like peptide-1-stimulated insulin secretion regulated by the cyclic adenosine monophosphate/protein kinase A pathway.8 Intriguingly, its function in PDAC is ambiguous.9,10 Zhang et al 9 believe that downregulation of miR-132 by promoter methylation contributes to pancreatic cancer development, while Park et al 10 consider that miR-132/212 is increased in pancreatic cancer and targets the retinoblastoma tumor suppressor.
In this study, we analyzed miR-132 expression in 50 pairs of PDACs and para-carcinoma tissues. Using the cell counting kit (CCK)-8 assay, flow cytometry, and bioinformatic analysis, we investigated the function and downstream effects of miR-132. Our findings help clarify our understanding of the relationship between miR-132 and PDAC.
Material and Methods
Patients and Tissue Samples
Pancreatic cancer and adjacent noncancerous tissues from patients who had not undergone chemotherapy or radiotherapy prior to surgery were collected during surgery in Shanghai Changhai Hospital, The Second military University (Shanghai, China) from October 10, 2012, to March 20, 2013. Imaging examinations found that the pancreas was occupied by a carcinoma, and patients who underwent surgery after multidisciplinary consultation were included in the study. Our study was approved by Shanghai Changhai Hospital Ethics Committee (approval no. CHEC2011-114). All patients provided written informed consent prior to enrollment in the study. After pathologic confirmation, 50 patients with PDAC were included and their clinical features were recorded. All patients received periodic follow-up (every 2 months following surgery). Overall survival (OS) was defined as the time from primary surgery until the patient was deceased.
Cell Lines and Culture
The human pancreatic cancer cell lines, Panc-1 and SW1990, were purchased from the American type tissue collection (Manassas, Virginia). Cells were grown in Dulbecco’s modified Eagle’s medium (Gibco, Gaithersburg, Maryland) with 10% fetal bovine serum (Gibco). Cells were incubated at 37°C under a humidified atmosphere with 5% carbon dioxide.
Extraction of RNA and the Real-Time Reverse Transcription Polymerase Chain Reaction
Total RNA was prepared using TRIzol reagent (Invitrogen, Carlsbad, California). Reverse transcription was conducted for 15 minutes at 37 patients p ng of purified total RNA in a 20 L volume of the reaction mixture (Takara Bio, California) according to the manufacturer’s protocol. Real-time reverse transcription polymerase chain reaction (RT-PCR) was performed using the standard Taqman miRNA assay protocol (Applied Biosystems, Foster City, California) on an ABI7500 RT-PCR detection system (Applied Biosystems) under the following conditions: 95°C for 15 minutes, followed by 40 cycles, and 95°C for 60 seconds followed by 40 cycles. U6 small nuclear RNA was used as an internal control. Each sample was measured in triplicate, and the quantity of miR-132 relative to that of U6 was calculated using the formula 2−ΔΔ2.
RT primers:
MiR-132: 5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAGAATTG-3′
U6: 5′-TGGTGTCGTGGAGTCG-3′
Quantitative RT-PCR primers:
MiR-132: forward: 5′-GCCCTGATTGTCCAAACGC-3′; reverse: 5′-GTGCAGGGTCCGAGGT-3′
U6: forward: 5′-CTCGCTTCGGCAGCACA-3′; reverse: 5′-AACGCTTCACGAATTTGCGT-3′
Cell Transfection
Cells (1 × 105/well) in 6-well plates were transfected with control and miR-132 mimics (Gene Pharma, Shanghai, China) by Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. After 8 hours, cells were incubated for a further 48 hours in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum. Cells were then collected for analyses.
Cell-Cycle Analysis
To determine the cell-cycle distribution, cells were collected after trypsinization into a single-cell suspension and fixed with cold ethanol at 4°C overnight. The fixed cells were washed and resuspended in 200 μL binding buffer containing RNase A and propidium iodide. After incubating for 20 minutes at room temperature, cells were analyzed for DNA content by flow cytometry (Miltenyi, Auburn, California). For each sample, 20 000 events were acquired, and cell-cycle distributions were determined using FlowJo software (Ashland, Oregon).
