Abstract
γ-aminobutyric acid type B (GABAB) receptors are broadly expressed in the nervous system and play an important role in neuronal excitability. GABAB receptors are G protein-coupled receptors that mediate slow and prolonged inhibitory action, via activation of Gαi/o-type proteins. GABAB receptors mediate their inhibitory action through activating inwardly rectifying K+ channels, inactivating voltage-gated Ca2+ channels, and inhibiting adenylate cyclase. Functional GABAB receptors are obligate heterodimers formed by the co-assembly of R1 and R2 subunits. It is well established that GABAB receptors interact not only with G proteins and effectors but also with various proteins. This review summarizes the structure, subunit isoforms, and function of GABAB receptors, and discusses the complexity of GABAB receptors, including how receptors are localized in specific subcellular compartments, the mechanism regulating cell surface expression and mobility of the receptors, and the diversity of receptor signaling through receptor crosstalk and interacting proteins.
Keywords: GABAB receptors, G protein-coupled receptors, di-/oligomerization, trafficking, interacting proteins, posttranslational modification
Introduction
γ-aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the central nervous system (CNS). As many as one-third of CNS neurons in the brain use GABA as their primary neurotransmitter.1,2) Most of these neurons are interneurons, which are capable of altering the excitability of neural circuits by regulating glutamatergic neurons and preventing hyperexcitation. GABA provides strong inhibitory effects by acting on two distinct classes of receptors based on their physiological and pharmacological properties. GABA type A (GABAA) receptor is a ligand-gated chloride channel which mediates fast inhibitory signals through rapid postsynaptic membrane hyperpolarization,2) whereas the metabotropic GABAB receptor produces slow and prolonged inhibitory signals via G proteins and second messengers.3) Altered GABAB receptor function has been reported in a variety of neurological and psychiatric disorders, including epilepsy, depression, drug addiction, cognition, and nociception. This review will summarize our current knowledge of GABAB receptor structure, function, and binding partners, and how GABAB receptor trafficking is modulated by posttranslational modification. In addition, the relevance of GABAB receptors in various diseases will be discussed, along with current therapeutic attempts with GABAB receptor drugs.
1. Structure of GABAB receptors
GABAB receptors were first identified by Dr. Norman Bowery in 1979 as a receptor that reduces norepinephrine release through bicuculline- and isoguvacine-insensitive receptors.4,5) The first GABAB receptor was only cloned in 1997, nearly 20 years after their discovery.6) GABAB receptors are members of class C G protein-coupled receptor (GPCR) family. GPCRs are commonly divided into four classes (A, B, C, and F) based on the sequence homology levels of their transmembrane domains,7) and class A is by far the largest and most studied GPCRs. Class C GPCRs are composed of metabotropic glutamate receptors (mGluRs), GABAB receptors, Ca2+-sensing receptors, taste receptors, pheromone receptors, and several orphan receptors.8) GABAB receptors and taste receptors are obligatory heterodimers, whereas others are traditionally considered to function as homodimers, although recent studies discovered the assembly of class C GPCRs with other classes of GPCRs.9,10)
GABAB receptors are prototypical heterodimers of R1 and R2 subunits.11–13) GABAB receptor subunits are composed of three domains: a long extracellular N-terminal domain called Venus flytrap domain (VFT), which contains the orthosteric binding site for GABA; a heptahelical transmembrane domain (7TM); and a C-terminal intracellular tail (Fig. 1). Among these domains, the three-dimensional structures have been solved for the extracellular N-terminal domain and a fragment of the C-terminal intracellular tail.14–16) The VFT of R1 subunits binds to orthosteric ligands but not R2 subunits, although R2 subunits share 54% similarity with R1 subunits.17,18) Instead, R2 subunits couple with G protein to produce G protein-mediated signaling.19–21) Therefore, it is necessary for GABAB receptors to form R1/R2 heterodimers to produce GABA-mediated GPCR functions. The VFT is a shared structural feature among all class C GPCRs and is also found in bacterial periplasmic binding proteins.8,22) The existence of numerous alternatively spliced variants of GABAB receptor subunits have been described.3) R1 subunits comprise several splice variants designated as R1a, R1b, R1c, R1e, R1j, R1k, R11, R1m, and R1n.23) In the human CNS, the two major splice variants are the R1a and R1b isoforms, which have been studied intensively and are known to provide the molecular diversity of GABAB receptors.3,24) Structurally, the isoforms differ in their N-terminal domain, with a pair of sushi domains present in R1a (961aa) but not in R1b (844aa) (Fig. 1).25) Sushi domains have been found in several GPCRs26) and can mediate protein interactions in a wide variety of adhesion proteins.27) Possibly due to the presence of these sushi domains, R1a subunit-containing GABAB receptors are preferentially targeted to the axon terminals of excitatory synapses. Postsynaptically, both R1a and R1b isoforms are found in dendrites, but only the R1b subunit seems to localize in spine heads.28,29) Other splice variants of R1 subunits also exhibit some unique features. The R1c isoform has a single sushi domain and is widely expressed in the brain.30,31) The R1e/g/h/i/j/l/m/n isoforms do not have the 7 transmembrane domains, G-protein coupling region nor the C-terminal tail; therefore, they are thought to be secreted from cells. The R1e isoform (578aa), which is mainly expressed in peripheral tissues, strongly interacts with R2 subunits and disturbs normal R1/R2 heterodimer formation.32) Purified sushi domains of the R1j isoform impairs the inhibitory effect of GABAB receptors on evoked and spontaneous glutamate release.33) The R1g/h/i isoforms show similar sequences to R1j (190aa) containing sushi domains followed by a unique C-terminal sequence,34) but their function remains to be elucidated. Other isoforms such as R1d/f are mostly found in transcription expression profiles and so far, no function has been confirmed.34) Taken together, R1 subunit alternative splicing provides a diverse range of structural and functional GABAB receptors, and further studies are necessary to understand the physiological role of these isoforms.
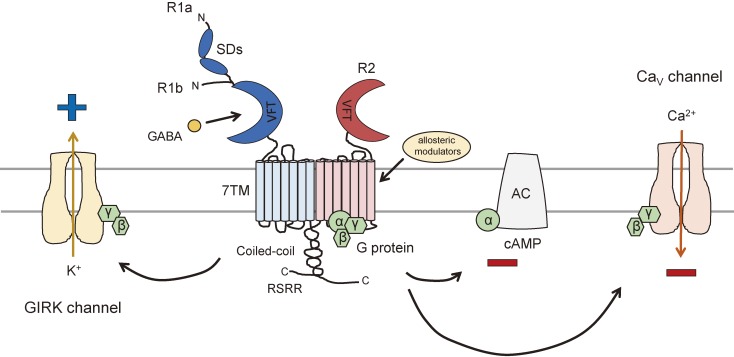
Figure 1.
Structural organization of GABAB receptors and the primary GABAB receptor effectors. Functional GABAB receptors form heterodimers composed of R1 and R2 subunits. Both subunits are heptahelical membrane proteins that have seven-transmembrane (7TM) domains with a large extracellular N-terminal domain containing a Venus flytrap (VFT) domain and a large intracellular C-terminal tail containing a coiled-coil protein–protein interaction module. The R1 subunit is responsible for ligand binding in the VFT domain, whereas the VFT of R2 subunit fails to bind any known ligands. Instead, the heptahelical domain of the R2 subunit contains a binding site for allosteric modulators, which affect the affinity of ligand binding to the R1 subunit. The interaction between the R1 and R2 subunits takes place at their C-terminus through the coiled-coil domains. The R1 subunit exists in two main isoforms. R1a is distinguished from R1b by the presence of two sushi domains (SDs). An endoplasmic reticulum (ER) retention signal (RSRR) is present distal to the coiled-coil domain in the R1 subunit and prevents the ER exit of R1 unless it is masked by an R2 subunit. The binding of GABA results in the recruitment and activation of Gαi/o proteins via the R2 subunit. The activated Gαi/o subunits inhibit adenylyl cyclase, resulting in lowered cAMP levels, while Gβγ subunits activate GIRK channels at postsynaptic sites and inhibit CaV channels at presynaptic sites, leading to neuronal inhibition.
The structure of a heterodimeric complex of R1b VFT and R2 VFT has been solved by X-ray crystallography.16) The two subunits bind in a side-by-side manner through non-covalent interactions between the N-terminal lobe structure within the VFT but facing the opposite direction.16) The VFT contains two lobes, LB1 and LB2, reaching further into the extracellular space, and LB1–LB1 interaction serves to facilitate heterodimer formation.15) In addition, GABAB receptor subunits lack the cysteine-rich region found at the C-terminal end of mGluR ectodomains, which are involved in the propagation of signals induced by the binding of orthosteric agonists to mGluRs.16,35)
Dimers, tetramers, or even higher-order oligomers of GABAB receptors can be detected both in heterologous systems and in native neurons.36–39) GABAB receptors are present in equilibrium between heterodimers and higher-order oligomers with a relative preference for tetramers (dimers of dimers) and octamers (tetramers of dimers).37) Of note, GABAB receptor heterodimers are stable due to strong non-covalent interactions, and the higher-order oligomers are the result of weaker and possibly transient interactions among heterodimers.37) Agonist stimulation does not alter receptor di-/oligomerization, but increases the lateral mobility of GABAB receptor complexes.37,40) Therefore, it is possible that the association and dissociation of GABAB receptors occurs in specific locations on the cell surface, and the dynamic re-arrangement of GABAB receptor complexes allow the expression of a variety of GABAB-mediated signaling pathways in various cellular locations.
2. GABAB receptor function
GABAB receptor-mediated signaling pathways involve one of three effector proteins: G protein-activated inwardly-rectifying K+ (GIRK) channels, voltage-gated Ca2+ (CaV) channels, and adenylyl cyclase (Fig. 1).3) The downstream effects of GABAB receptors include inhibition of neurotransmitter release and modulation of neuronal excitability.3) The GABAB receptors couple to pertussis toxin-sensitive G proteins (Gαi/o family).41) Following GABAB receptor activation, G proteins dissociate into their Gα and Gβγ subunits. The Gαi/o subunits inhibit adenylyl cyclase to reduce cyclic AMP (cAMP) levels, whereas Gβγ subunits inhibit Ca2+ channels and activate GIRK channels.42–44) It is known that Gαi/o proteins inhibit adenylyl cyclase types I, III, V, and VI, whereas Gβγ stimulates adenylyl cyclase types II, IV, and VII. This stimulation depends on the presence of Gαs, which results from the activation of GPCRs.45,46) GABAB receptor agonist stimulation inhibits basal and forskolin-stimulated neuronal adenylyl cyclase and reduces intracellular cAMP levels.47) However, it has been reported that the activation of GABAB receptors can enhance cAMP formation through Gs-coupled GPCR activation.48) Both the inhibition and enhancement of cAMP levels by GABAB receptors have been confirmed in vivo.49) GABAB receptors have been implicated in synaptic plasticity and memory formation.50) Because cAMP-dependent protein kinase (PKA) expresses a specific form of synaptic plasticity, which is associated with hippocampal long-term memory,51) the cAMP-PKA signaling pathways regulated through GABAB receptors are likely to be a mechanism for fine-tuning synaptic plasticity.
