Abstract
Oxygen plays a central role in cardiac energy metabolism. At high-altitude where the ambient oxygen level is low, we found EDNRB is associated with human hypoxia adaptation. Our subsequent study in global heterozygous knockout mice (Ednrb+/−) revealed that cardiac function was conserved in these mice when exposed to extreme hypoxia. The major goal of this study was i) to determine the functional role of cardiomyocyte EdnrB in maintaining cardiac function under hypoxic stress and ii) to validate the phenotypes we detected in Ednrb+/− mice using EDNRB blockers. Unlike the global knockouts, cardiac specific heterozygote (EdnrBflox/+) and homozygote (EdnrBflox/flox) EdnrB knockout mice were phenotypically normal. When treated with graded low levels of oxygen (10% and 5% O2), both EdnrBflox/+ and EdnrBflox/flox were hypoxia tolerant. The cardiac-index at 10% and 5% O2 for EdnrBflox/+ were significantly higher and lactate levels were significantly lower when compared to the cre-negative controls (P<0.05). Simultaneously, mice treated with BQ-788 (EDNRB specific) had a significantly higher cardiac-index (P<0.005) and significantly lower lactate levels (P<0.0001) than in control mice. A similar result was obtained with mice treated with Bosentan (non-specific). These data indicate that a lower level or complete lack of EdnrB in the cardiomyocytes significantly improves cardiac performance under extreme hypoxia, a novel role of cardiomyocyte EdnrB in the regulation of cardiac function. Furthermore, this rescue under extreme hypoxia can also be achieved using EDNRB specific pharmacological agents e.g., BQ-788. This systematically confirms, both genetically and pharmacologically, the protective role of a lower EDNRB under extreme hypoxia stress.
Keywords: EdnrB, Bosentan, BQ-788, high-altitude, hypoxia, cardiac index
Introduction
Humans adapted to high-altitude (HA) environment are anticipated to have certain favorable variants, accumulated in their genome due to their long habitation in harsh hypoxic environments. Through whole genome sequencing, we were able to identify DNA regions and candidate genes, under ‘selection’ [1, 2]. Since better therapeutic modalities for diseases involving hypoxia and ischemia in their pathogenesis can be developed by understanding mechanisms of HA adaptation, we have identified Endothelin receptor type B (EDNRB), as one of the candidate genes from the Ethiopian HA adapted population [1]. More recently, we have focused on validating the role of EDNRB in hypoxia tolerance in EdnrB knockout (KO) mice [3]. We found that a lower level of EdnrB contributes to the sustenance of a steady cardiovascular response to various degrees of hypoxia, even severe hypoxia of 5% O2. The heterozygote EdnrB (EdnrB−/+) mice were maintaining higher cardiac output (CO), peripheral perfusion and better O2 delivery to vital organs [3]. Together with the human study, EDNRB seems to play a key role in hypoxia tolerance.
In parallel, there has been considerable interest in the endothelin system because of its role in development of diseases and pathological conditions, and also because the receptors (EdnrA and EdnrB) are important target for developing drugs for treatment. Cardiac hypoxia tolerance in EdnrB−/+ mice is interesting from several points of view. First, the HA exposure itself is recognized as a cardiac stress [4] and a conventional approach for adaptation to such environment would be to cope with such stress. And as we noticed in the EdnrB−/+ mice, downregulating EDNRB is a good example. Second, endothelin-1 (Edn1), the ligand that binds to both receptors, increases during HA exposure [5] and lead to pulmonary hypertension. Third, the receptor antagonist Bosentan, although non-specific, is given to reduce pulmonary artery pressure [6]. Fourth, the improvements post receptor antagonist treatment likely occurs through blocking EdnrB because in spite of the higher ratio of EDNRA/EDNRB (4 to 1) in the heart [7], KO studies have ruled-out any important role of EdnrA in maintaining cardiac function [8]. And when Bosentan treatment during septic shock improves cardiac performance and microcirculatory blood flow [9, 10], the specific binding of this antagonist to EdnrB can be viewed as a major contributor. And lastly, the phenotype that describes EdnrB−/+ mice as ‘hypoxia tolerant’ were directly related to cardiac function, e.g. blood pressure (BP), CO, contractility etc., or its downstream mechanism/s e.g. lactate levels, O2 perfusion [3]. Based on this evidence, we hypothesized that by lowering EdnrB expression in the heart, either directly by tissue specific KO or using specific blockers; these mice would be able to demonstrate significant tolerance to acute hypoxia. In the current study, we systematically confirmed our hypothesis, first by generating cardiomyocyte-specific EdnrB KO mice (both heterozygote (EdnrB+/flox) and homozygote (EdnrBflox/flox)), exposing them to acute hypoxia and then measuring cardiac functions. Simultaneously, we also treated adult C57BL/6J mice with Bosentan (a dual endothelin receptor blocker which blocks both EDNRA and EDNRB) and BQ-788 (EDNRB specific blocker) and measured their cardiovascular tolerance to hypoxia.
