Abstract
Study Objectives:
To investigate the temporal association between chronic musculoskeletal pain (CMP) and sleep in women who are postmenopausal in a 10-day actigraphic study. This is a microlongitudinal study in which 52 participants were allocated to 4 groups women who are postmenopausal: control (CTRL, n = 10), chronic musculoskeletal pain (CMP, n = 12), insomnia (INS, n = 15) and chronic musculoskeletal pain+insomnia (CMP+INS, n = 15).
Methods:
All volunteers underwent a clinical interview and completed questionnaires, used an actigraph, and kept sleep diaries for 10 consecutive days.
Results:
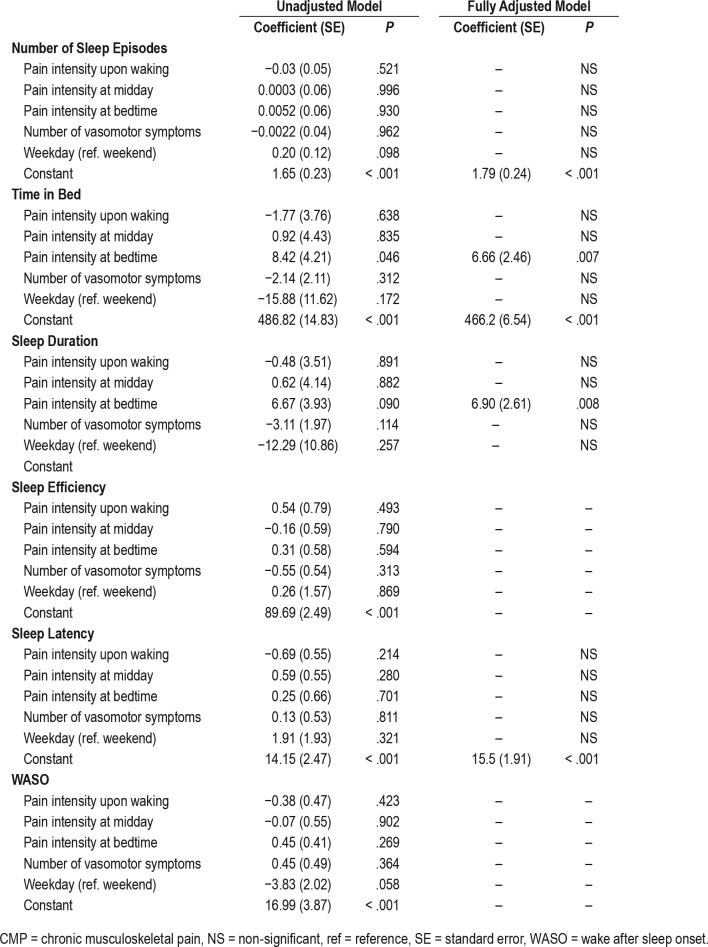
Women in the CMP+INS group presented more sleep episodes (mean of 1.02 episodes) and longer sleep latency (8.97 minutes), as well as higher pain intensity during the day compared to the other groups. Sleep duration recorded by actigraphy directly predicted pain intensity the following morning on waking, with a 1-unit increase in pain intensity, for every 6.9 minutes more of sleep. Higher pain intensity at bedtime was a significant predictor of both increased time in bed and sleep duration, meaning that for each 1-unit increase in pain intensity at bedtime, sleep duration increased by an average of 6.7 minutes.
Conclusions:
Data showed that the coexistence of insomnia and CMP results in greater pain intensity and alterations in sleep homeostasis. Collectively, the data indicate that there is a bidirectional and directly proportional relationship between sleep duration and pain intensity in women who are postmenopausal with insomnia. This result strongly suggests that both sleep and pain conditions should be targeted in the treatment of women who are postmenopausal.
Citation:
Frange C, Hachul H, Hirotsu C, Tufik S, Andersen ML. Temporal analysis of chronic musculoskeletal pain and sleep in postmenopausal women. J Clin Sleep Med. 2019;15(2):223–234.
Keywords: actigraphy, insomnia, musculoskeletal pain, postmenopause, sleep
BRIEF SUMMARY
Current Knowledge/Study Rationale: Pain and sleep are thought to share a reciprocal relationship. In sleep disorders such as insomnia, there is an increase in pain sensitivity and a decrease in pain thresholds. Although women who are postmenopausal often have insomnia and chronic pain, there is a lack of studies addressing these two factors in this specific population.
Study Impact: The findings highlight the association between chronic musculoskeletal pain and insomnia in women who are postmenopausal, as increased sleep duration was a predictor of increased pain intensity upon waking, and more pain at bedtime predicted both increased time in bed and sleep duration. Our results introduce a novel concept about the bidirectional relationship between pain and sleep in relation to women who are postmenopausal that should be further investigated in future studies.
INTRODUCTION
Many women who are postmenopausal have insomnia (INS) and chronic musculoskeletal pain (CMP), which, along with vasomotor symptoms, are considered hallmarks of menopausal symptomatology.1,2 Few studies have investigated the relationship between sleep and daily CMP, exploring the influence of sleep disorders on pain and vice versa, through temporal associations. These types of studies, called sequential analyses, temporal associations, or temporal pattern analyses, verified the interaction of variables (sleep and pain) in prediction models. Generally, they were longitudinal (ie, months to years) or microlongitudinal (hours to days) studies, so that the prediction models can be verified over time and the direction of causality can be postulated.
Actigraphy has been increasingly used to investigate the sleep-pain relationship by objectively estimating sleep variables and assessing temporal associations. The first studies were performed in women with fibromyalgia and showed a sequential relationship between sleep and pain: a night of poor sleep was followed by a painful day, and a more painful day preceded a night of poorer sleep.3 Later, an investigation of patients with INS and CMP of both sexes focused on the evaluation of their sleep-wake cycle for 2 consecutive days using actigraphy, sleep diaries, and questionnaires.4 Shorter sleep duration, frequent awakenings, and decreased sleep efficiency were associated with severe INS.4 In a study of the bidirectional relationship between sleep and pain in women with chronic pain, a disturbed night of sleep preceded the report of increased pain on the following day.5 In turn, after a day of higher pain perception, the following night of sleep was disturbed.5 Collectively, these data support the widely accepted idea that the relationship between sleep-pain is bidirectional, with pain disrupting sleep and sleep deprivation or disturbance increasing pain, one influencing the other and becoming difficult to dissociate in clinical practice.