CaV channels mediate calcium influx in response to membrane depolarization, thus regulating intracellular processes such as muscle contraction, release of hormones and neurotransmitters, excitation of neurons, and gene expression.52) One of the first confirmed ion channel effectors of the GABAB receptor is the CaV channel. GABAB receptors decrease calcium conductance in neuronal membranes, and this action appears to be linked primarily with presynaptic receptors.53) Presynaptic GABAB receptors inhibit the opening of CaV channels, mainly N-type (CaV2.2) and P/Q-type (CaV2.1), through Gβγ subunits to repress calcium influx and trigger neurotransmitter release.54) CaV channels are formed as a complex of several different subunits, α1, α2δ, β1-4, and γ. The structural subunit of CaV channels is α1, which forms an ion channel pore and regulates ion gating properties.55) The electrophysiological and pharmacological diversity of CaV channels also arises from the existence of α1 subunits, which encode at least 10 distinct genes that are further divided into three subfamilies (CaV1, CaV2, and CaV3).56) The CaV1 subfamily includes CaV1.1, CaV1.2, CaV1.3, and CaV1.4, which are known as L-type channels, are typically high voltage-activated and dihydropyridine-sensitive. CaV2.1, CaV2.2, and CaV2.3 are high voltage-activated and dihydropyridine-insensitive channels mediating P/Q-type, N-type, and R-type Ca2+ currents. CaV3 channels CaV3.1, CaV3.2, and CaV3.3 are low voltage-activated and dihydropyridine-sensitive channels, which are called T-type for their transient currents production.52) L-type and T-type CaV families are expressed in many cell types, whereas N-, P/Q-, and R-types are predominantly expressed in neurons. There is also some evidence suggesting that GABAB receptors inhibit N-type and P/Q-type CaV channel subtypes at postsynaptic sites.57) These channels are likely to have a role in the generation of dendritic spikes and the amplification of excitatory postsynaptic potentials.58)
GIRK channels are widely expressed within the CNS and constitute a key determinant of membrane excitability because they mediate the postsynaptic inhibitory effects of many neurotransmitters, including GABAB receptors.59) When activated, postsynaptic GABAB receptors increase potassium conductance in neuronal membranes by opening GIRK channels to promote K+ efflux (Fig. 1).60) This reaction occurs through Gβγ subunits, resulting in a hyperpolarization of the neuron that underlies slow and prolonged inhibitory postsynaptic potentials.59) There is also a convincing evidence that Gα subunits can directly interact with intracellular domains of GIRK channels and control their gating.61,62) In mammals, there are four different GIRK channel subunits (GIRK1, GIRK2, GIRK3, and GIRK4); each consists of two transmembrane spanning domains with both the N- and C-terminus on the intracellular side of the membrane, and a pore domain located between the two transmembrane domains.63) These GIRK channel subunits form functional homotetrameric or heterotetrameric channels.64,65) Three GIRK channel subunits (GIRK1, GIRK2, and GIRK3) exhibit broad distributions in the CNS, whereas GIRK4 expression is found primarily in the heart.66) Consisting of a functional interaction between GIRK channels and GABAB receptors, these two receptors are highly colocalized in dendritic spines,67) and the oligomerization of GABAB receptors with GIRK channels (GIRK1 and GIRK3) has also been reported.68)
3. Pharmacology of GABAB receptors
The ligands for GABAB receptors can be divided into three types, agonists, antagonists, and allosteric modulators. Baclofen, an analogue of GABA, was the first selective agonist, which was synthesized in 1962, to enhance blood-brain barrier (BBB) penetration.3) Up to now, baclofen is the only drug that targets GABAB receptors on the market, and it is used as a muscle relaxant to treat spasticity due to spinal cord injury, cerebral palsy, and multiple sclerosis.69) Phenibut, a deschloro analogue of baclofen, was developed in the Soviet Union in the 1960s and introduced as a neuropsychotropic drug.70) It is also a potent blocker of α2δ subunit-containing voltage-dependent calcium channels.71) Today, phenibut is marketed for medical use in Russia, Ukraine, and Latvia, but is not approved for clinical use in the United States and in most of European countries. The anticonvulsant and analgesic drug gabapentin interacts with the sushi domain of R1a/R2 heterodimer.72–74) However, the inhibitory effect of gabapentin was found to be independent of the activation of GABAB receptors.75–78) The phosphinic acid analogue of GABA, 3-aminopropyl-phosphonic acid (CGP27492) and its methyl homolog CGP35024 are more potent than baclofen.79–81) Of note, CGP27492 and CGP35024 also act as antagonists for GABAC receptors, the newly identified members of the Cl−-permeable ionotropic GABA receptors that mediate slow and sustained neural inhibition.82–84) Other methyl phosphinic acid-based agonists include CGP44532 and its (R)-(+)-enantiomer CGP44533. CGP44532 has a longer lasting inhibitory effect than CGP44533 with more potent analgesic response than baclofen.85)
There is a rapid transition from γ-aminopropyl-methyl-phosphinic acid CGP35024 acting as a GABAB receptor agonist to its homolog γ-aminopropyl-ethyl-phosphinic acid CGP36216 acting as a GABAB receptor antagonist.79,86,87) It has been shown that CGP47656 increases the release of GABA on presynaptic GABAB autoreceptors in the rat neocortex but it also acts as a full agonist at presynaptic GABAB heteroreceptors by inhibiting the release of somatostatin.88) γ-Hydroxy-butyric acid (GHB) is a minor metabolite of GABA synthesized by GABA transaminase and succinic semialdehyde reductase. GHB is known to act as a weak GABAB receptor partial agonist and is used to treat excessive daytime sleepiness and cataplexy in patients with narcolepsy.89)
GABAB receptor antagonists that block slow inhibitory signaling have been developed.90) Notably, the first available selective GABAB receptor antagonists are all baclofen analogues, namely phaclofen, sacrofen, and 2-hydroxy-sacrofen. Although these antagonists display low potency, they cannot penetrate the BBB. The first GABAB receptor antagonists capable of penetrating the BBB were 3-aminopropyl-diethoxymethyl-phosphinic acid (CGP35348), 3-aminopropyl-butylphosphinic acid (CGP36742), 3-aminopropyl-cyclohexylmethyl-phosphinic acid (CGP46381), and 3-amino-2(R)-hydroxypropyl-cyclohexylmethyl-phosphinic acid (CGP51176). These antagonists are called first-generation GABAB receptor antagonists.69) Currently there are numerous GABAB receptor antagonists, which display IC50 values from the nanomolar to micromolar range and their therapeutic potentials are currently being studied.
Allosteric modulators are molecules that bind to a site on a neurotransmitter receptor that is topographically distinct from the orthosteric binding pocket for agonists.91) Allosteric agents have little or no intrinsic agonistic activity on their own but induce conformational changes in the receptor, and affect the interaction of receptors with agonists and associated proteins. Allosteric modulators of GABAB receptors constitute a good pharmacological alternative to gain selectivity for the treatment of various disorders, because of their unique structure. The GABAB receptor positive allosteric modulator (PAM) acts by stabilizing the active conformation of the 7TM domain in R2 subunits and thus induces the closure of the VFT domain in the R1 subunit, which is associated with GABAB activity (Fig. 1). The first available PAMs for GABAB receptors were 2,6-di-tert-butyl-4-(3-hydroxy-2,2-dimethyl-propyl)-phenol (CGP7930) and N,N′-dicyclopentyl-2-methylsulfanyl-5-nitropyrimidine-4,6-diamine (GS39783), both of which enhance agonist-stimulated responses by binding at the 7TM domain of the R2 subunit.91) CGP7930 shows antidepressant-like effects and reduces alcohol intake in rodents.92) CGP7930 directly acts as a PAM and a partial agonist through R2 subunits, which can facilitate agonist responses at low concentrations, and activate the receptor at higher concentrations.93–95) A more potent compound has also been identified, GS39783, which enhances GABAB receptor-mediated inhibition of cAMP formation and shows anxiolytic-like effects and attenuates rewarding properties of the substances of abuse.96–98)
4. GABAB receptor interacting proteins
GPCR function can be attributed to receptor interacting molecules that are expressed and function in distinct cell types.99) A number of interacting proteins for GABAB receptors have been identified (Fig. 2).99) These proteins are important not only for regulating receptor activity but also for modulating receptor trafficking. This section summarizes the types of proteins that interact with GABAB receptors and discusses their roles in GABAB receptor function (Table 1).
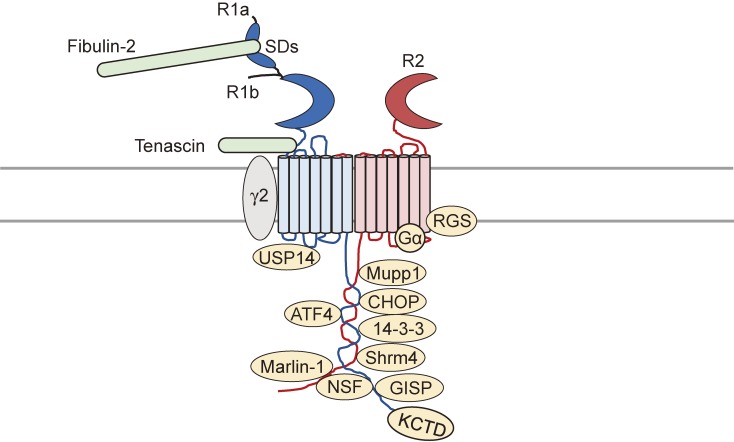
Figure 2.
GABAB receptor interacting proteins. A number of proteins have been found to interact with the C-terminus of GABAB receptor subunits. Among the interacting proteins are leucine-zipper transcription factors ATF4/CREB2 and CHOP, scaffolding and adaptor proteins 14-3-3, GISP, NSF, and PDZ domain-containing scaffold proteins Shrm4 and Mupp1. It is proposed that these proteins regulate receptor dimerization, intracellular trafficking, and synaptic localization. The C-terminus of the R2 subunit associates with KCTD proteins, which regulate CaV channel activity and GABAB receptor trafficking. The C-terminus of the R1 subunit associates with the brain-specific RNA binding protein Marlin-1 to target the cytoskeleton and regulate receptor transportation. Neurotransmitter receptors such as GABAA receptor γ2 subunit, mGluRs, and GIRK channels are also GABAB receptor binding partners although only the γ2 subunit has been identified to directly associate with R1 subunits so far. The N-terminus of R1 subunits also interact with proteins such as extracellular matrix protein fibulin-2 and tenascin. The extracellular sushi domains of the R1 subunit interact with fibulin-2, whereas tenascin binds to the extracellular domains of R1 subunits, possibly via the second transmembrane domain. Other proteins such as Gi/o proteins and RGS proteins bind to the R2 subunit to induce GPCR signaling.
Table 1.
Summary of GABAB receptor interacting proteins
| Proteins | Site of interaction | Function | References |
|---|---|---|---|
| Gi/o | R2 subunit second intracellular loop | Effector binding Essential for GABAB-mediated signaling | 3 101 |
| RGS | R2 subunit | GIRK channel signaling | 102–108 |
| ATF4/CREB2 | R1 subunit C-terminus | Receptor-mediated transcriptional regulation | 110 111 |
| CHOP | R2 subunit C-terminus R1a subunit | Receptor-mediated transcriptional regulation Accumulation of GABAB receptors in the ER | 112 |
| 14-3-3 | R1 subunit | Interfere R1/R2 heterodimerization | 118 |
| GISP | R1 subunit | Enhance GABAB receptor surface expression | 119 |
| NSF | C-terminus of R1 and R2 subunits | Enhance GABAB receptor signaling and trafficking | 120 |
| Shrm4 | R1 subunit C-terminus | Enhance GABAB receptor signaling and trafficking | 121 |
| Mupp1 | R2 subunit C-terminus | Modulate GABAB receptor stability and signaling | 122 |
| GABAA receptor γ2 subunit | R1 subunit | Enhance R1 subunit surface expression Enhance R1/R2 heterodimer internalization | 126 |
| mGluR1 | ND | GABAB receptor-mediated Ca2+ signaling Increase glutamate sensitivity of mGluR1 | 130 134 |
| GIRK channel | ND | Receptor signaling | 67 68 |
| KCTD | R2 subunit C-terminus | Reduces GABAB receptor internalization Increase S892 phosphorylation in R2 subunit | 145–148 |
| Marlin-1 | R1 subunit C-terminus | GABAB receptor transport? | 149 |
| Fibulin-2 | Sushi domain of the R1 subunit | Receptor anchoring | 25 |
| Tenascin | Extracellular domains of R1 subunit | Suppress postsynaptic GABAB receptor activity | 151 |
| USP14 | R1 subunit second intracellular loop | Regulates post-endocytic sorting of GABAB receptors | 170 |
ND: not determined.
G proteins and RGS proteins.
GABAB receptor-mediated signal transduction requires G proteins and G protein signaling (RGS) proteins.100) From biochemical studies, it is evident that GABAB receptors predominantly couple to Gαi- and Gαo-type G proteins.101) Activated receptors catalyze the exchange of guanosine diphosphate to guanosine triphosphate (GTP) on Gα subunits, promoting conformational changes of heterotrimeric G proteins (Gαβγ) and dissociation of Gα subunits from Gβγ subunits. The GTP-bound Gαi/o subunit then inhibits adenylyl cyclase, decreases intracellular cAMP levels and reduces PKA-mediated signaling.100) Gβγ subunits, on the other hand, couple with two types of ion channels, GIRK and Ca2+ channels. These channels modulate second messengers such as cAMP, diacylglycerol (DAG), or inositol trisphosphate (IP3) and regulate multiple signaling cascades.
It is well known that the family of RGS proteins are essential for GPCR-GIRK channel signaling pathway.102) RGS proteins negatively regulate GPCR signaling by serving as Gα GTPase-activating proteins.103) At least 37 RGS proteins have been identified in humans, with a conserved RGS homology domain that is crucial for GTPase activity.104) They are classified into eight subfamilies (RZ, R4, R7, R12, RA, GEF, GRK, and SNX) based on their structure and amino acid sequence similarities. RGS2 protein reduces GABAB-GIRK signaling sensitivity in dopaminergic neurons of the ventral tegmental area.105) RGS6 protein plays a role in motor coordination by modulating GABAB receptor signaling in the cerebellum.106) Furthermore, RGS4 protein directly associates with the R2 subunit of the GABAB receptor in the prefrontal cortex and hypothalamus.107) RGS4 protein also induces a faster form of desensitization within a second of agonist application in vitro.108)
Transcription factors.
Two transcription factors, activating transcription factor (ATF)/cAMP response element binding-protein (CREB) family and CCAAT/enhancer-binding protein (C/EBP) homologous protein (CHOP) have been reported to associate with GABAB receptor subunits.109–112) The C-terminus of R1 subunit interacts with the leucine zipper motif of ATF4/CREB2 and ATFx. Translocation of ATF4/CREB2 into or out of the nucleus is seen following GABAB receptor activation; however, the physiological significance of ATF4/CREB2 interaction with GABAB receptors is yet to be determined.110,111) The interaction of GABAB receptor and CHOP is reported to regulate GABAB receptor surface expression. The C-terminal leucine zipper of CHOP associates with the leucine zipper present in the C-terminal domain of R2 subunits, and the N-terminal domain of CHOP associates with an unidentified intracellular site of the R1a subunit.112) In HEK293 cells, overexpression of CHOP induces the accumulation of GABAB receptors in the endoplasmic reticulum (ER).112) Ischemia-mediated up-regulation of CHOP down-regulates cell surface GABAB receptors by preventing their trafficking from the ER to the plasma membrane, and disrupts GABAB receptor heterodimerization.113)
Nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) genes are thought to be the targets of GABAB-mediated transcriptional regulation. The production of both NGF and BDNF are enhanced following GABAB receptor antagonist stimulation.114) However, the linkage of GABAB receptor to the transcriptional factors such as a transduction signaling mechanism to the nucleus, still needs to be addressed.
Scaffolding proteins.
Scaffold proteins are crucial regulators of many key signaling pathways. It offers a simple and flexible mechanisms for regulating selectivity in signaling pathways, shaping output cellular behaviors, and achieving new responses.115) It is known that GPCRs also function as scaffolds for the recruitment of a variety of proteins that serve to modulate both G protein-dependent and -independent cellular signaling pathways, and regulate GPCR trafficking.116)
The C-terminus of the R1 subunit contains consensus motifs involved in binding to 14-3-3 proteins, small dimeric proteins (27–32 kDa) with seven highly conserved isoforms (β, γ, ζ, σ, ε, η, and τ).117) These proteins have been implicated in a variety of cellular processes, including regulation of synaptic transmission via K+ channels, GPCR-mediated signal transduction, and interactions with phosphoproteins.117) Only two isoforms of 14-3-3 proteins ζ and η interact with the R1 subunit of the GABAB receptors, and interfere with R1/R2 heterodimerization.118) A 130-kDa protein, GPCR interacting scaffolding protein (GISP), associates directly with the R1 subunit via a coiled-coil domain. GISP promotes GABAB receptor surface expression and enhances GIRK currents.119) The C-terminus of both R1 and R2 subunits interact with the scaffolding protein N-ethylmaleimide-sensitive factor (NSF), an ATPase that is critical for intracellular trafficking.120) Coordinated action of NSF and protein kinase C (PKC) regulates the activity of GABAB receptors.
