Honeybees forage more successfully by reducing their reliance on social information from the dance language.
Abstract
Honeybees use the waggle dance to share information about food-site locations with nestmates. However, the importance of this behavior in colony foraging success remains unclear. We tested whether spatial dance information affects colony foraging success in a human-modified temperate environment by comparing colonies with oriented and disoriented dances. Notably, colonies with disoriented dances had greater foraging success. Over time, bees exposed to disoriented dances showed reduced interest in dancing nestmates. This may explain why disoriented colonies had a higher foraging rate than oriented colonies, as bees did not waste time waiting for information. This change in information-use strategy suggests bees learn about the value of dance information. An agent-based model confirmed that, under challenging conditions, waiting for dance information reduces colony foraging success compared to foraging without social information. Our results raise the possibility that humans have created environments to which the waggle dance language is not well adapted.
INTRODUCTION
Foraging animals use various information sources when making decisions on how and where to forage. Animals can be led toward a food site by pheromones (1) and the presence of conspecifics (2) and heterospecifics (3), and spatial memories allow individuals to return to favored foraging sites (4). More generally, when setting off on a foraging trip, individuals have the option to (i) explore the environment for a new food site, (ii) return to a known food site using previously acquired memory (so-called private information), or (iii) use socially acquired information from another animal about a food site. Each strategy has costs and benefits associated with it (5–12). Thus, the relative benefit of social information is dependent on the current conditions, and therefore, individuals should adopt flexible strategies that dictate when to use a particular type of information (7).
Eusocial insects are known to rely heavily on social information to aid efficient running of the colony. In ants, termites, bees, and some stingless bees, trail pheromones play an important role in foraging (13–15), and trophallaxis between nestmates may enable communal control of colony phenotypes or food preferences (16, 17). Honeybees (Apis spp.) use a unique behavior that may have evolved more than 20 million years ago (18): The waggle dance communicates the location, odor, and presence of high-quality food (or nest) sites to nestmates (19). Finding a specific food site using this socially acquired information, as opposed to finding any food site, may increase the amount of time an individual worker spends searching for food, but it leads to the discovery of better food sources compared to bees that search for food sources by individual exploration (also called scouts) (20, 21). Given that dance communication has significant time costs (21), there are likely circumstances when it is more adaptive to either search for new foraging patches or return to known ones (12). Accordingly, Dechaume-Moncharmont et al. (22) used a mathematical model to show that there are a wide range of parameters that favor being a proactive worker (scout) as opposed to waiting for and using socially acquired information.
Several empirical studies [e.g., (20, 23–25)] have disrupted the honeybee waggle dance (see Materials and Methods for details) in an attempt to assess the benefit of the spatial dance information to the colony. Dance communication did not increase colony foraging success in most of the environments tested [temperate (25), spring and summer (23), and environments with low flower number and low species richness (24)]. In particular, dance information did not help honeybee colonies collect more nectar in temperate habitats (23) but may lead to a short-term boost of pollen collection (26). Furthermore, location information was beneficial when resources were unevenly distributed (25) and hard to find (23). These interpretations are consistent with computer simulations [e.g. (27)]. However, conclusive interpretation of the results of these dance-disruption experiments is complicated by the short time periods colonies would spend in a treatment [2-day (25), 3-day (24), 4-day (26), and (on average) ~11-day (23) treatment regimens]. These short treatment periods could lead to carryover of memory about a food site from one treatment to the next. Such carryover effects are possible because honeybees can remember foraging locations for several days (28). Simulations suggest that these carryover effects mask the long-term effects of dance information (29).
In general, the aforementioned studies suggest that a strategy that makes flexible use of private and social information might be the most adaptive, and there is increasing evidence for flexible information-use strategies in insects (5). However, it remains unclear whether the strategic use of social information is based on genetically encoded “rules of thumb” or whether this use is an outcome of learning itself (5). Recent research suggests that insect foragers might learn about the value of social information through experience and modify their reliance on social information accordingly (30). Here, we studied the importance of spatial dance information in a temperate Central European habitat. To avoid the memory carryover effects that might have confounded previous studies (25) and to allow long-term effects of dance communication (29), we used treatment periods of 18 days and allowed colonies to recover between treatment periods. We also tested the effects of a 3-day switching treatment, which allowed us to repeat previous work and test whether the experimental time period affected the results. We monitored the dance-following behavior of foraging bees to investigate their potential to respond to the value of information in the two treatments. We combined our empirical data with an agent-based simulation model (ABM) to better understand the environmental factors that are key to colony foraging success when the colony uses social information and when it does not.
RESULTS
Experiments were carried out using full-sized colonies (E1 and E3) and observation hives made up of two brood frames and one honey frame (E2). In E1 and E3, our experiments focused on colony-level changes, while in E2, we tracked individual bees.
Colony performance (E1, E2, and E3)
Overall, colonies lost weight during our experiments (Fig. 1A). It is known that summer can be a challenging period for colonies in temperate European habitats due to a scarcity of flowers (26, 31). Surprisingly, colonies with oriented dances lost significantly more weight than those that had disoriented dances over the 18-day experimental period (Fig. 1A). Treatment had a significant effect on colony weight change {Gaussian generalized linear mixed-effects model (glmm): oriented mean colony daily weight change [95% confidence interval (CI)], −0.134 kg (−0.173 to −0.0951); disoriented mean colony daily weight change (95% CI), −0.101 kg (−0.132 to −0.0693); mean difference between treatments (95% CI), 0.031 kg (−0.011 to 0.073); χ2 = 24.22, degree of freedom (df) = 1, P < 0.0001}, and day and treatment interacted significantly with each other (glmm: χ2 = 17.64, df = 1, P < 0.0001). Over the same period, a significant interaction was seen between day and treatment with regard to colony foraging activity [Gaussian glmm: oriented mean morning foraging activity (95% CI), 0.16 kg (0.134 to 0.186); disoriented mean morning foraging activity (95% CI), 0.197 kg (0.163 to 0.230); mean difference between treatments (95% CI), 0.037 kg (0.011 to 0.063); χ2 = 22.15, df = 1, P < 0.0001]. Over the experimental period, the foraging activity of colonies in the two treatments became increasingly different (Fig. 1B). In the 18-day oriented (OT) and disoriented (DT) treatments, the amount of pollen collected in a 30-min period did not differ [Gaussian glmm: oriented geometric mean (95% CI), 0.549 g (0.432 to 0.698); disoriented geometric mean (95% CI), 0.615 g (0.469 to 0.806); mean difference between treatments (95% CI), 0.066 g (−0.134 to 0.554); χ2 =0.02, df = 1, P = 0.884].