Materials and Methods:
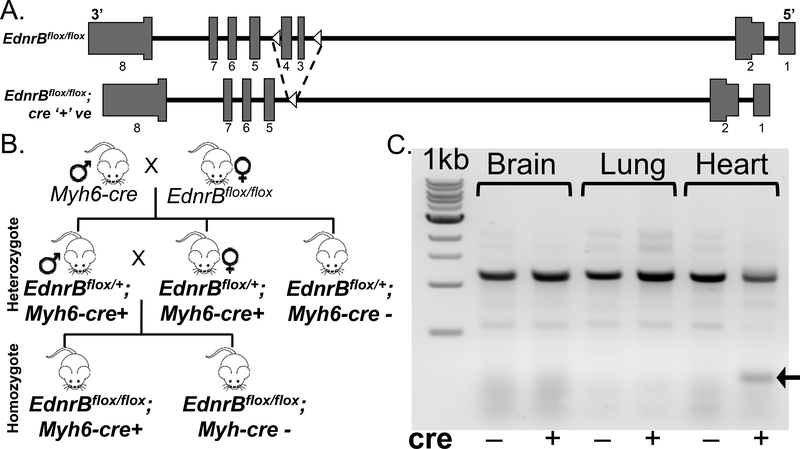
Generation of EdnrB KO and its validation
All animal care and handling was performed according to the NIH guidelines and protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the University of California San Diego (UCSD), US. Generation of EdnrB-LoxP (EdnrBflox/flox) mice has been previously detailed [11]. Prof. David Webb, Univ. Edinburgh and Prof. Donald Kohan, Univ. Utah, kindly provided these mice. Briefly, the LoxP sequence was inserted within intron 2 and 4 (flanking exon 3 and 4) of EdnrB gene (Fig. 1a). Earlier reports suggested that exon 3 was crucial as there was an amino acid encoded across a splice junction [12]. Subsequent sequencing confirmed no mutations within LoxP sites and no known phenotype related to EdnrB disruption e.g., abnormality of pigmentation, gut development, litter size, or mortality rate. These mice were crossed with αMHC-MerCreMer (stock# 005650, Jackson Lab) and selected for cre’+’ F1 progeny. Subsequently they are inbred to generate cardiac specific EdnrB homozygote and heterozygote KO mice. A schematic of the breeding strategy and its validation using PCR is provided in the Fig 1b and 1c. For the hypoxia experiments, we used cre’+’ mice, both EdnrBflox/+ and EdnrBflox/flox and compared with cre’-’ EdnrBflox/+ mice. All the mice were treated in advance with 3×30mg/kg of tamoxifen.
Fig 1:
Generation of cardiac specific EdnrB KO. a) EdnrB gene in mice before and after Cre-LoxP recombination. B) Schematic of the crosses between EdnrBflox/flox and Myh6-cre to generate heterozygote (EdnrBflox/+) and homozygote (EdnrBflox/flox) KO mice. b) Gel picture depicting tamoxifen induced exon 3–4 deletion in the Heart.
Pharmacological validation by EDNRB blockers
Studies were performed in adult (>3 months) male C57BL/6J mice (Stock# 000664, Jackson Lab). For the pharmacological inhibition studies, we used EDNRB specific blocker ‘BQ-788’ and endothelin receptor non-specific blocker ‘Bosentan’. We believe that the drug BQ-788 would mimic EdnrB KO mice and the use of Bosentan would help decipher the involvement of other subtype i.e., EdnrA. A pilot study with different doses of BQ-788 (at 0.5 and 1.0 mg/kg) and Bosentan (at 25 and 50 mg/kg) was initially conducted to estimate the dose-response activity. For the final experiment, we used 0.5 mg/kg of BQ-788 and 50 mg/kg Bosentan. Animals were randomly assigned to three different groups. Group I (n=5) was injected with carrier, Group II (n=6) with Bosentan (50 mg/kg) and Group III (n=5) with BQ-788 (0.5mg/kg). In total, all groups were injected four times at 48-hour interval with respective dosages. The baseline measurements i.e., 21% O2, was carried out after 2 hours of 4th dose injection.
Mice surgical preparation
The IACUC approved the experimental protocol (protocol S04052 and S11306). The animals were anesthetized with an i.p. injection of sodium pentobarbital (50 mg/kg). Mice were prepared for cardiac output (CO) measurements by first surgically implanting a jugular catheter (PE10 filled with heparin/saline solution 30 IU/mL) and a small thermocouple (IT18T75, Physitemp Instruments). A midline incision was made in the exposed area and the right jugular vein and carotid artery were exposed. The thermocouple probe was inserted approximately 0.7 cm into the carotid artery. The tip of the jugular vein catheter was inserted past the subclavian branch of the vein. The temperature probe and PE10 catheter were under the skin on the animal’s back. A short femoral catheter (PE10 filled with heparin/saline solution 30 IU/mL) was also implanted to monitor blood pressure and withdraw blood samples, which was secured underneath the skin. Mice recovered from catheter implantation for 24 hours before the hypoxia protocol. Mice were suitable for the experiments if: i) systemic parameters were within normal range, namely, heart rate (HR)>450 beat/min, mean arterial blood pressure (MAP)>90mmHg, systemic Hct>42%, and arterial O2 partial pressure (paO2)>80mmHg.
Cardiac Output Measurements
CO was measured by a modified thermodilution technique [13]. Briefly, a saline bolus indicator (0.1 mL at 25°C) was injected via the jugular vein catheter, and the change in temperature was detected at the aortic arch using the implanted thermocouple. This procedure was selected because in these small awake animals it is not feasible to inject the indicator directly into the right atrium. The conversion of the area under the indicator dilution temperature curve into flow was done with a fluid circuit that reproduced flow rates, volumes, and heat exchange properties of the mice circulation between the locations of saline injection and temperature measurements. Thermodilution curves peaked rapidly and decayed exponentially. The signal due to recirculation was eliminated by truncating the curve 30–50% after the maximum change in temperature. Thermodilution curves area is proportional to CO. Cardiac index (CI) was calculated as the CO divided by the animal body weight.
