Abstract
Study Objectives:
Previous research revealed a positive correlation between endothelial cell injury (indicated by albuminuria) and obstructive sleep apnea (OSA). However, little else has been revealed about acute kidney injury (AKI) in patients with OSA.
Methods:
This prospective study recruited consecutive patients undergoing overnight polysomnography for evaluation of sleep apnea. Patients in whom any major disease or recent infection had been previously diagnosed were excluded. Ultimately, data from 75 patients with apnea-hypopnea indices of 5 or more were analyzed. Baseline values for the urinary albumin-creatinine ratio (UACR), serum levels for three markers of AKI (cystatin C, neutrophil gelatinase-associated lipocalin [NGAL], interleukin-18 [IL-18]), and polysomnography data were recorded and analyzed. Patients then were followed for 6 months of continuous positive airway pressure (CPAP) treatment.
Results:
At baseline, UACRs were greater in patients with more severe OSA (P = .005, r = .329). All three serum markers of AKI (cystatin C, NGAL, and IL-18) studied were positively correlated with OSA severity, and two (cystatin C and IL-18) were positively correlated with the frequency of oxygen desaturation during sleep. However, none of the AKI markers had positive correlations with UACR. After 6 months of CPAP treatment, UACR and IL-18 were decreased significantly in patients with good adherence.
Conclusions:
Albuminuria and levels of three serum markers of AKI (cystatin C, NGAL, IL-18) were positively correlated with OSA severity, and good adherence with CPAP treatment decreased albuminuria and interleukin-18 levels. These results may provide additional tools for assessing early renal injury in patients with OSA.
Citation:
Chuang LP, Lin SW, Lee LA, Chang CH, Huang HY, Hu HC, Kao KC, Hsieh MJ, Yang CT, Li HY, Chen NH. Elevated serum markers of acute kidney injury in patients with obstructive sleep apnea. J Clin Sleep Med. 2019;15(2):207–213.
Keywords: albuminuria, cystatin c, interleukin-18, neutrophil gelatinase-associated lipocalin, obstructive sleep apnea
BRIEF SUMMARY
Current Knowledge/Study Rationale: Previous research demonstrated a positive correlation between albuminuria and the severity of obstructive sleep apnea. However, little else has been revealed about acute kidney injury in patients with obstructive sleep apnea.
Study Impact: We recruited 75 consecutive patients with obstructive sleep apnea and found that baseline levels of albuminuria and serum markers of acute kidney injury (cystatin C, neutrophil gelatinase-associated lipocalin, and interleukin-18), had positive correlations with the severity of obstructive sleep apnea. After 6 months of continuous positive airway pressure treatment, albuminuria and interleukin-18 were decreased significantly in patients with good adherence.
INTRODUCTION
Obstructive sleep apnea (OSA) has a prevalence of 2% to 4% in the general population.1 Recent studies have addressed the cardiovascular consequences of OSA, such as hypertension, ischemic heart disease or stroke, and neurocognitive deficits.2 Endothelial injury due to intermittent hypoxia may play a key role in systemic vascular damage, which leads to cardiovascular and neurocognitive diseases. Albumin-uria, indicated by an elevated urinary albumin-creatinine ratio (UACR), is a sensitive indicator of endothelial injury, and previous research showed that elevated UCAR was associated with increased cardiovascular risk for patients with diabetes mellitus (DM) or hypertension, and for the general population.3–6
Chronic kidney injury, such as end-stage renal disease and proteinuria, are closely associated with OSA.7 Moreover, lowering albuminuria is associated with better health outcomes, and is a target for treatment of patients with chronic kidney disease, with or without hypertension or diabetes.3,4 Our group previously demonstrated an increased UACR in patients with OSA without DM or hypertension,8 and that treatment of OSA by continuous positive airway pressure (CPAP) can reverse proteinuria in patients with OSA.9
Microalbuminuria is a sensitive biomarker of systemic endothelial injury. Recent studies have examined acute kidney injury (AKI) in patients with OSA. For example, a study of renal biopsies of patients with morbid obesity and OSA showed glomerulomegaly and focal segmental sclerosis, presumably from increased glomerular filtration and blood flow.10 Cystatin C, neutrophil gelatinase-associated lipocalin (NGAL), and interleukin-18 (IL-18) are the most commonly studied biomarkers during ischemic changes of the proximal tubules.11 Changes in levels of these markers provide an earlier sign of AKI than does serum creatinine (SCr) elevation.
