ABSTRACT
Background
The dietary insulin index (II) directly quantifies dietary effects on postprandial insulin secretion, whereas the empirical dietary index for hyperinsulinemia (EDIH), based on fasting C-peptide concentrations, is primarily reflective of insulin resistance. How these scores are related to nonfasting C-peptide in cohort studies has not been examined.
Objective
We investigated the extent to which EDIH and II scores predict plasma C-peptide concentrations, in cross-sectional analyses by postprandial duration at blood collection from 1 to ≥15 h.
Methods
Both EDIH and II scores were calculated from food-frequency questionnaire data reported by 3964 men in the Health Professionals Follow-up Study (1993–1995) and 6215 women in the Nurses’ Health Study (1989–1990) who were not diabetic. We constructed 12 multivariable-adjusted linear regression models separately in men and women, by postprandial duration, to calculate relative differences and absolute values of plasma C-peptide concentrations in dietary index quintiles.
Results
In both men and women, C-peptide concentrations were elevated 1–2 h after eating and declined with increasing postprandial duration. In men, percent differences in C-peptide concentration in the highest compared with lowest dietary index quintile were: EDIH: 0–1 h: 50%; 2 h: 22%; 14 h: 14%; ≥15 h: 30% (all P-trend< 0.05). II: 0–1 h: 19% (P-trend = 0.09); 2 h: 3% (P-trend = 0.09); 14 h: −6% (P-trend = 0.17); ≥15 h: −15% (P-trend = 0.02). Corresponding results among women were: EDIH: 0–1 h: 29% (P-trend = 0.002); 2 h: 33% (P-trend = 0.009); 14 h: 44% (P-trend < 0.0001); ≥15 h: 40% (P-trend < 0.0001). II: 0–1 h: −12% (P-trend = 0.09); 2 h: 17% (P-trend = 0.09); 14 h: −14% (P-trend = 0.009); ≥15 h: −3% (P-trend = 0.37).
Conclusion
The EDIH was superior to the II in predicting both fasting and nonfasting C-peptide concentrations, suggesting that the EDIH may be better in assessing dietary effects of hyperinsulinemia on disease risk in adult men and women.
Keywords: postprandial, fasting, dietary insulin index, dietary insulin load, empirical dietary index for hyperinsulinemia, C-peptide
Introduction
Hyperinsulinemia has been hypothesized to partly underlie the role of diet in major chronic disease development and/or progression (1, 2). Two dietary indexes have been developed to estimate how diet influences insulinemia. First, the insulinemic potential of diet has been estimated by the insulin index (II). The II is based on a similar concept to the more widely used glycemic index, which characterizes carbohydrate-containing foods according to their ability to raise blood glucose concentrations postprandially compared with a reference food (glucose or white bread) (3). Although the carbohydrate load is a key factor in the insulin response, noncarbohydrate factors such as protein can also stimulate insulin secretion. The II directly quantifies the postprandial insulinemic potential of a food and takes into account foods with low or no carbohydrate content (4). Based on the II of individual foods, the overall insulin load and average II of a diet can be estimated. Second, we developed the empirical dietary index for hyperinsulinemia (EDIH), a weighted dietary pattern score to assess the insulinemic potential of usual diets based on fasting plasma concentrations of C-peptide (insulin exposure) (5).
Conceptually, “chronic” insulin exposure involves both fasting and postprandial, during which most of the waking hours are spent. Neither the II nor the EDIH has been evaluated directly for chronic insulin exposure. Interestingly, the II was either not predictive of fasting C-peptide concentrations in our study populations or showed an unexpected inverse association with fasting (≥6 h after the last meal) C-peptide (4, 5). The lack of an association may not be surprising because the II is based on short-term increases in insulin after a meal, and may not be reflected in fasting C-peptide. In contrast, the EDIH is based on empirically predicting fasting C-peptide concentrations (5), but its influence on chronic (nonfasting plus fasting) C-peptide has not been evaluated. Thus, we designed the current study to examine the association of the II and the EDIH scores in relation to C-peptide concentrations over the course of postprandial duration; that is, time from nonfasting to fasting periods (1 to ≥15 h after eating). This analysis may provide a better indication of how these indexes assess chronic insulin exposure.
Methods
Study populations
The Nurses’ Health Study (NHS) and Health Professionals Follow-up Study (HPFS) are ongoing prospective cohorts established in 1976 and 1986, respectively. The NHS (n = 121,701) enrolled female registered nurses aged 30–55 y (6), and the HPFS (n = 51,529) enrolled male health professionals aged 40–75 y at baseline. Blood samples were collected from subpopulations of the NHS (n = 32,826) in 1989–1990, and HPFS (n = 18,225) from 1993 to 1995 (7). Blood collection was conducted using similar protocols for both cohorts. The procedures, including collection, handling, and storage, have been previously summarized (8). In the current study, we used data from previous matched case-control studies nested within each of the 2 cohorts that measured concentrations of plasma C-peptide. The current study included 3964 men from the HPFS and 6215 women from the NHS with C-peptide data. The Institutional Review Boards at Brigham and Women's Hospital and at Harvard T.H. Chan School of Public Health approved this study.
Assessment of dietary data and calculation of the insulin-related dietary indexes
Dietary data are updated every 4 y in the HPFS (since 1986) and in the NHS (since 1980), with a validated semiquantitative FFQ that assessed diet intake in the previous year (9–11). We used dietary data from the questionnaires closest to the blood draw, that is, the 1990 FFQ for the NHS and the 1994 FFQ for HPFS. Participants with excessive missing items (≥70) on the FFQs or implausibly low or high energy intake (<600 or >3500 kcal/d for women and <800 or >4200 kcal/d for men) were excluded (12). Also, all diabetic paticipants were excluded.
The EDIH score, described in detail elsewhere (5, 13), was developed in a sample of 5812 women in the NHS, to empirically create a score to measure the insulinemic potential of whole diets defined using food groups. Thirty-nine predefined food groups (servings per day) (12) were entered into stepwise linear regression models to identify a dietary pattern most predictive of fasting plasma C-peptide concentrations. The EDIH score is a weighted sum of 18 food groups, with higher (more positive) scores indicating higher insulinemic diets (hyperinsulinemia) and lower (more negative) scores indicating lower insulinemic diets. In the NHS, there was a 40% increase in C-peptide concentration among the women in EDIH quintile 5 compared with those in quintile 1 (adjusted relative concentration: 1.40; 95% CI: 1.32, 1.46) (5). The EDIH score was further evaluated for validity in 2 independent samples of women (NHS-II, n = 1717) and men (HPFS, n = 4002) and found to significantly predict fasting plasma C-peptide concentrations (5). The food groups contributing to higher EDIH scores are red meat, low-energy beverages (low-energy cola and other low-energy carbonated beverages), cream soups, processed meat, margarine, poultry, French fries, fish (other than dark-meat fish), high-energy beverages (cola and other carbonated beverages with sugar, fruit drinks), tomatoes, low-fat dairy, and eggs. The food groups contributing to lower EDIH score are intakes of wine, coffee, whole fruit, high-fat dairy products, and green leafy vegetables (5). We calculated EDIH scores for each participant based on self-administered FFQ data in 1990 in the NHS and in 1994 in the HPFS.
