The prevalence of primary hypertension in children has increased significantly, in parallel with the childhood obesity epidemic.(1, 2) School screening studies reveal that the current prevalence of hypertension in the US is 2 – 4% overall,(3) and as high as 10% in children who are overweight;(4) a remarkably high number given that nearly 20% of adolescents in the US are obese.(5) This striking prevalence of primary hypertension has important implications for both the short-term development of target-organ damage during youth itself and long-term development of premature hypertension related morbidity. Youth with hypertension commonly manifest adverse target-organ effects on the heart and vasculature, including left ventricular hypertrophy, increased carotid thickness, and increased arterial stiffness.(6) There is now emerging evidence for adverse effects on the brain as well in children with moderate hypertension,(7) findings with significant implications for the impact of primary hypertension on cognition both during childhood and later in life. This review will summarize the growing evidence for an association between early life hypertension and lower cognition both in mid-life and during childhood itself.
Early Life Primary Hypertension and Mid-life Cognition
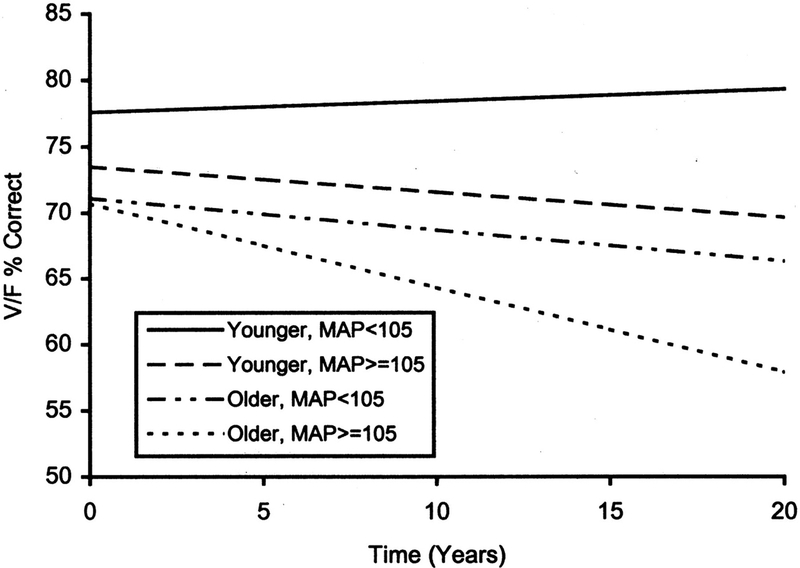
Studies relating elevated BP in early adulthood with subsequent mid-life cognition lend biological plausibility to the possibility that even earlier hypertension, in childhood and adolescence, may also impact cognition later in life. In one of the first studies to examine the impact of blood pressure on cognitive decline in young adults, investigators of the Maine-Syracuse Longitudinal Study determined the relation between blood pressure and cognitive decline over 20 years in 529 participants, consisting of both younger adults (mean age, 34.9 y; range, 18 – 46 y) and older adults (mean age, 58.1 y; range, 47 – 83 y) at enrollment.(8) Subjects underwent neurocognitive testing from 1 to 4 times with a mean time between tests of 5 years. Older and younger adult age groups were categorized by mean arterial blood pressure (MAP), either < 105 mm Hg or ≥ 105 mm Hg. The authors projected expected cognitive decline over 20 years (Figure 1). Both younger and older age groups with higher MAP showed substantially steeper cognitive decline compared with subjects with lower MAP, although the effect size was relatively small. The investigators concluded that young hypertensive adults are as susceptible to blood pressure-associated longitudinal decline in cognitive performance as are older adults and that the decline starts in young adulthood.
Figure 1.
Projected cognitive decline in hypertensive young adults. Linear regression lines for performance on a visualization/fluid intelligence composite score for older and younger adult age groups categorized by mean arterial blood pressure (MAP), either < 105 mm Hg or ≥ 105 mm Hg. Each regression line represents the estimated cognitive decline expected over 20 years for the two age groups by MAP category. Adapted with permission from reference 8.
In a more recent report, the potential effect of hypertension from young adulthood on cognition in mid-life was reported by the Coronary Artery Risk Development in Young Adults (CARDIA) Study.(9) The CARDIA study investigators enrolled 3,381 young adults, aged 18 to 30 years at baseline (mean age, 25.1 y), and completed follow-up examinations with blood pressure every 2 – 5 years. The subjects then underwent neurocognitive assessment at year 25 when the mean age of the cohort was 50.2 years old. After adjusting for age, sex, race, and education, higher 25-year cumulative exposure to systolic and diastolic blood pressure and fasting blood glucose from young adulthood were associated with lower neurocognitive test performance in mid-life on measures of verbal memory, processing speed, and executive function.
Most recently, the Cardiovascular Risk in Young Finns Study demonstrated that systolic hypertension in childhood and adolescence was associated with lower cognitive test performance in midlife.(10) Specifically, this study followed nearly 3,600 children, ages 3–18 years at study entry, for 31 years, with 2,026 available for follow-up between the ages of 34 and 49 years. They reported that the cumulative burden of early cardiovascular risk factors (systolic blood pressure, high total cholesterol, and smoking) from ages 6 to 24 years old were each significantly and independently related to poorer performance on measures of episodic memory and learning in midlife. The investigators defined high systolic blood pressure during youth as continuous area under the curve exposure greater than the 75th percentile. Importantly, this was one of the first studies to link hypertension during childhood itself to poorer cognitive performance in later adult life. The investigators also evaluated the additive effect of cardiovascular risk factors during youth on mid-life cognition, an important analysis as hypertension in youth often clusters with other cardiovascular risk factors that may have further negative effects on cognition, including metabolic syndrome, insulin resistance, and hyperlipidemia. They found that youth with 2 or 3 risk factors (systolic BP, total cholesterol, and/or smoking) above recommended clinical guidelines from 6 to 24 years of age performed progressively less well on neurocognitive testing in mid-life compared with the cognitive performance of youth with zero or only one risk factor above recommended guidelines.
