Abstract
Introduction:
This guideline establishes clinical practice recommendations for positive airway pressure (PAP) treatment of obstructive sleep apnea (OSA) in adults and is intended for use in conjunction with other American Academy of Sleep Medicine (AASM) guidelines in the evaluation and treatment of sleep-disordered breathing in adults.
Methods:
The AASM commissioned a task force of experts in sleep medicine. A systematic review was conducted to identify studies, and the Grading of Recommendations Assessment, Development and Evaluation (GRADE) process was used to assess the evidence. The task force developed recommendations and assigned strengths based on the quality of evidence, the balance of clinically significant benefits and harms, patient values and preferences, and resource use. In addition, the task force adopted recommendations from prior guidelines as “good practice statements” that establish the basis for appropriate and effective treatment of OSA. The AASM Board of Directors approved the final recommendations.
Good Practice Statements:
The following good practice statements are based on expert consensus, and their implementation is necessary for appropriate and effective management of patients with OSA treated with positive airway pressure:
Treatment of OSA with PAP therapy should be based on a diagnosis of OSA established using objective sleep apnea testing.
Adequate follow-up, including troubleshooting and monitoring of objective efficacy and usage data to ensure adequate treatment and adherence, should occur following PAP therapy initiation and during treatment of OSA.
Recommendations:
The following recommendations are intended as a guide for clinicians using PAP to treat OSA in adults. A STRONG (ie, “We recommend…”) recommendation is one that clinicians should follow under most circumstances. A CONDITIONAL recommendation (ie, “We suggest…”) reflects a lower degree of certainty regarding the outcome and appropriateness of the patient-care strategy for all patients. The ultimate judgment regarding any specific care must be made by the treating clinician and the patient, taking into consideration the individual circumstances of the patient, available treatment options, and resources.
We recommend that clinicians use PAP, compared to no therapy, to treat OSA in adults with excessive sleepiness. (STRONG)
We suggest that clinicians use PAP, compared to no therapy, to treat OSA in adults with impaired sleep-related quality of life. (CONDITIONAL)
We suggest that clinicians use PAP, compared to no therapy, to treat OSA in adults with comorbid hypertension. (CONDITIONAL)
We recommend that PAP therapy be initiated using either APAP at home or in-laboratory PAP titration in adults with OSA and no significant comorbidities. (STRONG)
We recommend that clinicians use either CPAP or APAP for ongoing treatment of OSA in adults. (STRONG)
We suggest that clinicians use CPAP or APAP over BPAP in the routine treatment of OSA in adults. (CONDITIONAL)
We recommend that educational interventions be given with initiation of PAP therapy in adults with OSA. (STRONG)
We suggest that behavioral and/or troubleshooting interventions be given during the initial period of PAP therapy in adults with OSA. (CONDITIONAL)
We suggest that clinicians use telemonitoring-guided interventions during the initial period of PAP therapy in adults with OSA. (CONDITIONAL)
Citation:
Patil SP, Ayappa IA, Caples SM, Kimoff RJ, Patel SR, Harrod CG. Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2019;15(2):335–343.
Keywords: obstructive sleep apnea, OSA, positive airway pressure, PAP
INTRODUCTION
Since the publication of the previous American Academy of Sleep Medicine (AASM) positive airway pressure (PAP) practice parameters,1–3 the scientific literature has continued to expand regarding the effects of PAP on clinical outcomes in adults with obstructive sleep apnea (OSA). In addition, research on improving PAP adherence and advancements in device technology have continued to evolve. Given these advancements, updating the prior practice parameters was considered timely.
The AASM commissioned a task force (TF) of content experts to update and consolidate previous AASM PAP practice parameters and reviews relevant to the treatment of adult OSA with PAP modalities.1,3,4 This guideline does not address the initiation and management of PAP in patients with obesity hypoventilation syndrome, sleep-related hypoventilation, or those with concurrent forms of obstructive and central sleep apnea. The efficacy of continuous PAP (CPAP), auto-adjusting PAP (APAP), bilevel PAP (BPAP), and other advanced PAP modalities for central sleep apnea and hypoventilation are addressed in other active AASM guidelines.5,6 Furthermore, several prior recommendations on the management of OSA with PAP were not readdressed in the present guideline. Nevertheless, the TF adopted and modified two prior statements as good practice statements, as they were considered essential to providing high quality care to patients with OSA who are treated with PAP.
This guideline, in conjunction with the accompanying systematic review,7 provides a comprehensive update of the available evidence and a synthesis of clinical practice recommendations.
METHODS
The AASM commissioned a TF of both board-certified sleep medicine specialists and experts with proficiency in the use of PAP in adults with OSA to develop this guideline. The TF was required to disclose all potential conflicts of interest (COI) per the AASM's COI policy prior to being appointed to the TF, and throughout the research and writing of this paper. In accordance with the AASM's conflicts of interest policy, TF members with a Level 1 conflict were not allowed to participate. TF members with a Level 2 conflict were required to recuse themselves from any related discussion or writing responsibilities. All relevant conflicts of interest are listed in the disclosure statement.