This relationship has already been explored in several experimental and clinical studies with polysomnography, and also in review studies.6–9 However, inconsistent data related to the changes in sleep pattern under CMP conditions emphasized the uncertainty of this bidirectional association.10,11
Recently, there has been an increased focus on the temporal associations between sleep and pain, the results of which have suggested that sleep disturbances are more significant predictors of pain than in the opposite direction.9,12,13 A study examining the influence of the previous night's sleep on pain reports estimated sleep variables through actigraphy for 7 days in a sample of both sexes with CMP and INS.14 Although daytime pain did not influence nighttime sleep variables, impaired sleep quality was a consistent predictor of pain during the following day. Presleep pain predicted self-reported poor sleep quality, but not as measured by actigraphy.14 Additionally, pain ratings upon waking and throughout the day were predicted by perceived worse sleep quality the previous night. This study adds to the evidence that sleep problems may be a more reliable and stronger predictor of pain than pain is of sleep problems.15 Therefore, the so-called bidirectional association appears to be stronger between sleep and pain, than that between pain and sleep—which challenges the often-assumed bidirectional relationship and demands that we reconsider this relationship.
A longer actigraphy-estimated sleep measures study investigated women with nighttime pain for 27 consecutive days along with the use of a sleep diary and questionnaires. Their sample consisted of women at different stages of female reproductive life (self-reported premenopausal, menopausal, and postmenopausal transition). The women were allocated into two groups: a group with night pain and a group with no complaints of night pain. Both groups had similar characteristics, except in respect of the reproductive stage: the group with pain had more women who were postmenopausal. The authors found that the report of night pain was associated with reduced sleep efficiency, feeling unrested after waking up and, contrary to most studies on sleep-pain, they found longer sleep duration in the group of women with nighttime pain. Women who reported vasomotor symptoms showed increased movement during sleep and greater sleep fragmentation, and also reduced sleep efficiency.16
There have been no temporal investigations into the relationship between CMP and sleep in women who are post-menopausal. Taking into consideration its complexity, the aim of this study was to investigate the temporal relationship between sleep and CMP in women who are postmenopausal over a 10-day period. Our previous study analyzed sleep patterns in women who are postmenopausal with CMP and identified a bidirectional relationship between sleep and pain using a cross-sectional design, meaning that it was not possible to indicate the direction of causality.17 In this microlongitudinal study, we hypothesized that reduced actigraphy estimated sleep variables would predict increased pain intensity at all moments of the day (on waking, at midday, and before bedtime). Additionally, we expected that women who are postmenopausal with both INS and CMP would be more affected than those with only one of these conditions in terms of both pain and sleep.
METHODS
Ethical Aspects
The study was approved by the Ethics Committee of our Institution (#786.299/2014). It was reviewed and carried out according to the Declaration of Helsinki. Recruitment took place between February 2015 and October 2016.
Participants
A total of 355 women were assessed for eligibility to take part in the study, with 52 being selected after applying the inclusion and exclusion criteria (Figure 1). The inclusion criteria were as follows: women age 50 to 65 years; presenting at least 1 year of amenorrhea; follicle-stimulating hormone (FSH) concentrations ≥ 30 mIU/mL; agreed to undergo a clinical diagnosis; no hormonal therapy in the previous 6 months; and a body mass index (BMI) < 30 kg/m2. Noninclusion criteria were: answering positively to three or more items in the STOP-Bang questionnaire, a screening tool for obstructive sleep apnea18; self-report of uncontrolled clinical diseases (diabetes, hypertension, renal and other cardiovascular diseases); self-report of neurologic disorders (neuropathies, stroke, Parkinson disease); psychiatric disorders; use of psychoactive drugs such as hypnotics, anti-depressants, anxiolytics, benzodiazepines and central nervous system stimulants; attending psychotherapy, physiotherapy, or treatments for INS and/or pain; shift work; and illiteracy. Exclusion criteria were: the presence of other sleep disorders diagnosed by polysomnogram examination (narcolepsy, obstructive sleep apnea evidenced by an apnea-hypopnea index (AHI) ≥ 15 events/h, periodic limb movements (PLMs) > 15 events/h and parasomnias), actigraphy misuse, malfunction, or missed recordings.
Figure 1. Study flowchart.
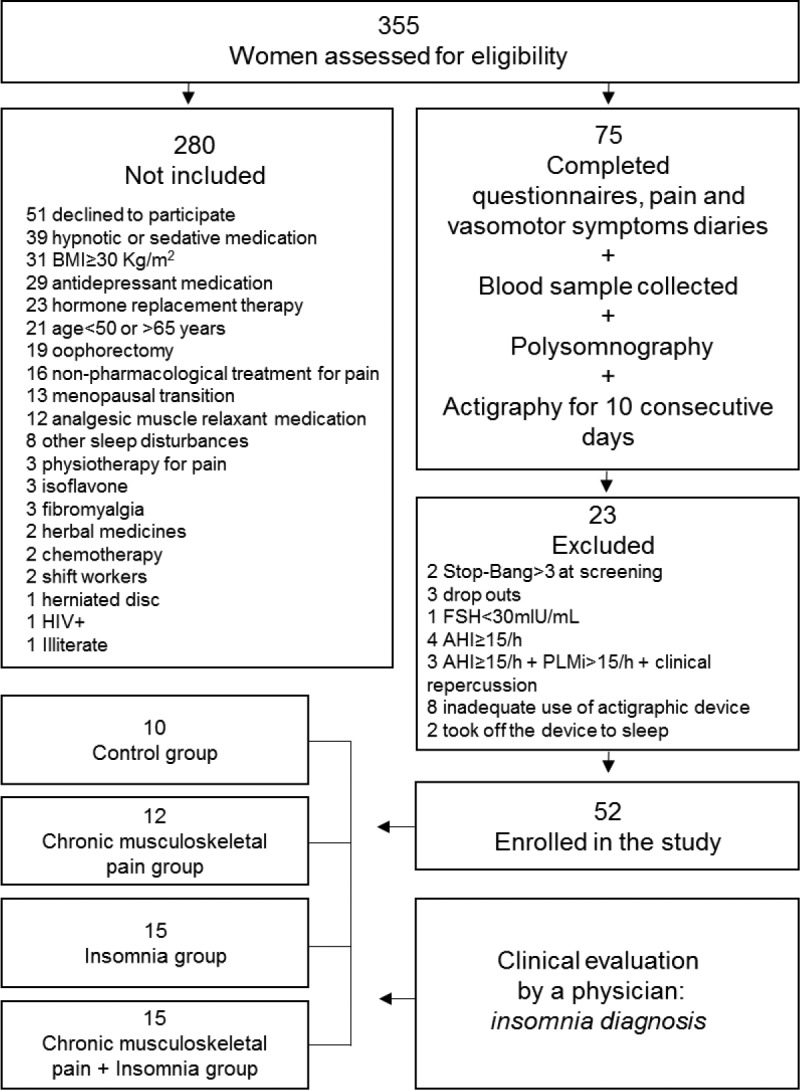
AHI = apnea-hypopnea index, BMI = body mass index, FSH = follicle-stimulating hormone, HIV+ = human immunodeficiency virus, PLMi = periodic limb movement index.