Apoptosis Assay
Apoptosis was quantified by Annexin V/FITC staining according to the supplier’s instructions (eBioscience, San Diego, California). After a 48-hour treatment, cells were collected and washed twice in cold phosphate-buffered saline. After centrifugation at 1000g, cells were resuspended in 100 μL of binding buffer and then stained with Annexin V/FITC (5 μL) for 15 minutes at room temperature in the dark. Before analysis by flow cytometry, cells were also stained with propidium iodide (10 μL). The results were analyzed by FlowJo software.
Cell Counting Kit -8 Assay
Cell counting was performed by the CCK-8 assay kit protocol (Dojindo, Shanghai, China). Briefly, cells (control, cells transfected with control small interfering RNA, and cells transfected with miR-132) were seeded into 96-well plates at 2 × 104 cells/ well and cultured overnight. At 0, 12, 24, 36, and 48 hours, cells were treated with 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium monosodium salt according to the manufacturer’s protocol. Absorbance values were measured at 450 nm by a microplate reader. All experiments were performed in triplicate.
Statistical Analyses
Data are expressed as means (standard deviations). Statistical analyses were conducted using SPSS software version 19.0 (IBM, Chicago, Illinois). Associations between miR-132 expression and various clinicopathologic parameters were examined by the χ2 and Fisher exact tests. Survival curves were generated using the Kaplan-Meier method; statistical significance between these curves was assessed by the log-rank test. The Cox proportional hazard regression model was performed to identify independent prognostic factors. Only variables of the defined value (P < .20) from the univariate analysis were entered into the Cox regression analysis. Student t test was used to compare normally distributed measurement data between any 2 groups. The miR-132 group was compared with the normal control group or control group separately. A value of P < .05 was considered statistically significant.
Results
Expression of MiR-132 and Clinicopathological Features in PDAC
Clinical features of the 50 patients of this study were recorded and miR-132 expression in PDAC tissues was tested by quantitative RT-PCR. Patients were divided into 2 groups by the median miR-132 expression of all 50 patients (mean value = 6.476). The relationship between miR-132 expression and clinical features was analyzed (Table 1). Age, gender, tumor location, CA199, carcinoembryonic antigen, tumor stage, tumor size, differentiation, vascular invasion, and surgical margin were not significantly different (P > .05) between the 2 groups.
Table 1.
Correlations Between miR-132 Expression and Clinical Features of Patients With Pancreatic Ductal Adenocarcinoma.
| Variable | Number of Cases | MiR-132 Expression | P Value | |
|---|---|---|---|---|
| Low, n = 24 | High, n = 26 | |||
| Age, years | .273 | |||
| <59 | 19 | 11 (57.89%) | 8 (42.11%) | |
| ≥59 | 31 | 13 (41.94%) | 18 (58.06%) | |
| Gender | .164 | |||
| Male | 32 | 13 (40.63%) | 19 (59.83%) | |
| Female | 18 | 11 (61.11%) | 7 (38.89%) | |
| Tumor location | .369 | |||
| Head | 33 | 14 (42.42%) | 19 (57.58%) | |
| Others | 17 | 10 (58.82%) | 7 (41.18%) | |
| CA199, U/L | .080 | |||
| <37 | 14 | 4 (28.57%) | 10 (41.18%) | |
| ≥37 | 36 | 20 (55.56%) | 16 (44.44%) | |
| CEA, ng/mL | .133 | |||
| <5 | 30 | 17 (56.67%) | 13 (43.33%) | |
| ≥5 | 20 | 7 (35.00%) | 13 (65.00%) | |
| TNMa | .469 | |||
| I+II | 47 | 22 (46.81%) | 25 (53.19%) | |
| III+IV | 3 | 2 (66.67%) | 1 (33.33%) | |
| T stage | .496 | |||
| T1+T2 | 26 | 13 (50.00%) | 13 (50.00%) | |
| T3+T4 | 24 | 11 (45.83%) | 13 (54.17%) | |
| N stage | .059 | |||
| N0 | 33 | 19 (57.58%) | 14 (42.42%) | |
| N1 | 17 | 5 (29.41%) | 12 (70.59%) | |
| Differentiation | .650 | |||
| Poor | 14 | 6 (42.86%) | 8 (57.14%) | |
| Moderate | 36 | 18 (50.00%) | 18 (50.00%) | |
| Tumor size, cmb | .775 | |||
| <3.35 | 27 | 14 (51.85%) | 13 (48.15%) | |
| ≥3.35 | 23 | 10 (43.48%) | 13 (56.52%) | |
| Vascular invasion | .273 | |||
| No | 31 | 13 (41.94%) | 18 (58.06%) | |
| Yes | 19 | 11 (57.89%) | 8 (42.11%) | |
| Surgical marginsc | 1.000 | |||
| Free | 41 | 20 (48.78%) | 21 (51.22%) | |
| Not free | 9 | 4 (44.44%) | 5 (55.56%) | |
Abbreviations: CEA, carcinoembryonic antigen; TNM, tumor node metastasis.
