Abstract
SETTING:
Four human immunodeficiency virus (HIV) clinics located at South African tertiary hospitals.
OBJECTIVE:
To assess the effectiveness of highly active antiretroviral therapy (HAART) in reducing incident tuberculosis (TB) in HIV-infected children.
DESIGN:
Retrospective cohort.
RESULTS
A total of 1132 children’s records were included in the study. At entry to the cohort, the median (interquartile range [IQR]) age, CD4%, CD4 count and viral load of all children was respectively 6.3 years (4.1– 8.8), 15% (9.0–22.2), 576 cells/mm3 (287–960) and 160 000 copies/ml (54 941.5–449 683); 75.9% were started on HAART. The male: female ratio was 1:1, and median follow-up time was 1.7 years. In children whose follow-up included both pre-HAART and on-HAART periods, the incidence of clinically diagnosed TB was respectively 21.1 per 100 person-years (py; 95%CI 18.2–24.4) and 6.4/100 py (95%CI 4.8–8.1), and when restricted to confirmed cases, respectively 3.1/100 py (95%CI 2.2–4.2) and 0.8/100 py (95% CI 0.5–1.4). Only 23% of all cases of TB were microbiologically confirmed. Multivariate analyses showed that HAART reduced incident TB by approximately 70%, both for confirmed and all TB cases.
CONCLUSIONS:
In this high TB burden country, the incidence of diagnosis of TB in HIV-infected children is at least as high as that of adults. HAART reduces incident TB, but further prospective TB preventive and diagnostic studies are urgently needed in children.
Keywords: paediatric, HIV, tuberculosis, HAART, cohort
HIGHLY ACTIVE antiretroviral therapy (HAART) has revolutionised the management of paediatric human immunodeficiency virus (HIV) disease,1–3 and children’s access to HAART is increasing due to the implementation of large treatment programmes in high HIV prevalence countries. In South Africa, from April 2004—when national HAART roll-out was formally started—to mid 2006, more than 21 500 children aged <14 years had been initiated on HAART.4 Tuberculosis (TB) is the leading cause of mortality and morbidity in HIV-infected adults, and several cohort studies from both industrialised and developing settings report the marked protective effect of HAART on incident TB.5–7 However, the magnitude of the TB burden in HIV-infected children is not as well described, due both to the emphasis of TB control programmes on smear-positive TB cases—a diagnostic category rare in children—and to difficulties in diagnosing TB in children. It is, however, estimated that children aged 0–14 years constitute between 16% and 21% of all TB notifications in high TB burden settings.8 Inadequate access to prevention of mother- to-child HIV transmission interventions of proven efficacy has resulted in two million HIV-infected children in Africa, with almost half of these in South- ern Africa.9 In 2002, HIV prevalence in South African children aged 2–9 years was 6.2%, and it was 4.7% in those aged 10–14 years.10 Although several cross- sectional studies from developing settings report the prevalence of HIV in children with TB and their clinical presentation,11–14 there are few longitudinal data describing the incidence of active TB in this group. We assessed the effectiveness of HAART in reducing incident TB in HIV-infected children.
STUDY POPULATION AND METHODS
A retrospective cohort was performed at four paediatric HIV clinics, three in Johannesburg (Rahima Moosa Mother and Child Hospital, the Perinatal
HIV Research Unit and Harriet Shezi Clinic—the latter two are located in the Chris Hani Baragwanath Hospital in Soweto) and one in Cape Town (Tygerberg Hospital), where the burden of childhood TB is extremely high.15
The ethics committees of the Universities of Witwatersrand and Stellenbosch approved the study.
HIV-infected children aged <15 years were eligible for the study. The clinical visit within 3 months of the date of the child’s first recorded CD4 estimation defined the beginning of follow-up, which ended at the last recorded visit prior to June 2006 when abstraction was complete. At three of the sites, all children eligible for the study were included. At Harriet Shezi, 500 records were randomly selected from approximately 1200 children by allocating random numbers to a list of all unique identification numbers of the clinic, which was then sorted by the allocated random number. The lowest 500 random numbers were selected. If the selected medical record was not eligible, the medical record of the subsequent clinic identification number was used. Demographic data, tuberculin skin test results, preventive treatment for latent TB infection and any episodes of TB, prior history of TB and TB preventive treatment were obtained from the entire record.
