Abstract
Long noncoding RNAs are capable of regulating gene expression at multiple levels. These RNA molecules are also involved in a variety of physiological and pathological processes. Emerging data demonstrate that a series of differentially expressed long noncoding RNAs are implicated in tumorigenesis. In the present study, we used microarray analysis to identify long noncoding RNAs that are dysregulated in non-small-cell lung cancer when compared to normal lung tissues. Accordingly, we performed quantitative real-time polymerase chain reaction to analyze the levels of long noncoding RNA and the cis target gene. We further found the oncogene property of long noncoding RNA that long noncoding RNA downexpression inhibits non-small-cell lung cancer cells proliferation and migration based on 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide and colony formation assays and wound healing as well as transwell assays. The influence of long noncoding RNA on cell cycle of non-small-cell lung cancer cells is also analyzed by flow cytometry. Among the dysregulated long noncoding RNAs, we identified INS-IGF2 readthrough, transcript variant 1, noncoding RNA (NR_003512.3) is upregulated in non-small-cell lung cancer tissues, the cis gene of which is insulin-like growth factor 2 gene hinted by bioinformatics analysis. We also observed that downregulation of INS-IGF2 readthrough, transcript variant 1, noncoding RNA reduces insulin-like growth factor 2 messenger RNA expression. Furthermore, INS-IGF2 readthrough, transcript variant 1, noncoding RNA downregulation suppresses non-small-cell lung cancer cell proliferation and migration. This downregulation results in a concomitant inhibition of the G1/S transition in non-small-cell lung cancer cells. Our findings suggest that INS-IGF2 readthrough, transcript variant 1, noncoding RNA may be an oncogene involved in the development of lung cancer. Therefore, we speculate that INS-IGF2 readthrough, transcript variant 1, noncoding RNA represents a potential therapeutic target for lung cancer.
Keywords: lncRNA, lncINS-IGF2, lung cancer, proliferation, migration
Introduction
Lung cancer, one of the most common malignancies worldwide, has become the leading cause of cancer-associated mortality, with approximately 1.8 million new cases reported annually throughout the world.1 Among all cases with lung cancer, non-small-cell lung cancer (NSCLC) constitutes approximately 85%.2 Despite considerable advances in curative protocols, the prognosis for lung cancer remains unfavorable, with patient 5-year survival rates of approximately 15%.3 With this in mind, it is imperative that we elucidate the mechanisms that underpin lung cancer development. It is also important that we attempt to exploit new diagnostic biomarkers as well as therapeutic strategies for the treatment of lung cancer.
Long noncoding RNAs (lncRNAs) are evolutionarily conserved transcripts consisting of more than 200 nucleotides (per transcript). These RNA molecules do not have the capacity to encode for proteins. Accumulating evidence has demonstrated that lncRNAs are involved in a wide range of biological functions, including cell-cycle progression, cell growth, cell proliferation, and apoptosis.4 Therefore, dysregulation of lncRNAs plays a pivotal role in a variety of human diseases, especially cancer, which are associated with the development, invasion, and metastasis of tumors.5 For instance, the upregulation of the lncRNA HOTAIR contributes to the metastasis of breast cancer cells following the transcriptional modulation of genes involved in cell development;6 the metastasis-associated lung adenocarcinoma transcript-1 (MALAT1) lncRNA is highly expressed in multiple types of cancers including lung adenocarcinoma and hepatocellular carcinomas. The latter transcript is also involved in the promotion of proliferation and metastasis of cancer cells, suggesting concomitant involvement in oncogenic effects.7 Undoubtedly, aberrant lncRNA expression also plays a regulatory role in lung cancer. It is widely reported that 2420 lncRNAs are differentially expressed between lung adenocarcinoma samples and normal lung tissue, suggesting that lncRNAs may be novel molecular biomarkers for diagnosing lung adenocarcinoma.8 At present, several lung cancer-related lncRNAs including MALAT1, ANRIL, H19, and HOTAIR,9 lncRNA GAS6-AS1 (GAS6 antisense RNA1),10 and lncRNA AK126698 have been identified.11 Further studies are required to identify additional lncRNAs implicated in lung cancer and their associated mechanisms of action.
In the present study, we analyzed the expression patterns of lncRNAs in lung cancer tissues and the corresponding patterns in adjacent nontumorous tissue by microarray analysis. Among the differentially expressed lncRNAs, we found that INS-IGF2 readthrough (INS-IGF2), transcript variant 1, noncoding RNA (lncINS-IGF2; NR_003512.3) was obviously elevated in lung cancer tissues compared to normal tissues, and the role of lncINS-IGF2 is unclear. Bioinformatics analysis further showed that the cis gene of lncINS-IGF2 was insulin-like growth factor 2 (IGF2) gene. The IGF2, a mitogenic peptide hormone, plays a regulatory role in cell growth, differentiation, and metabolism. Previous studies have shown that this hormone is abnormally expressed in various human malignancies, and overexpressed IGF2 is closely associated with poor prognosis in cancer.12–14 Collectively, we speculated that lncINS-IGF2 might potentially be important in lung cancer development. Next, we utilized small-interfering RNA (siRNA)-mediated silencing in NSCLC cells to determine the relationships between lncINS-IGF2 and IGF2 expression and to investigate the impact of lncINS-IGF2 on cell proliferation and metastasis of lung cancer cells. Our observations are likely to provide a useful platform for future investigations into the mechanisms that underpin lung cancer.