The TF conducted a systematic review of the scientific literature to answer 11 PICO (Patient, Population or Problem, Intervention, Comparison, and Outcomes) questions regarding the initiation of PAP therapy in patients with OSA that focus on patient-oriented, clinically relevant outcomes (see systematic review, Table 1).7 The purpose of the review was to determine the effectiveness of PAP, alternative PAP modes (ie, APAP, BPAP), and concurrent strategies designed to improve outcomes by enhancing acceptance and use of PAP for OSA treatment (eg, patient education and telemonitoring). The TF did not compare PAP against other treatment options (eg, oral appliance therapy, surgical therapy). Assessment of the evidence was performed according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) process.8 The TF assessed the following four components to determine the direction and strength of a recommendation: quality of evidence, balance of beneficial and harmful effects, patient values and preferences, and resource use. Details of these assessments can be found in the accompanying systematic review.7 Taking these major factors into consideration, each recommendation statement was assigned a strength (STRONG or CONDITIONAL). Additional information is provided in the form of remarks immediately following the recommendation statements, when deemed necessary by the TF. Remarks are based on the evidence evaluated during the systematic review and are intended to provide context for the recommendations to guide clinicians in the implementation of the recommendations in daily practice.
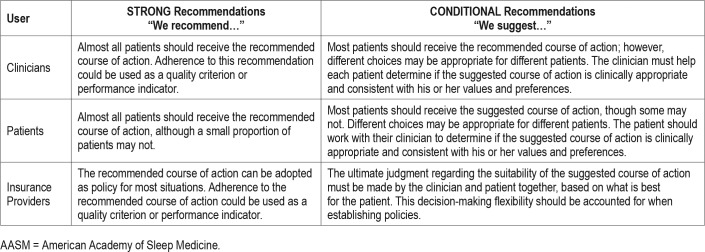
Table 1.
Implications of STRONG and CONDITIONAL recommendations for users of AASM clinical practice guidelines.
As this guideline focuses on the indications for PAP therapy for OSA in adults, rather than the use of specific components or accessories of the PAP device, recommendations for three of the PICO questions were not included. A summary of the systematic review and meta-analyses of the evidence for these PICO questions can be found in the additional considerations section, as these factors are still important for clinicians to consider in the context of their individual patient's circumstances, when initiating PAP therapy.
The AASM expects this guideline to have an impact on professional behavior, patient outcomes and, possibly, health care costs. This clinical practice guideline reflects the state of knowledge at the time of publication and will be reviewed and updated as new information becomes available.
GOOD PRACTICE STATEMENTS
The following are good practice statements, the implementation of which is necessary for appropriate and effective management of patients with OSA who are treated with PAP.
Treatment of OSA with PAP therapy should be based on a diagnosis of OSA established using objective testing.9
This good practice statement applies specifically to a new diagnosis of OSA, which should be established by either a home sleep apnea test or in-laboratory sleep testing prior to initiation of treatment for OSA. Patients with a previously established diagnosis of OSA who are currently on PAP therapy and have good symptom control should continue PAP therapy, even when prior testing results are not readily available.
Adequate follow-up, including troubleshooting and monitoring of objective efficacy and usage data to ensure adequate treatment and adherence, should occur following PAP therapy initiation and during treatment of OSA.
OSA is a chronic disease that rarely resolves except with substantial weight loss or successful corrective surgery. As with other chronic diseases, periodic follow-up by a qualified clinician (eg, physician or advanced practice provider) is necessary to confirm adequate treatment, assess symptom resolution, and promote continued adherence to treatment. Initial treatment of OSA requires close monitoring and early identification of difficulties with PAP use, as adherence over the first few days to weeks has been shown to predict long-term adherence.10,11 Objective monitoring of PAP therapy should be performed to complement patient reporting of difficulties with PAP use, as patients often overestimate their use of PAP treatment.12
The timing of adequate follow-up after treatment is initiated will vary depending on patient circumstances. However, patients should be followed in the initial weeks to months after PAP initiation to promote adherence and assess response to treatment. Subsequently, yearly evaluation by a trained health care provider is reasonable, although longer periods of follow-up may be appropriate for selected patients who are highly adherent to PAP therapy, have sustained resolution of OSA-related symptoms, and have no concerns regarding their PAP therapy. In contrast, patients with persistent or recurrent sleep-related complaints or persistent difficulties with PAP use should receive more frequent follow-up to address their issues. Routine sleep testing to re-evaluate OSA status in patients on PAP therapy with good symptom control and no change in clinical status (eg, significant weight loss or upper airway surgery) is considered low value care.
CLINICAL PRACTICE RECOMMENDATIONS
The following clinical practice recommendations are based on a companion systematic review, which evaluated the evidence using the GRADE methodology and should be read concurrently with this clinical practice guideline.7 The recommendations in this guideline define principles of practice that should meet the needs of most patients in most situations. A STRONG recommendation is one that clinicians should follow for almost all patients (ie, something that might qualify as a quality measure). A CONDITIONAL recommendation reflects a lower degree of certainty in the appropriateness of the patient-care strategy for all patients. It requires that the clinician use clinical knowledge and experience, while strongly considering the individual patient's values and preferences to determine the best course of action. The ultimate judgment regarding any specific care must be made by the treating clinician and the patient, taking into consideration the individual circumstances of the patient, available treatment options, and resources.