Early Life Primary Hypertension and Cognition in Early Life
In addition to the association between early life hypertension and downstream mid-life cognition, studies also indicate that early life hypertension can influence cognition much earlier, well before mid-life. In fact, young adults with primary hypertension, while not cognitively impaired, have decreased performance on neurocognitive testing compared with that of matched normotensive young adult subjects, particularly in the domains of attention, working memory, and executive function, a finding postulated to represent an early manifestation of hypertensive target organ damage.(11, 12) It is important to emphasize that most young adults with primary hypertension are not cognitively impaired. Instead, the differences in test scores between hypertensive and normotensive young adults occur within the broad normal range of the neurocognitive tests. In other words, hypertensive young adults have lower cognitive test performance only in comparison with that of normotensive controls.(13) Studies in young adult have controlled for other factors known to affect cognition in this age group including alcohol consumption, education, anxiety, and depression.
Over the last 15 years, database, single-center, and multicenter studies have provided evidence that elevated BP in children and adolescents is associated with lower performance on neurocognitive testing during youth itself. The relation between elevated blood pressure and neurocognitive test performance in children was first investigated in a cross-sectional analysis of 5,077 children 6–16 years old (mean age, 11.5 years) who participated in NHANES III, a nationally representative sample of noninstitutionalized US children and adults.(14) After adjusting for socioeconomic status, obesity, and other demographic factors, elevated SBP ≥90th percentile remained independently associated with lower Digit Span scores, a measure of verbal attention and working memory. The association between increased SBP and lower Digit Span scores was even more pronounced for children with SBP in the hypertensive range, suggesting a dose-response effect of elevated BP on cognition.
Another recent cross-sectional analysis of 5,853 young children (mean age, 6.2 years) who participated in the Generation R Study, a population-based birth cohort in Rotterdam, The Netherlands, evaluated the relation between blood pressure and a standardized nonverbal IQ score.(15) Blood pressure was not categorized into elevated and non-elevated groups but instead was evaluated as a continuous variable across the normal BP range. After controlling for age, birth weight, body mass index, physical activity, and other potential confounders, higher diastolic blood pressure remained independently associated with lower nonverbal IQ score in this very young cohort. The association remained statistically significant after excluding the top decile of diastolic BP, suggesting that the relation holds even in the normotensive range.
A single center report of 14-year-old boys participating in a longitudinal study of the development of aggression found that subjects with SBP in the high normal range had significantly lower performance on spatial learning and memory compared to subjects with lower SBP.(16) In addition, subjects with both a parental history of hypertension and high normal SBP had lower performance on verbal learning, also suggesting that lower neurocognitive test performance in children may be detectable even within the normotensive range, and that there may be a genetic predisposition to such deficits. In a subsequent prospective, single-center report, adolescents (median age, 15 y) with newly diagnosed primary hypertension had worse scores on parent ratings of executive function compared with matched normotensive controls.(17) Furthermore, the parent ratings of executive function of subjects with hypertension improved significantly after one year of antihypertensive therapy. By contrast, the parent ratings of the control subjects did not change.(18)
Secondary analysis of registry data to investigate the relation between blood pressure and cognition has several significant limitations. High blood pressure is usually determined by casual (office) blood pressure measurement, often on a single occasion, rather than ambulatory blood pressure monitoring (ABPM), thereby allowing subjects with white coat hypertension to be included as having elevated blood pressure. Registries often include a very limited number of cognitive measures that have been selected for an unrelated purpose. A prospective multicenter study of neurocognition in children and adolescents was specifically designed to address the limitations of previous studies.(19) Hypertension-associated reductions in neurocognitive test performance tend to be relatively small in magnitude, and absolute scores fall within the broad developmentally normal range. The multicenter study was specifically designed to minimize the possibility that subject characteristics known to more strongly influence performance on tests of cognition, such as socioeconomic status, would overshadow potential group differences due to hypertension.(20) The neurocognitive test performance of 75 untreated newly diagnosed 10 to 18-year-old subjects with primary hypertension was compared to that of 75 frequency matched normotensive control subjects. Both hypertension and control groups underwent baseline ABPM to confirm sustained hypertension and normotension, respectively.
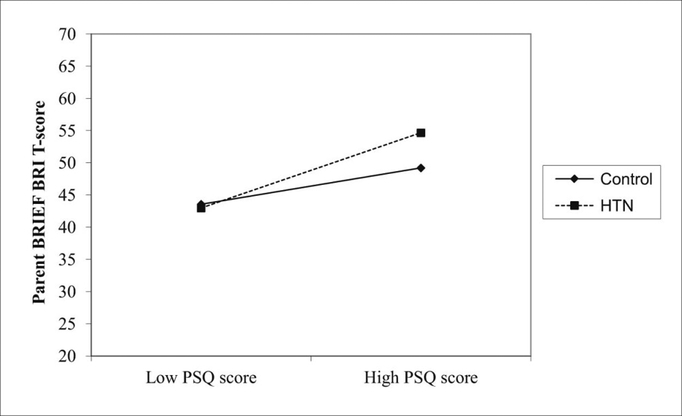
The results showed that hypertension was independently associated with decreased performance on neurocognitive measures of verbal learning, memory, fine-motor dexterity, and vocabulary (Table 1). The effect sizes of the group differences were modest but increased when the hypertension group was limited to subjects with severe ambulatory hypertension, suggesting a dose effect of hypertension on neurocognitive test performance. There were no measures where the control subjects performed worse than the hypertension subjects. It is important to emphasize that the differences in neurocognitive test performance between hypertensive and control subjects occurred within the normal range of the neurocognitive measures, similar to results in young adults. The subjects with hypertension were not cognitively impaired but instead had lower cognitive test performance only relative to the normotensive control group. The results also revealed a significant interaction between disordered sleep, as measured by the Pediatric Sleep Questionnaire (PSQ), and hypertension on ratings of executive function, such that hypertension heightened the expected association between increased disordered sleep and worse executive function(21) (Figure 2). This finding is significant as disordered sleep and primary hypertension in children are highly comorbid, and children with sleep apnea often have more severe BP elevation.(22) The study also showed that ABPM is superior to office BP in distinguishing hypertensive youth with lower neurocognitive test performance, underscoring the importance of using ABPM in studies of cognition and blood pressure in youth.(23)
Table 1.
