Abstract
Study Objectives:
Evaluation of apnea detection using a tracheal sound (TS) sensor during sleep in patients with obstructive sleep apnea.
Methods:
Polysomnographic recordings of 32 patients (25 male, mean age 66.7 ± 15.3 years, and mean body mass index 30.1 ± 4.5 kg/m2) were analyzed to compare the detection of apneas by four different methods of airflow signals: oronasal thermal airflow sensor (thermistor), nasal pressure transducer (NP), respiratory inductance plethysmography (RIPsum) and TS. The four used signals were scored randomly and independently from each other according to American Academy of Sleep Medicine rules. Results of apnea detection using NP, RIPsum and TS signals were compared to those obtained by thermistor as a reference signal.
Results:
The number of apneas detected by the thermistor was 4,167. The number of apneas detected using the NP was 5,416 (+29.97%), using the RIPsum was 2,959 (−29.71%) and using the TS was 5,019 (+20.45%). The kappa statistics (95% confidence interval) were 0.72 (0.71 to 0.74) for TS, 0.69 (0.67 to 0.70) for NP, and 0.57 (0.55 to 0.59) for RIPsum. The sensitivity/specificity (%) with respect to the thermistor were 99.23/69.27, 64.07/93.06 and 96.06/76.07 for the NP, RIPsum and TS respectively.
Conclusions:
With the sensor placed properly on the suprasternal notch, tracheal sounds could help detecting apneas that are underscored by the RIPsum and identify apneas that may be overscored by the NP sensor due to mouth breathing. In the absence of thermistor, TS sensors can be used for apnea detection.
Clinical Trial Registration:
Registry: German Clinical Trials Register (DRKS), Title: Using the tracheal sound probe of the polygraph CID102 to detect and differentiate obstructive, central, and mixed sleep apneas in patients with sleep disordered breathing, Identifier: DRKS00012795, URL: https://www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00012795
Citation:
Sabil A, Glos M, Günther A, Schöbel C, Veauthier C, Fietze I, Penzel T. Comparison of apnea detection using oronasal thermal airflow sensor, nasal pressure transducer, respiratory inductance plethysmography and tracheal sound sensor. J Clin Sleep Med. 2019;15(2):285–292.
Keywords: flow measurement, home sleep apnea test, obstructive sleep apnea, polysomnography, sleep-disordered breathing, tracheal sound
BRIEF SUMMARY
Current Knowledge/Study Rationale: Reliable recording of respiratory flow is needed for apnea detection. In patients with obstructive sleep apnea, tracheal sound monitoring by the PneaVox sensor was tested and its performance was compared to those of the nasal pressure and respiratory inductance plethysmography belts with respect to the thermistor.
Study Impact: Associated with nasal pressure, tracheal sound meet the oronasal flow evaluation required by the American Academy of Sleep Medicine for apnea detection. Tracheal sound can be used as a substitute for oral thermistors to reliably detect apneas.
INTRODUCTION
Obstructive sleep apnea (OSA) is the most common sleep-related breathing disorder. OSA is characterized by repetitive closure of the upper airway during sleep and it affects between 6% and 13% of the adult population.1,2 Polysomnographic diagnosis and assessment of severity of OSA depend on accurate measurement of respiratory airflow and reliable detection of respiratory events.
In adults, apneas are defined as a decrease of airflow by more than 90% from baseline over a period of more than 10 seconds.3 Pneumotachography has traditionally been considered the gold standard for flow measurement and detection of apneas.3 However, this technique is not suitable for routine sleep studies with a polysomnography (PSG) or a polygraphy. Alternative techniques to measure airflow include oronasal thermal airflow sensors (thermistors or thermocouples), nasal cannulas and respiratory inductance plethysmography (RIP).
Thermal airflow sensors use the difference between the temperature of exhaled and ambient air to estimate airflow and detect mouth breathing. The use of temperature as a surrogate for measurement of airflow works well for detecting apnea because it has the advantage to detect both nasal and oral airflow. Nasal cannulas are pressure sensors capable of detecting pressure changes during inspiration and expiration. Most sleep laboratories use signals from a thermistor and nasal pressure (NP) to assure an oronasal flow measurement. This sensor combination improves the identification of apneas that are missed by thermistors or overestimated by NP in the case of mouth breathing, for example. However, these two sensors can cause patients much discomfort and even affect their sleep.4 They are therefore often displaced or even removed by the patients during recording at night. The validity of thermistor and NP signals for more than 6 hours of recording is satisfactory for less than 60% for both children4 and adults.5
When the nasal pressure and the thermistor signals are missing or of bad quality, the RIP sum signal can be used as a surrogate respiratory flow.3 The RIP method uses two belts placed around the thorax and the abdomen and these sensors allow semi-quantitative assessment of volume changes through the measurement of thoracic and abdominal movements.6
Recordings of tracheal sound (TS) correlate well with respiratory flow, with no significant difference in the number of apneas detected with TS or reference sensors.7–10 Tracheal sounds, recorded at the sternal notch, reflect the superficial vibrations of the body set in motion by pressure fluctuations.11 Placed on the sternal notch, the TS sensors can detect these vibrations and thus, measure tracheal flow sound as well as snoring.