Materials and Methods
Patients and Specimens
The lung cancer samples and matched adjacent normal lung tissue samples were gathered from the Affiliated Hospital of Guangdong Medical University, China, between April 2012 and August 2013. None of the patients were subjected to preoperative chemotherapy and/or radiotherapy, and all patients were diagnosed with NSCLC according to histopathology. Of these samples, 3 were selected for lncRNA microarray analysis using an SBC-lncRNA (human 4 × 180 k) chip from Shanghai Biotechnology Co, Ltd (Shanghai, China). All of the extracted samples were immediately snap-frozen in liquid nitrogen after resection. This work was approved by the Ethics Committee of the Affiliated Hospital of Guangdong Medical University, and all patients provided written informed consent prior to study initiation.
Microarray Analysis
Three pairs of matched samples were used for microarray analysis. RNA was purified from total RNA after removal of ribosomal RNA (mRNA-ONLY eukaryotic messenger RNA [mRNA] isolation kit, Epicentre, Madison, Wisconsin). Then, each sample was transcribed into fluorescent complementary RNA (cRNA) along the entire length of the transcripts without 3′ bias utilizing a random priming method. The labeled cRNAs were hybridized onto the human lncRNA array v2.0 (8 × 60 k, Arraystar).
Cell Culture and Transfection
The human lung cancer cell line (A549 and H1299) was obtained from the Shanghai Cell Institute Country Cell Bank (Shanghai, China). All cell lines were cultured in RPMI-1640 medium with 10% fetal bovine serum (Gibco/BRL, Maryland) and grown at 37°C in a humidified atmosphere containing 5% CO2. Cell transfection was performed with Lipofectamine 2000 reagent (Invitrogen, California) according to the manufacturer’s instructions. The siRNA against lncINS-IGF2 and negative control siRNA were synthesized by Shanghai GenePharma Co Ltd (Shanghai, China). Oligonucleotides including negative control siRNA and lncINS-IGF2 siRNA were synthesized by GenePharma.
RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction
Total RNA was extracted from cultured cell lines using Trizol reagent (Invitrogen) according to the manufacturer’s protocol. A total of 2 μg of total RNA were subsequently reverse transcribed using a PrimeScript real-time (RT) reagent kit with genomic DNA Eraser (Takara, Otsu, Shiga, Japan); a final volume of 20 μL was used to synthesize cDNA according to the manufacturer’s protocol. Real-time quantitative polymerase chain reaction (qRT-PCR) was then conducted using the SYBR Premix Ex Taq II PCR kit (Takara) following the manufacturer’s instrument. The levels of lncINS-IGF2 transcript were measured using the following forward/reverse primer pair: 5′-CCAGCAATCGGAAGTGAGC-3′ and 5′-GGACGTGGAACCGAGAGATT-3′. The levels of IGF2 transcript were measured using the following forward/reverse primer pair: 5′-ATGCTGGTGCTTCTCACCTT-3′ and 5′-ACAGACGAACTGGAGGGTGT-3′. The human GAPDH gene was utilized as an endogenous control and amplified with the following forward/reverse primer pair: 5′-GACCTGACCTGCCGTCTA-3′ and 5′-AGGAGTGGGTGTCGCTGT-3′. Results were normalized to the content of GAPDH. All experiments were performed in triplicate. The gene expression fold-change values are represented using the 2−ΔΔCt method.
Cell Proliferation Assay
Cell proliferation was measured using 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assays following the manufacturer’s instructions. In brief, 5000 cells were plated into each well of a 96-well plate. The MTT reagent was prepared at 5 mg/mL in phosphate-buffered saline (PBS). The MTT stock solution (0.5 mg/mL) was then added to each well. Cells were cultured for an additional 4 hours, and dimethyl sulfoxide was subsequently added to dissolve the resultant crystals before reading the absorbance at a wavelength of 490 nm in a plate reader. All independent experiments were performed 3 times.
Colony Formation Assay
Five hundred cells were plated into each well in 6-well plates in triplicate. After 14 days of incubation, cells were washed with PBS, fixed with 4% paraformaldehyde, and subsequently stained using 0.1% Crystal violet (Sigma, St Louis). Eventually, the number of effective colonies was counted. Colonies consisting of more than 50 cells were defined as effective colonies.
