Abstract
Study Objectives:
This study has investigated the risk of major adverse cardiovascular events (MACEs), including acute myocardial infarction, coronary artery disease, peripheral artery disease, and acute stroke, among children and adolescents (age younger than 20 years) with obstructive sleep apnea (OSA).
Methods:
In this study, the population-based National Health Insurance Research Database of Taiwan was used to identify patients in whom OSA had been first diagnosed between 2000 and 2015. Children and adolescents with OSA (n = 6,535) were included with 1:3 ratio by age, sex, and index year of control participants without OSA (n = 19,605). The Cox proportional regression model was used to evaluate the risk of MACEs in this cohort study.
Results:
After a 15-year follow-up, the incidence rate of MACEs was higher in the OSA cohort when compared with the non-OSA control cohort (15.97 and 8.20 per 100,000 person-years, respectively). After adjusting for covariates, the risk of MACEs among children and adolescents with OSA was still significantly higher (hazard ratio = 2.050; 95% confidence interval = 1.312–3.107; P = .010). No MACEs were found in the children and adolescents with OSA who received continuous airway positive pressure treatment or pharyngeal surgery.
Conclusions:
This study found a significantly higher risk of MACEs in children and adolescents with OSA. These findings strongly suggest that clinicians should provide careful follow-up and medical treatment for children and adolescents with OSA.
Citation:
Tzeng NS, Chung CH, Chang HA, Chang CC, Lu RB, Yeh HW, Chiang WS, Kao YC, Chang SY, Chien WC. Obstructive sleep apnea in children and adolescents and the risk of major adverse cardiovascular events: a nationwide cohort study in Taiwan. J Clin Sleep Med. 2019;15(2):275–283.
Keywords: cohort study, major adverse cardiovascular events, National Health Insurance Research Database, sleep apnea
BRIEF SUMMARY
Current Knowledge/Study Rationale: To address a gap in knowledge, we investigated the association between obstructive sleep apnea (OSA) in children and adolescents, and the risk of major adverse cardiovascular events (MACEs) on the population database. In a nationwide, matched cohort study, the selection bias could be minimized.
Study Impact: OSA in children and adolescents is associated with increased MACEs on the population database. The children and adolescents with OSA were more likely to experience MACEs (hazard ratio = 2.050; 95% confidence interval = 1.312–3.107; P = .010) when adjusting for sex, age, monthly income, urbanization level, geographic region, and comorbidities.
INTRODUCTION
Obstructive sleep apnea (OSA) is a common disorder characterized by repeated partial or complete collapse of the upper airway with episodes of apnea and hypopnea during sleep.1,2 The prevalence of pediatric OSA in children ranges from 1% to 4% according to the varying criteria of diagnostic studies, with a male predominance,3 and one systematic review reports that sleep-disordered breathing is estimated at 4% to 11%.4 Previous studies had reported that OSA in children and adolescents (younger than 20 years) is associated with an increased risk of atopic dermatitis,5 depressive disorders,6 nocturnal enuresis,7 and postsurgical complications.7
In this study, we defined the major adverse cardiovascular events (MACEs) as acute myocardial infarctions (MIs), coronary artery disease (CAD) combined with stent implantation and/or percutaneous transluminal coronary angioplasty, peripheral artery disease (PAD) combined with percutaneous transluminal angioplasty, and acute stroke. Previous studies have reported that adult patients with OSA are associated with CAD, such as acute MI,8 stroke,9 PAD,10 and heart failure.11 One study found that OSA is associated with the risk of other MACEs, including heart failure (HF), and cardiometabolic mortality,12 However, the association between OSA in children and adolescents (younger than 20 years) and MACEs has not, as yet, been clarified. We hypothesize that the population-based study using a national representative database could examine as to whether OSA in children and adolescents is associated with the risk of MACEs. Therefore, we carried out this study to determine whether OSA in children and adolescents is associated with MACEs.
METHODS
Data Sources
The National Health Insurance (NHI) Program was launched in Taiwan in 1995, and as of June 2009, included contracts with 97% of the medical providers with approximately 23 million beneficiaries, or more than 99% of the entire population.13 The National Health Insurance Research Database (NHIRD) contains all the claims data of the beneficiaries; that is, approximately 23 million beneficiaries are registered in the NHIRD, including the NHI medical claims database that comprises ambulatory care, inpatient care, outpatient care, and prescription drugs. This database uses the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes, based on the World Health Organization's Ninth Revision, International Classification of Diseases, as the official system of assigning codes to diagnoses and procedures associated with hospital utilization in Taiwan. In order to protect individual privacy, the identities of individuals included in this database all were encrypted. The details of the program have been documented in previous studies.14–20
A subset of the NHIRD, the Longitudinal Health Insurance Database (LHID) of a randomized sample population of two million between 2000 and 2015, was used to study the association between OSA and the risk of MACEs. The current study used this subset from the NHIRD to identify patients younger than 20 years with a diagnosis of OSA, based on the ICD-9-CM codes, including 327.23, 780.51, 780.53, and 780.57, during the years 2000–2015. In Taiwan, nocturnal polysomnography (PSG) is used to confirm the diagnosis of OSA; therefore, PSG within 1 year before, or within 1 year after the index day of OSA diagnosis, could be used to make the diagnosis of OSA, with reference of one previous study.21 Furthermore, one recent study has validated the diagnosis of OSA recorded in the NHIRD. Briefly, in one sample from one hospital-based databank from a medical center, single or repeated polysomno-graphic data have confirmed that 3,333 of 3,363 patients with sleep apnea (99%) also have OSA.22,23 The Institutional Review Board of the Tri-Service General Hospital approved this study and waived the need for individual written informed consent (IRB No. 2-105-05-040).
