Abstract
Study Objectives:
Few population-based studies have explored how excessive sleepiness (ES) contributes to burden of illness among patients with obstructive sleep apnea (OSA).
Methods:
This study utilized data from the annual, cross-sectional 2016 US National Health and Wellness Survey. Respondents self-reporting an OSA diagnosis were categorized as having ES (Epworth Sleepiness Scale [ESS] score ≥ 11) or not having ES (ESS score < 11). Comorbidities, health-related quality of life (HRQoL), and productivity were examined in three groups: OSA with ES (n = 731), OSA without ES (n = 1,452), and non-OSA controls (n = 86,961).
Results:
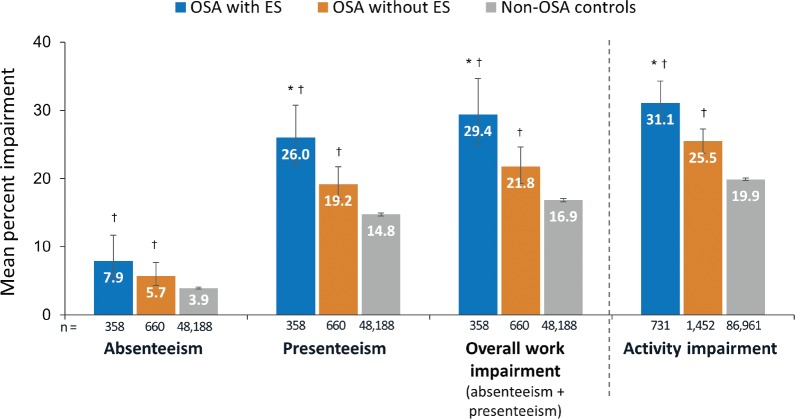
The OSA with ES group had significantly higher proportions of respondents reporting depression (62.4% versus 48.0%), gastroesophageal reflux disease (39.0% versus 29.4%), asthma (26.3% versus 20.7%), and angina (7.8% versus 6.7%) compared to the OSA without ES group (P < .05). After controlling for covariates, the OSA with ES group had significantly lower (worse) scores for mental component score (41.81 versus 45.65 versus 47.81), physical component score (46.62 versus 48.68 versus 51.36), and SF-6D (0.65 versus 0.69 versus 0.73) compared with OSA without ES and non-OSA controls (all P < .001). The OSA with ES group had significantly higher (greater burden) mean rates of presenteeism (25.98% impairment versus 19.24% versus 14.75%), work impairment (29.41% versus 21.82% versus 16.85%), and activity impairment (31.09% versus 25.46% versus 19.93%) compared with OSA without ES and non-OSA controls (all P < .01) after controlling for covariates.
Conclusions:
OSA with ES is associated with higher prevalence of comorbidities, reduced HRQoL, and greater impairment in productivity compared to OSA without ES and compared to non-OSA controls.
Citation:
Stepnowsky C, Sarmiento KF, Bujanover S, Villa KF, Li VW, Flores NM. Comorbidities, health-related quality of life, and work productivity among people with obstructive sleep apnea with excessive sleepiness: findings from the 2016 US National Health and Wellness Survey. J Clin Sleep Med. 2019;15(2):235–243.
Keywords: burden of illness, comorbidities, excessive sleepiness, health-related quality of life, obstructive sleep apnea
BRIEF SUMMARY
Current Knowledge/Study Rationale: It is well documented that obstructive sleep apnea (OSA) is associated with negative ramifications including decreased health-related quality of life (HRQoL) and increased prevalence of various comorbid illnesses. Although a growing body of evidence suggests that excessive sleepiness (ES) in individuals with OSA imparts incremental HRQoL, comorbidity, and work productivity burdens beyond those of OSA alone, few population-based studies have explored how ES contributes to burden of illness among patients with OSA.
Study Impact: Data from participants in the annual, cross-sectional 2016 US National Health and Wellness Survey found that those with OSA with ES (n = 731) had a higher prevalence of certain comorbidities, reduced HRQoL, and greater impairment in productivity compared to participants with OSA without ES (n = 1,452) and compared to non-OSA controls (n = 86,961), even after controlling for covariates. These data reinforce the importance of addressing ES as an independent and potentially treatable symptom associated with disease burden and hindered work productivity in patients with OSA.