Cell Cycle Analysis
Cells were plated onto a 6-well plate at a density of 5 × 105 cells/well and grown for 24 hours. The cells were then starved with serum-free culture medium for 24 hours to synchronize them at the G1/S boundary, followed by transfection. After 48 hours, the cells were collected, washed twice with ice-cold PBS, and fixed with 70% ice-cold ethanol at 4°C overnight. After rehydration in PBS for 15 minutes, the cells were stained for 30 minutes in the dark with propidium iodide solution and then analyzed by flow cytometry (BD Biosciences, New York, USA).
Wound-Healing Assays
Cells were seeded in 6-well plates at an initial density of 2 × 105 cells/well and grown to about 80% to 90% confluence. A vertical wound was created by scratching the monolayer with a sterile 200-μL pipette tip, and the cells were then washed 3 times with PBS to remove the floating cells. The monolayer was subsequently incubated in serum-free medium. At 0, 24, 48, or 72 hours following wound induction, photographs were taken with a microscope at 200× magnification (Nikon, Japan) at the same location in each well to monitor cell migration into the wounded area.
Transwell Assay
Cell migration was evaluated using 8-μm pore size polycarbonate filter transwell inserts on the upper chamber with noncoated membranes (Corning, New York, USA). After siRNA transfection for 24 hours, the cells were digested with trypsin and then 5 × 104 transfected cells were diluted with serum-free culture medium. The cells were plated into the upper chamber (to which 200 μL of Dulbecco’s modified Eagle’s medium without fetal bovine serum was added). The bottom chamber was supplemented with 600 μL of 10% fetal bovine serum medium. After incubating for 24 hours, the nonmigrated cells in the upper chamber were removed with a cotton swab, and the migrated cells in the lower chamber were fixed with 4% paraformaldehyde. All cells were stained using 0.1% Crystal violet (Sigma), and the cell numbers in each chamber were counted in 5 random fields under the phase-contrast microscope. The migrated cells were visualized by photographing at 200× magnification using an Leica DMI3000B microscope. Each experiment was repeated 3 times.
Statistical Analysis
Data were expressed as the mean (standard deviation). The significance of intergroup differences was evaluated using 1-way analysis of variance. Differences were considered significant at P < .05.
Results
lncINS-IGF2 is Upregulated in NSCLC Tissues
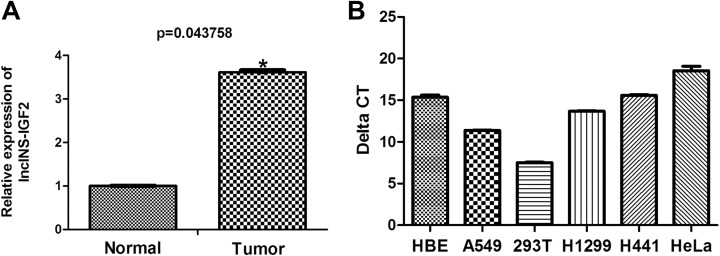
To explore the potential biological roles of lncRNAs in lung cancer, we examined lncRNA expression profiles in human lung cancer tissues using microarray analysis. We have primarily found the differential expression of several lncRNAs including lncINS-IGF2 (data not published). As illustrated in Figure 1A, the level of lncINS-IGF2 expression was approximately 4 times in lung cancer tissues more than nontumorous tissue, suggesting that lncINS-IGF2 expression may correlate with malignancy in lung cancer. Subsequently, we also measured the background expression of lncINS-IGF2 in HBE cells, 293T cells, HeLa cells as well as lung cancer cell lines including A549, H1299, and H441. We found that there existed background expression of lncINS-IGF2 in all 3 NSCLC cell lines: HBE cells, 293T as well as HeLa cells (Figure 1B). In addition, we selected A549 and H1299 cell lines for further study to determine whether lncINS-IGF2 expression may correlate with malignant behavior in lung cancer.
Figure 1.
Relative expression levels of lncINS-IGF2 in lung cancer tissues and cell lines. A, lncINS-IGF2 expression was assessed following microarray analysis of lung cancer tumor specimens (n = 3) and normal adjacent lung tissue (n = 3). B, The expression of lncINS-IGF2 was measured by qRT-PCR in A549, H1299, and H441 lung cancer cell lines, HBE cells, 293T cells, and HeLa cells. *P < .05 versus normal group. Results are expressed as the mean (SD) of the samples and each analysis was performed in triplicate. qRT-PCR indicates real-time quantitative polymerase chain reaction; SD, standard deviation.