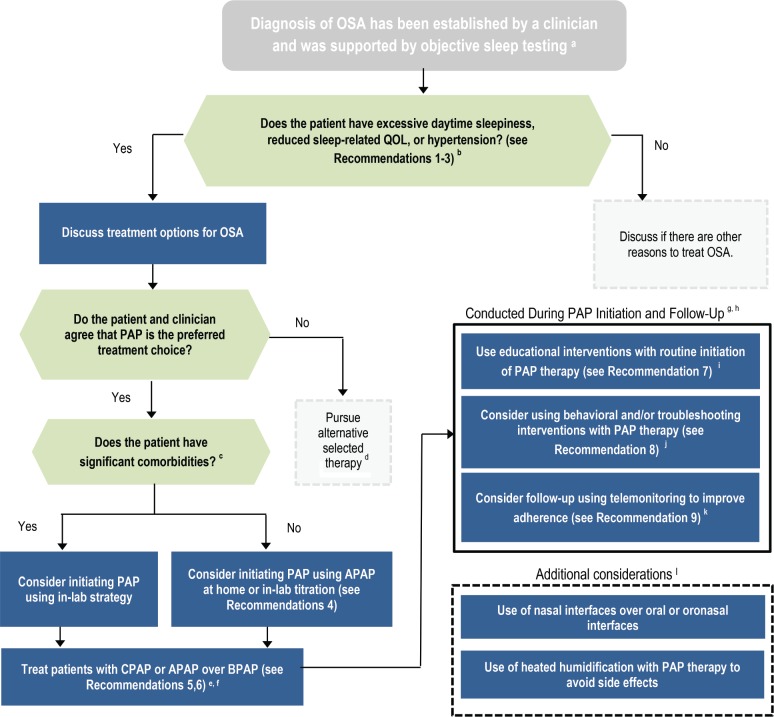
The implications of the strength of recommendations for clinicians, patients, and policymakers are summarized in Table 1. Remarks are provided to guide clinicians in the implementation of these recommendations. A flowchart for the implementation of the recommendations is presented in Figure 1.
Figure 1. Flow chart for implementation of clinical practice guideline.
a = Kapur et al., 2017.9 b = symptoms that can impair sleep-related QOL include but are not limited to snoring, sleep-related choking, insomnia, disruption of bedpartner's sleep, morning headaches, nocturia, impairments in productivity or social functioning, and daytime fatigue. c = comorbidities may include: congestive heart failure, chronic opiate use, significant lung disease such as chronic obstructive pulmonary disease, neuromuscular disease, history of uvulopalatopharyngoplasty, those with known sleep-related oxygen requirements or expected to have nocturnal arterial oxyhemoglobin desaturation due to conditions other than OSA including hypoventilation syndromes and central sleep apnea syndromes. d = alternative therapies may include, but are not limited to, weight loss, positional therapy, oral appliance therapy or surgical interventions. e = BPAP is defined as a respiratory assist device that delivers inspiratory and expiratory positive airway pressure. f = BPAP devices may need to be used for patients with therapeutic pressure requirements greater than can be provided with CPAP or APAP; the decision to use BPAP should be based on the clinician's clinical judgement and needs of the individual patient. g = PAP therapy should be performed in conjunction with adequate follow-up to ensure adequate treatment and adherence. h = recommendations included within these boxes should be considered concurrently. i = educational interventions include those focused primarily on providing information about what OSA is, downstream consequences of untreated OSA, what PAP therapy is, how to use it, and the potential benefits of PAP therapy. j = behavioral interventions include those focused on behavior change related to use of PAP therapy using strategies such as cognitive behavioral therapy or motivational enhancement. Troubleshooting interventions include those focused on close patient communication to identify PAP-related problems and to initiate potential solutions. k = telemonitoring interventions include those that remotely monitor data obtained from a PAP device to identify PAP-related problems and to initiate potential solutions. l = when implementing the above recommendations, providers should consider additional strategies that will maximize the individual patient's comfort and adherence. APAP = auto-adjusting positive airway pressure, BPAP = bilevel positive airway pressure, CPAP = continuous positive airway pressure, OSA = obstructive sleep apnea, PAP = positive airway pressure, QOL = quality of life.
PAP Therapy
Recommendation 1: We recommend that clinicians use positive airway pressure, compared to no therapy, to treat OSA in adults with excessive sleepiness. (STRONG)
The TF assessed whether PAP should be offered to adult patients with OSA, based on improvements in the critical outcome of sleepiness, compared to no therapy. The TF identified 38 randomized controlled trials (RCTs) that assessed the efficacy of PAP to reduce excessive sleepiness. Meta-analyses demonstrated a clinically significant improvement in sleepiness with the use of PAP to treat adults with OSA as compared with no treatment. The overall quality of evidence, based on the critical outcome of sleepiness, was high. While a benefit of PAP use includes a reduction in daytime sleepiness, the potential harms include side effects such as nasal dryness or irritation, dry mouth, sore throat, and sinus infection as well as loss of intimacy,4,9 all of which can be mitigated with appropriate interventions or are reversible with discontinuation of PAP. The potential burdens to the patient may include the costs of treatment and inconvenience such as maintaining the equipment and attending follow-up visits with the sleep clinician. The TF concluded that in adult patients with OSA and excessive sleepiness, the benefits of PAP therapy compared to no PAP therapy likely outweigh the potential harms and burdens, and that the majority of well-informed patients would choose the intervention over no treatment.
Recommendation 2: We suggest that clinicians use positive airway pressure, compared to no therapy, to treat OSA in adults with impaired sleep-related quality of life. (CONDITIONAL)
Remarks: Sleep-related quality of life (QOL) in adult patients with OSA may be adversely affected by OSA-related symptoms. Examples of such symptoms include: snoring, sleep-related choking, insomnia, disruption of bedpartner's sleep, morning headaches, nocturia, impairments in productivity or social functioning, and daytime fatigue.