Fig. 1. Weight data over the course of the experiment.
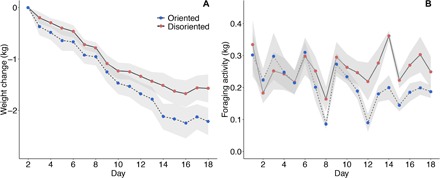
(A) Cumulative weight change over the 18-day experimental period (n = 12 colonies). (B) Foraging activity measured as colony weight change between 4 a.m. and 12 p.m. Each point is the mean difference for a treatment on a day. Solid line, disoriented colonies; dashed line, oriented colonies (shaded area, SE).
When colonies were switched every 3 days between oriented and disoriented, we did not see any difference in the distribution of colony weight changes between treatments [Gaussian glmm: oriented mean (95% CI), −0.066 kg (−0.128 to −0.004); disoriented mean morning foraging activity (95% CI), −0.098 kg (−0.166 to −0.029); mean difference between treatments (95% CI), 0.032 kg (−0.124 to 0.06); χ2 = 0.47, df = 1, P = 0.49; fig. S4]. There was no significant difference in colony activity between treatments when they were switched every day [Gaussian glmm: oriented mean (95% CI), 0.214 kg (0.168 to 0.26); disoriented mean morning foraging activity (95% CI), 0.229 kg (0.183 to 0.275); mean difference between treatments (95% CI), 0.015 kg (−0.05 to 0.08); χ2 = 0.52, df = 1, P = 0.47; fig. S5], and no interaction was found between day and treatment (Gaussian glmm: χ2 = 2.95, df = 1, P = 0.086).
Dance frequency was not affected by treatment or period in E1 [start: DT mean (95% CI), 2.44 dances per minute (dpm) (1.35 to 3.52) versus oriented treatment (OT) mean (95% CI), 2.64 dpm (1.24 to 4.05); z = 0.19, P = 0.85; end: DT mean (95% CI), 1.62 dpm (0.82 to 2.43) versus OT mean (95% CI), 2.15 dpm (1.05 to 3.25); z = 0.04, P = 0.97; DT: start versus end, z = −1.16, P = 0.25; OT: start versus end, z = −0.52, P = 0.61]. In E2, we did not see an interaction between period (first 4 days and last 4 days of the experiment) and treatment with respect to dance frequency [Poisson glmm: oriented mean (95% CI), 3.5 dances per 2 min (2.75 to 4.25); disoriented mean (95% CI), 3.9 dances per 2 min (3.29 to 4.5); mean difference between treatments (95% CI) = 0.4 dances per 2 min (−1.35 to 0.55); χ2 = 0.65, df = 1, P = 0.42]. However, dance frequency increased from the start to the end of the experiment (Poisson glmm: χ2 = 11.4, df = 1, P = 0.0007). These data suggest that the motivation to dance was not affected by whether bees were able to perform oriented versus disoriented dances.
In E3, we tested whether light on the first frame could be responsible for the changes in effort by studying eight colonies with vertical frames. Thus, foragers could perform oriented dances in all colonies, but some colonies had light on the dance floor. We found no interaction between day and treatment (light or dark) and no difference in foraging activity between treatments [Gaussian glmm: light mean (95% CI), 39.7 bees leaving per minute (32.8 to 46.7); dark mean (95% CI), 42.0 bees leaving per minute (35.8 to 48.2); mean difference between treatments (95% CI) = 2.3 bees leaving per minute (−10.52 to 12.37); χ2 = 0.34, df = 1, P = 0.56].
Individual performance (E1)
We did not find significant differences in the concentration or the volume of nectar collected by foragers in the two 18-day treatments [quality: Gaussian glmm: oriented geometric mean (95% CI), 19.1% (17.0 to 21.5); disoriented geometric mean (95% CI), 21.0% (18.9 to 23.3); mean difference between treatments (95% CI), 1.9% (−2.28 to 4.8); χ2 = 2.47, df = 1, P = 0.124; quantity: Gaussian glmm: oriented mean (95% CI), 12.7 μl (11.0 to 15.0); disoriented mean (95% CI), 13.4 μl (11.0 to 16.0); mean difference between treatments (95% CI), 0.7 μl (−2.0 to 4.0); χ2 = 0.1, df = 1, P = 0.882; Fig. 2]. There was also no difference in the weight of the average pollen load brought back by a returning forager between the two long-term treatments [Gaussian glmm: oriented geometric mean (95% CI), 6.0 mg (5.6 to 6.4); disoriented geometric mean (95% CI), 5.9 mg (5.6 to 6.2); mean difference between treatments (95% CI), 0.1 mg (−0.8 to 0.4); χ2 = 1.99, df = 1, P = 0.159; fig. S6]. Finally, the trip duration of foragers was significantly higher in disoriented colonies in the 18-day treatments [oriented geometric mean (95% CI), 32.8 min (29.5 to 36.4); disoriented geometric mean (95% CI), 40.7 min (36.4 to 45.4); mean difference between treatments (95% CI), 8.6 min (2.65 to 17.30); χ2 = 5.93, df = 1, P = 0.015; fig. S7].
Fig. 2. Individual foraging success.