Our study aimed to evaluate the use of a TS sensor, PneaVoX (Cidelec, France), for apnea detection. The results were compared to those obtained with thermistor, NP and RIPsum signals.
METHODS
Patients
Thirty-five recordings from 32 patients (25 male) with a clinical suspicion of OSA were included in the study. Patients were admitted to the Charité-Universitätsmedizin certified sleep laboratory. The study was approved by the local Ethics Committee (application number: EA1/009/13) of the university hospital in Berlin, and patients gave their written consent for participation in the study. Inclusion criteria were patients between 18 and 80 years old with either suspected OSA after clinical evaluation and before a sleep study or patients who had already been diagnosed with OSA, but who were readmitted to the sleep laboratory for control PSG. Exclusion criteria were drug use and excessive alcohol consumption, any medication intake that could influence sleep, the presence of any sleep disorder other than OSA, clinically unstable respiratory and patients who were incapable to read and understand the consent statement for any reason. Age, height, weight and neck circumference as well as medication and diagnoses of the patients were recorded.
Data Acquisition
After signing written consent for participation in the study, patients underwent PSG recordings using the SOMNOscreen plus system (SOMNOmedics GmbH, Randersacker, Germany). Recorded data included all electrophysiological signals for sleep evaluation as well as airflow by thermistor and nasal cannula, RIP belts, pulse oximetry, body position, limb movements, actigraphy and light. In addition to the laboratory routine, esophageal pressure (Pes) probe (Gaeltec, Isle of Sky, Scotland) as well as TS using the PneaVoX sensor with the CIDLXe polygraph (CIDELEC, Angers, France) were recorded.
The following specifications for recording of the respiratory signals were applied:
Thermistor: oronasal sensor from Somnomedics, effective range = ± 80 mV, frequency range = 0.1 Hz to 1 kHz, sampling rate = 32 Hz and software low-pass (LP) filtering at 1 Hz.
Nasal flow: nasal pressure transducer built-in device from Somnomedics, effective range = ± 48 mV, no square transformation, frequency range = 0.023 Hz to 1 kHz, sampling rate = 256 Hz and software LP filtering at 1 Hz
Effort: RIP sensors from Somnomedics, effective range = ± 170 mV, uncalibrated, frequency range = 0.2 Hz to 35 Hz, sampling rate = 32 Hz and no software filtering. The Somnomedics software provides the sum signal of thoracic and abdominal signals (RIPsum) which was used according to American Academy of Sleep Medicine (AASM) rules for apnea detection.
The PneaVoX sensor was placed on the skin above the sternal notch and then secured in place using a double-sided ring tape and an adhesive tape. Correct positioning of 1 cm right above the sternal notch with a well-sealed contact surface of the transducer is an essential element to obtain a good quality signal. For later synchronization of recordings, the nasal pressure sensor was connected to both systems using a Y-piece connector so that both the SOMNOscreen system and the CIDELEC system share the same NP signal. Thus, accurate synchronization of the separate recordings was made possible. The presence and quality of all signals were monitored throughout the night.
All respiratory signals from the SOMNOscreen system were imported into the CIDELEC system in European Data Format and a new anonymized polygraph file was created for each patient. Sections where respiratory signals necessary to the scoring were missing or of poor quality and sections which could not be synchronized via the NP signal were not validated. Each synchronized recording was then visualized and scored using the CIDELEC software.
Tracheal Sounds
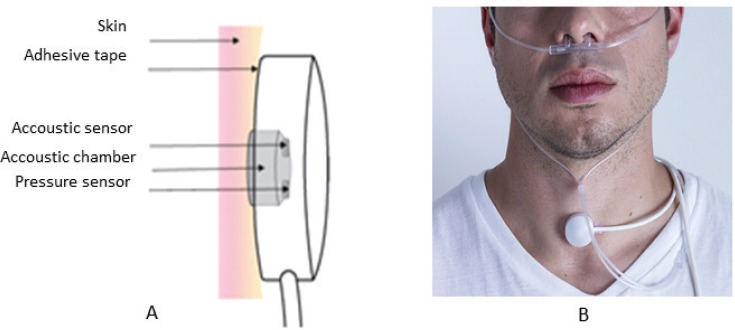
The PneaVoX is a stethoscope-like transducer with an acoustic sensor and a pressure sensor inserted inside a 28-mm diameter and 15-mm thick protective housing. The surface of the transducer attached to the skin contains a 2-mm thick cuff, designed to ensure an airtight cavity between the skin and the transducers (Figure 1).12–15
Figure 1. Presentation of a tracheal sound transducer.