Study Design and Sampled Participants
This study was of a population-based, matched-cohort design. Patients younger than 20 years in whom OSA was newly diagnosed (ICD-9-CM codes: 327.23, 780.51, 780.53, and 780.57) were selected from the LHID from January 1, 2000, to December 31, 2015. The patients with OSA before the year 2000 were excluded. In addition, the patients in whom congenital heart diseases or MACEs were diagnosed, including acute MI, CAD combined with stent implantation and/or percutaneous transluminal coronary angioplasty, PAD combined with percutaneous transluminal angioplasty, and acute stroke, before the year 2000, or before the first visit for OSA were also excluded. The definition of MACEs has been modified from previous studies.11,24 A total of the patients who were enrolled, including 6,535 patients with OSA and 19,605 controls participants without OSA, were matched for age, sex, and index date (Figure S1). The index date was defined as the date at which the patient received a diagnosis of OSA, or the corresponding index date for the matched comparison cohort members enrolled.
Covariates
The covariates included sex, age groups (0–11, and 12–19 years), geographical area of residence (north, center, south, and east of Taiwan), urbanization level of residence (levels 1 to 4), and monthly income (in New Taiwan dollars [NT$]; < 18,000, 18,000–34,999, ≥ 35,000). The division of the age groups is according to the laws in Taiwan.25 The urbanization level of residence was defined according to the population and various indicators of the level of development. Level 1 was defined as a population less than 1,250,000, and a specific designation as political, economic, cultural, and metropolitan development. Level 2 was defined as a population between 500,000 and 1,249,999, and as playing an important role in the politics, economy, and culture. Urbanization levels 3 and 4 were defined as a population between 149,999 and 499,999, and less than 149,999, respectively.
In Taiwan, the continuous positive airway pressure (CPAP) for the patients with OSA is not reimbursed in the NHI policy. However, nocturnal PSG testing 6 months after the CPAP usage is needed for the CPAP titration for adequately testing the response among patients. Therefore, repeated PSG, which was recorded 6 months after the first PSG, could confirm that the CPAP treatment has been used, with details documented in a previous study.21 The pharyngeal surgery including the tonsil-lectomy, adenoidectomy, adenotonsillectomy, or uvulopalatopharyngoplasty were also recorded.
Comorbidity
Comorbidities were assessed using the Charlson Comorbidity Index (CCI), which categorizes the comorbidities using the ICD-9-CM codes, scores each comorbidity category,26–28 and combines all the scores to calculate a single comorbidity score. A score of zero indicates that no comorbidities were found, and higher scores indicate higher comorbidity burdens.29 Other comorbidities included hypertension (ICD-9-CM codes 401–405), diabetes mellitus (DM, ICD-9-CM codes 250), insomnia (ICD-9-CM code 307.4, and 780.5), attention deficit hyperactivity disorder (ADHD, ICD-9-CM codes 314), asthma (ICD-9-CM codes 493), and obesity (ICD-9-CM code 278.0).
Major Outcomes
All of the study participants were followed from the index date until one of the following events occurred: the diagnosis of the onset of acute MI (ICD-9-CM codes 410.xx), CAD combined with stent implantation and/or percutaneous transluminal coronary angioplasty (treatment codes 33076A, 33076B, 33077A, 33077B, 33078A, 33078B), PAD (ICD-9-CM codes 440.2x, 440.3x, 444.2x, 444.8x,) combined with percutaneous transluminal angioplasty (treatment codes 33074A, 33074B, 33115B), and acute stroke (ICD-9-CM codes 430.xx-436.xx), withdrawal from the NHI program, or the end of 2015.
Statistical Analysis
All statistical analyses were performed using SPSS for Windows, version 22.0 (IBM Corp., Armonk, New York, United States). χ2 and t tests were used to evaluate the distributions of the categorical and continuous variables, respectively, with a Fisher exact test. The multivariate Cox proportional hazards regression analysis was used to determine the risk of MACEs, and the results were presented as a HR with a 95% CI. The difference in the risk of MACEs, between the study and control groups, was estimated using the Kaplan-Meier method with the log-rank test. A two-tailed value of P < .05 was considered to indicate statistical significance.
RESULTS
Baseline Characteristics of the Study Population
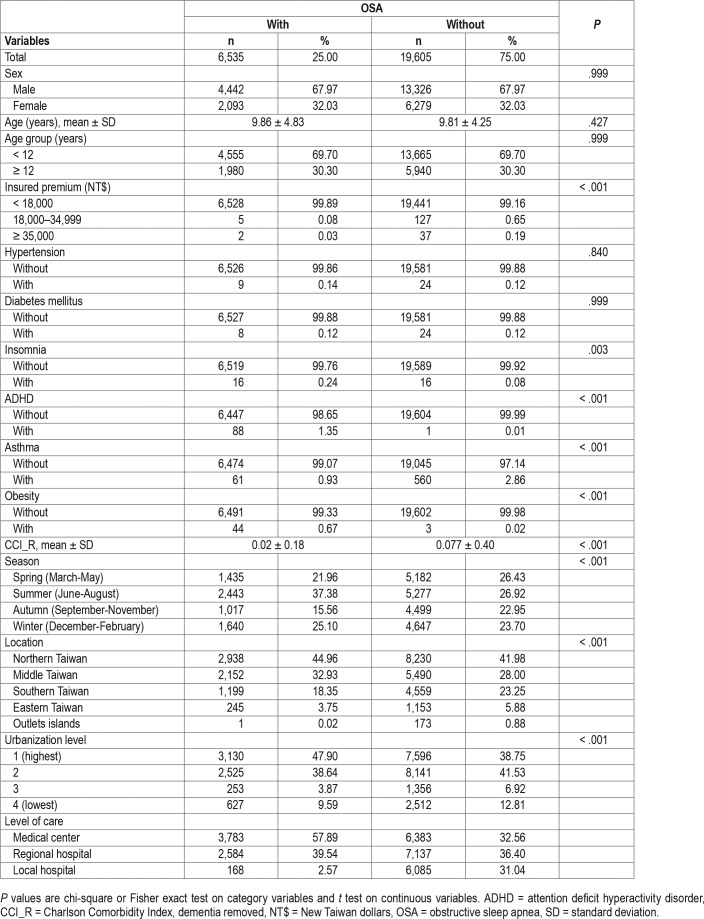
Baseline characteristics of the study population are as depicted in Table 1. There were 6,535 individuals in the OSA group and 19,605 in the control group without OSA, with a similar distribution of sex, age, and monthly insured premiums. The mean CCI (standard deviation, [SD]) for the study participants was 0.02 (0.18), and 0.077 (0.040) for the OSA and controls groups, respectively. The OSA group tended to have higher rates in insomnia, ADHD, and obesity, and a lower rate in asthma. The study participants had more medical visits in the summer and winter, with residences in northern and middle Taiwan, living in level 1 urbanization, or seeking help in medical centers and regional hospitals than the control group.
