Abstract
OBJECTIVE:
To assess the economic effect and cost effectiveness of a targeted catheter-associated urinary tract infection (CAUTI) prevention intervention in the nursing home (NH) setting.
DESIGN:
Randomized clinical trial.
SETTING:
Community-based NHs (N=12).
PARTICIPANTS:
NH residents with indwelling urinary catheters (N=418).
INTERVENTION:
Standard care versus infection prevention program involving barrier precautions, active surveillance, and NH staff education.
MEASUREMENTS:
Costs of the intervention, costs of disease, and health outcomes were used to calculate an incremental cost-effectiveness ratio for the intervention. Data came from intervention results and the literature and outcomes were analyzed over one year.
RESULTS:
A 120-bed NH would have program costs of $20,279/year. The cost of disease treatment would be reduced by $54,316 per year, resulting in a $34,037 net cost savings. Most of this savings would come from fewer CAUTI hospitalizations ($39,180), with $15,136 in savings from CAUTI care within the NH. The intervention also yielded a gain of 0.197 quality-adjusted life-years (QALYs). Taking into account uncertainty in all parameters suggests there is an 85% chance that the intervention is cost-saving.
CONCLUSIONS:
The CAUTI prevention program is expected to benefit payers by reducing costs and improving health outcomes. Because the savings accrue to payers and not to NHs, payers such as Medicare and private insurers may want to provide incentives for NHs to implement such programs.
Keywords: cost-effectiveness, drug resistance, multiple, catheter-related infections, nursing homes
At any given time, there are more people in nursing homes (NHs) in the United States than in hospitals, making NHs a crucial part of the healthcare system.1 Because NHs have a predominantly frail older population with a high acuity of illness, nosocomial infections are common and often severe in NHs, resulting in frequent hospital transfers and an estimated $2 billion in hospital expenditures per year.2
NH residents at risk of infection share several characteristics, including indwelling devices, prior antimicrobial usage, recent hospitalization, and functional impairment.3,4 Recent national data show that approximately 12% to 15% of individuals newly admitted to a NH have an indwelling urinary catheter and that 5% to 10% have a chronic urinary catheter.5,6 Thus, on any given day, 80,000 to 150,000 of the 1.5 million NH residents have an indwelling urinary catheter.7 Unlike acute care settings with shorter lengths of stay, even appropriately placed urinary catheters in NH residents often remain in place for prolonged periods, with an average duration of 105 days.8 These catheters are responsible for the largest institutional reservoir of multidrug-resistant organisms (MDROs), and their presence doubles the risk of symptomatic infections.3,9 More than half of episodes of fever in individuals with chronic indwelling urinary catheters are from a urinary source, with an incidence of approximately 1 per 100 catheterized resident-days.3 Some of these catheter-associated urinary tract infections (CAUTIs) will lead to bacteremia, sepsis, and death.3,4 The Centers for Medicare and Medicaid Services (CMS) recent reform of the requirements for nursing facilities requires that facilities have an infection prevention and control program and designate an infection preventionist. The estimated cost of this infection control preventionist is expected to be $19,032 per facility per year.10 CMS does not have an estimate of the benefits of this type of program.
In a recent interventional study, we evaluated the effectiveness of a 3-year targeted infection prevention (TIP) multimodal intervention program in reducing MDRO prevalence and device-associated infections in a group of 12 NHs in southeast Michigan.8 The intervention included a structured interactive educational program for NH staff, hand hygiene promotion, preemptive barrier precautions when assisting with high-risk activities of daily living, and active surveillance for MDROs and infections, with an infection preventionist supporting monthly data feedback. This intervention led to a 23% reduction in overall MDRO prevalence density rate and a 31% reduction in all clinically diagnosed CAUTIs.8 In addition, we documented 30% fewer hospitalizations of residents with CAUTIs.8 To further enhance the usefulness of our findings, we sought to determine whether the targeted intervention was cost effective for an average NH. Previous studies reporting on the effectiveness of CAUTI reduction interventions in hospital settings have showed cost reductions and significant decreases in morbidity,11–13 but few studies have included cost-effectiveness analyses to make a business case to devote resources to infection-prevention programs in NHs and other long-term care settings.14–16 Our primary aim was to examine the economic effect of our intervention program.