aThe seventh edition of the TNM Classification of Malignant Tumors.
b3.35 cm is a mean of tumor diameters.
c Surgical margin free is defined when there was no tumor cell inside 0.5 cm of incisal edge.
Survival Analysis
Multivariate Cox regression analysis was used to identify the factors that impacted survival (P < .20, Table 2). This analysis revealed that miR-132 expression (relative risk: 0.309; 95% confidence interval, 0.098-0.970, P = .044) was an independent prognostic marker of 1-year survival in patients with PDAC (Table 2).
Table 2.
Univariate and Multivariate Analyses of Survival in 50 Patients with Pancreatic Ductal Adenocarcinoma.
| Variable | Number of Cases | Univariate Log-Rank Test P Value | Cox Multivariable Analysis | |
|---|---|---|---|---|
| P Value | HR (95% CI) | |||
| Age, years | .265 | |||
| <59 | 19 | |||
| ≥59 | 31 | |||
| Gender | .648 | |||
| Male | 32 | |||
| Female | 18 | |||
| Tumor location | .890 | |||
| Head | 33 | |||
| Others | 17 | |||
| CA19-9, U/L | .137 | .168 | ||
| <37 | 14 | 1 (reference) | ||
| ≥37 | 36 | 2.852 (0.643-12.648) | ||
| CEA, ng/mL | .887 | |||
| <5 | 30 | |||
| ≥5 | 20 | |||
| TNMa | .819 | |||
| I+II | 47 | |||
| III+IV | 3 | |||
| T stage | .449 | |||
| T1+T2 | 26 | |||
| T3+T4 | 24 | |||
| N stage | .448 | |||
| N0 | 33 | |||
| N1 | 17 | |||
| Differentiation | .943 | |||
| Poor | 14 | |||
| Moderate | 36 | |||
| Tumor size, cmb | .061 | .081 | ||
| <3.35 | 27 | 1 (reference) | ||
| ≧3.35 | 23 | 0.360 (0.115 -1.134) | ||
| Vascular invasion | .054 | .071 | ||
| No | 31 | 1 (reference) | ||
| Yes | 19 | 2.586 (0.920-7.265) | ||
| Surgical marginsc | .867 | |||
| No | 41 | |||
| Yes | 9 | |||
| Adjuvant therapy | .418 | |||
| No | 31 | |||
| Yes | 19 | |||
| MiR-132 | .028 | .044 | ||
| Low | 24 | 1 (reference) | ||
| High | 26 | 0.309 (0.098-0.970) | ||
Abbreviations: CEA, carcinoembryonic antigen; CI, confidence interval; HR, hazard ratio; miR-132, microRNA-132; TNM, tumor node metastasis.
a The seventh edition of the TNM Classification of Malignant Tumors.
b3.35 cm is a mean of tumor diameters.
c Surgical margin free is defined when there was no tumor cell inside 0.5 cm of incisal edge.