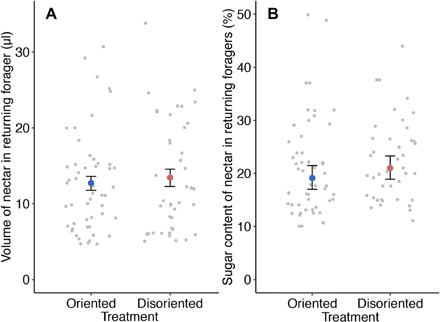
(A) Mean volume of nectar in returning foragers in the two treatments ± SE (microliter). (B) Geometric mean sugar concentration of nectar in returning foragers ± CI (%). In both plots, raw data are represented by gray points.
Dance-following behavior (E1 and E2)
We observed colony dance-following behavior over a period of 18 days in full-sized colonies (E1) and individual dance-following behavior over a period of 12 days in observation hives (E2). A total of 579 dance following events were recorded in E1 and 2341 dance following events in E2. We did not see a difference between treatments in dance-following behavior at the start of the experiment; however, at the end of the experiment, we find that dance-following is significantly lower in disoriented colonies compared to oriented colonies. In E1 at the start of the experiment, there was no difference between treatments in the number of bees following dances [DT mean (95% CI), 4.6 bees (4.16 to 5.04) versus OT mean (95% CI), 5.02 bees (4.6 to 5.44); mean difference between treatments (95% CI), 0.42 bees (−0.59 to 0.59); z = 1.38, P = 0.50]. On average, a disoriented dance at the end of the experiment was followed by 20% fewer bees [DT mean (95% CI), 3.67 bees (3.24 to 4.1) versus OT mean (95% CI), 4.58 bees (4.09 to 5.08); mean difference between treatments (95% CI), 0.91 bees (−0.65 to 1.18); z = 2.94, P = 0.013; Fig. 3A]. The number of waggle runs followed by a bee was not significantly different between treatments at the start of the experiment (χ2 = 1.18, df = 1, P = 0.28). However, followers observed on average 25% fewer waggle runs in the DT than in the OT at the end of the experiment (χ2 = 6.21, df = 1, P = 0.012; Fig. 3B). Similarly, when observing individually marked bees in observation hives (E2), we found that, over the period of the experiment, bees in the DT followed fewer runs per dance following event than those in the OT. An interaction was seen between day of experiment and treatment [Gaussian glmm: oriented mean (95% CI), 7.85 runs followed per dance (7.53 to 8.17); disoriented mean (95% CI), 6.95 runs followed per dance (6.77 to 7.19); mean difference between treatments (95% CI), 0.9 runs followed per dance (−0.35 to 1.38); χ2 = 7.22, df = 2, P = 0.0072], indicating that interest in dances diverged between the two treatments during the course of the experiment (Fig. 3C). In E2, we also observed follower behavior after following a dance. We saw that, over the course of 12 days, there was no difference between treatments in whether bees would leave or stay in the hive within 40 s of following a dance (χ2 = 0.69, df = 1, P = 0.41), and there was no interaction of treatment with day (χ2 = 1.81, df = 1, P = 0.18).
Fig. 3. Following behavior change in E1 and E2 for oriented colonies (blue) and disoriented colonies (red).
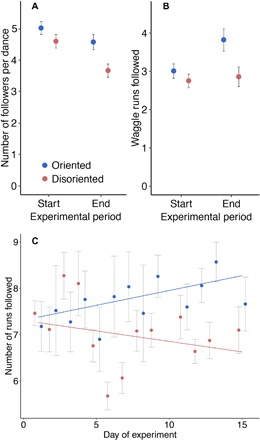
(A) Average number of dance followers per dance in the first 2 days (start) and in the last 2 days (end) in E1 (mean ± SE). (B) Average number of waggle runs followed by dance followers at the start and end in E1 (mean ± SE). (C) Number of runs followed by a dance follower in E2 (mean ± SE). Lines were drawn using linear model function.
Model results
The ABM was modified from a model developed by Schürch and Grüter (29) and simulates colonies of virtual bees foraging in a virtual environment offering food patches of varying quality. On the basis of our empirical findings, the data presented in the main results used a set patch molarity of 0.5 mol, a patch yield of 25 μl, and a patch age of 5 or 15 days. When the virtual environment is more ephemeral (patch age, 5 days), patch density significantly interacts with foraging mode (either dance following or scouting; F9,9 = 163.53, P < 0.001). When patch density (0.1) and variation (SD = mean/4) are high, scouting colonies collected 22.59 ± 11.1% more energy than dancing colonies (Fig. 4A). However, when patch density is low (0.01), the scouting colonies collect only 12.9 ± 20.8% as much energy as dancing colonies (Fig. 4C). Patch variation does not interact with the foraging condition (F9,9 = 3.652, P > 0.05).
Fig. 4. Total energy intake in colonies that rely on either dance information or scouting when foraging in different environmental conditions (mean ± CI).
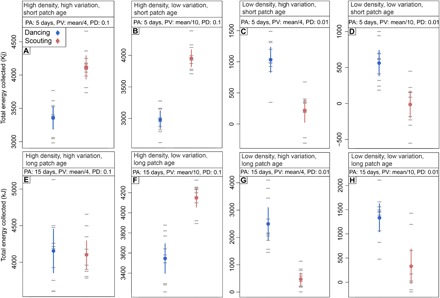
(A to H) In each of these simulations, patch molarity is 0.5 and patch yield is 25 μl. We manipulated the patch density (PD) and patch molarity variance. PA, patch age; PV, patch variation.
When the environment is more constant (patch age, 15 days), patch density again interacts with foraging mode (F9,9 = 64.74, P < 0.001). Foraging mode also interacts with patch variation (F9,9 = 14.273, P < 0.001). When patch density is high (0.1) and patch variance is low (SD = mean/10), scouting colonies collected 17.5 ± 9.7% more energy over the 18-day simulation (Fig. 4F). When patch density is low and variability is high, scouting colonies collected just 18.96 ± 16.7% of the energy that the dancing colonies collect (Fig. 4G). Last, when variation is low and density is low, scouting colonies collected just 24.88 ± 43.6% of the amount of energy that dancing colonies collected.