INTRODUCTION
Obstructive sleep apnea (OSA) is characterized by recurrent episodes of partial or complete airway collapse during sleep and is commonly associated with loud snoring, oxygen desaturation, and various manifestations of sleep disturbance.1 It is not unusual for patients with OSA to have evidence of concomitant central sleep apnea (complex sleep apnea); however, central sleep apnea by itself occurs very infrequently, except in patients with heart failure.2–4 OSA, with or without central sleep apnea, is a highly prevalent condition, estimated to affect 10% to 17% of men and 3% to 9% of women older than 30 years in the United States, with trends over past decades indicative of increasing prevalence.5 Patients with OSA commonly experience fragmented, poor-quality sleep, which can lead to low energy, cognitive deficits, mood changes, and excessive sleepiness (ES).6–11
ES is one of the most common symptoms of OSA12,13 and often persists despite treatment with continuous positive airway pressure (CPAP).14–16 In one study of 149 patients with severe OSA, 62.5% of patients highly compliant with CPAP (≥ 7 h/night) continued to demonstrate pathologic sleepiness based objectively on the multiple sleep latency test, yet only 7% demonstrated residual sleepiness based on the subjective self-reported Epworth Sleepiness Scale (ESS), suggesting that patients may not always recognize or report ES as a problem.14 ES has been associated with health detriments ranging from impaired cognition17 to increased mortality in elderly patients,18 and contributes to an increased risk of falls,19 motor vehicle accidents,20,21 and work-related injury and fatalities.22,23
It is well documented that OSA is associated with decreased health-related quality of life (HRQoL)24–27 and an increase in comorbid illnesses including cardiovascular disease and metabolic disorders.1,28–30 A growing number of studies suggest that ES imparts an incremental burden of illness beyond that of OSA alone, including increased comorbidities (eg, coronary artery disease, depression, diabetes),31–34 greater negative HRQoL effect,16,24,35–37 and more substantial deficits in work productivity measures.7,38–40 Existing research in this area has been limited primarily to small sample sizes and/or clinic-based studies involving patients referred for sleep diagnostic testing. Few population-based studies have explored the burden in patients with OSA and ES. This study was designed to evaluate comorbidity prevalence, HRQoL, and work productivity in individuals who have OSA with ES, compared to those having OSA without ES and non-OSA controls using data from a large, United States national survey.
METHODS
Data Source
This study utilized data from the 2016 US National Health and Wellness Survey (NHWS) (n = 97,503), an annual, representative, cross-sectional, general health survey. NHWS is a self-administered, internet-based questionnaire that uses a randomly stratified sampling framework to ensure that the demographic composition with respect to age, sex, and race/ ethnicity is representative of the United States adult population based on governmental statistics.41–43
Study Sample
The sample for this analysis included survey respondents who self-reported a diagnosis of sleep apnea and who completed the ESS as a component of the survey. The survey item did not specify type of sleep apnea. Given that sleep apnea almost always includes an obstructive component and central sleep apnea alone is rare,2,3 the term OSA is used hereafter. By survey design, approximately one-third of respondents reporting a diagnosis of OSA were randomly selected to complete the ESS, an eight-item questionnaire that scores respondents on how likely they are to doze off during certain routine daily activities (eg, sitting and reading, watching TV, etc). Scores for each item (from 0 = no chance of dozing to 3 = high chance of dozing) were totaled. ESS scores range from 0 to 24, with higher ESS score indicating worse daytime sleepiness.44 For this study, a cutoff ESS score was used to categorize ES status; those who scored 11 or higher were considered to have ES. Respondents who self-reported a narcolepsy diagnosis were excluded.
Demographics and health characteristics for all participants were summarized, including: age, sex, race/ethnicity, education, employment, annual household income, smoking status, alcohol use, exercise, body mass index, and Charlson Comorbidity Index (CCI). The CCI represents a weighted sum of multiple comorbid conditions predictive of mortality (see footnote, Table 1), with higher scores indicating greater comorbidity burden.45 Patients were asked to report having a diagnosis of comorbid conditions not included in the CCI including: depression, high blood pressure (hypertension), atrial fibrillation, high cholesterol, angina, unstable angina, asthma, and gastroesophageal reflux disease (GERD).
Table 1.
Demographics and clinical characteristics by OSA/ES status.
Quality of Life Measures
HRQoL was evaluated using the revised Medical Outcomes Study 36-Item Short-Form Survey Instrument, version 2 (SF-36v2).46 The SF-36v2 is a multipurpose self-administered health survey consisting of 36 questions covering eight domains: physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role-emotional, and mental health. Two summary scores were calculated: physical component summary (PCS) and mental component summary (MCS) scores. For the purpose of the current analysis, the PCS and MCS summary scores were utilized as normed scores, as recommended in the SF-36 user's manual to simplify interpretation of the data.47 This was achieved by transforming the raw scores for the items to a mean of 50 and a standard deviation of 10 for the United States population. Normed scores were calculated for both the eight SF-36v2 scales as well as for the PCS and MCS summary scores. Scores were interpreted relative to a population average score of 50 as well as with other comparison groups of interest. Higher scores indicate better quality of life. In addition to generating profile and summary PCS and MCS scores, the SF-36v2 was used to generate health state utilities. This was achieved through application of the SF-6D algorithm which is based on six domains from the SF-36v2. The SF-6D is a preference-based single index measure for health utilities using general population values.48 The SF-6D index yields summary scores ranging from 0 (worst health state) to 1 (best health state).
