Abstract
Mantle cell lymphoma (MCL) is a heterogeneous disease with high relapse rates. Limited data guide the use of surveillance imaging following treatment. We constructed a retrospective cohort from two academic institutions of patients with MCL who completed first-line therapy and underwent follow-up for relapse, analyzing the effect of surveillance imaging on survival. Of 217 patients, 102 had documented relapse, with 38 (37%) diagnosed by surveillance imaging and 64 (63%) by other methods. Relapse diagnosis by surveillance imaging had no significant advantage in overall survival from diagnosis date (hazard ratio [HR] = 0.80, p= .39) or relapse date (HR = 0.72, p= .22). Of 801 surveillance images, PET/CT had a positive predictive value (PPV) of 24% and number needed-to-scan/treat (NNT) of 51 to detect one relapse, and CT had a PPV of 49% and NNT of 24. For MCL after first-line therapy, relapse detection by surveillance imaging was not associated with improved survival and lacks clinical benefit.
Keywords: Mantle cell lymphoma, relapse, surveillance imaging, survival, PET/CT, CT
Introduction
Mantle cell lymphoma (MCL) is an uncommon subtype of non-Hodgkin lymphoma (NHL) characterized by a heterogeneous presentation and disease course, ranging from indolent disease warranting watchful waiting to a highly aggressive malignancy with poorer prognosis. Although there is no standard approach to initial therapy, intensive cytarabine-containing induction chemotherapy followed by autologous stem cell transplantation (ASCT) is associated with prolonged progression-free survival (PFS), with up to 75% of patients remaining in remission at 5 years [1–3]. Despite these results with initial induction therapy, most patients ultimately relapse. Some patients relapse shortly after therapy and have a poor prognosis [4,5], while others may relapse as late as 15 years after remission [2]. This heterogeneous pattern of relapse makes identification of a routine surveillance approach challenging for treating clinicians.
For MCL patients in first remission, one approach to surveillance involves routine imaging with positron emission tomography (PET) studies or computed tomography (CT) studies, but this modality lacks sufficient data supporting its routine use. In a series of 164 PET/CT images for 32 patients in first remission of MCL, it was estimated that the positive predictive value (PPV) for a PET/CT images is only 8% in first remission [6]. In addition, while some smaller studies have provided evidence against the predictive capabilities of PET/CT imaging in first remission [6–8], no existing study has evaluated whether surveillance imaging improves patient outcomes such as overall survival (OS). Even with limited data suggesting a lack of clinical utility of PET/CT and CT in monitoring for relapse [6–8], many patients with MCL likely continue to receive surveillance imaging following first remission.
Evaluation of the role of surveillance imaging in other lymphoma subtypes suggests no clear benefit for patients in first remission. For example, a recent ASH Choosing Wisely statement and a focused evidence-based review in Blood noted that surveillance imaging for the detection of asymptomatic relapse in diffuse large B-cell lymphoma (DLBCL) does not confer a survival benefit, with the overwhelming majority of relapses identified via development of clinical signs and symptoms rather than by detection on a routine scan [9–11]. As a result of these findings, guidelines for the management of DLBCL recommend against routine imaging [12,13], while guidelines on surveillance imaging in MCL are conflicting [12,13].
Given the lack of substantive data to guide surveillance approaches in MCL, we performed a multi-center study to describe the pattern of relapse in MCL patients in first remission, evaluated the performance of surveillance imaging in detection of relapse, and tested the impact of routine surveillance on patient survival.
Methods
Included patients
We included patients diagnosed with MCL between 1993 and 2015 who achieved a documented complete response, partial response, or stable disease after first treatment as assessed by radiologic or clinical assessment at either Emory University or the Ohio State University. Remission was determined by the treating clinician at the time using available criteria based on the imaging modality used to assess treatment response. Patients were excluded if they had incomplete or missing outcome data after their first treatment, if they had progressive disease after initial therapy, or if they never received therapy. This study was approved by the institutional review boards at both institutions, and consent was obtained where required.