(A) Diagram of the PneaVoX sensor that uses both an acoustic sensor and a pressure sensor. The sensors are inserted in a protective plastic housing to ensure an airtight acoustic chamber between the skin and the transducer. (B) Placement of tracheal sound sensor right above the sternal notch using a double-faced tape. If necessary, an adhesive bandage could be used over the sensor to hold it in place.
Filtering techniques are used to separate the high pitch (200 to 2000 Hz) tracheal flow sound from the low pitch (20 to 200 Hz) snoring sound.16 The intensity of the tracheal sound at high pitch allows the measurement of respiratory flow and the detection of apneas.17 When the signal's amplitude is decreased to more than 90% or in the absence of flow for more than 10 seconds, it can be assumed that there is no airflow through the trachea and therefore an apnea can be scored.
Data Analysis
For the detection of apneas, only one of the four respiratory flow signals was displayed and analyzed at a time, while the other flow signals were masked. The AASM definition of apnea in terms of signal amplitude decrease and duration was applied to all four signals (Figure 2). Four apnea scorings were performed, using each of the following signals separately and in random order: thermistor, NP, RIPsum or TS signal. The four scorings were performed sequentially for each study. The number of detected events and the duration of each event was measured and compared for the four signals. The apnea detection results using the NP, RIPsum and TS were compared to those of the thermistor being the reference sensor.
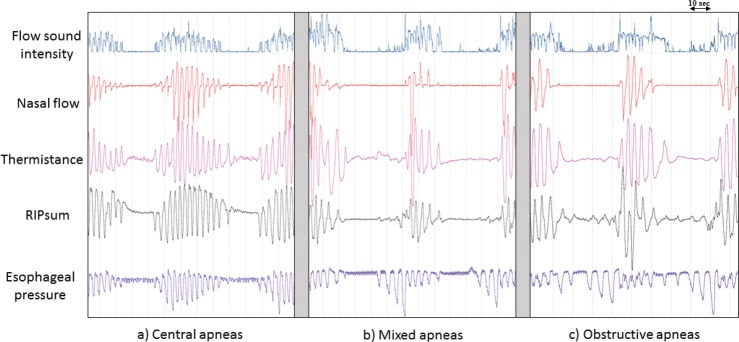
Figure 2. Example of apneas detected separately on four different signals.
Example of apneas—central (A), mixed (B) and obstructive (C)—detected separately on four different signals (flow sound intensity, nasal pressure, thermistor and RIPsum). The American Academy of Sleep Medicine definition of apnea in terms of signal amplitude decrease and duration was applied to all four signals. The esophageal pressure signal confirms the characterization of the illustrated apneas. Note how obstructive apneas could be mistaken for hypopneas when using the RIPsum signal. The duration of the detected apneas could also vary from one signal to the other. RIP = respiratory inductance plethysmography.
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics version 22.0 (IBM Corp, Armonk, New York, United States). Values are presented as mean ± standard deviation. The Cohen kappa, sensitivity and specificity, as well as positive predictive value (PPV), and negative predictive value (NPV) for apnea detection were calculated for all patients using thermistor as a reference signal. Bland-Altman analysis was also performed for this study.
RESULTS
Patients
Patients had a mean age of 66.7 ± 15.3 years, a mean body mass index of 30.1 ± 4.6 kg/m2 and a mean neck circumference of 42.8 ± 4.1 cm. The apnea-hypopnea index (AHI) was 36.1 ± 25.1 events/h with an apnea index (AI) of 25.7 ± 24.3 events/h. The mean total sleep time was 317.4 ± 77.5 minutes. Five patients had mild OSA (AHI 5 to < 15 events/h), 12 patients had moderate OSA (AHI 5 to < 30 events/h) and 18 patients had severe OSA (AHI ≥ 30 events/h).
Detection of Apneas With TS, NP, Thermistor, and RIPsum
The total number of apneas detected using NP was the highest, with 5,416 apneas. We detected 5,019 apneas with TS, 4,167 apneas with thermistor and only 2,959 apneas using RIPsum. There were five patients with relatively few apneas. This patient-to-patient variability exists because there was no minimum AHI imposed in the exclusion criteria. These patients have an AHI < 5 events/h with more hypopneas than apneas.