Measurements of individual AKI markers in patients with OSA have been reported previously, but to our knowledge there has been no comprehensive study in these patients to evaluate their use to assess AKI.12–17 The current study examines the use of three serum markers of AKI—cystatin C, NGAL, and IL-18—to evaluate the effects of CPAP treatment of patients with OSA.
METHODS
Study Population
The study protocol was approved by the Research and Ethics Committee of the Chang Gung Memorial Hospital (CGMH) in Taiwan (CGMH IRB no.97-0231C, no.98-2149B, and no.99-0940C). Each patient provided informed consent before participating. This study was funded by the National Institute of Science (MOST 104-2314-B-182A-119-MY3 and MOST 106-2314-B-182A-073) and Chang Gung Memorial Hospital (CMRPG3D1011 and CMRPG391231-3).
Adults age 20 years or older who visited our sleep clinics because of snoring and sleepiness between 2008 and 2012 were invited on presentation to enroll in the study. Medical records were reviewed for baseline data. Patients were excluded for a previous diagnosis of OSA, DM (defined as use of a DM medication, fasting glucose of at least 126 mg/dL, or glycated hemoglobin (HbA1C) of at least 6.5% at baseline screening18), abnormal renal function (defined as SCr higher than 1.4 mg/ dL), liver cirrhosis, chronic obstructive pulmonary disease, hematologic disease, autoimmune disease, cancer, heart failure, or recent infection. Patients taking angiotensin-converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), or diuretics were also excluded.
Polysomnography
All patients underwent standard in-laboratory overnight polysomnography (PSG) (Embla N7000, Medcare, Reykjavik, Iceland) performed using conventional methods. The variables recorded were as follows: four channels of the electroencephalogram (C3/A2, C4/A1, O1/A2, O2/A1); bilateral electro-oculogram; chin, left, and right anterior tibial electro-myogram; electrocardiogram; airflow (measured by pressure sensors and thermistors); chest and abdominal wall movement (measured by inductive respiratory plethysmographic bands); snoring (measured by a neck microphone); and arterial oxygen saturation (SpO2) (measured by pulse oximetry). Video recording was used to assess the behavior of all participants while sleeping. All measurements were collected on a computerized sleep system (Somnologica Studio 3.0, Med-care, Reykjavik, Iceland). Apnea was defined as a cessation of airflow for at least 10 seconds. Hypopnea was defined as an abnormal respiratory event with at least 30% reduction of airflow (relative to baseline) for at least 10 seconds, with at least 4% oxygen desaturation and/or arousal. The apnea-hypopnea index (AHI) is the number of events of apnea plus hypopnea per hour of total sleep time. All patients with an AHI of 5 or more were invited to participate further in the study. The oxygen desaturation index (ODI) is the number of times per hour during sleep that the blood oxygen level dropped by 4% or more from baseline.
CPAP Use and Adherence Evaluation
Participating patients with moderate or severe OSA used CPAP during 6 consecutive months of follow-up evaluation. Adherence with CPAP treatment was determined by downloading data directly from the CPAP machine's recorder. The adherence rate was defined as the number of nights during which CPAP was used for at least 4 hours divided by the total number of follow-up days.
Specimen Sampling and Enzyme-Linked Immunosorbent Assay
Morning blood and urine samples were collected from all study participants. Peripheral venous blood (20 mL) was sampled at 6:00 am on the morning after PSG and at the end of the follow-up period, with the patient in the supine position and before eating. Samples were collected in heparin rinse tubes, and centrifuged (3000 rpm × 20 minutes) within 30 minutes of collection. The levels of cystatin C, NGAL, and IL-18 were determined by enzyme-linked immunosorbent assay kits (R&D System, Inc., Minneapolis, Minnesota, United States). Briefly, a microplate was first coated with 100 μL per well of the diluted capture antibody overnight at room temperature. After washing, the microplate was blocked with 300 μL of a reagent diluent for 1 hour. Standards and samples were diluted in the reagent diluent, added to the microplate, and incubated for 2 hours. After washing, 100 μL of detection antibody was added, and incubated for 2 hours. After washing, 100 μL of streptavidin horseradish peroxidase was added, and incubated for 20 minutes. Then, 100 μL of substrate solution was added, and incubated for 20 minutes. The reaction was stopped by adding 50 μL of the stop solution. The concentration of each marker was determined by absorbance at 450 nm, using a microplate reader.