Procedures
This investigation comprised four appointments. In the first one, participants provided written informed consent and sociodemographic data on age, marital status, ethnicity, monthly income, and educational level. They also completed:
The Insomnia Severity Index (ISI), which assesses sleep onset, sleep maintenance and terminal INS, dissatisfaction with current sleep pattern, interference with daily functioning, and degree of distress or concern caused by the sleep problem19;
The Nordic Musculoskeletal Questionnaire (NMQ), which identifies participants who have had chronic CMP complaints for more than 12 months20;
The morningness-eveningness questionnaire, which establishes the circadian preference of the participants as morning, intermediate, or evening type.21,22
They were also instructed on how to keep the pain intensity diary, which participants had to complete 3 times a day upon waking, at midday, and before bedtime for 10 consecutive days using a visual analog scale (0 “no pain”, 10 “worst imaginable pain”)23; and the vasomotor symptoms diary, which participants had to complete before going to bed, recording the number of hot flashes and night sweats of the previous day and night (from 0 to 9 or more than 10) for 10 days.
A clinical evaluation was also performed in which weight and height were measured (allowing for the calculation of BMI; kg/m2), and information was collected on tobacco and alcohol consumption, the presence of self-reported comorbidi-ties such as hypertension, diabetes, and osteoporosis, and the type of menopause (spontaneous or surgical) and its duration Postmenopausal stage was classified according to the Stages of Reproductive Aging Workshop criteria.24 The participants were also given instructions on how to use the actigraph.
At the second appointment, the participants returned their actigraphy device, sleep diary, and pain and vasomotor symptoms diaries. At the third appointment, the participants underwent basal polysomnography (PSG) to exclude sleep disorders and to compare sleep patterns among the groups. On the morning after PSG recording, blood was drawn from a peripheral forearm vein to evaluate fasting hormone levels after an overnight fast of 12 hours. The blood was collected in appropriate tubes according to the required laboratory tests and FSH was measured using the acridinium-ester chemiluminescence method (Advia Centaur; Siemens Healthcare Diagnostics Inc, Tarrytown, New York, United States). Only those participants with an ISI ≥ 15 were invited for a fourth appointment, for a clinical consultation to confirm the INS diagnosis.
Wrist Actigraphy and Sleep Diaries
Actigraphy can provide unique information regarding temporal interrelations between sleep and pain, and has become a leading modality to assess the state of sleep and objective INS. While not appropriate for the diagnosis of sleep-disordered breathing or PLMs, multiday actigraphy is highly appropriate for examining nightly variability in INS of patients in their home environment, with their regular habits and routine. Reduced night-to-night variability obtained from actigraphy can reflect improved sleep stability and be an objective measure of sleep quality. In addition, actigraphy is useful to investigate individual preferences regarding sleep and waking times, which is hypothesized to be a behavioral manifestation of underlying circadian rhythms. Morningness (early sleep timing) and eveningness (late sleep timing) are the extremes of these individual preferences.
An actigraph is a wristwatch-like device loaded with a miniaturized acceleration sensor25–27 that quantifies physical motions. The resulting sleep-wake pattern of actigraphy shows sleep status including sleep onset time, considered as 10 consecutive minutes of no arm movement (hour and minutes), and sleep offset time (hour and minutes). Time in bed was considered as sleep duration plus awakenings, from initial sleep onset to sleep offset (in minutes); sleep duration was the total amount of sleep without awakenings (in minutes); sleep efficiency was calculated as sleep duration divided by time in bed (as a percentage); sleep latency was measured as the time between bedtime and falling asleep (in minutes); and wake after sleep onset (WASO) was the time spent awake between initial sleep onset and final sleep offset (in minutes).28 Sleep episodes were defined as awakenings that lasted for at least 1 minute.
The actigraph (Motionlogger Watch, Ambulatory Monitoring, Inc., United States) was worn on the non-dominant wrist (de Souza et al.29) by the participants, concurrently with the completion of sleep diaries,26,29 to provide a behavioral assessment in their home environment.
Actigraphy data were collected in 1-minute sampling epochs, using the zero-crossing mode,29 and sleep-wake variables were calculated using the Motionlogger WatchWare software 1.45.0.5 (Ambulatory Monitoring Inc., United States). The analyses were performed using ActionW 2.6 software (Ambulatory Monitoring Inc.) by a blinded investigator (CF).
Participants were requested to wear the actigraph for 10 consecutive days, only removing it during bathing. Participants were instructed to push the event button at bedtime and wake time to create a marker in the data to be scored. Sleep diaries were used to set the parameters for total time in bed in the actigraphy software. When the participant did not push the event button, sleep onset/offset was considered to occur after 10 minutes of no arm movement from the time recorded in the sleep diary.29
Actigraphy has been validated by demonstrating its agreement with polysomnographic recordings.25,30 About 5 to 7 nights are considered reliable for estimating total sleep time.31 We used 10 days to compare weekdays and weekends. All variables related to sleep were calculated using the Cole-Kripke algorithm.32 The number of episodes of sleep was not included in the ActionW software package; this was calculated using the SPSS 18 package.