METHODS
We used a cost-effectiveness analysis framework. Our goal was to determine whether the benefits from the TIP intervention program exceeded the costs and, if not, how much was being paid per unit of health outcome gained. This retrospective analysis of the program was conducted to assist NH administrators and policy-makers in evaluating its success and to facilitate future implementation. This TIP study is a multicenter randomized clinical trial evaluating a multimodal, practical, evidence-based program to reduce CAUTIs in nursing homes.8 This cluster-randomized study, which the University of Michigan institutional review board approved, was conducted in 12 (6 control, 6 intervention) community-based NHs in Michigan. Intervention and control sites had similar infection prevention programs at baseline.8 The intervention took place over 3 years. Although only approximately half of residents with indwelling devices at the NHs provided consent and were enrolled in the program, we predict that the benefits of the intervention will apply to the care of all NH residents at the facility, including short- and long-stay residents.
We conducted the cost-effectiveness analysis from the healthcare system perspective. We examined the effect of the intervention at a representative NH over 1 year, applying reductions in adverse events observed in the trial to all residents with indwelling urinary catheters. The intervention resulted in a reduction of CAUTIs from 9.2 per 1,000 device-days to 5.9 per 1,000 device days.8 There were 3.7 hospitalizations for CAUTIs per 1,000 devicedays in controls and 2.6 per 1,000 device days in intervention participants.8 These outcomes are modeled in this analysis and affect costs and quality-adjusted life-years (QALYs) lost (Figure 1).
Figure 1.
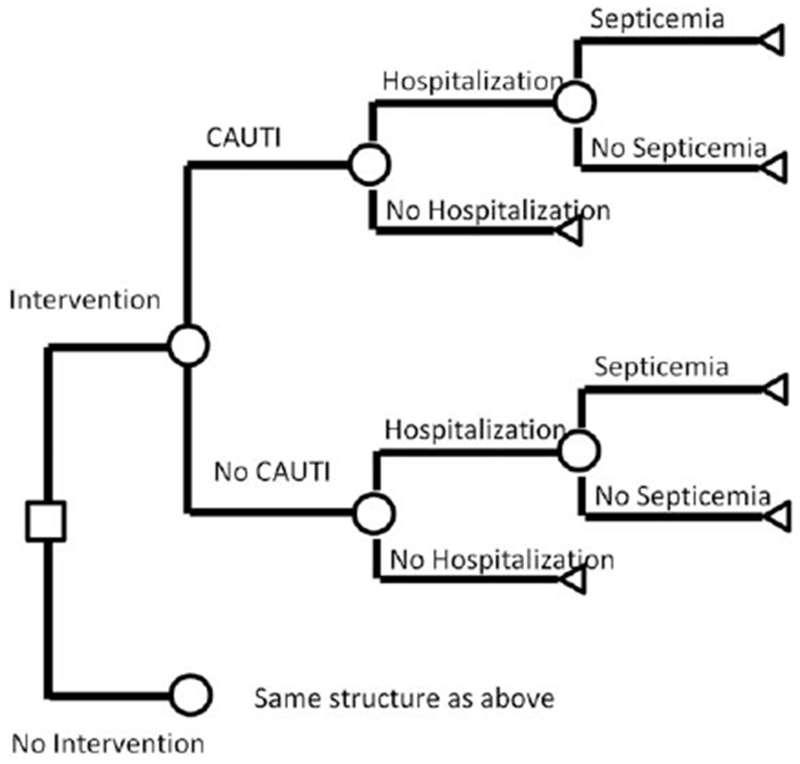
Schematic of decision tree model. The figure should be read from left to right. The square represents the intervention decision. Circles represent uncertain outcomes for nursing home residents: whether they develop a catheter-associated urinary tract infection (CAUTI), whether they are hospitalized, and whether they have septicemia, given hospitalization.
In addition to the base-case cost-effectiveness analysis, costs and outcomes were calculated from the perspective of just the NH (ignoring costs outside the NH such as hospitalization) to assess how the intervention might affect the NH alone. Only the costs of the intervention and the costs of health outcomes that the intervention affected were used in our calculations; the costs of standard NH care were not included and were assumed to be equal for the intervention and control NHs (Table 1). Costs and outcomes were calculated for a representative 120-bed NH with 6% of residents having indwelling urinary catheters,5 using the annual rates observed over the 3-year intervention and applied to all residents with indwelling urinary catheters.
Table 1.