Statistical Analysis
The t test was used to compare mean values of two groups, and one-way analysis of variance to compare differences among more than two groups. Correlations of AKI markers, UACR, and parameters of PSG were determined by linear regression. All values are expressed as mean ± standard error of the mean. All analyses were two-tailed, and a value of P < .05 was considered statistically significant. Statistical analyses were performed using Statistical Package for Social Sciences 20.0 for Windows (SPSS Inc., Chicago, Illinois, United States).
RESULTS
A total of 172 consecutive adult outpatients who received initial diagnoses of OSA and agreed to use CPAP continuously were enrolled in the study. Excluded were 70 individuals due to elevated fasting blood sugar or HbA1C, and 27 individuals were lost to follow-up. Thus, the final study cohort consisted of 75 patients with OSA (Table 1). We divided these patients into four groups according to the severity of OSA, as indicated by the number of respiratory events per hour of sleep (AHI). These four groups had no significant differences in age, fasting glucose, HbA1C, SCr, cholesterol, or triglycerides. However, the four groups had significant differences in body mass index (BMI) and AHI.
Table 1.
Baseline characteristics and polysomnography parameters of enrolled patients.
Analysis of UACR (an indicator of early endothelial injury) revealed a positive association with baseline AHI (Figure 1). More specifically, UACR was higher in groups with higher AHI scores, and there was positive correlation between AHI score and UACR among all patients (P = .005, r = .329).
Figure 1. Relationship of UACR with AHI at baseline.

(A) When patients were placed into four groups according to AHI, UACR increased as AHI increased. (B) There was positive correlation between AHI and UACR (P = .005, r = .329). AHI = apnea-hypopnea index, UACR = urinary albumin-creatinine ratio.
Figure 2 shows that there were positive correlations of AHI with plasma cystatin C (P < .001, r = .529), NGAL (P = .038, r = .240), and serum IL-18 (P < .001, r = .702) levels.