DISCUSSION
Our empirical results show that colonies with disoriented dances lost, on average, 29% less weight than those with oriented dances over the 18-day experimental period. This outcome does not appear to be the result of improved foraging success by individual bees in the DT, as we did not see differences in the quality or quantity of nectar in returning foragers between treatments (Fig. 2) and foraging journey times were longer in the disoriented treatment (fig. S7). In contrast with Nürnberger (26), we also did not see differences in the quantity of pollen collected at the individual (fig. S6A) or colony (fig. S6B) level. Our data suggest that a switch in foraging strategy by bees in the DTs is responsible for this improved foraging success. When analyzing the follower behavior in both full-sized colonies (E1) and observation hives (E2), we found a reduction in the number of waggle runs that a bee follows per dance over the course of the experiment in disoriented colonies compared to oriented colonies (Fig. 3, B and C). In full-sized colonies, we also saw a reduction in the number of bees following dances over the course of the experiment in disoriented colonies compared to oriented colonies (Fig. 3A). Thus, bees in the DTs seem to have changed their foraging strategy to one that relies less on social information from the dance. Reducing reliance on social information may allow bees to forage more because bees spend less time waiting for dancers. We found that the foraging activity of colonies, measured as the mass of foragers leaving the colony in the morning, was, on average, 23% higher in the DT than in the OT. In E1, this difference in foraging activity became apparent after around 10 days (Fig. 1B), which coincides with the appearance of a difference in weight change between the two conditions (Fig. 1A). In E2, we saw that dance following became different in the two treatments from around day 6. This reduction in latency to change foraging behavior was likely due to tracking individual bee behavior in E2 as opposed to colony level behavior in E1. Overall, the data suggest that colonies in the DT performed better because of a greater foraging effort, i.e., more trips were performed at the colony level (Fig. 1B).
Studies on honeybees (32) and bumble bees (30) have suggested that an individual’s past experience affects its social information use. Beekman and colleagues (33) have shown that foragers that fail to find a recruitment dance are more likely to become scouts. The change in following behavior that we observed in both E1 and E2 may be the result of bees learning the value of spatial information in the dance. Bees could be learning that disoriented dances lack informative value while following them, or they could respond to the lack of positive reinforcement if they are not locating the resource after dance following. We believe that the absence of differences in post following behavior in E2 is more consistent with the second explanation. Furthermore, if bees were comprehending the dances as being nonsense while following them, then we might expect to see an immediate difference in the number of runs they follow compared to the OT (Fig. 3). Dechaume-Moncharmont and colleagues (22) have shown that eschewing social information can theoretically offer fitness benefits to a colony. If the costs in energy and time incurred by using social information are high and do not offer significant benefits, then the strategy of waiting for social information becomes counterproductive. However, as far as we know, our study is the first empirical study to provide support for this prediction.
The number of waggle runs followed increased over the course of the experiments in the OT. This could suggest that bees were learning to follow dances for longer. However, given that dancing did not seem to be very beneficial in the study area, it is possible that temporal changes in resource distribution and availability have affected the dance-following behavior of bees in this treatment. Another potential explanation is that nutritional stress from an environment in which there are few high-quality resources has resulted in an increase in motivation to receive information. That this change in dance-following behavior was not seen in colonies with disoriented dances suggests that bees in these colonies have learned about the value of the dance information. More research is needed to better understand the role of environmental and nutritional factors for dance following motivation.
Our experiment with 3-day switching periods supported the results of previous studies, which suggested that there is no effect of spatial information in the dance in many environments (23–26). In contrast with our 18-day experiment but in agreement with the aforementioned studies, we found no difference in colony foraging success (fig. S4) or colony foraging activity (fig. S5) between treatments. This corroborates simulations suggesting that short treatment periods underestimate the effects of spatial information (29), be they negative (this study) or positive (34).
Bees returned to the colony with an average nectar sugar concentration of ~21%, which is very low compared to other studies [e.g., (31)]. Furthermore, bees returned with small nectar loads (mean, 13 ± 6.9 μl; honeybees can carry more than 50 μl). These data, in combination with the colony weight loss over the 18-day experiment (Fig. 1A), suggest that this was an environment without many high-quality resources. Accordingly, both Couvillon et al. (31) and Nürnberger et al. (26) found that bees struggle to find high-quality food sites during the summer in southern England and southern Germany, respectively. Our model reveals that poor foraging conditions can favor the scouting strategy even if food sources persist for several days (Fig. 4, A, B, and F). The benefits of not using dance information were most obvious when patch age was short (5 days), patch variation was low, and patch density was high (Fig. 4B). A 5-day patch age could reflect the environment in our study. Primack (35) found that in temperate habitats containing herbs and shrubs, species that flower in spring, early summer, and late summer do so on average for 6.8, 5.7, and 2.5 days, respectively. In agreement with our simulations, a model developed by Dornhaus et al. (27) found that in high resource density conditions, recruitment can be detrimental to colony foraging success because bees do not require social information to be successful [see also (24)]. Our model suggests that patch density is an important factor influencing whether spatial information in the dance is beneficial to colony foraging success. In all cases, when resources were at low density, dancing colonies perform better than scouting colonies (Fig. 4, C, D, G, and H).
If there is no benefit of dance communication in temperate climates, then why do bees dance? First, the dance might still be beneficial to foraging success in our study area during other time periods, e.g., in spring. Bee colonies may gain weight during only a few weeks per year. For this reason, it is critically important that the colony can exploit the high-quality resources available while there are good foraging conditions; the dance is likely to play an important role in maximizing foraging efficiency during such periods. Second, encoded spatial information is only one part of the dance. For example, forage odor plays an important role in honeybee foraging, and incoming dancers will distribute this information to followers during their dance displays (19, 28, 36). Dancers can also reactivate foraging at a patch by stimulating experienced foragers to revisit foraging sites (8).
Thus, while the spatial information contained in the dance will likely have a fluctuating value over the seasons, the other cues may mean that dancing remains an important feature of the honeybee’s foraging success. Furthermore, it is important to note that while the dance offers vector information to the dance following bee, it has been suggested that there is additional recruitment in the field through the use of “buzzing flights” that take place close to the feeding site (37, 38). The importance of this recruitment is not well understood and deserves further study.