Table 1.
Characteristics at baseline.
Table S1 depicts the sample characteristics at the end of the study. The study participants with OSA tended to have higher rates of insomnia, ADHD, and obesity, and a lower rate of asthma. The rate of asthma had increased from 0.93% to 1.44% in the OSA cohort, with that of the control group increasing to 3.41%, at the end of the study.
OSA Associated With MACEs
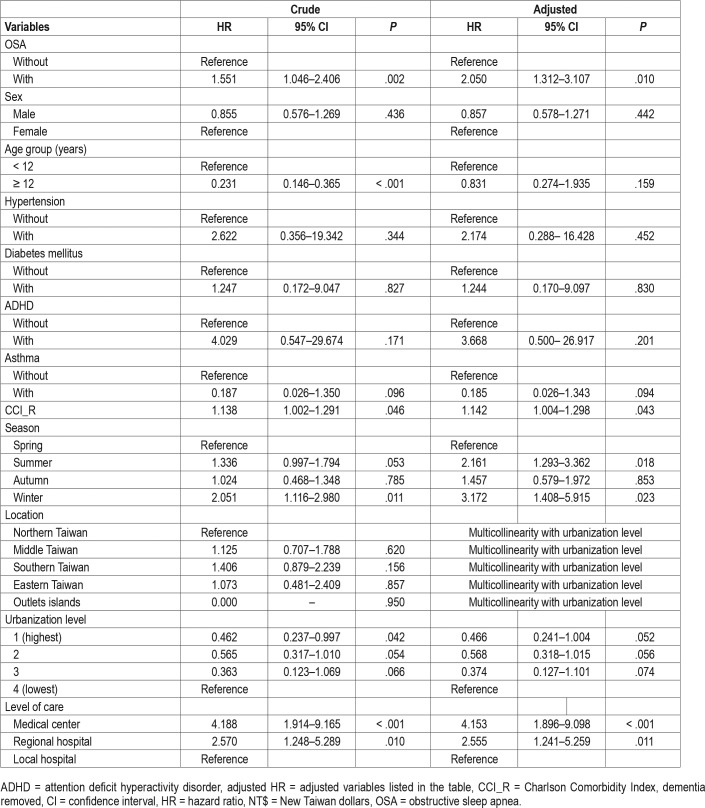
Of the study participants, MACEs developed in 13 (0.199%, or 15.97 per 100,000 person-years) compared to 20 (0.102%, or 8.20 per 100,000 person-years) in the control group, after the 15-year follow-up. We have also examined the risk of MACEs associated with OSA: after adjusting for age, sex, CCI scores, and all the covariates listed in Table 1, the Cox proportional hazards regression analysis revealed that the adjusted HR for MACEs was 2.050 for the study participants (95% CI = 1.312– 3.107; P = .01) when compared with the control group (Table 2). Figure 1 shows the Kaplan–Meier analysis for the cumulative incidence of MACEs in the study participants and control groups (log-rank test, P < .001). Furthermore, each single increased score of CCI was associated with a 14.2% increased risk of MACEs, and the study participants who sought medical help in summer (adjusted HR: 2.161, P = .018) and winter (adjusted HR: 3.172, P = .023) were associated with a lower risk of MACEs, than those in the spring. Study participants who sought medical help in the medical centers (adjusted HR: 4.153, P < .001), and regional hospitals (adjusted HR: 2.555, P = .011) were associated with a lower risk of MACEs than those in local hospitals. It took a mean of 2.57 years (SD = 3.01) for the study participants with OSA, and a mean of 6.49 years (SD = 5.37) to the MACEs for the control group without OSA, respectively (Table S2).
Table 2.
Factors of major cardiovascular adverse effects by using Cox regression.
Figure 1. Kaplan-Meier analysis for cumulative incidence of MACEs in individuals younger than 20 years stratified by sleep apnea with log-rank test.
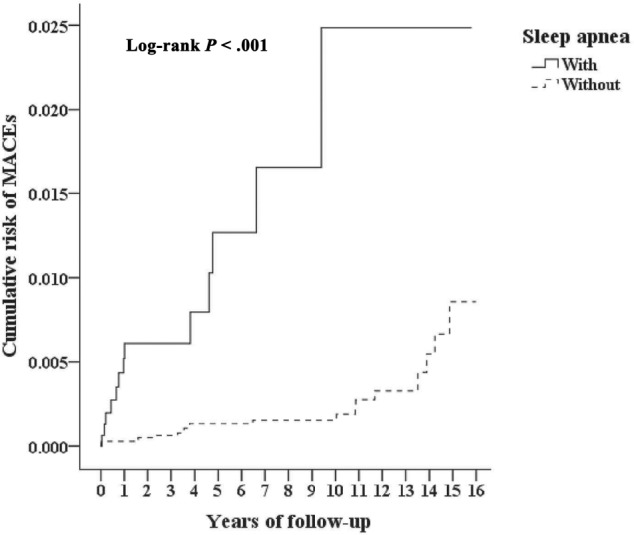
MACE = major adverse cardiovascular event.