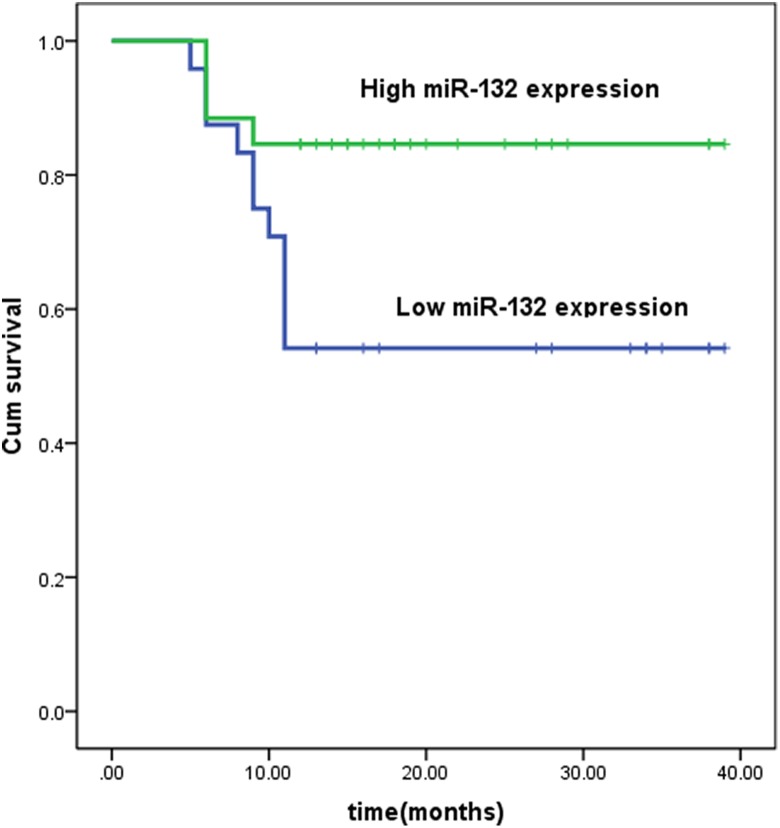
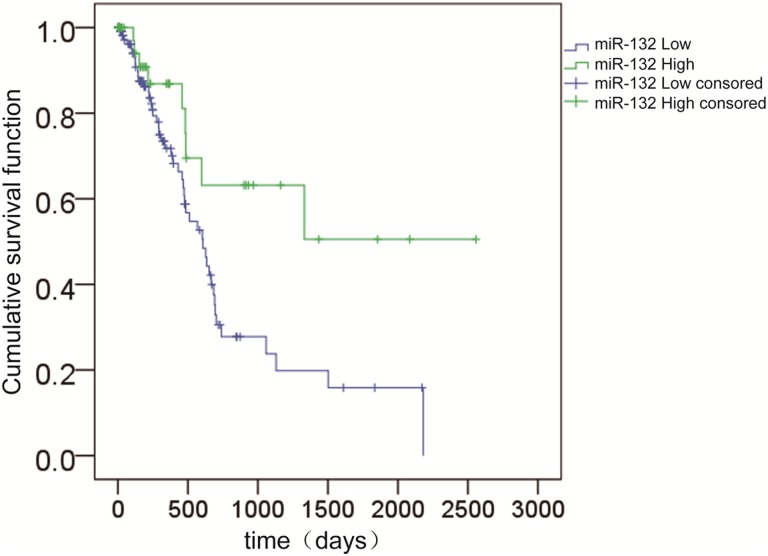
Based on these results, we analyzed the correlation between patient survival and the expression of miR-132. Using the Kaplan-Meier method and log-rank test, the 1-year survival rate of patients with high miR-132 expression was greater than that of patients with low miR-132 expression (Figure 1). To further confirm our results, we downloaded PDAC data from The Cancer Genome Atlas (TCGA) and performed a similar analysis. The results showed that patients with low expression of miR-132 had a lower OS rate (Figure 2).
Figure 1.
Survival curves for 2 groups of patients with pancreatic ductal adenocarcinoma defined by low and high expression of miR-132. Low miR-132 expression is significantly associated with a shorter 1-year survival outcome (P < .028, log-rank test). miR-132 indicates microRNA-132.
Figure 2.
Overall survival curves for 2 groups of patients with pancreatic ductal adenocarcinoma defined by low and high expression of miR-132. Low miR-132 expression is significantly associated with a shorter overall survival (P = .013, log-rank test). miR-132 indicates microRNA-132.