Input Parameters
| Parameter | Base | Minimum | Maximum | Source |
|---|---|---|---|---|
| Intervention effectiveness | ||||
| Residents with catheter, % | 6 | 5 | 7 | Rogers et al. 5 |
| Clinically defined CAUTI per 1,000 days of catheter use | ||||
| Control | 9.2 | 7.38 | 11.57 | Mody et al.8 |
| Intervention | 5.9 | 4.59 | 7.72 | Mody et al.8 |
| Hospitalizations per 1,000 days of catheter use | ||||
| Control | 3.7 | 2.60 | 5.28 | Mody et al.8 |
| Intervention | 2.6 | 1.76 | 3.85 | Mody et al.8 |
| Intervention cost parameters | ||||
| Full-time equivalents required | 0.21 | 0.17 | 0.25 | Mody et al.8 |
| Infection control annual salary, $ | 55,000 | 41,250 | 68,750 | Mody et al.8 |
| Educational activities | ||||
| Nurse hourly wages, $ | 32 | 24 | 40 | Mody et al.8 |
| Nurse hours for intervention training | 16.67 | 12.50 | 20.83 | Mody et al.8 |
| Nursing aide hourly wages, $ | 12 | 9 | 15 | Mody et al.8 |
| Nursing aide hours for intervention training | 50 | 37.50 | 62.50 | Mody et al.8 |
| Additional resident time | ||||
| Gown and glove donning time (minutes) per resident visit for intimate activities of daily living (with indwelling device) | 0.63 | 0.27 | 1.00 | Martin et al.17 |
| Resident visits for intimate activities of daily living per day | 6.00 | 4.00 | 8.00 | Assumption |
| Supplies, $ | 2,000 | 1,500 | 2,500 | Mody et al.8 |
| Printing, $ | 361 | 271 | 451 | Mody et al.8 |
| Costs of disease, $ | ||||
| Cost of CAUTI in nursing home | 1,745 | 1,500 | 2,000 | Maki et al.21 |
| Cost of hospitalization | ||||
| Cost of hospitalization due to CAUTI | 7,193 | 5,395 | 8,992 | OIG report19 |
| Cost of hospitalization from septicemia | 19,914 | 14,936 | 24,893 | OIG report19 |
| Fraction of CAUTI leading to septicemia | 0.5 | 0.25 | 0.75 | OIG reports18‘19 |
| Utilities | ||||
| Length of CAUTI symptoms | 14 | 7 | 14 | Hout et al. 22 |
| Utility loss per CAUTI | 0.09 | 0.04 | 0.14 | Maxwell et al.24 |
| Long-term utility loss due to CAUTI | 0.02 | 0 | 0.05 | Hout et al.22 |
The Base column contains values used for the base case analysis. The Minimum and Maximum columns are used for sensitivity analysis. In the probabilistic sensitivity analysis, the parameters are assumed to be normally distributed, with the base representing the mean, and the minimum and maximum representing a 4–standard deviation spread (roughly 95% of the distribution). Additional details on how these parameters were calculated are in the supplemental text.
CAUTI = catheter-associated urinary tract infection.
Intervention Costs
All costs are expressed in 2015 U.S. dollars. The intervention costs include the cost of an infection prevention specialist to lead and oversee the intervention program, nursing personnel costs to attend educational activities, nursing time for donning protective equipment, supplies for hand hygiene activities and barrier precautions, and interactive educational materials (Table 1). We did not include costs of active surveillance for MDROs, which were used for research evaluation purposes. Resource use for the 6 intervention NH sites were evenly divided to calculate per-NH resource use. Additional details on the calculations of these intervention costs are in Supplemental Appendix S1.
Disease Costs
The cost of CAUTI treatment in a NH, the cost of hospitalization for a CAUTI, and the cost of hospitalization for CAUTI-associated septicemia were incorporated into the disease cost analysis.17–20 The cost of CAUTI treatment in a NH was estimated to be $1,745 per infection episode.21 The Office of the Inspector General of the Department of Health and Human Services has reported on the costs specifically related to hospitalizations from NHs, reporting that the average cost of hospitalization for a CAUTI was $13,554 in 2011 and that the cost of hospitalization for septicemia was reported to be $19,914 per episode.18 A weighted average of the cost of hospitalization for a CAUTI and the cost of hospitalization for CAUTI-related septicemia was used to calculate the overall average cost of CAUTI care in a hospital. We assumed that half of NH hospitalizations for CAUTI had a primary diagnosis of septicemia (Table 1).20 Additional details on hospitalization cost calculations can be found in Supplemental Appendix S1. Because NH residents are usually not employed, opportunity costs of infection and hospitalization were not included.