For this analysis, a case of TB was defined as documentation of a diagnosis of TB in the medical record. For those without laboratory confirmation, this had to be accompanied by treatment with multidrug anti- tuberculosis treatment or referral to a TB clinic for initiation of TB treatment. The basis for the decision to initiate TB treatment and the duration of treatment was extracted from the clinic notes. A case of TB was confirmed if Mycobacterium tuberculosis was cultured, if acid-fast bacilli (AFB) were seen on microscopy of samples of any specimen or if an open or fine needle biopsy result was suggestive of TB.
Incident TB was defined as a case that occurred >7 weeks after the first study visit. Children diagnosed with incident TB were temporarily censored for the duration of TB treatment because they were not at risk of another TB episode; however, at the end of TB treatment, they were again considered to be at risk. Likewise, those with prevalent TB, diagnosed either at the clinic visit that defined the start of their follow-up time or within 7 weeks of that visit, were temporarily censored to the end of their TB treatment. Subsequent episodes of TB were included if they occurred >9 months after the date of diagnosis of the previous episode. If no end date was available for TB treatment, it was imputed to have lasted 8 months— the duration of second-line anti-tuberculosis treatment in South Africa.16
The dates of initiating and stopping individual antiretroviral agents (ARVs) were abstracted. HAART was defined as triple antiretroviral therapy (ART) received for >2 weeks. To avoid complicating the analysis with unusual treatment scenarios, follow-up time was censored if fewer than three ARVs were pre- scribed or if HAART treatment was interrupted for 2–52 weeks.
Statistical considerations
Two analyses were conducted: the first, stratified by whether or not the episode of TB was confirmed, and in the second, TB incidence was assessed using all follow-ups of children, irrespective of whether or not they received HAART; the analysis was then repeated but this time restricted only to children whose follow-up included both time before receiving HAART and time on HAART (‘dual’ children). The latter analysis was performed to reduce potential confounding of socio-economic class, maternal education, maternal age and other unmeasured variables.
Cox proportional hazards models were used to determine predictors for incident TB after adjusting for potential confounders. Second episodes of TB were included in the analysis, but care was taken to ensure that follow-up time was not counted twice and that CD4 counts were time-dependent—not just using the baseline value. If the P value for a hazard ratio of a risk factor in the univariate analysis was >0.2, it was not included in the multivariate model.
RESULTS
Data on a total of 1132 children were included: 499 (44.1%) from Harriet Shezi Clinic at Chris Hani Baragwanath Hospital in Soweto, 233 (20.6%) from Tygerberg Hospital, 266 (23.5%) from Rahima Moosa Hospital and 134 (11.8%) from the Perinatal HIV Re- search Unit Clinic in Soweto. The first study visit was in September 1994 and the last in June 2006. The me- dian age of all children at entry into the cohort was 6.3 years (interquartile range [IQR] 4.1–8.8), and the median CD4 count was 576.5 cells/mm3 (IQR 287– 960; Table 1). Using the Centers for Disease Prevention and Control’s revised paediatric HIV staging sys- tem,17 respectively 24.9%, 17.4% and 13.2% were staged as B3, B2 and C3. Overall, 859 (75.9%) children ever received HAART in follow-up (3.1% of these were receiving HAART at their first study visit) and 787/ 859 were considered ‘dual’ children. Total follow-up time was 2415.3 person-years (py), of which 126.6 py (5.2%) were censored while children were on TB treatment. The median follow-up time of all children was 1.7 py (IQR 0.9–2.9); median follow-up while not receiving HAART was 9.3 months (IQR 3.0– 23.5), and while receiving HAART it was 9.7 months (IQR 5.6–17.9).
Table 1.
Characteristics of the entire multi-site South African cohort*
| Characteristic | %, or median (IQR) |
|---|---|
| Proportion girls, % | 50.3 |
| Age at entry, years | 6.3 (4.1–8.8) |
| Weight for age, Z-score† | −4.18 (−6.3–−2.7) |
| Height for age, Z-score† | −4.92 (−6.5–−3.5) |
| Weight for height, Z-score† | −0.13 (−1.0–0.7) |
| CD4 count (IQR), cells/mm3 | 576.5 (287.0–960.0) |
| CD4, % | 15.0 (9.0–22.2) |
| Viral load at entry, copies/ml‡ | 160000 (54 941.5–449 683) |
| Taking ARVs at entry to study, % | 3.1 |
| Previous history of TB treatment, % | 39.5 |
| Proportion ever taking cotrimoxazole prophylaxis, % | 96.7 |
Prevalent TB was defined as either receiving TB treatment at the first study visit or diagnosed with TB at the first study visit or within 48 days of the first visit.