The II value for each food item compares the postprandial plasma insulin response induced by that food relative to that of a reference food (glucose or white bread). Insulin index values for foods that appeared in the FFQ were obtained either from published estimates (31 foods) (14) or from direct testing of food items at the University of Sydney, Australia (73 foods) (4). Various US food samples, which were selected to be representative for FFQ items, were shipped for analysis to the laboratory in Sydney, Australia, for testing as previously described (15). Briefly, each person consumed a variety of test foods on separate days, with blood insulin measured every 15 min for 2 h after consumption. The food II value was calculated by dividing the area under the insulin response curve for 1000 kJ of a test food by the area under the insulin response curve for 1000 kJ of the reference food. The II value for each food represented the mean responses of 11–13 participants (15). We then calculated the average dietary insulin load for each participant by multiplying the II value of each food by the total energy intake contributed by that food, and summing values for all reported food items. The dietary II for the overall diet, which is the weighted mean of the II values for each of the component foods, was calculated by dividing the insulin load by total energy intake (4). Higher dietary II scores represent higher insulinemic potential of the overall diet, whereas lower scores represent lower insulinemic potential of the diet.
C-peptide measurement
C-peptide has been shown to be a better measure of β-cell secretory activity than insulin, as it is not extracted by the liver, has a slower metabolic clearance rate, and does not cross-react with antibodies to insulin (16). Procedures for the measurement of plasma C-peptide concentrations in the HPFS and NHS have been described (17, 18). C-peptide was measured by ELISA (Diagnostic Systems Laboratories/Beckman Coulter). The average intra-assay coefficient of variation from blinded quality control samples was <12% across batches. In the nested case-control studies in which C-peptide was measured, samples from cases and their matched controls were analyzed in the same batch. Quality control samples were randomly interspersed among the case-control samples, and laboratory personnel were blinded to quality control and case-control status for all assays. These measurements were conducted in multiple batches over several years, therefore we conducted statistical calibration to adjust for batch-related variability according to the methods of Rosner et al. (19). Briefly, a batch effect correction factor was calculated using linear regression to model the association between assay batch and natural log-transformed values of C-peptide. All values were corrected by the batch-specific factor to normalize values across the batches.
Assessment of covariate data
Both cohorts collected nondietary data (e.g., medical history and lifestyle factors) and updated the data every 2 y through self-administered questionnaires. For this cross-sectional study, we used covariate data collected closest to blood draw, that is, 1990 for NHS and 1994 for HPFS. Age was defined as the participant's age at the time of blood draw. We calculated participants’ BMI (kg/m2) using height (meters) reported at baseline for each cohort, and weight (kilograms) reported on the questionnaire closest to blood draw. Participants reported smoking status (never, former, current), and we calculated physical activity, expressed in metabolic equivalent h/wk (MET-h/wk) by summing the average MET-h/wk for the following activities: tennis/squash/racquetball, rowing, calisthenics, walking, jogging, running, bicycling, and swimming. The reproducibility and validity of the physical activity questionnaire have been evaluated (20, 21). Regular use of medication [aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs)] was defined as use of ≥2 standard tablets (325 mg) of aspirin or ≥2 tablets of NSAIDs/wk. Data on diabetes (yes/no), hypertension (yes/no), hypercholesterolemia (yes/no), arthritis (yes/no), cancer (yes/no), and heart disease (yes/no) were also collected. Women were asked about menopausal status (pre-/postmenopausal) and current and past use of hormone therapy (yes/no).
Statistical analysis
We described participants’ characteristics using means ± SDs) for continuous variables, and frequencies (percentages) for categorical variables across quintiles of the insulin-related dietary scores. Given that C-peptide values were log transformed to normalize the distributions before analyses, we back-transformed C-peptide values to the original units (i.e., ex, where x is the natural log-transformed C-peptide concentration) (22, 23).
To assess the association of the dietary scores with plasma C-peptide concentrations by postprandial duration, we conducted 12 multivariable-adjusted linear regression analyses by postprandial duration from 1 to ≥15 h after the last meal before blood donation; to model the natural log of C-peptide concentration as the outcome (or dependent variable) and then back-transformed to obtain an estimate of the absolute concentration in each quintile of the dietary score, or to obtain the relative change (as percentage of difference) in C-peptide concentrations in higher dietary index quintiles compared with the lowest quintile as reference. One multivariable-adjusted linear regression model was fit for each postprandial hour before blood donation, separately in men and in women, for a total of 12 models (i.e., 0–1 to ≥15 h postprandial). The sample size for each model was unique and separate from all other models. We combined participants at some timepoints to have ≥200 participants at each postprandial duration. Therefore, we combined men at 5–7 and 8–9 h and women at 4–5 and 6–8 h. In sensitivity analyses to determine whether the association of the II was different from the insulin load, we replaced the II with the insulin load in each of the 12 linear regression models.
All models were adjusted for the potential confounding variables listed in the covariate section, and the model-based absolute and relative biomarker concentrations were calculated at the mean values of the continuous variables and at the reference category of the categorical variables. Covariates were selected based on previous studies of the insulinemic potential of diet and C-peptide concentrations (4, 5). Both dietary scores were adjusted for total energy intake using the residual method (24), therefore energy intake was not directly included in the multivariable models. We did not include BMI in the primary analyses but adjusted for BMI (continuous) in sensitivity analyses. In addition, we examined trends in unadjusted C-peptide concentrations by postprandial duration in quintiles of the dietary indexes. Given that the sample sizes for each analysis (at each distinct timepoint) differed, the precision of the estimates for each timepoint (i.e., width of the 95% CI) may be affected. Also, the sample size at each timepoint was distributed into fifths across index quintiles using cutpoints based on the total study population to facilitate comparisons across distinct timepoints.
We also conducted analyses of unadjusted C-peptide concentrations stratified by BMI categories (<25 and ≥25), to examine potential influence by body weight. In the subgroup analyses we used the same dietary index quintile cutpoints as for the primary analysis, to enhance comparability of findings across subgroups. For analyses of linear trend, we used the continuous dietary index in multivariable-adjusted analyses. All analyses were conducted using SAS version 9.4, and all tests were 2-sided. Tests of trend with P < 0.05 were considered statistically significant, whereas for the relative change models, 95% CIs not including 0 were considered to indicate statistically significant findings.
Results
Table 1 shows participant characteristics according to quintiles of the dietary indexes. Participants consuming the most hyperinsulinemic diets (classified in EDIH quintile 5) had higher C-peptide concentrations, higher BMI, lower physical activity, and lower intakes of dietary fiber, calcium, and whole grains than did those consuming low insulinemic diets (EDIH quintile 1). In contrast, the II score did not appear to be associated with these characteristics or tended to be correlated either inversely with BMI or directly with nutrients (Table 1).
TABLE 1.