Figure 2. Relationship of serum markers of acute kidney injury with AHI at baseline.
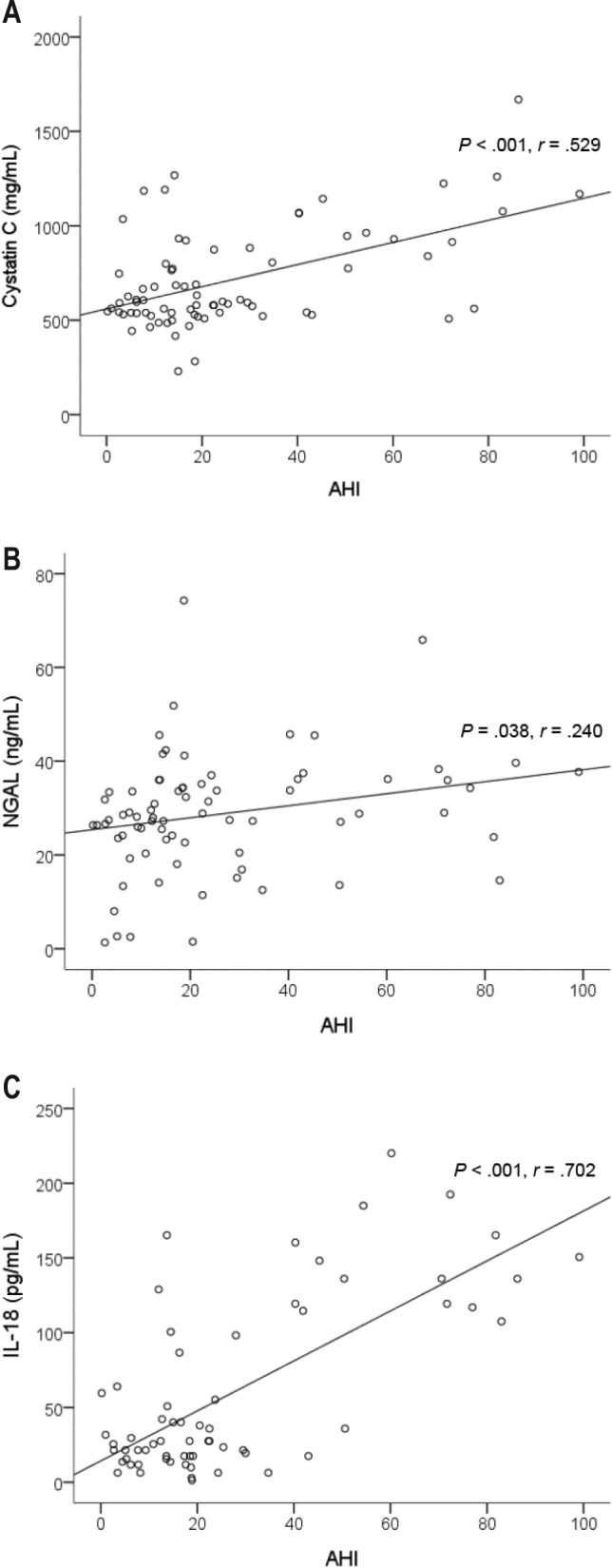
There were positive correlations of AHI with (A) plasma cystatin C, (B) serum NGAL, and (C) IL-18. AHI = apnea-hypopnea index, IL-18 = interleukin-18, NGAL = neutrophil gelatinase-associated lipocalin.
The ODI indicates the frequency of intermittent hypoxia and is an integral characteristic of OSA. Figure 3 shows that there were positive correlations of ODI with levels of cystatin C (P = .001, r = .383) and IL-18 (P < .001, r = .573), but not NGAL (P = .45).
Figure 3. Relationship of serum markers of acute kidney injury with ODI.

There were positive correlations of the ODI with (A) serum cystatin C and (C) serum IL-18, but not with (B) serum NGAL. IL-18 = interleukin-18, NGAL = neutrophil gelatinase-associated lipocalin, ODI = oxygen desaturation index.
Possible correlations of the three serum markers of AKI with endothelial injury and PSG parameters (Table 2) were assessed. UACR had no significant correlation with any of the AKI markers studied. However, the AHI correlated significantly with cystatin C (P < .001), NGAL (P < .05), and Il-18 (P < .001). In addition, the ODI correlated with cystatin C (P < .05) and IL-18 (P < .001), but not with NGAL levels.
Table 2.
R values from linear regression of serum markers of acute kidney injury with PSG parameters and albuminuria.

Among the 75 study patients, 55 with moderate to severe OSA were followed during 6 consecutive months of CPAP treatment. A CPAP adherence rate higher than 70% was recorded for 21 patients, who were defined as the “good adherence” group. No significant differences in demographic parameters were found between the poor and good adherence groups (Table 3). However, for the good adherence group, UACR decreased significantly after 6 months of CPAP treatment. Additionally, of the AKI markers studied, IL-18 was found to be decreased significantly in the good CPAP adherence group (Table 3).
Table 3.
Demographic data and change in acute kidney injury markers stratified by CPAP adherence.