Neurocognitive test performance of youth with primary HTN relative to that of matched normotensive control subjects. Shown are multivariate results for individual neurocognitive subtests for measures where HTN subjects performed less well than controls.
| Neurocognitive Subtest | Cognitive Domain | β | β SE | P value* |
|---|---|---|---|---|
| RAVLT Trial 1 | Attention | −0.58 | 0.27 | 0.034 |
| RAVLT Total | Learning, Verbal Memory | −3.25 | 1.24 | 0.009 |
| RAVLT Short delay | Learning, Verbal Memory | −1.12 | 0.44 | 0.013 |
| CogState Maze Delayed Recall | Memory | 3.7 | 1.2 | 0.002 |
| WASI Vocabulary | General Intelligence | −3.7 | 1.5 | 0.016 |
| Grooved Pegboard, dominant hand | Manual Dexterity | 4.5 | 2.3 | 0.04 |
Adapted with permission from reference 19.
RAVLT = Rey Auditory Verbal Learning Test; WASI = Wechsler Abbreviated Scale of Intelligence
adjusted for age, maternal education, household income, African American race, Hispanic ethnicity, PSQ-SRBD score, sex, triglyceride level, and glucose level.
Figure 2.
The effect of hypertension on the relation between disordered sleep as measured by PSQ score and executive dysfunction as measured by the Parent Behavior Rating Inventory of Executive Function Behavior Regulation Index (BRIEF BRI) score. Higher scores on the PSQ and BRIEF represent worse parent ratings of disordered sleep and executive function, respectively. HTN heightened the association between increased disordered sleep and worse executive function, interaction term, P = .04. Adapted with permission from reference 19.
The same study evaluated the effect of antihypertensive therapy on neurocognitive test performance.(24) Both the hypertension and control groups had repeat ABPM and neurocognitive testing at 1-year. During that year, the hypertension subjects received standard of care antihypertension treatment consisting of lifestyle modification and antihypertensive medication (angiotensin converting enzyme inhibitor), when indicated. Overall neurocognitive test performance of the hypertension subjects improved compared with baseline, particularly on measures of verbal learning and memory, manual dexterity, and executive function. However, the control group also improved in the same measures to the same degree, suggesting improvements with age or practice effects (due to repeated neurocognitive testing), rather than improvement with treatment. However, while 69% of the hypertension subjects who returned for the 1-year assessment had successful improvement of their hypertension, 31% had persistent ambulatory hypertension, largely due to the presence of masked hypertension. Secondary analysis revealed differences in neurocognitive test performance of the hypertension subjects according to the effectiveness of the antihypertensive treatment. The hypertension subjects with successful treatment had a similar improvement as controls in neurocognitive test scores at 1-year (analogous to results of the primary analysis). In contrast, the hypertension subjects with poor control of their hypertension did not show improvement in scores at 1-year on measures of verbal learning and memory or fine motor dexterity, suggesting that undertreated hypertension in youth may contribute to lower cognitive functioning. It appeared that effective antihypertensive therapy was necessary for improvement in cognition over the 1-year time course; implying that undertreated hypertension in youth may contribute to lower cognitive reserve and the beginnings of cognitive decline.
The real world impact during childhood of the modestly lower neurocognitive test performance in youth with primary hypertension is unclear. However, a retrospective study of the prevalence of learning disabilities in hypertensive youth sheds light on this question.(25) Two hundred and one consecutive children aged 10 – 18 years referred to a pediatric hypertension clinic for elevated BP were ultimately diagnosed with either sustained primary hypertension (n = 100) or prehypertension (n = 101). Children with secondary causes of hypertension were excluded from the analysis. The hypertensive children were more likely than those with prehypertension to be receiving special education services at school for a learning disability (28 vs. 9%, p < 0.001) and were more likely to be receiving medication for attention deficit disorder (20 vs. 7%, p = 0.007). In adjusted analysis, the odds of the diagnosis of learning disability were four times higher in the hypertensive children compared with the prehypertension comparison group. These results suggest that children with primary hypertension may manifest real world learning and attention problems.
Hypertension and Cognition in Children with Chronic Kidney Disease
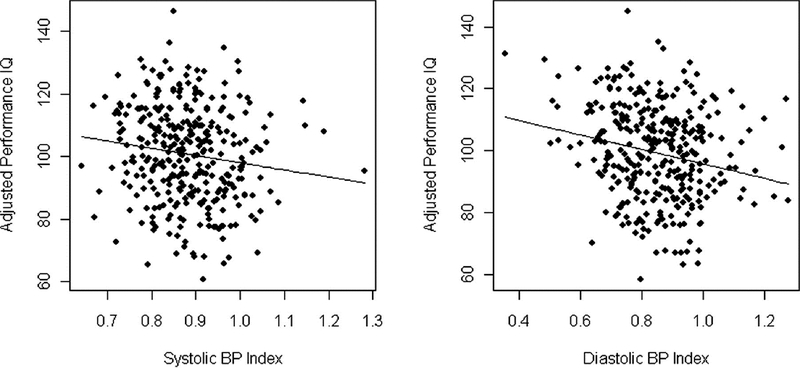
Children with chronic kidney disease (CKD) are at risk for cognitive dysfunction, and over half have hypertension.(26) The etiology of neurocognitive dysfunction in children with CKD is likely multifactorial and may include effects of the renal disease itself as well as effects of associated comorbidities such as anemia, hyperlipidemia, and hypertension.(27) A cross-sectional analysis of children 6 to 17 years enrolled in the Chronic Kidney Disease in Children (CKiD) study reported on the relation between auscultatory blood pressure and neurocognitive test performance.(28) The CKiD study is a longitudinal, observational cohort study of children with mild-to-moderate CKD (estimated glomerular filtration rate, 30 to 90 ml/min per 1.73 m2) being conducted at 46 pediatric nephrology centers in North America.(29) Subjects with elevated systolic and diastolic BP (≥ 90th percentile for age, sex, and height) had worse Performance IQ (PIQ) scores on the Wechsler Abbreviated Scales of Intelligence compared with subjects with normal BP (92.4 vs. 96.1, p = 0.03). Elevated BP remained independently associated with lower PIQ score, after adjusting for severity of CKD and other potential confounders (Figure 3). The investigators concluded that children with CKD may have difficulties with visual-spatial organization and visuoconstructive abilities that are related, in part, to elevated BP.