US National Health and Nutrition Examination Survey (NHANES) growth charts were used to calculate Z-scores.
Few children had viral load recorded in their files.
IQR = interquartile range; ARV = antiretroviral; TB = tuberculosis.
The median age at HAART initiation was 5.2 years (IQR 2.7–7.5), while median CD4 count and CD4% were respectively 360.5 cells/mm3 (IQR 143.0–652.5) and 11% (IQR 6.2–17.7). Frequently prescribed initial HAART regimens were efavirenz, lamivudine (LMV) and stavudine (STV) in 60.2% of the children, lopinavir/ ritonavir, LMV and STV in 12.1% and zidovudine, LMV and nevirapine in 7.8%. Three children received two- or single-drug ART; 12.6% of the children receiving HAART were switched to another ARV regimen during follow-up. Treatment interruptions of >2 weeks occurred in 36 children, of whom five had treatment interruptions of >1 year. Of 1132 children, 235 (21%) had prevalent TB; 183/235 (77%) were on TB treatment at their first study follow-up visit, while 52/235 (22%) were diagnosed with TB either at their first study visit or within 7 weeks of that visit.
Incident TB
Overall, there were 281 incident TB episodes in 255 children. Of these, 65 (23.1%) were confirmed by AFB smear (n = 30), TB culture (n = 24) or biopsy (n = 11). The overall incidence of all cases of TB in all children in the study (Table 2) was 12.3/100 py (95% confidence interval [CI] 9.8–12.6) and that of confirmed TB in all children 2.8/100 py (95%CI 2.2– 3.7). Of those children not on HAART, median base- line CD4 count and CD4% of 210 children who had TB were respectively 459 cells/mm3 (IQR 206–815) and 13% (IQR 7.4–20), whereas the median baseline CD4 count and CD4% of 756 children who did not have incident TB were respectively 609.5 cells/mm3 (IQR 335–967.5) and 16% (IQR 10–23; P = 0.007 and P < 0.001, respectively). In a graphic representation of incident TB with follow-up time before and during HAART, the incidence rates of all cases of TB and of confirmed TB were calculated by time interval before or after HAART initiation, including only follow-up of ‘dual’ children (Figure).
Table 2.
Incidence of TB (per 100 person-years) before HAART and when on HAART in South African children
| Not treated with HAART (95%CI) |
On HAART (95%CI) |
Incidence rate ratio (95%CI) |
|
|---|---|---|---|
| Any TB episode | |||
| All children | 16.4 (14.3–18.7) | 6.3 (4.9–8.2) | 0.4 (0.3–0.5) |
| Dual children* | 21.1 (18.2–24.4) | 6.4 (4.8–8.1) | 0.3 (0.2–0.4) |
| Confirmed TB† | |||
| All children | 3.1 (2.2–4.2) | 2.5 (1.6–3.7) | 0.8 (0.5–1.4) |
| Dual children* | 3.8 (2.6–5.4) | 2.7 (1.7–4.0) | 0.7 (0.4–1.22) |
Children who had follow-up time recorded both before and during HAART.
AFB smear and/or culture and/or biopsy-confirmed TB.
TB = tuberculosis; HAART = highly active antiretroviral therapy; CI = confi-denceinterval; AFB = acid-fast bacilli.
Figure.
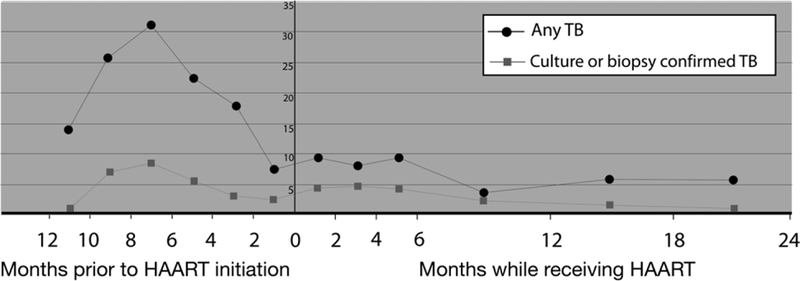
Incidence of TB (per 100 person-years) stratified by period before or during HAART. Re- stricted to children who had follow-up time before and during HAART (‘dual’ children). HAART = highly active antiretroviral therapy; TB = tuberculosis.