DISCUSSION
It is important to identify individuals with kidney damage before there is detectable change in renal function, so that treatment can begin early. Our previous study of patients with OSA demonstrated that albuminuria is associated with AHI, and that albuminuria can be reversed by CPAP treatment.9 Such treatments are especially important in cases of AKI, which is clinically quiescent and irreversible after progression to a terminal stage.19 Other than the well-known presentation of endothelial injury with microalbuminuria, there is little known about the effect of OSA on AKI. An abrupt change in SCr, the most common indicator of AKI, correlates strongly with poor outcomes in multiple clinical settings.20 SCr is a standard indicator of kidney injury: however, SCr alone cannot meaningfully identify different types of AKI, the time of onset, rate of progression, or recovery. This has hindered the successful implementation of promising therapeutics.21
Recent study reports have proposed the use of several novel plasma and urine biomarkers to characterize the cause and course of AKI. Some of the more promising biomarkers are uri-nary or plasma NGAL, IL-18, and cystatin C.22–24 Recent data suggest that some of these new biomarkers represent important parameters of acute tubular necrosis, and are reliable predictors of the development and prognosis of AKI.25 In addition, monitoring these markers may help with early diagnosis and tracking of disease progression or recovery, not only in patients with various forms of AKI and other renal diseases, but also in patients with cardiorenal syndrome, heart failure, cardio-pulmonary bypass, and cardiothoracic surgical interventions.26
Cystatin C is mainly used as a biomarker of kidney function. The serum level of cystatin C is a more precise indicator of kidney function (as indicated by the glomerular filtration rate) than is SCr.27,28 There is some evidence that cystatin C can be used to predict the risk of the development of chronic kidney disease, in that it signals a state of preclinical kidney dysfunction.29 Cystatin C seems to have a stronger correlation with long-term outcome than does glomerular filtration rate, so cystatin C might also be linked to mortality factors independent of kidney function.30 Among patients with OSA, an increased level of cystatin C indicates clinically latent renal dysfunction,12 and treatment with a CPAP ventilator reduces the serum level of cystatin C in patients with OSA.13
NGAL has a role in innate immunity, in that it sequesters iron and therefore limits bacterial growth.31 NGAL expression occurs in neutrophils, and also at low levels in the kidney, prostate, and epithelia of the respiratory and alimentary tracts.32 Clinicians also use NGAL as a biomarker of kidney injury. In the case of AKI, there are high levels of NGAL in the blood and urine within 2 hours of renal injury.33 NGAL is a more precise and sensitive biomarker of AKI than is SCr. In fact, the increased urinary excretion of NGAL is due to tubular alterations that occur before any kidney damage can be detected by other methods. Therefore, monitoring NGAL levels can shorten delays in the diagnosis and treatment of AKI.34 For patients with OSA, the level of urinary NGAL was reported to be proportional to the severity of OSA and intermittent hypoxia14: however, a more recent paper reported no differences of uri-nary NGAL among patients with OSA.17
IL-18 is a cytokine in the IL-1 superfamily that is produced by macrophages and other cells.35 IL-18 mediates ischemic proximal tubular injury in mice and proinflammatory responses. Following ischemic injury, proximal tubules produce IL-18, which is then activated by caspase 1 and excreted into the urine.36 Urinary IL-18 is a useful biomarker of AKI, with moderate predictive value in all clinical settings.37 Serum IL-18 is an inflammatory marker in patients with OSA,15 and treatment with adenotonsillectomy can reduce serum IL-18 levels in children with OSA.16 The current study demonstrates for the first time a significant decrease in serum IL-18 levels in patients with OSA with good CPAP adherence.
The current study and several previous studies indicate a significant positive correlation between albuminuria and OSA severity.8,38,39 Microalbuminuria, indicated by an elevated UACR, is a sensitive indicator of endothelial injury and a risk factor for cardiovascular disease in patients with diabetes or hypertension, and in the general population.3–6,40 Cardiovascular complications are the major adverse effects of OSA. Because UACR correlates positively with OSA severity and negatively with CPAP adherence, it is not only a marker for cardiovascular risk, but is also a sensitive marker for treatment efficacy. The results presented here also demonstrate no significant correlation between albuminuria and the three tested AKI markers. Albuminuria is a well-known clinical biomarker for glomerular injury, and NGAL is also a marker of tubular injury.41 Thus, these markers might be useful for assessing the onset or extent of glomerular and tubular injury in patients with OSA.
Although OSA is obviously a chronic disorder, nighttime events such as intermittent hypoxia may have acute effects that contribute to this disease. According to the published paper by Tamaki et al., just 1 night of hypoxic stress can activate the invasiveness of monocytes in patients with OSA.42 Additionally, our previously published reports of studies assessing serum MMP-9 expression in patients with OSA revealed the same phenomenon; 1 night of hypoxia events can increase MMP-9 expression after sleep.43 It seems that some aspects of OSA-related injury occur on a nightly basis. However, during the events-free daytime, some injury may recover to a certain extent, depending on the severity of the injury itself and the host reparative ability.44 Thus, studies of patients with OSA using different measurement time points, such as before or after sleep, may yield different results for some injury markers.