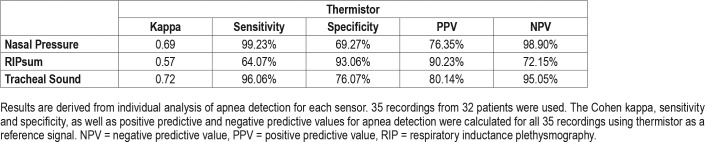
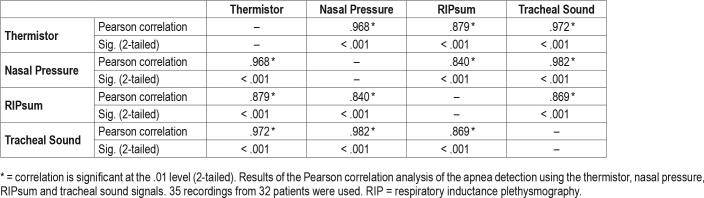
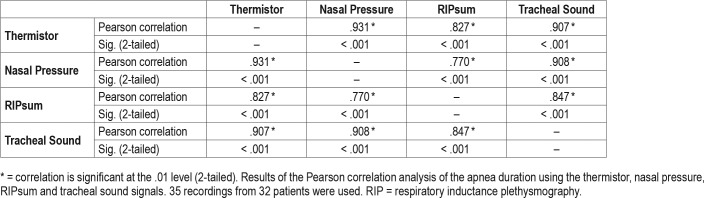
The number of common apneas with the thermistor was 4,135 for the NP, 2,670 for the RIPsum, and 4,022 for the TS. However, in comparison with the thermistor as a reference sensor, the NP and TS had an overdetection of 1,281 and 997 apneas respectively while the RIPsum had an underdetection of 1,497 events. For each patient, the sensitivity, specificity, PPV and NPV for apnea detection were calculated using the thermistor signal as a reference. The results are presented for each sensor in Table 1. With the thermistor as a reference detection, the kappa statistics (95% confidence interval) were 0.69 (0.67 to 0.70) for NP, 0.57 (0.55 to 0.59) for RIPsum and 0.72 (0.71 to 0.74) for TS. The results of the Pearson correlation analysis for apnea detection using the four different sensors are presented in Table 2. There was a strong positive correlation between thermistor and NP (r = .968, n = 35, P < .001) and between thermistor and TS (r = .972, n = 35, P < .001); and a less important correlation between thermistor and RIPsum (r = .879, n = 35, P < .001).
Table 1.
Statistical results for apnea detection.
Table 2.
Pearson correlation results for apnea detection.
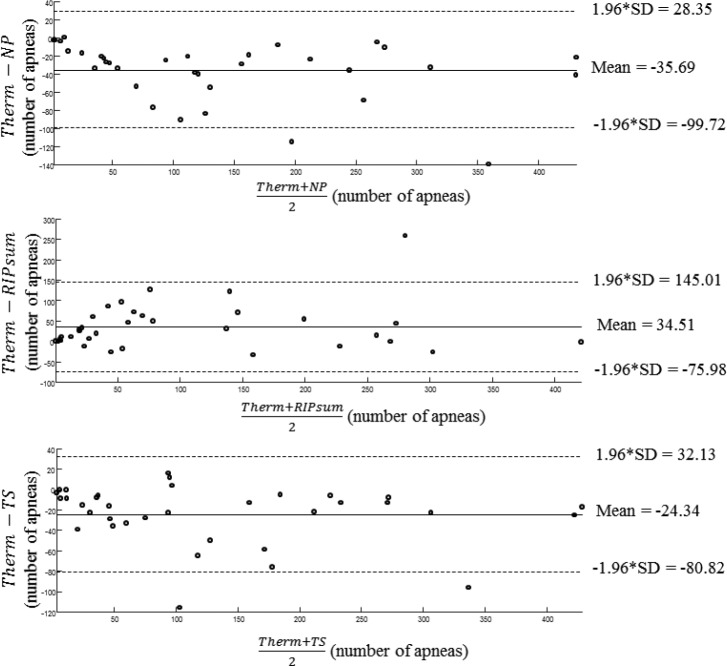
Figure 3 displays the results of the Bland-Altman analysis for apnea detection outcomes considering the thermistor method as the reference. The mean difference value of the number of detected apneas between the thermistor and the TS was smaller than between the thermistor and the NP and between the thermistor and the RIPsum. However, for all recordings, both the NP and TS overestimated the number of events in average by 35.7 and 24.3 apneas respectively with the limits of agreements from −99.72 to 28.35 events for NP and from −80.82 to 32.13 events for TS. In the contrary, the RIPsum underestimated the number of events in average by 34.5 apneas with limits of agreement from −75.98 to 145.01 events.
Figure 3. Results of the Bland-Altman analysis for apnea detection outcomes considering the thermistor method as the reference.
The mean difference value of the number of detected apneas between the thermistor and the tracheal sounds was smaller than between the thermistor and the nasal pressure and between the thermistor and the RIPsum. NP = nasal pressure, RIP = respiratory inductance plethysmography, SD = standard deviation, Therm = thermistor, TS = tracheal sound.