Utilities
The health outcomes of the intervention were calculated in terms of QALYs lost related to CAUTI (Table 2). Utility scores were taken from the literature.22–24 These studies averaged utilities over a mix of residents who were hospitalized and not hospitalized. The utility score decrement during the course of a CAUTI was estimated to be 0.09, and the average duration of a CAUTI was estimated to be 14 days in the base case.23,24 Long-term utility loss for a CAUTI was estimated to be 0.02.22 The long-term utility loss was assumed to occur for the remainder of the year.
Table 2.
One-Year Health and Cost Outcomes for a Representative 120-Bed Nursing Home
| Outcome | Intervention | Control |
|---|---|---|
| Health | ||
| CAUTI (events) | 15.5 | 24.2 |
| Hospitalizations due to CAUTI (events) | 6.8 | 9.7 |
| Quality-adjusted life-years lost from CAUTI | 0.35 | 0.55 |
| Costs, $ | ||
| Intervention costs | 20,279 | 0 |
| Disease costs | ||
| CAUTI care in nursing home | 27,061 | 42,197 |
| CAUTI care in hospital | 92,608 | 131,789 |
| Disease subtotal | 119,669 | 173,986 |
| Total costs | 139,948 | 173,986 |
| Interpretation | Cost savings of 34,037 | |
CAUTI = catheter-associated urinary tract infection.
Evaluating the Value of the TIP Intervention Program
If the TIP intervention program results in lower NH costs and fewer QALYs lost from disease than in the control group, it is said to be cost saving. In that case, we recorded the costs saved and QALYs gained. In the case in which the TIP intervention would lead to higher costs and fewer QALYs lost than in the control group, the important consideration is whether the QALYs saved are worth the extra cost. In that case, we computed an incremental cost-effectiveness ratio (ICER) defined as: (cost of intervention—cost of control) / (QALYs lost with control–QALYs lost with intervention). We have included the Consolidated Health Economic Evaluation Reporting checklist (Supplemental Appendix S1) to help readers understand and find many of the details of this analysis.
Sensitivity Analysis
We conducted univariate sensitivity analysis on all variables in our model and conducted a probabilistic sensitivity analysis using Monte Carlo simulation to simultaneously vary parameter assumptions to quantify overall uncertainty in the results in cost-effectiveness acceptability curves. Assumptions about the variability of the parameters used for the univariate and probabilistic sensitivity analyses are shown in Table 1.
RESULTS
Base-Case Analysis
Our analyses show that the implementation of a TIP intervention program would lead to 8.7 fewer CAUTIs and 2.9 fewer resident hospitalizations per NH per year. The intervention would cost $20,279 for the NH over the course of a year, with disease costs under the intervention of $119,669, for a total of $139,948. This is lower than the $173,986 in disease costs for the control group in the TIP study. The intervention saved $15,136 in NH CAUTI care and $39,180 in hospital care for CAUTIs and CAUTI-associated septicemia, for a total net savings of $34,037 for the healthcare system, as well as 0.2 more QALYs than in the control group (Table 2).
The intervention costs were estimated to be $11,458 for the time of an infection control specialist, $1,133 for other staff in-service time, $5,326 for staff time donning protective equipment, and $2,361 in supplies and printing. The largest costs were disease-related costs. A majority of the costs were for CAUTI care in a hospital. If the NH was responsible for the costs of the intervention and CAUTI care within the NH but not for costs of CAUTI care in the hospital, the NH would have a net cost increase of $5,143.
Sensitivity Analysis
We first varied all input parameter assumptions one at a time to see how they affected the value of the TIP intervention program. The variables that most substantially affected the costs saved were the rates of hospitalization for CAUTIs (Supplemental Table S2). If the intervention reduced the rate of hospitalizations from 3.7 to 3.55 per 1,000 device-days (9.7 per year to 9.3 per year for the NH), the TIP intervention would be cost saving (Supplemental Figure S1). Taking into account uncertainty in all parameter values simultaneously in the probabilistic sensitivity analysis, we conclude that the TIP intervention is 85% likely to be cost saving and 96% likely to be cost effective at a threshold of $200,000/QALY (Supplemental Figure S2).