The TF assessed whether PAP compared to no therapy should be offered to adult patients with OSA to improve the critical outcome of sleep-related QOL. OSA-related symptoms that can reduce sleep-related QOL include, but are not limited to; snoring, nocturnal choking, insomnia, disruption of their partner's sleep, morning headaches, nocturia, impairments in productivity or social functioning, and daytime fatigue. The TF identified 19 RCTs that assessed the efficacy of PAP to improve sleep-related QOL. Meta-analyses of sleep-related QOL, as assessed by the Calgary Sleep Apnea QOL Index (SAQLI) and the Functional Outcomes of Sleep Questionnaire (FOSQ), demonstrated a clinically significant improvement. However, meta-analyses of global QOL, as assessed by the SF-36 component scores, demonstrated no clinically significant improvement. The overall quality of evidence, based on the critical outcome of sleep-related QOL, was moderate due to imprecision. The benefits and potential harms of PAP for patients with impaired sleep-related QOL are the same as for patients with sleepiness.4,9 The TF concluded that in adult patients with OSA and impaired sleep-related QOL, the benefits of PAP therapy compared to no PAP therapy likely outweigh the potential harms and burdens, and that the majority of well-informed patients would choose the intervention over no treatment.
Recommendation 3: We suggest that clinicians use positive airway pressure, compared to no therapy, to treat OSA in adults with comorbid hypertension. (CONDITIONAL)
The TF assessed whether PAP should be offered to adult patients with hypertension and OSA, compared to no therapy to reduce the critical outcome of blood pressure (BP). The TF identified 5 RCTs that reported on the efficacy of PAP therapy on BP in this patient population. Meta-analyses demonstrated clinically significant BP reductions in nocturnal, daytime, and 24-hour systolic and diastolic BP when all patients in the studies were considered, with the largest effects seen for nocturnal measurements. When stratified by resistant hypertensive, hypertensive, and normotensive status, BP reduction was clinically significant in the meta-analyses for the group with hypertension and most BP measures in the group with resistant hypertension. The overall quality of evidence, based on the critical outcome of mean arterial BP, was moderate due to imprecision. The TF notes that the majority of studies evaluating the impact of PAP on BP recruited patients with predominantly moderate to severe OSA. (Note: studies did not systematically report BP based on OSA severity, which limited the ability to make recommendations specific to OSA severity.) The benefits and potential harms of PAP for patients with comorbid hyper-tension are the same as for patients with excessive sleepiness.4,9 The TF recognized that patients experiencing symptoms of OSA (eg, excessive sleepiness) may be more accepting of PAP therapy, with the possibility of secondary benefits related to blood pressure reduction. Non-sleepy patients with OSA, however, may have a more nuanced view of whether to pursue treatment of OSA, particularly given the efficacy of standard antihypertensive treatments. The TF recognized that some non-sleepy patients will place a high value on any intervention that potentially reduces blood pressure, including PAP therapy. Nevertheless, the TF concluded that in adult patients with OSA and comorbid hypertension, the benefits of PAP therapy compared to no PAP therapy likely outweigh the potential harms and burdens, and that the majority of well-informed patients would choose the intervention over no treatment.
There is insufficient and inconclusive evidence to either recommend or withhold PAP to treat non-sleepy adults with OSA as a means to reduce cardiovascular events or mortality.
The TF assessed whether PAP compared to no therapy should be offered to adult patients with OSA to improve the critical outcomes of cardiovascular (CV) event and mortality risk. The TF identified 17 studies (11 observational studies and 6 RCTs) that assessed the impact of PAP therapy on cardiovascular events, and 13 studies (9 observational studies and 4 RCTs) that assessed the impact of PAP therapy on all-cause mortality. Meta-analyses of observational studies suggested a reduction in CV events and mortality with PAP therapy. In contrast, meta-analyses of randomized controlled trials demonstrated no clinically significant improvements in CV events or mortality. The quality of evidence for incident CV events and mortality ranged from very low to moderate due to study type and imprecision. Thus, the TF judged that the meta-analyses demonstrated insufficient and inconclusive findings regarding the impact of PAP therapy on incident CV events and mortality. Therefore, no recommendation is made regarding the use of PAP based on reduced incident CV events and mortality.
Some patients may, however; place a high value on any intervention that potentially reduces CV risk even when they are non-sleepy. In this situation, the patient and clinician should have a balanced discussion about the current state of the evidence about CV risk reduction with PAP therapy for OSA and the potential harms of PAP therapy when there are no other indications to treat the patient's OSA. Conversely, the uncertainty of any CV benefit, may lead some non-sleepy patients with OSA to decline treatment of their OSA. In these patients, conservative management of OSA, with monitoring for development of OSA symptoms over time, may be appropriate.
Initiation of PAP Therapy
Recommendation 4: We recommend positive airway pressure therapy be initiated using either APAP at home or in-laboratory PAP titration in adults with OSA and no significant comorbidities. (STRONG)
Remarks: When APAP is initiated in the home setting, therapy is maintained over the long-term by either using a fixed, continuous pressure setting determined from PAP monitoring data or continuing in the auto-adjusting mode. In-laboratory titration refers to both full-night and split-night titration. The choice of PAP initiation in the home or lab should be based on access, cost-effectiveness, patient preference, sleep clinician judgement, and other factors.