Finally, the average duration of apneas detected with NP was the longest with 21.0 ± 7.2 seconds. It was slightly lower for the TS, 19.4 ± 5.9 seconds and the thermistor, 18.7 ± 5.9 seconds. The duration of apneas detected using the RIPsum was the lowest with 17.6 ± 4.8 seconds. The results of the Pearson correlation analysis for apnea duration using the four different sensors are presented in Table 3.
Table 3.
Pearson correlation results for apnea duration.
DISCUSSION
This is the first study to compare, in an adult population, the detection of apneas using four different signals: thermistor, nasal pressure, thoracoabdominal RIPsum, and tracheal sound. Provided that the TS sensor is well placed and that the scorer is familiar with the tracheal flow sound signal, the intensity of the TS at high pitch allows the detection of apneas.
As expected, the NP signal detected the largest number of apneas (7.3% less with TS, 23.1% less with thermistor and 45.4% less with RIPsum). Previous studies comparing different sensors show that NP identifies more apneas than thermistor signal18, as it may overestimate the extent of airflow amplitude reduction and classify certain hypopneas as apneas.18 Oral respiration, especially in pediatric patients and in case of nasal obstruction, can have a significant impact on the classification of respiratory events when using a NP signal.19 However, our data shows that most apneas detected by the other three sensors were also detected by NP (99. 9% for thermistor, 99.2% for RIPsum, and 96.4% for TS). These results confirm that the NP is a very sensitive sensor for apnea detection.
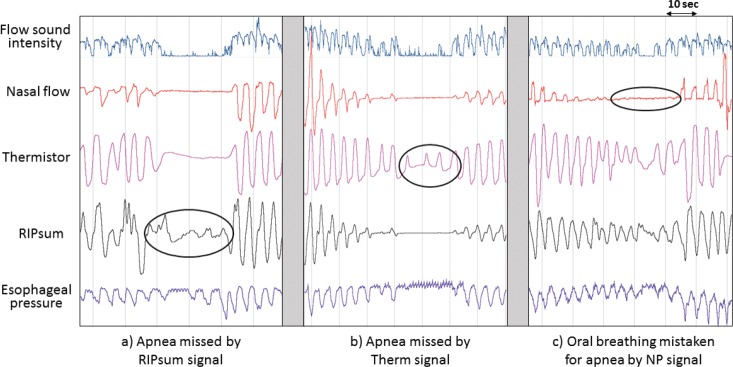
The RIPsum signal detected the least apneas with 29% less than thermistor. Furthermore, almost all the central and mixed apneas and only a third of obstructive apneas were detected by RIPsum when compared to the thermistor detection. These results suggest that the RIPsum signal is excellent for detecting central apneas but not suitable for detecting obstructive and mixed apneas. The excursions of the RIPsum and RIPflow signals usually have minimal amplitude during apnea.20,21 However, during an obstructive apnea, significant excursions in the RIPsum or RIPflow signals may be seen if the thorax and abdominal belt signals do not exactly sum up to zero (Figure 4A). This problem is minimized by calibration of the RIP signals; however, even the calibrated RIPsum may not remain accurate overnight due to changes in patient position and/or belt movements.22 Detecting apneas using the RIPsum signal is not reliable and may underestimate the AI. Thus, the RIPsum signal may have an impact on the clinical diagnosis of the OSA and undermines the severity of the disease.
Figure 4. Examples of apnea detection errors by different signals.
(A) Obstructive apnea missed by the RIPsum signal. Sometimes during obstructive apnea, the RIPsum signal is not reduced more than 90% as it should during apneas because the thoracic and abdominal signals are not necessary in paradoxical movements. Thus, the thorax and abdominal belt signals do not exactly sum up to zero. (B) Central apnea missed by the thermistor signal. Based on the thermistor signal, there is flow and an apnea should not be scored. However, when examining the esophageal pressure, there was no respiratory effort which is interpreted as a presence of central apnea. Note that the event is clearly identified by the nasal pressure, tracheal sound and RIPsum signals. This discrepancy could be due to high sensitivity of the thermal flow sensor. (C) Oral breathing mistaken for apnea by the nasal pressure signal. In the absence of the thermistor and based solely on the nasal pressure, one could score an apnea given that the nasal flow amplitude is reduced more that 90%. Note that respiratory cycles persist on the thermistor, tracheal sound and RIPsum signals. NP = nasal pressure, RIP = respiratory inductance plethysmography.