Hypoxia protocol
The awake animals were placed in a restraining tube within seal acrylic box (4” x 4” x 10”). The inlet of the box was connected to gas tanks with different O2 concentrations: 1) compressed air; 2) 15% O2 balance N2; 3) 10% O2 balance N2; and 4) 5% O2 balance N2. The gas flow rate into the box was kept at 0.2 L/min using a gas flow meter. Air coming into the box was warmed to 25°C and diffused using a cotton filter. O2 concentration in the box was measured continuously using an O2 gas sensor (Vernier Corp. Beaverton, OR). Mice were first exposed to compress air and given 20 min to adjust to the experimental environment before baseline measurements were completed. Then, the compressed air was replaced by 15% O2, and mice were given 15 min to adjust to the change in the gas environment before measurements. Similarly, the 15% O2 gas was replaced for the 10% O2 gas, and another 15 min were given to the mice before measurements. Lastly, the 10% O2 gas was replaced for the 5% O2 gas, and another 10–15 min were given to the mice before measurements. At each time point, systemic parameters and blood gas analysis were performed which also included lactate level measurements. Briefly, a capillary blood sample was taken from the femoral artery catheter ~15 mins after animals were exposed to specific oxygen concentrations. Blood samples were immediately analyzed in a blood gas analyzer (ABL90 FLEX analyzer Radiometer America Inc., Brea, CA). To prevent animal stress or discomfort, hypoxia was stopped if BP dropped below 40 mmHg, and the animal was excluded from the study.
Data analysis
Results are presented as means ± standard deviations. The Grubbs’ method was used to assess closeness for all measured parameters values at baseline. Data comparison between groups was analyzed using two-way analysis of variance (ANOVA) with genotype as a between factor and hypoxia treatment as a repeated measure. Data within each group was analyzed using Kruskal-Wallis one-way ANOVA. When appropriate, post-hoc analyses were performed with the Dunn’s multiple comparison test and Bonferroni post-tests comparison. All statistics were calculated using Analyse-it for Microsoft excel 4.18.1 (Analyse-it Software, Ltd.). Changes were considered statistically significant if P<0.05.
Results
Generation of cardiac specific EdnrB KO
We hypothesized that decreasing functional EDNRB specifically in the heart would be advantageous in hypoxia, and hence we generated cardiac-specific KO mice of this gene and then studied its phenotype in both normoxia and hypoxia. While the generation of EdnrB-LoxP (EdnrBflox/flox) mice has been previously reported [11], a detailed schematic of the crosses to generate EdnrB KOs in the cardiomyocytes is presented in Fig 1B. Both homozygous EdnrBflox/flox;Myh6-cre’+’ (EdnrBflox/flox) as well as the heterozygotes EdnrBflox/+;Myh6-cre’+’ (EdnrBflox/+) mice were injected with tamoxifen (3×30mg/kg) 10 days prior to the hypoxia experiment. Simultaneously, the control EdnrBflox/+;Myh6-cre’-’ (cre negative) mice were also injected with equal dosages i.e., 3×30mg/kg of tamoxifen (Fig 1b and 1c). At the end of experiment brain, lung and heart were collected for DNA isolation and PCR confirmation of the cardiac-specific EdnrB KO (EdnrBflox/+and EdnrBflox/flox). Genotype of brain, lung and heart confirms exon 3–4 deletion in the heart and not in other tissues or the heart of tamoxifen-treated cre-negative mice (Fig 1C).
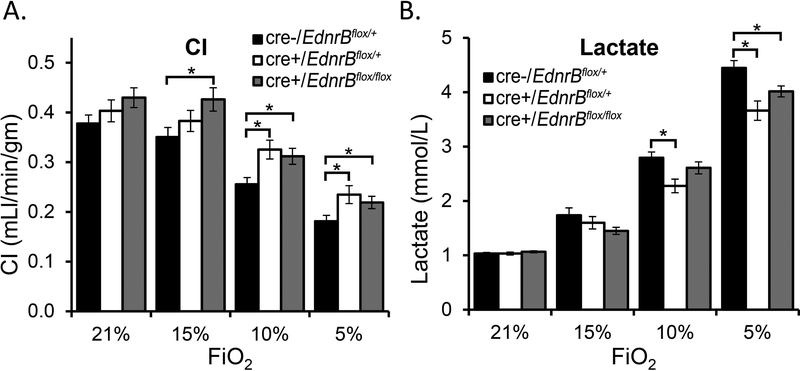
Hypoxia tolerance in cardiac specific EdnrBflox/+ and EdnrBflox/flox KO
Three sizeable groups of mice i.e., tamoxifen injected EdnrBflox/+ (n=12), EdnrBflox/flox (n=9), and the cre-negative controls (n=11), were analyzed utilizing modified thermodilution technique after exposing to acute hypoxia of different O2 i.e., 21%, 15%, 10% and 5% (see Methods and Stobdan et al. 2015) [3]. Both EdnrBflox/+ and EdnrBflox/flox mice, similar to EdnrB−/+ (global heterozygote) [3], maintained a higher cardiac performance at various levels of hypoxia, including a severe one. Although the CI dropped as the O2 decreased from room air (21% O2) to 5% O2 (Fig 2a), the CI in EdnrBflox/+ and EdnrBflox/flox were significantly higher at 10% and 5% O2 when compared to the control mice (Fig 2a, P<0.05). The CI at 10% and 5% O2 were 0.33±0.02 and 0.22±0.02 mL/min/gm in EdnrBflox/+ and 0.31±0.02 and 0.21±0.01 mL/min/gm in EdnrBflox/flox, which is significantly higher than the cre-negative control group with 0.26±0.02 and 0.18±0.02 mL/min/gm (Fig 2a). When we analyzed the percentage drop in CI from its baseline values, the cre-negative CI drop at 5% O2 was ~52% of the levels at 21% O2. The drop at the same O2 level was 42% in the EdnrBflox/+. Similarly, no drop was detected at 15% O2 in EdnrBflox/flox and 4.8% in EdnrBflox/+ but a 7.3% drop in the controls. When we analyze CO, especially under extreme hypoxia (10% and 5% O2), EdnrBflox/+ mice maintained a higher CO (supplementary Fig 1S) and the relative drop from its 21% O2 was significant when compared with the controls (supplementary Fig 1S). Additionally, the lactate levels strongly correlated with the hypoxia tolerance observed in EdnrBflox/+ and EdnrBflox/flox (Fig 2b). The lactate levels at 5% O2 in both EdnrBflox/+ and EdnrBflox/flox (P<0.05) and at 10% O2 in EdnrBflox/+ (P<0.05) were significantly lower than the cre-negative mice at respective O2 levels. This is also supported by the trends between the three groups with levels in cre-negative > EdnrBflox/flox > EdnrBflox/+ both at 10% and 5% O2, which is inversely related to the CI and CO measurements (Fig 2b and supplementary Fig 1S).