Temperate habitats have changed drastically in the last decades, coinciding with a loss of honeybee colonies in some areas (39). These changes in landscape are also suspected to have played a role in a pollinator decline over the past few decades (39, 40). Human-modified temperate habitats are often characterized by few large floral patches (mass-flowering crops) that may be easy to find and profitable in spring (41); however, once these have finished flowering, the environment becomes bereft of isolated high-quality foraging sites (26, 34) and the dance’s value may be diminished. In these environments, there are likely to be many foraging sites; however, their quality is such that the cost of recruitment to these sites may outweigh the benefits. Thus, data presented in this paper raise the possibility that human impact may have created landscapes and temporal periods to which the honeybee “dance language” is not well adapted.
MATERIALS AND METHODS
Study site and study animals
Experiments were carried out during the periods of June to August 2014 (E1), May to August 2016 (E2), and May to June 2017 (E3) at the University of Lausanne, Switzerland (46.5225°N, 6.5794°E). The area within an 8-km radius of our colonies consisted of 27.9% settlement and urban areas, 23.6% agricultural areas, 9.2% wooded areas, and 39.3% unproductive areas (ArcGIS data). Twelve colonies made up of 15,000 to 20,000 workers of Apis mellifera (Buckfast), kept in a 10-frame Dadant brood box, were used for E1. Four colonies made up of around 3000 workers, kept in an observation hive made up of two brood frames and a honey frame, were used for E2. Eight colonies made up of 15,000 to 25,000 workers were used for E3. No colonies were used in more than one experiment. All colonies were queen-right and had a naturally mated queen.
Experimental procedure (E1): Colony performance
The colonies were divided into three blocks, each of four colonies. All colonies were used in each treatment, and blocks were split after each treatment, so treatment histories were balanced. The three treatments were as follows: (i) oriented: horizontal frames with polarized light (view of sky) for 18 days; (ii) disoriented: horizontal frames with no light for 18 days; and (iii) switch: switching between oriented and disoriented conditions every 3 days for the 18-day treatment period. Custom-built hive boxes were designed to ensure that bees would walk across the first frame before descending into the hive; therefore, most dances would take place there. Seeley and Towne (42) found that 94% of dances take place within one frame width of the hive entrance. Each hive had a window revealing the first frame, so upon entering the bees are visible until they reach the opposite side. All hives were given shade from the sun during the hottest periods of the day while still allowing bees on the top frame to see a part of the sky and, thereby, orientate their dances.
To quantify foraging success, colonies were weighed every hour using BeeWatch hive scales (accuracy of ±20 g). Weight readings were collected by taking the mean weight of a colony from 12 a.m. to 3 a.m. To confirm that the treatments (oriented and disoriented) were working, the orientation of dances was compared between the two. In the DT, a red acetate sheet (LEE filter: type “Bright Red 026”) was used to limit the amount of visible light that could get to the bees. We found that 58.5% of recorded dances were disoriented when filming them through the red light filter (n = 74). A disoriented dance consisted of runs that were in random directions. That dances were not 100% disoriented is likely to be the result of some visible light getting through the acetate sheet, which was necessary for filming. We expect that when the window was fully covered, as was the case during DTs, 100% of the dances were disoriented. In the OT, 98.3% of dances were oriented (n = 67; Pearson’s χ2, P < 0.0001).
Foraging activity was calculated by taking the weight of the colonies at 12 p.m. and comparing it to their initial weight for that day at 4 a.m. On good foraging days, bees leave the colony in the morning and the weight decreases. This loss of weight is a good indication of foraging activity. The time of 12 p.m. was chosen because this was commonly the time at which the colonies had the lowest weight for the day. To validate the robustness of these data, we also collected daily foraging activity by manually counting the number of foragers leaving the colonies at three time points per day. We carried out a regression with colony activity from weight data and manual counts taken over the day. There was strong correlation, suggesting that they were both good representations of colony foraging activity (fig. S2). Once per colony per experimental period, during days 13 to 17, pollen was collected from returning foragers, then dried, and weighed. Pollen traps were placed over hive entrances of each hive in every treatment for 30 min. To confirm that the presence of light on the first frame per se did not affect the foraging activity of a colony over 18 days, we carried out a paired experiment (E3) using eight colonies in a vertical orientation with and without light on the first frame.
Dance frequency
On 4 days of E1 (first 2 days and last 2 days), we observed the colonies for 2 min and counted the number of dances taking place (count data). In E2, we observed colonies for 2 min in the morning and 2 min in the afternoon and counted unique dances in this time. This was carried out for the first 4 days and last 4 days of data collection, giving us dance frequency during the “start” and “end” periods.
Experimental procedure (E1): Individual performance
Individual foraging trip duration was measured by individually marking 50 bees (Opalith number plates) in each colony during the last 2 to 5 days of the two 18-day treatments. We focused on this period because potential treatment effects were expected to be more obvious at the end of the treatments. Entrances were filmed for 95 min between 12 p.m. and 3 p.m., and the time spent on a foraging trip was noted. Up to four trips per bee were recorded. The foraging durations for a unique bee were averaged, and the times were compared between treatments.
The quality of forage (sugar concentration and quantity) collected was assessed through the extraction of nectar from returning workers on day 9 or 10 of the two 18-day treatments. This corresponds to the time when differences between oriented and disoriented colonies in colony weights and foraging activity started to become more pronounced (Fig. 1). Workers were caught just before entering the hive and immediately cooled on ice. The nectar was extracted by gently squeezing the abdomen of the bee and holding a capillary tube to its proboscis. The volume of nectar that the bee collected was then calculated. The nectar was then moved from the capillary tube to a hand refractometer (Krüss HR 25/800) to obtain the sugar concentration. If the sugar concentration was below 8% or the volume was below 5 μl, then the data were removed from the analysis as they were considered to be either water, an orientation flight, or an unsuccessful trip.