Distribution of participant characteristics in quintiles of the insulin-related dietary indexes1
| Dietary index quintiles2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nurses’ Health Study | Health Professionals Follow-up Study | |||||||||
| Characteristic | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 |
| EDIH score | ||||||||||
| n | 1238 | 1245 | 1245 | 1245 | 1242 | 788 | 795 | 796 | 795 | 790 |
| Plasma C-peptide, ng/mL | 1.9 ± 0.9 | 2.1 ± 0.8 | 2.1 ± 0.8 | 2.3 ± 0.9 | 2.6 ± 0.9 | 2.0 ± 0.9 | 2.2 ± 0.9 | 2.2 ± 0.9 | 2.4 ± 0.9 | 2.4 ± 0.9 |
| BMI, kg/m2 | 24.1 ± 3.9 | 25.0 ± 4.1 | 25.0 ± 4.1 | 26.3 ± 4.9 | 27.7 ± 5.4 | 25.2 ± 2.9 | 25.3 ± 2.9 | 25.7 ± 3.1 | 26.0 ± 3.2 | 26.9 ± 3.9 |
| Overweight/obese (BMI ≥25), % | 33.5 | 42.6 | 45.1 | 53.5 | 63.5 | 48.2 | 52.6 | 57.3 | 60.9 | 67.1 |
| Age at screening, y | 59.5 ± 6.6 | 58.9 ± 6.6 | 58.9 ± 6.6 | 57.6 ± 7.1 | 56.3 ± 7.5 | 62.6 ± 8.2 | 62.5 ± 8.3 | 62.4 ± 8.4 | 61.9 ± 8.3 | 60.8 ± 8.4 |
| Physical activity, MET-h/wk | 18.9 ± 20.1 | 17.3 ± 25.5 | 17.3 ± 25.5 | 15.0 ± 21.8 | 13.2 ± 21.3 | 45.0 ± 40.0 | 38.0 ± 34.1 | 38.8 ± 39.0 | 34.1 ± 34.6 | 34.6 ± 48.5 |
| Alcohol,3 servings/wk | 1.3 ± 1.8 | 0.6 ± 0.9 | 0.6 ± 0.9 | 0.4 ± 0.6 | 0.3 ± 0.8 | 2.4 ± 2.3 | 1.5 ± 1.6 | 1.1 ± 1.4 | 1.0 ± 1.2 | 0.9 ± 1.2 |
| Total energy intake, kcal/d | 1907 ± 487 | 1739 ± 479 | 1739 ± 479 | 1678 ± 489 | 1859 ± 552 | 2207 ± 617 | 1982 ± 574 | 1958 ± 599 | 1920 ± 568 | 2176 ± 640 |
| Dietary fiber, g/d | 20.9 ± 6.5 | 19.7 ± 5.2 | 19.7 ± 5.2 | 17.8 ± 5.0 | 16.2 ± 4.2 | 27.2 ± 8.1 | 24.8 ± 7.3 | 22.5 ± 6.0 | 21.0 ± 5.6 | 18.6 ± 5.3 |
| Dietary calcium, mg/d | 765 ± 293 | 780 ± 278 | 780 ± 278 | 741 ± 283 | 684 ± 253 | 830 ± 277 | 832 ± 285 | 840 ± 308 | 804 ± 286 | 750 ± 270 |
| Dietary vitamin D, IU/d | 208 ± 117 | 221 ± 113 | 221 ± 113 | 224 ± 122 | 203 ± 112 | 257 ± 135 | 269 ± 139 | 273 ± 128 | 269 ± 126 | 257 ± 121 |
| Dietary lycopene, mg/d | 5.8 ± 3.6 | 6.3 ± 3.7 | 6.3 ± 3.7 | 6.4 ± 3.6 | 6.7 ± 4.2 | 7.4 ± 4.6 | 7.6 ± 4.7 | 7.5 ± 4.7 | 7.8 ± 5.9 | 7.9 ± 5.6 |
| Whole grains, g/d | 24.5 ± 19.2 | 23.2 ± 15.4 | 23.2 ± 15.4 | 19.9 ± 14.6 | 14.8 ± 11.8 | 37.3 ± 21.6 | 35.5 ± 21.1 | 31.8 ± 19.8 | 27.8 ± 16.3 | 22.2 ± 14.8 |
| Aspirin/NSAID user, % | 33.8 | 31.4 | 35.3 | 37.8 | 40.5 | 18.4 | 21.1 | 17.3 | 17.4 | 22.7 |
| Current smoker, % | 10.9 | 10.8 | 11.5 | 11.2 | 12.2 | 2.0 | 4.3 | 3.5 | 4.9 | 7.7 |
| II score | ||||||||||
| n | 1243 | 1243 | 1242 | 1243 | 1244 | 789 | 792 | 799 | 795 | 789 |
| Plasma C-peptide, ng/mL | 2.2 ± 0.9 | 2.2 ± 0.9 | 2.2 ± 0.9 | 2.2 ± 0.9 | 2.1 ± 0.9 | 2.3 ± 0.9 | 2.3 ± 0.9 | 2.4 ± 0.9 | 2.2 ± 0.9 | 2.2 ± 0.9 |
| BMI, kg/m2 | 25.6 ± 4.8 | 26.2 ± 4.9 | 25.8 ± 4.7 | 25.8 ± 4.7 | 25.1 ± 4.3 | 26.1 ± 3.4 | 26.1 ± 3.3 | 25.9 ± 3.3 | 25.7 ± 3.2 | 25.3 ± 3.1 |
| Overweight/obese (BMI ≥25), % | 47.2 | 50.6 | 48.4 | 48.3 | 43.7 | 61.1 | 60.4 | 58.2 | 56.1 | 50.3 |
| Age at screening, y | 57.7 ± 6.9 | 58.0 ± 7.2 | 57.7 ± 7.2 | 58.3 ± 7.0 | 59.2 ± 6.7 | 61.9 ± 8.1 | 61.7 ± 8.4 | 62.3 ± 8.2 | 61.8 ± 8.6 | 62.5 ± 8.5 |
| Physical activity, MET-h/wk | 17.4 ± 23.6 | 16.4 ± 20.8 | 14.9 ± 16.2 | 17.0 ± 32.1 | 15.7 ± 24.0 | 36.8 ± 36.6 | 40.9 ± 47.7 | 37.7 ± 39.1 | 37.1 ± 37.9 | 37.9 ± 35.8 |
| Alcohol,3 servings/wk | 1.6 ± 1.9 | 0.6 ± 0.9 | 0.4 ± 0.6 | 0.3 ± 0.5 | 0.2 ± 0.4 | 2.9 ± 2.3 | 1.6 ± 1.4 | 1.1 ± 1.2 | 0.8 ± 1.0 | 0.5 ± 0.7 |
| Total energy intake, kcal/d | 1765 ± 541 | 1820 ± 528 | 1800 ± 497 | 1770 ± 484 | 1693 ± 451 | 2001 ± 622 | 2105 ± 627 | 2028 ± 645 | 2006 ± 564 | 2001 ± 584 |
| Dietary fiber, g/d | 17.8 ± 6.3 | 18.3 ± 5.5 | 18.7 ± 5.0 | 19.1 ± 5.2 | 19.6 ± 5.4 | 20.6 ± 7.4 | 22.2 ± 6.9 | 22.7 ± 7.0 | 23.8 ± 6.8 | 24.8 ± 7.2 |
| Dietary calcium, mg/d | 668 ± 266 | 736 ± 293 | 752 ± 270 | 783 ± 278 | 791 ± 264 | 717 ± 254 | 792 ± 292 | 806 ± 252 | 846 ± 292 | 897 ± 329 |
| Dietary vitamin D, IU/d | 206 ± 125 | 220 ± 123 | 219 ± 114 | 221 ± 109 | 215 ± 105 | 245 ± 128 | 267 ± 125 | 265 ± 122 | 273 ± 136 | 276 ± 138 |
| Dietary lycopene, mg/d | 6.9 ± 4.6 | 6.5 ± 3.8 | 6.2 ± 3.3 | 6.2 ± 3.4 | 5.6 ± 3.2 | 8.1 ± 5.2 | 8.3 ± 5.8 | 7.4 ± 4.4 | 7.6 ± 4.9 | 6.9 ± 5.3 |
| Whole grains, g/d | 15.9 ± 16.9 | 19.8 ± 15.2 | 20.7 ± 14.9 | 22.2 ± 15.0 | 25.0 ± 17.0 | 24.1 ± 17.9 | 28.7 ± 18.0 | 31.3 ± 19.6 | 33.5 ± 18.5 | 37.2 ± 21.0 |
| Aspirin/NSAID user, % | 36.6 | 37.2 | 35.8 | 36.0 | 33.4 | 22.2 | 21.0 | 20.2 | 17.4 | 16.2 |
| Current smoker, % | 20.4 | 12.4 | 9.5 | 7.2 | 7.2 | 8.9 | 4.3 | 4.9 | 2.1 | 2.3 |
Values are percentages or means ± SDs. All biomarker values were back-transformed (ex) as biomarker data were ln-transformed before analyses. EDIH, empirical dietary index for hyperinsulemia; II, dietary insulin index; MET-h, metabolic equivalent hours; NSAID, nonsteroidal anti-inflammatory drug.