Figure 3.
Partial residual plots showing that higher systolic and diastolic BP index are associated with lower WASI Performance IQ in children with mild-to-moderate CKD. Adapted with permission from reference 28.
Alterations in BP can have negative cardiovascular effects, not only through elevations of mean BP, but also through increases in blood pressure variability (BPV), a factor associated with the development of cardiovascular damage, renal dysfunction, and lower neurocognitive test scores in adults.(30) Blood pressure variability can be assessed in the short term, as measured by variability in measurements within a 24 hour period during ambulatory BP monitoring (ambulatory BPV), or in the long term as variability in office visit measurements over time (visit-to-visit BPV).(31) Children with CKD and hypertension have been shown to demonstrate increased ambulatory BPV.(32) In a separate analysis of the CKiD cohort, the neurocognitive test performance of children with CKD and increased BPV was compared to that of children with CKD and lower BPV.(31) Visit-to-visit BPV was assessed using the standard deviation of visit BPs and average real variability. Ambulatory BPV was assessed using the standard deviation of sleep and wake BP on ABPM. Subjects with systolic visit-to-visit BP variability in the upper tertile scored lower on the Delis–Kaplan Executive Function System (D-KEFS) Verbal Category Switching test compared with subjects with BP variability in the lower tertile, after controlling for mean BP, demographic characteristics, and disease-related variables. Ambulatory BPV was not independently associated with any cognitive measure. However, the ABPM sessions in CKiD were obtained 6 – 12 months apart from the neurocognitive testing, limiting inference regarding the association between ABPM parameters and cognition. Category Switching is a measure of set-shifting, a component of executive function.(33) These results suggest that the difficulties with executive function demonstrated in children with CKD may be, in part, related to increased visit-to-visit BP variability.
The Neurocognitive Assessment and Magnetic Resonance Imaging Analysis of Children and Young Adults With Chronic Kidney Disease (NiCK) Study, a more recent single center study of cognition in children and young adults with CKD, extended the evaluation of the role of hypertension by including 24-hr ABPM concomitantly with neurocognitive testing.(34, 35) The neurocognitive test performance of 90 subjects with CKD aged 8 – 25 years was compared to that of 70 control subjects. After adjusting for sociodemographic characteristics and estimated glomerular filtration rate, increased diastolic BP load (the percent of diastolic BP readings ≥ 95th percentile for pediatric ABPM norms) was associated with worse performance on tests of language and verbal memory and blunted diastolic nocturnal dipping was associated with lower scores on measures of attention. Taken together, results of the CKiD and NiCK studies suggest that that hypertension may be an important and potentially modifiable risk factor for decreased neurocognitive function in youth with CKD.
Potential Mechanisms of Altered Neurocognition in Primary Hypertension
Although the physiologic basis of the decreased performance in neurocognitive testing in hypertensive youth has not yet been elucidated, there is evidence for altered cerebrovascular reactivity in the young.(36–39) Cerebrovascular reactivity is an important physiologic mechanism for maintaining a constant cerebral flow and is defined as the capacity of the cerebral blood vessels to dilate (or constrict) in response to different stimuli. In addition, cerebrovascular reactivity may be an important biomarker for brain vascular reserve.
Hypertension can affect small vessels resulting in vascular remodeling and impairment of cerebral blood flow regulation. As cognitive processing elicits regional distribution of blood flow, providing metabolic support to active neural areas, hypertension may interfere with this normal distribution of blood flow and/or decrease the ability to enhance cerebral blood flow in response to increased neuronal activity. This has been called the “vascular hypothesis” of cognitive dysfunction; this altered process might underlie the cognitive deficits of hypertensive individuals.(40, 41)
Different imaging methodologies (e.g., transcranial Doppler, magnetic resonance imaging) using different reactivity stimuli (e.g., carbon dioxide, hyperventilation) have been utilized to assess cerebral hemodynamics and to characterize the physiological association between hypertension and cerebrovascular reactivity. These methods have demonstrated impairment in the cerebrovascular response to changes in the arterial pressure of carbon dioxide in both hypertensive animals and hypertensive human subjects compared to normotensive controls.(42)
Studies of Cerebrovascular Reactivity in Children
There are several studies that have evaluated the effects of hypertension on cerebrovascular reactivity with transcranial Doppler assessing changes in cerebral blood flow in response to different stimuli in children (Table 2). Transcranial Doppler is a non‐invasive ultrasonographic technique that utilizes a pulsed Doppler transducer for assessment of intracerebral blood flow. The middle cerebral artery is identified by placing the transducer in the temporal window, between the angle of the eye and the pinna above the zygomatic ridge. The transducer emits waves, and then receives their reflections off the surfaces of red blood cells within the intracranial vasculature. This information is analyzed by a computer that provides numerical and visual output, which are useful for inferring the flow characteristics of the artery.
Table 2.