Fig 2:
Cardiac index and blood lactate level comparisons between cardiac specific EdnrB homozygous (EdnrBflox/flox), heterozygous (EdnrBflox/+) and cre-negative control mice. a) Both EdnrBflox/flox and EdnrBflox/+ mice maintains a higher CI under extreme hypoxia of 10% and 5% O2. b) Lactate levels were significantly lower in EdnrBflox/+ mice at 10% and 5% O2 when compared with the cre-negative group and in EdnrBflox/flox at 5% O2. The trends were inversely related to the CI where it is high in the cre-negative controls, medium in the EdnrBflox/flox group and low in the EdnrBflox/+ group. Error bar indicates ± standard error.
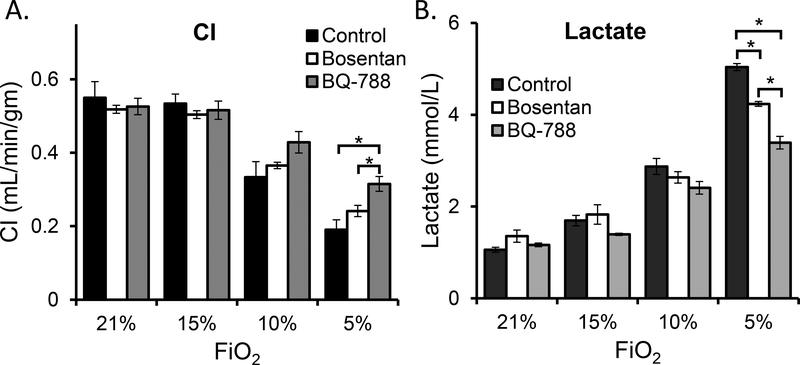
EDNRB specific blocker depict maximum hypoxia tolerance
In order to systematically evaluate the protective role of relatively lower EDNRB in a hypoxic environment, we utilized both non-specific (Bosentan, blocks both EDNRA and EDNRB) and specific (BQ-788) blockers of EDNRB. Adult C57BL/6J mice were randomly assigned to three different groups. Group I (n=5) was treated with carrier, Group II (n=5) was treated with Bosentan (50 mg/kg) and Group III (n=5) was treated with BQ-788 (0.5mg/kg). Cardiovascular functions were measured utilizing modified thermodilution technique (materials and methods). Remarkably, both Bosentan and BQ-788 rescue mice by maintaining better cardiac functions under extreme hypoxia. Under ambient conditions i.e., at 21% O2, and also at 15% O2, the CI was comparable in all the three groups (Fig 3a). However, at 10% and 5% O2 a clear trend was seen where the CI was in the ascending order from controls<Bosentan<BQ-788. Under extreme hypoxia of 5% O2 the CI was highest for BQ-788 treated mice with P-value <0.005 when compared to the controls and P-value <0.05 when compared with Bosentan treated mice (Fig 3a). On the contrary and as anticipated, the lactate levels at 10% O2 followed a reverse order with BQ-788<Bosentan<controls. The differences between the groups were further cumulated at 5% O2 (P<0.0001, Fig 3b). At this O2 the mice treated with BQ-788 had only 44.2% increase in the lactate levels from the level detected at 21% O2. This level was lower than Bosentan treated mice with 62.3% increase and control mice with 77.4% increase. Of note, the BQ-788 treated mice had higher mean arterial pressure, higher heart rate and lower stroke volume (supplementary Fig 2S) and these trends are similar to our findings in EdnrB−/+ mice.
Fig 3:
Cardiac index and blood lactate level comparisons between BQ-788, Bosentan treated and control mice. a) Both Bosentan and BQ-788 treated mice maintains a relative higher CI under extreme hypoxia of 10% and 5% O2. However, the level in BQ-788 treated mice was significantly higher at 5% O2 when compared to both Bosentan and control mice. b) Lactate levels at 10% O2 show a trend towards where it is high in the controls, medium in the Bosentan treated mice and low in the BQ-788 treated mice. The difference is significant (P<0.005) between all the three groups. The error bar indicates ± standard error.