The Expression of IGF2 Was Reduced After lncINS-IGF2 Downregulation in NSCLC Cells
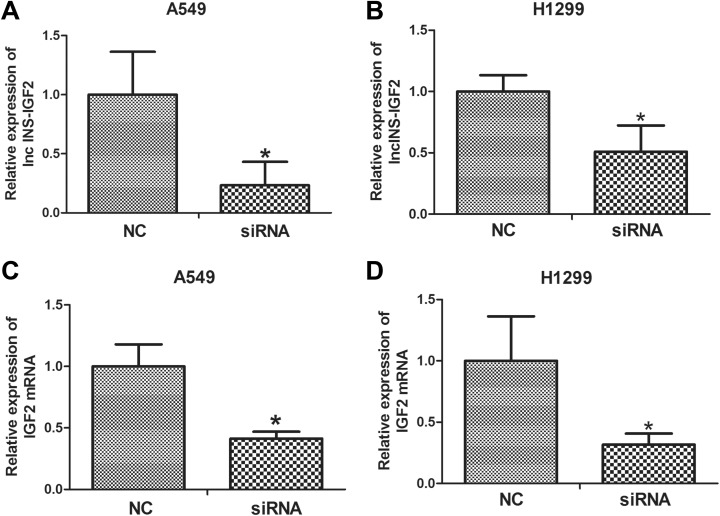
First, to clarify the relationship between the expression of IGF2 and lncINS-IGF2, RNA interference analysis was conducted in A549 as well as H1299 cells. The lncINS-IGF2 siRNA was transiently transfected into NSCLC cells to facilitate a reduction in the expression of lncINS-IGF2. The knockdown efficiency was verified by qRT-PCR assay, and the results revealed that lncINS-IGF2 expression was significantly reduced following transfection in A549 cells (Figure 2A) and H1299 cells (Figure 2B). The levels of IGF2 mRNA in these 2 cell lines were further detected. Our results showed that downregulation of lncINS-IGF2 obviously reduced IGF2 mRNA levels (Figure 2C and D) in NSCLC cells, indicating that lncINS-IGF2 has positive regulation effect on IGF2 mRNA expression levels in NSCLC cells.
Figure 2.
lncINS-IGF2 knockdown results in downregulation of IGF2 in NSCLC cells. A549 and H1299 cells were transfected with control siRNA or lncINS-IGF2 siRNA for 48 hours. The cells were subsequently harvested to assess knockdown effects (A and B). A549 and H1299 cells were transfected with NC and siRNA of lncINS-IGF2. IGF2 mRNA levels were measured by qRT-PCR assay (C and D). *P < .05 versus NC. Data are expressed as the mean (SD) and each experiment was performed in triplicate. IGF2, insulin-like growth factor 2; NSCLC, non-small-cell lung cancer; siRNA, small interfering RNA; qRT-PCR, real-time quantitative polymerase chain reaction; NC, normal control; SD, standard deviation.
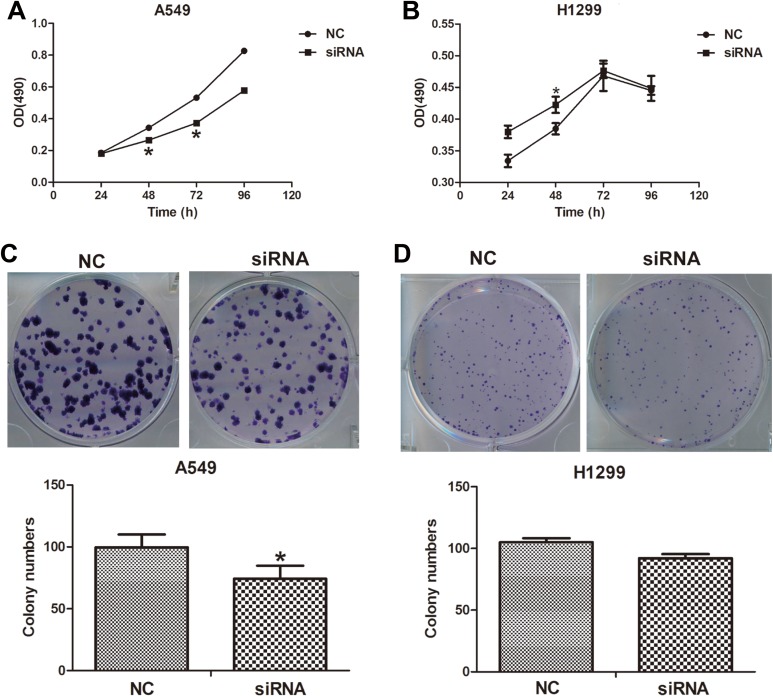
Downregulation of lncINS-IGF2 Inhibits Cell Proliferation and Colony Formation of NSCLC Cells
To further explore the role of lncINS-IGF2 in lung cancer, we subsequently explored the impact of lncINS-IGF2 knockdown on the proliferation of NSCLC cells. As illustrated in Figure 3A, proliferation of lncINS-IGF2 siRNA-transfected A549 and H1299 cells were obviously repressed upon comparison with the control groups (Figure 3A and B). Additionally, we also studied the effect of lncINS-IGF2 downregulation on colony formation in NSCLC cells. Our results demonstrated that the number of colonies was decreased in siRNA lncINS-IGF2-transfected A549 cells (Figure 3C) and was tendency to reduce in lncINS-IGF2-downregulated H1299 cells (Figure 4D). These data suggest that knockdown of lncINS-IGF2 inhibits cell proliferation of NSCLC cells.