Association Between OSA and Risk of Different MACEs
We have analyzed the association between the OSA and the different MACEs, and found that the OSA in children and adolescents was associated with an increased risk of acute MI (adjusted HR = 7.945, P = .003), CAD combined with stent implantation and/or percutaneous transluminal coronary angioplasty (adjusted HR = 2.108, P = .015), and acute stroke (adjusted HR = 1.577, P = .047) (Table S3).
Table 3 showed the distributions of treatment or surgery that the patients with OSA received in this study. In these patients with OSA, 42.41% received neither CPAP nor pharyngeal surgery. The rate of CPAP usage was 0.72% (n = 47). Most of the treatments that the patients with OSA received were pharyngeal surgery only (n = 3,716, 56.87%), and surgery for the removal of tonsils or adenoids predominated. Among those patients who underwent CPAP, pharyngeal surgery, or both, no MACEs were found (data not shown).
Table 3.
Distribution of treatment surgery.
Table S4 depicts the subgroup analysis between the OSA cohort and the risk of MACE, in comparison with the cohort without OSA. In addition, in our study, the patients with OSA and who had obesity did not experience MACE at the end of the follow-up period.
DISCUSSION
Association Between OSA and the Risk of MACEs
In this study, we examined the association between OSA and the risk of MACEs. After adjusting the covariates, the adjusted HR was 2.05 for the study participants (95% CI = 1.312–3.107; P = .01) when compared with the control group. In other words, the youth patients with OSA had a 2.05-fold increased risk of developing MACEs. The Kaplan-Meier analysis revealed that the study participants had a significantly higher 15-year cumulative MACE incidence rate than the control group. Furthermore, for the cohort with OSA who underwent CPAP treatment or pharyngeal surgery, no MACEs were found. Previous studies on the treatments for mental health diagnoses, such as antipsychotic agents,30 antimanic agents,31 and antidepressant agents32 in children and adolescents, are associated with the risk of cardiovascular events. However, in this study, no MACEs were found in the OSA children and adolescents who underwent CPAP treatment or pharyngeal surgery. This study therefore showed that CPAP and pharyngeal surgery could be helpful for children and adolescents with OSA. Recent studies have shown doubtful effects of the CPAP treatment on the prevention of cardiovascular diseases in adults.33 Several studies on the outcomes of adenotonsillectomy might improve the autonomic functions in children with OSA. We recommend that long-term follow-up studies for the children and adolescents with OSA with CPAP or pharyngeal surgery might well be needed to evaluate the effects of these treatments in the prevention of cardiovascular diseases, especially after they have become adults. To the best of our knowledge, this is the first study on the topic for new-onset OSA in children and adolescents that is associated with an increased risk of the development of MACEs.
Comparison of this Study to Previous Literature
In comparison to the previous studies about OSA in adults and the risk of cardiovascular or cerebrovascular diseases in Taiwan, the incidence of MACEs, for example, acute stroke, 7.23 per 100,000 individual-years, is lower than that in the adult patients, being 546 per 100,000 individual-years.34 The overall incidence of MACEs (0.126%) is also lower than that in another study (6.3%).11 This finding could be due to the shorter durations (maximum 15 years, or those near age 35 years) between new-onset OSA and MACEs, and the age of the study participants who were far younger than the mean of the onset of MACEs. In one previous study, OSA in adults was associated with PAD,10 but in this study, the association was insignificant. Furthermore, OSA in patients with obesity was not associated with the risk of MACEs; however, studies have consistently shown that OSA is associated with an increased cardiovascular mortality, independent of obesity.35
Possible Mechanisms for the Increased Risk of MACEs in Patients With OSA
The mechanisms underlying the association between OSA in children and adolescents and the risk of MACEs remain unclear. Scholars have summarized that chronic, intermittent hypoxia from OSA in adults would induce damage to the vascular endothelium, and consequent cardiovascular and cerebrovascular diseases, through oxidative stress, inflamma-tory responses, platelet activation, cell apoptosis, insulin resistance/neuroendocrine disruptions,36 increase in sympathetic tone, cortical arousal, and changes in the adipokine levels.37 However, further study is needed for the clarification of the mechanisms underlying OSA in children and adolescents and the risk of MACEs.
Additionally, we have included several psychosocial factors in the analysis, such as monthly insurance premiums, urbanization level, and residence. In comparison to the patients with OSA who sought medical help in the medical centers and regional hospitals, the patients with OSA who sought medical care in the local hospitals were associated with a higher risk of MACEs. In addition, even though people younger than 20 years pay fewer insurance premiums in Taiwan's NHI system,38 there was still a statistical difference in monthly insurance premiums, and the OSA cohort had a slightly higher rate in the subgroup of NT$ < 18,000. These findings suggest that socioeconomic factors might well contribute to the risk of MACEs. Furthermore, one Taiwanese study has shown that the burden of diseases, including ischemic heart disease and stroke, was attributable to ambient fine particulate matter exposure in Taiwan, and these air pollutants were higher in middle and southern Taiwan, and in the current study, the patients in the OSA cohort lived in northern and middle Taiwan, but the Cox proportional regression model revealed that there were no significant differences in the risk of MACE in the areas in Taiwan (Table 2). However, the influences of socioeconomic factors, such as monthly insured premiums, urbanization, and areas of Taiwan, on the risk of MACE in the patients with OSA require additional study.
Potential Effect of Nonadherence to CPAP
One review article reported that, using a minimum of 4 hours per night to define adherence, 29% to 83% of patients are non-adherent with CPAP therapy,39 and one study in Taiwan found that only 53% of the patients were in the good adherence group, defined as adherence rates higher than 50%.40 One study suggested that surgical interventions such as maxillomandibular advancement and uvulopalatopharyngoplasty should be the surgical treatment option of choice for most patients with moderate to severe OSA who are unable to adequately adhere to CPAP.41 However, the records of the preference of patients or the consideration of surgeons were not included in the NHIRD.