The C-terminus of the R1 subunit contains a putative PDZ domain-binding consensus sequence. A recent study identified that Shrm4, a protein expressed only in polarized tissues and whose mutations have been linked to epilepsy and intellectual disability, interacts with the C-terminus of the R1 subunit and controls their cell surface expression and intracellular trafficking via a dynein-dependent mechanism.121) Shrm4 associates with both R1a and R1b subunits, and Shrm4 knockdown reduced the levels of both isoforms in dendrites. Because the R1a subunit preferentially localizes to axons via its sushi domains, only an R1a subunit that could escape from axonal targeting may associate with Shrm4 in the Golgi apparatus and be re-directed to dendrites.121) GABAB receptor R2 subunits possess a C-terminal motif VSGL that has the potential to interact with PDZ-domain-containing scaffold proteins. Biochemical analysis confirmed that Multi-PDZ domain protein 1 (Mupp1) interacts with R2 subunits and regulates GABAB receptor signaling as well as receptor stability.122)
Neurotransmitter receptors.
GABAB receptors interact with several neurotransmitter receptors and regulate receptor activity. Examples of such receptors are ionotropic GABAA receptors. Twenty-one GABAA receptor subunits have been cloned from the mammalian CNS. These have been divided into eight classes based on sequence identity: α(1–6), β(1–3), γ(1–3), δ, ε(1–3), π, θ, and ρ(1–3).123) The majority of GABAA receptor subtypes in the brain are composed with a likely stoichiometry of 2α:2β:1γ.124) To a lesser extent, δ/ε/π subunits replace the γ subunit to form benzodiazepine-insensitive receptor subtypes.125) The γ2 subunit of GABAA receptors was found to interact with the R1 subunits of GABAB receptors and promote R1 subunit surface expression in the absence of R2 subunits.126) On the other hand, the γ2 subunit associates with functional R1/R2 heterodimers and enhances GABAB receptor internalization in response to agonist stimulation.126) In contrast, the activation of GABAB receptors promotes BDNF secretion through increased phospholipase C (PLC)/DAG/PKC activation, and enhances GABAA receptor cell surface expression.127) Signaling crosstalk between GABAB and GABAA receptors has also been identified. In developing hypothalamic neurons, GABAB receptor activation depresses GABAA receptor-mediated Ca2+ elevation, both by reducing the presynaptic release of GABA and decreasing postsynaptic Ca2+ responses.128) In dentate gyrus granule cells, GABAB receptors colocalize with GABAA receptors on postsynaptic dendritic and somatic membranes, and GABAB receptor activity enhances tonic inhibition induced by extrasynaptic GABAA receptors.129)
mGluR1 is another receptor found to interact with GABAB receptors.130) mGluR1 belongs to class C type GPCRs as a GABAB receptor. It couples with Gq protein to increase IP3 production and Ca2+ signaling when activated by glutamate.131) Both receptors exhibit a high co-localization in the dendritic spine of Purkinje cells but no oligomerization of GABAB receptor and mGluR1a is observed, suggesting the existence of a GABAB-mGluR1 receptor complex but with no physical contact.132,133) Extracellular Ca2+ interacts with GABAB receptors in cerebellar Purkinje cells, leading to an increase in the glutamate sensitivity of mGluR1, and that extracellular Ca2+-mediated crosstalk is not mediated via Gαi/o proteins.134) Precise control of these two receptors is thought to be important for the balance of neuronal inhibition and excitation.
Although no physical contact or complex formation has been reported, there is functional crosstalk between GABAB receptors and ionotropic glutamate receptors. The major synaptic Ca2+ signals in the brain are mediated via N-methyl-D-aspartate (NMDA) receptors, which are crucial for activity-dependent changes in synaptic plasticity.135,136) Ca2+ influx via NMDA receptors is inhibited by GABAB receptor activation.137) This effect on NMDA receptors is independent of GIRK channel or CaV channel activation. There are several reports, including from the author, that NMDA receptors can regulate GABAB receptor endocytosis, trafficking and, degradation.138–140) NMDA receptor activation promotes GABAB receptor phosphorylation and dephosphorylation depending on the length of NMDA receptor activation, and regulates GABAB receptor cell surface expression.139) NMDA receptor-mediated regulation of GABAB receptors may be important in conditions of neurological diseases, such as epilepsy and ischemia. Another type of ionotropic glutamate receptor, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors are the main fast synaptic transduction elements and crucial for synaptic plasticity. It was found that enhanced GABAB receptor activity increases the number of excitatory synapses and cell surface AMPA receptors.50)
Functional crosstalk between GABAB receptors and receptor for tyrosine kinases (RTKs) has also been reported. GABAB receptors trigger the secretion of BDNF and subsequent activation of the tropomyosin-related receptor kinase B (TrkB) receptor signaling pathway to promote the development of GABAergic synapses.141) The GABAB receptor can also transactivate insulin-like growth factor-1 (IGF-1) receptor (IGF-1R) to induce Akt (protein kinase B) phosphorylation and protect cerebellar granule cells from apoptosis.142) Upon activation of GABAB receptors, Gαi/o and Gβγ subunits are released from GABAB receptors, followed by recruitment of focal adhesion kinase 1, IGF-1R, and Akt to GABAB receptors. This dynamic regulation of GABAB receptor-associated complex formation is critical for signal transduction and transactivation-dependent neuronal survival.143)
Other important binding proteins.
A recent study showed that GABAB receptors form macromolecular complexes with members of a subfamily of the potassium channel tetramerization domain-containing (KCTD) proteins. KCTD proteins consist of 26 members that share sequence similarity with the cytoplasmic domain of voltage-gated K+ (Kv) channels and have roles in various biological processes including transcriptional repression and cytoskeleton regulation.144) The KCTD protein family members KCTD8, KCTD12, KCTD12b, and KCTD16 are tightly associated with the C-terminus of GABAB receptor R2 subunit.145,146) This co-assembly changes the properties of GABAB receptors in a KCTD subtype-specific manner. For instance, KCTD16 and KCTD8 lead to the persistent inhibition of CaV channel activity, whereas KCTD12 and KCTD12b receptors transiently decrease CaV channel activity.146,147) Furthermore, KCTD12 reduces the constitutive receptor internalization to increase the magnitude of receptor signaling.148)
The C-terminus of the GABAB receptor R1 subunit associates with brain-specific RNA-binding protein Marlin-1, also designated as Jamip-1 or Jakmip1.149) The association of GABAB receptor and Marlin-1 was found in cytoskeleton, thus it is thought to regulate receptor transport.150) The N-terminus of the GABAB receptors also interacts with proteins such as fibulin-2. Fibulin-2 is an extracellular matrix protein that binds to the sushi domain of R1a subunit, but not R1b subunit.25) Because R1a and R1b isoforms of the R1 subunit have been shown to preferentially localize to different subcellular compartments, fibulin-2 may provide evidence for the existence of subtype-specific interacting proteins. Finally, there is some evidence that the HNK-1 carbohydrate carried by many neural extracellular matrix proteins, such as tenascin-R and tenascin-C, binds to an extracellular domain of R1 subunits.151) HNK-1 carbohydrate may be involve in homeostatic regulation of GABAA receptor-mediated perisomatic inhibition by suppressing postsynaptic GABAB receptor activity.151)
5. Posttranslational modification of GABAB receptors
Posttranslational modifications (PTMs) of proteins play an important role in cellular functions. PTM is the covalent addition of certain functional groups to proteins. More than 40 PTMs have been identified, and their relation to the diseases such as cancer and neurological disorders have been proposed. This section summarizes two major PTMs found in GABAB receptors, phosphorylation and ubiquitination, and their role in regulating GABAB receptor function.
Phosphorylation.
Protein phosphorylation is the most common and best studied PTM, in which protein function is regulated in response to extracellular stimuli both inside and outside the nervous system.152) Regulation of protein phosphorylation requires protein kinases, protein phosphatases, and substrate proteins. Phosphorylation is involved in almost every cellular process, and it modulates the activity of target proteins at various cellular locations, and controls the activity of signaling networks. Protein phosphorylation is achieved by protein kinases that transfer a phosphate group from adenosine triphosphate (ATP) to serine, threonine, and/or tyrosine residues of a target protein, and this can be reversed by a reaction called dephosphorylation by protein phosphatases. Disruption or enhancement of protein phosphorylation is implicated in the progression of various serious diseases including cancer, neurodegeneration, and immune diseases.153)
Prolonged agonist stimulation of GPCRs often leads to phosphorylation of multiple intracellular residues, which is largely dependent upon the activity of G protein-coupled receptor kinases (GRKs). In general, phosphorylation of GPCRs by GRKs induces the desensitization of the receptor followed by an interaction with cytosolic cofactor protein β-arrestin, which uncouples G proteins from the receptor and removes them from the plasma membrane via clathrin-dependent endocytosis to terminate receptor signaling.154) This is considered as an important mechanism for GPCRs to regulate receptor signaling efficacy. Emerging evidence for GABAB receptors suggests that this GPCR does not conform to this type of regulation. Multiple studies using both native and recombinant receptors have demonstrated that GABAB receptors do not undergo agonist-induced internalization and are not GRK substrates.155) Although GRKs did not appear to be GABAB receptor kinases, GRK4 and GRK5 have been reported to play a role in agonist-induced desensitization.156,157) The suppression of GRK4 levels in cerebellar granule cells strongly inhibits GABAB receptor desensitization.156) Similarly, in Xenopus oocytes and baby hamster kidney cells, expression of GABAB receptors and GIRKs does not result in desensitization unless co-expressed with GRK4 or GRK5.157) Thus, unlike most GPCRs, GRKs may function as anchoring proteins that regulate GABAB receptor activity but not phosphorylation. Biochemical studies have revealed that GABAB receptors are phosphorylated by various kinases on multiple serine and threonine residues within the cytoplasmic domains of both R1 and R2 subunits.158) Moreover, GABAB receptor exhibits significant levels of basal phosphorylation that are not due to agonist stimulation and undergo clathrin-dependent constitutive endocytosis followed by receptor recycling.155)
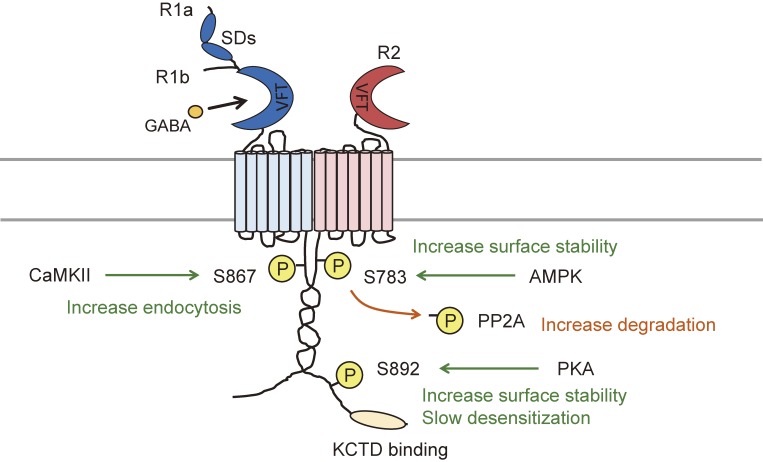
So far, five phosphorylation sites have been identified: serine 867 (S867) and S917/923 on the R1 subunit, and S783 and S892 on the R2 subunit (Fig. 3). S867 on the R1 subunit is subject to phosphorylation by calcium/calmodulin-dependent protein kinase II (CaMKII). S867 phosphorylation promotes dynamin-dependent GABAB receptor endocytosis particularly to the receptors that cluster with GIRK channels.140) Phosphorylation of S917/923 on the R1 subunit and S783 on the R2 subunit are all mediated by 5′AMP-dependent protein kinase (AMPK).159) AMPK acts as an energy sensor to regulate cellular metabolism and directly associates with the R1 subunit via residues 910–925 within the coiled-coil domain. The role of S783 phosphorylation in GABAB receptors has been studied intensively by the author in both native and recombinant receptors. The physiological relevance of all three AMPK substrates have been examined by measuring AMPK-mediated GIRK channel activity, and so far, only S783 phosphorylation is evident in enhancing the cell surface stability of GABAB receptors.159) Termination of S783 phosphorylation has also been studied. Activation of NMDA-type glutamate receptors rapidly increase S783 phosphorylation followed by a slower protein phosphatase 2A activity, which transiently switches the state of S783 phosphorylation. Dephosphorylated GABAB receptors undergo clathrin-mediated endocytosis and divert from a recycling to a proteasomal degradation pathway to attenuate GABAB receptor signaling.139) It is evident from the studies using S783A mutant knock-in mice that S783 phosphorylation does not significantly impact presynaptic GABAB receptor function at glutamatergic neurons but modulate postsynaptic GABAB receptor activity.50) S892 on the R2 subunit is a PKA substrate.160) S892 phosphorylation enhances the membrane stability of GABAB receptors, and prolonged activation of GABAB receptors via activation of Gαi/o protein, leads to the inhibition of adenylyl cyclase to reduce PKA levels, and consequently a reduction in the phosphorylation of S892.155) The phosphorylation of S892 can be promoted by the assembly of KCTD12 with R2 subunits.161) The assembly of receptors with KCTD12 increases basal S892 phosphorylation and stabilizes receptors on the cell surface.148) Increased tonic S892 phosphorylation attenuates KCTD12-induced fast desensitization. Phosphorylation of S783 and S892 has also been detected in astrocytes, which are the most abundant cells in the CNS and play essential roles in synaptic transmission. ATP-mediated P2Y receptor (P2YR) signaling elevates intracellular calcium levels and enhances both S783 and S892 phosphorylation.162) S783 phosphorylation is mediated via P2YR-Ca2+/CaM-dependent protein kinase kinase (CaMKK)-AMPK signaling, and S892 phosphorylation is induced by pertussis toxin-sensitive P2YRs. These phosphorylation on astrocytic GABAB receptors are likely to act as a detector to fine-tune astrocyte activity.