Figure 3.
lncINS-IGF2 knockdown inhibits cell proliferation and colony formation in NSCLC cells. A549 and H1299 cells transfected with control siRNA or lncINS-IGF2 siRNA were trypsinized and plated into 96-well plates for the MTT assay. The asterisk shows the significant differences on the proliferation rate between 2 groups (A and B). Colony formation of A549 and H1299 cells at 14 days after transfection with the indicated oligonucleotides (C and D). *P < .05 versus NC. Data are expressed as the mean (SD) and each experiment was performed in triplicate. NSCLC indicates non-small-cell lung cancer; siRNA, small interfering RNA; MTT, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide; NC, normal control; SD, standard deviation.
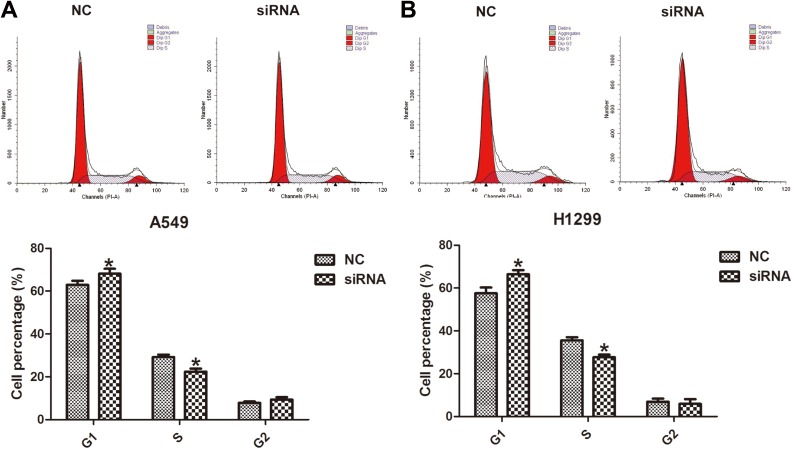
Figure 4.
Downregulation of lncINS-IGF2 retards G1/S transition in NSCLC cells. A549 and H1299 cells were transfected with control siRNA or lncINS-IGF2 siRNA for 48 hours. Cell populations at the G1, S, and G2/M phases were detected by flow cytometry. *P < .05 versus NC. Data are expressed as the mean (SD) and the experiments were performed in triplicate. NSCLC indicates non-small-cell lung cancer; siRNA, small interfering RNA; NC, normal control; SD, standard deviation.
Downregulation of lncINS-IGF2 Inhibited G1/S Transition of NSCLC Cells
To investigate the possible mechanisms underpinning lncINS-IGF2-induced inhibition of cell proliferation in NSCLC cells, we explored the effect of lncINS-IGF2 knockdown on the cell cycle of lung cancer cells by flow cytometry. As shown in Figure 4A, knockdown of lncINS-IGF2 resulted in an increase in the proportion of A549 cells in the G1 phase with a concomitant reduction in the proportion of A549 cells in the S phase. The similar results were observed in H1299 cells (Figure 4B). These observations suggested that lncINS-IGF2 knockdown may block G1/S transition.
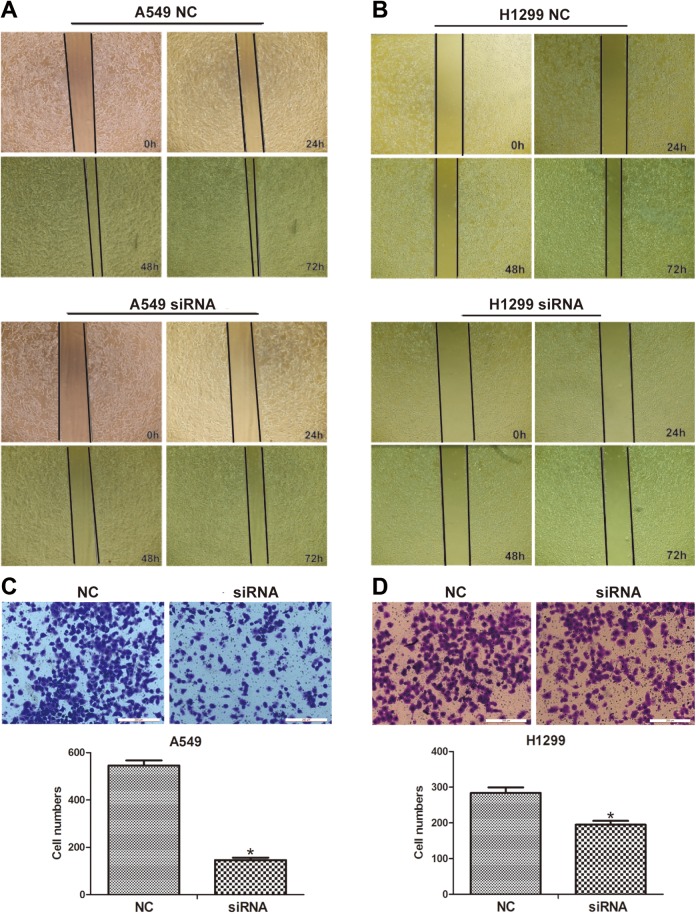
lncINS-IGF2 siRNA Inhibits Cell Migration of NSCLC Cells
One of the features of metastasis, the main cause of death among patients diagnosed with cancer, is the conversion to a malignant cell phenotype.15 Thus, we also explored the effect of lncINS-IGF2 depletion on metastasis of NSCLC cells using wound-healing and transwell assays following transfection with lncINS-IGF2-specific siRNA. We observed that lncINS-IGF2 downregulation leads to an increase in the area of wound healing (Figure 5A) and a decrease in the number of migratory A549 cells (Figure 5C). The depressed migration ability was also presented in lncINS-IGF2 siRNA-transfected H1299 cells (Figure 5B and D). These data suggest that depletion of lncINS-IGF2 inhibits the migration capacity of NSCLC cells.