Potential Effect of Asthma and OSA
Previous studies report the association between asthma and OSA,42–44 but the OSA cohort had a lower rate of comorbidity of asthma than the control group without OSA, in the current study. The reasons for this discrepancy remain unclear. However, at the end of the study, the rate of asthma had increased from 0.93% to 1.44% in the OSA cohort, though that in the control group increased to 3.41% (Table S1). Because one review article revealed that the relationship between OSA and asthma could be bidirectional,45 and one study found that 29% of patients have had the initial diagnosis of asthma at the age of 25 to 44 years, and asthma developed in 21% of patients age 45 to 65 years old in Taiwan,46 more than 15 years of follow-up may be required for the patients with OSA to catch up, or even have higher rates of asthma, than the control group. In addition, perhaps this finding also reflects the behaviors of physicians in coding only one diagnosis at one time. Additional studies are needed to clarify the association between OSA and asthma in the patients younger than 20 years in Taiwan.
Potential Effect of Obesity in the Risk of MACEs Associated With OSA
In Taiwan, the International Obesity Task Force criteria were used to determine normal, overweight, and obesity classifications at each age and for each sex.47 Nonetheless, in this study, the rate of obesity is less than 1%, which is lower than the 7.2% obesity rate in adolescents.48 This observation is similar to that in other studies using the NHIRD,14,49–52 and this might be related to the fact that physicians seldom code obesity in the medical records. The actual reason why they undercode obesity is not known, but we speculate that body image issues and even obesity discrimination in our society might be one of the reasons.53 Previous studies have shown that the highest cardiovascular risk was found in patients with OSA and obesity, but OSA plays a role in the development of cardiovascular disorders.54,55 Some studies also found that patients without obesity and with OSA are at a risk of the development of cardiovascular diseases.54,56,57 In addition, in our study, in the patients with OSA and obesity MACEs did not develop at the end of the follow-up period (Table S4). Therefore, the findings in the current study are related to OSA, not only obesity.
Limitations of This Study
The current study has several limitations that warrant consideration. First, similar to previous studies using the NHIRD on OSA as mentioned, not all the data were recorded in the NHIRD, and we were unable to evaluate the severity, weakness severity, laboratory parameters, or lung function examinations in the patients with apnea. Second, other factors, such as body mass index and genetic, psychosocial, and environmental factors, were not included in the dataset. The lack of data of the severity of OSA would limit the generalization of the results of this study. Although the diagnosis of OAS requires overnight PSG to detect the frequency of apneic and hypopneic events,58 the NHIRD does not contain the records of overnight oximetry screening prior to deciding about PSG, which may produce a 17% false-negative rate.59 Third, the CPAP treatment is not reimbursed in the NHI policy; we therefore could only use indirect methods to confirm the usage of the CPAP treatment. Fourth, although the adherence of CPAP,60,61 smoking,62 and the severity of OSA63,64 play important roles in the risk of the development of MACE or mortality in the patients with OSA, and alcohol consumption may aggravate sleep apnea,65–67 the records of these factors were not available in the NHIRD. Fifth, the use of oral appliances, such as the mandibular advancement devices, is not reimbursed in the NHI program; therefore, we could not evaluate the effects of these appliances on the MACEs in the children and adolescents with OSA. Sixth, one study reported that among middle-aged people, snoring and not OSA predicted hypertension, but the records of snoring were not included in the NHIRD.68 Finally, the NHI program started in 1995, but in this study, the NHIRD we used contained a database of 15 years only. We highly recommend that a longer follow-up study is needed in the future.
CONCLUSIONS
We have evaluated the risk of MACEs in association with OSA in Taiwan's population, using the representative population-based data. We have demonstrated that the children and adolescents with OSA are at a twofold risk of MACEs than the control group. We sincerely hope that this study will provide information for the earlier intervention of patients with OSA.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. This study was funded by Tri-Service General Hospital Research Foundation (TSGH-C106-106, TSGH-C107-106, and TSGH-C107-004) and Medical Affairs Bureau (MAB-107-084). The funders have not had a role in the design, concept, data collection and interpretation, analysis, drafting, or other process in this paper. Nian-Sheng Tzeng is an active-duty Army lieutenant colonel in Taiwan's Army Medical Corps. Chi-Hsiang Chung is the secretary of Taiwanese Injury Prevention and Safety Promotion Association. Hsin-An Chang is an active-duty Navy commander and flight surgeon in Taiwan's Navy Medical Corps. Chuan-Chia Chang is an active-duty Navy commander and undersea-hyperbaric medical officer in Taiwan's Navy Medical Corps. Ru-Band Lu is a senior physician-scientist in Taiwan and also a retired Army colonel and in Taiwan's Army Medical Corps. Hui-Wen Yeh is a retired Army Major in Taiwan's Army Nursing Corps. Wei-Shan Chiang is a research associate in Department of Psychiatry, Tri-Service General Hospital, School of Medicine, National Defense Medical Center, Taipei, Taiwan. Yu-Chen Kao is an active-duty Air Force lieutenant colonel and flight surgeon in Taiwan's Air Force Medical Corps. Shan-Yueh Chang is an active-duty Army major in Taiwan's Army Medical Service Corps. Wu-Chien Chien is a retired Army lieutenant colonel in Taiwan's Military Medical Service Corp. The authors declare no conflicts of interest from Taiwan's Ministry of Defense and other institutions.