Both dietary index scores were adjusted for total energy intake using the residual method. Lower dietary index scores indicate low insulinemic diets, whereas higher scores indicate high insulinemic diets.
Alcohol servings was the sum of servings of: beer (1 glass, 1 bottle, or 1 can), wine (4-oz glass of red wine, white wine), and liquor (1 drink or 1 shot whiskey, gin, etc.).
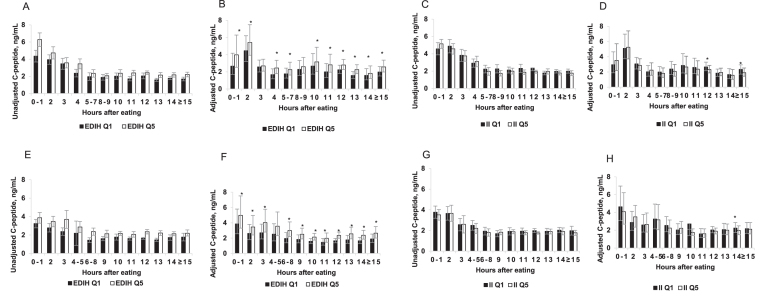
Figure 1 shows the trend of insulin response over time by postprandial duration for the unadjusted and adjusted C-peptide concentrations in the highest and lowest quintiles of the dietary indexes. As expected, we showed that there was a high insulin response, as reflected by high C-peptide concentrations 1–4 h after the last meal, in both men and women. In multivariable analyses examining trends over time, the EDIH showed greater differences between participants across quintiles (Q) than the II. For example, the adjusted concentrations among women were: 0–1 h: Q1 = 3.86 ng/mL, Q5 = 4.98 ng/mL; 2 h: Q1 = 2.61 ng/mL, Q5 = 3.46 ng/mL; …; 14 h: Q1 = 1.65 ng/mL, Q5 = 2.38 ng/mL; ≥15 h: Q1 = 1.90 ng/mL, Q5 = 2.65 ng/mL (Figure 1 E, F). For the II score in men and women, there did not appear to be differences in C-peptide concentrations between participants in the lowest and highest II quintiles. For example, adjusted concentrations among women were: 0–1 h: Q1 = 4.63 ng/mL, Q5 = 4.09 ng/mL; 2 h: Q1 = 2.87 ng/mL, Q5 = 3.50 ng/mL; …; 14 h: Q1 = 2.24 ng/mL, Q5 = 1.98 ng/mL; ≥15 h: Q1 = 2.18 ng/mL, Q5 = 2.12 ng/mL (Figure 1 G, H). A similar trend over time by postprandial duration was observed among the men (Figure 1 C, D).
FIGURE 1.
Plasma absolute unadjusted and adjusted C-peptide concentrations among men (A, B, C, D) and women (E, F, G, H) classified in the lowest (Q1) and highest (Q5) quintiles of the EDIH score (A, B, E, F) and the dietary II score (C, D, G, H). Values are means and 95% CIs. *Significant linear trend across quintiles, P-trend < 0.05. The unadjusted concentrations represent observed C-peptide concentrations not statistically transformed. The adjusted concentrations were obtained from 12 separate multivariable-adjusted linear regression models, 1 model for each postprandial duration; controlling for age at blood draw, aspirin/NSAID use, smoking status, chronic diseases/conditions (hypertension, hypercholesterolemia, heart disease, arthritis), and case-control status. Diabetic patients were excluded and both dietary scores were adjusted for energy intake using the residual method. The sample sizes at each postprandial duration were as follows: (Men) 0–1 h, n = 287; 2h, n = 348; 3 h, n = 244; 4 h, n = 258; 5–7 h, n = 254; 8–9 h, n = 200; 10 h, n = 333; 11 h, n = 205; 12 h, n = 833; 13 h, n = 256; 14 h, n = 420; and ≥15 h, n = 326. (Women) 0–1 h, n = 240; 2 h, n = 289; 3 h, n = 205; 4–5 h, n = 225; 6–8 h, n = 272; 9 h, n = 304; 10 h, n = 799; 11 h, n = 469; 12 h, n = 1656; 13 h, n = 516; 14 h, n = 753; and ≥15 h, n = 487. EDIH, empirical dietary index for hyperinsulinemia; II, insulin index; NSAID, nonsteroidal anti-inflammatory drug; Q, quintile.
The cross-sectional associations of the dietary indexes and C-peptide concentrations by postprandial duration are shown in Table 2 for men and in Table 3 for women. For the EDIH in both men and women, we observed significant cross-sectional associations in EDIH quintiles comparing higher quintiles to the lowest quintile as reference. There were also significant linear trends across the quintiles at all postprandial durations, but there was no clear trend in the magnitude of the associations given the differing sample sizes. For example, among men, percentage of differences in C-peptide concentration in the highest compared with the lowest EDIH quintiles were: 0–1 h: 50% (95% CI: 15%, 95%); 2 h: 22% (−8%, 60%); …; 14 h: 14% (−6%, 39%); ≥15 h: 30% (5%, 61%) (all P-trend < 0.05) (Table 2). A similar pattern was observed among women (Table 3). In contrast, for the II, there was no association during postprandial period and weak inverse associations were observed during the fasting period in both men and women. For example, among men, percentage of differences in C-peptide concentration in the highest compared with lowest II quintiles were: 0–1 h: 19% (95% CI: −8%, 55%; P-trend = 0.09); 2 h: 3% (−23%, 36%; P-trend = 0.09); …; 14 h: −6% (−23%, 15%; P-trend = 0.17); ≥15 h: −15% (−31%, 5%; P-trend = 0.02) (Table 2), with a similar trend observed among women (Table 3). Also, results for the insulin load were not materially different from those for the II (Supplemental Table 1). Although associations were slightly attenuated when additionally adjusted for BMI, the trends did not change materially (Supplemental Tables 2and3). With the weak inverse associations observed for the II, we further explored the potential influence of sample size by combining all participants who fasted for ≤5 h (n = 1391 in men and 1231 in women) and those who fasted for ≥10 h (n = 1003 in men and 1756 in women). The percentage of differences in C-peptide concentrations in the highest compared with the lowest II quintiles were as follows: 3% (−11%, 19%; P-trend = 0.72) postprandial and −7% (−17%, 5%; P-trend = 0.03) during fasting among men; and −6% (−17%, 11%; P-trend = 0.42) postprandial and −7% (−16%, 3%; P-trend = 0.04) during fasting among women.
TABLE 2.