Discussion
EDNRB was one of the top candidate genes we identified in our whole-genome sequence analysis for hypoxia adaptation in Ethiopian highlanders [1]. This was primarily marked by a strong signature of positive selection where a large block containing SNPs were fixed in the highlanders but were at a significantly lower frequency in nearby lowlander populations. Subsequently, our findings in EdnrB−/+ mice exquisitely depicted that these mice were resistant to various levels of hypoxia, maintained higher CO, peripheral perfusion and better O2 delivery to vital organs, even in severe hypoxia [3]. In other words, by downregulating EdnrB, mice were able to conserve cardiac function when exposed to extreme hypoxia. There is other evidence also demonstrating the advantages of lower EDNRB in the heart. For example, a lower EDNRB in patients with ischemic heart disease is thought to be beneficial [14, 15].
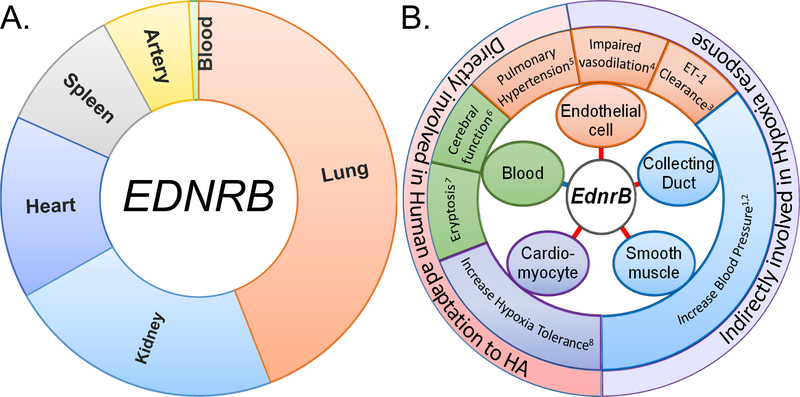
The EDNRB receptor is a G protein-coupled receptor that activates a phosphatidylinositol - calcium (Ca2+) second messenger system [16]. The gene is express in most tissues (Fig.4A). Functionally, EDNRB is involved in vasodilatation, opposing EDNRA, which is involved in vasoconstriction [17]. However, the role of EDNRB is much more complex than was originally anticipated. During early development, it plays a critical role throughout neural crest cells migration, proliferation and differentiation [18]. Its disruption leads to phenotypes like Hirschsprung’s disease and megacolon [19, 20]. Recent studies specifically involving spatiotemporal KO of EdnrB in mice have depicted a variety of roles it can play in different tissues (Fig. 4b). Interestingly, most of the phenotypes affected are directly or indirectly related to cardiovascular function or hypoxia response (Fig. 4b). For example, its direct involvement in blood pressure (BP) or sodium retention was depicted in the collecting duct-specific KO mice [21] and not in the endothelial cell-specific KO [11], unlike previously anticipated. The contribution of endothelial cells’ EdnrB in BP regulation, however, is indirect as it mediates nitric oxide release and responsible for ET-1 clearance from the circulation [22, 23]. At the same time, the EdnrB in smooth muscle contributes to an increased BP but surprisingly had no role in vascular contraction [24]. Even in our previous study on EdnrB−/+ mice the BP was consistently higher but we did not observe any increase in circulating ET-1 [3]. To summarize, an overview of whether EDNRB is expressed in the particular tissues and major phenotype observed when the gene is KO in that specific tissue is simplified in Fig 4.
Fig 4:
EDNRB expression and phenotype associated with tissue specific KO of this gene. a) Tissues wise EDNRB expression in humans. RPKM value for EDNRB were obtained from GTEx portal (https://gtexportal.org/)). b) Diverse role of EdnrB identified using tissue specific KO mice (red line) and levels measured in the whole blood/serum (blue line). Middle zone depicts the phenotype related to the tissue specific KO study (color-coded). For the role of EdnrB in blood, ‘eryptosis’ was retrieved from rescued global EdnrB KO mice. The ‘cerebral function’ was indicated by ‘cerebral vasoconstriction reactions’ and the EDNRB level measured in the blood serum of CMS patients. All indicate a direct or indirect involvement in hypoxia adaptation. The respective numbers indicate the related references: 1, Ge et al. (2006); 2, Miller et al. (2017); 3, Kelland et al. (2010a); 4, Bagnall et al. (2006); 5, Kelland et al. (2010b); 6, Wu et al. (2016); 7, Foller et al. (2010); 8, Present study.
EdnrBs’ involvement in cardiac function, especially in extreme hypoxic stress, has been an important issue because of the maintenance of cardiac function in EdnrB−/+. Available literature suggests that all the major components of the endothelin system, Edn1, EdnrA and EdnrB are expressed in cardiomyocytes. Although Edn1 expression is reportedly necessary for cardiomyocyte’s survival and maintenance of normal cardiac function, even during stressful conditions [25], we did not observe any difference in its level in the EdnrB−/+ mice at baseline [3]. Of the two receptors, only EdnrB was reportedly present on the cardiomyocytes’ nuclear membrane and was associated with regulation of nuclear Ca2+ signaling [26]. This may be an indication of the important role it plays in contractility. In addition, another study just confirmed the role of nuclear membranes’ EDNRB in Ca2+ signaling [27], but the authors noted this in endocardial endothelial cells of human left ventricles. From our present study, it is clear that the EdnrB in cardiomyocyte has an important role in maintaining cardiac contractility especially under acute stressful condition i.e., severe hypoxia. We used inducible cardiomyocyte specific driver because our intention was to KO EdnrB only in the adult cardiomyocytes to avoid the spatiotemporal complexities related to its diverse role (as depicted in Fig. 4b). The cardiomyocyte’s specific EdnrB KO is viable and therefore we could test hypoxia tolerance in both heterozygote (EdnrBflox/+) as well as homozygote (EdnrBflox/flox) KO mice. Remarkably, similar to EdnrB−/+, cardiac specific EdnrB KO mice, both EdnrBflox/+ and EdnrBflox/flox, withstood various levels of hypoxia with a significant cardiac tolerance. For example, CI was better maintained at 10% and 5% O2 by both EdnrBflox/+ and EdnrBflox/flox (Fig. 2a) with lower lactate levels (Fig. 2b), and the differences were profoundly visible particularly at 5% O2. Interestingly, the current study also indicates that, while the cardiomyocytes’ EdnrB makes a significant contribution to hypoxia tolerance by protecting the heart during hypoxia by maintaining CI, it does not fully account for the whole phenotype that we observed in the EdnrB−/+. For example the higher MAP is seen in EdnrB−/+ but not in the cardiomyocyte-specific EdnrB down-regulation. Only at 10% O2 was the SPB and DBP differs between EdnrBflox/+ and controls.