Figure 3.
Phosphorylation of GABAB receptors and their functional modulation. Five phosphorylation sites have been identified so far: serine 867 (S867) and S917/923 on the R1 subunit, and S783 and S892 on the R2 subunit. Calcium/calmodulin-dependent protein kinase II (CaMKII) phosphorylates S867 on R1 subunit and promotes dynamin-dependent receptor endocytosis. 5′AMP-dependent protein kinase (AMPK) has been found to phosphorylate S917/923 on the R1 subunit and S783 on the R2 subunit. However, only S783 phosphorylation is evident in native tissue. S783 phosphorylation stabilizes GABAB receptors on the plasma membrane, thereby enhancing GIRK channel activity. The termination of S783 phosphorylation is due to dephosphorylation by protein phosphatase 2A (PP2A), which promotes clathrin-mediated endocytosis of GABAB receptors followed by proteasomal degradation. S892 phosphorylation by PKA enhances GABAB receptor cell surface stability, promotes potassium channel tetramerization domain-containing (KCTD) 12 (KCTD12) protein assembly with the R2 subunit and attenuates KCTD12-induced desensitization.
PKC is also known to phosphorylate GABAB receptor R1 subunits, although the phosphorylation site has not been identified.120) In Chinese hamster ovary cells, GABAB receptor activity promotes PKC recruitment to the plasma membrane and induces R1 subunit phosphorylation. Phosphorylation of the R1 subunit fosters the dissociation of NSF protein from GABAB receptors and enhances desensitization. PKC phosphorylation does not trigger GABAB receptor internalization similar to PKA phosphorylation.120)
Ubiquitination.
Ubiquitination is a posttranslational modification that generally directs proteins for degradation by proteasomes or by lysosomes, and this modification functions to regulate the number of cellular processes including inflammation, stress responses, and DNA repair. An 8.5 kDa protein ubiquitin associates with the lysine (Lys) residues of target proteins by a sequential reaction of three enzymes: ubiquitin activating enzymes (E1), ubiquitin-conjugation enzymes (E2), and ubiquitin ligases (E3). Ubiquitination of GPCRs and the mechanisms for regulating receptor to undergo lysosomal degradation are well established.163) Furthermore, recent findings have provided strong evidence for the additional role of ubiquitin in other cellular mechanisms such as receptor trafficking, β-arrestin- and G protein-mediated signaling.164–166)
Ubiquitination has been reported to regulate the amount of newly synthesized GABAB receptors that traffic to the plasma membrane via endoplasmic reticulum-associated degradation machinery.167) The Lys-48-linked polyubiquitination of lysines 767/771 in the C-terminal domain of the R2 subunit targets receptors to proteasomes for degradation, and inactivation of these ubiquitination sites increases receptor levels in the plasma membrane as well as GABAB receptor-mediated signaling. Another type of GABAB receptor ubiquitination, Lys-63-linked ubiquitination of R1 subunit is known to promote surface receptor degradation.168) Cell surface GABAB receptor degradation has been reported upon activation of glutamate receptors, possibly through CaMKII-mediated phosphorylation of S867 on the R1 subunit.138,169) Lys-63-linked ubiquitination of the R1 subunit is mediated by the E3 ligase Mind Bomb-2.169) PKC-induced ubiquitination of GABAB receptors has also been proposed recently, and the de-ubiquitination enzyme USP14 (ubiquitin-specific protease 14), which associates with the R1 subunit via the second intercellular loop, regulates post-endocytic ubiquitination of the GABAB receptors.170)
6. GABAB receptor trafficking and cell surface mobility
Cell-surface trafficking of GABAB receptors is controlled by an ER retention sequence (RSRR) in the C-terminus of R1 subunits, thus the R1 subunit cannot reach the plasma membrane by itself and is retained in the ER. The C-terminal tail of R2 subunit masks ER retention sequences in the R1 subunit via their coiled-coil domain interaction and escort the R1 subunit to the cell surface.171)
Control of cell surface GABAB receptor expression plays an important role in the regulation of receptor efficacy. GABAB receptor cell surface expression is remarkably stable, and baclofen treatment does not induce conventional β-arrestin recruitment.155,160) However, the GABAB receptor undergoes constitutive endocytosis via clathrin-mediated pathways.172) In basal conditions, GABAB receptors internalize as heterodimers via clathrin- and dynamin-dependent mechanisms and localize to Rab11-positive recycling endosomes. After constitutive endocytosis, large numbers of GABAB receptors recycle back to the plasma membrane to maintain steady-state cell surface numbers.138,155) Of note, endocytosis is detected only in dendrites and not in axons.138) The balance between insertion and degradation after receptor internalization as well as a rapid recycling processes maintain GABAB receptor cell surface expression levels.173) As mentioned earlier, phosphorylation of GABAB receptors dramatically regulate cell surface stability of the receptors. Exposure to glutamate promotes phosphorylation/dephosphorylation of GABAB receptors and regulates cell surface number of the receptors.
Lipid rafts are dynamic assemblies of proteins and lipids that float freely within the liquid-disordered bilayer of cellular membranes. These highly dynamic raft domains are essential in signaling processes and also form sorting platforms for targeted protein trafficking. GABAB receptors and their downstream effectors, Gαi/o proteins, are all localized in lipid rafts.174,175) Notably, GABAB receptors in raft-enriched fractions exhibited lower GTPγS response to agonist binding than in whole membranes, suggesting that changes in the membrane environment may regulate receptor function.175) Furthermore, studies of the dynamic lateral diffusion of GABAB receptors at the cell surface revealed that the restricted mobility of GABAB receptors is regulated by the C-terminal region in R2 subunits. After activation by baclofen, the levels of mobile receptors are increased significantly.40) By using single-molecule analysis of fluorescence-labeled GABAB receptor, it is evident that agonist stimulation increases the mobility of large oligomers of GABAB receptors on the cell surface.176) These data suggests the possibility of GABAB receptor mobility between lipid raft and non-lipid raft domains. Given that the level of cell surface GABAB receptors is stable after agonist stimulation, lateral diffusion of GABAB receptors may provide a mechanism for controlling inhibitory strength.
7. GABAB receptors and diseases
Impaired GABAB receptor-mediated synaptic transmission underlies a variety of neurological and psychiatric disorders. This section will discuss several diseases in which GABAB receptors are known to be involved together with some promising indications for treatment using GABAB receptor drugs.
Anxiety and depression.
GABAB receptors have been implicated in the pathophysiology of emotional disorders such as anxiety and depression.177) Interest in the role of GABAB receptors in anxiety has emerged because R1 subunit-deficient mice are more anxious than their wild-type counterparts in several anxiety-related tests, such as the light-dark box and staircase tests.178) The role of GABAB receptors in emotional behavior was also suggested by the elevated levels of GABAB receptor expression in the limbic system.178) Supporting these observations, baclofen has been shown to have an anxiolytic effect and GABAB receptor PAMs were found to be promising compounds in the treatment of anxiety disorders.179) The antagonism of GABAB receptors may also be a potential therapeutic strategy for depression. R1 subunit-deficient mice display an antidepressant-like phenotype in forced swim tests, and these phenotypes were recapitulated in studies using the GABAB receptor antagonist CGP56433A.178) In support of these data, baclofen attenuates the decrease in immobility caused by antidepressants.180,181)
Addiction.
Over the years, a number of clinical observations suggested that baclofen may offer benefit in the treatment of alcohol use and substance use disorders.182–185) Multiple preclinical studies have demonstrated the ability of baclofen to suppress alcohol drinking, oral alcohol self-administration, and intravenous self-administration of cocaine, nicotine, amphetamine, methamphetamine, morphine, and heroin in rodents.186) Some randomized controlled trials and case reports support the efficacy of baclofen in suppressing alcohol consumption, craving for alcohol, and alcohol withdrawal symptomatology in alcohol-dependent patients.186) Baclofen attenuates the reinforcing effects of abused drugs by influencing the mesolimbic dopamine system.187) Recently, interest in testing high doses of baclofen in alcohol use disorder treatment has emerged; however, side-effects such as somnolence, insomnia, dizziness, and paresthesia pose a principle limitation to its administration in alcohol addiction.188,189) Preclinical research has then extended the anti-addictive properties of baclofen to PAM. In light of their more favorable side-effect profile compared to baclofen, PAMs may represent a major step forward in GABAB receptor-based pharmacotherapy of alcohol use and substance use disorders.186)
Epilepsy.
GABAB receptors have been implicated into the etiology of epilepsies.3) The G1465A polymorphism in the gene for the R1 subunit has been linked to the risk of temporal lobe epilepsy as well as the severity of the disease.189) mRNA expression and immunoreactivity of GABAB receptors, as well as GABAB-mediated pre- and postsynaptic responses, are decreased in discrete cortical and hippocampal areas of epileptic patients.189–192) In addition, R1 subunit-deficient mice exhibited generalized seizure activities.193,194) The role of GABAB receptor-mediated mechanisms in the pathogenesis of seizures depends on neural networks that involve GABAB receptors, which determine the seizure type. GABAB receptor agonists have been shown to diminish seizure activity in mouse models of both generalized convulsive and focal seizures; however, generalized non-convulsive seizures such as typical and atypical absence seizures are exacerbated by GABAB receptor agonists and blocked by GABAB receptor antagonists.195–197) This dichotomy is likely due to the involvement of thalamic circuitry in both typical and atypical absence seizures. Therefore, GABAB receptor-mediated mechanisms can be pro- or anti-convulsant depending on the nature of the pathological neuronal networks. Recent studies reported that GABAB receptor PAMs offer anti-convulsive actions in animal models.198–200) Considering that PAMs offer beneficial behavioral effects without overt side-effects, PAMs may serve as a clinically relevant strategy for the management of epileptic seizures.69,201,202)
Cognition.
GABAB receptors are highly expressed in brain regions implicated in learning and memory.203) Post-mortem GABAB receptor expression studies in Alzheimer’s disease brains has suggested an increase in R1 subunit expression in the hippocampus that correlates with the extent of neurofibrillary tangle pathology.204) Alternative splicing of GABAB receptors and GIRK expression have also been suggested a possible changes in GABAB receptor signaling in Alzheimer’s disease.205) In this respect, a clinical trial using GABAB receptor antagonist SGS742 (formerly known as CGP36742) was progressed to Phase II in an attempt to treat mild cognitive impairment.206) SGS742 was administered orally at 600 mg three times a day for eight weeks in a double-blind trial in 75 patients. SGS742 significantly improved working memory and attention, suggesting that GABAB receptor antagonism can promote cognitive performance. However, SGS742 failed to progress to a Phase III clinical trial, and currently there are no other GABAB receptor antagonists in development for cognitive diseases.207) Identifying novel targets to develop specific drugs for GABAB receptors will be necessary.
8. Conclusion
This review summarizes how one single receptor, GABAB receptor, generates multiple functions such as: (1) the presence of heterodimers and large oligomers increase the complexity in cellular localization and function; (2) splice variants of the R1 subunit contribute to the functional diversity of this receptor; (3) interacting proteins for GABAB receptors provide a vast amount of receptor function by regulating receptor localization, signaling specificity, and pharmacological profiles; (4) crosstalk between various receptors helps to balance neuronal inhibition and excitation as well as signal transduction and transactivation-dependent neuronal survival; (5) PTMs such as phosphorylation and ubiquitination regulate receptor trafficking and the amount of receptors on the plasma membrane; and (6) receptor localization in lipid rafts is involved in regulating the efficacy of receptor signaling. These complexities generated from a single GPCR still need to be clarified, but future studies will help to confirm the mechanisms regulating GABAB receptor function and reasons for the interactions with multiple proteins. GABAB receptors are excellent therapeutic targets, because drugs acting on these receptors have the potential to treat a wide variety of neurological diseases. The full therapeutic benefits of GABAB receptors need to be elucidated using various methods.
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AMPK
5′AMP-dependent protein kinase
- ATF
activating transcription factor
- ATP
adenosine triphosphate
- BBB
blood-brain barrier
- BDNF
brain-derived neurotrophic factor
- CaMKII
calcium/calmodulin-dependent protein kinase II
- cAMP
cyclic AMP
- CaV
voltage-gated Ca2+
- CGP35348
3-Aminopropyl-(diethoxymethyl) phosphinic acid
- CGP36742
3-Aminopropyl-butylphosphinic acid
- CGP46381
(3-Aminopropyl)(cyclohexylmethyl)phosphinic acid
- CGP51176
3-amino-2(R)-hydroxypropyl-cyclohexylmethyl-phosphinic acid
- CGP7930
2,6-di-tert-butyl-4-(3-hydroxy-2,2-dimethyl-propyl)-phenol
- CHOP
C/EBP-homologous protein
- CNS
central nervous system
- CREB
cAMP response element-binding protein
- C/EBP
CCAAT/enhancer-binding protein
- DAG
diacylglycerol
- ER
endoplasmic reticulum
- GABA
γ-aminobutyric acid
- GABAA
GABA typeA
- GABAB
GABA typeB
- GDP
guanosine diphosphate
- GHB
γ-hydroxy-butyric acid
- GIRK
G protein-activated inwardly-rectifying K+
- GISP
GPCR interacting scaffolding protein
- GPCR
G protein coupled receptor
- GRKs
G protein-coupled receptor kinases
- GS39783
N,N′-dicyclopentyl-2-methylsulfanyl-5-nitropyrimidine-4,6-diamine
- GTP
guanosine triphosphate
- IGF-1
insulin-like growth factor-1
- IP3
inositol trisphosphate
- KCTD
Potassium Channel Tetramerization Domain-containing
- mGluRs
metabotropic glutamate receptors
- Mupp1
Multi-PDZ domain protein 1
- NGF
nerve growth factor
- NMDA
N-methyl-D-aspartate
- NSF
N-ethylmaleimide-sensitive factor
- PAM
positive allosteric modulator
- PKA
cAMP-dependent protein kinase
- PKC
protein kinase C
- PLC
phospholipase C
- PTM
posttranslational modification
- RGS
G protein signaling
- RTKs
receptor for tyrosine kinases
- TrkB
tropomyosin-related receptor kinase B
- VFT
Venus flytrap domain
- 7TM
heptahelical transmembrane domain
Profile
Miho Terunuma graduated from the Department of Dentistry at Kyushu University in 2000 and received her Ph.D. from Kyushu University Graduate School of Dentistry in 2004. In 2003, she became a JSPS Research Fellow (DC2) and studied the role of novel inositol 1,4,5-triphosphate-binding protein (PRIP) in the central nervous system. She carried out her postdoctoral research with Professor Stephen J. Moss at the Department of Neuroscience in the University of Pennsylvania from 2005 to 2008, and at Tufts University from 2008 to 2013, where she studied the phosphorylation and functional modulation of GABA receptors and their behavioral effect. She joined the University of Leicester in the United Kingdom as a Neuroscience lecturer in the Department of Cell Physiology & Pharmacology in October, 2013. She was appointed as Professor and Chair at the Department of Oral Biochemistry in Niigata University in August, 2016.