Multivariable-adjusted percentage of difference (95% CI) in C-peptide concentrations across quintiles of the EDIH and the II scores, according to time since the last meal before blood donation in men1
| Dietary index quintiles | |||||||
|---|---|---|---|---|---|---|---|
| Hours after eating | Sample size | Quintile 1 (reference) | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P-trend2 |
| EDIH | |||||||
| 0–1 | 287 | 0 | 12 (−15, 45) | 16 (−10, 50) | 17 (−10, 51) | 50 (15, 95) | 0.0001 |
| 2 | 348 | 0 | 10 (−17, 44) | 15 (−13, 51) | 16 (−12, 53) | 22 (−8, 60) | 0.046 |
| 3 | 244 | 0 | 11 (−19, 50) | −1 (−27, 35) | 24 (−9, 69) | 4 (−28, 41) | 0.52 |
| 4 | 258 | 0 | 16 (−14, 57) | 43 (6, 92) | 11 (−18, 50) | 46 (8, 99) | 0.0006 |
| 5–7 | 254 | 0 | 7 (−20, 42) | 3 (−23, 37) | 21 (−9, 62) | 28 (−4, 71) | 0.004 |
| 8–9 | 200 | 0 | 7 (−20, 44) | 4 (−23, 40) | 17 (−13, 57) | 14 (−16, 54) | 0.10 |
| 10 | 333 | 0 | 1 (−19, 26) | −2 (−22, 23) | 13 (−11, 41) | 18 (−6, 48) | 0.03 |
| 11 | 205 | 0 | 13 (−13, 48) | 11 (−14, 46) | 17 (−10, 53) | 41 (7, 86) | 0.009 |
| 12 | 833 | 0 | 4 (−9, 19) | 13 (−1, 29) | 16 (2, 33) | 25 (9, 43) | <0.0001 |
| 13 | 256 | 0 | 18 (−5, 47) | 22 (−2, 50) | 35 (8, 68) | 44 (15, 80) | <0.0001 |
| 14 | 420 | 0 | 5 (−14, 27) | 9 (−10, 32) | 10 (−9, 33) | 14 (−6, 39) | 0.03 |
| ≥15 | 326 | 0 | −3 (−22, 20) | 9 (−12, 34) | 18 (−4, 46) | 30 (5, 61) | 0.0008 |
| II | |||||||
| 0–1 | 287 | 0 | 3 (−20, 34) | 19 (−9, 55) | 3 (−21, 35) | 19 (−8, 55) | 0.09 |
| 2 | 348 | 0 | −2 (−26, 29) | −1 (−25, 31) | −5 (−28, 25) | 3 (−23, 36) | 0.79 |
| 3 | 244 | 0 | −25 (−45, 2) | 1 (−26, 37) | −3 (−28, 32) | −6 (−31, 27) | 0.84 |
| 4 | 258 | 0 | 1 (−20, 50) | 6 (23, 46) | 3 (−24, 41) | 11 (−19, 52) | 0.49 |
| 5–7 | 254 | 0 | −2 (−27, 31) | −2 (−27, 31) | −9 (−32, 22) | −10 (−33, 21) | 0.81 |
| 8–9 | 200 | 0 | 7 (−21, 43) | 14 (−15, 52) | 8 (−20, 45) | −13 (−35, 16) | 0.32 |
| 10 | 333 | 0 | 8 (−13, 36) | 3 (−18, 29) | −7 (−25, 17) | −7 (−26, 16) | 0.12 |
| 11 | 205 | 0 | −9 (−31, 19) | −8 (−30, 22) | 7 (−19, 40) | −9 (−31, 20) | 0.50 |
| 12 | 833 | 0 | −2 (−15, 12) | 6 (−8, 20) | −7 (−19, 7) | −13 (−24, −1) | 0.01 |
| 13 | 256 | 0 | 18 (−5, 47) | 7 (−14, 33) | 5 (−15, 31) | 5 (−16, 31) | 0.87 |
| 14 | 420 | 0 | 5 (−13, 28) | 10 (−9, 34) | −5 (−22, 16) | −6 (−23, 15) | 0.17 |
| ≥15 | 326 | 0 | 7 (−14, 33) | 0 (−19, 23) | −2 (−21, 22) | −15 (−31, 5) | 0.02 |
Dietary indexes were adjusted for energy intake using the residual method. Models were adjusted for age at blood draw, aspirin/NSAID use, smoking status, previous chronic conditions (hypertension, hypercholesterolemia, heart disease, arthritis), and case-control status. Values are percentage changes in concentrations of biomarkers (i.e., the relative differences in C-peptide concentrations between higher dietary index quintiles relative to quintile 1 as the reference; e.g., C-peptide concentration in quintile 5 minus the concentration in quintile 1). All values were back-transformed (ex), because C-peptide data were ln-transformed before analyses. For the index quintiles, higher EDIH and II scores indicate high insulinemic diets, whereas lower scores indicate low insulinemic diets. Given that the sample sizes for each analysis (at each distinct timepoint) differed, the precision of the estimates for each timepoint (i.e., width of the 95% CI) may be affected. Also, the sample size stated in the second column was distributed into fifths across index quintiles using cutpoints based on the total study population, to facilitate comparisons across distinct timepoints. EDIH, empirical dietary index for hyperinsulinemia; II, dietary insulin index; NSAID, nonsteroidal anti-inflammatory drug.
P-trend values represent the P values of the dietary index as a continuous variable adjusted for all covariates listed in footnote 2.
TABLE 3.