Effects of miR-132 on the Cell Cycle, Apoptosis, and Cell Proliferation in PDAC Cell Lines
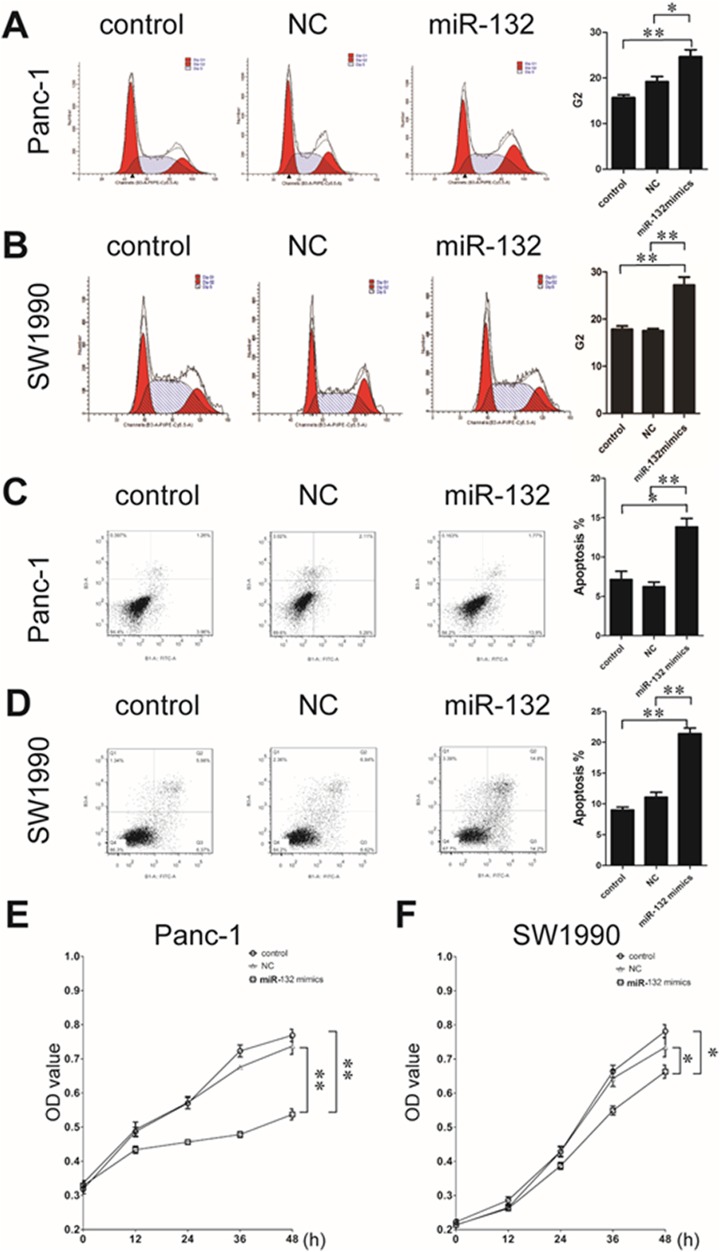
To analyze the specific mechanism by which miR-132 suppressed tumor progression, we used the SW1990 and Panc-1 PDAC cell lines. Cells were transfected with control or miR-132 mimics; cells in the blank group were treated with Lipofectamine 2000 only. Then, we examined the cell cycle and apoptosis by flow cytometry. Both Panc-1 and SW1990 cells transfected with the miR-132 mimic were paused in the G2 period. These cells also exhibited increased apoptosis and attenuated cell proliferation compared to the control and blank cells (Figure 3).
Figure 3.
Cell cycle, apoptosis, and cell proliferation changes after miR-132 upregulation in pancreatic ductal adenocarcinoma cells. SW1990 and Panc-1 cells were transfected with control (NC) and miR-132 mimics. Lipofectamine 2000 alone was used as a blank control group. In both cell lines, increased miR-132 expression results in cells resting in the G2 phase (A, Panc-1; B, SW1990). Upregulated miR-132 expression also increases apoptosis (C, Panc-1; D, SW1990) and reduces cell proliferation (E, Panc-1; F, SW1990) in both cell lines. **P < .01; *P < .05; miR-132 group was compared with the NC group or control group separately. miR-132 indicates microRNA-132.