Work Productivity
Work productivity was measured using the Work Productivity and Activity Impairment General Health (WPAI-GH) questionnaire, a six-item validated instrument consisting of: absenteeism (percentage of work time missed because of one's health in the past 7 days), presenteeism (percentage of impairment while at work in the past 7 days because of one's health), overall work productivity loss (an overall impairment percentage estimate combining absenteeism and presenteeism), and activity impairment (percentage of impairment in daily activities because of one's health in the past 7 days).49
Statistical Analysis
Outcomes were analyzed in the three study groups (OSA with ES, OSA without ES, and non-OSA controls). Bivariate comparison used one-way analyses of variance (continuous outcomes) and chi-square tests (categorical outcomes) to examine the effect of OSA/ES status on HRQoL and WPAI-GH. Multivariable analysis used generalized linear models controlling for covariates to examine the effect of OSA/ES status on HRQoL and WPAI-GH.15 Stepwise selection was performed to select significant covariates that predict absenteeism, MCS, PCS, SF-6D, presenteeism, overall work impairment, and activity impairment.
RESULTS
Study Population
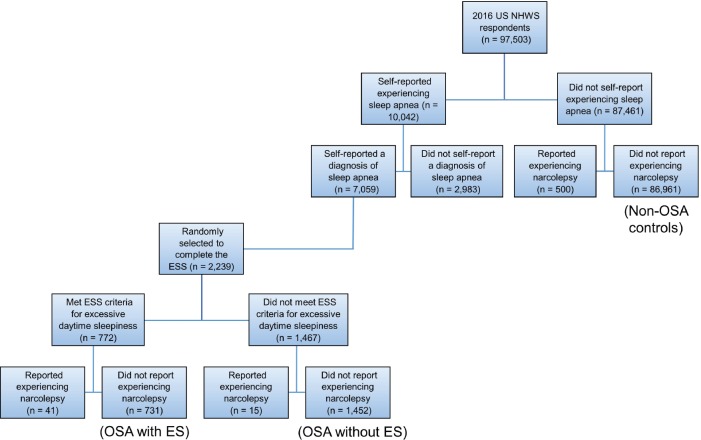
The 2016 NHWS survey included 97,503 participants, of whom 7,059 (7.2%) reported having a diagnosis of OSA. From the participants reporting a diagnosis of sleep apnea, 2,239 were randomly selected to complete the ESS (Figure 1). After excluding 56 participants who self-reported narcolepsy, the OSA group included 2,183 participants. Of these, 731 (33.5%) had ESS scores consistent with having OSA with ES and 1,452 (66.5%) had OSA without ES. The non-OSA control group was composed of 86,961 participants who did not self-report OSA or narcolepsy.
Figure 1. Study sample flowchart.
ES = excessive sleepiness, ESS = Epworth Sleepiness Scale, OSA = obstructive sleep apnea, US NHWS = United States National Health and Wellness Survey.
Sociodemographic and clinical characteristics between the three study groups (OSA with ES, OSA without ES, and non-OSA controls) were significantly different for all variables evaluated (Table 1). Compared to non-OSA controls, both OSA groups (OSA with ES and OSA without ES) were characterized by older age, higher percentages of males, higher non-Hispanic White representation, more overweight and obese, fewer “never smokers,” and lower proportions of individuals who exercised 20 minutes or longer at least once in the past month. Among participants with OSA, the OSA with ES group had a number of significant differences compared with the OSA without ES group, including younger mean age, lower percentage of males, less non-Hispanic White, lower proportion of those who earned $75,000 or more, higher prevalence of obesity, and significantly less “never smokers.” The OSA with ES group had a significantly higher proportion of participants who do not drink alcohol compared to non-OSA controls, and a lower proportion of participants who had a 4-year degree or higher compared to both OSA without ES and non-OSA control groups.
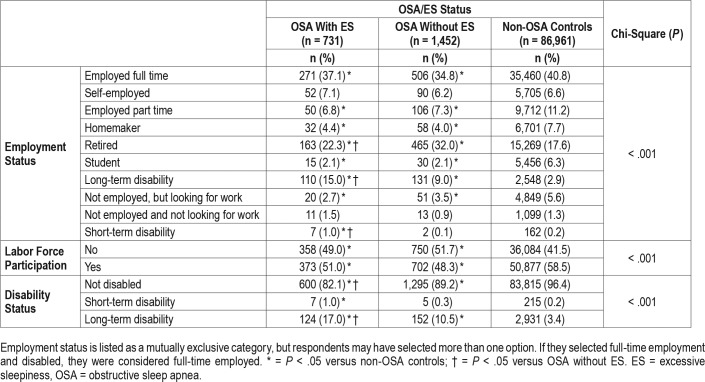
Significantly more participants in the non-OSA control group were employed full-time compared with both OSA groups (P < .05) (Table 2). The percentage of participants on long-term disability was significantly greater in both OSA groups compared with non-OSA controls, and greater in the OSA with ES cohort compared with the OSA without ES cohort.