References
- 1).Bloom F.E., Inersen L.L. (1971) Localizing 3H-GABA in nerve terminals of rat cerebral cortex by electron microscopic autoradiography. Nature 229, 628–630. [DOI] [PubMed] [Google Scholar]
- 2).Vithlani M., Terunuma M., Moss S.J. (2011) The dynamic modulation of GABA(A) receptor trafficking and its role in regulating the plasticity of inhibitory synapses. Physiol. Rev. 91, 1009–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Bettler B., Kaupmann K., Mosbacher J., Gassmann M. (2004) Molecular structure and physiological functions of GABA(B) receptors. Physiol. Rev. 84, 835–867. [DOI] [PubMed] [Google Scholar]
- 4).Bowery N.G., Hudson A.L. (1979) gamma-Aminobutyric acid reduces the evoked release of [3H]-noradrenaline from sympathetic nerve terminals. Br. J. Pharmacol. 66, 108P. [PMC free article] [PubMed] [Google Scholar]
- 5).Bowery N.G., Brown D.A. (1997) The cloning of GABA(B) receptors. Nature 386, 223–224. [DOI] [PubMed] [Google Scholar]
- 6).Kaupmann K., Huggel K., Heid J., Flor P.J., Bischoff S., Mickel S.J., et al. (1997) Expression cloning of GABA(B) receptors uncovers similarity to metabotropic glutamate receptors. Nature 386, 239–246. [DOI] [PubMed] [Google Scholar]
- 7).Lagerström M.C., Schiöth H.B. (2008) Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat. Rev. Drug Discov. 7, 339–357. [DOI] [PubMed] [Google Scholar]
- 8).Pin J.P., Galvez T., Prézeau L. (2003) Evolution, structure, and activation mechanism of family 3/C G-protein-coupled receptors. Pharmacol. Ther. 98, 325–354. [DOI] [PubMed] [Google Scholar]
- 9).Chun L., Zhang W.H., Liu J.F. (2012) Structure and ligand recognition of class C GPCRs. Acta Pharmacol. Sin. 33, 312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Møller T.C., Moreno-Delgado D., Pin J.P., Kniazeff J. (2017) Class C G protein-coupled receptors: reviving old couples with new partners. Biophys. Rep. 3, 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Jones K.A., Borowsky B., Tamm J.A., Craig D.A., Durkin M.M., Dai M., et al. (1998) GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature 396, 674–679. [DOI] [PubMed] [Google Scholar]
- 12).Kaupmann K., Malitschek B., Schuler V., Heid J., Froestl W., Beck P., et al. (1998) GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature 396, 683–687. [DOI] [PubMed] [Google Scholar]
- 13).White J.H., Wise A., Main M.J., Green A., Fraser N.J., Disney G.H., et al. (1998) Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature 396, 679–682. [DOI] [PubMed] [Google Scholar]
- 14).Burmakina S., Geng Y., Chen Y., Fan Q.R. (2014) Heterodimeric coiled-coil interactions of human GABAB receptor. Proc. Natl. Acad. Sci. U.S.A. 111, 6958–6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Geng Y., Xiong D., Mosyak L., Malito D.L., Kniazeff J., Chen Y., et al. (2012) Structure and functional interaction of the extracellular domain of human GABA(B) receptor GBR2. Nat. Neurosci. 15, 970–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Geng Y., Bush M., Mosyak L., Wang F., Fan Q.R. (2013) Structural mechanism of ligand activation in human GABA(B) receptor. Nature 504, 254–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Galvez T., Parmentier M.L., Joly C., Malitschek B., Kaupmann K., Kuhn R., et al. (1999) Mutagenesis and modeling of the GABAB receptor extracellular domain support a venus flytrap mechanism for ligand binding. J. Biol. Chem. 274, 13362–13369. [DOI] [PubMed] [Google Scholar]
- 18).Galvez T., Prezeau L., Milioti G., Franek M., Joly C., Froestl W., et al. (2000) Mapping the agonist-binding site of GABAB type 1 subunit sheds light on the activation process of GABAB receptors. J. Biol. Chem. 275, 41166–41174. [DOI] [PubMed] [Google Scholar]
- 19).Margeta-Mitrovic M., Jan Y.N., Jan L.Y. (2001) Function of GB1 and GB2 subunits in G protein coupling of GABA(B) receptors. Proc. Natl. Acad. Sci. U.S.A. 98, 14649–14654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Robbins M.J., Calver A.R., Filippov A.K., Hirst W.D., Russell R.B., Wood M.D., et al. (2001) GABA(B2) is essential for G-protein coupling of the GABA(B) receptor heterodimer. J. Neurosci. 21, 8043–8052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Duthey B., Caudron S., Perroy J., Bettler B., Fagni L., Pin J.P., et al. (2002) A single subunit (GB2) is required for G-protein activation by the heterodimeric GABA(B) receptor. J. Biol. Chem. 277, 3236–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Sack J.S., Saper M.A., Quiocho F.A. (1989) Periplasmic binding protein structure and function. Refined X-ray structures of the leucine/isoleucine/valine-binding protein and its complex with leucine. J. Mol. Biol. 206, 171–191. [DOI] [PubMed] [Google Scholar]
- 23).Lee C., Mayfield R.D., Harris R.A. (2010) Intron 4 containing novel GABAB1 isoforms impair GABAB receptor function. PLoS One 5, e14044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Pinard A., Seddik R., Bettler B. (2010) GABAB receptors: physiological functions and mechanisms of diversity. Adv. Pharmacol. 58, 231–255. [DOI] [PubMed] [Google Scholar]
- 25).Blein S., Ginham R., Uhrin D., Smith B.O., Soares D.C., Veltel S., et al. (2004) Structural analysis of the complement control protein (CCP) modules of GABAB receptor 1a. J. Biol. Chem. 279, 48292–48306. [DOI] [PubMed] [Google Scholar]
- 26).Grace C.R., Perrin M.H., DiGruccio M.R., Miller C.L., Rivier J.E., Vale W.W., et al. (2004) NMR structure and peptide hormone binding site of the first extracellular domain of a type B1 G protein-coupled receptor. Proc. Natl. Acad. Sci. U.S.A. 101, 12836–12841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Lehtinen M.J., Meri S., Jokiranta T.S. (2004) Interdomain contact regions and angles between adjacent short consensus repeat domains. J. Mol. Biol. 344, 1385–1396. [DOI] [PubMed] [Google Scholar]
- 28).Vigot R., Barbieri S., Brauner-Osborne H., Turecek R., Shigemoto R., Zhang Y.P., et al. (2006) Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron 50, 589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Biermann B., Ivankova-Susankova K., Bradaia A., Abdel Aziz S., Besseyrias V., Kapfhammer J.P., et al. (2010) The sushi domains of GABAB receptors function as axonal targeting signals. J. Neurosci. 30, 1385–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Pfaff T., Malitschek B., Kaupmann K., Prezeau L., Pin J.P., Bettler B., et al. (1999) Alternative splicing generates a novel isoform of the rat metabotropic GABA(B)R1 receptor. Eur. J. Neurosci. 11, 2874–2882. [DOI] [PubMed] [Google Scholar]
- 31).Isomoto S., Kaibara M., Sakurai-Yamashita Y., Nagayama Y., Uezono Y., Yano K., et al. (1998) Cloning and tissue distribution of novel splice variants of the rat GABAB receptor. Biochem. Biophys. Res. Commun. 253, 10–15. [DOI] [PubMed] [Google Scholar]
- 32).Schwarz D.A., Barry G., Eliasof S.D., Petroski R.E., Conlon P.J., Maki R.A. (2000) Characterization of gamma-aminobutyric acid receptor GABAB(1e), a GABAB(1) splice variant encoding a truncated receptor. J. Biol. Chem. 275, 32174–32181. [DOI] [PubMed] [Google Scholar]
- 33).Tiao J.Y., Bradaia A., Biermann B., Kaupmann K., Metz M., Haller C., et al. (2008) The sushi domains of secreted GABA(B1) isoforms selectively impair GABA(B) heteroreceptor function. J. Biol. Chem. 283, 31005–31011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Jiang X., Su L., Zhang Q., He C., Zhang Z., Yi P., et al. (2012) GABAB receptor complex as a potential target for tumor therapy. J. Histochem. Cytochem. 60, 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Niswender C.M., Conn P.J. (2010) Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 50, 295–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Maurel D., Comps-Agrar L., Brock C., Rives M.L., Bourrier E., Ayoub M.A., et al. (2008) Cell-surface protein-protein interaction analysis with time-resolved FRET and snap-tag technologies: application to GPCR oligomerization. Nat. Methods 5, 561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Calebiro D., Rieken F., Wagner J., Sungkaworn T., Zabel U., Borzi A., et al. (2013) Single-molecule analysis of fluorescently labeled G-protein-coupled receptors reveals complexes with distinct dynamics and organization. Proc. Natl. Acad. Sci. U.S.A. 110, 743–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Schwenk J., Metz M., Zolles G., Turecek R., Fritzius T., Bildl W., et al. (2010) Native GABA(B) receptors are heteromultimers with a family of auxiliary subunits. Nature 465, 231–235. [DOI] [PubMed] [Google Scholar]
- 39).Comps-Agrar L., Kniazeff J., Nørskov-Lauritsen L., Maurel D., Gassmann M., Gregor N., et al. (2011) The oligomeric state sets GABA(B) receptor signalling efficacy. EMBO J. 30, 2336–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Pooler A.M., McIlhinney R.A. (2007) Lateral diffusion of the GABAB receptor is regulated by the GABAB2 C terminus. J. Biol. Chem. 282, 25349–25356. [DOI] [PubMed] [Google Scholar]
- 41).Knight A.R., Bowery N.G. (1996) The pharmacology of adenylyl cyclase modulation by GABAB receptors in rat brain slices. Neuropharmacology 35, 703–712. [DOI] [PubMed] [Google Scholar]
- 42).Bowery N.G., Bettler B., Froestl W., Gallagher J.P., Marshall F., Raiteri M., et al. (2002) International union of pharmacology. XXXIII. Mammalian gamma-aminobutyric acid(B) receptors: structure and function. Pharmacol. Rev. 54, 247–264. [DOI] [PubMed] [Google Scholar]
- 43).Ulrich D., Bettler B. (2007) GABA(B) receptors: synaptic functions and mechanisms of diversity. Curr. Opin. Neurobiol. 17, 298–303. [DOI] [PubMed] [Google Scholar]
- 44).Chalifoux J.R., Carter A.G. (2011) GABAB receptor modulation of voltage-sensitive calcium channels in spines and dendrites. J. Neurosci. 31, 4221–4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Simonds W.F. (1999) G protein regulation of adenylate cyclase. Trends Pharmacol. Sci. 20, 66–73. [DOI] [PubMed] [Google Scholar]
- 46).Tang W.J., Gilman A.G. (1991) Type-specific regulation of adenylyl cyclase by G protein beta gamma subunits. Science 254, 1500–1503. [DOI] [PubMed] [Google Scholar]
- 47).Calver A.R., Michalovich D., Testa T.T., Robbins M.J., Jaillard C., Hill J., et al. (2003) Molecular cloning and characterisation of a novel GABAB-related G-protein coupled receptor. Brain Res. Mol. Brain Res. 110, 305–317. [DOI] [PubMed] [Google Scholar]
- 48).Enna, S.J. (2000) GABAB Receptor Signaling Pathways. In Pharmacology of GABA and Glycine Neurotransmission (ed. Möhler, H.). Springer-Verlag, Berlin, pp. 329–342. [Google Scholar]
- 49).Hashimoto T., Kuriyama K. (1997) In vivo evidence that GABA(B) receptors are negatively coupled to adenylate cyclase in rat striatum. J. Neurochem. 69, 365–370. [DOI] [PubMed] [Google Scholar]
- 50).Terunuma M., Revilla-Sanchez R., Quadros I.M., Deng Q., Deeb T.Z., Lumb M., et al. (2014) Postsynaptic GABAB receptor activity regulates excitatory neuronal architecture and spatial memory. J. Neurosci. 34, 804–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51).Nguyen P.V., Woo N.H. (2003) Regulation of hippocampal synaptic plasticity by cyclic AMP-dependent protein kinases. Prog. Neurobiol. 71, 401–437. [DOI] [PubMed] [Google Scholar]
- 52).Catteral W.A., Goldin A.L., Waxman S.G. (2005) International union of pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol. Rev. 57, 397–409. [DOI] [PubMed] [Google Scholar]
- 53).Bettler B., Tiao J.Y. (2006) Molecular diversity, trafficking and subcellular localization of GABAB receptors. Pharmacol. Ther. 110, 533–543. [DOI] [PubMed] [Google Scholar]
- 54).Wu L.G., Saggau P. (1997) Presynaptic inhibition of elicited neurotransmitter release. Trends Neurosci. 20, 204–212. [DOI] [PubMed] [Google Scholar]