Biological Changes Induced in PDAC Cells by Upregulating MiR-132 Expression
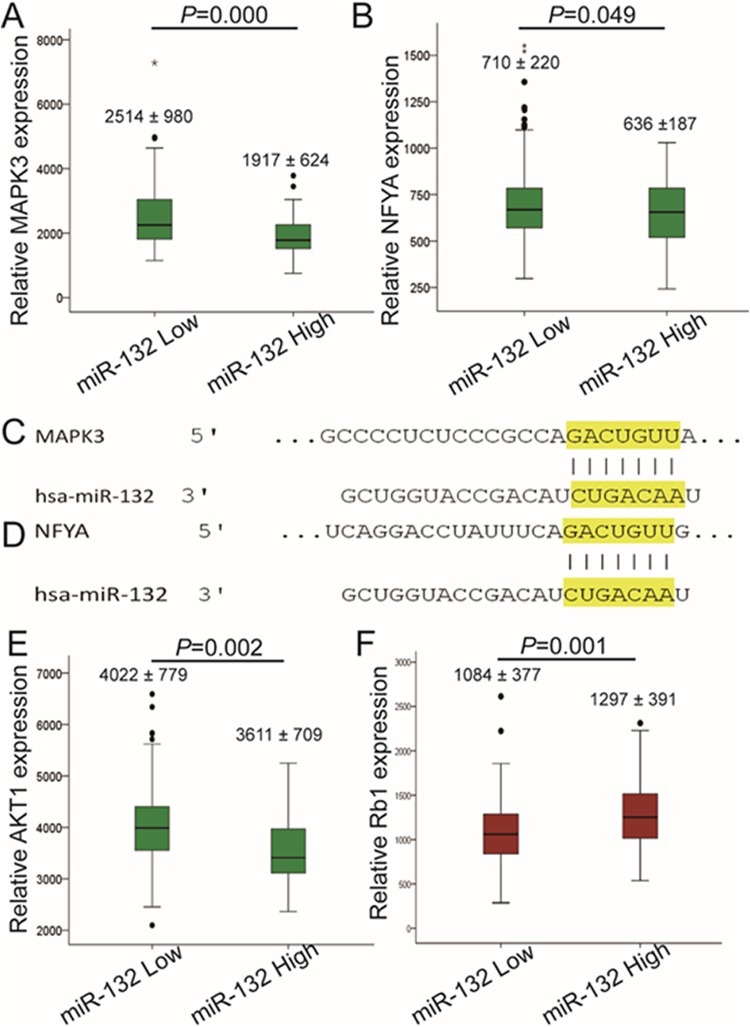
The molecular mechanisms of miR-132 were still unclear. To determine the mechanism, we used PDAC data from TCGA to analyze the correlation between messenger RNA (mRNA) levels of genes that were predicted to be targets of miR-132 by Target Scan, and miR-132 expression. The results showed that samples with high miR-132 expression had significantly lower mitogen-activated protein kinase 3 (MAPK3) and nuclear transcription factor Y subunit α (NFYA) expression (Figure 4A–D). This suggested that miR-132 may exert its effects on cells through downregulating MAPK3 and NFYA. Although AKT1 was not a predicted target gene of miR-132, it also had lower expression in the miR-132 high expression group (Figure 4E). Considering the complicated relationship between AKT1 and MAPK3/NFYA, this finding may provide additional evidence for the effects of miR-132. Furthermore, we also identified one predicted target gene, Rb1, which was also found in a recent study, that had higher expression in the high miR-132 group (Figure 4F).
Figure 4.
Possible mechanisms by which miR-132 attenuates tumor progression. Gene expression differences between miR-132 high and low expression pancreatic ductal adenocarcinoma groups were compared (data from The Cancer Genome Atlas). Mitogen-activated protein kinase 3 (MAPK3) (A) and nuclear transcription factor Y subunit α (NFYA) (B) are expressed at lower levels in the miR-132 high group (MAPK3: miR-132 low, 2514 [980]; miR-132 high, 1917 [624]; NFYA: miR-132 low, 710 [220]; miR-132 high, 636 [187]). Both MAPK3 (C) and NFYA (D) had binding sites for miR-132 (data from Target Scan). The tumor suppressor, Rb1 (E), has higher expression in the miR-132 high group (Rb1: miR-132 low, 1084 [377]; miR-132 high, 1297 [391]). AKT1 (F) is expressed at a lower level in the miR-132 high group (AKT1: miR-132 low, 4022 [779]; miR-132 high, 3611 [709]). miR-132 indicates microRNA-132.