Apneas are defined as events where the respiratory flow is absent or reduced by more than 90% of the reference value for at least 10 seconds.3,23,24 They are easily detected on TS signals with the same definitions (Figure 2). Furthermore, using a TS sensor, apnea could be identified by the cessation of TS and/or the absence of definite respiratory cycles during monitoring.9,14,17,25 A first generation PneaVoX has been already validated against a pneumotachograph for apnea detection and there were no differences in apnea number and duration recorded by the tracheal sound method and the pneumotachograph.17 Our data showed that the TS signal detects less apneas than the NP signal and more apneas the RIPsum, placing the TS signal as the closest signal to the thermistor in term of apnea detection in comparison to the performance of the NP and the RIPsum signals. Our data also showed that the TS signal detected more apneas than the thermistor signal. TS may indeed overestimate the number of detected apneas in comparison to the thermistor signal detection; however, some of these extra apneas detected by the TS may simply be events that were missed by the thermistor signal. TS may record the presence of apneas while the thermistor shows air movement (Figure 4B) during periods of thermistor drift or when airflow is so slow that upper airway aerodynamic sounds are not produced. This difference could also be due to the high sensitivity of thermistors which may cause false positive events.9
Tracheal sounds are well correlated with respiratory flow and can be used as an additional flow indicator for the analysis of respiratory events during sleep.15 The recording of TS eliminates the need for oral respiration sensors (Figure 4C). Because they are recorded directly on the sternal notch, TS reflects total ventilation, whether oral or nasal. Detection of oral respiration is important during sleep and the AASM recommends its detection with thermistors. During exclusive oral breathing, the nasal pressure signal is zero while the thermistor signal detects respiratory variations. TS sensors could also be used as auxiliary airflow sensors to reliably detect oral breathing (Figure 4C). The TS signal is recognized in the French clinical practice recommendations as a valid signal, associated with nasal pressure, to detect oronasal respiration.23
The Bland Altman plots show important patient to patient variability. This is mainly due to the patient to patient variability presence of central apneas. The more central apneas present in the recording, the higher is the number of apneas in common and the better is the correlation between the sensors. The quality of the recording may also partially have contributed to this variability. In fact, for four patients with only obstructive apneas, no apneas were detected on the RIPsum signal. However, these results show that the mean difference value of the number of detected apneas between the thermistor and the TS was smaller than between the thermistor and the NP and between the thermistor and the RIPsum. These results suggest that with respect to the thermistor as a reference signal for apnea detection, the TS signal has a better performance than the recommended NP and the RIPsum signals. Further statistical analysis supports the higher performance of the TS signal comparing to the NP and the RIPsum. The Kappa score for the TS was the highest at 0.72 which falls within the range of good agreement (> 0.60). The sensitivity of the TS (96.06%) for apnea detection was as good as that of the NP (99.23%) with a slightly better specificity(76.07 for TS and 69.27% for the NP). However, while the RIP-sum has a lower kappa (0.57) and a lower sensitivity (64.07), its specificity was excellent (93.06) compared to the TS and the NP.
A Pearson correlation was run to determine the relationship between each pair of signals. Using the thermistor signal as a reference for apnea detection, there was a very strong positive correlation between thermistor and TS (r = .972, n = 35, P < .001), between thermistor and NP (r = .968, n = 35, P < .001) and between thermistor and RIPsum (r = .879, n = 35, P < .001).
Finally, the NP had the longest average apnea duration in comparison to the TS and the thermistor, but the difference was not clinically significant. The average duration of apneas detected with RIPsum was the lowest. This could be explained by the fact that for most mixed apneas, only the central part of the event was detected as an apnea with the RIPsum signal which reduced the length of the real apneas. Some mixed apneas were not detected by the RIPsum signal for the same reason. By limiting the length of the event to the central part only, the total duration of the event did not meet the minimum 10 second requirement for apnea detection. Finally, some obstructive apneas lasted close to the necessary 10 seconds duration when scored on the NP or TS but they were shorter when evaluated with the thermistor or the RIPsum. Thus, some borderline apneas were detected using some sensors but not others and this may have contributed to the higher number of apneas detected by the NP and the TS. Pearson's correlation was also performed for apnea duration with each sensor. There was a strong positive correlation between thermistor and TS (r = .907, n = 35, P < .001), between thermistor and NP (r = .931, n = 35, P < .001) and between thermistor and RIPsum (r = .827, n = 35, P < .001).
One limitation of our study is that the pneumotach, the gold standard sensor for respiratory flow measurement, was not used. However, this technique is not suitable for routine sleep studies with a PSG as it requires the use of a full-face mask which could influence the quality of sleep. Our data was collected during routine diagnosis recording at the sleep laboratory where procedures didn't include the pneumotach to avoid altering the results of the diagnosis. We used the thermistor signal as the reference which is the AASM recommended sensor for routine sleep recording. Another limitation is that the TS signal used in this study cannot be generated and recorded by generic PSG equipment and the sensor is limited to a particular PSG system. However, while the study evaluates only one TS device with certain specifications, it opens the door for other devices to be tested as well. The principle remains the same for all TS devices which is the use of an acoustic sensor placed just above the sternal notch with the apnea defined as the absence of respiratory sound for at least 10 seconds. Finally, an overall visual quality validation for all signals was performed but this validation was not quantified. More studies are necessary to validate and assess the applicability of TS devices. Another limitation of this study is that we cannot determine in how far this new sensor contributes to the clarification of the severity of the disease and adds to the clinical diagnosis of sleep apnea. Advantages in the application of the new sensor and follow-up studies based on the new signals may ultimately show whether a significant contribution to clinical diagnosis and severity of the disease can be derived.