We reviewed all included patients for any evidence of relapse of disease as well as for the method of relapse detection using clinician notes and radiologist reports. Among those patients with a detected relapse, the means of relapse detection were categorized as: clinical presentation; detection by surveillance imaging without associated signs or symptoms; or incidental finding following the methods described by Thompson et al. [10]. Detection of relapse by clinical presentation was defined as initial evidence of relapse stemming from a patient-reported symptom, physical exam finding, or abnormal lab value performed as part of a clinic visit. Relapse detection by surveillance imaging was defined as relapse identified by concerning findings on routine imaging in patients without any documented clinical signs or symptoms. Relapse detection by incidental finding was determined if testing or imaging performed for an unrelated complaint showed findings concerning for relapse, with subsequent testing confirming the presence of a true relapse. Neither of the two institutions participating had an institutional policy regarding surveillance imaging, and the schedule for such imaging was left to the discretion of the treating clinician. Data regarding the planned surveillance schedule employed for each patient were not consistently available for analysis, but typically imaging was more frequent in the two years after remission consistent with NCCN guidelines [13].
Review of surveillance images
We reviewed reports from all imaging studies available at either academic institution performed after the documented date of treatment response and before the documented date of relapse or before the last date of follow up if no relapse of disease was documented. Imaging studies were determined to be either for surveillance purposes, as a response to a clinical sign/symptom, or as an evaluation for an unrelated purpose based on the radiology report and clinician notes. In this same manner, studies were categorized by imaging type, study indication, and final radiologic diagnosis.
Statistical considerations
There were three primary goals of the study. The first was to describe the disease course for MCL in first remission including patterns of relapse and methods of relapse detection. The second was to describe the basic performance characteristics of radiology imaging by assessing the rate of positive studies and the PPV of PET/CT and CT surveillance strategies. As MCL is such a heterogeneous disease and because surveillance imaging in remission is not well studied or understood, no data are available to guide in the definition of a false negative surveillance image. As such, this information was not collected and therefore imaging characteristics including negative predictive value, specificity, and sensitivity were not determined. The third goal was to determine whether any method of relapse detection was associated with a difference in OS from the date of first diagnosis of disease as well as from date of relapse detection.
Patients included in the study were stratified by whether they relapsed and by the method of relapse diagnosis. Demographic and clinical variables were tested between these subgroups using two-sided Student’s t-test for binary variables and by the Mann–Whitley U test for categorical ordinal variables with significant defined at an alpha = 0.05. Patients were stratified into two groups based on method of relapse diagnosis, and survival analysis was performed using the Kaplan–Meier plots and hazard ratio (HR) analysis with significant defined at an alpha = 0.05. No adjustments for multiple testing were made as only one primary outcome involved statistical testing.
Results
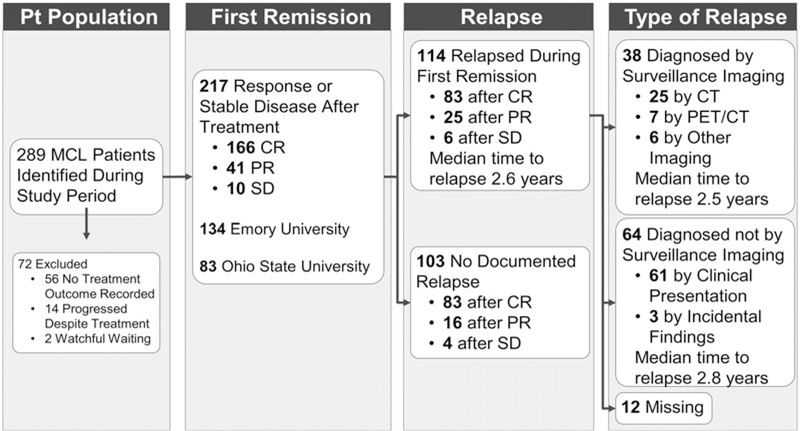
A total of 289 MCL patients were initially identified, and 217 were included in the study. Of the 72 patients excluded, 56 were excluded because of inadequate treatment outcome data, 14 were excluded because they progressed on initial therapy, and two were excluded because they underwent watchful waiting with no treatment recorded. A total of 134 patients were from Emory University and 83 from the Ohio State University. The pattern of disease and relapse is further illustrated in Figure 1.