Experimental procedure (E1): Dance following behavior
Colonies were filmed to obtain data on dance following in 2 of the first 3 days and in 2 of the last 3 days (10 min per colony per day). Filming took place between 12 p.m. and 4 p.m. Videos were taken through the glass window, which was covered with red acetate filters. We recorded the number of dance followers per dance and the number of waggle runs followed by each dance follower. Followers were identified as bees facing the dancer with their heads within antennal length during the waggle run and who followed the movement of the dancer during at least one waggle run phase of a dance (43). Data on the number of bees following a dance were collected from the third waggle run (WR3) for three runs. The score for a dance was the average number of dances from these three runs. We counted the number of waggle runs followed per observer present at WR3 to estimate the motivation of observers to follow an individual dance. To quantify overall dancing activity in a colony, we also carried out scan samples of the first comb, counting the number of dances taking place during 1 min and measured four times per experimental period (twice in start period and twice in end period).
Experimental procedure (E2): Dance following behavior
All colonies were kept in a wooden shed for protection from the weather. Disoriented colonies were kept in artificial light during filming, and oriented horizontal colonies were kept with a view of the sky. This was achieved through a window on the vertical wall (50 cm2) and three windows on the roof (30, 30, and 40 cm2). Each time a dance following event occurred, we noted the orientation for the dance. We found that 62.8% of dances were oriented in the OT and 14.8% of dances were oriented in the DT. A higher proportion of oriented dances in the OT were not seen for two reasons: (i) thick cloud cover sometimes limited the bees’ access to polarized light and (ii) to remove glare when filming the frame we would sometimes cover one window; this cover was removed when filming was complete. To test each colony in both treatments, three experimental periods were used (EP1, 10 May 2016 to 22 May 2016; EP2, 22 June 2016 to 4 July 2016; EP3, 9 August 2016 to 21 August 2016). Colonies were prepared for an experimental period in vertical positions. About 500 newly emerged bees taken from other colonies in our apiary were added to each observation colony. Introduced bees were tagged with unique color and number combinations (Opalith number plates). A few days before the start of filming, the colonies were standardized in the amount of food and brood they had and were rotated into their treatment orientations. Dance filming began when the bees were aged between 10 and 17 days. This is shortly before most bees start to perform their first foraging trips (19). Each colony was then filmed for the next 8 or 9 days of good foraging weather (EP1, 9 days in 13; EP2, 8 days in 15; EP3, 8 days in 13). Filming took place in the morning for 3 hours from 10 a.m. and in the afternoon for 3 hours from 2 p.m. Data were extracted from the videos using the VLC player (v2.2.6). Each time a dance following event was observed, the following data were recorded: date and time, dance orientation, follower ID, number of runs followed, and behavior for 40 s after dance following. There were five possible post-dance following behaviors: leave colony, stay in colony, climb on glass, follow another dance, and leave the frame of the camera. If a bee left or climbed on the glass, then it was considered to be leaving the hive, and if it carried out any other behavior, then it was considered to be staying in the hive.
Model description
We used the spatially explicit ABM developed by Schürch and Grüter (29) (see the Supplementary Materials for a more detailed description of the model). The major difference between our model and this previous model was that rather than switching colonies between OT and DT, we created colonies that either could use social information from the waggle dance [called “pure SI strategy” in (29)] or had no access to social information [called “pure NI strategy” in (29)]. In the latter case, all agents had a high probability to leave the hive and search for novel patches or use private information, depending on their previous experience. Another difference between models was that, on the basis of our empirical findings, we used, on average, lower food qualities (0.5 M instead of 1.0 M) and quantities (25 μl instead of both 25 and 50 μl; see Results).
To replicate the empirical methods, we again used an 18-day experimental period in the simulations. For a list of all model parameters used, see table S2. We explored the potential benefits of foraging with or without social information focusing on a few key environmental factors. Each factor combination was simulated 10 times. The key environmental factors were (i) patch quality (0.5 M versus 1 M) and yield (25 μl versus 50 μl), (ii) the variability in patch quality (SD = mean/10 or SD = mean/4), (iii) the density of patches (0.1 or 0.01), and (iv) patch age (on average, 5 or 15 days).
Statistical analyses
E1 and E3: Colony performance
We used R 3.1.0 (44), “lme4” (45), and “lmerTest” to perform glmms on (i) weight change (continuous), (ii) foraging activity (continuous), (iii) foraging journey time (continuous), and (iv) nectar data (continuous). For (i) and (ii), fixed effects were day of experiment and dance treatment (with interaction term). Random effects for (i) were colony and experimental period (first, second, or third), and for (ii), we also used date. In (ii), we square root–transformed foraging activity data. For (iii), the fixed effect was dance treatment and the random effect was colony. The response variable was log-transformed in both models (total pollen weight and average grain weight). In (iv), we analyzed both nectar volume (continuous, log-transformed) and nectar sugar concentration (continuous, log-transformed). In both cases, dance treatment was the fixed effect. Random effects for sugar concentration were collection date and colony. For volume, random effects were experimental period (first, second, or third) and colony. In E3, we analyzed foraging activity (continuous data) using lme4 (45) and lmerTest to perform glmms. The response variable was square root–transformed, and colony was kept as a random effect. For all analyses, visual inspection of residual plots did not reveal any obvious deviations from homoscedasticity or normality. P values were obtained by likelihood ratio χ2 tests of the full model with the effect in question against the model without the effect in question. Means and geometric means and their CIs were calculated using groupwiseMean and groupwiseGeometric from the rCompanion package. CIs between treatments were calculated using t test.
E1: Dance frequency and dance follower behavior
Dance frequency (count data) data were zero-inflated, so we ran a zero-inflated model with a Poisson distribution using pscl. Our fixed effect was treatment, and our random effects were colony and date. Follower behavior in E1 was analyzed using lme4 (45) and lmerTest to perform glmms. We looked at number of followers per dance (v, continuous data) and number of runs followed by a dance follower (vi, count data). In (v), the fixed effect was treatment/period (start or end) combination with colony kept as a random effect. Multiple comparisons were performed using Tukey’s method and Holm P value correction in the “multcomp” package (46). To do multiple comparisons, we ran another model with four treatments (oriented and disoriented start and end) and used colony, period, and treatment as random effects. In (vi), P values were obtained by likelihood ratio χ2 tests of the full model with the effect in question against the model without the effect in question. The response variable was log-transformed. Again, means and geometric means and their CIs were calculated using groupwiseMean and groupwiseGeometric from the rCompanion package. CIs between treatments were calculated using t test.