CONCLUSIONS
In conclusion, visual detection of apneas was performed in 35 PSG recordings using four different noninvasive methods. We found that apneas could be identified by the cessation of TS during continuous monitoring. The TS device used in this study provides a sensitive, reliable, technically simple, and easily applied noninvasive means to monitor respiration during sleep. While NP tends to overestimate the number of apneas and cannot detect oral breathing, TS can detect apneas seen by a thermistor and/or a RIPsum, as well as additional events that could be missed by these two sensors. Lastly, combined with NP, TS allow the detection of oral breathing and can reclassify as hypopneas, apneas that would be incorrectly detected by NP alone. TS can therefore be used as a substitute for oral thermistors to reliably detect apneas and associated with NP. TS meets the oro-nasal flow evaluation required by the AASM for apnea detection.
In our study the TS signal was of good quality for all the recordings, which indicates good applicability of the sensor. In addition, the TS sensor can easily be placed on patients, is well tolerated, does not disturb sleep, and once installed properly, it is not susceptible to move or be displaced during sleep. We propose that the advantages of this kind of systems justify their routine use in nocturnal PSG as well as in home polygraphy. However, prospective evaluation in a larger group of patients with analysis of hypopneas as well as apneas is needed to establish a larger clinical utility of this approach.
DISCLOSURE STATEMENT
The study was financially supported by an unrestricted grant by CIDELEC, France. Thomas Penzel has received research grants from Heinen & Löwenstein, Itamar, Philips / Respironics, Resmed, Somnodent. He received speaker fees and travel support from Bayer, Itamar, Inspire, Somnodent, UCB, Weinmann. He is a shareholder of Advanced Sleep Research GmbH, The Siestagroup GmbH, Somnico GmbH. He was supported by by the project no. LQ1605 from the National Program of Sustainability II (MEYS CR) and by the project FNUSA-ICRC no. CZ.1.05/1.1.00/02.0123 (OP VaVpI). Ingo Fietze has received research grants from Actelion, Eisai, Heinen & Löwenstein, Jazz Pharmaceuticals, Philips / Respironics, Resmed, Somnodent, UCB, Vanda. At the time of the study AbdelKebir Sabil was fully employed by Cidelec. The other authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the help of the sleep technicians at both laboratories as well as the CIDELEC company for lending their CID-LXe system to record the tracheal sound and the suprasternal pressure.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- AI
apnea index
- NP
nasal pressure
- NPP
negative predictive value
- OSA
obstructive sleep apnea
- PPV
positive predictive value
- PSG
polysomnography
- RIP
respiratory inductance plethysmography
- TS
tracheal sound
REFERENCES
- 1.Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310–318. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodwin JL, Enright PL, Kaemingk KL, et al. Feasibility of using unattended polysomnography in children for research--report of the Tucson Children's Assessment of Sleep Apnea study (TuCASA) Sleep. 2001;24(8):937–944. doi: 10.1093/sleep/24.8.937. [DOI] [PubMed] [Google Scholar]
- 5.Redline S, Sanders MH, Lind BK, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21(7):759–767. [PubMed] [Google Scholar]
- 6.Eberhard A, Calabrese P, Baconnier P, Benchetrit G. Comparison between the respiratory inductance plethysmography signal derivative and the airflow signal. Adv Exp Med Biol. 2001;499:489–494. doi: 10.1007/978-1-4615-1375-9_79. [DOI] [PubMed] [Google Scholar]
- 7.Beckerman RC, Wegmann MJ. A comparison of tracheal breath sounds, airflow, and impedance pneumography in the detection of childhood apnea. Sleep. 1985;8(4):342–346. doi: 10.1093/sleep/8.4.342. [DOI] [PubMed] [Google Scholar]
- 8.Beckerman RC, Wegmann MJ, Waring WW. Tracheal breath sounds for detection of apnea in infants and children. Crit Care Med. 1982;10(6):363–366. doi: 10.1097/00003246-198206000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Cummiskey J, Williams TC, Krumpe PE, Guilleminault C. The detection and quantification of sleep apnea by tracheal sound recordings. Am Rev Respir Dis. 1982;126(2):221–224. doi: 10.1164/arrd.1982.126.2.221. [DOI] [PubMed] [Google Scholar]