Sleep Pattern By PSG Examination
In order to assess objective sleep patterns and to exclude volunteers with other comorbid sleep disorders we performed a full-night basal PSG examination. We used a digital system (EMBLA N7000, Embla Systems Inc., Broomfield, Colorado, United States) during their usual sleep time. The following physiological variables were evaluated: electroencephalogram, electrooculogram (bilateral), electromyogram, electrocardiogram (derivation D2 modified), airflow detection by a thermocouple and by nasal pressure, respiratory effort using thoracic and abdominal x-trace belts, snoring and body position by EMBLA sensors, and pulse rate by an EMBLA oximeter. All PSG were visually scored by a registered and trained PSG technologist, blinded to group allocation. All sleep stages, electroencephalogram arousals, leg movements, and respiratory events were scored according to the guidelines of the American Academy of Sleep Medicine.33,34
Definition of Groups
The presence of CMP was established through the application of the NMQ, with the participants categorized into those with no symptoms and those presenting symptoms (CMP at ≥ 1 body site). The presence of INS was established through application of the ISI, with those with a score ≥ 15 having a clinical consultation with a gynecologist specializing in sleep medicine. A diagnosis of INS was confirmed in all cases, according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition criteria. Participants were then categorized into four groups based on complaints of CMP and INS diagnosis: CTRL, healthy postmenopausal controls without CMP and without INS; CMP, participants presenting CMP complaints without insomnia; INS, participants presenting an insomnia disorder diagnosis without CMP; and CMP+INS, participants having both conditions.
Statistical Analysis
The demographic and clinical characteristics of the participants as well as sleep variables were summarized using descriptive statistics. One-way analysis of variance followed by Tukey post hoc test was used to compare PSG variables among the groups. Generalized Estimation Equations (GEE)35 analyses were performed to test predictive models for (1) previous night sleep pattern (number of sleep episodes, sleep duration, sleep time, sleep efficiency, sleep latency, and WASO) on pain intensity (on waking, at midday, and before bedtime); and (2) pain intensity (on waking, at midday, and at bedtime) on each of the aforementioned sleep variables. For sleep duration and sleep efficiency of the previous night, the assumption of normality in the data distribution was not violated as for the other sleep and pain variables, and thus the mixed linear model,36 also known as the multilevel model or hierarchical linear model, was used. Models were run using parameter estimation and inference to account for the correlations in the data due to repeated assessments across days within individuals.
Our choice of a GEE approach allowed the examination of temporal associations among variables while taking into account the correlations among outcome measurements within individuals. Furthermore, GEE is a semiparametric approach and relies less on explicit distributional assumptions. GEE allows the incorporation of the dependency between the observations of the same individual resulting from the repeated measurements carried out over time. In this study, the functions of identity bonding, normal marginal distribution, and an autoregressive dependence structure of order 1 between the observations of the same patient were adopted. Although this model admits normal marginal distribution, the GEE allows the relaxation of the normality assumption in the distribution of the dependent variables. The mixed linear model36 integrates the effect of each patient as a random effect, accepting a possible dependence between the observations of the same individual. Unlike GEE, whose intercept is a constant, this model presents a random intercept that incorporates the specific characteristics of each participant. This model assumes normality in the data, which was verified using the Kolmogorov-Smirnov test. Initially, all predictor variables were included in the model, then the nonsignificant variables at 5% were excluded one by one in order of significance (backward method). Data analyses were conducted using the STATA command ‘‘xtgee’’ (STATA version 12 SE, StataCorp, College Station, Texas, United States), and the Statistical Package for the Social Sciences (SPSS) Version 22.0 (SPSS 22.0). Statistical significance was set at P ≤ .05.
RESULTS
Sociodemographic and Clinical Information
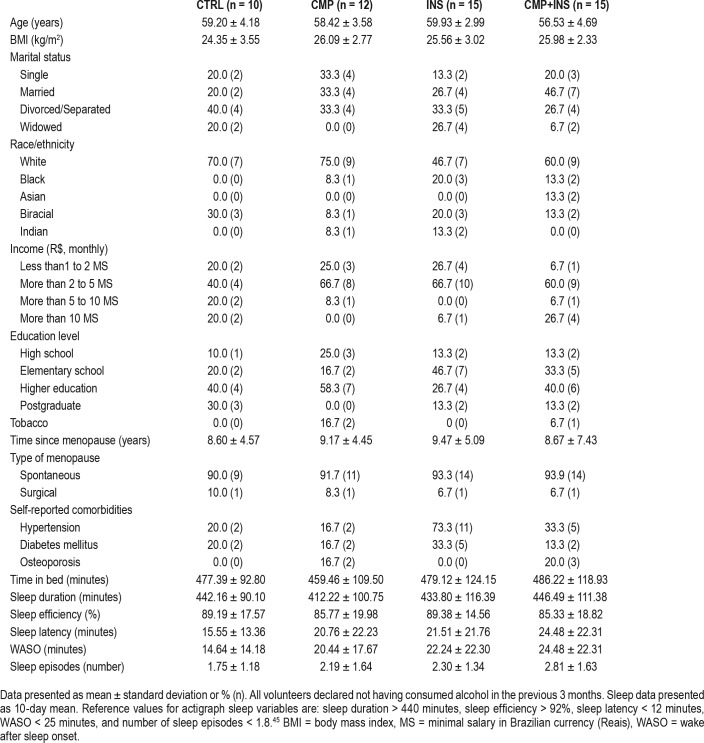
From a total of 75 women who entered the protocol, 52 were eligible and were allocated into four groups: CTRL, CMP, INS, and CMP+INS (Figure 1). Table 1 depicts the homogeneity of the sample.
Table 1.
Sociodemographic, clinical characteristics, and actigraphy variables of the control (CTRL), chronic musculoskeletal pain (CMP), insomnia (INS), and chronic musculoskeletal pain+insomnia (CMP+INS) groups.
Actigraphy Sleep and Wake Time Characteristics
Analyzable actigraphy data were obtained from 52 participants, and a total of 82.7% of women had data for all 10 nights, and 96.2% for 6 nights or more, including weekdays and weekends.
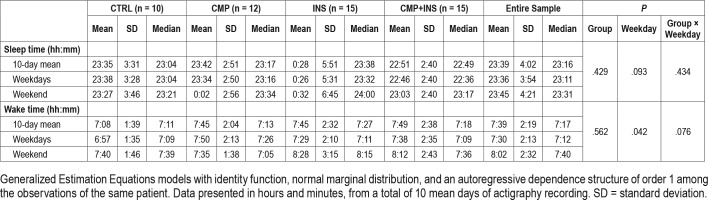
To assess whether the groups differed in respect of rhythmic behaviors of sleep and wake times we performed the GEE models including for the effects of the interaction between groups and weekdays/weekends. The groups presented statistically similar rhythmic behaviors in respect of bedtime on weekdays (P = .434), bedtime per group (P = .429), weekdays effect (P = .093) and interaction between groups and weekdays (P = .434). Wake times on weekdays were not different between the groups (P = .076). When analyzing pooled data, participants woke up 35 minutes earlier on weekdays than at weekends (P = .042) (Table 2).