Figure 1.
Flowchart of MCL patient selection and disease course of selected patients following first remission.
Patient characteristics are summarized in Table 1. Consistent with previously described demographics in MCL, most patients were male, and median age was 59 years (range: 33–81). Eighty-five (29%) patients received R-HyperCVAD (rituximab, hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone alternating with methotrexate and cytarabine), and 118 (54%) patients underwent ASCT in first complete or partial remission. Patients had observations for a median of 4.5 years (range 0.3–21.7) after date of diagnosis. Cellular characteristics were not available for the majority of patients. Among those for whom it was, MIPI risk score was equally distributed between low, intermediate, and high risk while half of patients had a high Ki-67 proliferative index.
Table 1.
Characteristics of patients identified with documented remission after first treatment.
| All | Relapse recorded during first remission | No relapse recorded during first remission | Relapse diagnosed by surveillance imaging | Relapse not diagnosed by surveillance imaging | |
|---|---|---|---|---|---|
| N = 217 | N = 114 | N = 103 | N = 38 | N = 64 | |
| Median age at diagnosis and range (years)* | 60.6 (33–81) | 59.8 (33–81) | 61.6 (36–81) p = .298 |
59.8 (34–73) | 59.6 (33–80) p = .566 |
| Gender* | |||||
| Male | 170 (78%) | 91 (80%) | 79 (77%) | 29 (76%) | 53 (83%) |
| Female | 47 (22%) | 23 (20%) | 24 (23%) p = .579 |
9 (24%) | 11 (17%) p = .429 |
| Ethnicity* | |||||
| White | 111 (86%) | 60 (90%) | 51 (82%) | 15 (83%) | 38 (93%) |
| Non-White | 18 (14%) | 7 (10%) | 11 (18%) | 3 (17%) | 3 (7%) |
| Missing | 88 | 47 | 41 p = .236 |
20 | 23 p = .282 |
| ECOG Score† | |||||
| 0 | 79 (48%) | 37 (43%) | 42 (55%) | 14 (47%) | 19 (40%) |
| 1 | 73 (45%) | 41 (48%) | 32 (42%) | 14 (47%) | 24 (50%) |
| 2 | 9 (6%) | 7 (8%) | 2 (3%) | 1 (3%) | 5 (10%) |
| 3 | 2 (1%) | 1 (1%) | 1 (1%) | 1 (3%) | 0 (0%) |
| 4 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Missing | 54 | 28 | 26 p = .097 |
8 | 16 p = .497 |
| Ann Arbor Score† | |||||
| 1 | 5 (2%) | 3 (3%) | 2 (2%) | 0 (0%) | 3 (5%) |
| 2 | 7 (3%) | 2 (2%) | 5 (5%) | 1 (3%) | 0 (0%) |
| 3 | 13 (6%) | 9 (8%) | 4 (4%) | 1 (3%) | 6 (10%) |
| 4 | 182 (88%) | 96 (87%) | 86 (89%) | 33 (94%) | 54 (86%) |
| Missing | 10 | 4 | 6 p = .812 |
3 | 1 p = .201 |
| B-symptoms at diagnosis* | |||||
| Yes | 62 (33%) | 40 (42%) | 22 (24%) | 12 (38%) | 25 (45%) |
| No | 124 (67%) | 56 (58%) | 68 (76%) | 20 (62%) | 31 (54%) |
| Missing | 31 | 18 | 13 p = .013 |
6 | 8 p = .519 |
| Splenomegaly* | |||||
| Yes | 85 (55%) | 53 (70%) | 32 (41%) | 16 (62%) | 32 (76%) |
| No | 70 (45%) | 23 (30%) | 47 (59%) | 10 (38%) | 10 (34%) |
| Missing | 62 | 38 | 24 p< .001 |
12 | 22 p = .203 |
| Bulky disease (any lymph node >5 cm present)* | |||||
| Yes | 40 (27%) | 27 (41%) | 13 (16%) | 13(50%) | 11 (33%) |
| No | 107 (73%) | 39 (59%) | 68 (84%) | 13 (50%) | 22 (67%) |
| Missing | 70 | 48 | 22 p<.001 |
12 | 31 p =.202 |
Variables tested for variation with two-sample t-test.