- 55).Dolphin A.C. (2006) A short history of voltage-gated calcium channels. Br. J. Pharmacol. 147 (Suppl 1), S56–S62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).Buchanan P.J., McCloskey K.D. (2016) CaV channels and cancer: canonical functions indicate benefits of repurposed drugs as cancer therapeutics. Eur. Biophys. J. 45, 621–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57).Harayama N., Shibuya I., Tanaka K., Kabashima N., Ueta Y., Yamashita H. (1998) Inhibition of N- and P/Q-type calcium channels by postsynaptic GABAB receptor activation in rat supraoptic neurones. J. Physiol. 509, 371–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58).Chalifoux J.R., Carter A.G. (2011) GABAB receptor modulation of voltage-sensitive calcium channels in spines and dendrites. J. Neurosci. 31, 4221–4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59).Lüscher C., Jan L.Y., Stoffel M., Malenka R.C., Nicoll R.A. (1997) G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron 19, 687–695. [DOI] [PubMed] [Google Scholar]
- 60).Kaupmann K., Schuler V., Mosbacher J., Bischoff S., Bittiger H., Heid J., et al. (1998) Human gamma-aminobutyric acid type B receptors are differentially expressed and regulate inwardly rectifying K+ channels. Proc. Natl. Acad. Sci. U.S.A. 95, 14991–14996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61).Peleg S., Varon D., Ivanina T., Dessauer C.W., Dascal N. (2002) G(alpha)(i) controls the gating of the G protein-activated K(+) channel, GIRK. Neuron 33, 87–99. [DOI] [PubMed] [Google Scholar]
- 62).Clancy S.M., Fowler C.E., Finley M., Suen K.F., Arrabit C., Berton F., et al. (2005) Pertussis-toxin-sensitive Galpha subunits selectively bind to C-terminal domain of neuronal GIRK channels: evidence for a heterotrimeric G-protein-channel complex. Mol. Cell. Neurosci. 28, 375–389. [DOI] [PubMed] [Google Scholar]
- 63).Bichet D., Haass F.A., Jan L.Y. (2003) Merging functional studies with structures of inward-rectifier K(+) channels. Nat. Rev. Neurosci. 4, 957–967. [DOI] [PubMed] [Google Scholar]
- 64).Krapivinsky G., Gordon E.A., Wickman K., Velimirović B., Krapivinsky L., Clapham D.E. (1995) The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K(+)-channel proteins. Nature 374, 135–141. [DOI] [PubMed] [Google Scholar]
- 65).Kubo Y., Reuveny E., Slesinger P.A., Jan Y.N., Jan L.Y. (1993) Primary structure and functional expression of a rat G-protein-coupled potassium channel. Nature 364, 802–806. [DOI] [PubMed] [Google Scholar]
- 66).Wickman K., Karschin C., Karschin A., Picciotto M.R., Clapham D.E. (2000) Brain localization and behavioral impact of the G-protein-gated K+ channel subunit GIRK4. J. Neurosci. 20, 5608–5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67).Margeta-Mitrovic M., Mitrovic I., Riley R.C., Jan L.Y., Basbaum A.I. (1999) Immunohistochemical localization of GABA(B) receptors in the rat central nervous system. J. Comp. Neurol. 405, 299–321. [DOI] [PubMed] [Google Scholar]
- 68).Ciruela F., Fernández-Dueñas V., Sahlholm K., Fernández-Alacid L., Nicolau J.C., Watanabe M., et al. (2010) Evidence for oligomerization between GABAB receptors and GIRK channels containing the GIRK1 and GIRK3 subunits. Eur. J. Neurosci. 32, 1265–1277. [DOI] [PubMed] [Google Scholar]
- 69).Froestl W. (2010) Chemistry and pharmacology of GABAB receptor ligands. Adv. Pharmacol. 58, 19–62. [DOI] [PubMed] [Google Scholar]
- 70).Lapin I. (2001) Phenibut (beta-phenyl-GABA): A tranquilizer and nootropic drug. CNS Drug Rev. 7, 471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71).Zvejniece L., Vavers E., Svalbe B., Veinberg G., Rizhanova K., Liepins V., et al. (2015) R-phenibut binds to the α2-δ subunit of voltage-dependent calcium channels and exerts gabapentin-like anti-nociceptive effects. Pharmacol. Biochem. Behav. 137, 23–29. [DOI] [PubMed] [Google Scholar]
- 72).Bertrand S., Morin F., Lacaille J.C. (2003) Different actions of gabapentin and baclofen in hippocampus from weaver mice. Hippocampus 13, 525–528. [DOI] [PubMed] [Google Scholar]
- 73).Bertrand S., Nouel D., Morin F., Nagy F., Lacaille J.C. (2003) Gabapentin actions on Kir3 currents and N-type Ca2+ channels via GABAB receptors in hippocampal pyramidal cells. Synapse 50, 95–109. [DOI] [PubMed] [Google Scholar]
- 74).Ng G.Y., Bertrand S., Sullivan R., Ethier N., Wang J., Yergey J., et al. (2001) Gamma-aminobutyric acid type B receptors with specific heterodimer composition and postsynaptic actions in hippocampal neurons are targets of anticonvulsant gabapentin action. Mol. Pharmacol. 59, 144–152. [PubMed] [Google Scholar]
- 75).Lanneau C., Green A., Hirst W.D., Wise A., Brown J.T., Donnier E., et al. (2001) Gabapentin is not a GABAB receptor agonist. Neuropharmacology 41, 965–975. [DOI] [PubMed] [Google Scholar]
- 76).Jensen A.A., Mosbacher J., Elg S., Lingenhoehl K., Lohmann T., Johansen T.N., et al. (2002) The anticonvulsant gabapentin (neurontin) does not act through gamma-aminobutyric acid-B receptors. Mol. Pharmacol. 61, 1377–1384. [DOI] [PubMed] [Google Scholar]
- 77).Cheng J.K., Lee S.Z., Yang J.R., Wang C.H., Liao Y.Y., Chen C.C., et al. (2004) Does gabapentin act as an agonist at native GABA(B) receptors? J. Biomed. Sci. 11, 346–355. [DOI] [PubMed] [Google Scholar]
- 78).Shimizu S., Honda M., Tanabe M., Ono H. (2004) GABAB receptors do not mediate the inhibitory actions of gabapentin on the spinal reflex in rats. J. Pharmacol. Sci. 96, 444–449. [DOI] [PubMed] [Google Scholar]
- 79).Froestl W., Mickel S.J., Hall R.G., von Sprecher G., Strub D., Baumann P.A., et al. (1995) Phosphinic acid analogues of GABA. 1. New potent and selective GABAB agonists. J. Med. Chem. 38, 3297–3312. [DOI] [PubMed] [Google Scholar]
- 80).Bittiger H., Reymann N., Hall R., Kane P. (1988) CGP27492, a new potent and selective radioligand for GABAB receptors. Eur. J. Neurosci. 116 (Suppl), 10. [Google Scholar]
- 81).Patel S., Naeem S., Kesingland A., Froestl W., Capogna M., Urban L., et al. (2001) The effects of GABA(B) agonists and gabapentin on mechanical hyperalgesia in models of neuropathic and inflammatory pain in the rat. Pain 90, 217–226. [DOI] [PubMed] [Google Scholar]
- 82).Chebib M., Mewett K.N., Johnston G.A.R. (1998) GABAC receptor antagonists differ entiate between human ρ1 and ρ2 receptors expressed in Xenopus oocytes. Eur. J. Pharmacol. 357, 227–234. [DOI] [PubMed] [Google Scholar]
- 83).Chebib M., Vandenberg R.J., Froestl W., Johnston G.A.R. (1997) Unsaturated phosphinic analogues of γ-aminobutyric acid as GABAC receptor antagonists. Eur. J. Pharmacol. 329, 223–229. [PubMed] [Google Scholar]
- 84).Zhang D., Pan Z.H., Awobuluyi M., Lipton S.A. (2001) Structure and function of GABA(C) receptors: a comparison of native versus recombinant receptors. Trends Pharmacol. Sci. 22, 121–132. [DOI] [PubMed] [Google Scholar]
- 85).Ong J., Bexis S., Marino V., Parker D.A., Kerr D.I., Froestl W. (2001) Comparative activities of the enantiomeric GABA(B) receptor agonists CGP 44532 and 44533 in central and peripheral tissues. Eur. J. Pharmacol. 412, 27–37. [DOI] [PubMed] [Google Scholar]
- 86).Ong J., Bexis S., Marino V., Parker D.A., Kerr D.I., Froestl W. (2001) CGP 36216 is a selective antagonist at GABA(B) presynaptic receptors in rat brain. Eur. J. Pharmacol. 415, 191–195. [DOI] [PubMed] [Google Scholar]
- 87).Froestl W., Mickel S.J., von Sprecher G., Diel P.J., Hall R.G., Maier L., et al. (1995) Phosphinic acid analogues of GABA. 2. Selective, orally active GABAB antagonists. J. Med. Chem. 38, 3313–3331. [DOI] [PubMed] [Google Scholar]
- 88).Gemignani A., Paudice P., Bonanno G., Raiteri M. (1994) Pharmacological discrimination between gamma-aminobutyric acid type B receptors regulating cholecystokinin and somatostatin release from rat neocortex synaptosomes. Mol. Pharmacol. 46, 558–562. [PubMed] [Google Scholar]
- 89).Bernasconi R., Mathivet P., Bischoff S., Marescaux C. (1999) Gamma-hydroxybutyric acid: an endogenous neuromodulator with abuse potential? Trends Pharmacol. Sci. 20, 135–141. [DOI] [PubMed] [Google Scholar]
- 90).Vergnes M., Boehrer A., Simler S., Bernasconi R., Marescaux C. (1997) Opposite effects of GABAB receptor antagonists on absences and convulsive seizures. Eur. J. Pharmacol. 332, 245–255. [DOI] [PubMed] [Google Scholar]
- 91).Urwyler S., Gjoni T., Koljatić J., Dupuis D.S. (2005) Mechanisms of allosteric modulation at GABAB receptors by CGP7930 and GS39783: effects on affinities and efficacies of orthosteric ligands with distinct intrinsic properties. Neuropharmacology 48, 343–353. [DOI] [PubMed] [Google Scholar]
- 92).Carai M.A., Colombo G., Froestl W., Gessa G.L. (2004) In vivo effectiveness of CGP7930, a positive allosteric modulator of the GABAB receptor. Eur. J. Pharmacol. 504, 213–216. [DOI] [PubMed] [Google Scholar]
- 93).Urwyler S., Mosbacher J., Lingenhoehl K., Heid J., Hofstetter K., Froestl W., et al. (2001) Positive allosteric modulation of native and recombinant gamma-aminobutyric acid(B) receptors by 2,6-Di-tert-butyl-4-(3-hydroxy-2,2-dimethyl-propyl)-phenol (CGP7930) and its aldehyde analog CGP13501. Mol. Pharmacol. 60, 963–971. [PubMed] [Google Scholar]
- 94).Onali P., Mascia F.M., Olianas M.C. (2003) Positive regulation of GABA(B) receptors dually coupled to cyclic AMP by the allosteric agent CGP7930. Eur. J. Pharmacol. 471, 77–84. [DOI] [PubMed] [Google Scholar]
- 95).Binet V., Goudet C., Brajon C., Le Corre L., Acher F., Pin J.P., et al. (2004) Molecular mechanisms of GABA(B) receptor activation: new insights from the mechanism of action of CGP7930, a positive allosteric modulator. Biochem. Soc. Trans. 32, 871–872. [DOI] [PubMed] [Google Scholar]
- 96).Gjoni T., Desrayaud S., Imobersteg S., Urwyler S. (2006) The positive allosteric modulator GS39783 enhances GABA(B) receptor-mediated inhibition of cyclic AMP formation in rat striatum in vivo. J. Neurochem. 96, 1416–1422. [DOI] [PubMed] [Google Scholar]
- 97).Maccioni P., Pes D., Orrù A., Froestl W., Gessa G.L., Carai M.A., et al. (2007) Reducing effect of the positive allosteric modulator of the GABA(B) receptor, GS39,783, on alcohol self-administration in alcohol-preferring rats. Psychopharmacology (Berl.) 193, 171–178. [DOI] [PubMed] [Google Scholar]
- 98).Paterson N.E., Vlachou S., Guey A., Kapumann K., Froestl W., Markou A. (2008) Positive modulation of GABAB receptors decreased nicotine self-administration and counteracted nicotine-induced enhancement of brain reward function in rats. J. Pharmacol. Exp. Ther. 326, 306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99).Pin J.P., Bettler B. (2016) Organization and functions of mGlu and GABAB receptor complexes. Nature 540, 60–68. [DOI] [PubMed] [Google Scholar]
- 100).Couve A., Calver A.R., Fairfax B., Moss S.J., Pangalos M.N. (2004) Unravelling the unusual signalling properties of the GABA(B) receptor. Biochem. Pharmacol. 68, 1527–1536. [DOI] [PubMed] [Google Scholar]
- 101).Padgett C.L., Slesinger P.A. (2010) GABAB receptor coupling to G-proteins and ion channels. Adv. Pharmacol. 58, 123–147. [DOI] [PubMed] [Google Scholar]
- 102).Doupnik C.A., Davidson N., Lester H.A., Kofuji P. (1997) RGS proteins reconstitute the rapid gating kinetics of Gbetagamma-activated inwardly rectifying K+ channels. Proc. Natl. Acad. Sci. U.S.A. 94, 10461–10466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103).De Vries L., Zheng B., Fischer T., Elenko E., Farquhar M.G. (2000) The regulator of G protein signaling family. Annu. Rev. Pharmacol. Toxicol. 40, 235–271. [DOI] [PubMed] [Google Scholar]
- 104).Siderovski D.P., Willard F.S. (2005) The GAPs, GEFs, and GDIs of heterotrimeric G-protein alpha subunits. Int. J. Biol. Sci. 1, 51–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105).Labouèbe G., Lomazzi M., Cruz H.G., Creton C., Luján R., Li M., et al. (2007) RGS2 modulates coupling between GABAB receptors and GIRK channels in dopamine neurons of the ventral tegmental area. Nat. Neurosci. 10, 1559–1568. [DOI] [PubMed] [Google Scholar]
- 106).Maity B., Stewart A., Yang J., Loo L., Sheff D., Shepherd A.J., et al. (2012) Regulator of G protein signaling 6 (RGS6) protein ensures coordination of motor movement by modulating GABAB receptor signaling. J. Biol. Chem. 287, 4972–4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107).Kim G., Jung S., Son H., Kim S., Choi J., Lee D.H., et al. (2014) The GABAB receptor associates with regulators of G-protein signaling 4 protein in the mouse prefrontal cortex and hypothalamus. BMB Rep. 47, 324–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108).Mutneja M., Berton F., Suen K.F., Lüscher C., Slesinger P.A. (2005) Endogenous RGS proteins enhance acute desensitization of GABA(B) receptor-activated GIRK currents in HEK-293T cells. Pflugers Arch. 450, 61–73. [DOI] [PubMed] [Google Scholar]