E2: Dance follower behavior
Data on the number of runs followed by marked bees (count data) from E2 were analyzed using lme4 (45) and lmerTest to perform glmms. The response variable was log10-transformed, and colony was kept as a random effect. Post following behavior was analyzed with binomial family and “logit” link function. We kept experimental period and colony as random effects. In all cases, P values were obtained by likelihood ratio χ2 tests of the full model with the effect in question against the model without the effect in question.
ABM: Colony performance
Agent-based model analyses were also carried out in R 3.1.0 (44). Models were run using the lm function. For each model, we set the average patch age (5 or 15), the average patch molarity (0.5), and the average patch yield (25 μl). This meant that the model contained foraging strategy, patch variation, and patch density and their interactions as their explanatory variables and colony energy intake as the response variable (continuous). We used stepwise removal of nonsignificant interactions to find the best model. Sensitivity analysis of the model can be found in the study of Schürch and Grüter (29).
Supplementary Material
Acknowledgments
We would like to thank L. Keller and his group for valuable insight and advice. We also thank A. Parreño for colony maintenance, M. Wüst for help with video analysis, and R. Fernandes for GIS assistance. Funding: This work was funded by a Swiss National Science Foundation Ambizione fellowship to C.G. (grant no. PZOOP3_142628/1). Author contributions: R.I.P. and C.G. contributed to design, experiments, and writing. R.I.P., N.D., N.V., and C.V. contributed to video analysis. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/2/eaat0450/DC1
Methods
Fig. S1. Hive in horizontal orientation and hive in vertical orientation, both with glass windows.
Fig. S2. Correlation of scale foraging effort against morning manual foraging counts.
Fig. S3. Test for effect of light on the first frame of vertical colonies.
Fig. S4. Daily weight change of hives that were switched every 3 days.
Fig. S5. Foraging activity in hives that were switched every 3 days.
Fig. S6. Colony pollen collection in 18-day treatments.
Fig. S7. Foraging journey time of foragers in the 18-day treatments (geometric mean ± CI).
Table S1. Colony treatment order—numbers represent colony ID.
Table S2. Overview of all model parameters and the values used in our simulations for the two conditions (colonies with dancing or without dancing).
REFERENCES AND NOTES
- 1.Sumpter D. J. T., Beekman M., From nonlinearity to optimality: Pheromone trail foraging by ants. Anim. Behav. 66, 273–280 (2003). [Google Scholar]
- 2.Leadbeater E., Chittka L., The dynamics of social learning in an insect model, the bumblebee (Bombus terrestris). Behav. Ecol. Sociobiol. 61, 1789–1796 (2007). [Google Scholar]
- 3.Dawson E. H., Chittka L., Conspecific and heterospecific information use in bumblebees. PLOS ONE 7, e31444 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collett M., Chittka L., Collett T. S., Spatial memory in insect navigation. Curr. Biol. 23, R789–R800 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Grüter C., Leadbeater E., Insights from insects about adaptive social information use. Trends Ecol. Evol. 29, 177–184 (2014). [DOI] [PubMed] [Google Scholar]
- 6.R. L. Kendal, I. Coolen, K. N. Laland, Adaptive trade-offs in the use of social and personal information, in Cognitive Ecology II, R. Dukas, J. M. Ratcliffe, Eds. (The University of Chicago Press, 2009), chap. 13, pp. 249–271. [Google Scholar]
- 7.Laland K. N., Social learning strategies. Learn. Behav. 32, 4–14 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Grüter C., Balbuena M. S., Farina W. M., Informational conflicts created by the waggle dance. Proc. Biol. Sci. 275, 1321–1327 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rendell L., Boyd R., Cownden D., Enquist M., Eriksson K., Feldman M. W., Fogarty L., Ghirlanda S., Lillicrap T., Laland K. N., Why copy others? Insights from the social learning strategies tournament. Science 328, 208–213 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grüter C., Leadbeater E., Ratnieks F. L. W., Social learning: The importance of copying others. Curr. Biol. 20, R683–R685 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Mullon C., Lehmann L., Invasion fitness for gene-culture co-evolution in family-structured populations and an application to cumulative culture under vertical transmission. Theor. Popul. Biol. 116, 33–46 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Giraldeau L.-A., Valone T. J., Templeton J. J., Potential disadvantages of using socially acquired information. Philos. Trans. R Soc. Lond. B. Biol. Sci. 357, 1559–1566 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Czaczkes T. J., Grüter C., Ratnieks F. L., Trail pheromones: An integrative view of their role in social insect colony organization. Annu. Rev. Entomol. 60, 581–599 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Nieh J. C., Recruitment communication in stingless bees (Hymenoptera, Apidae, Meliponini). Apidologie 35, 159–182 (2004). [Google Scholar]
- 15.B. Hölldobler, E. O. Wilson, The Ants (The Belknap Press of Harward University, 1990). [Google Scholar]
- 16.LeBoeuf A. C., Waridel P., Brent C. S., Gonçalves A. N., Menin L., Ortiz D., Riba-Grognuz O., Koto A., Soares Z. G., Oral transfer of chemical cues, growth proteins and hormones in social insects. eLife 5, e20375 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.W. M. Farina, C. Grüter, Trophallaxis: A mechanism of information transfer, in Food Exploitation by Social Insects: Ecological, Behavioral, and Theoretical Approaches, S. Jarau, M. Hrncir, Eds. (CRC Press, 2009), chap. 10, pp. 173–187. [Google Scholar]
- 18.I’Anson Price R., Grüter C., Why, when and where did honey bee dance communication evolve? Front. Ecol. Evol. 3, 125 (2015). [Google Scholar]