Table 2.
Employment status and labor force participation by OSA status.
Comorbidities
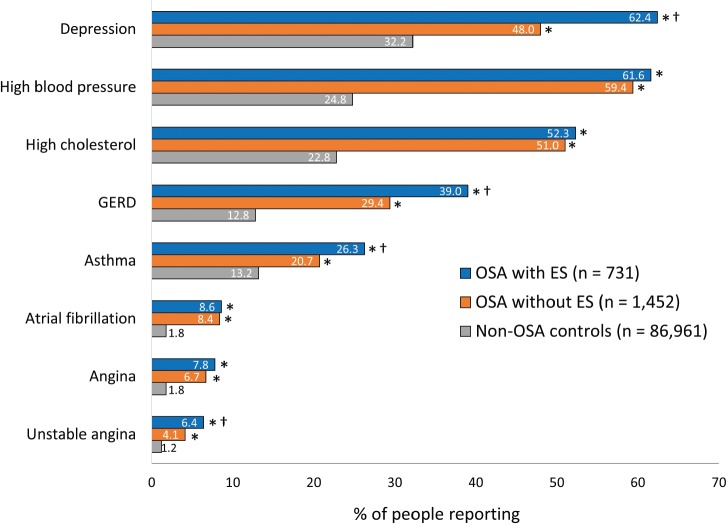
The mean CCI score was highest in the OSA with ES group (1.38), and was significantly higher compared with the OSA without ES group (1.12) and the non-OSA control group (0.36) (both P < .05) (Table 1). The OSA with ES group had a significantly higher prevalence in four of eight comorbidities not reflected in the CCI score (depression, GERD, asthma, unstable angina) compared to the OSA without ES group (all P < .05), and a significantly higher prevalence of all eight comorbidities compared to non-OSA controls (all P < .05) (Figure 2).
Figure 2. Comorbidities by OSA status (with or without ES) and compared with controls not having OSA.
Comorbidities were scored if they were ever experienced and were self-reported. * P < .05 versus non-OSA controls; † = P < .05 versus OSA without ES. ES = excessive sleepiness, GERD = gastroesophageal reflux disease, OSA = obstructive sleep apnea.
HRQoL
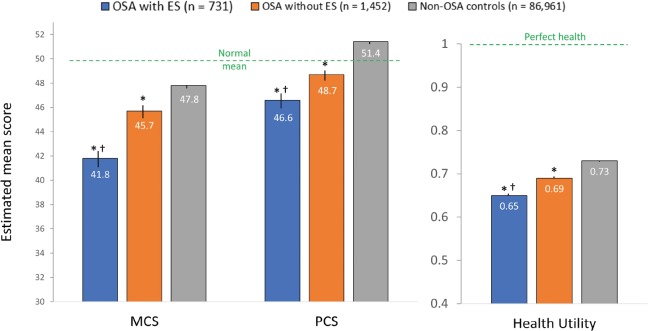
At a significance level of P < .05, all covariates were found significant for MCS, PCS, and SF-6D in the stepwise selection; thus, all covariates were entered into each multivariable regression model. After controlling for covariates, the OSA with ES group had significantly lower mean scores for MCS and PCS summaries and mean SF-6D health state utility score compared with the OSA without ES group and non-OSA controls (all P < .001) (Figure 3).
Figure 3. SF-36 health-related quality of life measures by OSA status, controlling for covariates.
Covariates included age, sex, race/ethnicity, income, education, BMI, smoking status, alcohol use, exercise activity, and Charlson Comorbidity Index. At a significance level P < .05, sex and alcohol use were not significant for absenteeism, and sex and education were not significant for presenteeism or for overall work impairment; thus, these covariates were not entered into the multivariable regression model for the respective outcomes. All covariates were significant for activity impairment, and therefore, all covariates were entered into the multivariable regression model for activity impairment. * = P < .05 versus non-OSA controls; † = P < .05 versus OSA without ES. Error bars represent 95% confidence intervals. BMI = body mass index, CI = confidence interval, ES = excessive sleepiness, MCS = Mental Component Summary, OSA = obstructive sleep apnea, PCS = Physical Component Summary.
Work Productivity and Impairment
The work productivity analysis involved 49,206 participants who reported being employed, 1,018 of whom reported a diagnosis of OSA. Among employed respondents with OSA, 35% (358/1,018) had ES. Except for absenteeism, which was statistically similar between the OSA with ES and OSA without ES groups, the OSA with ES group demonstrated greater impairment in work productivity and activity outcomes compared to the OSA without ES and non-OSA groups after controlling for covariates (Figure 4).
Figure 4. WPAI by OSA status after controlling for covariates.