Variables tested with the Mann–Whitley U test. Ki-67 percent, cytogenetic abnormalities, and MIPI risk group were also analyzed but were not presented due to high rates of missing data.
p < .05 are marked in bold.
One hundred forty-one (65%) patients underwent at least one surveillance imaging exam at either academic institution, with a median of five imaging studies (range 1–19) obtained during monitoring after first remission. Patients received a median of 1.6 scans (range 0.1–4.7) per year of observation while in first remission. Rates were not significantly different between the two academic institutions and did not vary greatly over time based on date of original diagnosis.
One hundred fourteen (53%) patients suffered a documented relapse during clinical monitoring. The method of relapse detection could not be determined for 12 of those patients. Those who relapsed during observation were significantly more likely at diagnosis to have B-symptoms, splenomegaly, or bulky disease (largest lymph node >5 cm in largest dimension). Otherwise, there were no other significant differences in demographic or clinical variables for patients who relapsed (Table 1). Over the study’s time period, date of original diagnosis was associated with type of relapse diagnosis, with patients diagnosed prior to 2000 experiencing lower rates of diagnosis by surveillance imaging. However, this difference was not statistically significant.
Relapse was identified by surveillance imaging alone without any other documented clinical suspicion of relapse in 38 (37%) of the 102 patients with known method of relapse, while 64 (63%) presented initially with symptoms or signs concerning for relapse. There were no differences in assessed baseline characteristics among those patients whose relapse was detected by surveillance as compared to patients with a clinical presentation of relapse (Table 1).
Methods of surveillance and performance of radiographic studies
Among all patients, 863 radiographic studies were available for review (Table 2). Routine surveillance was the indication for 801 of the studies; 59 were obtained following clinical presentation concerning for relapse; and three were performed for other indications with incidental findings concerning for MCL relapse. Of the 801 studies obtained for routine surveillance, 587 (73%) were CT scans and 205 (26%) were PET/CT scans. For CT scans, 49 out of 587 (8%) scans were read as concerning or definitive for relapse, and 24/49 led to a diagnosis of relapsed disease confirmed by biopsy or further imaging, leading to a PPV of 49%. Only 4% of scans obtained for surveillance purposes ultimately resulted in a diagnosis of relapse, with a number needed-to-scan/treat (NNT) of 24 surveillance CT scans to detect one asymptomatic relapse. For PET/CT scans, 18 out of 205 (8%) PET/CT scans were read as concerning or definitive for relapse, and 4/18 eventually led to a true diagnosis of relapsed disease for a PPV of 22%. The other 14/18 failed to show evidence of true relapse by biopsy (six patients), by repeat PET/CT imaging (four patients), by colonoscopy (one patient), or by watchful waiting (three patients) as ordered by the treating clinician. Watchful waiting for these three patients included two patients who went at least 2 years of observation without a diagnosis of relapse and one patient who died from causes unrelated to MCL 10 months after their PET/CT scan without a diagnosis of relapse. Only 2% of scans obtained for surveillance resulted in a diagnosis of relapse, with a NNT of 51 surveillance PET/CT scans to detect one asymptomatic relapse.
Table 2.