- 19.K. von Frisch, The Dance Language and Orientation of Bees (Harvard Univ. Press, 1967). [Google Scholar]
- 20.Donaldson-Matasci M. C., DeGrandi-Hoffman G., Dornhaus A., Bigger is better: Honeybee colonies as distributed information-gathering systems. Anim. Behav. 85, 585–592 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.T. D. Seeley, The Wisdom of the Hive: The Social Physiology of Honey Bee Colonies (Harward Univ. Press, 1995). [Google Scholar]
- 22.Dechaume-Moncharmont F. X., Dornhaus A., Houston A. I., McNamara J. M., Collins E. J., Franks N. R., The hidden cost of information in collective foraging. Proc. Biol. Sci. 272, 1689–1695 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sherman G., Visscher P. K., Honeybee colonies achieve fitness through dancing. Nature 419, 920–922 (2002). [DOI] [PubMed] [Google Scholar]
- 24.Donaldson-Matasci M. C., Dornhaus A., How habitat affects the benefits of communication in collectively foraging honey bees. Behav. Ecol. Sociobiol. 66, 583–592 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dornhaus A., Chittka L., Why do honey bees dance? Behav. Ecol. Sociobiol. 55, 395–401 (2004). [Google Scholar]
- 26.Nürnberger F., Steffan-Dewenter I., Härtel S., Combined effects of waggle dance communication and landscape heterogeneity on nectar and pollen uptake in honey bee colonies. PeerJ 5, e3441 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dornhaus A., Klügl F., Oechslein C., Puppe F., Chittka L., Benefits of recruitment in honey bees: Effects of ecology and colony size in an individual-based model. Behav. Ecol. 17, 336–344 (2006). [Google Scholar]
- 28.Grüter C., Farina W. M., The honeybee waggle dance: Can we follow the steps? Trends Ecol. Evol. 24, 242–247 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Schürch R., Grüter C., Dancing bees improve colony foraging success as long-term benefits outweigh short-term costs. PLOS ONE 9, e104660 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dawson E. H., Avarguès-Weber A., Chittka L., Leadbeater E., Learning by observation emerges from simple associations in an insect model. Curr. Biol. 23, 727–730 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Couvillon M. J., Schürch R., Ratnieks F. L., Waggle dance distances as integrative indicators of seasonal foraging challenges. PLOS ONE 9, e93495 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wray M. K., Klein B. A., Seeley T. D., Honey bees use social information in waggle dances more fully when foraging errors are more costly. Behav. Ecol. 23, 125–131 (2012). [Google Scholar]
- 33.Beekman M., Gilchrist A. L., Duncan M., Sumpter D. J. T., What makes a honeybee scout? Behav. Ecol. Sociobiol. 61, 985–995 (2007). [Google Scholar]
- 34.Couvillon M. J., Fensome K. A., Quah S. K., Schürch R., Summertime blues: August foraging leaves honey bees empty-handed. Commun. Integr. Biol. 7, e28821 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Primack R. B., Longevity of individual flowers. Annu. Rev. Ecol. Syst. 16, 15–37 (1985). [Google Scholar]
- 36.Kirchner W. H., Grasser A., The significance of odor cues and dance language information for the food search behavior of honeybees (Hymenoptera: Apidae). J. Insect Behav. 11, 169–178 (1998). [Google Scholar]
- 37.von Frisch K., Über die “Sprache” der Bienen, eine tierpsychologische Untersuchung. Zool. Jahrb. Abt. Allg. Zool. Physiol. Tiere 40, 1–186 (1923). [Google Scholar]
- 38.Tautz J., Sandeman D. C., Recruitment of honeybees to non-scented food sources. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 189, 293–300 (2003). [DOI] [PubMed] [Google Scholar]
- 39.Levy S., The pollinator crisis: What’s best for bees. Nature 479, 164–165 (2011). [DOI] [PubMed] [Google Scholar]
- 40.Winfree R., Bartomeus I., Cariveau D. P., Native pollinators in anthropogenic habitats. Annu. Rev. Ecol. Evol. Syst. 42, 1–22 (2011). [Google Scholar]
- 41.Holzschuh A., Dainese M., González-Varo J. P., Mudri-Stojnić S., Riedinger V., Rundlöf M., Scheper J., Wickens J. B., Wickens V. J., Bommarco R., Kleijn D., Potts S. G., Roberts S. P. M., Smith H. G., Vilà M., Vujić A., Steffan-Dewenter I., Mass-flowering crops dilute pollinator abundance in agricultural landscapes across Europe. Ecol. Lett. 19, 1228–1236 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seeley T. D., Towne W. F., Tactics of dance choice in honey bees: Do foragers compare dances? Behav. Ecol. Sociobiol. 30, 59–69 (1992). [Google Scholar]
- 43.Grüter C., Ratnieks F. L. W., Honeybee foragers increase the use of waggle dance information when private information becomes unrewarding. Anim. Behav. 81, 949–954 (2011). [Google Scholar]
- 44.R Development Core Team, R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2011).
- 45.Bolker B. M., Brooks M. E., Clark C. J., Geange S. W., Poulsen J. R., Stevens M. H., White J. S., Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135 (2009). [DOI] [PubMed] [Google Scholar]
- 46.Hothorn T., Bretz F., Westfall P., Simultaneous inference in general parametric models. Biom. J. 50, 346–363 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/2/eaat0450/DC1
Methods
Fig. S1. Hive in horizontal orientation and hive in vertical orientation, both with glass windows.
Fig. S2. Correlation of scale foraging effort against morning manual foraging counts.
Fig. S3. Test for effect of light on the first frame of vertical colonies.
Fig. S4. Daily weight change of hives that were switched every 3 days.
Fig. S5. Foraging activity in hives that were switched every 3 days.
Fig. S6. Colony pollen collection in 18-day treatments.
Fig. S7. Foraging journey time of foragers in the 18-day treatments (geometric mean ± CI).
Table S1. Colony treatment order—numbers represent colony ID.
Table S2. Overview of all model parameters and the values used in our simulations for the two conditions (colonies with dancing or without dancing).