- 109).Nehring R.B., Horikawa H.P., El Far O., Kneussel M., Brandstätter J.H., Stamm S., et al. (2000) The metabotropic GABAB receptor directly interacts with the activating transcription factor 4. J. Biol. Chem. 275, 35185–35191. [DOI] [PubMed] [Google Scholar]
- 110).White J.H., McIllhinney R.A., Wise A., Ciruela F., Chan W.Y., Emson P.C., et al. (2000) The GABAB receptor interacts directly with the related transcription factors CREB2 and ATFx. Proc. Natl. Acad. Sci. U.S.A. 97, 13967–13972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111).Vernon E., Meyer G., Pickard L., Dev K., Molnar E., Collingridge G.L., et al. (2001) GABA(B) receptors couple directly to the transcription factor ATF4. Mol. Cell. Neurosci. 17, 637–645. [DOI] [PubMed] [Google Scholar]
- 112).Sauter K., Grampp T., Fritschy J.M., Kaupmann K., Bettler B., Mohler H., et al. (2005) Subtype-selective interaction with the transcription factor CCAAT/enhancer-binding protein (C/EBP) homologous protein (CHOP) regulates cell surface expression of GABA(B) receptors. J. Biol. Chem. 280, 33566–33572. [DOI] [PubMed] [Google Scholar]
- 113).Maier P.J., Zemoura K., Acuña M.A., Yévenes G.E., Zeilhofer H.U., Benke D. (2014) Ischemia-like oxygen and glucose deprivation mediates down-regulation of cell surface γ-aminobutyric acidB receptors via the endoplasmic reticulum (ER) stress-induced transcription factor CCAAT/enhancer-binding protein (C/EBP)-homologous protein (CHOP). J. Biol. Chem. 289, 12896–12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114).Heese K., Otten U., Mathivet P., Raiteri M., Marescaux C., Bernasconi R. (2000) GABA(B) receptor antagonists elevate both mRNA and protein levels of the neurotrophins nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) but not neurotrophin-3 (NT-3) in brain and spinal cord of rats. Neuropharmacology 39, 449–462. [DOI] [PubMed] [Google Scholar]
- 115).Good M.C., Zalatan J.G., Lim W.A. (2011) Scaffold proteins: hub for controlling the flow of cellular information. Science 332, 680–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116).Walther C., Ferguson S.S.G. (2015) Role of intracellular scaffolding proteins in the regulation of endocrine G protein-coupled receptor signaling. Mol. Endocrinol. 29, 814–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117).Fu H., Subramanian R.R., Masters S.C. (2000) 14-3-3 proteins: Structure, function, and regulation. Annu. Rev. Pharmacol. Toxicol. 40, 617–647. [DOI] [PubMed] [Google Scholar]
- 118).Couve A., Kittler J.T., Uren J.M., Calver A.R., Pangalos M.N., Walsh F.S., et al. (2005) Association of GABA(B) receptors and members of the 14-3-3 family of signaling proteins. Mol. Cell. Neurosci. 17, 317–328. [DOI] [PubMed] [Google Scholar]
- 119).Kantamneni S., Corrêa S.A., Hodgkinson G.K., Meyer G., Vinh N.N., Henley J.M., et al. (2007) GISP: a novel brain-specific protein that promotes surface expression and function of GABA(B) receptors. J. Neurochem. 100, 1003–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120).Pontier S.M., Lahaie N., Ginham R., St-Gelais F., Bonin H., Bell D.J., et al. (2006) Coordinated action of NSF and PKC regulates GABAB receptor signaling efficacy. EMBO J. 25, 2698–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121).Zapata J., Moretto E., Hannan S., Murru L., Longatti A., Mazza D., et al. (2017) Epilepsy and intellectual disability linked protein Shrm4 interaction with GABABRs shapes inhibitory neurotransmission. Nat. Commun. 8, 14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122).Balasubramanian S., Fam S.R., Hall R.A. (2007) GABAB receptor association with the PDZ scaffold Mupp1 alters receptor stability and function. J. Biol. Chem. 282, 4162–4171. [DOI] [PubMed] [Google Scholar]
- 123).Whiting P.J., Bonnert T.P., McKernan R.M., Farrar S., Le Bourdellès B., Heavens R.P., et al. (1999) Molecular and functional diversity of the expanding GABA-A receptor gene family. Ann. N. Y. Acad. Sci. 868, 645–653. [DOI] [PubMed] [Google Scholar]
- 124).Tretter V., Ehya N., Fuchs K., Sieghart W. (1997) Stoichiometry and assembly of a recombinant GABAA receptor subtype. J. Neurosci. 17, 2728–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125).Sieghart W., Sperk G. (2002) Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr. Top. Med. Chem. 2, 795–816. [DOI] [PubMed] [Google Scholar]
- 126).Balasubramanian S., Teissére J.A., Raju D.V., Hall R.A. (2004) Hetero-oligomerization between GABAA and GABAB receptors regulates GABAB receptor trafficking. J. Biol. Chem. 279, 18840–18850. [DOI] [PubMed] [Google Scholar]
- 127).Kuczewski N., Fuchs C., Ferrand N., Jovanovic J.N., Gaiarsa J.L., Porcher C. (2011) Mechanism of GABAB receptor-induced BDNF secretion and promotion of GABAA receptor membrane expression. J. Neurochem. 118, 533–545. [DOI] [PubMed] [Google Scholar]
- 128).Obrietan K., van den Pol A.N. (1998) GABAB receptor-mediated inhibition of GABAA receptor calcium elevations in developing hypothalamic neurons. J. Neurophysiol. 79, 1360–1370. [DOI] [PubMed] [Google Scholar]
- 129).Tao W., Higgs M.H., Spain W.J., Ransom C.B. (2013) Postsynaptic GABAB receptors enhance extrasynaptic GABAA receptor function in dentate gyrus granule cells. J. Neurosci. 33, 3738–3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130).Kantamneni S. (2015) Cross-talk and regulation between glutamate and GABAB receptors. Front. Cell. Neurosci. 9, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131).Masilamoni G.J., Smith Y. (2018) Metabotropic glutamate receptors: targets for neuroprotective therapies in Parkinson disease. Curr. Opin. Pharmacol. 38, 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132).Kamikubo Y., Tabata T., Kakizawa S., Kawakami D., Watanabe M., Ogura A., et al. (2007) Postsynaptic GABAB receptor signalling enhances LTD in mouse cerebellar Purkinje cells. J. Physiol. 585, 549–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133).Rives M.L., Vol C., Fukazawa Y., Tinel N., Trinquet E., Ayoub M.A., et al. (2009) Crosstalk between GABAB and mGlu1a receptors reveals new insight into GPCR signal integration. EMBO J. 28, 2195–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134).Tabata T., Araishi K., Hashimoto K., Hashimotodani Y., van der Putten H., Bettler B., et al. (2004) Ca2+ activity at GABAB receptors constitutively promotes metabotropic glutamate signaling in the absence of GABA. Proc. Natl. Acad. Sci. U.S.A. 101, 16952–16957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135).Bliss T.V., Collingridge G.L. (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361, 31–39. [DOI] [PubMed] [Google Scholar]
- 136).Malenka R.C., Bear M.F. (2004) LTP and LTD: an embarrassment of riches. Neuron 44, 5–21. [DOI] [PubMed] [Google Scholar]
- 137).Chalifoux J.R., Carter A.G. (2010) GABAB receptors modulate NMDA receptor calcium signals in dendritic spines. Neuron 66, 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138).Vargas K.J., Terunuma M., Tello J.A., Pangalos M.N., Moss S.J., Couve A. (2008) The availability of surface GABA B receptors is independent of gamma-aminobutyric acid but controlled by glutamate in central neurons. J. Biol. Chem. 283, 24641–24648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139).Terunuma M., Vargas K.J., Wilkins M.E., Ramírez O.A., Jaureguiberry-Bravo M., Pangalos M.N., et al. (2010) Prolonged activation of NMDA receptors promotes dephosphorylation and alters postendocytic sorting of GABAB receptors. Proc. Natl. Acad. Sci. U.S.A. 107, 13918–13923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140).Guetg N., Abdel Aziz S., Holbro N., Turecek R., Rose T., Seddik R., et al. (2010) NMDA receptor-dependent GABAB receptor internalization via CaMKII phosphorylation of serine 867 in GABAB1. Proc. Natl. Acad. Sci. U.S.A. 107, 13924–13929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141).Fiorentino H., Kuczewski N., Diabira D., Ferrand N., Pangalos M.N., Porcher C., et al. (2009) GABA(B) receptor activation triggers BDNF release and promotes the maturation of GABAergic synapses. J. Neurosci. 29, 11650–11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142).Tu H., Xu C., Zhang W., Liu Q., Rondard P., Pin J.P., et al. (2010) GABAB receptor activation protects neurons from apoptosis via IGF-1 receptor transactivation. J. Neurosci. 30, 749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143).Lin X., Li X., Jiang M., Chen L., Xu C., Zhang W., et al. (2012) An activity-based probe reveals dynamic protein-protein interactions mediating IGF-1R transactivation by the GABA(B) receptor. Biochem. J. 443, 627–634. [DOI] [PubMed] [Google Scholar]
- 144).Liu Z., Xiang Y., Sun G. (2013) The KCTD family of proteins: structure, function, disease relevance. Cell Biosci. 3, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145).Bartoi T., Rigbolt K.T., Du D., Köhr G., Blagoev B., Kornau H.C. (2010) GABAB receptor constituents revealed by tandem affinity purification from transgenic mice. J. Biol. Chem. 285, 20625–20633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146).Schwenk J., Metz M., Zolles G., Turecek R., Fritzius T., Bildl W., et al. (2010) Native GABA(B) receptors are heteromultimers with a family of auxiliary subunits. Nature 465, 231–235. [DOI] [PubMed] [Google Scholar]
- 147).Seddik R., Jungblut S.P., Silander O.K., Rajalu M., Fritzius T., Besseyrias V., et al. (2012) Opposite effects of KCTD subunit domains on GABA(B) receptor-mediated desensitization. J. Biol. Chem. 287, 39869–39877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148).Ivankova K., Turecek R., Fritzius T., Seddik R., Prezeau L., Comps-Agrar L., et al. (2013) Up-regulation of GABA(B) receptor signaling by constitutive assembly with the K+ channel tetramerization domain-containing protein 12 (KCTD12). J. Biol. Chem. 288, 24848–24856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149).Couve A., Restituito S., Brandon J.M., Charles K.J., Bawagan H., Freeman K.B., et al. (2004) Marlin-1, a novel RNA-binding protein associates with GABA receptors. J. Biol. Chem. 279, 13934–13943. [DOI] [PubMed] [Google Scholar]
- 150).Vidal R.L., Ramírez O.A., Sandoval L., Koenig-Robert R., Härtel S., Couve A. (2007) Marlin-1 and conventional kinesin link GABAB receptors to the cytoskeleton and regulate receptor transport. Mol. Cell. Neurosci. 35, 501–512. [DOI] [PubMed] [Google Scholar]
- 151).Saghatelyan A.K., Snapyan M., Gorissen S., Meigel I., Mosbacher J., Kaupmann K., et al. (2003) Recognition molecule associated carbohydrate inhibits postsynaptic GABA(B) receptors: a mechanism for homeostatic regulation of GABA release in perisomatic synapses. Mol. Cell. Neurosci. 24, 271–282. [DOI] [PubMed] [Google Scholar]
- 152).Humphrey S.J., James D.E., Mann M. (2015) Protein phosphorylation: a major switch mechanism for metabolic regulation. Trends Endocrinol. Metab. 26, 676–687. [DOI] [PubMed] [Google Scholar]
- 153).Cohen P. (2001) The role of protein phosphorylation in human health and disease. The Sir Hans Krebs Medal Lecture. Eur. J. Biochem. 268, 5001–5010. [DOI] [PubMed] [Google Scholar]
- 154).von Zastrow M. (2003) Mechanisms regulating membrane trafficking of G protein-coupled receptors in the endocytic pathway. Life Sci. 74, 217–224. [DOI] [PubMed] [Google Scholar]
- 155).Fairfax B.P., Pitcher J.A., Scott M.G., Calver A.R., Pangalos M.N., Moss S.J., et al. (2004) Phosphorylation and chronic agonist treatment atypically modulate GABAB receptor cell surface stability. J. Biol. Chem. 279, 12565–12573. [DOI] [PubMed] [Google Scholar]
- 156).Perroy J., Adam L., Qanbar R., Chénier S., Bouvier M. (2003) Phosphorylation-independent desensitization of GABA(B) receptor by GRK4. EMBO J. 22, 3816–3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157).Kanaide M., Uezono Y., Matsumoto M., Hojo M., Ando Y., Sudo Y., et al. (2007) Desensitization of GABA(B) receptor signaling by formation of protein complexes of GABA(B2) subunit with GRK4 or GRK5. J. Cell. Physiol. 210, 237–245. [DOI] [PubMed] [Google Scholar]