Discussion
The PDAC is a complicated, multifactorial disease, resulting from the mutation of multiple susceptibility genes and the impact of various environmental factors. Diagnosis of PDAC is often made at an advanced or terminal stage, and treatment of PDAC is still a major worldwide problem. This study provided a new understanding of the relationship between miRNA-132 and PDAC. Kaplan-Meier survival curves showed that high expression of miR-132 was a significant prognostic factor for 1-year survival of patients with PDAC (P = .028). Multivariate analysis for OS indicated that high expression of miR-132 was an independent prognostic factor for patients with PDAC (P = .044). In vitro, ectopic expression of miR-132 significantly inhibited the proliferation of PDAC cell lines and promoted apoptosis. Bioinformatic analysis indicated that miR-132 may exert its effects on PDAC by downregulating MAPK3 and NFYA.
The miRNAs are conserved noncoding RNA sequences 18 to 25 nucleotides long that regulate gene expression by interacting with the 3′-untranslated region of target mRNAs, thereby repressing post-transcriptional translation or/and degradation of these mRNAs. There is mounting evidence that deregulation of miRNAs contributes to the occurrence and development of many human malignancies, including PDAC. Recent studies reported that miR-100, miR-125b, and miR-34a are closely related to the development of PDAC.11,12 There have been many previous studies on miR-132 and PDAC, but the results varied. Zhang et al 9 reported that miR-132 was downregulated in pancreatic cancer, and ectopic expression of miR-132 inhibited cell colony formation, suggesting a tumor suppressor role. Park et al 10 reported that overexpression of miR-132 and miR-212 reduced the pRb protein in pancreatic cancer cells and that the increased cell proliferation from overexpression of these miRNAs was likely due to increased expression of several E2F target genes. Zhang et al 13 postulated that miR-132 promoted the proliferation, invasion, and migration of human pancreatic cancer by inhibiting phosphatase and tensin homolog, and could be a tumor oncogene in the development and progression of pancreatic carcinoma. Furthermore, miR-132 might be a candidate prognostic biomarker and promising new target for treating human pancreatic cancer. In our study, we analyzed the expression of miR-132 in 50 pairs of PDAC and para-carcinoma tissue. We found that miR-132 was an independent prognostic factor in patients with PDAC. Specifically, patients with high expression of miR-132 had a higher survival rate. Importantly, this finding was consistent with TCGA data analysis results.
The NFYA is a sequence-specific DNA-binding subunit of the Nfy complex, which forms a histone-like structure binding to DNA and promotes chromatin accessibility.14 The NFYA shows various functions in tumor development.15 For example, NFYA promotes the proliferation of ovarian cancer cells by inducing expression of EZH2.16 It also acts as a transcription factor of SATB1 in endometrial cancer.15 According to bioinformatic analysis, we found a binding site of miR-132 in the 3′-untranslated region of NFYA. The expression of NFYA was negatively correlated with miR-132. Rb1 encodes a nuclear protein whose phosphorylation status is linked closely to the cell cycle. Rb1 gene mutations that inactivate its protein were detected in virtually all retinoblastomas and most small-cell lung carcinomas.17 In our study, Rb1 was one of the predicted target genes for miR-132.
In conclusion, the results of this study indicate that miR-132 could be a tumor suppressor in the development and progression of PDAC. Importantly, miR-132 might be a candidate prognostic biomarker for PDAC.
Abbreviations
- CCK
cell counting kit
- MAPK
mitogen-activated protein kinase
- miRNA-132
microRNA-132
- mRNA
messenger RNA
- NFYA
nuclear transcription factor Y subunit α
- OS
overall survival
- PDAC
pancreatic ductal adenocarcinoma
- RT-PCR
real-time reverse transcription polymerase chain reaction
- TCGA
The Cancer Genome Atlas
Footnotes
Author’s Note: Chen Y, Zhu H. Y., and Wang Y. Q. contributed as co-first author to this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr Zhu Huiyun is supported by Innovative Fund for PhD granted by The Second Military Medical University/Naval Medical University. Dr Du Yiqi is supported by the National Natural Science Foundation of China (grant no. 81770643) and 1255 Research Project of Changhai Hospital.