Cerebrovascular reactivity prospective studies in young hypertensive subjects
| Authors (year) |
Subjects | Main findings |
|---|---|---|
| Settakis et al. (2003) (36) | 58 normotensive and 113 hypertensive adolescents |
Systolic blood flow velocity percent change = 4.2 ± 14 vs. 4.7 ± 11.7; p = 0.82 (hypertensive vs. control) Mean blood flow velocity percent change = p<0.01 (hypertensive vs. control) Diastolic blood flow velocity percent change = p<0.01 (hypertensive vs. control) Note: for diastolic and mean velocities only p values without exact figures are shown on reference (data only in graph form) Percent change in diastolic and mean blood flow velocities after breath-holding were lower in hypertensives compared to normotensive controls. |
| Settakis et al. (2006) (37) | 58 normotensive and 113 hypertensive adolescents |
Systolic blood flow velocity percent change = 21.0±19.0 vs. 25.9±12.5, p<0.05 (hypertensive vs. control) Mean blood flow velocity percent change = 32.3±14.7 vs. 35.6±35.6, p = 0.18 (hypertensive vs. control) Diastolic blood flow velocity percent change = 40.4±18.1 vs. 45.5±15.2, p<0.05 (hypertensive vs. control) Systolic and diastolic blood flow velocities percent change after hyperventilation was lower in hypertensives compared to normotensive controls |
| Katona et al.(2006) (43) | 45 normotensive 61 hypertensive adolescents |
Blood flow velocity percent change after breath holding = 8.1±2.1 vs. 12.1±1.7 (hypertensive vs. control) Blood flow velocity percent change after hyperventilation = 31.0± 16.0 vs. 35±15 (hypertensive vs. control) Cerebral blood flow velocities after breath-holding and after hyperventilation were similar among hypertensives and normotensive adolescents |
| Páll et al. (2011) (38) | 59 normotensive, 47 white-coat hypertension and 73 hypertensive adolescents | Mean blood flow velocity percent change = 5.3±3.1 vs. 9.5±2.6 vs. 12.1±2.2% (white coat hypertension vs. hypertensive vs. normotensive controls) Blood flow velocity percent change was lower in white-coat hypertensives and hypertensives compared to normotensive controls |
| Wong et al. (2011) (39) | 9 normotensive, 9 elevated blood pressure, 18 white-coat hypertension, 13 untreated hypertension, 7 treated hypertension children and adolescents |
Time-averaged maximum mean velocity/end-tidal carbon dioxide = 2.556±1.832 cm/sec/mm Hg vs. 4.256±1.334 cm/sec/mm Hg (p<0.05) (untreated hypertensives vs. normotensive controls) Cerebrovascular reactivity was lower in untreated hypertensives compared to normotensive controls |
| Ostrovskaya et al. (2013) (48) | 4 elevated blood pressure 10 hypertensives |
Reactivity slopes showed significant inverse correlation with BRIEF scores ([Behavioral Regulation Index (r = −.60, P = .02), Metacognition Index (r = −.40, P = .05), and Global Executive Component (r = −.53, P = .05)]. Blunted cerebrovascular reactivity was associated with worse parental ratings of executive function |
In the first study that investigated cerebral vasoreactivity in hypertensive adolescents, 113 hypertensive (mean age 16.4 years) and 58 normotensive (mean age 15.8 years) adolescents from the population-based cohort of the Debrecen Hypertension Study underwent transcranial Doppler measurements of the middle cerebral artery at rest and after 30 seconds of breath-holding as a vasodilatory stimulus.(36) Hypertension was defined by casual BP measurements on three different occasions with an oscillometric device. Cerebrovascular reactivity was expressed as percent change to the resting cerebral blood flow velocity. These investigators found that hypertensive adolescents have higher cerebral blood flow velocities at rest compared with normotensives. After inducing temporary hypercapnia by breath holding, there was a smaller change in mean and diastolic blood flow velocities in hypertensives compared to normotensive subjects, possibly reflecting decreased cerebrovascular reactivity among hypertensives compared to normotensive controls.
The same hypertensive and normotensive groups were assessed utilizing transcranial Doppler at rest and after 60 seconds of voluntary hyperventilation as a vasoconstrictory stimulus.(37) After assessment before and after hyperventilation, it was found that hyperventilation induced a less pronounced change in systolic and diastolic blood flow velocities in the hypertensive subjects compared to the controls, indicating a diminished capacity of cerebral arterioles to vasoconstrict consistent with decreased cerebrovascular reactivity in hypertensive adolescents. Limitations of these initial studies were the lack of measuring end-tidal pressure of carbon dioxide in the study groups and BP classification based on casual blood pressure readings.
Another group of 106 adolescents (61 hypertensive and 45 normotensive) from the same cohort described above underwent transcranial Doppler measurements of cerebral blood flow velocities in a similar manner, but in this study, cerebrovascular reactivity was similar between the hypertensive and the normotensive control groups after both breathholding and hyperventilation. These authors did not provide an explanation for their negative results in regards to changes in cerebrovascular reactivity after the different stimuli compared to their own previous positive results.(43)
In a subsequent study, young subjects were divided according to 24-hour ABPM assessment. Seventy-three subjects with ambulatory hypertension (mean age 16.5 years), 47 with white-coat hypertension (mean age 16.3 years) and 59 normotensive controls (mean age 15.8 years) underwent transcranial Doppler at rest and after breath-holding test. Cerebrovascular reactivity was expressed as the percent change to the resting cerebral blood flow velocity value. Decreased vasodilatory reaction to carbon dioxide was present in both white-coat and hypertensive subjects, compared to controls, suggestive of abnormal cerebrovascular reactivity involving the cerebral arterioles.(38)
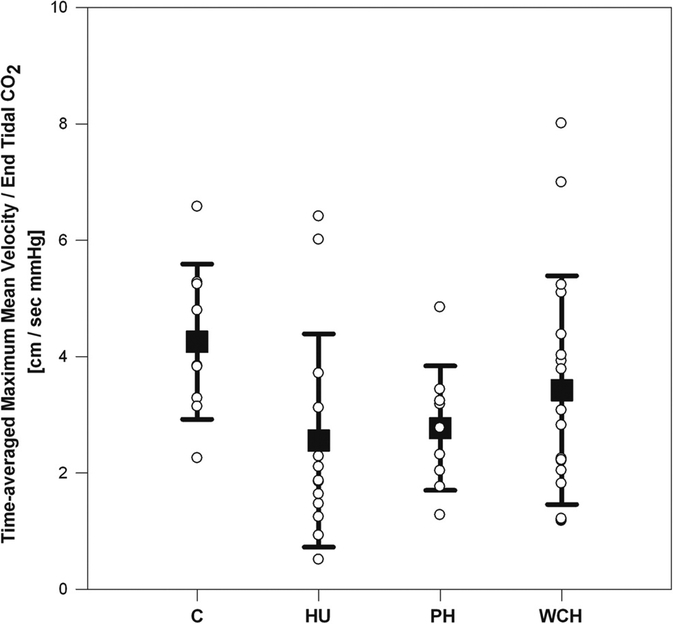
Another group of investigators evaluated 56 children and adolescents, from 7 to 20 years of age (mean age 15.3 years), and classified them according to 24-hr ABPM parameters as hypertensive, prehypertensive or white-coat hypertensive, and compared to normotensive controls.(39) All subjects were evaluated by transcranial Doppler examination of the middle cerebral artery while rebreathing carbon dioxide with a plastic bag. This study used a capnometer to measure end-tidal carbon dioxide. Cerebrovascular reactivity during hypercapnia was quantified by time-averaged maximum mean cerebral blood flow velocity and end-tidal carbon dioxide. This study found that untreated hypertensive children and adolescents had significantly lower cerebrovascular reactivity in response to an hypercapnic stimulus compared to normotensive controls (Figure 4). In addition, these researchers found that baseline diastolic blood pressure was inversely related to cerebral vasoreactivity. The authors postulated that these results suggest disordered cerebrovascular autoregulation, perhaps due to down-stream effects of hypertension on small vessels.(44)
Figure 4.