Finally, our current study using EDNRB blocker comprehensively illustrates the protective role of lower EDNRB under extreme hypoxia. Data from the literature show that both Bosentan and specific blockers of EDNRA prevent the increase in pulmonary artery pressure during hypoxia and help in the restoration of oxygen saturation [28–31]. Since CI is maintained during hypoxia and lactate levels are lower in the EDNRB specific blocker (BQ-788)-treated mice, we suggest that both EDNRA and EDNRB are involved in hypoxia tolerance. However, in this study, which focuses on cardiac measurements, we demonstrate that cardiac specific EDNRB (EdnrBflox/+ and EdnrBflox/flox) are involved in maintaining cardiac output.
Given that EDNRB was first identified in human HA dwellers from Ethiopia known to be best adapted to HA [32], it is interesting to note a recent study showing that the level of EDNRB in serum was significantly higher in chronic mountain sickness (CMS) patients [33]. Furthermore, the cerebrovascular reactivity (CRV), i.e., the change in cerebral blood flow in response to a stimulus, was reportedly lower in these patients pretty much similar to patients with cardiac failure [34]. This is an interesting observation because (i) we anticipated lower EdnrB to be protective at HA and higher as seen in CMS is deleterious. (ii) This was a study done in Tibetan HA population and we discovered EDNRB as a candidate gene in the Ethiopian HA natives where no case of CMS was ever reported. Therefore, it will be interesting to explore the differences in genetic variants at this locus between these two populations. (iii) A lower CRV, as depicted in cardiac failure patients, possibly indicates that the heart in these CMS patients is less efficient. And we know from our study, both global and cardiac specific EdnrB KO mice, that a lower EdnrB under hypoxic environment could lead to a better cardiac function. Furthermore, similar to our EdnrB study, the ET-1 levels were similar when CMS subjects were compared to controls. Alternatively, the higher EDNRB in CMS can also be correlated with the inhibitory role of EDNRB in suicidal erythrocyte deaths and therefore preventing ‘eryptosis’ [35]. In other words, a higher EDNRB in CMS may be inducing polycythemia while a lower level plausibly promoting eryptosis and lowering the abnormally high hematocrit levels.
In summary, it is reasonable to conclude that by lowering EdnrB, genetically or pharmacologically, plays an important role in hypoxia tolerance. Specifically, the cardiomyocyte EdnrB plays the major role in hypoxia tolerance in the EdnrB−/+ mice model. And when we connect to a comprehensive role of EDNRB at HA, it appears that the protective role of a lower EDNRB is potentiated through its two-pronged hypoxia-tolerance-strategy, i.e., by (i) sustaining cardiac function, as seen in EdnrB KO and BQ-788 treated mice under extreme hypoxia and (ii) preventing hypoxia-induced excessive erythrocytosis. This may indicate better adaptation in the HA Ethiopians or lack of CMS thereof. An important question that remains unclear is related to the molecular mechanisms leading to protection against hypoxic injury. Since we now have the crystal structures of EDNRB in both ligand-free form and in complex with EDN1 [36], progress towards novel therapies is possible.
Supplementary Material
Supplementary Fig S1: Cardiac output comparison between cardiac specific EdnrB homozygous (EdnrBflox/flox), heterozygous (EdnrBflox/+) and controls mice. EdnrBflox/+ mice maintains a higher CO under hypoxia. Error bar indicates ± standard error.
Supplementary Fig S2: Cardiac output comparison between BQ-788, Bosentan treated and control mice. Both BQ-788 and Bosentan treated mice maintains a relative higher CO under extreme hypoxia of 10% and 5% O2. However, CO in BQ-788 treated mice was significantly higher at 5% O2 when compared to both Bosentan and control mice. Error bar indicates ± standard error.
Acknowledgements
We thank Dr. Donald Kohan (Univ. of Utah) and Dr. David Webb (Univ. Of Edinburgh) for providing the Lox-P mice.
Sources of Funding
This work was supported by National Institutes of Health (NIH) grant (R01 HL127403–02) to GGH. TS was supported by the Emerald Foundation Inc. This work was partially supported by NIH grants from the Heart Lung and Blood Institute (P01-HL110900, R01-HL126945, and T32-HL007444) PC.
Footnotes
Disclosures
None.