Characteristics and performance metrics of radiology studies available at study sites for patients during first remission, stratified by image type and indication.
| Surveillance imaging only, all types | Surveillance imaging, CT | Surveillance imaging, PET/CT | Surveillance imaging, MRI | |
|---|---|---|---|---|
| N = 801 | N = 587 | N = 205 | N = 9 | |
| Radiologist diagnosis of study | ||||
| No concerning findings | 726 (91%) | 534 (91%) | 184 (90%) | 8 (89%) |
| Concerning or positive for relapse | 68 (8%) | 49 (8%) | 18 (9%) | 1 (11%) |
| Non-diagnostic | 5 (1%) | 2 (<1%) | 3 (1%) | 0 (0%) |
| Other | 2 (<1%) | 1 (<1%) | 0 (0%) | 0 (0%) |
| Total true positive studies | 29 (4%) | 24 (4%) | 4 (2%) | 1 (11%) |
| Positive predictive value | 43% | 49% | 22% | 100% |
CT: computerized tomography; PET/CT: positron emission tomography with computerized tomography overlay.
Outcomes for relapsed patients based on method of detection
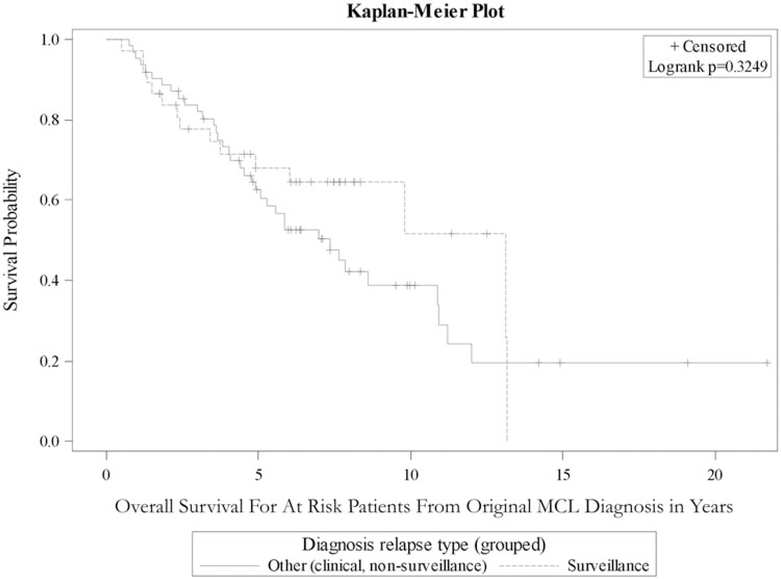
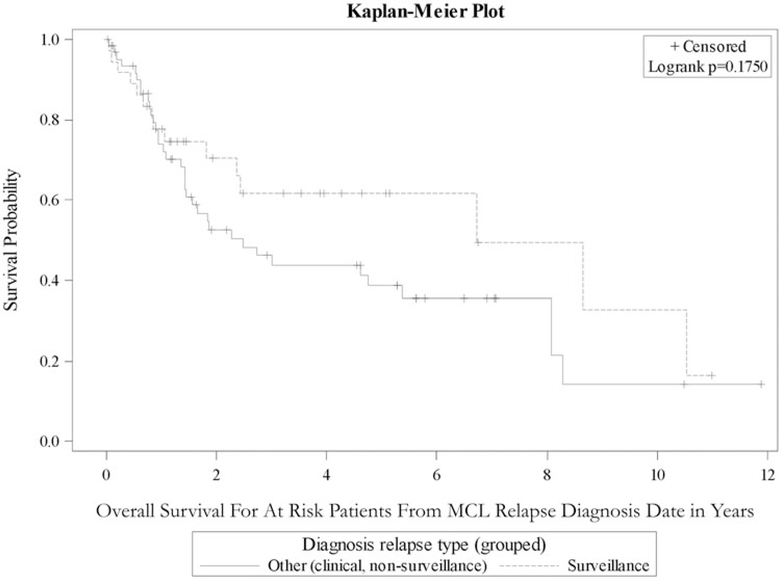
Table 3 outlines survival analysis from time of original MCL diagnosis for demographic and clinical variables for patients who had a documented relapse during the study period. Among patients whose MCL relapsed during first remission, there was not a statistically significant difference in OS between those diagnosed by surveillance imaging and those diagnosed by clinical presentation or incidental finding in the Emory cohort, the OSU cohort, or the combined cohort (HR 0.74, 95% CI 0.40–1.35; Figure 2). Additionally, there was no significant difference in OS from relapse diagnosis date based on method of relapse detection (Figure 3).