Associations with incident TB
Crude incident rate ratios (IRRs) suggested a 70% reduction in incident TB when children were on HAART if the analysis was restricted to ‘dual’ children whose follow-up time included before and during HAART (Table 2).
Preliminary multivariate modelling, including both weight-for-age and height-for-age Z-scores, yielded identical hazard ratios (HRs) to one decimal place; because weight is more commonly measured than height, we opted to include weight-for-age Z-scores. Cox models appeared to confirm the protective effect of HAART on any episode of incident TB after adjusting for a variety of potential confounders (Table 3). The direction and magnitude of the associations shown in Table 3 were similar in a model including all children’s person time (data not shown) as well as a model restricted to children who had follow-up time before and during HAART (dual children). A third model (data not shown), replacing the categorical HAART variable with time on HAART, measured in months, including covariates identical to those in Table 3, suggested a 12% reduction in risk of any TB per additional month of HAART (adjusted HR 0.88, 95%CI 0.85–0.91), the other HRs remaining identical to the second decimal place. A previous episode of TB (con- firmed or not), either before or during follow-up, was associated with a 30% increase in risk of any case of TB, and almost a six-fold increase in the risk of con- firmed TB. Increasing age was protective for TB; each year of age was associated with 11% and 17% reduction in risk of an episode of any case and confirmed case of TB, respectively. Replacing absolute CD4 count with absolute CD4% suggested a statistically significant reduction in risk of 0.04% for every unit increase in CD4% in both univariate and multivariate models.
Table 3.
Cox proportional hazards for time to TB in South African HIV-infected children—restricted to those children who had follow-up time before HAART initiation and on HAART (‘dual’ children)*
| Univariate odds ratio (95%CI) |
Multivariate odds ratio (95%CI) |
|
|---|---|---|
| All cases of TB | ||
| Age, years | 0.82 (0.77–0.87) | 0.89 (0.83–0.95) |
| CD4 count, cells/mm3 | ||
| <200 | 1 | 1 |
| 200–350 | 1.2 (0.8–1.6) | 0.99 (0.53–1.46) |
| >350 | 0.6 (0.3–0.9) | 0.84 (0.49–1.18) |
| Weight for age, Z-score | 0.75 (0.67–0.83) | 0.86 (0.78–0.94) |
| HAART | ||
| No | 1 | 1 |
| Yes | 0.12 (0.00–0.45) | 0.15 (0.00–0.51) |
| Previous history of TB | ||
| No | 1 | 1 |
| Yes | 1.19 (0.91–1.47) | 1.33 (1.04–1.63) |
| Sex | ||
| Female | 1 | |
| Male | 0.80 (0.52–1.08) | — |
| Confirmed cases of TB | ||
| Age, years | 0.86 (0.75–0.97) | 0.83 (0.71–0.95) |
| CD4 count, cells/mm3 | ||
| <200 | 1 | 1 |
| 200–350 | 1.28 (0.42–2.14) | 1.17 (0.19–2.15) |
| >350 | 0.44 (0.00–1.04) | 0.89 (0.15–1.62) |
| Weight for age, Z-score | 0.68 (0.52–0.84) | 0.79 (0.63–0.95) |
| HAART | ||
| No | 1 | 1 |
| Yes | 0.17 (0.00–0.77) | 0.21 (0.00–0.88) |
| Previous history of TB | ||
| No | 1 | 1 |
| Yes | 5.98 (5.21–6.75) | 4.82 (4.04–5.60) |
| Sex | ||
| Female | 1 | |
| Male | 0.80 (0.20–1.40) | — |
Patient age, CD4 count, Z-score (weight for age), HAART status and previous history of TB are time-dependent. The model includes provision for multiple TB events to take place per child.
TB = tuberculosis; HIV = human immunodeficiency virus; HAART = highly active antiretroviral therapy; CI = confidence interval.