Covariates included for each outcome: absenteeism (age, ethnicity, income, education, BMI, smoking status, exercise activity, and CCI), presenteeism (age, ethnicity, income, BMI, smoking status, exercise activity, alcohol use, and CCI), overall work impairment (age, ethnicity, income, BMI, smoking status, alcohol use, exercise activity, and CCI), and activity impairment (age, sex, ethnicity, income, education, BMI, smoking status, alcohol use, exercise activity, and CCI). Questions only applicable to those working full-time, part-time, or self-employed: OSA with ES = 358, OSA without ES = 660, and non-OSA controls = 48,188. Total is 49,206. * = P < .05 versus non-OSA controls; † = P < .05 versus OSA without ES. Error bars represent 95% confidence intervals. BMI = body mass index, CCI = Charlson Comorbidity Index, CI = confidence interval, ES = excessive sleepiness, OSA = obstructive sleep apnea, WPAI = Work Productivity and Activity Impairment.
DISCUSSION
To our knowledge, this is the first population-based study, as well as one of the largest cohorts, in which the effect of ES on comorbidity prevalence, HRQoL, and work productivity in individuals with OSA with or without ES has been evaluated. The findings suggest that OSA with ES is associated with a wide range of measurable burdens relative to controls that also extend beyond those exacted by OSA alone, including higher comorbidity rates, lower HRQoL scores, and greater work and productivity impairment.
In the current study, mean CCI score was significantly greater in the OSA with ES group relative to those with OSA without ES, indicating a generally higher overall burden of comorbid disease in the presence of ES. In addition, four comorbidities not reflected in the CCI score (depression, GERD, asthma, and unstable angina) were noted in significantly higher proportions of individuals with OSA and ES compared with OSA without ES or non-OSA controls. A number of prior studies have also reported higher rates of depression and anxiety in OSA subgroups with ES.8,9,16,31,34,50 Gasa et al. found that patients with ES had significantly higher mean values of depression (P = .0067) and fatigue (P = .0099) (Pichot depression and fatigue scales) and reported worse overall health status than those without ES.8 Pepin et al. reported significantly poorer emotional health and energy levels (Nottingham Health Profile scale) in patients with ES compared with those without ES (both P < .003).16 Lee et al. noted anxiety in 54.5% of patients with OSA with ES compared with 42.9% of patients without ES (State-Trait Anxiety Inventory-State Scale); ESS ≥ 10 was significantly associated with the presence of anxiety in patients with OSA.9
Although ES has been reported to correlate positively with blood pressure in patients with severe OSA,33 our findings did not suggest that ES was significantly associated with a higher prevalence of hypertension among individuals with OSA. A higher risk of diabetes mellitus has been previously reported in patients with OSA and sleepiness (ESS ≥ 10) compared with OSA without sleepiness (odds ratios, 2.59 and 1.16, respectively)32; diabetes mellitus was not evaluated as an independent comorbidity in the current study.
In the current study, impairment in HRQoL as assessed by the SF-36 mental component and physical component summaries, as well as health state utility scores, were significantly worse in individuals reporting OSA with ES, even after adjusting for a number of covariates, including comorbidity burden (CCI score). These findings corroborate prior research in sleep clinic populations that identified sleepiness as a significant contributor to multiple facets of HRQoL in patients with OSA as measured by the SF-1224 and SF-36.35,36,51
Based on WPAI results in the current study, the presence of ES in individuals with OSA was associated with a measurable negative effect on work productivity compared to both OSA without ES and controls without OSA. Individuals with ES reported significantly higher rates of work-related impairment as well impairment in non-work-related activities. A study by Dean et al.7 noted a WPAI presenteeism rate (% of impairment while working) of 32.4% in people with OSA and ES, which is similar to the adjusted rate observed in the current study in the OSA with ES group (26.0%) and higher than the OSA without ES group (19.2%). These rates of work impairment are similar to those reported for patients with other chronic diseases such as diabetes, depression, and arthritis (22% to 33%).52 Mulgrew et al.38 found a clear relationship between ES and decreased work performance was found in a group of 428 blue-and white-collar workers being evaluated for sleep-disordered breathing. For all scales of the Work Limitations Questionnaire except physical output, each 1-point increase in ESS correlated with an additional 1% of time spent at suboptimal work performance. These and other studies showing similar findings39,40 suggest that ES may be an independent risk factor for work productivity impairment.