Cerebrovascular reactivity as measured by Transcranial Doppler. Untreated hypertensive adolescents had blunted cerebrovascular reactivity to hypercapnia compared with normotensive controls. Groups: C, control; HU, hypertension untreated; PH, prehypertension; WCH, white coat hypertension. Adapted with permission from reference 39.
In adults, it has been hypothesized that altered cerebrovascular reactivity is associated with lower executive function as hypertension is associated with both a decline in cognitive function and decreased responsiveness to carbon dioxide.(45) In children, it has also been suggested that the deficits in neurocognitive test performance described in hypertension may be secondary to abnormal cerebrovascular reactivity, as alterations in cerebral blood flow and possible neurocognitive deficits have been described in other diseases (e.g., sickle cell disease and mild-disordered breathing).(46, 47) A recent study evaluated the relation between cerebrovascular reactivity and executive function in hypertensive youth.(48) These investigators correlated the results of transcranial reactivity slopes with executive function as measured by the parent Behavior Rating Inventory of Executive Function (BRIEF) in a small subset of children (n = 14). The transcranial Doppler reactivity slopes had a significant inverse relationship with BRIEF scores. While the sample size was small, these preliminary results suggest that children with elevated BP may have decreased executive function that is associated with blunted cerebrovascular reactivity, same as in adults. Taken together, the described studies have shown that children and adolescents with hypertension have abnormal response to various reactivity stimuli, suggesting abnormal cerebrovascular reactivity as a result of elevated BP. All of these studies have limitations, especially the low numbers of subjects.
Implications and Future Directions
The finding of lower neurocognitive test performance in hypertensive youth suggests that treatment of hypertension from adolescence may represent an opportunity to ameliorate subsequent cognitive decline and thereby improve downstream cognitive health. Efforts at primordial prevention in early childhood to decrease the development of cardiovascular risk factors, including elevated BP, are warranted.(49)
The studies described in this review suggest that hypertension in early life is associated with decreased neurocognitive test performance only relative to that of normotensive controls. Youth and young adults with hypertension are not cognitively impaired. Studies are needed to determine whether treating hypertension from adolescence would result in a lower incidence of cognitive decline and vascular dementia later in life, and if so, what target BP level would achieve these important goals. In fact, a recent scientific statement from the American Heart Association (AHA) on the impact of hypertension on cognitive function identified as a critical question whether treatment as early in life as possible, such as treatment in adolescence, would improve subsequent cognitive outcomes.(50) Yet randomized clinical trials of the impact of hypertension treatment from adolescence through midlife and beyond on cognitive decline and cerebrovascular outcomes would be prohibitively impractical. Early biomarkers of target-organ damage (TOD) to the brain are needed to serve as surrogates of longer term cognitive outcomes in order to conduct shorter term, pragmatic clinical studies. Neurocognitive testing alone as a method of detecting an effect of hypertension treatment on TOD to the brain would be limited by the relatively wide variability in neurocognitive test scores with relatively small differences in scores between groups. Research to establish robust neuroimaging biomarkers of hypertensive TOD in youth is needed to bolster and extend the findings obtained with neurocognitive testing. While hypertensive youth demonstrate altered cerebrovascular reactivity on TCD, that technique is highly operator dependent with wide inter-observer measurement variability, limiting its use as a neuroimaging biomarker for future clinical trials. White matter hyperintensities (WMH) on MRI are the hallmark of hypertension-associated cerebral small vessel disease; these late findings occur primarily in the elderly. With the advent of diffusion tensor imaging (DTI), it has become clear that hypertension causes more subtle damage to white matter microstructural integrity well before the development of WMH. A report from the Framingham Heart Study showed a strong relation between elevated systolic BP and decreased white matter integrity on DTI in young adults,(51) indicating that impaired white matter integrity is demonstrable in the early stages of hypertension. Studies to establish robust neuroimaging markers of hypertensive TOD in youth, such as DTI, would allow clinical trials to optimize the effect of antihypertensive treatment from adolescence on cerebrovascular health and thereby ultimately improve downstream cognitive outcomes.
A confounding challenge to the study of neurocognitive test performance in hypertension is that the cognitive domains and the specific tests affected often vary widely between studies. These differences are in part related to the inconsistency in the neurocognitive test battery administered, which in turn complicates comparison of results across studies. As a consequence, the recent Scientific Statement from the AHA identified the need for applying uniform cognitive outcomes across studies.(50) Future studies to establish standardized neurocognitive testing that distinguishes hypertensive subjects across the lifespan are needed for longitudinal assessment of the effects of hypertension and its treatment on cognitive health, starting from its earliest stages.
Supplementary Material
Acknowledgments
Sources of funding: NIH grant R01HL098332 (PI, M Lande) supported some of the research reported in this review.
Footnotes
Disclosures: Drs. Lande and Kupferman authored some of the articles included in this review.