DISCUSSION
This multi-site, retrospective cohort study of HIV- infected children in South Africa suggests that HAART is associated with a 70% reduction in risk of incident childhood TB—an association that persisted after adjusting for a variety of confounders. Crude IRRs did not provide evidence for a similar protective association with confirmed TB (IRR 0.8, 95%CI 0.5–1.4), but after adjustment, a strong protective association of HAART with incident, confirmed TB was seen (adjusted HR 0.2, 95%CI 0.0–0.88). Our data highlight 1) the critical absence of an accurate TB diagnosis, as only 23% of all cases of childhood TB in this study were confirmed, and 2) the high risk of incident TB conferred by a previous episode. Autopsy and hospital studies from sub-Saharan Africa suggest that TB, although not the leading pathology in HIV-infected children, is an important cause of mortality and morbidity, particularly in Southern Africa;18–20 however, outcomes of TB treatment in children receiving HAART are relatively good.21 A previous study reported on 98 children from Abidjan, Cote d’Ivoire, where incident TB decreased from 0.71 per 100 untreated child months to 0.16 per 100 treated child months.22 At that time, the TB notification rate in Cote d’Ivoire was 107/100 000, one fifth that in South Africa.23 In contrast, data from North America, where all-case TB notification rates are 4/100 000, the TB incidence in HIV-infected children not treated with HAART is 0.1–0.4/100 py, and after HAART initiation no cases were reported in 364 children followed for 1101 py.24,25 The peak in all cases of TB before HAART initiation (see Figure) is likely attributable to South African paediatric antiretroviral guidelines recommending that HAART not be initiated before the completion of TB treatment,26 due to bias by indication (the diagnosis of TB ensuring that an HIV-infected child is started on HAART), and/or to the attending doctors’ reluctance to treat either immune reconstitution TB or worsening TB after HAART initiation. Furthermore, it is possible that children who did not receive treatment for TB were never started on HAART, as wide- spread access to HAART was only available from 2004. Our data suggest that, as in adults, immune re- constitution TB or unmasking existing TB27 in children may be a problem in the first 6 months after HAART initiation. The weaker protective association of confirmed TB with HAART reported here may also be as a result of the higher rate of smear-positive disease with increasing CD4 count.28
The limitations of this study are the following: as this is a retrospective review of clinical records, important potentially confounding data were not collected. Furthermore, there were likely regional variations in the eligibility criteria for initiation of HAART and case definitions of TB, possibly resulting in misclassification. This analysis may have been strongly affected by survivor bias, as neither accurate mortality nor loss to follow-up data were available, with only six deaths abstracted from clinical records. Moreover, the median age of children was >5 years, albeit similar to that reported from Durban; 29 our results therefore reflect HIV-infected children who survived to access HAART in settings where the majority of paediatric HIV-1 infections are from mother-to-child transmission, and mortality in young HIV-infected children not receiving HAART is extremely high.30–32 Our results likely underestimate TB, as our data suggest that TB incidence is higher in younger children. Survivor bias may be compounded by including follow-up time before HAART was widely available, although all sites did have some patients receiving HAART prior to national roll-out, from either donors, pharmaceutical trials or out-of-pocket payments. To address this bias, we defined the start of follow-up as when a CD4 count was first recorded in the notes. We also attempted to deal with confounding by indication by performing two analyses, first on all children, and then only among children who received HAART. Finally, although TB treatment is recommended for 8 months, in practice it may be extended under some circumstances. One such circumstance is drug-resistant TB; we did not, however, collect data on mycobacterial drug susceptibility. Although 6 months of daily isoniazid preventive treatment (IPT) is recommended for children in close contact with an infectious case,26 no children in this study were recorded as having received IPT.
While the eradication of paediatric HIV by effectively preventing mother-to-child transmission is achievable, in high HIV prevalence settings a large proportion of admissions to paediatric wards remain HIV-related.33 This retrospective study suggests that, as in HIV-infected adults, incident TB in HIV-infected children is reduced by HAART. We hope that our results will inform urgent prospective research including young children that assesses novel TB diagnostics, effective TB treatment and effective TB preventive interventions in HIV-infected and non-infected children.