These data are subject to certain limitations. Data from the NHWS are self-reported and cross-sectional. Thus, there is no way to independently verify reported variables such as medical diagnoses, including OSA status and comorbidities. However, the survey offers little incentive to misrepresent one's experiences. It is worth noting that the prevalence of OSA in the survey results (7.2%) is generally consistent with prevalence rates reported in other United States studies.53 The ESS has limitations in that it is based on self-reported sleep propensity, and suitability has not been established in certain populations, such as the cognitively impaired. Also, given the cross-sectional nature of the data, statements of causality cannot be made from the study results. For example, the higher rates of some comorbidities (eg, GERD, asthma) could be a cause of ES, rather than a consequence. Some previous studies have stratified populations by severity of OSA based on apnea-hypopnea index; this was not possible in the current study, limiting the potential to make OSA severity-based comparisons. However, given that our data are expected to be reflective of all severities of OSA, the results likely err on the side of underestimating the effect of ES in persons with higher OSA severity. The data were gathered from a United States cohort and may not be generalizable to non-United States populations.
CONCLUSIONS
The results of this large national survey are consistent with findings from smaller and practice-centered cohort studies suggesting that the presence of ES increases the burden of illness in patients with OSA. These data reinforce the importance of addressing ES as an independent and potentially treatable symptom associated with disease burden and hindered work productivity in patients with OSA.
DISCLOSURE STATEMENT
This study (data collection and analysis) was funded by Jazz Pharmaceuticals. Editorial assistance with manuscript preparation was provided by Judith Adams, PharmD and Sandra Westra, PharmD of Churchill Communications (Maplewood, NJ). These data were presented at SLEEP 2017, the 31st Annual Meeting of the Associated Professional Sleep Societies, Boston, MA, June 3–7, 2017. All authors have seen and approved of this manuscript. Dr. Kathleen Sarmiento is on the advisory board for Jazz Pharmaceuticals. Dr. Shay Bujanover is an employee of Jazz Pharmaceuticals, and holds stock and/or stock options in Jazz Pharmaceuticals, plc. Dr. Kathleen Villa is an employee of Jazz Pharmaceuticals, and holds stock and/ or stock options in Jazz Pharmaceuticals, plc. Dr. Vicki Li is an employee of Kantar Health which was contracted by Jazz Pharmaceuticals for this work. Dr. Natalia Flores was a full-time employee at Kantar Health at the time when the company was contracted by Jazz Pharmaceuticals for this work. Dr. Carl Stepnowsky has no conflicts to report.
ABBREVIATIONS
- CCI
Charlson Comorbidity Index
- CPAP
continuous positive airway pressure
- ESS
Epworth Sleepiness Scale
- ES
excessive sleepiness
- GERD
gastroesophageal reflux disease
- HRQoL
health-related quality of life
- SF-36v2
Medical Outcomes Study 36-Item Short-Form Survey Instrument, version 2
- MCS
Mental Component Summary
- NHWS
National Health and Wellness Survey
- OSA
obstructive sleep apnea
- PCS
Physical Component Summary
- WPAI-GH
Work Productivity and Activity Impairment General Health
REFERENCES
- 1.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thor Soc. 2008;5(2):136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morganthaler TI, Kagramanov V, Hanak V, Decker PA. Complex sleep apnea syndrome: is it a unique clinical syndrome? Sleep. 2006;29(9):1203–1209. doi: 10.1093/sleep/29.9.1203. [DOI] [PubMed] [Google Scholar]
- 3.Kaul S, Meena AK, Murthy JM. Sleep apnea syndromes: clinical and polysomnographic study. Neurol India. 2001;49(1):47–50. [PubMed] [Google Scholar]
- 4.Sands SA, Owens RL. Congestive heart failure and central sleep apnea. Crit Care Clin. 2015;31(3):473–495. doi: 10.1016/j.ccc.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bixler EO, Vgontzas AN, Lin HM, Calhoun SL, Vela-Bueno A, Kales A. Excessive daytime sleepiness in a general population sample: the role of sleep apnea, age, obesity, diabetes, and depression. J Clin Endocrinol Metab. 2005;90(8):4510–4515. doi: 10.1210/jc.2005-0035. [DOI] [PubMed] [Google Scholar]
- 7.Dean B, Aguilar D, Shapiro C, et al. Impaired health status, daily functioning, and work productivity in adults with excessive sleepiness. J Occup Environ Med. 2010;52(2):144–149. doi: 10.1097/JOM.0b013e3181c99505. [DOI] [PubMed] [Google Scholar]
- 8.Gasa M, Tamisier R, Launois SH, et al. Residual sleepiness in sleep apnea patients treated by continuous positive airway pressure. J Sleep Res. 2013;22(4):389–397. doi: 10.1111/jsr.12039. [DOI] [PubMed] [Google Scholar]