DISCUSSION
This cost-effectiveness analysis showed that the TIP intervention program was expected to save $34,000 per year and improve health outcomes by 0.2 QALYs. A systematic review of the cost-effectiveness analysis literature showed that only 20% of prevention interventions evaluated in cost-effectiveness analyses are found to be cost saving,25 meaning that they improve health and reduce costs. Finding interventions that are cost saving is difficult because the right interventions must be applied in the correct settings to the appropriate residents. The TIP program is one of the few interventions that saves money and improves health outcomes by reducing infections and related hospitalizations in a high-risk setting and population.
When viewed solely from the financial perspective of the NH alone, the intervention may not appear to have net cost savings. We show that a focused risk factor–based intervention would cost the NH approximately $20,000 per year but result in approximately $15,000 in annual savings from reduced CAUTI care in the NH, for a net cost to the NH of approximately $5,000. Most of the benefits to the overall healthcare system result from $40,000 of reduced CAUTI care in hospitals, which are savings that payers probably capture. This suggests that payers, who benefit the most from an intervention like TIP, may find it financially worthwhile to provide incentives to NHs to conduct similar infection control programs that promote consistent application of evidence-based practices in high-risk populations. CMS estimates that the cost of its new broad infection control program would be $19,032 per NH per year, which is a significant underestimation because it includes only the cost of the infection preventionist.10 In addition, CMS fails to account for the benefits to the NH and the health system of the reduced infections and hospitalizations.10
These findings should be placed in context with recent infection prevention initiatives in other healthcare settings. A systematic review of hospital-based infection prevention interventions found 7 recent studies in the United States evaluating the net economic effect of the programs.26 All 7 found that the programs had cost savings in excess of intervention costs. Since that review was conducted, several additional studies have been published.27–29 A recent study27 of a multifaceted quality improvement program to reduce central line-associated bloodstream infections in intensive care units (ICUs) found that the intervention prevented 42 central line-associated bloodstream infections and 6 deaths and saved $249,000 per 1,000 patients. Another study28 of infection precautions to prevent methicillin-resistant Staphylococcus aureus (MRSA) transmission in U.S. ICUs found that universal decolonization would prevent 246 infections and save $2.81 million per 10,000 ICU admissions and that universal contact precautions plus decolonization would prevent an additional 66 infections per 10,000 admissions at a cost of $9,007 per incremental infection prevented. In a third study,29 an evaluation of multifaceted infection prevention programs designed to decrease central line-associated bloodstream infections and ventilator-associated pneumonia in ICUs found that the intervention improved health and saved initial inpatient costs, but even though long-term costs increased (partially because of longer life expectancy after discharge), the intervention was still cost effective, at $23,278/QALY gained. These studies provide evidence that multifaceted programs to reduce infections in healthcare settings can be cost saving or cost effective, but they were all in ICU settings. Our results build on this evidence and show that, in a NH setting, similar interventions can be cost saving and improve health outcomes for NH residents.
Our study has several limitations. In the analysis of a representative NH, we assumed that NH residents who did not consent to participate in the TIP intervention program would have similar reductions in rates of CAUTI. We felt this was a reasonable assumption, given that the program was geared toward all residents. Furthermore, the intervention in the trial included active surveillance for MDROs to evaluate study outcomes. Because this is neither practical for a typical NH nor the current standard of care, we did not include that as part of the cost-effectiveness analysis, although it is possible the active surveillance may have had an additional effect on the study results.
The analysis may also have underestimated the effect of CAUTI prevention in several ways. First, although the results indicated a reduction in MRSA colonization, we did not incorporate these costs into the analysis because we had limited information on the cost effectiveness of preventing MRSA colonization. Presumably, though, the decrease in colonization of pathogens should lead to fewer infections in NH populations and would extend the cost savings of this program. Second, we did not evaluate the benefits of reduction in MDRO transmission to other NH residents, which could lead to further infection reductions in MDROs, as well as other pathogens. It is possible that reductions in infections could also lead to reductions in mortality, although the study was not powered to detect differences in mortality, so we did not include it as an outcome measure. This may have led to an underestimate of the benefits of the intervention. Finally, we did not measure the effect of this type of intervention on other aspects of broad-based infection prevention. For example, greater adherence to hand hygiene guidelines may reduce overall nosocomial infections in NH residents without indwelling urinary catheters.
