Abstract
It is well established that inadequate nutrition during fetal life followed by postnatal overabundance programs adiposity and glucose intolerance. Studies addressing sexual dimorphism in developmental responses to a dietary mismatch are limited; the effect on blood pressure and renal function are understudied. Therefore, this study tested the hypothesis that a mismatch of pre- and postnatal nutrition heightens cardiorenal and metabolic risk, outcomes that may vary by sex. Male and female offspring from sham-operated (control) or reduced uterine perfusion dams (growth-restricted) were fed regular chow or a diet high in fat and sugar (enriched-diet) from weaning until 6 months of age. Male and female offspring were assessed separately; 2-way Analysis of Variance was used to investigate interactions between intrauterine growth restriction and enriched-diet. Blood pressure was increased in all enriched-diet groups, but did not differ in enriched-diet male or female growth-restricted versus same-sex control counterparts. Glomerular filtration rate was reduced in male growth-restricted regardless of diet; a decrease exacerbated by the enriched-diet suggesting the pathogenesis of increased blood pressure induced via an enriched-diet differs between male growth-restricted versus male control. An enriched-diet was associated with glucose intolerance in male and female control but not male growth-restricted; the enriched-diet exacerbated glucose intolerance in female growth-restricted. Thus, these findings indicate male growth-restricted are resistant to impaired glucose homeostasis whereas female growth-restricted are susceptible to metabolic dysfunction regardless of postnatal diet. Hence, moderation of fat and sugar intake may be warranted in those born low birth weight to ensure minimal risk for chronic disease.
Keywords: Sex differences, intrauterine growth restriction, postnatal nutrition, GFR, blood pressure
Summary
This study demonstrated that IUGR programs sex-specific dysregulation in renal function and metabolic risk in offspring exposed to a diet enriched in fat and sugar. Moderation of fat and sugar intake may be warranted in those born low birth weight to ensure minimal risk for chronic disease.
INTRODUCTION
The use of corn syrup and its major ingredient, fructose, is increasing in the Western diet1 and this increase parallels the rise in obesity.2 A direct link between dietary sugar intake and the development of obesity and obesity-related chronic disease is not yet clear.3–5 However, the addition of fructose to the diet is thought to be a major contributory factor to development of obesity and components of the metabolic syndrome, all risk factors for cardiovascular and renal diseases. 2,6-8 Dietary fat intake can also contribute to increased risk for chronic disease7,9,10 although this is mainly limited to intake of saturated fats.11 In the United States, intake of a suboptimal diet and the risk for chronic disease is related to age, race/ethnicity, education level and socioeconomic status.12 The incidence of low birth weight, a crude indicator for poor fetal growth, parallel these same factors.13–16 Low birth weight is associated with an increase in blood pressure (BP),17 and an increased risk for type 2 diabetes18 and chronic kidney disease.19 Experimental studies report that when a fetal environment of undernutrition is mismatched to a post-natal diet of adequate nutritional resources, adaptions of the fetus result in an increased risk for adiposity, hypertension, and diabetes mellitus in later life.20,21 When post-natal nutrition is overabundant following intrauterine growth restriction (IUGR), the risk for impaired metabolic health is amplified.22,23 The physiological responses to a mismatch of pre- and postnatal nutrition also results in sex differences in weight gain, adiposity and glucose/lipid profiles.22,23 Yet, the investigation into the effect of a mismatch of pre- and post-natal nutrition on blood pressure or renal function including sex-specific influences is not clear
Although numerous studies utilize different models of maternal nutrient restriction as a prenatal insult to examine the pathophysiology of diseases that have their origins in early life,22–25 placental insufficiency is the most common causative factor within the Western world.26 The highest rates of low birth weight in addition to obesity are concentrated within the southern United States. Using a model of IUGR induced by placental insufficiency in the rat, our laboratory reports that BP at 4 months of age is increased in male but not female IUGR maintained on regular chow; yet, glomerular filtration rate (GFR) is not decreased in male IUGR offspring despite the increase in blood pressure.27 However, in response to a second hit such as acute angiotensin II (ANG II), GFR is reduced in male IUGR relative to male control at 4 months of age28, whereas the renal response to acute ANG II does not differ in female rats regardless of birth weight.29 BP remains elevated in male30 but not female offspring by 6 months of age31 whereas only female offspring exhibit glucose intolerance at 6 months of age.32,33. Therefore, this study tested the hypothesis that poor fetal growth induced by placental insufficiency followed by chronic exposure to a postnatal diet rich in fat and a sugar-sweetened beverage would cause a greater increase in adiposity and fat mass in IUGR offspring exacerbating the developmental programming of increased blood pressure, impaired renal function and metabolic risk established by IUGR.