Acknowledgements
The authors thank the children whose records were used, and L Kalete, D Jacobs and L Moulton for their valuable input. The Perinatal HIV Research Unit funded this study. The South African Government, the Elizabeth Glaser Pediatric AIDS Foundation and the President’s Emergency Plan for AIDS Relief (PEPFAR) funded the children’s care and treatment.
References
- 1.Resino S, Bellon JM, Resino R, et al. Extensive implementation of highly active antiretroviral therapy shows great effect on survival and surrogate markers in vertically HIV-infected children. Clin Infect Dis 2004; 38: 1605–1612. [DOI] [PubMed] [Google Scholar]
- 2.Patel K, Hernan MA, Williams PL, et al. Long-term effective- ness of highly active antiretroviral therapy on the survival of children and adolescents with HIV infection: a 10-year follow- up study. Clin Infect Dis 2008; 46: 507–515. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez JM, Ramos Amador JT, Fernandez de Miguel S, et al. Impact of highly active antiretroviral therapy on the morbid- ity and mortality in Spanish human immunodeficiency virus- infected children. Pediatr Infect Dis J 2003; 22: 863–867. [DOI] [PubMed] [Google Scholar]
- 4.Meyers T, Moultrie H, Naidoo K, Cotton M, Eley B, Sherman [DOI] [PubMed]
- 5.Challenges G to pediatric HIV care and treatment in South Africa. J Infect Dis 2007; 196 (Suppl 3): S474–S481. [DOI] [PubMed] [Google Scholar]
- 6.Girardi E, Sabin CA, d’Arminio Monforte A, et al. Incidence of tuberculosis among HIV-infected patients receiving highly active antiretroviral therapy in Europe and North America. Clin Infect Dis 2005; 41: 1772–1782. [DOI] [PubMed] [Google Scholar]
- 7.Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet 2002; 359: 2059–2064. [DOI] [PubMed] [Google Scholar]
- 8.San-Andres FJ, Rubio R, Castilla J, et al. Incidence of acquired immunodeficiency syndrome-associated opportunistic diseases and the effect of treatment on a cohort of 1115 patients infected with human immunodeficiency virus, 1989–1997. Clin Infect Dis 2003; 36: 1177–1185. [DOI] [PubMed] [Google Scholar]
- 9.Marais BJ, Obihara CC, Warren RM, Schaaf HS, Gie RP, Donald PR. The burden of childhood tuberculosis: a public health perspective. Int J Tuberc Lung Dis 2005; 9: 1305–1313. [PubMed] [Google Scholar]
- 10.Joint United Nations Program on HIV/AIDS. 2006. Report on the global AIDS epidemic. Geneva, Switzerland: UNAIDS, 2006. [Google Scholar]
- 11.Connolly C, Shisana O, Colvin M, Stoker D. Epidemiology of HIV in South Africa—results of a national, community-based survey. S Afr Med J 2004; 94: 776–781. [PubMed] [Google Scholar]
- 12.Madhi SA, Huebner RE, Doedens L, Aduc T, Wesley D, Cooper PA. HIV-1 co-infection in children hospitalised with tubercu- losis in South Africa. Int J Tuberc Lung Dis 2000; 4: 448–454. [PubMed] [Google Scholar]
- 13.Soeters M, de Vries AM, Kimpen JL, Donald PR, Schaaf HS. Clinical features and outcome in children admitted to a TB hos- pital in the Western Cape—the influence of HIV infection and drug resistance. S Afr Med J 2005; 95: 602–606. [PubMed] [Google Scholar]
- 14.Mukadi YD, Wiktor SZ, Coulibaly IM, et al. Impact of HIV infection on the development, clinical presentation and out- come of tuberculosis among children in Abidjan, Cote d’Ivoire. AIDS 1997; 11: 1151–1158. [DOI] [PubMed] [Google Scholar]
- 15.Palme IB, Gudetta B, Bruchfeld J, Muhe L, Giesecke J. Impact of human immunodeficiency virus 1 infection on clinical presentation, treatment outcome and survival in a cohort of Ethiopian children with tuberculosis. Pediatr Infect Dis J 2002; 21: 1053–1061. [DOI] [PubMed] [Google Scholar]
- 16.van Rie A, Beyers N, Gie RP, Kunneke M, Zietsman L, Donald PR. Childhood tuberculosis in an urban population in South Africa: burden and risk factor. Arch Dis Child 1999; 80: 433–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Department of Health, Republic of South Africa. The South African tuberculosis control programme practical guidelines. Pretoria, South Africa: Department of Health, Republic of South Africa, 2000. [Google Scholar]