References
- 1.Udpa N, Ronen R, Zhou D, Liang J, Stobdan T, Appenzeller O, et al. Whole genome sequencing of Ethiopian highlanders reveals conserved hypoxia tolerance genes. Genome biology. 2014;15(2):R36 Epub 2014/02/22. doi: 10.1186/gb-2014-15-2-r36. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou D, Udpa N, Ronen R, Stobdan T, Liang J, Appenzeller O, et al. Whole-genome sequencing uncovers the genetic basis of chronic mountain sickness in Andean highlanders. American journal of human genetics. 2013;93(3):452–62. Epub 2013/08/21. doi: 10.1016/j.ajhg.2013.07.011. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stobdan T, Zhou D, Ao-Ieong E, Ortiz D, Ronen R, Hartley I, et al. Endothelin receptor B, a candidate gene from human studies at high altitude, improves cardiac tolerance to hypoxia in genetically engineered heterozygote mice. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(33):10425–30. Epub 2015/08/05. doi: 10.1073/pnas.1507486112. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naeije R Physiological adaptation of the cardiovascular system to high altitude. Progress in cardiovascular diseases. 2010;52(6):456–66. Epub 2010/04/27. doi: 10.1016/j.pcad.2010.03.004. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 5.Goerre S, Wenk M, Bartsch P, Luscher TF, Niroomand F, Hohenhaus E, et al. Endothelin-1 in pulmonary hypertension associated with high-altitude exposure. Circulation. 1995;91(2):359–64. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 6.Kojonazarov B, Isakova J, Imanov B, Sovkhozova N, Sooronbaev T, Ishizaki T, et al. Bosentan reduces pulmonary artery pressure in high altitude residents. High altitude medicine & biology. 2012;13(3):217–23. Epub 2012/09/22. doi: 10.1089/ham.2011.1107. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 7.Kuc RE, Maguire JJ, Davenport AP. Quantification of endothelin receptor subtypes in peripheral tissues reveals downregulation of ET(A) receptors in ET(B)-deficient mice. Exp Biol Med (Maywood). 2006;231(6):741–5. Epub 2006/06/03. PubMed PMID: . [PubMed] [Google Scholar]
- 8.Kedzierski RM, Grayburn PA, Kisanuki YY, Williams CS, Hammer RE, Richardson JA, et al. Cardiomyocyte-specific endothelin A receptor knockout mice have normal cardiac function and an unaltered hypertrophic response to angiotensin II and isoproterenol. Molecular and cellular biology. 2003;23(22):8226–32. Epub 2003/10/31. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krejci V, Hiltebrand LB, Erni D, Sigurdsson GH. Endothelin receptor antagonist bosentan improves microcirculatory blood flow in splanchnic organs in septic shock. Critical care medicine. 2003;31(1):203–10. Epub 2003/01/25. doi: 10.1097/01.CCM.0000038205.54367.0A. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 10.Wanecek M, Weitzberg E, Alving K, Rudehill A, Oldner A. Effects of the endothelin receptor antagonist bosentan on cardiac performance during porcine endotoxin shock. Acta anaesthesiologica Scandinavica. 2001;45(10):1262–70. Epub 2001/12/12. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 11.Bagnall AJ, Kelland NF, Gulliver-Sloan F, Davenport AP, Gray GA, Yanagisawa M, et al. Deletion of endothelial cell endothelin B receptors does not affect blood pressure or sensitivity to salt. Hypertension. 2006;48(2):286–93. Epub 2006/06/28. doi: 10.1161/01.HYP.0000229907.58470.4c. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 12.Druckenbrod NR, Powers PA, Bartley CR, Walker JW, Epstein ML. Targeting of endothelin receptor-B to the neural crest. Genesis. 2008;46(8):396–400. Epub 2008/08/12. doi: 10.1002/dvg.20415. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cabrales P, Acero C, Intaglietta M, Tsai AG. Measurement of the cardiac output in small animals by thermodilution. Microvasc Res. 2003;66(2):77–82. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 14.Dimitrijevic I, Edvinsson ML, Chen Q, Malmsjo M, Kimblad PO, Edvinsson L. Increased expression of vascular endothelin type B and angiotensin type 1 receptors in patients with ischemic heart disease. BMC cardiovascular disorders. 2009;9:40 Epub 2009/08/27. doi: 10.1186/1471-2261-9-40. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dagassan PH, Breu V, Clozel M, Kunzli A, Vogt P, Turina M, et al. Up-regulation of endothelin-B receptors in atherosclerotic human coronary arteries. Journal of cardiovascular pharmacology. 1996;27(1):147–53. Epub 1996/01/01. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 16.Tykocki NR, Watts SW. The interdependence of endothelin-1 and calcium: a review. Clin Sci (Lond). 2010;119(9):361–72. Epub 2010/07/29. doi: 10.1042/CS20100145. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider MP, Boesen EI, Pollock DM. Contrasting actions of endothelin ET(A) and ET(B) receptors in cardiovascular disease. Annual review of pharmacology and toxicology. 2007;47:731–59. Epub 2006/09/28. doi: 10.1146/annurev.pharmtox.47.120505.105134. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee HO, Levorse JM, Shin MK. The endothelin receptor-B is required for the migration of neural crest-derived melanocyte and enteric neuron precursors. Developmental biology. 2003;259(1):162–75. Epub 2003/06/19. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 19.Hosoda K, Hammer RE, Richardson JA, Baynash AG, Cheung JC, Giaid A, et al. Targeted and natural (piebald-lethal) mutations of endothelin-B receptor gene produce megacolon associated with spotted coat color in mice. Cell. 1994;79(7):1267–76. Epub 1994/12/30. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 20.Gariepy CE, Williams SC, Richardson JA, Hammer RE, Yanagisawa M. Transgenic expression of the endothelin-B receptor prevents congenital intestinal aganglionosis in a rat model of Hirschsprung disease. The Journal of clinical investigation. 