- 9.Lee SA, Han SH, Ryu HU. Anxiety and its relationship to quality of life independent of depression in patients with obstructive sleep apnea. J Psychosom Res. 2015;79(1):32–36. doi: 10.1016/j.jpsychores.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Werli KS, Otuyama LJ, Bertolucci PH, et al. Neurocognitive function in patients with residual excessive sleepiness from obstructive sleep apnea: a prospective, controlled study. Sleep Med. 2016;26:6–11. doi: 10.1016/j.sleep.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 11.Zhou J, Camacho M, Tang X, Kushida CA. A review of neurocognitive function and obstructive sleep apnea with or without daytime sleepiness. Sleep Med. 2016;23:99–108. doi: 10.1016/j.sleep.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 12.American Academy of Sleep Medicine. International Classification of Sleep Disorders: Diagnostic and Coding Manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 13.Dongol EM, Williams EJ. Residual excessive sleepiness in patients with obstructive sleep apnea on treatment with continuous positive airway pressure. Curr Opin Pulm Med. 2016;22(6):589–594. doi: 10.1097/MCP.0000000000000324. [DOI] [PubMed] [Google Scholar]
- 14.Weaver TE, Maislin GM, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30(6):711–719. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koutsourelakis I, Perraki E, Economou NT, et al. Predictors of residual sleepiness in adequately treated obstructive sleep apnea patients. Eur Respir J. 2009;34(3):687–693. doi: 10.1183/09031936.00124708. [DOI] [PubMed] [Google Scholar]
- 16.Pepin JL, Viot-Blanc V, Escourrou P, et al. Prevalence of residual excessive sleepiness in CPAP-treated sleep apnoea patients: the French multicentre study. Eur Respir J. 2009;33(5):1062–1067. doi: 10.1183/09031936.00016808. [DOI] [PubMed] [Google Scholar]
- 17.Engleman HM, Douglas NJ. Sleep. 4: Sleepiness, cognitive function, and quality of life in obstructive sleep apnoea/hypopnoea syndrome. Thorax. 2004;59(7):618–622. doi: 10.1136/thx.2003.015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gooneratne NS, Richards KC, Joffe M, et al. Sleep disordered breathing with excessive daytime sleepiness is a risk factor for mortality in older adults. Sleep. 2011;34(4):435–442. doi: 10.1093/sleep/34.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onen F, Higgins S, Omen SH. Falling-asleep-related injured falls in the elderly. J Am Med Dir Assoc. 2009;10(3):207–210. doi: 10.1016/j.jamda.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Tregear S, Reston J, Schoelles K, Phillips B. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. J Clin Sleep Med. 2009;5(6):573–581. [PMC free article] [PubMed] [Google Scholar]
- 21.Ward KL, Hillman DR, James A, et al. Excessive sleepiness increases the risk of motor vehicle crash in obstructive sleep apnea. J Clin Sleep Med. 2013;9(10):1013–1021. doi: 10.5664/jcsm.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garbarino S, Guglielmi O, Sanna A, Mancardi GL, Magnavita N. Risk of occupational accidents in workers with obstructive sleep apnea: systematic review and meta-analysis. Sleep. 2016;39(6):1211–1218. doi: 10.5665/sleep.5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uehli K, Mehta AJ, Miedinger D, et al. Sleep problems and work injuries: a systematic review and meta-analysis. Sleep Med Rev. 2014;18(1):61–73. doi: 10.1016/j.smrv.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Iacono Isidoro S, Salvaggio A, Lo Bue A, Romano S, Marrone O, Insalaco G. Quality of life in patients at first time visit for sleep disorders of breathing at a sleep centre. Health Qual Life Outcomes. 2013;11:207. doi: 10.1186/1477-7525-11-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D'Ambrosio C, Bowman T, Mohsenin V. Quality of life in patients with obstructive sleep apnea: effect of nasal continuous positive airway pressure – a prospective study. Chest. 1999;115(1):123–129. doi: 10.1378/chest.115.1.123. [DOI] [PubMed] [Google Scholar]
- 26.Lacasse Y, Godbout C, Sériès F. Health-related quality of life in obstructive sleep apnoea. Eur Respir J. 2002;19(3):499–503. doi: 10.1183/09031936.02.00216902. [DOI] [PubMed] [Google Scholar]
- 27.Diamanti C, Manali E, Ginieri-Coccossis M, et al. Depression, physical activity, energy consumption, and quality of life in OSA patients before and after CPAP treatment. Sleep Breath. 2013;17(4):1159–1168. doi: 10.1007/s11325-013-0815-6. [DOI] [PubMed] [Google Scholar]
- 28.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283(14):1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 29.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172(12):1590–1595. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 31.Ronksley PE, Hemmelgarn BR, Heitman SJ, et al. Excessive daytime sleepiness is associated with increased health care utilization among patients referred for assessment of OSA. Sleep. 2011;34(3):363–370. doi: 10.1093/sleep/34.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ronksley PE, Hemmelgarn BR, Heitman SJ, et al. Obstructive sleep apnoea is associated with diabetes in sleepy subjects. Thorax. 2009;64(10):834–839. doi: 10.1136/thx.2009.115105. [DOI] [PubMed] [Google Scholar]