References
- 1.Rosner B, Cook NR, Daniels S, Falkner B. Childhood blood pressure trends and risk factors for high blood pressure: the NHANES experience 1988–2008. Hypertension. 2013. August;62(2):247–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosner B, Cook N, Portman R, Daniels S, Falkner B. Blood pressure differences by ethnic group among United States children and adolescents. Hypertension. 2009. September;54(3):502–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell CS, Samuel JP, Samuels JA. Prevalence of Hypertension in Children. Applying the New American Academy of Pediatrics Clinical Practice Guideline. Hypertension. 2019;73(DOI: 10.1161/HYPERTENSIONAHA.118.11673). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McNiece KL, Poffenbarger TS, Turner JL, Franco KD, Sorof JM, Portman RJ. Prevalence of hypertension and pre-hypertension among adolescents. J Pediatr. 2007. June;150(6):640, 4,, 644.e1. [DOI] [PubMed] [Google Scholar]
- 5.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, et al. Trends in Obesity Prevalence Among Children and Adolescents in the United States, 1988–1994 Through 2013–2014. JAMA. 2016. June 07;315(21):2292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics. 2017. September;140(3). [DOI] [PubMed] [Google Scholar]
- 7.Urbina EM, Lande MB, Hooper SR, Daniels SR. Target Organ Abnormalities in Pediatric Hypertension. J Pediatr. 2018. November;202:14–22. [DOI] [PubMed] [Google Scholar]
- 8.Elias PK, Elias MF, Robbins MA, Budge MM. Blood pressure-related cognitive decline: does age make a difference?. Hypertension. 2004. November;44(5):631–6. [DOI] [PubMed] [Google Scholar]
- 9.Yaffe K, Vittinghoff E, Pletcher MJ, Hoang TD, Launer LJ, Whitmer R, et al. Early adult to midlife cardiovascular risk factors and cognitive function. Circulation. 2014. April 15;129(15):1560–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rovio SP, Pahkala K, Nevalainen J, Juonala M, Salo P, Kahonen M, et al. Cardiovascular Risk Factors From Childhood and Midlife Cognitive Performance: The Young Finns Study. J Am Coll Cardiol. 2017. May 09;69(18):2279–89. [DOI] [PubMed] [Google Scholar]
- 11.Waldstein SR.Hypertension and neuropsychological function: a lifespan perspective. Exp Aging Res. 1995. Oct-Dec;21(4):321–52. [DOI] [PubMed] [Google Scholar]
- 12.Waldstein SR, Jennings JR, Ryan CM, Muldoon MF, Shapiro AP, Polefrone JM, et al. Hypertension and neuropsychological performance in men: interactive effects of age. Health Psychol. 1996. March;15(2):102–9. [DOI] [PubMed] [Google Scholar]
- 13.Elias MF, Goodell AL, Dore GA. Hypertension and cognitive functioning: a perspective in historical context. Hypertension. 2012. August;60(2):260–8. [DOI] [PubMed] [Google Scholar]
- 14.Lande MB, Kaczorowski JM, Auinger P, Schwartz GJ, Weitzman M. Elevated blood pressure and decreased cognitive function among school-age children and adolescents in the United States. J Pediatr. 2003. December;143(6):720–4. [DOI] [PubMed] [Google Scholar]
- 15.Lamballais S, Sajjad A, Leening MJG, Gaillard R, Franco OH, Mattace-Raso FUS, et al. Association of Blood Pressure and Arterial Stiffness With Cognition in 2 Population-Based Child and Adult Cohorts J Am Heart Assoc. 2018;7(e009847):DOI: 10.1161/JAHA.118.009847). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ditto B, Seguin JR, Tremblay RE. Neuropsychological characteristics of adolescent boys differing in risk for high blood pressure. Ann Behav Med. 2006;31(3):231, 232–237. [DOI] [PubMed] [Google Scholar]
- 17.Lande MB, Adams H, Falkner B, Waldstein SR, Schwartz GJ, Szilagyi PG, et al. Parental Assessments of Internalizing and Externalizing Behavior and Executive Function in Children with Primary Hypertension. J Pediatr. 2009;154(2):207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lande MB, Adams H, Falkner B, Waldstein SR, Schwartz GJ, Szilagyi PG, et al. Parental assessment of executive function and internalizing and externalizing behavior in primary hypertension after anti-hypertensive therapy. J Pediatr. 2010. July;157(1):114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lande MB, Batisky DL, Kupferman JC, Samuels J, Hooper SR, Falkner B, et al. Neurocognitive Function in Children with Primary Hypertension. J Pediatr. 2017. January;180:148, 155.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lande MB, Adams HR, Kupferman JC, Hooper SR, Szilagyi PG, Batisky DL. A multicenter study of neurocognition in children with hypertension: methods, challenges, and solutions. J Am Soc Hypertens. 2013. Sep-Oct;7(5):353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Owens JA. Neurocognitive and behavioral impact of sleep disordered breathing in children. Pediatr Pulmonol. 2009. May;44(5):417–22. [DOI] [PubMed] [Google Scholar]
- 22.Hinkle J, Connolly HV, Adams HR, Lande MB. Severe obstructive sleep apnea in children with elevated blood pressure. J Am Soc Hypertens. 2018. March;12(3):204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kupferman JC, Batisky DL, Samuels J, Adams HR, Hooper SR, Wang H, et al. Ambulatory blood pressure monitoring and neurocognitive function in children with primary hypertension. Pediatr Nephrol. 2018. October;33(10):1765–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lande MB, Batisky DL, Kupferman JC, Samuels J, Hooper SR, Falkner B, et al. Neurocognitive Function in Children with Primary Hypertension after Initiation of Antihypertensive Therapy. J Pediatr. 2018. February 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams HR, Szilagyi PG, Gebhardt L, Lande MB. Learning and attention problems among children with pediatric primary hypertension. Pediatrics. 2010. December;126(6):e1425–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flynn JT, Mitsnefes M, Pierce C, Cole SR, Parekh RS, Furth SL, et al. Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study. Hypertension. 2008. October;52(4):631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hooper SR, Gerson AC, Butler RW, Gipson DS, Mendley SR, Lande MB, et al. Neurocognitive functioning of children and adolescents with mild-to-moderate chronic kidney disease. Clinical Journal of The American Society of Nephrology: CJASN. 