There are several limitations of the current study that could potentially bias the results. The sample size was relatively small. However, we still found significant correlations of all three AKI markers with severity of OSA. Another limitation is that we did not specifically exclude patients with hypertension, and this could have biased the results. However, we did exclude patients who took ACEIs, ARBs, or diuretics, drugs that can affect the levels of AKI markers.34 Body weight was one of the confounding factors in the previous studies of albuminuria, but recent research indicated that body weight has no significant association with NGAL levels, so the body weight factor can be ignored in the current study.45 Finally, the relatively small number of patients assessed after CPAP use, especially in the mild (AHI 5 to 15) OSA group, and the lack of a non-CPAP control group, are other limitations of the current study.
In summary, the current study demonstrated that the serum levels of three AKI markers—cystatin C, NGAL, and IL-18— had positive correlations with the severity of OSA, and also confirmed the previously reported correlation of OSA severity with albuminuria. Our findings suggest that, in addition to endothelial injury, as assessed by albuminuria, multiple causes of AKI should be considered as consequences of OSA. Clinical measurement of AKI markers, such as cystatin C, NGAL, and IL-18, may provide improved screening for early renal injury in patients with OSA.
DISCLOSURE STATEMENT
Work for this study was performed at Sleep Center, Chang Gung Memorial Hospital, Taoyuan, Taiwan. All authors have seen and approved the manuscript. This study was funded by Ministry of Science and Technology (MOST 104-2314-B-182A-119-MY3 and MOST 106-2314-B-182A-073) and Chang Gung Memorial Hospital (CMRPG3F0071, CMRPG3G0421 and CORPG3F0861). The funders had no role in performing the study or in preparation of the manuscript. The authors report no conflicts of interest.
ABBREVIATIONS
- ACEI
angiotensin-converting enzyme inhibitor
- AHI
apnea-hypopnea index
- AKI
acute kidney injury
- ARB
angiotensin II receptor blocker
- BMI
body mass index
- CPAP
continuous positive airway pressure
- DM
diabetes mellitus
- HbA1C
glycated hemoglobin
- IL-18
interleukin-18
- NAGL
neutrophil gelatinase-associated lipocalin
- ODI
oxygen desaturation index
- OSA
obstructive sleep apnea
- PSG
polysomnography
- SCr
serum creatinine
- UACR
urinary albumin-creatinine ratio
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Newman AB, Nieto FJ, Guidry U, et al. Relation of sleep-disordered breathing to cardiovascular disease risk factors: the Sleep Heart Health Study. Am J Epidemiol. 2001;154(1):50–59. doi: 10.1093/aje/154.1.50. [DOI] [PubMed] [Google Scholar]
- 3.KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3(1):1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 4.Jarraya F, Lakhdar R, Kammoun K, et al. Microalbuminuria: a useful marker of cardiovascular disease. Iran J Kidney Dis. 2013;7(3):178–186. [PubMed] [Google Scholar]
- 5.Romundstad S, Holmen J, Kvenild K, Hallan H, Ellekjaer H. Microalbuminuria and all-cause mortality in 2,089 apparently healthy individuals: a 4.4-year follow-up study. The Nord-Trondelag Health Study (HUNT), Norway. Am J Kidney Dis. 2003;42(3):466–473. doi: 10.1016/s0272-6386(03)00742-x. [DOI] [PubMed] [Google Scholar]
- 6.Wang TJ, Evans JC, Meigs JB, et al. Low-grade albuminuria and the risks of hypertension and blood pressure progression. Circulation. 2005;111(11):1370–1376. doi: 10.1161/01.CIR.0000158434.69180.2D. [DOI] [PubMed] [Google Scholar]
- 7.Hanly PJ, Ahmed SB. Sleep apnea and the kidney: is sleep apnea a risk factor for chronic kidney disease? Chest. 2014;146(4):1114–1122. doi: 10.1378/chest.14-0596. [DOI] [PubMed] [Google Scholar]
- 8.Chou YT, Lee PH, Yang CT, et al. Obstructive sleep apnea: a stand-alone risk factor for chronic kidney disease. Nephrol Dial Transplant. 2011;26(7):2244–2250. doi: 10.1093/ndt/gfq821. [DOI] [PubMed] [Google Scholar]