ABBREVIATIONS
- CAD
coronary artery disease
- CCI
Charlson Comorbidity Index
- CPAP
continuous positive airway pressure
- ICD
International Classification of Diseases
- MACE
major adverse cardiovascular event
- MI
myocardial infarction
- NHI
National Health Insurance
- NHIRD
National Health Insurance Research Database
- OSA
obstructive sleep apnea
- PAD
peripheral artery disease
- PSG
polysomnography
- SD
standard deviation
REFERENCES
- 1.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360(9328):237–245. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 2.Chan AS, Phillips CL, Cistulli PA. Obstructive sleep apnoea--an update. Intern Med J. 2010;40(2):102–106. doi: 10.1111/j.1445-5994.2009.02069.x. [DOI] [PubMed] [Google Scholar]
- 3.Ferini-Strambi L, Fantini ML, Castronovo C. Epidemiology of obstructive sleep apnea syndrome. Minerva Med. 2004;95(3):187–202. [PubMed] [Google Scholar]
- 4.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):242–252. doi: 10.1513/pats.200708-135MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tien KJ, Chou CW, Lee SY, et al. Obstructive sleep apnea and the risk of atopic dermatitis: a population-based case control study. PloS One. 2014;9(2):e89656. doi: 10.1371/journal.pone.0089656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang CH, Chen SJ, Liu CY. Pediatric sleep apnea and depressive disorders risk: a population-based 15-year retrospective cohort study. PloS One. 2017;12(7):e0181430. doi: 10.1371/journal.pone.0181430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsai JD, Chen HJ, Ku MS, et al. Association between allergic disease, sleep-disordered breathing, and childhood nocturnal enuresis: a population-based case-control study. Pediatr Nephrol. 2017;32(12):2293–2301. doi: 10.1007/s00467-017-3750-0. [DOI] [PubMed] [Google Scholar]
- 8.Gessner V, Bitter T, Horstkotte D, Oldenburg O, Fox H. Impact of sleep-disordered breathing in patients with acute myocardial infarction: a retrospective analysis. J Sleep Res. 2017;26(5):657–664. doi: 10.1111/jsr.12540. [DOI] [PubMed] [Google Scholar]
- 9.Chang CC, Chiu CC, Chiang CH, et al. Obstructive sleep apnea and the risk of ischemic stroke in patients with atrial fibrillation. Int J Cardiol. 2015;181:144–146. doi: 10.1016/j.ijcard.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 10.Chen JC, Koo M, Hwang JH. Risks of peripheral arterial occlusive disease in patients with obstructive sleep apnoea: a population-based case-control study. Clin Otolaryngol. 2015;40(5):437–442. doi: 10.1111/coa.12393. [DOI] [PubMed] [Google Scholar]
- 11.Lin YS, Liu PH, Chu PH. Obstructive sleep apnea independently increases the incidence of heart failure and major adverse cardiac events: a retrospective population-based follow-up study. Acta Cardiol Sin. 2017;33(6):656–663. doi: 10.6515/ACS20170825A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiang CL, Chen YT, Wang KL, et al. Comorbidities and risk of mortality in patients with sleep apnea. Ann Med. 2017;49(5):377–383. doi: 10.1080/07853890.2017.1282167. [DOI] [PubMed] [Google Scholar]
- 13.Ho Chan W. Taiwan's healthcare report 2010. EPMA J. 2010;1(4):563–585. doi: 10.1007/s13167-010-0056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chien WC, Chung CH, Lin FH, Chang HA, Kao YC, Tzeng NS. Is weight control surgery associated with increased risk of newly onset psychiatric disorders? A population-based, matched cohort study in Taiwan. J Med Sci. 2017;37(4):137–149. [Google Scholar]
- 15.Kao LC, Chien WC, Chung CH, et al. The association between newly-diagnosed amnestic disorders and dementia: a nationwide, population-based, historical cohort study in Taiwan. Taiwanese J Psychiatry. 2018;32(1):18–28. [Google Scholar]
- 16.Yang YJ, Chien WC, Chung CH, et al. Risk of erectile dysfunction after traumatic brain injury: a nationwide population-based cohort study in Taiwan. Am J Mens Health. 2018;12(4):913–925. doi: 10.1177/1557988317750970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tzeng NS, Chung CH, Lin FH, et al. Anti-herpetic medications and reduced risk of dementia in patients with herpes simplex virus infections-a nationwide, population-based cohort study in Taiwan. Neurotherapeutics. 2018;15(2):417–429. doi: 10.1007/s13311-018-0611-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang SY, Chien WC, Chung CH, et al. Risk of dementia after charcoal-burning suicide attempts: a nationwide cohort study in Taiwan. J Invest Med. 2018;66(7):1070–1082. doi: 10.1136/jim-2018-000759. [DOI] [PubMed] [Google Scholar]
- 19.Tzeng NS, Chang HA, Chung CH, et al. Increased risk of psychiatric disorders in allergic diseases: a nationwide, population-based, cohort study. Front Psychiatry. 2018;9:133. doi: 10.3389/fpsyt.2018.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeh HW, Chien WC, Chung CH, Hu JM, Tzeng NS. Risk of psychiatric disorders in irritable bowel syndrome-a nationwide, population-based, cohort study. Int J Clin Pract. 2018;72(7):e13212. doi: 10.1111/ijcp.13212. [DOI] [PubMed] [Google Scholar]
- 21.Hang LW, Chen CF, Wang CB, Wu TN, Liang WM, Chou TC. The association between continuous positive airway pressure therapy and liver disease development in obstructive sleep apnea/hypopnea syndrome patients: a nationwide population-based cohort study in Taiwan. Sleep Breath. 2017;21(2):461–467. doi: 10.1007/s11325-016-1439-4. [DOI] [PubMed] [Google Scholar]
- 22.Su VY, Liu CJ, Wang HK, et al. Sleep apnea and risk of pneumonia: a nationwide population-based study. CMAJ. 2014;186(6):415–421. doi: 10.1503/cmaj.131547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shiao TH, Liu CJ, Luo JC, et al. Sleep apnea and risk of peptic ulcer bleeding: a nationwide population-based study. Am J Med. 2013;126(3):249–255. 55.e1. doi: 10.1016/j.amjmed.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 24.Yang CW, Tzeng NS, Yin YJ, et al. Angiotensin receptor blockers decrease the risk of major adverse cardiovascular events in patients with end-stage renal disease on maintenance dialysis: a nationwide matched-cohort study. PloS One. 2015;10(10):e0140633. doi: 10.1371/journal.pone.0140633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ministry of Justice. Civil Code. [Accessed May 2, 2018]. https://law.moj.gov.tw/Eng/LawClass/LawContent.aspx?PCODE=B0000001. Updated June 10, 2015.