- 158).Terunuma M., Pangalos M.N., Moss S.J. (2010) Functional modulation of GABAB receptors by protein kinases and receptor trafficking. Adv. Pharmacol. 58, 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159).Kuramoto N., Wilkins M.E., Fairfax B.P., Revilla-Sanchez R., Terunuma M., Tamaki K., et al. (2007) Phospho-dependent functional modulation of GABA(B) receptors by the metabolic sensor AMP-dependent protein kinase. Neuron 53, 233–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160).Couve A., Thomas P., Calver A.R., Hirst W.D., Pangalos M.N., Walsh F.S., et al. (2002) Cyclic AMP-dependent protein kinase phosphorylation facilitates GABA(B) receptor-effector coupling. Nat. Neurosci. 5, 415–424. [DOI] [PubMed] [Google Scholar]
- 161).Adelfinger L., Turecek R., Ivankova K., Jensen A.A., Moss S.J., Gassmann M., et al. (2014) GABAB receptor phosphorylation regulates KCTD12-induced K+ current desensitization. Biochem. Pharmacol. 91, 369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162).Terunuma M., Haydon P.G., Pangalos M.N., Moss S.J. (2015) Purinergic receptor activation facilitates astrocytic GABAB receptor calcium signalling. Neuropharmacology 88, 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163).Dores M.R., Trejo J. (2012) Ubiquitination of G protein-coupled receptors: functional implications and drug discovery. Mol. Pharmacol. 82, 563–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164).Kennedy J.E., Marchese A. (2015) Regulation of GPCR trafficking by ubiquitin. Prog. Mol. Biol. Transl. Sci. 132, 15–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165).Kommaddi R.P., Shenoy S.K. (2013) Arrestins and protein ubiquitination. Prog. Mol. Biol. Transl. Sci. 118, 175–204. [DOI] [PubMed] [Google Scholar]
- 166).Cottrell G.S. (2013) Roles of proteolysis in regulation of GPCR function. Br. J. Pharmacol. 168, 576–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167).Zemoura K., Schenkel M., Acuña M.A., Yévenes G.E., Zeilhofer H.U., Benke D. (2013) Endoplasmic reticulum-associated degradation controls cell surface expression of γ-aminobutyric acid, type B receptors. J. Biol. Chem. 288, 34897–34905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168).Zemoura K., Trümpler C., Benke D. (2016) Lys-63-linked ubiquitination of γ-aminobutyric acid (GABA), type B1, at multiple sites by the E3 ligase mind bomb-2 targets GABAB receptors to lysosomal degradation. J. Biol. Chem. 291, 21682–21693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169).Zemoura K., Balakrishnan K., Grampp T., Benke D. (2018) Ca2+/calmodulin-dependent protein kinase II (CaMKII) β-dependent phosphorylation of GABAB1 triggers lysosomal degradation of GABAB receptors via Mind Bomb-2 (MIB2)-mediated Lys-63-linked ubiquitination. Mol. Neurobiol., doi:10.1007/s12035-018-1142-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170).Lahaie N., Kralikova M., Prézeau L., Blahos J., Bouvier M. (2016) Post-endocytotic deubiquitination and degradation of the metabotropic γ-aminobutyric acid receptor by the ubiquitin-specific protease 14. J. Biol. Chem. 291, 7156–7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171).Galvez T., Duthey B., Kniazeff J., Blahos J., Rovelli G., Bettler B., et al. (2001) Allosteric interactions between GB1 and GB2 subunits are required for optimal GABA(B) receptor function. EMBO J. 20, 2152–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172).Grampp T., Sauter K., Markovic B., Benke D. (2007) Gamma-aminobutyric acid type B receptors are constitutively internalized via the clathrin-dependent pathway and targeted to lysosomes for degradation. J. Biol. Chem. 282, 24157–24165. [DOI] [PubMed] [Google Scholar]
- 173).Grampp T., Notz V., Broll I., Fischer N., Benke D. (2008) Constitutive, agonist-accelerated, recycling and lysosomal degradation of GABA(B) receptors in cortical neurons. Mol. Cell. Neurosci. 39, 628–637. [DOI] [PubMed] [Google Scholar]
- 174).Becher A., White J.H., McIlhinney R.A. (2001) The gamma-aminobutyric acid receptor B, but not the metabotropic glutamate receptor type-1, associates with lipid rafts in the rat cerebellum. J. Neurochem. 79, 787–795. [DOI] [PubMed] [Google Scholar]
- 175).Becher A., Green A., Ige A.O., Wise A., White J.H., McIlhinney R.A. (2004) Ectopically expressed gamma-aminobutyric acid receptor B is functionally down-regulated in isolated lipid raft-enriched membranes. Biochem. Biophys. Res. Commun. 321, 981–987. [DOI] [PubMed] [Google Scholar]
- 176).Calebiro D., Rieken F., Wagner J., Sungkaworn T., Zabel U., Borzi A., et al. (2013) Single-molecule analysis of fluorescently labeled G-protein-coupled receptors reveals complexes with distinct dynamics and organization. Proc. Natl. Acad. Sci. U.S.A. 110, 743–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177).Cryan J.F., Kaupmann K. (2005) Don’t worry ‘B’ happy!: a role for GABA(B) receptors in anxiety and depression. Trends Pharmacol. Sci. 26, 36–43. [DOI] [PubMed] [Google Scholar]
- 178).Mombereau C., Kaupmann K., Froestl W., Sansig G., van der Putten H., Cryan J.F. (2004) Genetic and pharmacological evidence of a role for GABA(B) receptors in the modulation of anxiety- and antidepressant-like behavior. Neuropsychopharmacology 29, 1050–1062. [DOI] [PubMed] [Google Scholar]
- 179).Cryan J.F., Kelly P.H., Chaperon F., Gentsch C., Mombereau C., Lingenhoehl K., et al. (2004) Behavioral characterization of the novel GABAB receptor-positive modulator GS39783 (N,N′-dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine): anxiolytic-like activity without side effects associated with baclofen or benzodiazepines. J. Pharmacol. Exp. Ther. 310, 952–963. [DOI] [PubMed] [Google Scholar]
- 180).Nakagawa Y., Ishima T., Ishibashi Y., Yoshii T., Takashima T. (1996) Involvement of GABA(B) receptor systems in action of antidepressants: baclofen but not bicuculline attenuates the effects of antidepressants on the forced swim test in rats. Brain Res. 709, 215–220. [DOI] [PubMed] [Google Scholar]
- 181).Nakagawa Y., Ishima T., Ishibashi Y., Tsuji M., Takashima T. (1996) Involvement of GABAB receptor systems in experimental depression: baclofen but not bicuculline exacerbates helplessness in rats. Brain Res. 741, 240–245. [DOI] [PubMed] [Google Scholar]
- 182).Morley K.C., Baillie A., Fraser I., Furneaux-Bate A., Dore G., Roberts M., et al. (2018) Baclofen in the treatment of alcohol dependence with or without liver disease: multisite, randomised, double-blind, placebo-controlled trial. Br. J. Psychiatry 212, 362–369. [DOI] [PubMed] [Google Scholar]
- 183).Rose A.K., Jones A. (2018) Baclofen: its effectiveness in reducing harmful drinking, craving, and negative mood. A meta-analysis. Addiction 113, 1396–1406. [DOI] [PubMed] [Google Scholar]
- 184).Shoptaw S., Yang X., Rotheram-Fuller E.J., Hsieh Y.C., Kintaudi P.C., Charuvastra V.C., et al. (2003) Randomized placebo-controlled trial of baclofen for cocaine dependence: preliminary effects for individuals with chronic patterns of cocaine use. J. Clin. Psychiatry 64, 1440–1448. [DOI] [PubMed] [Google Scholar]
- 185).Young K.A., Franklin T.R., Roberts D.C., Jagannathan K., Suh J.J., Wetherill R.R., et al. (2014) Nipping cue reactivity in the bud: baclofen prevents limbic activation elicited by subliminal drug cues. J. Neurosci. 34, 5038–5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186).Agabio R., Colombo G. (2014) GABAB receptor ligands for the treatment of alcohol use disorder: preclinical and clinical evidence. Front. Neurosci. 8, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187).Dewey S.L., Brodie J.D., Gerasimov M., Horan B., Gardner E.L., Ashby C.R., Jr. (1999) A pharmacologic strategy for the treatment of nicotine addiction. Synapse 31, 76–86. [DOI] [PubMed] [Google Scholar]
- 188).Reynaud M., Aubin H.J., Trinquet F., Zakine B., Dano C., Dematteis M., et al. (2017) A randomized, placebo-controlled study of high-dose baclofen in alcohol-dependent patients-the ALPADIR study. Alcohol Alcohol. 52, 439–446. [DOI] [PubMed] [Google Scholar]
- 189).Gambardella A., Manna I., Labate A., Chifari R., La Russa A., Serra P., et al. (2003) GABA(B) receptor 1 polymorphism (G1465A) is associated with temporal lobe epilepsy. Neurology 60, 560–563. [DOI] [PubMed] [Google Scholar]
- 190).Muñoz A., Arellano J.I., DeFelipe J. (2002) GABABR1 receptor protein expression in human mesial temporal cortex: changes in temporal lobe epilepsy. Comp. Neurol. 449, 166–179. [DOI] [PubMed] [Google Scholar]
- 191).Princivalle A.P., Duncan J.S., Thom M., Bowery N.G. (2002) Studies of GABA(B) receptors labelled with [(3)H]-CGP62349 in hippocampus resected from patients with temporal lobe epilepsy. Br. J. Pharmacol. 136, 1099–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192).Teichgräber L.A., Lehmann T.N., Meencke H.J., Weiss T., Nitsch R., Deisz R.A. (2009) Impaired function of GABA(B) receptors in tissues from pharmacoresistant epilepsy patients. Epilepsia 50, 1697–1716. [DOI] [PubMed] [Google Scholar]
- 193).Schuler V., Lüscher C., Blanchet C., Klix N., Sansig G., Klebs K., et al. (2001) Epilepsy, hyperalgesia, impaired memory, and loss of pre- and postsynaptic GABA(B) responses in mice lacking GABA(B(1)). Neuron 31, 47–58. [DOI] [PubMed] [Google Scholar]
- 194).Vienne J., Bettler B., Franken P., Tafti M. (2010) Differential effects of GABAB receptor subtypes, {gamma}-hydroxybutyric acid, and baclofen on EEG activity and sleep regulation. J. Neurosci. 30, 14194–14204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195).Crunelli V., Leresche N. (1991) A role for GABAB receptors in excitation and inhibition of thalamocortical cells. Trends Neurosci. 14, 16–21. [DOI] [PubMed] [Google Scholar]
- 196).Hosford D.A., Clark S., Cao Z., Wilson W.A., Jr., Lin F.H., Morrisett R.A., et al. (1992) The role of GABAB receptor activation in absence seizures of lethargic (lh/lh) mice. Science 257, 398–401. [DOI] [PubMed] [Google Scholar]
- 197).Seidenbecher T., Pape H.C. (2001) Contribution of intralaminar thalamic nuclei to spike-and-wave-discharges during spontaneous seizures in a genetic rat model of absence epilepsy. Eur. J. Neurosci. 13, 1537–1546. [DOI] [PubMed] [Google Scholar]
- 198).Pacey L.K., Tharmalingam S., Hampson D.R. (2011) Subchronic administration and combination metabotropic glutamate and GABAB receptor drug therapy in fragile X syndrome. J. Pharmacol. Exp. Ther. 338, 897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199).Mareš P. (2012) Anticonvulsant action of GABAB receptor positive modulator CGP7930 in immature rats. Epilepsy Res. 100, 49–54. [DOI] [PubMed] [Google Scholar]
- 200).Mareš P., Tichá K., Mikulecká A. (2013) Anticonvulsant and behavioral effects of GABA(B) receptor positive modulator CGP7930 in immature rats. Epilepsy Behav. 28, 113–120. [DOI] [PubMed] [Google Scholar]
- 201).Adams C.L., Lawrence A.J. (2007) CGP7930: a positive allosteric modulator of the GABAB receptor. CNS Drug Rev. 13, 308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202).Pin J.P., Prézeau L. (2007) Allosteric modulators of GABA(B) receptors: mechanism of action and therapeutic perspective. Curr. Neuropharmacol. 5, 195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203).Bowery N.G., Hudson A.L., Price G.W. (1987) GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience 20, 365–383. [DOI] [PubMed] [Google Scholar]
- 204).Iwakiri M., Mizukami K., Ikonomovic M.D., Ishikawa M., Hidaka S., Abrahamson E.E., et al. (2005) Changes in hippocampal GABABR1 subunit expression in Alzheimer’s patients: association with Braak staging. Acta Neuropathol. 109, 467–474. [DOI] [PubMed] [Google Scholar]
- 205).Massone S., Vassallo I., Fiorino G., Castelnuovo M., Barbieri F., Borghi R., et al. (2010) 17A, a novel non-coding RNA, regulates GABA B alternative splicing and signaling in response to inflammatory stimuli and in Alzheimer disease. Neurobiol. Dis. 41, 308–317. [DOI] [PubMed] [Google Scholar]
- 206).Froestl W., Gallagher M., Jenkins H., Madrid A., Melcher T., Teichman S., et al. (2004) SGS742: the first GABA(B) receptor antagonist in clinical trials. Biochem. Pharmacol. 68, 1479–1487. [DOI] [PubMed] [Google Scholar]
- 207).Serrats J., Cunningham M.O., Davies C.H. (2017) GABAB receptor modulation-to B or not to be B a pro-cognitive medicine? Curr. Opin. Pharmacol. 35, 125–132. [DOI] [PubMed] [Google Scholar]