Table 3.
Covariate analysis of overall survival from time of diagnosis for patients with a documented relapse during first remission.
| Covariate | Level | N | Hazard ratio (95% confidence interval) | p Value |
|---|---|---|---|---|
| Age at diagnosis | Risk per year | 98 | 1.03 (1.01–1.06) | .023 |
| Gender | Female | 20 | 1.10 (0.56–2.14) | .789 |
| Male | 79 | |||
| Ethnicity | White | 50 | 1.28 (0.37–4.45) | .692 |
| Non-white | 6 | |||
| Splenomegaly | Yes | 48 | 1.72 (0.80–3.69) | .164 |
| No | 20 | |||
| Bulky disease (any lymph node >5 cm) | Yes | 24 | 0.74 (0.34–1.57) | .426 |
| No | 35 | |||
| Ki67% | >30% | 15 | 0.61 (0.18–2.10) | .433 |
| MIPI risk group | ≤30% High |
9 9 |
2.02 (0.70–5.82) | .193 |
| Intermediate | 15 | 0.95 (0.33–2.70) | .918 | |
| Low | 13 | |||
| Relapse diagnosis type | Surveillance | 37 | 0.74 (0.40–1.35) | .327 |
| Non-surveillance | 62 |
p< .05 are marked in bold.
Figure 2.
The Kaplan–Meier curve showing overall survival from date of first diagnosis in MCL patients who suffered relapse after first remission, stratified by method of relapse diagnosis.
Figure 3.
The Kaplan–Meier curve showing overall survival from date of confirmed relapse in MCL patients who suffered relapse after first remission, stratified by method of relapse diagnosis.
All of the findings above were also separately analyzed in each individual academic institution with similar results. In both institutions, OS and survival from date of relapse were not significantly associated with method of relapse diagnosis, and both PET/CT and CT imaging modalities had low PPV and high NNT to detect one additional asymptomatic relapse (see Supplement).
Discussion
We examined the clinical utility of surveillance CT and PET/CT imaging in a large cohort of patients with MCL at an academic medical center and validated these findings in a second large cohort. We conclude that there is no survival advantage when relapse after first-line therapy is detected by surveillance imaging and that the use of routine surveillance imaging in MCL in first remission should be discouraged. Most patients experienced relapse within the study period with over one-third of these were diagnosed by surveillance imaging. Nonetheless, this provided no significant survival benefit for these patients and was likely associated with significant financial and emotional cost. Thus, the role of imaging should be limited to judicious use to confirm potential relapse when clinically suspected and to restaging patients with documented relapse, when appropriate.
When comparing the metrics of the different imaging modalities for surveillance, both performed poorly with PET/CT performing more poorly than CT alone. Due to a higher number of false positive results, PET/CT imaging had a relatively low PPV of 22% compared to 49% for CT imaging alone. In addition, the NNT to detect one asymptomatic relapse was 51 surveillance PET/CT studies compared to 24 for CT alone. Given the excess cost in time and money associated with PET/CT imaging, our results suggest that the role for routine PET/CT in the surveillance of MCL patients is limited in the absence of a unique clinical indication for a particular patient. Like for other forms of NHL and for many other malignancies, PET/CT should be reserved for staging/restaging and response assessment.