Table 2.
Actigraphy mean sleep time and wake time of control (CTRL), chronic musculoskeletal pain (CMP), insomnia (INS), and chronic musculoskeletal pain+insomnia (CMP+INS) groups.
Circadian Preferences
No statistical differences were found in circadian preferences between the groups (χ2 = 5.257; df = 6; P = .511), with no differences between evening-type, intermediate and morning-type in our sample.
Vasomotor Symptoms
No statistical differences were found in vasomotor symptoms between the groups (P = .421), as our entire sample comprised women in the late postmenopausal stage with more than 8 years postmenopause.24
Self-Reported Pain Etiology
CMP was a result of the following disorders: plantar fasciitis, elbow and shoulder tendinitis, hip and shoulder bursitis, carpal tunnel syndrome, low back pain, cervical spine pain, ankylosing spondylitis, temporomandibular disorder, and hand, shoulder, arm, back, knee, hip, leg, and foot pain.
Sleep Pattern (PSG)
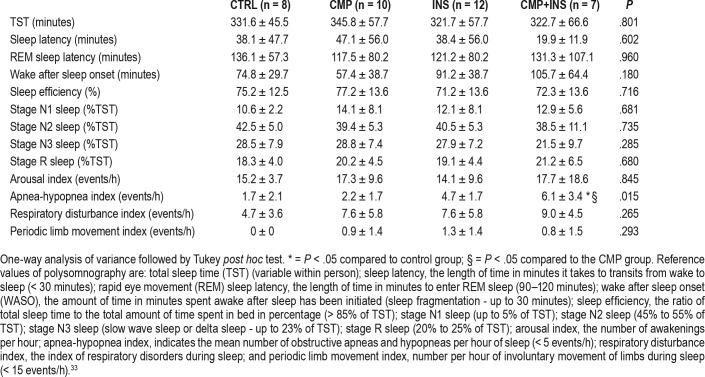
From the 52 volunteers who had actigraphy examination, 37 underwent PSG examination. We observed an increased apnea-hypopnea index (AHI) in the group with both comorbidities, indicating mild obstructive sleep apnea37 (OSA), compared to the control and to CMP group. Clinically, all the groups in our sample of women who are postmenopausal had impaired sleep with a short sleep duration of < 360 minutes; reduced stage N2 sleep, indicating attempts to maintain sleep; and increased WASO, evidence of sleep fragmentation. All groups, except the CMP+INS group, had poor sleep efficiency (< 85%), indicating nonrestorative sleep and increased stage N3 sleep, indicating more time in deep sleep. All groups, except the CMP group, had increased rapid eye movement sleep latency, indicating more time to enter rapid eye movement sleep stage (Table 3).
Table 3.
Polysomnography mean ± standard deviation variables among control (CTRL), chronic musculoskeletal pain (CMP), insomnia (INS), and chronic musculoskeletal pain+insomnia (CMP+INS) groups (n = 37).
Actigraphy Measured Nighttime Sleep Variables as a Predictor of Pain Intensity
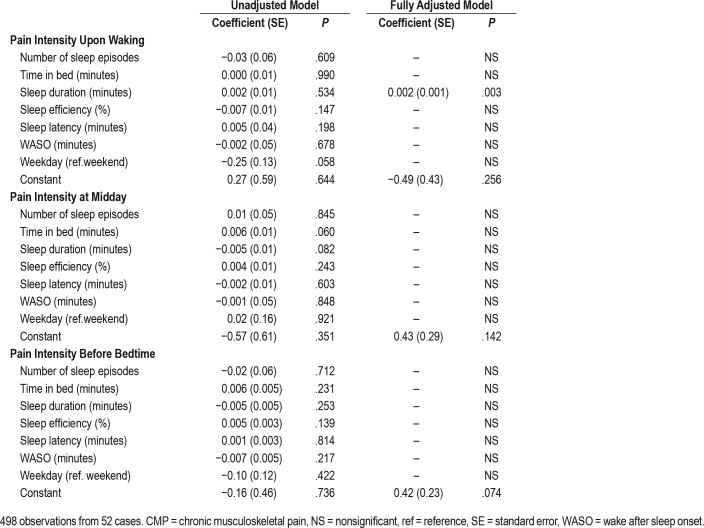
For pain intensity upon waking, at midday and before bedtime, according to the adjusted model of GEE, we found increased pain intensity at all moments for the CMP+INS group (Figure 2). For pain intensity upon waking, GEE models evaluated the previous night's sleep (number of sleep episodes, time in bed, sleep duration, sleep efficiency, sleep latency, WASO) as a predictor of pain intensity on the following morning. Three models showed statistically significant temporal associations between increased sleep duration and pain (P = .003) (Table 4). Both groups with CMP, the CMP group (P = .002) and the CMP+INS group, (P = .002) remained significant in the final model, meaning that they were associated with higher reports of pain intensity on the following morning. For each additional minute of sleep, participants reported a 0.002 increase in pain intensity upon waking. Only the CMP and CMP+INS groups presented increased risk of pain (1.90 more) compared to the CTRL group, with no significant difference in pain between the CTRL and INS groups.
Figure 2. Actigraphy nighttime sleep as a predictor of pain intensity upon waking, at midday and before bedtime in the adjusted model of Generalized Estimation Equations (P < .05).
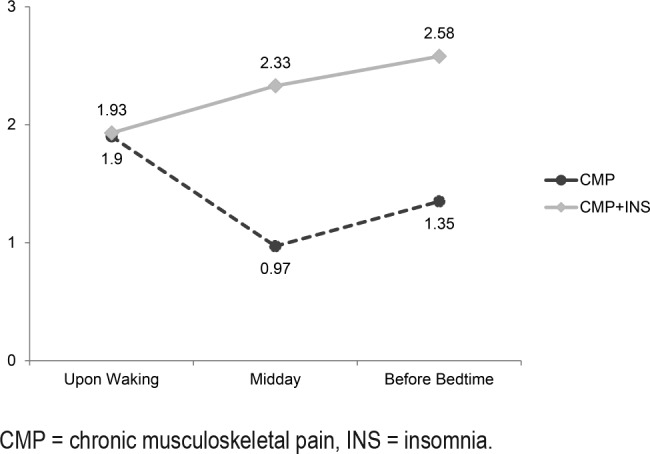
CMP = chronic musculoskeletal pain, INS = insomnia.
Table 4.
Actigraphy measured nighttime sleep variables as a predictor of pain intensity upon waking, at midday and before bedtime.