Multivariable-adjusted percentage of difference (95% CI) in plasma C-peptide concentrations across quintiles of the EDIH and the II scores, according to time since the last meal before blood donation in women1
| Dietary index quintiles | |||||||
|---|---|---|---|---|---|---|---|
| 5 h after eating | Sample size | Quintile 1 (reference) | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P-trend2 |
| EDIH | |||||||
| 0–1 | 240 | 0 | 18 (−12, 58) | 12 (−16, 50) | 28 (−4, 71) | 29 (−4, 74) | 0.002 |
| 2 | 289 | 0 | 11 (−17, 48) | 0 (−25, 33) | 17 (−12, 57) | 33 (0, 77) | 0.009 |
| 3 | 205 | 0 | −10 (−35, 23) | −3 (−30, 33) | 14 (−18, 58) | 49 (8, 106) | 0.0003 |
| 4–5 | 225 | 0 | 19 (−15, 66) | 21 (−14, 70) | 38 (−2, 93) | 41 (0, 100) | 0.004 |
| 6–8 | 272 | 0 | 1 (−22, 29) | 20 (−6, 54) | 21 (−6, 55) | 54 (19, 100) | 0.0001 |
| 9 | 304 | 0 | 1 (−21, 28) | 23 (−3, 56) | 28 (1, 64) | 37 (7, 76) | 0.0002 |
| 10 | 799 | 0 | 11 (−3, 27) | 25 (9, 43) | 27 (10, 45) | 35 (18, 55) | <0.0001 |
| 11 | 469 | 0 | 7 (−11, 29) | 10 (−9, 32) | 23 (2, 48) | 35 (12, 63) | 0.002 |
| 12 | 1656 | 0 | 15 (5, 27) | 15 (4, 27) | 26 (14, 39) | 42 (29, 57) | <0.0001 |
| 13 | 516 | 0 | 1 (−15, 19) | 13 (−5, 34) | 18 (−1, 40) | 44 (21, 72) | <0.0001 |
| 14 | 753 | 0 | 13 (−3, 30) | 11 (−4, 28) | 28 (11, 48) | 44 (24, 67) | <0.0001 |
| ≥15 | 487 | 0 | 8 (−12, 33) | 4 (−16, 29) | 10 (11, 36) | 40 (13, 73) | <0.0001 |
| II | |||||||
| 0–1 | 240 | 0 | −5 (−29, 27) | −4 (−29, 29) | −6 (−29, 26) | −12 (−35, 20) | 0.09 |
| 2 | 289 | 0 | −1 (−26, 33) | 11 (−17, 50) | −3 (−27, 29) | 17 (−12, 54) | 0.09 |
| 3 | 205 | 0 | 30 (−8, 84) | 20 (−15, 67) | 11 (−21, 56) | 2 (−27, 42) | 0.79 |
| 4–5 | 225 | 0 | −1 (−30, 39) | −15 (−40, 21) | −8 (−34, 29) | −3 (−32, 39) | 0.22 |
| 6–8 | 272 | 0 | 2 (−21, 32) | 3 (−20, 32) | 13 (−15, 50) | −12 (−32, 15) | 0.35 |
| 9 | 304 | 0 | 5 (−13, 32) | 15 (−10, 49) | 13 (−13, 46) | 9 (−16, 42) | 0.28 |
| 10 | 799 | 0 | 11 (−3, 29) | 22 (6, 40) | 13 (−2, 30) | 2 (−11, 18) | 0.99 |
| 11 | 469 | 0 | 14 (−7, 39) | −1 (−19, 20) | −3 (−20, 19) | 1 (−18, 22) | 0.51 |
| 12 | 1656 | 0 | 3 (−6, 14) | 0 (−9, 10) | 2 (−17, 13) | −7 (−16, 3) | 0.35 |
| 13 | 516 | 0 | 5 (−12, 25) | −10 (−25, 8) | −10 (−25, 8) | −3 (−19, 15) | 0.90 |
| 14 | 753 | 0 | −9 (−22, 5) | −7 (−20, 8) | −14 (−26, −1) | −14 (−26, 0) | 0.009 |
| ≥15 | 487 | 0 | 7 (−12, 30) | 1 (−18, 26) | −7 (−24, 15) | −3 (−22, 21) | 0.37 |
Dietary indexes were adjusted for energy intake using the residual method. Models were adjusted for: age at blood draw, aspirin/NSAID use, smoking status, previous chronic conditions (hypertension, hypercholesterolemia, heart disease, arthritis), case–control status, menopausal status, and postmenopausal hormone use. Values are percentage changes in concentrations of biomarkers (i.e., the relative differences in C-peptide concentrations between higher dietary index quintiles relative to quintile 1 as the reference; e.g., C-peptide concentration in quintile 5 minus the concentration in quintile 1). All values were back-transformed (ex), because C-peptide data were ln-transformed before analyses. For the index quintiles, higher EDIH and II scores indicate high insulinemic diets, whereas lower scores indicate low insulinemic diets. Given that the sample sizes for each analysis (at each distinct timepoint) differed, the precision of the estimates for each timepoint (i.e., width of the 95% CI) may be affected. Also, the sample size stated in the second column was distributed into fifths across index quintiles using cutpoints based on the total study population, to facilitate comparisons across distinct timepoints. EDIH, empirical dietary index for hyperinsulinemia; II, dietary insulin index; NSAID, nonsteroidal anti-inflammatory drug.
P-trend values represent the P values of the dietary index as a continuous variable adjusted for all covariates listed in footnote 2.
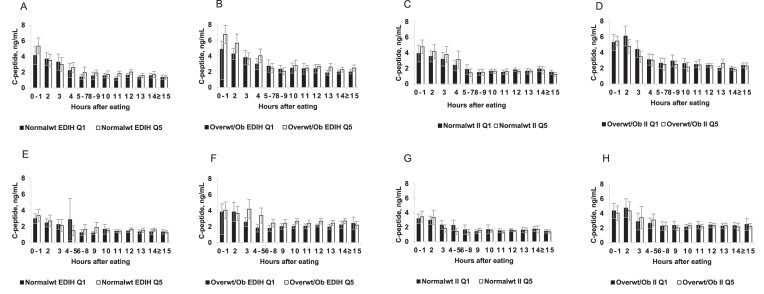
In Figure 2, we present the unadjusted C-peptide concentrations stratified by BMI category. The pattern of higher C-peptide concentrations during the nonfasting period that declines with increasing duration of fasting was observed for both dietary indexes among both normal weight and overweight or obese men and women. For the EDIH, higher C-peptide concentrations were observed among overweight or obese participants than among normal weight participants. Also, among overweight or obese participants, C-peptide concentrations were higher among those classified in the highest EDIH quintiles than those in the lowest quintile. These differences were not evident for the II (Figure 2).
FIGURE 2.
Plasma absolute observed C-peptide concentrations among men (A, B, C, D) and women (E, F, G, H) classified in the lowest (Q1) and highest (Q5) quintiles, of the EDIH (A, B, E, F) and the II (C, D, G, H) scores. Normal weight participants have a BMI < 25 kg/m2, whereas overweight or obese participants have BMI ≥ 25 kg/m2. The C-peptide values were the unadjusted observed values without statistical transformation. Diabetic patients were excluded and both dietary scores were adjusted for energy intake using the residual method. The sample size for each fasting duration was: normal weight: 0–1 h, n = 126; 2 h, n = 157; 3 h, n = 100; 4 h, n = 114; 5–7 h, n = 111; 8–9 h, n = 100; 10 h, n = 156; 11 h, n = 79; 12 h, n = 333; 13 h, n = 130; 14 h, n = 169; ≥15 h, n = 126. Among overweight/obese: 0–1 h, n = 148; 2 h, n = 176; 3 h, n = 131; 4 h, n = 134; 5–7 h, n = 131; 8–9 h, n = 96; 10 h, n = 169; 11 h, n = 126; 12 h, n = 475; 13 h, n = 119; 14 h, 235; ≥15 h, 193 in men; and in women: normal weight: 0–1 h, 135; 2 h, 165; 3 h, 110; 4–5 h, 117; 6–8 h, 127; 9 h, 151; 10 h, 416; 11 h, 236; 12 h, 877; 13 h, 288; 14 h, 386; ≥15 h, 246. Among overweight/obese: 0–1 h, 105; 2 h, 124; 3 h, 95; 4–5 h, n = 108; 6–8 h, n = 145; 9 h, n = 153; 10 h, n = 383; 11 h, n = 233; 12 h, n = 779; 13 h, n = 228; 14 h, n = 367; ≥15 h, n = 228. EDIH, empirical dietary pattern for hyperinsulinemia; II, dietary insulin index; Normalwt, normal weight; Overwt/ob, overweight/obese; Q, quintile.
Discussion
Daily average insulin concentrations may be a relevant exposure for diseases such as cancer (13) and possibly cardiovascular disease. Because the absolute concentrations of insulin are much higher in the postprandial than in the fasting state, and because individuals spend much of their waking hours in a postprandial state, average insulin exposure (i.e., AUC) will largely reflect the postprandial state (25). Two variables have been used to capture the dietary effect on insulin exposure in population-based studies. The II captures the direct influence of foods characteristic of a person's diet immediately after a meal on secretion of insulin, whereas the EDIH is calculated empirically based on fasting C-peptide, which captures insulin resistance rather than secretion. Because postprandial insulin stimulation and the underlying insulin resistance could both determine cumulative daily insulin secretion, it is not obvious whether the EDIH or the II would better predict long-term average postprandial insulin secretion. Ideally, this comparison would be made on individuals providing multiple measures of circulating insulin or C-peptide over the course of a day (25) (or multiple days) or 24-h urine samples for excreted C-peptide.