2011. August;6(8):1824–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lande MB, Gerson AC, Hooper SR, Cox C, Matheson M, Mendley SR, et al. Casual blood pressure and neurocognitive function in children with chronic kidney disease: a report of the children with chronic kidney disease cohort study. Clinical Journal of The American Society of Nephrology: CJASN. 2011. August;6(8):1831–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, et al. Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol. 2006. September;1(5):1006–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Epstein NU, Lane KA, Farlow MR, Risacher SL, Saykin AJ, Gao S, et al. Cognitive dysfunction and greater visit-to-visit systolic blood pressure variability. J Am Geriatr Soc. 2013. December;61(12):2168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lande MB, Mendley SR, Matheson MB, Shinnar S, Gerson AC, Samuels JA, et al. Association of blood pressure variability and neurocognition in children with chronic kidney disease. Pediatr Nephrol. 2016. June 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barletta GM, Flynn J, Mitsnefes M, Samuels J, Friedman LA, Ng D, et al. Heart rate and blood pressure variability in children with chronic kidney disease: a report from the CKiD study. Pediatr Nephrol. 2014. June;29(6):1059–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.A compendium of neuropsychological tests: administration, norms, and commentary. Straus E; Sherman EMS; Spreen O. 3rd ed ed. New York: Oxford University Press; 2006. [Google Scholar]
- 34.Hartung EA, Laney N, Kim JY, Ruebner RL, Detre JA, Liu H, et al. Design and methods of the NiCK study: neurocognitive assessment and magnetic resonance imaging analysis of children and young adults with chronic kidney disease. BMC Nephrol. 2015;16:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruebner RL, Laney N, Kim JY, Hartung EA, Hooper SR, Radcliffe J, et al. Neurocognitive Dysfunction in Children, Adolescents, and Young Adults With CKD. Am J Kidney Dis. 2016. April;67(4):567–75. [DOI] [PubMed] [Google Scholar]
- 36.Settakis G, Pall D, Molnar C, Bereczki D, Csiba L, Fulesdi B. Cerebrovascular reactivity in hypertensive and healthy adolescents: TCD with vasodilatory challenge. Journal of Neuroimaging. 2003. April;13(2):106–12. [PubMed] [Google Scholar]
- 37.Settakis G, Pall D, Molnar C, Katona E, Bereczki D, Fulesdi B. Hyperventilation-induced cerebrovascular reactivity among hypertensive and healthy adolescents. Kidney Blood Press Res. 2006;29(5):306–11. [DOI] [PubMed] [Google Scholar]
- 38.Pall D, Lengyel S, Komonyi E, Molnar C, Paragh G, Fulesdi B, et al. Impaired cerebral vasoreactivity in white coat hypertensive adolescents. European Journal of Neurology. 2011. April;18(4):584–9. [DOI] [PubMed] [Google Scholar]
- 39.Wong LJ, Kupferman JC, Prohovnik I, Kirkham FJ, Goodman S, Paterno K, et al. Hypertension impairs vascular reactivity in the pediatric brain. Stroke. 2011. July;42(7):1834–8. [DOI] [PubMed] [Google Scholar]
- 40.Jennings JR, Zanstra Y. Is the brain the essential in hypertension? Neuroimage. 2009. September;47(3):914–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jennings JR, Muldoon MF, Ryan C, Price JC, Greer P, Sutton-Tyrrell K, et al. Reduced cerebral blood flow response and compensation among patients with untreated hypertension. Neurology. 2005. April 26;64(8):1358–65. [DOI] [PubMed] [Google Scholar]
- 42.Maeda H, Matsumoto M, Handa N, Hougaku H, Ogawa S, Itoh T, et al. Reactivity of cerebral blood flow to carbon dioxide in hypertensive patients: evaluation by the transcranial Doppler method. J Hypertens. 1994. February;12(2):191–7. [PubMed] [Google Scholar]
- 43.Katona E, Settakis G, Varga Z, Juhasz M, Paragh G, Bereczki D, et al. Both nitric oxide and endothelin-1 influence cerebral blood flow velocity at rest and after hyper- and hypocapnic stimuli in hypertensive and healthy adolescents. Kidney Blood Press Res. 2006;29(3):152–8. [DOI] [PubMed] [Google Scholar]
- 44.Sharma M, Kupferman JC, Brosgol Y, Paterno K, Goodman S, Prohovnik I, et al. The effects of hypertension on the paediatric brain: a justifiable concern. Lancet neurol. 2010. September;9(9):933–40. [DOI] [PubMed] [Google Scholar]
- 45.Hajjar I, Marmerelis V, Shin DC, Chui H. Assessment of cerebrovascular reactivity during resting state breathing and its correlation with cognitive function in hypertension. Cerebrovasc Dis. 2014;38(1):10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kral MC, Brown RT, Nietert PJ, Abboud MR, Jackson SM, Hynd GW. Transcranial Doppler ultrasonography and neurocognitive functioning in children with sickle cell disease. Pediatrics. 2003. August;112(2):324–31. [DOI] [PubMed] [Google Scholar]
- 47.Hill CM, Hogan AM, Onugha N, Harrison D, Cooper S, McGrigor VJ, et al. Increased cerebral blood flow velocity in children with mild sleep-disordered breathing: a possible association with abnormal neuropsychological function. Pediatrics. 2006. October;118(4):e1100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ostrovskaya MA, Rojas M, Kupferman JC, Lande MB, Paterno K, Brosgol Y, et al. Executive function and cerebrovascular reactivity in pediatric hypertension. J Child Neurol. 2015. April;30(5):543–6. [DOI] [PubMed] [Google Scholar]
- 49.Lloyd-Jones DM, Allen NB. Childhood Cardiovascular Risk Factors and Midlife Cognitive Performance: Time to Act on Primordial Prevention. J Am Coll Cardiol. 2017. May 9;69(18):2290–2. [DOI] [PubMed] [Google Scholar]
- 50.Iadecola C, Yaffe K, Biller J, Bratzke LC, Faraci FM, Gorelick PB, et al. Impact of Hypertension on Cognitive Function: A Scientific Statement From the American Heart Association. Hypertension. 2016. December;68(6):e67–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maillard P, Seshadri S, Beiser A, Himali JJ, Au R, Fletcher E, et al. Effects of systolic blood pressure on white-matter integrity in young adults in the Framingham Heart Study: a cross-sectional study. Lancet neurol. 2012. December;11(12):1039–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.