For pain intensity at midday, only the CMP (P = .049) and CMP+INS (P < .001) groups remained significant in the final model. Therefore, CMP and CMP+INS presented more pain (0.97 and 2.33 more, respectively) than the CTRL group. There was no statistical difference in pain intensity at midday between the CTRL and INS groups.
As shown in Table 4, no nighttime sleep variables were a significant predictor of pain intensity at bedtime. Only CMP (P = .010) and CMP+INS (P < .001) remained signifi-cant in the final model. The CMP and CMP+INS groups presented a higher risk of pain intensity (1.35 and 2.58 higher, respectively) than control groups. There was no statistical difference in pain intensity at bedtime between the CTRL and INS groups.
Pain Intensity as a Predictor of Actigraphy Measured Nighttime Sleep Variables
Higher pain intensity during the day was not a significant predictor of sleep episodes (Table 5). Only the CMP+INS group presented more pain (1.02 more, on average) than the CTRL group (P = .002). No statistically significant differences were found between other groups.
Table 5.
Pain intensity upon waking, at midday, and before bedtime as a predictor of actigraphy measured nighttime sleep variables.
Pain intensity at bedtime was a significant predictor of time in bed (P = .007), meaning that the greater the pain at bedtime, the greater the time in bed (6.7 minutes longer for each 1-unit increase in pain intensity). Higher pain intensity at bedtime also predicted increased sleep duration (P = .007). The greater the pain intensity at bedtime, the greater the sleep duration (6.9 minutes longer for each 1-unit increase in pain intensity). The group with the highest pain intensity at bedtime was the CMP group (P = .020), which presented decreased sleep duration, 36.2 minutes less than other groups.
Sleep efficiency and WASO were not predicted by pain intensity at any of the three times, suggesting that pain before bed had little effect on sleep efficiency and WASO in the 10-night period. However, a marginal significance was observed for WASO (P = .058), with all groups presenting reduced WASO time on weekdays, although this was not statically significant (Table 5).
Pain intensity during the day was not a significant predictor of sleep latency, and remained significant in the final model only in the CMP+INS group (P = .006), meaning that CMP+INS presented higher sleep latency, 8.97 minutes more on average than the CTRL group. There were no significant differences in pain between the CMP P = .120) and INS (P = 0.106) groups compared to the CTRL group (Table 5).
DISCUSSION
To the best of our knowledge, this is the first study to explore the relationship between CMP and sleep, particularly in relation to INS, using nightly objective measures in women who are postmenopausal. In our sample, increased sleep duration predicted higher pain intensity upon waking. Pain upon waking and at midday did not predict sleep variables, but higher pain intensity at bedtime predicted more time in bed and increased sleep duration, creating a cyclical relationship. These findings were contrary to our expectations. We had hypothesized that reduced sleep duration would predict higher pain intensity at all moments of the day (on waking, at midday, and before bed), and that this higher pain intensity would have in turn predicted reduced sleep duration.
Although there is more evidence linking reduced sleep or sleep deprivation with increased pain,11,38–41 there are some studies that question this.14,15 In the Study of Women Across the Nation, the authors investigated women with and without pain during sleep.16 Higher nighttime pain severity was associated with longer sleep duration and reduced sleep efficiency. Women reporting nighttime pain averaged 5.2 to 12.1 minutes more sleep than women with no pain.16 In our study, for each additional minute of sleep duration there was a reported 0.002 increase in pain intensity upon waking. Although this finding was statistically significant, it is important to note that the strength of the sleep-pain association was small.
Our findings are in accordance with other studies that show that increased awakenings and decreased sleep efficiency were related to INS.4 Increased pain during the day was reported after an impaired night of sleep, and a day of increased pain perception predicted a disturbed night of sleep.5 As in our investigation, greater pain intensity during the day did not predict nighttime sleep variables, but impaired sleep quality was a predictor of pain during the next day.16 Consequently, we notice that the quality of sleep is affected, as well as its quantity.
There has not yet been an explanation of the link between increased sleep duration and increased pain and a description of the mechanism that could be responsible for such a relationship. Our findings raise the question of whether increased sleep duration could be the result of an (unsuccessful?) attempt by the body to reduce pain. We might expect that individuals with chronic pain could obtain relief through more sleep, and that this was, therefore, an adaptation strategy to cope with pain. Another possible hypothesis is that the increase in sleep duration reflects a lack of restorative sleep, because those with higher pain levels had difficulty maintaining sleep, which resulted in increased sleep on the following night (recovery sleep).
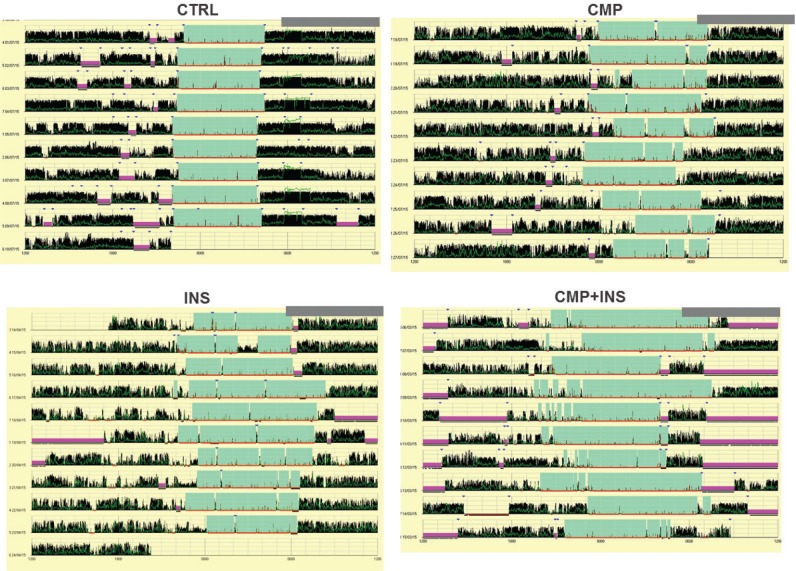
In respect of the other sleep variables from our actigraphy data, for all groups we found decreased sleep efficiency, increased WASO, and increased sleep episodes in line with recent evidence in women in midlife.16 These examples of sleep fragmentation may be caused by extended time in bed or long sleep, which have been linked to increased WASO and sleep latency.42–44 Part of our sample was composed of longer sleepers, as the CMP and CMP+INS groups had sleep durations (hours:minutes) of 8:03 and 8:58, respectively, compared to the 7:33 of the CTRL group, and the 7:17 of the INS groups. Another possibility is that dysregulation of circadian rhythms could increase sleep pressure during the day and increase sleep duration over 24 hours. However, actigraphy data showed similar rhythmic behaviors over the 10 days (Figure 3). In addition, we also found no statistically significant differences in circadian preferences or in actigraphy wake and bedtimes.