- 26.van den Berg B, Walgaard C, Drenthen J, Fokke C, Jacobs BC, van Doorn PA. Guillain-Barre syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol. 2014;10(8):469–482. doi: 10.1038/nrneurol.2014.121. [DOI] [PubMed] [Google Scholar]
- 27.Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barre syndrome: surveillance and cost of treatment strategies - authors' reply. Lancet. 2017;389(10066):253–254. doi: 10.1016/S0140-6736(17)30055-7. [DOI] [PubMed] [Google Scholar]
- 28.Randomised trial of plasma exchange, intravenous immunoglobulin, and combined treatments in Guillain-Barré syndrome. Plasma Exchange/Sandoglobulin Guillain-Barré Syndrome Trial Group. Lancet. 1997;349(9047):225–230. [PubMed] [Google Scholar]
- 29.Needham DM, Scales DC, Laupacis A, Pronovost PJ. A systematic review of the Charlson comorbidity index using Canadian administrative databases: a perspective on risk adjustment in critical care research. J Crit Care. 2005;20(1):12–19. doi: 10.1016/j.jcrc.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 30.McIntyre RS, Jerrell JM. Metabolic and cardiovascular adverse events associated with antipsychotic treatment in children and adolescents. Arch Pediatr Adolesc Med. 2008;162(10):929–935. doi: 10.1001/archpedi.162.10.929. [DOI] [PubMed] [Google Scholar]
- 31.Jerrell JM. Neurological and cardiovascular adverse events associated with antimanic treatment in children and adolescents. CNS Neurosci Ther. 2010;16(1):25–31. doi: 10.1111/j.1755-5949.2009.00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jerrell JM, McIntyre RS. Cardiovascular and neurological adverse events associated with antidepressant treatment in children and adolescents. J Child Neurol. 2009;24(3):297–304. doi: 10.1177/0883073808323523. [DOI] [PubMed] [Google Scholar]
- 33.McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 34.Chang CC, Chuang HC, Lin CL, et al. High incidence of stroke in young women with sleep apnea syndrome. Sleep Med. 2014;15(4):410–414. doi: 10.1016/j.sleep.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 35.Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol. 2013;62(7):569–576. doi: 10.1016/j.jacc.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma L, Zhang J, Liu Y. Roles and mechanisms of obstructive sleep apneahypopnea syndrome and chronic intermittent hypoxia in atherosclerosis: evidence and prospective. Oxid Med Cell Longev. 2016;2016:8215082. doi: 10.1155/2016/8215082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen CY, Chen CL, Yu CC, Chen TT, Tseng ST, Ho CH. Association of inflammation and oxidative stress with obstructive sleep apnea in ischemic stroke patients. Sleep Med. 2015;16(1):113–118. doi: 10.1016/j.sleep.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 38.Ministry of Health and Welfare. Handbook of Taiwan's National Health Insurance (2015-2016) Taipei City, Taiwan: National Health Insurance Administration; 2016. [Google Scholar]
- 39.Wang Y, Gao W, Sun M, Chen B. Adherence to CPAP in patients with obstructive sleep apnea in a Chinese population. Respir Care. 2012;57(2):238–243. doi: 10.4187/respcare.01136. [DOI] [PubMed] [Google Scholar]
- 40.Chen NH, Chou YT, Lee PH, et al. Reversibility of albuminuria and continuous positive airway pressure compliance in patients of obstructive sleep apnea syndrome. Medicine (Baltimore) 2016;95(26):e4045. doi: 10.1097/MD.0000000000004045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boyd SB, Walters AS, Song Y, Wang L. Comparative effectiveness of maxillomandibular advancement and uvulopalatopharyngoplasty for the treatment of moderate to severe obstructive sleep apnea. J Oral Maxillofac Surg. 2013;71(4):743–751. doi: 10.1016/j.joms.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gutierrez MJ, Zhu J, Rodriguez-Martinez CE, Nino CL, Nino G. Nocturnal phenotypical features of obstructive sleep apnea (OSA) in asthmatic children. Pediatr Pulmonol. 2013;48(6):592–600. doi: 10.1002/ppul.22713. [DOI] [PubMed] [Google Scholar]
- 43.Li L, Xu Z, Jin X, et al. Sleep-disordered breathing and asthma: evidence from a large multicentric epidemiological study in China. Respir Res. 2015;16:56. doi: 10.1186/s12931-015-0215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teodorescu M, Polomis DA, Hall SV, et al. Association of obstructive sleep apnea risk with asthma control in adults. Chest. 2010;138(3):543–550. doi: 10.1378/chest.09-3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanchez T, Castro-Rodriguez JA, Brockmann PE. Sleep-disordered breathing in children with asthma: a systematic review on the impact of treatment. J Asthma Allergy. 2016;9:83–91. doi: 10.2147/JAA.S85624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsu JY, King SL, Kuo BI, Chiang CD. Age of onset and the characteristics of asthma. Respirology. 2004;9(3):369–372. doi: 10.1111/j.1440-1843.2004.00572.x. [DOI] [PubMed] [Google Scholar]
- 47.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320(7244):1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liou YM, Liou TH, Chang LC. Obesity among adolescents: sedentary leisure time and sleeping as determinants. J Adv Nurs. 2010;66(6):1246–1256. doi: 10.1111/j.1365-2648.2010.05293.x. [DOI] [PubMed] [Google Scholar]