- 18.Centers for Disease Control and Surveillance. 1994 Revised classification system for human immunodeficiency virus infection in children less than 13 years of age. MMWR 1994; 43: 1–10. [Google Scholar]
- 19.Rennert WP, Kilner D, Hale M, Stevens G, Stevens W, Crewe- Brown H. Tuberculosis in children dying with HIV-related lung disease: clinical-pathological correlations. Int J Tuberc Lung Dis 2002; 6: 806–813. [PubMed] [Google Scholar]
- 20.Lucas SB, Hounnou A, Koffi K, Beaumel A, Andoh J, De Cock KM. Pathology of paediatric human immunodeficiency virus infections in Cote d’Ivoire. East Afr Med J 1996; 73 (Suppl): S7–S8. [PubMed] [Google Scholar]
- 21.Chintu C, Mudenda V, Lucas S, et al. Lung diseases at necropsy in African children dying from respiratory illnesses: a descrip- tive necropsy study. Lancet 2002; 360: 985–990. [DOI] [PubMed] [Google Scholar]
- 22.Walters E, Cotton MF, Rabie H, Schaaf HS, Walters LO, Marais BJ. Clinical presentation and outcome of tuberculosis in human immunodeficiency virus infected children on anti- retroviral therapy. BMC Pediatr 2008; 8: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kouakoussui A, Fassinou P, Anaky MF, et al. Respiratory manifestations in HIV-infected children pre- and post-HAART in Abidjan, the Ivory Coast. Paediatr Respir Rev 2004; 5: 311–315. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. WHO report 2006. Global tuberculosis control: surveillance, planning, financing WHO/HTM/ TB/2006.362. Geneva, Switzerland: WHO, 2006. [Google Scholar]
- 25.Nesheim SR, Kapogiannis BG, Soe MM, et al. Trends in opportunistic infections in the pre- and post-highly active antiretroviral therapy eras among HIV-infected children in the Perinatal AIDS Collaborative Transmission Study, 1986–2004. Pediatrics 2007; 120: 100–109. [DOI] [PubMed] [Google Scholar]
- 26.Dankner WM, Lindsey JC, Levin MJ. Correlates of opportunistic infections in children infected with the human immuno- deficiency virus managed before highly active antiretroviral therapy. Pediatr Infect Dis J 2001; 20: 40–48. [DOI] [PubMed] [Google Scholar]
- 27.Department of Health, Republic of South Africa. Guidelines for the management of HIV infection—2005. Pretoria, South Africa: Department of Health, Republic of South Africa, 2005. [Google Scholar]
- 28.Meintjes G, Lawn SD, Scano F, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis 2008; 8: 516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mugusi F, Villamor E, Urassa W, Saathoff E, Bosch RJ, Fawzi WW. HIV co-infection, CD4 cell counts and clinical correlates of bacillary density in pulmonary tuberculosis. Int J Tuberc Lung Dis 2006; 10: 663–669. [PubMed] [Google Scholar]
- 30.Reddi A, Leeper SC, Grobler AC, et al. Preliminary outcomes of a paediatric highly active antiretroviral therapy cohort from KwaZulu-Natal, South Africa . BMC Pediatr 2007; 7: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newell ML, Coovadia H, Cortina-Borja M, et al. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet 2004; 364: 1236–1243. [DOI] [PubMed] [Google Scholar]
- 32.Harambat J, Fassinou P, Becquet R, et al. 18-month occurrence of severe events among early diagnosed HIV-infected children before antiretroviral therapy in Abidjan, Cote d’Ivoire: a cohort study. BMC Public Health 2008; 8: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hussey GD, Reijnhart RM, Sebens AM, Burgess J, Schaaf S, Potgieter S. Survival of children in Cape Town known to be ver- tically infected with HIV-1. S Afr Med J 1998; 88: 554–558. [PubMed] [Google Scholar]
- 34.Schneider H, Kellerman R, Oyedele S. HIV impact surveillance system Summary report: design and data collection. Johannes- burg, South Africa: School of Public Health, University of the Witwatersrand and Gauteng Department of Health, 2005 [Google Scholar]