Figure 3. Examples of the actograms for control (CTRL), chronic musculoskeletal pain (CMP), insomnia (INS), and chronic musculoskeletal pain+insomnia (CMP+INS) groups.
Our study has some limitations. First, actigraphy data estimate the sleep-wake cycle parameters and may have overemphasized the sleep duration. In addition, PSG data must be interpreted with caution as they might not reflect the typical daily sleep of the participants and may reflect a period of adaptation that is unrepresentative of usual sleep patterns. Another limitation is that pain was self-reported, which could have underestimated or overestimated the results, as we did not have clinical diagnoses of the pain etiology. We believe that a combination of both measures (objective and subjective ones) would better represent the dimensions of musculoskeletal pain. The final limitation of our study is that there was an altered objective sleep pattern indicative of impaired sleep quality across the sample, even in the CTRL group. The PSG examination of the sleep patterns in the women who are postmenopausal found that they had short sleep duration, decreased sleep efficiency, disturbed sleep stages, and sleep fragmentation. Furthermore, we found a slightly elevated AHI in the CMP+INS group, but with no diurnal repercussions, as the participants presented no complaints during a physician consultation, which means that OSA was not diagnosed, despite the higher AHI. Pain and OSA are commonly associated. AHI is a potential variable that could influence sleep fragmentation and therefore the sleep-pain relationship. Patients, in general, who presented with both chronic pain and OSA, seem to have worse clinical symptoms. In addition, both OSA and INS predispose individuals to chronic pain or to the worsening of painful conditions. Our investigation raises the question of the possible importance of a mild but probably significant sleep related breathing disorder, as shown by the increased AHI, but without a diagnosis of OSA due to the moderate level of the AHI lack of associated symptoms in women postmenopause.
We analyzed actigraphy data collected for 10 consecutive days in women who are postmenopausal and took intraindividual and interindividual variability into account. The large number of observations (498 observations) provided by this cross-sectional study delivered sufficient statistical power to control for the effects of some covariates known or suspected to be associated with sleep and pain. Our findings refute our previous hypothesis that reduced estimated sleep variables would predict increased pain intensity at all moments of the day. Instead, we found increased estimated sleep duration predicting pain upon waking, and increased pain at bedtime predicting increased time in bed and sleep duration. Our findings do, however, corroborate our hypothesis that women who are postmenopausal and with CMP+INS would be more affected in terms of both pain and sleep. These results are significant and should be explored in future studies. They reflect the need to investigate pain and sleep disorders when treating women who are postmenopausal and call for a reevaluation of current thinking on this subject.
DISCLOSURE STATEMENT
The final version of the manuscript has been read and approved by all authors, who have each provided the attention necessary to ensure the integrity of the work. The authors alone are responsible for the content and writing of the manuscript. This work was supported by the Associação Fundo de Incentivo à Pesquisa (AFIP); Conselho Nacional de Desenvolvimento Científico e Tecnológico (MLA and HH are recipients of CNPq fellowships); and São Paulo Research Foundation (FAPESP #2014/18722-5 to CF). The sponsors had no role in the design or conduct of this research. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors are immensely grateful to Paul Davis for his help editing the manuscript, The authors thank the Associacao Fundo de Incentivo à Pesquisa (AFIP), Sao Paulo Research Foundation (FAPESP), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for their financial support.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CMP
chronic musculoskeletal pain
- CTRL
control
- CMP+INS
chronic musculoskeletal pain plus insomnia
- FSH
follicle-stimulating hormone
- GEE
Generalized Estimation Equations
- INS
insomnia
- ISI
Insomnia Severity Index
- NMQ
Nordic Musculoskeletal Questionnaire
- PLM
periodic limb movement
- PSG
polysomnography
- WASO
wake after sleep onset
REFERENCES
- 1.Dugan SA, Powell LH, Kravitz HM, Everson Rose SA, Karavolos K, Luborsky J. Musculoskeletal pain and menopausal status. Clin J Pain. 2006;22(4):325–331. doi: 10.1097/01.ajp.0000208249.07949.d5. [DOI] [PubMed] [Google Scholar]
- 2.Yang D, Haines CJ, Pan P, et al. Menopausal symptoms in mid-life women in southern China. Climacteric. 2008;11(4):329–336. doi: 10.1080/13697130802239075. [DOI] [PubMed] [Google Scholar]
- 3.Affleck G, Urrows S, Tennen H, Higgins P, Abeles M. Sequential daily relations of sleep, pain intensity, and attention to pain among women with fibromyalgia. Pain. 1996;68(2–3):363–368. doi: 10.1016/s0304-3959(96)03226-5. [DOI] [PubMed] [Google Scholar]
- 4.Wilson KG, Watson ST, Currie SR. Daily diary and activity monitoring of sleep in patients with insomnia associated with chronic musculoskeletal pain. Pain. 1998;75(1):75–84. doi: 10.1016/S0304-3959(97)00207-8. [DOI] [PubMed] [Google Scholar]
- 5.O'Brien EM, Waxenberg LB, Atchison JW, et al. Intraindividual variability in daily sleep and pain ratings among chronic pain patients: bidirectional association and the role of negative mood. Clin J Pain. 2011;27(5):425–433. doi: 10.1097/AJP.0b013e318208c8e4. [DOI] [PubMed] [Google Scholar]
- 6.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8(2):119–132. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 7.Lautenbacher S, Kundermann B, Krieg JC. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10(5):357–369. doi: 10.1016/j.smrv.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Smith MT, Klick B, Kozachik S, et al. Sleep onset insomnia symptoms during hospitalization for major burn injury predict chronic pain. Pain. 2008;138(3):497–506. doi: 10.1016/j.pain.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersen ML, Araujo P, Frange C, Tufik S. Sleep disturbance and pain: a tale of two common problems. Chest. 2018;154(5):1249–1259. doi: 10.1016/j.chest.2018.07.019. [DOI] [PubMed] [Google Scholar]