This study also has several strengths. Our results are based on a randomized, controlled intervention study of 12 NHs in southeast Michigan. We believe the results are generalizable to other NH populations. The intervention was comprehensive and designed to affect all NH residents, not just those at high risk. We evaluated the results from several perspectives and found that the multicomponent intervention may be viewed differently from the perspective of the NH itself and that of the payer and the healthcare system as a whole. Because the program is expected to have a net cost to the NH (cost of the program outweigh CAUTI savings within the NH), there may be a need to provide incentives to NHs to enact these types of interventions. The overall benefits to the health system of infection prevention interventions along with the benefits to payers provide sufficient value and cost benefits to be shared with NHs. In addition, new organizational structures such as accountable care organizations may be ideal platforms to align incentives between NHs, hospitals, residents, and payers because these organizations may share benefits and savings. NHs that implement these types of interventions may be more attractive to hospitals looking for preferred locations for postacute care in their networks.
Supplementary Material
Text S1. Detailed Intervention Costs
Table S1. CHEERS Checklist
Table S2. Sensitivity to Parameters
Figure S1. Sensitivity on Rate of Hospitalization
Figure S2. Cost Effectiveness Acceptability for a Representative Nursing Home
ACKNOWLEDGMENTS
Financial Disclosure: This study was supported by RO1AG032298 from the National Institute on Aging (NIA) (Mody, Krein, Saint), K24 AG050685 from the National Institutes of Health (Mody), RO1AG041780 from the NIA (Mody), the Claude D. Pepper Older American Independence Centers funding from the NIA (Mody), and a Veterans Affairs Health Services Research and Development Service Research Career Scientist Award (Krein)
Sponsor’s Role: The sponsor was not involved in the study design, methods, subject recruitment, data collections, analysis, or preparation of the paper. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Footnotes
TRIAL REGISTRATION: clinicaltrials.gov Identifier: NCT01062841 J Am Geriatr Soc 2018.
Conflict of Interest: None.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.Harris-Kojetin L, Sengupta M, Park-Lee E et al. Long-term care services in the United States: 2013 overview. National Center for Health Statistics. Vital Health Stat 2013;3:1–107. [PubMed] [Google Scholar]
- 2.Montoya A, Mody L. Common infections in nursing homes: A review of current issues and challenges. Aging health 2011;7:889–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicolle LE. Catheter associated urinary tract infections. Antimicrob Resist Infect Control 2015;3:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mody L, Maheshwari S, Galecki A et al. Indwelling device use and antibiotic resistance in nursing homes: Identifying a high-risk group. J Am Geriatr Soc 2007;55:1921–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogers MA, Mody L, Kaufman S et al. Use of urinary collection devices in skilled nursing facilities in five states. J Am Geriatr Soc 2008;56:854–861. [DOI] [PubMed] [Google Scholar]
- 6.Crnich CJ, Drinka P. Medical device-associated infections in the long-term care setting. Infect Dis Clin North Am 2012;26:143–164. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Medicare and Medicaid Services. Nursing Home Data Compendium. 2015. [on-line]. Available at https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/CertificationandComplianc/Downloads/nursinghomedatacompendium_508-2015.pdf Accessed August 29, 2016.