Figure 5.
lncINS-IGF2 knockdown induces inhibition of cell migration in NSCLC cells. A, B, The migration capacity of A549 and H1299 cells was determined using a wound-healing assay. Photographs were taken at ×100 magnification at 0, 24, 48, and 72 hours after wounding; the photographs were taken at the same location in each well. C, D, Representative images of the migration transwell assay; the number of migrated cells was randomly measured by photograph at ×200 magnification in 5 different fields for each chamber. *P < .05 versus NC. Data are defined as mean (SD) following 3 independent experiments. NSCLC indicates non-small-cell lung cancer; NC, normal control; SD, standard deviation.
Discussion
Lung cancer is the most common cause of cancer-associated mortalities around the world, accounting for more than 25% of the total number of cases with cancer.16 The development of effective therapeutics for the treatment of lung cancer remains a challenge due to properties including uncontrollable proliferation, metastasis, invasion, and defects the apoptotic pathways of cancer cells.17 It is well known that one of the main reasons for poor therapeutic efficacy in lung cancer is late-stage diagnosis. Therefore, the identification of higher specificity and sensitivity biomarkers would be extremely useful in this regard.
The lncRNA molecules are usually more than 200 nt long and frequently range up to 100 kb.18 It is well known that lncRNAs are involved in a variety of cellular processes including proliferation,19 apoptosis,20 cell cycle,21 survival,22 and migration.6 An ever-increasing number of studies have demonstrated that aberrant lncRNAs play crucial roles in many complicated human diseases including cancer.7–24 Furthermore, dysregulated lncRNAs that have been observed in cancer always function as oncogenes or tumor suppressor genes.25,26 However, the mechanism of action, biological activity, and associated signaling pathways are yet to be elucidated for most lncRNAs.
In the current study, we observed that lncINS-IGF2 was upregulated in NSCLC tissues. In addition, our results demonstrate that downregulation of lncINS-IGF2 following siRNA transfection decreases IGF2 mRNA levels and inhibits cell proliferation as well as migration; this results in the concomitant inhibition of the G1/S transition. First, we analyzed the expression profiles of lncRNA in lung cancer tissues in order to characterize the potential biological function of lncRNAs in the pathogenesis of lung cancer. The results of gene chip analyses revealed a group of differentially expressed lncINS-IGF2. As part of this analysis, results generated from gene chip analyses revealed that lncINS-IGF2 is overexpressed in NSCLC tissues compared to adjacent normal tissues. These observations suggest that lncINS-IGF2 might play a role in tumorigenesis. In order to better understand the biological role of lncINS-IGF2 in NSCLC cell lines, we used siRNA-mediated knockdown of lncINS-IGF2 following transient transfection in A549 and H1299 cells. Interestingly, bioinformatics analysis further showed that the cis gene regulated by lncINS-IGF2 was IGF2 gene. Insulin-like growth factor 2 (IGF2) is a mitogenic peptide hormone that is expressed in many tissues including lung tissue.27,28 The IGF2 plays a regulatory role in cell growth, differentiation, and metabolism.29 Accumulating evidence suggests that abnormalities in IGF2 expression occur during cancer development and progression.30 In esophageal cancer, increased IGF2 expression is closely associated with decreased disease-free survival.31 Furthermore, it is reported that IGF2 downregulation decreases survival and adhesion of colorectal cancer cells.32 Interestingly, IGF2 gene was identified as the cis gene of lncINS-IGF2 revealed by the bioinformatics analysis. Therefore, we first explored the association between the expression of IGF2 and lncINS-IGF2 by determining the impact of lncINS-IGF2 silencing on IGF2 mRNA expression. We observed that knockdown of lncINS-IGF2 resulted in a concomitant reduction in the levels of IGF2 mRNA. These data suggest that lncINS-IGF2 positively regulates IGF2 expression in NSCLC cells, which might be associated with its modulation on the flanking sequence such as the promoter of IGF2 gene expression that affects gene expression. However, the precise mechanism underpinning lncINS-IGF2-mediated modulation of IGF2 remains unclear and requires further study.