- 10.Bjurstrom MF, Irwin MR. Polysomnographic characteristics in nonmalignant chronic pain populations: a review of controlled studies. Sleep Med Rev. 2016;26:74–86. doi: 10.1016/j.smrv.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schrimpf M, Liegl G, Boeckle M, Leitner A, Geisler P, Pieh C. The effect of sleep deprivation on pain perception in healthy subjects: a meta-analysis. Sleep Med. 2015;16(11):1313–1320. doi: 10.1016/j.sleep.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 12.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14(12):1539–1552. doi: 10.1016/j.jpain.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Afolalu EF, Ramlee F, Tang NKY. Effects of sleep changes on pain-related health outcomes in the general population: a systematic review of longitudinal studies with exploratory meta-analysis. Sleep Med Rev. 2018;39:82–97. doi: 10.1016/j.smrv.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang NK, Goodchild CE, Sanborn AN, Howard J, Salkovskis PM. Deciphering the temporal link between pain and sleep in a heterogeneous chronic pain patient sample: a multilevel daily process study. Sleep. 2012;35(5):675–687. doi: 10.5665/sleep.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choy EH. The role of sleep in pain and fibromyalgia. Nat Rev Rheumatol. 2015;11(9):513–520. doi: 10.1038/nrrheum.2015.56. [DOI] [PubMed] [Google Scholar]
- 16.Kravitz HM, Zheng H, Bromberger JT, Buysse DJ, Owens J, Hall MH. An actigraphy study of sleep and pain in midlife women: the Study of Women's Health Across the Nation Sleep Study. Menopause. 2015;22(7):710–718. doi: 10.1097/GME.0000000000000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frange C, Hachul H, Hirotsu C, Tufik S, Andersen ML. Insomnia with musculoskeletal pain in postmenopause: associations with symptoms, mood, and quality of life. J Menopausal Med. 2018;24(1):17–28. doi: 10.6118/jmm.2018.24.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung F, Yegneswaran B, Liao P, et al. STOP Questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108(5):812–821. doi: 10.1097/ALN.0b013e31816d83e4. [DOI] [PubMed] [Google Scholar]
- 19.Bastien CH, Vallières A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 20.Kourinka I, Jonsson B, Kilbom A, et al. Standardised Nordic questionnaires for the analysis of musculoskeletal symptoms. Appl Ergon. 1987;18(3):233–237. doi: 10.1016/0003-6870(87)90010-x. [DOI] [PubMed] [Google Scholar]
- 21.Benedito-Silva AA, Menna-Barreto L, Marques N, Tenreiro S. A self-assessment questionnaire for the determination of morningness-eveningness types in Brazil. Prog Clin Biol Res. 1990;341B:89–98. [PubMed] [Google Scholar]
- 22.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97–110. [PubMed] [Google Scholar]
- 23.Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17(1):45–56. doi: 10.1016/0304-3959(83)90126-4. [DOI] [PubMed] [Google Scholar]
- 24.Harlow SD, Gass M, Hall JE, et al. STRAW + 10 Collaborative Group. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97(4):1159–1168. doi: 10.1210/jc.2011-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 26.Sadeh A, Hauri PJ, Kripke DF, Lavie P. The role of actigraphy in the evaluation of sleep disorders. Sleep. 1995;18(4):288–302. doi: 10.1093/sleep/18.4.288. [DOI] [PubMed] [Google Scholar]
- 27.Sadeh A. The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev. 2011;15(4):259–267. doi: 10.1016/j.smrv.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Lichstein KL, Stone KC, Donaldson J, et al. Actigraphy validation with insomnia. Sleep. 2006;29(2):232–239. [PubMed] [Google Scholar]
- 29.de Souza L, Benedito-Silva AA, Pires ML, Poyares D, Tufik S, Calil HM. Further validation of actigraphy for sleep studies. Sleep. 2003;26(1):81–85. doi: 10.1093/sleep/26.1.81. [DOI] [PubMed] [Google Scholar]
- 30.Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2002;2(5):389–396. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 31.Acebo C, Sadeh A, Seifer R, et al. Estimating sleep patterns with activity monitoring in children and adolescents: how many nights are necessary for reliable measures? Sleep. 1999;22(1):95–103. doi: 10.1093/sleep/22.1.95. [DOI] [PubMed] [Google Scholar]
- 32.Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15(5):461–469. doi: 10.1093/sleep/15.5.461. [DOI] [PubMed] [Google Scholar]
- 33.Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SF for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 34.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 35.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 36.Skrondal A, Rabe-Hesketh S. Generalized Latent Variable Modeling: Multilevel, Longitudinal and Structural Equation Models. Boca Raton, FL: Chapman & Hall/CRC; 2004. [Google Scholar]
- 37.American Academy of Sleep Medicine. International Classification of Sleep Disorders: Diagnostic and Coding Manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 38.Onen SH, Alloui A, Gross A, Eschallier A, Dubray C. The effects of total sleep deprivation, selective sleep interruption and sleep recovery on pain tolerance thresholds in healthy subjects. J Sleep Res. 2000;10(1):35–42. doi: 10.1046/j.1365-2869.2001.00240.x. [DOI] [PubMed] [Google Scholar]
- 39.Roehrs T, Roth T. Sleep and pain: interaction of two vital functions. Semin Neurol J. 2005;25(1):106–116. doi: 10.1055/s-2005-867079. [DOI] [PubMed] [Google Scholar]
- 40.Roehrs T, Hyde M, Blaisdell B, Greenwald M, Roth T. Sleep loss and REM sleep loss are hyperalgesic. Sleep. 2006;29(2):145–151. doi: 10.1093/sleep/29.2.145. [DOI] [PubMed] [Google Scholar]
- 41.Smith MT, Edwards RR, McCann UD, Haythornwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30(4):494–505. doi: 10.1093/sleep/30.4.494. [DOI] [PubMed] [Google Scholar]
- 42.Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation's updated sleep duration recommendations: final report. Sleep Health. 2015;1(4):233–243. doi: 10.1016/j.sleh.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 43.Dew MA, Hoch CC, Buysse DJ, et al. Healthy older adults' sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Med. 2003;65(1):63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- 44.Youngstedt SD, Kripke DF. Long sleep and mortality: rationale for sleep restriction. Sleep Med Rev. 2004;8(3):159–174. doi: 10.1016/j.smrv.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 45.Steptoe A, Peacey V, Wardle J. Sleep duration and health in young adults. Arch Intern Med. 2006;166(16):1689–1692. doi: 10.1001/archinte.166.16.1689. [DOI] [PubMed] [Google Scholar]