- 10.Nakano H, Hayashi M, Ohshima E, Nishikata N, Shinohara T. Validation of a new system of tracheal sound analysis for the diagnosis of sleep apneahypopnea syndrome. Sleep. 2004;27(5):951–957. doi: 10.1093/sleep/27.5.951. [DOI] [PubMed] [Google Scholar]
- 11.Beck R, Rosenhouse G, Mahagnah M, Chow RM, Cugell DW, Gavriely N. Measurements and theory of normal tracheal breath sounds. Ann Biomed Eng. 2005;33(10):1344–1351. doi: 10.1007/s10439-005-5564-7. [DOI] [PubMed] [Google Scholar]
- 12.Amaddeo A, Fernandez-Bolanos M, Olmo Arroyo J, Khirani S, Baffet G, Fauroux B. Validation of a suprasternal pressure sensor for sleep apnea classification in children. J Clin Sleep Med. 2016;12(12):1641–1647. doi: 10.5664/jcsm.6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glos M, Sabil A, Jelavic KS, Schobel C, Fietze I, Penzel T. Characterization of respiratory events in obstructive sleep apnea using suprasternal pressure monitoring. J Clin Sleep Med. 2018;14(3):359–369. doi: 10.5664/jcsm.6978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meslier N, Simon I, Kouatchet A, Ouksel H, Person C, Racineux JL. Validation of a suprasternal pressure transducer for apnea classification during sleep. Sleep. 2002;25(7):753–757. doi: 10.1093/sleep/25.7.753. [DOI] [PubMed] [Google Scholar]
- 15.Penzel T, Sabil A. The use of tracheal sounds for the diagnosis of sleep apnoea. Breathe (Sheff) 2017;13(2):e37–e45. doi: 10.1183/20734735.008817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Penzel T, Sabil A. Breath Sounds: From Basic Science To Clinical Practice. Cham, Switzerland: Springer; 2018. Physics and Applications for Tracheal Sound Recordings in Sleep Disorders. In: Priftis KN, Hadjileontiadis LJ, Everard ML, eds; pp. 83–104. [Google Scholar]
- 17.Meslier N, Racineux JL. Sleep Related Disorders and Internal Diseases. Berlin, Heidelberg: Springer; 1987. Use of Tracheal Sound Recordings to Monitor Airflow During Sleep. In: Peter JH, Podszus T, von Wichert P, eds. [Google Scholar]
- 18.Thornton AT, Singh P, Ruehland WR, Rochford PD. AASM criteria for scoring respiratory events: interaction between apnea sensor and hypopnea definition. Sleep. 2012;35(3):425–432. doi: 10.5665/sleep.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernandez L, Ballester E, Farre R, et al. Performance of nasal prongs in sleep studies: spectrum of flow-related events. Chest. 2001;119(2):442–450. doi: 10.1378/chest.119.2.442. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan V, Zhang JN, Russi EW, Bloch KE. Detection of inspiratory flow limitation during sleep by computer assisted respiratory inductive plethysmography. Eur Respir J. 2000;15(3):570–578. doi: 10.1034/j.1399-3003.2000.15.24.x. [DOI] [PubMed] [Google Scholar]
- 21.Farre R, Montserrat JM, Navajas D. Noninvasive monitoring of respiratory mechanics during sleep. Eur Respir J. 2004;24(6):1052–1060. doi: 10.1183/09031936.04.00072304. [DOI] [PubMed] [Google Scholar]
- 22.Whyte KF, Gugger M, Gould GA, Molloy J, Wraith PK, Douglas NJ. Accuracy of respiratory inductive plethysmograph in measuring tidal volume during sleep. J Appl Physiol (1985) 1991;71(5):1866–1871. doi: 10.1152/jappl.1991.71.5.1866. [DOI] [PubMed] [Google Scholar]
- 23.Escourrou P, Meslier N, Raffestin B, et al. [Which clinical approach and which diagnostic procedures for obstructive sleep apnea syndrome?] Rev Mal Respir. 2010;27(Suppl 3):S115–S123. doi: 10.1016/S0761-8425(10)70017-6. [DOI] [PubMed] [Google Scholar]
- 24.Mayer G, Arzt M, Braumann B, et al. German S3 Guideline Nonrestorative Sleep/Sleep Disorders, chapter “Sleep-Related Breathing Disorders in Adults,” short version: German Sleep Society (Deutsche Gesellschaft fur Schlafforschung und Schlafmedizin, DGSM) Somnologie (Berl) 2017;21(4):290–301. doi: 10.1007/s11818-017-0136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krumpe PE, Cummiskey JM. Use of laryngeal sound recordings to monitor apnea. Am Rev Respir Dis. 1980;122(5):797–801. doi: 10.1164/arrd.1980.122.5.797. [DOI] [PubMed] [Google Scholar]