With regard to lymphoma, research efforts aimed at reducing or eliminating surveillance imaging have been directed at diseases with good outcomes in relapse. Our study is unique in that MCL has poor outcomes in relapse. In particular, the results of this study are comparable to similar studies in DLBCL but still show some fundamental differences. The MCL patients studied had a much higher incidence of relapse detection by surveillance imaging compared to DLBCL, likely related to the propensity for MCL to relapse at a higher rate than DLBCL even in patients achieving a CR or PR. Despite this, MCL patients who experienced relapse did not gain a survival benefit from surveillance imaging. Further investigation into this cohort would be required to fully understand why, but it could reflect that treatment options for relapsed MCL are limited and may not affect survival. It could also be that early detection of asymptomatic relapse may not be critical because it detects a more indolent subset of relapsed patients that would be reasonably managed by observation and who would not benefit from early initiation of salvage therapy.
Our study is limited by the heterogeneous therapies implemented over the 22-year timeline covered by this study, which could affect the treatment each patient received, how remission was diagnosed, and the methods and criteria for diagnosis relapse. In addition, these patients experienced a variety of surveillance imaging schedules and modalities, reflecting the lack of a standard approach to surveillance in this disease. The cohort followed is also only 217 total patients with only 102 experiencing a relapse during observation. Although this is the largest cohort to date evaluated in this way, the size of the cohort likely makes it difficult to detect minor differences in survival, and this study would benefit from validation in other cohorts. Additionally, the paucity of prognostic information for patients at the time of initial diagnosis, specifically the lack of Ki67 percentages and MIPI scores, does not allow the cohort to be evaluated for significant differences in baseline characteristics relative to method of relapse diagnosis method.
Another potential limitation of this design evaluating a screening test is lead-time bias, where the survival after relapse diagnosis appears improved after earlier detection because relapsed disease is diagnosed in an earlier stage. However, we evaluated OS from time of original diagnosis, which would not be susceptible to this bias (Figure 2). In addition, such a lead-time bias would have been apparent in an improved OS from time of relapse diagnosis (Figure 3). While Figure 3 shows some visual separation, it still shows an insignificant difference in survival in agreement with Figure 2.
In summary, while great progress has been made in the diagnosis and treatment of MCL producing high remission rates and prolonged remission periods, successfully treated patients remain at significant risk of relapse even 15 years after treatment, as shown by this study and by others [2]. Prolonged radiographic surveillance is expensive, of unclear benefit, and associated with known risks of radiation and patient anxiety [9]. Even if relapse can be diagnosed, there is a need for improved therapies before meaningful advances in survival after relapse can be achieved. As the options for salvage therapy change in the future, the issue of early detection of asymptomatic relapse will likely change. However, in the current landscape of MCL in remission, the results of our study provide clinicians evidence to change practice patterns and to avoid routine surveillance radiologic imaging in patients with MCL in favor of alternative surveillance strategies with better supporting data. One such alternative approach is molecular monitoring of minimal residual disease in the blood or bone marrow, which has been demonstrated to predict OS in MCL patients in first remission [14–16] but which has not been widely adopted yet. While further evaluation is required to confirm our findings and to determine the optimal surveillance strategy, this analysis generated the largest retrospective study of patients with MCL undergoing surveillance and still provided no evidence for clinical benefit for surveillance imaging. This is the strongest support yet for a recommendation to avoid imaging as a method of surveillance for patients with MCL in first remission.
Supplementary Material
Acknowledgements
Thanks to Dr. David Guidot for his contributions in reviewing the manuscript.
This work was previously presented in poster format at the American Society of Hematology annual meeting on 3 December 2016.
Funding
This study is supported in part by grants from the American Society of Hematology and Lymphoma Research Foundation (JBC). Research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article online at https://doi.org/10.1080/10428194.2017.1361032.