This recommendation is based on studies that excluded patients with the following comorbidities or conditions: congestive heart failure, chronic opiate use, significant lung disease such as chronic obstructive pulmonary disease, neuromuscular disease, history of uvulopalatopharyngoplasty, sleep-related oxygen requirements, or expectation for nocturnal arterial oxyhemoglobin desaturation due to conditions other than OSA, including hypoventilation syndromes and central sleep apnea syndromes.
This recommendation is based on the clinical trials reviewed, in which mask fittings and education on PAP use at a sleep center and/or close follow-up by trained staff during the treatment period were provided to the home APAP group. In some studies, daytime acclimatization to PAP was included.
The TF examined whether initiation of PAP using APAP at home (ie, without an in-laboratory titration) versus in-laboratory titration improved the critical outcomes of adherence, sleepiness, and QOL. The TF did not specifically assess patient outcomes with split-night titration testing as this was recently assessed by an AASM clinical practice guideline on diagnostic sleep testing and was deemed to be a reasonable approach under certain circumstances.9 The guideline assumes that a diagnosis of OSA has already been established. The TF identified 10 RCTs that compared initiation of PAP using home APAP versus an in-laboratory PAP titration. Meta-analyses demonstrated no clinically significant differences in adherence, sleepiness, or QOL between APAP at home and in-laboratory PAP titration. The overall quality of evidence for this recommendation was high.
Potential benefits of using APAP in the home setting include lower cost, reduced time away from home, faster initiation of treatment, and greater access to care, whereas the potential harms may include inadequate patient education and the inability to identify and rectify problems related to mask fit, leak, or other PAP-related issues on the night of APAP use. The potential benefits of an in-laboratory PAP titration include providing education by a trained sleep technologist, real-time visual identification of efficacy of therapy, and the ability to provide immediate interventions to make PAP treatment more comfortable for the patient, whereas the potential harms include the need for an overnight stay away from home at the testing facility with the associated costs and patient time and the potential delay in initiation of therapy.
The TF concluded that the majority of well-informed adult patients with OSA and without significant comorbidities would prefer initiation of PAP using the most rapid, convenient and cost-effective strategy. This recommendation assumes that adequate education on PAP use and mask fittings with or without daytime acclimatization by trained staff are available. Furthermore, when APAP is implemented, the clinician is strongly encouraged to monitor the clinical response and PAP usage and therapy data within the first few weeks to make necessary PAP adjustments when indicated. Independent of payor restrictions, home APAP will be more rapid and convenient for most patients and has been shown to be more cost-effective.13–15 Nevertheless, final determination of which strategy is ideal for an individual patient should be based on patient preferences and abilities, the sleep clinician's judgment, anticipated or known previous difficulty with PAP treatment, and availability of resources and cost of each strategy in a particular region.
PAP Modalities
Recommendation 5: We recommend that clinicians use either APAP or CPAP for ongoing treatment of OSA in adults. (STRONG)
Remarks: This recommendation is based on studies that mostly excluded patients with the following comorbidities or conditions: congestive heart failure, chronic opiate use, significant lung disease such as chronic obstructive pulmonary disease, neuromuscular disease, history of uvulopalatopharyngoplasty, sleep-related oxygen requirements or expectation of nocturnal arterial oxyhemoglobin desaturation due to conditions other than OSA, including hypoventilation syndromes and central sleep apnea syndromes.
The TF examined whether APAP versus CPAP improved the critical outcomes of adherence, sleepiness, and QOL. The TF identified 26 RCTs that investigated the effects of ongoing treatment with APAP compared with fixed CPAP. Meta-analyses demonstrated no clinically significant differences between APAP versus CPAP in adherence, self-reported and objective sleepiness, or QOL. The overall quality of evidence for this recommendation was moderate due to imprecision.
The TF judged that the benefits and harms of APAP and CPAP are similar, and the balance of effects does not favor either intervention. The main potential benefit of APAP to patients is the ability to automatically adjust pressure requirements over time in response to acute and chronic changes (eg, alcohol consumption, body position, or weight changes). No substantial differences in harms were identified for APAP versus CPAP use. Although meta-analyses demonstrated a lack of clinically significant differences in treatment adherence and outcomes, and patient preference varied between studies, the TF determined that individual patient tolerance of PAP, adherence, and symptom response may differ for one form of PAP or the other.
The TF concluded that either APAP or CPAP should be used for ongoing treatment of adult OSA, with the choice of therapy being tailored to patient tolerance and symptom response.
Recommendation 6: We suggest that clinicians use CPAP or APAP over BPAP in the routine treatment of OSA in adults. (CONDITIONAL)
Remarks: This recommendation is based on BPAP defined as a respiratory assist device that delivers inspiratory and expiratory positive airway pressure. This recommendation applies to all BPAP devices including flexible, modified, and auto-adjusting BPAP.