- 9.Chen NH, Chou YT, Lee PH, et al. Reversibility of albuminuria and continuous positive airway pressure compliance in patients of obstructive sleep apnea syndrome. Medicine (Baltimore) 2016;95(26):e4045. doi: 10.1097/MD.0000000000004045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fletcher EC. Obstructive sleep apnea and the kidney. J Am Soc Nephrol. 1993;4(5):1111–1121. doi: 10.1681/ASN.V451111. [DOI] [PubMed] [Google Scholar]
- 11.Siew ED, Ware LB, Ikizler TA. Biological markers of acute kidney injury. J Am Soc Nephrol. 2011;22(5):810–820. doi: 10.1681/ASN.2010080796. [DOI] [PubMed] [Google Scholar]
- 12.Kato K, Takata Y, Usui Y, et al. Severe obstructive sleep apnea increases cystatin C in clinically latent renal dysfunction. Respir Med. 2011;105(4):643–649. doi: 10.1016/j.rmed.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 13.Zhang XB, Jiang XT, Lin QC, Chen X, Zeng HQ. Effect of continuous positive airway pressure on serum cystatin C among obstructive sleep apnea syndrome patients. Int Urol Nephrol. 2014;46(10):1997–2002. doi: 10.1007/s11255-014-0779-x. [DOI] [PubMed] [Google Scholar]
- 14.Murase K, Mori K, Yoshimura C, et al. Association between plasma neutrophil gelatinase associated lipocalin level and obstructive sleep apnea or nocturnal intermittent hypoxia. PLoS One. 2013;8(1):e54184. doi: 10.1371/journal.pone.0054184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minoguchi K, Yokoe T, Tazaki T, et al. Increased carotid intima-media thickness and serum inflammatory markers in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172(5):625–630. doi: 10.1164/rccm.200412-1652OC. [DOI] [PubMed] [Google Scholar]
- 16.Kheirandish-Gozal L, Gileles-Hillel A, Alonso-Alvarez ML, et al. Effects of adenotonsillectomy on plasma inflammatory biomarkers in obese children with obstructive sleep apnea: a community-based study. Int J Obes (Lond) 2015;39(7):1094–1100. doi: 10.1038/ijo.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maski MR, Thomas RJ, Karumanchi SA, Parikh SM. Urinary neutrophil gelatinase-associated lipocalin (NGAL) in patients with obstructive sleep apnea. PLoS One. 2016;11(5):e0154503. doi: 10.1371/journal.pone.0154503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34(Suppl 1):S62–S69. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicholl DD, Hanly PJ, Poulin MJ, et al. Evaluation of continuous positive airway pressure therapy on renin-angiotensin system activity in obstructive sleep apnea. Am J Respir Crit Care Med. 2014;190(5):572–580. doi: 10.1164/rccm.201403-0526OC. [DOI] [PubMed] [Google Scholar]
- 20.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 21.Lewis J, Salem MM, Chertow GM, et al. Atrial natriuretic factor in oliguric acute renal failure. Anaritide Acute Renal Failure Study Group. Am J Kidney Dis. 2000;36(4):767–774. doi: 10.1053/ajkd.2000.17659. [DOI] [PubMed] [Google Scholar]
- 22.Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365(9466):1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 23.Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL. Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kidney Dis. 2004;43(3):405–414. doi: 10.1053/j.ajkd.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 24.Ahlstrom A, Tallgren M, Peltonen S, Pettila V. Evolution and predictive power of serum cystatin C in acute renal failure. Clin Nephrol. 2004;62(5):344–350. doi: 10.5414/cnp62344. [DOI] [PubMed] [Google Scholar]
- 25.Ho J, Tangri N, Komenda P, et al. Urinary, plasma, and serum biomarkers' utility for predicting acute kidney injury associated with cardiac surgery in adults: a meta-analysis. Am J Kidney Dis. 2015;66(6):993–1005. doi: 10.1053/j.ajkd.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 26.Medić B, Rovcanin B, Vujovic KS, Obradovic D, Duric D, Prostran M. Evaluation of novel biomarkers of acute kidney injury: the possibilities and limitations. Curr Med Chem. 2016;23(19):1981–1997. doi: 10.2174/0929867323666160210130256. [DOI] [PubMed] [Google Scholar]