References
- [1].Nastoupil LJ, Shenoy PJ, Ambinder A, et al. Intensive chemotherapy and consolidation with high dose therapy and autologous stem cell transplant in patients with mantle cell lymphoma. Leuk Lymphoma. 2015;56:383–389. [DOI] [PubMed] [Google Scholar]
- [2].Eskelund CW, Kolstad A, Jerkeman M, et al. 15-Year follow-up of the second Nordic mantle cell lymphoma trial (MCL2): prolonged remissions without survival plateau. Br J Haematol. 2016;175:410–418. [DOI] [PubMed] [Google Scholar]
- [3].Delarue R, Haioun C, Ribrag V, et al. CHOP and DHAP plus rituximab followed by autologous stem cell transplantation in mantle cell lymphoma: a phase 2 study from the Groupe d’Etude des Lymphomes de l’Adulte. Blood. 2013;121:48–53. [DOI] [PubMed] [Google Scholar]
- [4].Cohen JB, Ruppert AS, Heerema NA, et al. Complex karyotype is associated with aggressive disease and shortened progression-free survival in patients with newly diagnosed mantle cell lymphoma. Clin Lymph Myeloma Leuk. 2015;15:278–285. [DOI] [PubMed] [Google Scholar]
- [5].Cheah C, Seymour JF, Wang ML. Mantle cell lymphoma. J Clin Oncol. 2016;34:1256–1269. [DOI] [PubMed] [Google Scholar]
- [6].Hosein PJ, Pastorini VH, Paes FM, et al. Utility of positron emission tomography scans in mantle cell lymphoma. Am J Hematol. 2011;86:841–845. [DOI] [PubMed] [Google Scholar]
- [7].Brepoels L, Stroobants S, De Wever W, et al. Positron emission tomography in mantle cell lymphoma. Leuk Lymphoma. 2008;49:1693–1701. [DOI] [PubMed] [Google Scholar]
- [8].Gill S, Wolf M, Prince HM, et al. [18F]fluorodeoxyglucose positron emission tomography scanning for staging, response assessment, and disease surveillance in patients with mantle cell lymphoma. Clin Lymph Myeloma. 2008;8:159–165. [DOI] [PubMed] [Google Scholar]
- [9].Cheung MC, Mittmann N, Earle C, et al. Are we choosing wisely in lymphoma? Excessive use of surveillance CT imaging in patients with diffuse large b-cell lymphoma (DLBCL) in long-term remission. Blood. 2016;128:692. [DOI] [PubMed] [Google Scholar]
- [10].Thompson CA, Ghesguieres H, Maurer MJ, et al. Utility of routine post-therapy surveillance imaging in diffuse large B-cell lymphoma. JCO. 2014;32:3506–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cohen JB, Behera M, Thompson CA, et al. Evaluating surveillance imaging for diffuse large B-cell lymphoma and Hodgkin lymphoma. Blood. 2017;129(5):561–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano Classification. JCO. 2014;32: 3048–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zelenetz AD, Gordon LI, Wierda WG, et al. Non-Hodgkin’s lymphomas, version 4. J Natl Comprehens Cancer Netw. 2014;12:1282–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kolstad A, Pedersen LB, Eskulund CW, et al. Molecular monitoring after autologous stem cell transplantation and preemptive rituximab treatment of molecular relapse; results from the Nordic mantle cell lymphoma studies (MCL2 and MCL3) with median follow-up of 8.5 years. Biol Blood Bone Marrow Transplant. 2017;23:428–435. [DOI] [PubMed] [Google Scholar]
- [15].Holster E, Pott C. Minimal residual disease in mantle cell lymphoma: insights into biology and impact on treatment. Hematology Am Soc Hematol Educ Prog. 2016;2016(1):437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cowan AJ, Stevenson PA, Cassaday RD, et al. Pretransplantation minimal residual disease predicts survival in patients with mantle cell lymphoma undergoing autologous stem cell transplantation in complete remission. Biol Blood Bone Marrow Transplant. 2016;22:380–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.