As an alternative approach to compare II and EDIH, we used a convenience sample of a single measure of C-peptide from almost 10,000 adult men and women, who had provided their sample with a range of hours since the last meal from 1 h (nonfasting) to >15 h (fasting). In this cross-sectional analysis, we found that: 1) the EDIH score, although formulated to predict fasting C-peptide concentrations, is also predictive of postprandial C-peptide concentrations; 2) the dietary II showed no association postprandially and a modest decrease of fasting C-peptide. The patterns are amplified in overweight/obese participants, as expected because of their more insulin-resistant state. Our results are consistent with previous analyses for fasting C-peptide in these cohorts (4, 5). As expected because it was empirically created to predict fasting C-peptide, the EDIH score has shown direct associations with fasting plasma C-peptide concentrations (5). In the study by Nimptsch et al. (4), II was not positively associated with fasting plasma C-peptide, and in contrast, an unexpected inverse association was observed. Tabung et al. (5) also found an inverse association between the II and fasting plasma C-peptide concentrations among men, with a relative concentration difference of 0.94 ng/mL (95% CI: 0.89, 1.00; P-trend = 0.03) comparing extreme II quintiles, although there was no association among women.
Although it may be expected that the EDIH is a better predictor of fasting C-peptide than the II, it might appear surprising that it is also superior to the II in assessing postprandial C-peptide concentrations. The EDIH was developed to incorporate dietary factors that influence C-peptide concentrations beyond immediate postprandial effects on insulin secretion. The EDIH is thus responsive to factors that influence insulin resistance. For example, an item such as coffee does not contribute calories and thus cannot influence the glycemic index or II score. Yet, coffee may improve insulin sensitivity, and thus may lower insulin concentrations, both postprandial and at fasting. Because the EDIH is made empirically by factors related to C-peptide concentrations, it incorporates factors such as coffee and thus may represent a more realistic and comprehensive estimate of the influence of the whole diet on insulin concentrations. Of note, individuals with greater insulin resistance will experience greater postprandial insulin secretion for a given insulinemic stimulus. For cumulative insulin secretion, the underlying state of insulin sensitivity as well as the direct insulinemic potential of foods are both important, which perhaps may explain why EDIH appears more predictive of some chronic disease outcomes [e.g., colorectal cancer (13)] than the II (26–28).
The inverse association between the II score and fasting C-peptide concentrations may appear paradoxical but is consistent with physiology. Diets with a high insulinemic potential may provoke an elevated blood glucose concentration along with elevated concentrations of gut hormones including glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide within the first 2 h of the meal, which stimulate insulin release from pancreatic β-cells and inhibits glucagon release from α-cells (29). This then results in a high insulin-to-glucagon ratio which would exaggerate the normal anabolic responses to eating, including uptake of nutrients by insulin-responsive tissues, stimulation of glycogenesis and lipogenesis, and suppression of gluconeogenesis and lipolysis. Between 2 and 4 h after a high insulinemic meal, there is a decline in nutrient absorption from the gut but the biological effects of the high insulin-to-glucagon ratio persist (29). Consequently, blood glucose concentration falls rapidly, often into the hypoglycemic range, along with a decline in blood insulin concentrations. In the late postprandial period (∼4–6 h after a high insulinemic meal), the low circulating concentrations of metabolic fuels trigger a counter-regulatory hormone response that restores normal glucose concentration by stimulating glycogenolytic and gluconeogenic pathways and elevating free fatty acid concentrations (29). This combination of elevated counter-regulatory hormone and free fatty acid concentrations resembles a state of fasting normally reached only after many hours without food (30), and may potentially further lower the concentrations of circulating insulin, which may explain the inverse association between the II score and C-peptide concentrations during fasting.
An important aspect of our study design is that the apparent shape of the postprandial response is based on a population rather than formulated from individuals with multiple measures after a meal. For example, a subgroup of the population contributed to the data for 1 h postprandial, a separate subgroup to 2 h postprandial, and so on. The pattern of C-peptide concentrations over time that we have described in the current results is for habitual diets and not necessarily for the specific meal that preceded the blood donation. Yet, the population average C-peptide for each hour after a meal should provide a reasonable unbiased estimate of the mean C-peptide for this population. Although the individual meals before the sample would represent only 1 meal for the individual, because of the large sample size the mean for the population should be a robust measure. This concept is similar to the observation that a large number of single 24-h dietary recalls from many individuals in a population generates a valid estimate of mean dietary intakes for that population (31, 32). As demonstrated in Figures 1 and 2, the shape of the average population C-peptide by hours since the last meal reflects the physiologic changes over time since the last meal in circulating glucose and insulin concentrations for an individual (29).
Our study is not without limitations. Self-reported dietary intake has inevitable measurement error, but previous validation studies in these cohorts have evaluated the relative validity of FFQ data and have shown reasonably good correlations between FFQs and diet records, which suggests that dietary intake is generally well measured (10, 11). However, with an FFQ we do not know what foods are consumed together and this can potentially influence the insulin response. Our study participants were mostly European-American health professionals, but the distributions of most participant characteristics are generally similar to those of the larger US multiracial/ethnic population (33, 34). Although our C-peptide data were pooled from both cases and controls, these data were pooled from nested case-control studies in which participants provided blood samples before any disease development. Moreover, previous studies of biomarker data from controls only yielded results that were not materially different from when cases and controls were included (5, 35). Participants were also eligible for blood donation only if they were free of diagnosed chronic diseases including coronary heart disease, stroke, cancer, or diabetes. In addition, we adjusted for case-control status in all analyses, while also adjusting for a large number of other potential confounding variables; however, the possibility of confounding by unmeasured variables cannot be completely ruled out.
In summary, the current study results provide 2 important clarifications on the findings from previous studies: 1) although the EDIH was developed using fasting plasma C-peptide, it is predictive of nonfasting C-peptide (insulin secretion), and 2) the II is not predictive of postprandial insulin secretion and may be inversely related to long-term insulin exposure. Therefore, the EDIH represents a more realistic characterization of long-term hyperinsulinemia and its effect on diseases in adult men and women. Although these findings are useful for population-level studies, future studies should assess integrated exposure by EDIH and II concentration for individuals using multiple blood samples after a meal or 24-h urinary C-peptide concentration as an integrated measure of total insulin secreted in a day.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—FKT, KN, and ELG: designed research; FKT: conducted research and performed statistical analysis; FKT, KN, and ELG: analyzed and interpreted the data and provided critical input; FKT and ELG: wrote the paper; ELG: provided study oversight; and all authors: read and approved the final paper.
Notes
Supported by National Cancer Institute grant #K99CA207736 and #R00CA207736 (to FKT). The HPFS cohort was supported by grant #UM1CA167552 and the NHS cohort by grant #UM1CA176726.
FKT, KM, and ELG, no conflicts of interest.
Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: EDIH, empirical dietary index for hyperinsulinemia; HPFS, Health Professionals Follow-up Study; II, insulin index; MET-h/wk, metabolic equivalent hours per week; NHS, Nurses’ Health Study; NSAID, nonsteroidal anti-inflammatory drug.