BPAP devices may need to be used for patients with higher therapeutic pressure requirements than can be provided by CPAP or APAP devices. The decision to use BPAP should be based on the clinician's judgement and needs of the individual patient. Furthermore, this recommendation is for the initial treatment of OSA and does not address management of patients who have previously failed CPAP or APAP. In addition, treatment of other forms of sleep-related breathing disorders associated with hypercapnia, which may require the use of BPAP, are covered in other AASM guidelines.5,16
To improve PAP adherence, BPAP has been used as an alternative to CPAP, in part due to issues of patient intolerance of high CPAP settings.17 The TF examined whether BPAP versus CPAP improves the critical outcomes of adherence, sleepiness, and QOL (Note: while no direct evidence was available for the comparison of APAP to BPAP, the TF considers APAP to be equivalent to CPAP for the implementation of this recommendation. See recommendation 5 and Figure 1. The TF identified 5 RCTs that compared the use of BPAP to CPAP. Meta-analyses demonstrated no clinically significant differences in adherence, self-reported sleepiness, and residual OSA with BPAP compared to CPAP. Studies reporting on sleep-related QOL and sleep quality also demonstrated no clinically significant differences. The overall quality of evidence for this recommendation was very low due to publication bias from industry funding and imprecision associated with small sample size.
The main potential benefit of BPAP over CPAP or APAP is improved comfort by lowering the pressure during exhalation, which may then increase adherence. The potential harms of BPAP over CPAP or APAP are a sub-optimally low expiratory pressure level that fails to prevent the occurrence of obstructive breathing events and the higher cost of BPAP. Furthermore, the historically perceived benefits of BPAP are less likely to be relevant since modified pressure profile technology, which also lowers expiratory pressures, has been integrated into modern PAP devices. The TF judged that although the benefits of treatment with BPAP versus CPAP or APAP are similar, the low quality of evidence and potential harms or burdens did not favor the regular use of BPAP for the routine treatment of OSA. Therefore, the TF concluded that the majority of well-informed adult patients with OSA would prefer initiation of treatment with CPAP or APAP over BPAP. However, there is a small subset of patients that require PAP treatment with pressures higher than 20 cm H2O, which CPAP units are not typically capable of delivering. In these situations, BPAP devices may be needed for optimal treatment and can be utilized during an initial or subsequent in-laboratory PAP titration study. In addition, for specific patients who are unable to tolerate CPAP or APAP due to high pressure requirements and despite the use of modified pressure profiles, a trial of BPAP may be offered either during the initial in-laboratory titration or following a period of demonstrated non-acceptance.
Educational and Behavioral Interventions With PAP
Recommendation 7: We recommend that educational interventions be given prior to initiation of PAP therapy in adults with OSA. (STRONG)
Recommendation 8: We suggest that behavioral and/ or troubleshooting interventions be given during the initial period of PAP therapy in adults with OSA. (CONDITIONAL)
Remarks: These recommendations are based on interventions defined as follows:
Educational interventions: Interventions focused primarily on providing information prior to initiation of PAP about what OSA is, its downstream consequences, what PAP therapy is, and the potential benefits of PAP therapy.
Behavioral interventions: Interventions focused on behavior change prior to and during the initiation and subsequent use of PAP therapy using strategies such as cognitive behavioral therapy or motivational enhancement.
Troubleshooting interventions: Interventions focused on close patient communication to identify PAP-related problems and to initiate potential solutions during the initial period of PAP therapy.
The intervention period may include interactions prior to, during and after PAP titration and follow-up.
The TF examined whether an educational, behavioral, or troubleshooting intervention versus no intervention prior to or during PAP treatment improves the critical outcome of adherence. QOL was initially considered a critical outcome; however, none of the accepted studies reported on this outcome. The TF identified 18 RCTs that evaluated the use of some combination of an educational, behavioral, or troubleshooting intervention as an adjunct to initiation of PAP therapy compared to PAP therapy with usual care. Meta-analyses demonstrated a clinically significant improvement in PAP adherence with all three types of interventions. The overall quality of evidence was moderate due to imprecision.
The potential harms of each of these interventions are minimal. The potential burdens to the patient are negligible for educational interventions but include the time required to receive the intervention and the cost of the additional care for the more intensive behavioral and troubleshooting interventions.
The TF judged that the benefits of educational interventions outweigh potential harms and burdens, while the benefits of behavioral and troubleshooting interventions likely outweigh the potential harms and burdens in most patients. As such, the TF concluded that the vast majority of well-informed adult patients with OSA would prefer that an educational intervention be provided with initiation of PAP therapy over initiation of PAP without education. In addition, the TF concluded that the majority of well-informed adult patients with OSA would likely prefer that a behavioral and/or troubleshooting intervention be given during the initial period of PAP therapy over no such intervention.
Monitoring During Treatment
Recommendation 9: We suggest that clinicians use telemonitoring-guided interventions during the initial period of PAP therapy in adults with OSA. (CONDITIONAL)
Remarks: This recommendation is based on interventions defined as follows: Telemonitoring includes the remote monitoring of PAP parameters such as PAP use, residual OSA severity, unintentional mask leaks, and PAP settings during treatment initiation and follow-up.