- 49.Tzeng NS, Chung CH, Lin FH, et al. Headaches and risk of dementia. Am J Med Sci. 2017;353(3):197–206. doi: 10.1016/j.amjms.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 50.Tzeng NS, Chung CH, Liu FC, et al. Fibromyalgia and risk of dementia-a nationwide, population-based, cohort study. Am J Med Sci. 2018;355(2):153–161. doi: 10.1016/j.amjms.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 51.Tzeng NS, Chung CH, Yeh CB, et al. Are chronic periodontitis and gingivitis associated with dementia? A nationwide, retrospective, matched-cohort study in Taiwan. Neuroepidemiology. 2016;47(2):82–93. doi: 10.1159/000449166. [DOI] [PubMed] [Google Scholar]
- 52.Tzeng NS, Chung CH, Lin FH, et al. Magnesium oxide use and reduced risk of dementia: a retrospective, nationwide cohort study in Taiwan. Curr Med Res Opin. 2017:1–7. doi: 10.1080/03007995.2017.1385449. [DOI] [PubMed] [Google Scholar]
- 53.Lin YC, Latner JD, Fung XCC, Lin CY. Poor health and experiences of being bullied in adolescents: self-perceived overweight and frustration with appearance matter. Obesity (Silver Spring) 2018;26(2):397–404. doi: 10.1002/oby.22041. [DOI] [PubMed] [Google Scholar]
- 54.Ramar K, Caples SM. Cardiovascular consequences of obese and nonobese obstructive sleep apnea. Med Clin North Am. 2010;94(3):465–478. doi: 10.1016/j.mcna.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kendzerska T, Leung RS, Gershon AS, Tomlinson G, Ayas N. The interaction of obesity and nocturnal hypoxemia on cardiovascular consequences in adults with suspected obstructive sleep apnea. a historical observational study. Ann Am Thorac Soc. 2016;13(12):2234–2241. doi: 10.1513/AnnalsATS.201604-263OC. [DOI] [PubMed] [Google Scholar]
- 56.Thunstrom E, Glantz H, Fu M, et al. Increased inflammatory activity in nonobese patients with coronary artery disease and obstructive sleep apnea. Sleep. 2015;38(3):463–471. doi: 10.5665/sleep.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Testelmans D, Tamisier R, Barone-Rochette G, et al. Profile of circulating cytokines: impact of OSA, obesity and acute cardiovascular events. Cytokine. 2013;62(2):210–216. doi: 10.1016/j.cyto.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 58.Park JG, Ramar K, Olson EJ. Updates on definition, consequences, and management of obstructive sleep apnea. Mayo Clinic Proc. 2011;86(6):549–54. doi: 10.4065/mcp.2010.0810. quiz 554–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zeidler MR, Santiago V, Dzierzewski JM, Mitchell MN, Santiago S, Martin JL. Predictors of obstructive sleep apnea on polysomnography after a technically inadequate or normal home sleep test. J Clin Sleep Med. 2015;11(11):1313–1318. doi: 10.5664/jcsm.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abuzaid AS, Al Ashry HS, Elbadawi A, et al. Meta-analysis of cardiovascular outcomes with continuous positive airway pressure therapy in patients with obstructive sleep apnea. Am J Cardiol. 2017;120(4):693–699. doi: 10.1016/j.amjcard.2017.05.042. [DOI] [PubMed] [Google Scholar]
- 61.Khan SU, Duran CA, Rahman H, Lekkala M, Saleem MA, Kaluski E. A meta-analysis of continuous positive airway pressure therapy in prevention of cardiovascular events in patients with obstructive sleep apnoea. Eur Heart J. 2018;39(24):2291–2297. doi: 10.1093/eurheartj/ehx597. [DOI] [PubMed] [Google Scholar]
- 62.Trenchea M, Deleanu O, Suta M, Arghir OC. Smoking, snoring and obstructive sleep apnea. Pneumologia. 2013;62(1):52–55. [PubMed] [Google Scholar]
- 63.Shamsuzzaman A, Amin RS, Calvin AD, Davison D, Somers VK. Severity of obstructive sleep apnea is associated with elevated plasma fibrinogen in otherwise healthy patients. Sleep Breath. 2014;18(4):761–766. doi: 10.1007/s11325-014-0938-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bironneau V, Goupil F, Ducluzeau PH, et al. Association between obstructive sleep apnea severity and endothelial dysfunction in patients with type 2 diabetes. Cardiovasc Diabetol. 2017;16(1):39. doi: 10.1186/s12933-017-0521-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scanlan MF, Roebuck T, Little PJ, Redman JR, Naughton MT. Effect of moderate alcohol upon obstructive sleep apnoea. Eur Respir J. 2000;16(5):909–913. doi: 10.1183/09031936.00.16590900. [DOI] [PubMed] [Google Scholar]
- 66.Issa FG, Sullivan CE. Alcohol, snoring and sleep apnea. J Neurol Neurosurg Psychiatry. 1982;45(4):353–359. doi: 10.1136/jnnp.45.4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taasan VC, Block AJ, Boysen PG, Wynne JW. Alcohol increases sleep apnea and oxygen desaturation in asymptomatic men. Am J Med. 1981;71(2):240–245. doi: 10.1016/0002-9343(81)90124-8. [DOI] [PubMed] [Google Scholar]
- 68.Khazaie H, Negahban S, Ghadami MR, Sadeghi Bahmani D, Holsboer-Trachsler E, Brand S. Among middle-aged adults, snoring predicted hypertension independently of sleep apnoea. J Int Med Res. 2018;46(3):1187–1196. doi: 10.1177/0300060517738426. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.