- 33.Feng J, He QY, Zhang XL, Chen BY Sleep Breath Disorder Group, Society of Respiratory Medicine. Epworth Sleepiness Scale may be an indicator for blood pressure profile and prevalence of coronary artery disease and cerebrovascular disease in patients with obstructive sleep apnea. Sleep Breath. 2012;16(1):31–40. doi: 10.1007/s11325-011-0481-5. [DOI] [PubMed] [Google Scholar]
- 34.Bjornsdottir E, Benediktsdottir B, Pack AI, et al. The prevalence of depression among untreated obstructive sleep apnea patients using a standardized psychiatric interview. J Clin Sleep Med. 2016;12(1):105–112. doi: 10.5664/jcsm.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee W, Lee SA, Ryu HU, Chung YS, Kim WS. Quality of life in patients with obstructive sleep apnea: relationship with daytime sleepiness, sleep quality, depression, and apnea severity. Chron Respir Dis. 2016;13(1):33–39. doi: 10.1177/1479972315606312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silva GE, Goodwin JL, Vana KD, Quan SF. Obstructive sleep apnea and quality of life: comparison of the SAQLI, FOSQ, and SF-36 questionnaires. Southwest J Pulm Crit Care. 2016;13(3):137–149. doi: 10.13175/swjpcc082-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silva GE, An MW, Goodwin JL, et al. Longitudinal evaluation of sleep-disordered breathing and sleep symptoms with change in quality of life: the Sleep Heart Health Study (SHHS) Sleep. 2009;32(8):1049–1057. doi: 10.1093/sleep/32.8.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mulgrew AT, Ryan CF, Fleetham JA, et al. The impact of obstructive sleep apnea and daytime sleepiness on work limitation. Sleep Med. 2007;9(1):42–53. doi: 10.1016/j.sleep.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Omachi TA, Claman DM, Blanc PD, Eisner MD. Obstructive sleep apnea: a risk factor for work disability. Sleep. 2009;32(6):791–798. doi: 10.1093/sleep/32.6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nena E, Steiropoulos P, Constantinidis TC, Perantoni E, Tsara V. Work productivity in obstructive sleep apnea patients. J Occup Med. 2010;52(6):622–625. doi: 10.1097/JOM.0b013e3181e12b05. [DOI] [PubMed] [Google Scholar]
- 41.Bolge SC, Doan JF, Kannan H, Baran RW. Association of insomnia with quality of life, work productivity, and activity impairment. Qual Life Res. 2009;18(4):415–422. doi: 10.1007/s11136-009-9462-6. [DOI] [PubMed] [Google Scholar]
- 42.DiBonaventura MD, Wagner JS, Girman CJ, et al. Multinational Internet-based survey of patient preference for newer oral or injectable type 2 diabetes medication. Patient Prefer Adherence. 2010;4:397–406. doi: 10.2147/PPA.S14477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Finkelstein EA, Allaire BT, DiBonaventura MD, Burgess SM. Direct and indirect costs and potential cost savings of laparoscopic adjustable gastric banding among obese patients with diabetes. J Occup Environ Med. 2011;53(9):1025–1029. doi: 10.1097/JOM.0b013e318229aae4. [DOI] [PubMed] [Google Scholar]
- 44.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 45.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 46.Maruish ME. User's manual for the SF-36v2 Health Survey. 3rd ed. Lincoln, RI: Quality Metric Incorporated; 2011. [Google Scholar]
- 47.Ware JE, Kosinski M, Dewey JE. How to Score Version 2 of the SF-36® Health Survey (Standard & Acute Forms) 2nd ed. Lincoln, RI: Quality Metric Incorporated; 2001. [Google Scholar]
- 48.Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care. 2004;42(9):851–859. doi: 10.1097/01.mlr.0000135827.18610.0d. [DOI] [PubMed] [Google Scholar]
- 49.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–365. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- 50.Jacobsen JH, Shi L, Mokhlesi B. Factors associated with excessive daytime sleepiness in patients with severe obstructive sleep apnea. Sleep Breath. 2013;17(2):629–635. doi: 10.1007/s11325-012-0733-z. [DOI] [PubMed] [Google Scholar]
- 51.Sforza E, Janssens JP, Rochat T, Ibanez V. Determinants of altered quality of life in patients with sleep-related breathing disorders. Eur Respir J. 2003;21(4):682–687. doi: 10.1183/09031936.03.00087303. [DOI] [PubMed] [Google Scholar]
- 52.Schultz AB, Edington DW. Employee health and presenteeism: a systematic review. J Occup Rehabil. 2007;17(3):547–579. doi: 10.1007/s10926-007-9096-x. [DOI] [PubMed] [Google Scholar]
- 53.Lee W, Nagubadi S, Kryger MH, Mokhlesi B. Epidemiology of obstructive sleep apnea: a population-based perspective. Expert Rev Respir Med. 2008;2(3):349–364. doi: 10.1586/17476348.2.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]