The TF examined whether behavioral, educational and troubleshooting interventions guided by remote monitoring of PAP therapy versus no remote monitoring improved the critical outcomes of adherence, sleepiness, and side effects. QOL was initially considered a critical outcome; however, none of the accepted studies reported on this outcome. The TF identified 5 RCTs that evaluated the use of remote monitoring of PAP variables to trigger early interventions versus no such system as an adjunct to PAP therapy. Meta-analyses demonstrated a clinically significant improvement in adherence, but not in sleepiness, with use of telemonitoring. PAP-associated side effect severity scores tended to be lower with a telemonitoring-guided intervention; however, these differences were not clinically significant. The overall quality of evidence was moderate due to imprecision. The potential harms of remote monitoring interventions are small, primarily related to potential loss of privacy. How tele-monitoring guided interventions are implemented could lead to substantial increases in costs to health-care systems, or conversely, may reduce costs if reductions in healthcare utilization substantially offset the investment in tele-monitoring systems. Nevertheless, the TF determined that the benefits of telemonitoring-guided adherence interventions likely outweigh the potential harms and burdens in most patients. Based on clinical experience, the TF concluded that the improvement in adherence would be valued by most patients. The TF concluded that the majority of well-informed adult patients with OSA would prefer enrollment in such a system compared to treatment without such an intervention.
ADDITIONAL CONSIDERATIONS
The AASM supports patient-centered care in which an individual patient's specific health needs and desired health outcomes are the driving force behind all health care decisions. When implementing the recommendations in this guideline, clinicians should consider strategies that will maximize the individual patient's comfort and adherence. The TF performed a systematic review of several interventions that aim to improve comfort and adherence including modified pressure profile, mask interfaces, and humidification. These are summarized below. A detailed evidence review can be found in the accompanying systematic review.7
Modified Pressure Profile PAP
Many PAP devices have now integrated modified pressure profiles, which lower the treatment pressure used during expiration. The potential benefit of using modified pressure profile PAP is increased patient comfort, while the potential harms of modified pressure profile PAP are similar to standard PAP. The TF identified 7 RCTs that investigated the use of modified pressure profile PAP to improve clinical outcomes and reduce side effects. Meta-analyses demonstrated no clinically significant differences in adherence, sleepiness, and QOL with modified pressure profile PAP versus standard PAP. Insufficient, standardized data were available to perform a meta-analysis on side effects; however, the reported data demonstrated no clinically significant differences. The data suggest that there are no systematic benefits to the routine initiation of treatment with modified pressure profile PAP, compared to standard PAP for OSA, despite perceived minimal potential harms and burdens. However, modified pressure profile PAP may have value in some patients in other contexts (eg, poorly adherent patients or patients with difficulty tolerating CPAP during in-laboratory titration studies) that have not yet been well studied.
Mask Selection
Appropriate mask selection will benefit patients by reducing side effects such as air leak and discomfort, which may then potentially improve adherence and subsequently patient outcomes. The TF identified 11 studies (3 observational studies and 8 RCTs) that evaluated the effects of different PAP interfaces on reducing the apnea-hypopnea index (AHI); improving adherence, sleepiness and QOL; and reducing side effects. Meta-analyses demonstrated a clinically significant improvement in adherence with nasal PAP versus oronasal interfaces, but there was no clinically significant difference in adherence between nasal or intra-nasal interfaces. With regards to self-reported sleepiness, meta-analyses demonstrated no clinically significant differences between interfaces. Studies reporting on QOL demonstrated no clinically significant differences between intra-nasal versus nasal interfaces. Finally, the studies analyzed indicated that there were fewer side effects with nasal compared with oronasal and oral interfaces. These data suggest that, for the routine initiation of PAP therapy in adults with OSA, clinicians should generally use nasal or intranasal mask interfaces over oronasal or oral interfaces. However, individual patient factors or preferences vary; therefore, the mask interface that minimizes side effects and optimizes efficacy and adherence should be used.
Humidified PAP
The use of humidification could also potentially reduce side effects from PAP therapy. The TF identified 9 RCTs that evaluated the use of PAP with humidification versus PAP without humidification to improve adherence, sleepiness, QOL, or PAP-related side effects. Meta-analyses demonstrated a clinically significant reduction in several side effects associated with the use of PAP, including dry mouth/throat, nasal discharge, nasal congestion, dry nose, bleeding nose, sinus pain or headache, sore throat, hoarse voice, and reduced smell. However, meta-analyses demonstrated no clinically significant improvement in PAP adherence, sleepiness, or QOL with the use of humidification compared with no humidification. The possibility of humidification causing “rain out” (ie, condensation of water into the PAP circuit, face, and nose or mouth of the patient) may deter its use. While the use of humidification may increase the ongoing costs (eg, purchasing distilled water, heated hoses) and maintenance requirements, these data suggest clinicians should generally use heated humidification with PAP devices to reduce side effects that may occur while treating adults with OSA.
SUMMARY
This clinical practice guideline provides recommendations for the use of PAP, approaches to the initiation of PAP treatment, and interventions to promote PAP adherence in adults with OSA. The recommendations are the result of the task force interpretation of evidence collected for the systematic review and application to the clinical care of adults with OSA. Four recommendations are strongly suggested and include: (1) using PAP to treat excessive sleepiness, (2) initiating PAP therapy with either APAP at home or an in-laboratory CPAP titration, (3) continuing PAP therapy for OSA with either CPAP or APAP, and (4) using educational interventions to initiate PAP therapy in adults with OSA. All other recommendations were conditional and include using PAP to treat impaired sleep-related QOL or concomitant hypertension; implementing CPAP or APAP over BPAP in the routine treatment of OSA; and utilizing behavioral, troubleshooting, and telemonitoring interventions during the initial period of PAP therapy. The TF determined that a recommendation could not be made for the use or withholding of PAP therapy to treat non-sleepy patients to reduce incident cardiovascular disease. When implementing the recommendations, providers should consider additional strategies that will maximize the individual patient's comfort and adherence such as nasal/intranasal over oronasal mask interface and heated humidification, as discussed in the additional considerations section. Readers are strongly encouraged to read the companion systematic review7 for a more detailed presentation and evaluation of the evidence. This clinical practice guideline reflects the state of knowledge at the time of publication and will be reviewed and updated as new information becomes available.