ORCID iD: Yiqi Du, MD, PhD  https://orcid.org/0000-0003-4580-8700
https://orcid.org/0000-0003-4580-8700
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2. Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350(12):1200–1210. [DOI] [PubMed] [Google Scholar]
- 3. Sultana A, Tudur Smith C, Cunningham D, et al. Systematic review, including meta-analyses, on the management of locally advanced pancreatic cancer using radiation/combined modality therapy. Br J Cancer. 2007;96(8):1183–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Simianu VV, Zyromski NJ, Nakeeb A, Lillemoe KD. Pancreatic cancer: progress made. Acta Oncol. 2010;49(4):407–417. [DOI] [PubMed] [Google Scholar]
- 5. Zhang B, Pan X, Cobb GP, Anderson TA. MicroRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302(1):1–12. [DOI] [PubMed] [Google Scholar]
- 6. Liu X, Yan S, Pei C, Cui Y. Decreased microRNA-132 and its function in human non-small cell lung cancer. Mol Med Rep. 2015;11(5):3601–3608. [DOI] [PubMed] [Google Scholar]
- 7. Wang F, Wang J, Ju L, Chen L, Cai W, Yang J. Diagnostic and prognostic potential of serum miR-132/212 cluster in patients with hepatocellular carcinoma. Ann Clin Biochem. 2018. doi:4563218755815. [DOI] [PubMed] [Google Scholar]
- 8. Shang J, Li J, Keller MP, et al. Induction of miR-132 and miR-212 expression by glucagon-like peptide 1 (GLP-1) in rodent and human pancreatic beta-cells. Mol Endocrinol. 2015;29(9):1243–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang S, Hao J, Xie F, et al. Downregulation of miR-132 by promoter methylation contributes to pancreatic cancer development. Carcinogenesis. 2011;32(8):1183–1189. [DOI] [PubMed] [Google Scholar]
- 10. Park JK, Henry JC, Jiang J, et al. MiR-132 and miR-212 are increased in pancreatic cancer and target the retinoblastoma tumor suppressor. Biochem Biophys Res Commun. 2011;406(4):518–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ottaviani S, Stebbing J, Frampton AE, et al. TGF-beta induces miR-100 and miR-125b but blocks let-7a through LIN28B controlling PDAC progression. Nat Commun. 2018;9(1):1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gibori H, Eliyahu S, Krivitsky A, et al. Amphiphilic nanocarrier-induced modulation of PLK1 and miR-34a leads to improved therapeutic response in pancreatic cancer. Nat Commun. 2018;9(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang H, Liu A, Feng X, et al. MiR-132 promotes the proliferation, invasion and migration of human pancreatic carcinoma by inhibition of the tumor suppressor gene PTEN. Prog Biophys Mol Biol. 2017. (IN PRESS). [DOI] [PubMed] [Google Scholar]
- 14. Lu F, Liu Y, Inoue A, Suzuki T, Zhao K, Zhang Y. Establishing chromatin regulatory landscape during mouse preimplantation development. Cell. 2016;165(6):1375–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pan X, Li D, Huo J, Kong F, Yang H, Ma X. LINC01016 promotes the malignant phenotype of endometrial cancer cells by regulating the miR-302a-3p/miR-3130-3p/NFYA/SATB1 axis. Cell Death Dis. 2018;9(3):303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garipov A, Li H, Bitler BG, Thapa RJ, Balachandran S, Zhang R. NF-YA underlies EZH2 upregulation and is essential for proliferation of human epithelial ovarian cancer cells. Mol Cancer Res. 2013;11(4):360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chang KW, Laconi S, Mangold KA, Hubchak S, Scarpelli DG. Multiple genetic alterations in hamster pancreatic ductal adenocarcinomas. Cancer Res. 1995;55(12):2560–2568. [PubMed] [Google Scholar]