- 27.Roos JF, Doust J, Tett SE, Kirkpatrick CM. Diagnostic accuracy of cystatin C compared to serum creatinine for the estimation of renal dysfunction in adults and children--a meta-analysis. Clin Biochem. 2007;40(5–6):383–391. doi: 10.1016/j.clinbiochem.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 28.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40(2):221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 29.Shlipak MG, Katz R, Sarnak MJ, et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145(4):237–246. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]
- 30.Stevens LA, Levey AS. Chronic kidney disease in the elderly--how to assess risk. N Engl J Med. 2005;352(20):2122–2124. doi: 10.1056/NEJMe058035. [DOI] [PubMed] [Google Scholar]
- 31.Yang J, Goetz D, Li JY, et al. An iron delivery pathway mediated by a lipocalin. Mol Cell. 2002;10(5):1045–1056. doi: 10.1016/s1097-2765(02)00710-4. [DOI] [PubMed] [Google Scholar]
- 32.Friedl A, Stoesz SP, Buckley P, Gould MN. Neutrophil gelatinase-associated lipocalin in normal and neoplastic human tissues. Cell type-specific pattern of expression. Histochem J. 1999;31(7):433–441. doi: 10.1023/a:1003708808934. [DOI] [PubMed] [Google Scholar]
- 33.Bennett M, Dent CL, Ma Q, et al. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol. 2008;3(3):665–673. doi: 10.2215/CJN.04010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blazquez-Medela AM, Garcia-Sanchez O, Blanco-Gozalo V, et al. Hypertension and hyperglycemia synergize to cause incipient renal tubular alterations resulting in increased NGAL urinary excretion in rats. PLoS One. 2014;9(8):e105988. doi: 10.1371/journal.pone.0105988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okamura H, Tsutsi H, Komatsu T, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378(6552):88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 36.Melnikov VY, Ecder T, Fantuzzi G, et al. Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J Clin Invest. 2001;107(9):1145–1152. doi: 10.1172/JCI12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y, Guo W, Zhang J, et al. Urinary interleukin 18 for detection of acute kidney injury: a meta-analysis. Am J Kidney Dis. 2013;62(6):1058–1067. doi: 10.1053/j.ajkd.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 38.Faulx MD, Storfer-Isser A, Kirchner HL, Jenny NS, Tracy RP, Redline S. Obstructive sleep apnea is associated with increased urinary albumin excretion. Sleep. 2007;30(7):923–929. doi: 10.1093/sleep/30.7.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ursavas A, Karadag M, Gullulu M, et al. Low-grade urinary albumin excretion in normotensive/non-diabetic obstructive sleep apnea patients. Sleep Breath. 2008;12(3):217–222. doi: 10.1007/s11325-008-0169-7. [DOI] [PubMed] [Google Scholar]
- 40.Mauer M, Fioretto P, Woredekal Y, Firedman E. Diseases of the Kidney and Urinary Tract. Philadelphia, PA: Lippincott Williams and Wilkins; 2001. Diabetic Nephropathy. In: Schrier R, ed; pp. 2083–2127. [Google Scholar]
- 41.Chou KM, Lee CC, Chen CH, Sun CY. Clinical value of NGAL, L-FABP and albuminuria in predicting GFR decline in type 2 diabetes mellitus patients. PLoS One. 2013;8(1):e54863. doi: 10.1371/journal.pone.0054863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tamaki S, Yamauchi M, Fukuoka A, et al. Nocturnal hypoxic stress activates invasive ability of monocytes in patients with obstructive sleep apnoea syndrome. Respirology. 2009;14(5):689–694. doi: 10.1111/j.1440-1843.2009.01540.x. [DOI] [PubMed] [Google Scholar]
- 43.Chuang LP, Chen NH, Lin SW, Chang YL, Chao IJ, Pang JH. Increased matrix metalloproteinases-9 after sleep in plasma and in monocytes of obstructive sleep apnea patients. Life Sci. 2013;93(5–6):220–225. doi: 10.1016/j.lfs.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 44.Mills PJ, Natarajan L, von Kanel R, Ancoli-Israel S, Dimsdale JE. Diurnal variability of C-reactive protein in obstructive sleep apnea. Sleep Breath. 2009;13(4):415–420. doi: 10.1007/s11325-009-0268-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen CN, Chou CH, Jeng SF, et al. Urinary Neutrophil Gelatinase-Associated Lipocalin Levels in Neonates. Pediatr Neonatol. 2016;57(3):207–212. doi: 10.1016/j.pedneo.2015.09.003. [DOI] [PubMed] [Google Scholar]