To further investigate the function of lncINS-IGF2 on the proliferation of NSCLC cells, an MTT assay and a colony formation assay subsequently revealed that the downregulation of lncINS-IGF2 markedly contributes to the inhibition of cell proliferation in A549 cells. Dysregulation of the cell cycle is a common occurrence during tumorigenesis. It is well known that defective cell cycle progression plays a significant role in cancer development.33,34 Therefore, we investigated whether the impact of lncINS-IGF2 knockdown on NSCLC cells proliferation was mediated through regulation of the cell cycle. Our results revealed that lncINS-IGF2 knockdown retarded cell cycle as evidenced by an increased proportion of NSCLC cells in the G1 phase. Conversely, we observed a reduction in the proportion of NSCLC cells in the S phase after lncINS-IGF2 silencing. These data revealed that this reduction in lncINS-IGF2 expression results in dysregulation of the cell cycle. Thus, we speculate that the effect of lncINS-IGF2 knockdown on NSCLC cells proliferation might be caused by lncINS-IGF2 knockdown-caused dysregulation of the cell cycle. Metastasis, one of the properties that defines the malignant cell phenotype, is the main cause of death in patients diagnosed with cancer.15 In this report, wound-healing and transwell assay analyses revealed that the downregulation of lncINS-IGF2 obviously inhibited the migration of NSCLC cells. Collectively, these results indicate that lncINS-IGF2 promotes cell proliferation and migration of NSCLC cells.
In conclusion, we observed upregulation of lncINS-IGF2 in NSCLC tissues and found that downregulation of lncINS-IGF2 decreases IGF2 mRNA expression and inhibits cell proliferation and metastasis of NSCLC cells. According to these findings, we propose that lncINS-IGF2 and IGF2 may represent important markers or targets in relation to NSCLC. However, more detail studies are required to further investigate the mechanisms underlying lncINS-IGF2-modulated IGF2 gene transcription as well as the potential importance of the interaction between these 2 factors in the development of lung cancer.
Abbreviations
- cRNA
complementary RNA
- IGF2
insulin-like growth factor 2
- lncRNAs
long noncoding RNAs
- lncINS-IGF2
INS-IGF2 readthrough (INS-IGF2), transcript variant 1, noncoding RNA
- MALAT1
metastasis-associated lung adenocarcinoma transcript-1
- MTT
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide
- mRNA
messenger RNA
- NSCLC
non-small-cell lung cancer
- PBS
phosphate-buffered saline
- qRT-PCR
real-time quantitative polymerase chain reaction
- siRNA
small interfering RNA
Footnotes
Authors’ Note: Shenglan Gao, MS, Ziying Lin, MS, and Chunyan Li, MS, contributed equally to this work.
Ethics Statements: This study was approved by the Affiliated Hospital of Guangdong Medical College Ethics Committee (No: PJ2012132).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Natural Science Foundation of China (grant nos. NSFC81570062, 81600049, and NSFC81172615), Guangdong Natural Science Foundation (grant no. 2016A030313681), and Guangdong medical University Scientific Research Fund (grant nos. M2016001, M2016007, and M2016022). Guangdong medical University Scientific Research Fund (GDMUM201801).