DISCLOSURE STATEMENT
Dr. Indu Ayappa disclosed that she receives royalties from patents held on some PAP devices. Dr. Ayappa's potential conflict was managed by requesting that she refrain from participation on any discussions pertaining to devices for which she may hold a patent. She was also asked to refrain from voting on recommendations pertaining to those devices. Dr. Sanjay Patel disclosed that he receives compensation from Bayer Pharmaceuticals to assess the impact of OSA and its treatment on pulmonary hypertension and other cardiovascular outcomes. He was asked to refrain from participation on any discussion pertaining to the use of PAP to treat hypertension. He was also asked to refrain from voting on recommendations pertaining to the use of PAP to treat hypertension. Mr. Harrod is employed by the American Academy of Sleep Medicine. No other task force members had any relevant conflicts of interest to disclose.
ACKNOWLEDGMENTS
The task force thanks Dr. Romola Bucks (University of Western Australia) and Dr. Gerry Taylor (Case Western Reserve University) for lending their expertise in determining how neurocognitive outcomes should be addressed in this guideline.
REFERENCES
- 1.Kushida CA, Littner MR, Hirshkowitz M, et al. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep. 2006;29(3):375–380. doi: 10.1093/sleep/29.3.375. [DOI] [PubMed] [Google Scholar]
- 2.Kushida CA, Chediak A, Berry RB, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4(2):157–171. [PMC free article] [PubMed] [Google Scholar]
- 3.Morgenthaler TI, Aurora RN, Brown T, et al. Practice parameters for the use of autotitrating continuous positive airway pressure devices for titrating pressures and treating adult patients with obstructive sleep apnea syndrome: an update for 2007. An American Academy of Sleep Medicine report. Sleep. 2008;31(1):141–147. doi: 10.1093/sleep/31.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gay P, Weaver T, Loube D, et al. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep. 2006;29(3):381–401. doi: 10.1093/sleep/29.3.381. [DOI] [PubMed] [Google Scholar]
- 5.Berry RB, Chediak A, Brown LK, et al. Best clinical practices for the sleep center adjustment of noninvasive positive pressure ventilation (NPPV) in stable chronic alveolar hypoventilation syndromes. J Clin Sleep Med. 2010;6(5):491–509. [PMC free article] [PubMed] [Google Scholar]
- 6.Aurora RN, Bista SR, Casey KR, et al. Updated adaptive servo-ventilation recommendations for the 2012 AASM guideline: “The Treatment of Central Sleep Apnea Syndromes in Adults: Practice Parameters with an Evidence-Based Literature Review and Meta-Analyses”. J Clin Sleep Med. 2016;12(5):757–761. doi: 10.5664/jcsm.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patil SP, Ayappa IA, Caples SM, Kimoff RJ, Patel SR, Harrod CG. Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 2019;15(2):301–334. doi: 10.5664/jcsm.7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morgenthaler TI, Deriy L, Heald JL, Thomas SM. The evolution of the AASM clinical practice guidelines: another step forward. J Clin Sleep Med. 2016;12(1):129–135. doi: 10.5664/jcsm.5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Budhiraja R, Parthasarathy S, Drake CL, et al. Early CPAP use identifies subsequent adherence to CPAP therapy. Sleep. 2007;30(3):320–324. [PubMed] [Google Scholar]
- 11.Weaver TE, Kribbs NB, Pack AI, et al. Night-to-night variability in CPAP use over the first three months of treatment. Sleep. 1997;20(4):278–283. doi: 10.1093/sleep/20.4.278. [DOI] [PubMed] [Google Scholar]
- 12.Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147(4):887–895. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 13.McArdle N, Singh B, Murphy M, et al. Continuous positive airway pressure titration for obstructive sleep apnoea: automatic versus manual titration. Thorax. 2010;65(7):606–611. doi: 10.1136/thx.2009.116756. [DOI] [PubMed] [Google Scholar]
- 14.Hui DS, Ng SS, Tam WWS. Home-based approach is non-inferior to hospital-based approach in managing patients with suspected obstructive sleep apnoea syndrome. Am J Respir Crit Care Med. 2018;197(9):1233–1234. doi: 10.1164/rccm.201711-2185LE. [DOI] [PubMed] [Google Scholar]
- 15.Rosen CL, Auckley D, Benca R, et al. A multisite randomized trial of portable sleep studies and positive airway pressure autotitration versus laboratory-based polysomnography for the diagnosis and treatment of obstructive sleep apnea: the HomePAP study. Sleep. 2012;35(6):757–767. doi: 10.5665/sleep.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aurora RN, Chowdhuri S, Ramar K, et al. The treatment of central sleep apnea syndromes in adults: practice parameters with an evidence-based literature review and meta-analyses. Sleep. 2012;35(1):17–40. doi: 10.5665/sleep.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanders MH, Kern N. Obstructive sleep apnea treated by independently adjusted inspiratory and expiratory positive airway pressures via nasal mask. Physiologic and clinical implications. Chest. 1990;98(2):317–324. doi: 10.1378/chest.98.2.317. [DOI] [PubMed] [Google Scholar]