References
- 1. Giovannucci E. Insulin and colon cancer. Cancer Causes Control. 1995;6(2):164–79. [DOI] [PubMed] [Google Scholar]
- 2. Nead KT, Sharp SJ, Thompson DJ, Painter JN, Savage DB, Semple RK, Barker A, The Australian National Endometrial Cancer Study Group, Perry JRB, Attia J et al.. Evidence of a causal association between insulinemia and endometrial cancer: a mendelian randomization analysis. J Natl Cancer Inst. 2015;107(9):djv178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, Bowling AC, Newman HC, Jenkins AL, Goff DV. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34(3):362–6. [DOI] [PubMed] [Google Scholar]
- 4. Nimptsch K, Brand-Miller JC, Franz M, Sampson L, Willett WC, Giovannucci E. Dietary insulin index and insulin load in relation to biomarkers of glycemic control, plasma lipids, and inflammation markers. Am J Clin Nutr. 2011;94(1):182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tabung FK, Wang W, Fung TT, Hu FB, Smith-Warner SA, Chavarro JE, Fuchs CS, Willett WC, Giovannucci EL. Development and validation of empirical indices to assess the insulinaemic potential of diet and lifestyle. Br J Nutr. 2016:8::1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Colditz G, Hankinson SE. The Nurses' Health Study: lifestyle and health among women. Nat Rev Cancer. 2005;5(5):388–96. [DOI] [PubMed] [Google Scholar]
- 7. Pai J, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, Curhan GC, Rifai N, Cannuscio CC, Stampfer MJ, Rimm EB. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351(25):2599–610. [DOI] [PubMed] [Google Scholar]
- 8. Hankinson SE, Willett WC, Manson JE, Hunter DJ, Colditz GA, Stampfer MJ, Longcope C, Speizer FE. Alcohol, height, and adiposity in relation to estrogen and prolactin levels in postmenopausal women. J Natl Cancer Inst. 1995;87(17):1297–302. [DOI] [PubMed] [Google Scholar]
- 9. Willett W, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. [DOI] [PubMed] [Google Scholar]
- 10. Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93(7):790–6. [DOI] [PubMed] [Google Scholar]
- 11. Rimm E, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–26. [DOI] [PubMed] [Google Scholar]
- 12. Hu FB, Rimm E, Smith-Warner SA, Feskanich D, Stampfer MJ, Ascherio A, Sampson L, Willett WC. Reproducibility and validity of dietary patterns assessed with a food frequency questionnaire. Am J Clin Nutr. 1999;69(2):243–9. [DOI] [PubMed] [Google Scholar]
- 13. Tabung FK, Wang W, Fung TT, Smith-Warner SA, Keum N, Wu K, Fuchs CS, Hu FB, Giovannucci EL. Association of dietary insulinemic potential and colorectal cancer risk in men and women. Am J Clin Nutr. 2018;108(2):363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holt SH, Miller JC, Petocz P. An insulin index of foods: the insulin demand generated by 1000-kJ portions of common foods. Am J Clin Nutr. 1997;66(5):1264–76. [DOI] [PubMed] [Google Scholar]
- 15. Bao J, de Jong V, Atkinson F, Petocz P, Brand-Miller JC. Food insulin index: physiologic basis for predicting insulin demand evoked by composite meals. Am J Clin Nutr. 2009;90(4):986–92. [DOI] [PubMed] [Google Scholar]
- 16. Bonser A, Garcia-Webb P. C-peptide measurement: methods and clinical utility. Crit Rev Clin Lab Sci. 1984;19(4):297–352. [DOI] [PubMed] [Google Scholar]
- 17. Shai I, Rimm EB, Hankinson SE, Curhan G, Manson JE, Rifai N, Stampfer MJ, Ma J. Multivariate assessment of lipid parameters as predictors of coronary heart disease among postmenopausal women: potential implications for clinical guidelines. Circulation. 2004;110(18):2824–30. [DOI] [PubMed] [Google Scholar]
- 18. Willett W, Stampfer M, Chu NF, Spiegelman D, Holmes M, Rimm E. Assessment of questionnaire validity for measuring total fat intake using plasma lipid levels as criteria. Am J Epidemiol. 2001;154(12):1107–12. [DOI] [PubMed] [Google Scholar]
- 19. Rosner B, Cook N, Portman R, Daniels S, Falkner B. Determination of blood pressure percentiles in normal-weight children: some methodological issues. Am J Epidemiol. 2008;167(6):653–66. [DOI] [PubMed] [Google Scholar]
- 20. Chasan-Taber S, Rimm EB, Stampfer MJ, Spiegelman D, Colditz GA, Giovannucci E, Ascherio A, Willett WC. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996;7(1):81–6. [DOI] [PubMed] [Google Scholar]
- 21. Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, Rosner B, Kriska A, Willett WC. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23(5):991–9. [DOI] [PubMed] [Google Scholar]
- 22. Bland JM, Altman DG. Statistics notes: transformations, means, and confidence intervals. Br Med J. 1996;312(7038):1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bland JM, Altman DG. Statistics notes: logarithms. Br Med J. 1996;312(7032):700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Willett W, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4):1220S–8S. [DOI] [PubMed] [Google Scholar]
- 25. Polonsky KS, Given BD, Van Cauter E. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J Clin Invest. 1988;81(2):442–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bao Y, Nimptsch K, Meyerhardt JA, Chan AT, Ng K, Michaud DS, Brand-Miller JC, Willett WC, Giovannucci E, Fuchs CS. Dietary insulin load, dietary insulin index, and colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2010;19(12):3020–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bao Y, Nimptsch K, Wolpin BM, Michaud DS, Brand-Miller JC, Willett WC, Giovannucci E, Fuchs CS. Dietary insulin load, dietary insulin index, and risk of pancreatic cancer. Am J Clin Nutr. 2011;94(3):862–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Prescott J, Bao Y, Viswanathan AN, Giovannucci EL, Hankinson SE, De Vivo I. Dietary insulin index and insulin load in relation to endometrial cancer risk in the Nurses’ Health Study. Cancer Epidemiol Biomarkers Prev. 2014;23(8):1512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287(18):2414–23. [DOI] [PubMed] [Google Scholar]
- 30. Cahil J, George F. Starvation in man. N Engl J Med. 1976;5:397–415. [Google Scholar]
- 31. Willett W. Nutritional Epidemiology. 2nd ed New York: Oxford University Press; 1998. p. 137–8. [Google Scholar]
- 32. Hébert JR, Hurley TG, Steck SE, Miller DR, Tabung FK, Peterson KE, Kushi LH, Frongillo EA. Considering the value of dietary assessment data in informing nutrition-related health policy. Adv Nutr. 2014;5(4):447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Woloshin S, Schwartz LM. Distribution of C-reactive protein values in the United States. N Engl J Med. 2005;352(15):1611–3. [DOI] [PubMed] [Google Scholar]
- 34. Tabung FK, Giovannucci EL, Giulianini F, Liang L, Chandler PD, Balasubramanian R, Manson JE, Cespedes Feliciano EM, Hayden KM, Van Horn L et al.. An empirical dietary inflammatory pattern score is associated with circulating inflammatory biomarkers in a multi-ethnic population of postmenopausal women in the United States. J Nutr. 2018;148(5):771–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tabung FK, Smith-Warner SA, Chavarro JE, Wu K, Fuchs CS, Hu FB, Chan AT, Willett WC, Giovannucci EL. Development and validation of an empirical index of dietary inflammatory potential. J Nutr. 2016;146(8):1560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.