- 8.Mody L, Krein SL, Saint S et al. A targeted infection prevention intervention in nursing home residents with indwelling devices: A randomized clinical trial. JAMA Intern Med 2015;175:714–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, Lansing B, Symons K et al. Infection rate and colonization with antibiotic-resistant organisms in skilled nursing facility residents with indwelling devices. Eur J Microbiol Infect Dis 2012; 31:1797–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medicare and Medicaid Programs; Reform of Requirements for Long-Term Care Facilities, 42 C.F.R. § 483.80, 2016. [PubMed] [Google Scholar]
- 11.Clarke K, Tong D, Pan Y et al. Reduction in catheter-associated urinary tract infections by bundling interventions. Int J Qual Health Care 2013;25: 43–49. [DOI] [PubMed] [Google Scholar]
- 12.Sutherland T, Beloff J, McGrath C et al. A single-center multidisciplinary initiative to reduce catheter-associated urinary tract infection rates: quality and financial implications. Health Care Manag (Frederick) 2015;34: 218–224. [DOI] [PubMed] [Google Scholar]
- 13.Ternavasio-de la Vega HG, Barbosa Ventura A, Castano-Romero F et al. Assessment of a multi-modal intervention for the prevention of catheter-associated urinary tract infections. J Hosp Infect 2016;94:175–181. [DOI] [PubMed] [Google Scholar]
- 14.Duffy LM, Cleary J, Ahem S et al. Clean intermittent catheterization: Safe, cost-effective bladder management for male residents of VA nursing homes. J Am Geriatr Soc 1995;43:865–870. [DOI] [PubMed] [Google Scholar]
- 15.Church DL, Davies HD, Mitton C et al. Clinical and economic evaluation of rapid influenza a virus testing in nursing homes in Calgary, Canada. Clin Infect Dis 2002;34:790–795. [DOI] [PubMed] [Google Scholar]
- 16.Trick WE, Weinstein RA, DeMarais PL et al. Comparison of routine glove use and contact-isolation precautions to prevent transmission of multidrug-resistant bacteria in a long-term care facility. J Am Geriatr Soc 2004;52: 2003–2009. [DOI] [PubMed] [Google Scholar]
- 17. Martin EM, Russell D, Rubin Z et al. Elimination of routine contact precautions for endemic methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus: A retrospective quasi-experimental study. Infect Control Hosp Epidemiol 2016;37:1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levinson DR. Adverse Events in Skilled Nursing Facilities: National Incidence Among Medicare Beneficiaries. Department of Health and Human Services, Office of Inspector General [on-line] Available at https://oig.hhs.gov/oei/reports/oei-06-11-00370.pdf Accessed August 29, 2016. [Google Scholar]
- 19.Levinson DR. Medicare Nursing Home Resident Hospitalization Rates Merit Additional Monitoring. Department of Health and Human Services, Office of Inspector General [on-line] Available at https://oig.hhs.gov/oei/reports/oei-06-11-00040.pdf Accessed June 14, 2017. [Google Scholar]
- 20.Elixhauser A, Friedman B, Stranges E. Septicemia in U.S. hospitals, 2009 HCUP Statistical Brief #122. October 2011. Agency for Healthcare Research and Quality, Rockville, MD: [on-line]. Available at http://www.hcup-us.ahrq.gov/reports/statbriefs/sb122.pdf Accessed August 29, 2016. [Google Scholar]
- 21.Maki PG, Tambyah PA. Engineering out the risk for infection with urinary catheters. Emerg Infect Dis 2001;7:342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hout WB, Caljouw MA, Putter H, Cools HJ, Gussekloo J. Cost-effectiveness of cranberry capsules to prevent urinary tract infection in long-term care facilities: Economic evaluation with a randomized controlled trial. J Am Geriatr Soc 2014;62:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bermingham SL, Ashe JF. Systematic review of the impact of urinary tract infections on health-related quality of life. BJU Int 2012;110:E830–E836. [DOI] [PubMed] [Google Scholar]
- 24.Maxwell CJ, Kang J, Walker JD et al. Sex differences in the relative contribution of social and clinical factors to the Health Utilities Index Mark 2 measure of health-related quality of life in older home care clients. Health Qual Life Outcomes 2009;7:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen JT, Neumann PJ, Weinstein MC. Does preventive care save money? Health economics and the presidential candidates. N Engl J Med 2008; 358:661–663. [DOI] [PubMed] [Google Scholar]
- 26.Arefian H, Vogel M, Kwetkat A, Hartmann M. Economic evaluation of interventions for prevention of hospital acquired infections: A systematic review. PloS One 2016;11:e0146381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herzer KR, Niessen L, Constenia DO, Ward WJ, Pronovost PJ. Cost-effectiveness of a quality improvement programme to reduce central line-associated bloodstream infections in intensive care units in the USA. BMJ Open 2014;4:e006065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gidengil CA, Gay C, Huang SS, Platt R, Yokoe D, Lee GM. Cost-effectiveness of strategies to prevent methicillin-resistant Staphylococcus aureus transmission and infection in an intensive care unit. Infect Control Hosp Epidemiol 2015;36:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dick AW, Perencevich EN, Pogorzelska-Maziarz M, Zwanziger J, Larson EL, Stone PW. A decade of investment in infection prevention: A cost-effectiveness analysis. Am J Infect Control 2015;43:4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Text S1. Detailed Intervention Costs
Table S1. CHEERS Checklist
Table S2. Sensitivity to Parameters
Figure S1. Sensitivity on Rate of Hospitalization
Figure S2. Cost Effectiveness Acceptability for a Representative Nursing Home