1998;102(6):1092–101. doi: 10.1172/JCI3702. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ge Y, Bagnall A, Stricklett PK, Strait K, Webb DJ, Kotelevtsev Y, et al. Collecting duct-specific knockout of the endothelin B receptor causes hypertension and sodium retention. American journal of physiology Renal physiology. 2006;291(6):F1274–80. doi: 10.1152/ajprenal.00190.2006. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 22.Kelland NF, Kuc RE, McLean DL, Azfer A, Bagnall AJ, Gray GA, et al. Endothelial cell-specific ETB receptor knockout: autoradiographic and histological characterisation and crucial role in the clearance of endothelin-1. Canadian journal of physiology and pharmacology. 2010;88(6):644–51. Epub 2010/07/16. doi: 10.1139/Y10-041. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 23.Fukuroda T, Fujikawa T, Ozaki S, Ishikawa K, Yano M, Nishikibe M. Clearance of circulating endothelin-1 by ETB receptors in rats. Biochemical and biophysical research communications. 1994;199(3):1461–5. doi: 10.1006/bbrc.1994.1395. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 24.Miller E, Czopek A, Duthie KM, Kirkby NS, van de Putte EE, Christen S, et al. Smooth Muscle Endothelin B Receptors Regulate Blood Pressure but Not Vascular Function or Neointimal Remodeling. Hypertension. 2017;69(2):275–85. doi: 10.1161/HYPERTENSIONAHA.115.07031. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao XS, Pan W, Bekeredjian R, Shohet RV. Endogenous endothelin-1 is required for cardiomyocyte survival in vivo. Circulation. 2006;114(8):830–7. doi: 10.1161/CIRCULATIONAHA.105.577288. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 26.Merlen C, Farhat N, Luo X, Chatenet D, Tadevosyan A, Villeneuve LR, et al. Intracrine endothelin signaling evokes IP3-dependent increases in nucleoplasmic Ca(2)(+) in adult cardiac myocytes. Journal of molecular and cellular cardiology. 2013;62:189–202. Epub 2013/06/13. doi: 10.1016/j.yjmcc.2013.05.021. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jules F, Avedanian L, Al-Khoury J, Keita R, Normand A, Bkaily G, et al. Nuclear Membranes ETB Receptors Mediate ET-1-induced Increase of Nuclear Calcium in Human Left Ventricular Endocardial Endothelial Cells. Journal of cardiovascular pharmacology. 2015;66(1):50–7. doi: 10.1097/FJC.0000000000000242. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 28.Pham I, Wuerzner G, Richalet JP, Peyrard S, Azizi M. Endothelin receptors blockade blunts hypoxia-induced increase in PAP in humans. European journal of clinical investigation. 2010;40(3):195–202. Epub 2010/04/27. doi: 10.1111/j.1365-2362.2010.02254.x. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 29.Radiloff DR, Zhao Y, Boico A, Wu C, Shan S, Palmer G, et al. The combination of theophylline and endothelin receptor antagonism improves exercise performance of rats under simulated high altitude. J Appl Physiol (1985). 2012;113(8):1243–52. doi: 10.1152/japplphysiol.01622.2011. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 30.Schroeder T, Piantadosi CA, Natoli MJ, Autmizguine J, Cohen-Wolkowieczs M, Hamilton KL, et al. Safety and Ergogenic Properties of Combined Aminophylline and Ambrisentan in Hypoxia. Clin Pharmacol Ther. 2018;103(5):888–98. doi: 10.1002/cpt.860. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naeije R, Huez S, Lamotte M, Retailleau K, Neupane S, Abramowicz D, et al. Pulmonary artery pressure limits exercise capacity at high altitude. The European respiratory journal. 2010;36(5):1049–55. doi: 10.1183/09031936.00024410. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 32.Beall CM, Decker MJ, Brittenham GM, Kushner I, Gebremedhin A, Strohl KP. An Ethiopian pattern of human adaptation to high-altitude hypoxia. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(26):17215–8. Epub 2002/12/10. doi: 10.1073/pnas.252649199. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu S, Hao G, Zhang S, Jiang D, Wuren T, Luo J. Cerebral vasoconstriction reactions and plasma levels of ETBR, ET-1, and eNOS in patients with chronic high altitude disease. Mol Med Rep. 2016;14(3):2497–502. doi: 10.3892/mmr.2016.5555. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Georgiadis D, Sievert M, Cencetti S, Uhlmann F, Krivokuca M, Zierz S, et al. Cerebrovascular reactivity is impaired in patients with cardiac failure. Eur Heart J. 2000;21(5):407–13. doi: 10.1053/euhj.1999.1742. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 35.Foller M, Mahmud H, Qadri SM, Gu S, Braun M, Bobbala D, et al. Endothelin B receptor stimulation inhibits suicidal erythrocyte death. FASEB J. 2010;24(9):3351–9. doi: 10.1096/fj.10-159483. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 36.Shihoya W, Nishizawa T, Okuta A, Tani K, Dohmae N, Fujiyoshi Y, et al. Activation mechanism of endothelin ETB receptor by endothelin-1. Nature. 2016;537(7620):363–8. doi: 10.1038/nature19319. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig S1: Cardiac output comparison between cardiac specific EdnrB homozygous (EdnrBflox/flox), heterozygous (EdnrBflox/+) and controls mice. EdnrBflox/+ mice maintains a higher CO under hypoxia. Error bar indicates ± standard error.
Supplementary Fig S2: Cardiac output comparison between BQ-788, Bosentan treated and control mice. Both BQ-788 and Bosentan treated mice maintains a relative higher CO under extreme hypoxia of 10% and 5% O2. However, CO in BQ-788 treated mice was significantly higher at 5% O2 when compared to both Bosentan and control mice. Error bar indicates ± standard error.