ORCID iD: Gang Liu, PhD  https://orcid.org/0000-0002-4536-8453
https://orcid.org/0000-0002-4536-8453
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. Cancer J Clin. 2014;64(1):9–29. [DOI] [PubMed] [Google Scholar]
- 2. Seve P, Reiman T, Dumontet C. The role of betaIII tubulin in predicting chemoresistance in non-small cell lung cancer. Lung Cancer. 2010;67(2):136–143. [DOI] [PubMed] [Google Scholar]
- 3. Raungrut P, Wongkotsila A, Lirdprapamongkol K, et al. Prognostic significance of 14-3-3gamma overexpression in advanced non-small cell lung cancer. Asian Pac J Cancer Prev. 2014;15(8):3513–3518. [DOI] [PubMed] [Google Scholar]
- 4. Gutschner T, Diederichs S. The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol. 2012;9(6):703–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kazemzadeh M, Safaralizadeh R, Orang AV. LncRNAs: emerging players in gene regulation and disease pathogenesis. J Genet. 2015;94(4):771–784. [DOI] [PubMed] [Google Scholar]
- 6. Gupta RA, Shah N, Wang KC, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464(7291):1071–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21(11):1253–1261. [DOI] [PubMed] [Google Scholar]
- 8. Xu G, Chen J, Pan Q, et al. Long noncoding RNA expression profiles of lung adenocarcinoma ascertained by microarray analysis. PLoS One. 2014;9(8):e104044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Mol Cancer. 2011;10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Han L, Kong R, Yin DD, et al. Low expression of long noncoding RNA GAS6-AS1 predicts a poor prognosis in patients with NSCLC. Med Oncol. 2013;30(4):694. [DOI] [PubMed] [Google Scholar]
- 11. Yang Y, Li H, Hou S, et al. The noncoding RNA expression profile and the effect of lncRNA AK126698 on cisplatin resistance in non-small-cell lung cancer cell. PLoS One. 2013;8(5):e65309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bergman D, Halje M, Nordin M, et al. Insulin-like growth factor 2 in development and disease: a mini-review. Gerontology. 2013;59(3):240–249. [DOI] [PubMed] [Google Scholar]
- 13. Ribarska T, Bastian KM, Koch A, et al. Specific changes in the expression of imprinted genes in prostate cancer – implications for cancer progression and epigenetic regulation. Asian J Androl. 2012;14(3):436–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dong Y, Li J, Han F, et al. High IGF2 expression is associated with poor clinical outcome in human ovarian cancer. Oncol Rep. 2015;34(2):936–942. [DOI] [PubMed] [Google Scholar]
- 15. Yu M, Bardia A, Wittner BS, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339(6119):580–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. Cancer J Clin. 2016;66(1):10–29. [DOI] [PubMed] [Google Scholar]
- 17. Fu X, Li H, Liu C, et al. Long noncoding RNA AK126698 inhibits proliferation and migration of non-small cell lung cancer cells by targeting Frizzled-8 and suppressing Wnt/beta-catenin signaling pathway. Onco Targets Ther. 2016;9:3815–3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10(3):155–159. [DOI] [PubMed] [Google Scholar]
- 19. Zhao X, Liu M, Zhang J, et al. Long noncoding RNA CPS1-IT1 suppresses cell proliferation and metastasis in human lung cancer. Oncol Res. 2016;25(3):373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. She K, Huang J, Zhou H, et al. lncRNA-SNHG7 promotes the proliferation, migration and invasion and inhibits apoptosis of lung cancer cells by enhancing the FAIM2 expression. Oncol Rep. 2016;36(5):2673–2680. [DOI] [PubMed] [Google Scholar]
- 21. Meola N, Pizzo M, Alfano G, et al. The long noncoding RNA Vax2os1 controls the cell cycle progression of photoreceptor progenitors in the mouse retina. RNA. 2012;18(1):111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hung T, Wang Y, Lin MF, et al. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat Genet. 2011;43(7):621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Niland CN, Merry CR, Khalil AM. Emerging roles for long non-coding RNAs in cancer and neurological disorders. Front Genet. 2012;3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang H, Chen Z, Wang X, et al. Long non-coding RNA: a new player in cancer. J Hematol Oncol. 2013;6:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qi P, Du X. The long non-coding RNAs, a new cancer diagnostic and therapeutic gold mine. Mod Pathol. 2013;26(2):155–165. [DOI] [PubMed] [Google Scholar]
- 26. Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Discov. 2011;1(5):391–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Livingstone C. IGF2 and cancer. Endocr Relat Cancer. 2013;20(6):R321–R339. [DOI] [PubMed] [Google Scholar]
- 28. Boo HJ, Min HY, Jang HJ, et al. The tobacco-specific carcinogen-operated calcium channel promotes lung tumorigenesis via IGF2 exocytosis in lung epithelial cells. Nat Commun. 2016;7:12961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. O’Dell SD, Day IN. Insulin-like growth factor II (IGF-II). Int J Biochem Cell Biol. 1998;30(7):767–771. [DOI] [PubMed] [Google Scholar]
- 30. Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst. 2000;92(18):1472–1489. [DOI] [PubMed] [Google Scholar]
- 31. Zhao R, DeCoteau JF, Geyer CR, et al. Loss of imprinting of the insulin-like growth factor II (IGF2) gene in esophageal normal and adenocarcinoma tissues. Carcinogenesis. 2009;30(12):2117–2122. [DOI] [PubMed] [Google Scholar]
- 32. Rogers MA, Kalter V, Strowitzki M, et al. IGF2 knockdown in two colorectal cancer cell lines decreases survival, adhesion and modulates survival-associated genes. Tumour Biol. 2016;37(9):12485–12495. [DOI] [PubMed] [Google Scholar]
- 33. Curtin NJ. DNA repair dysregulation from cancer driver to therapeutic target. Nat Rev Cancer. 2012;12(12):801–817. [DOI] [PubMed] [Google Scholar]
- 34. Visconti R, Della Monica R, Grieco D. Cell cycle checkpoint in cancer: a therapeutically targetable double-edged sword. J Exp Clin Cancer Res. 2016;35(1):153. [DOI] [PMC free article] [PubMed] [Google Scholar]