METHODS
All experimental procedures were conducted in accordance with National Institutes of Health guidelines for the Care and Use of Laboratory Animals with approval by the Animal Care and Use Committee at the University of Mississippi Medical Center. All other details related to Methods are located in the online-only Data Supplement.
Animals.
First time, timed-pregnant Sprague Dawley rats were purchased from Envigo Laboratories, Inc. (Indianapolis, IN) and housed in a temperature-controlled room (23°C) with a 12:12-hour light/dark cycle with a standard rat chow diet (regular chow or RC) (29% protein, 55% carbohydrate, and 16% fat; total energy value 3.1 kcal/g), and water available ad libitum. At day 14 of gestation rats were randomly selected for the sham (control) or reduced uterine perfusion procedure to induce IUGR offspring. At birth, each sham or reduced uterine perfusion dam was randomly selected to be: (1) Maintained on regular chow with water ad libitum (RC) or (2) switched to a diet high in fat (Harlan, Cat. No. TD.06415: 19% protein, 36% carbohydrate, and 45% fat; total energy value 4.6 kcal/g) plus a choice of water or a commercially available sugar-sweetened beverage that contains high fructose corn syrup ad libitum (High Fat High Sugar, HFHS). All dams delivered at term (21–22 days of gestation) with birth weight recorded within 12 hours of delivery. Offspring were culled 48 hours after birth to 8 pups (4 male and 4 female) per dam to ensure equal nutrient access for all offspring. Pups were weaned at postnatal day 21. Offspring were maintained on the respective maternal diets after weaning until the end-point of study at 24 weeks of age. Timed pregnant groups included offspring from 13 control pregnant on regular chow (RC control), 14 control pregnant on the enriched high fat, high sugar diet (HFHS control), 14 reduced uterine perfusion pregnant rats on regular chow (RC IUGR), and 14 reduced uterine perfusion pregnant rats on the enriched high fat, high sugar diet (HFHS IUGR). To ensure diversity and that programmed influences were not representative of a litter effect, only one male and one female offspring were used per litter per study parameter with littermates used for measurement of MAP and GFR or glucose testing. All animals undergoing surgical procedures were anesthetized using 2–5% isoflurane by inhalation. Systemic, renal and metabolic experimental endpoints were measured at 6 months of age followed by the harvest of visceral fat, kidney, pancreas, liver and gastrocnemius muscle. For all studies, animals were randomly selected from each study group; investigators were blinded to group identification for final study.
Data Availability and the Transparency and Openness Promotion Guidelines Statement.
Upon acceptance, we will make all data underlying the findings described in our manuscript fully available without restriction in compliance with Hypertension’s policy.
Statistics.
Graphpad PRISM version 4 (Graph Pad Software, San Diego, CA) was used for all statistical analysis. Differences between groups were evaluated by two-way analysis of variance (ANOVA) followed by Bonferroni posttest with IUGR and the high fat, high fructose diet as sources of variation. Unpaired student t-test was used for comparison of birth weight. Differences were considered statistically significant at P<0.05. All results are presented as mean±SE.
RESULTS
The effect of intrauterine growth restriction and an enriched post-natal diet on body composition and kidney weight.
By 6 months of age, the enriched-diet was associated with a higher body weight in all groups relative to same-sex counterparts (Fig 1a, 1b). Yet, body weight was significantly reduced in male IUGR relative to male control counterparts regardless of the post-natal diet; body weight was only reduced in female IUGR on the HFHS relative to their female control counterparts (Fig 1a, 1b). Lean mass was significantly increased in HFHS relative to same-sex regular chow (RC) counterparts (Fig 1c, 1d) but no significant difference was observed after normalization of lean mass (grams) to body weight (grams) (Supplemental Materials). Total body fat mass was significantly increased in HFHS control and HFHS IUGR offspring relative to same-sex counterparts on regular chow (Fig 1e, 1f). However, total fat mass was significantly higher in male (Fig 1e) but not female (Fig 1f) HFHS IUGR relative to same-sex HFHS control. These finding also persisted after normalization of total fat mass (grams) to body weight (grams) (Results, Supplemental Materials). Total visceral fat per body weight was also increased in the HFHS offspring relative to same-sex regular chow counterparts but did not differ upon comparison of control to IUGR indicating that IUGR did not program a differential distribution of visceral fat deposition in response to the enriched-diet. (Results, Supplemental Materials). Kidney weight also did not differ among groups (Results, Supplemental Materials).
Figure 1.
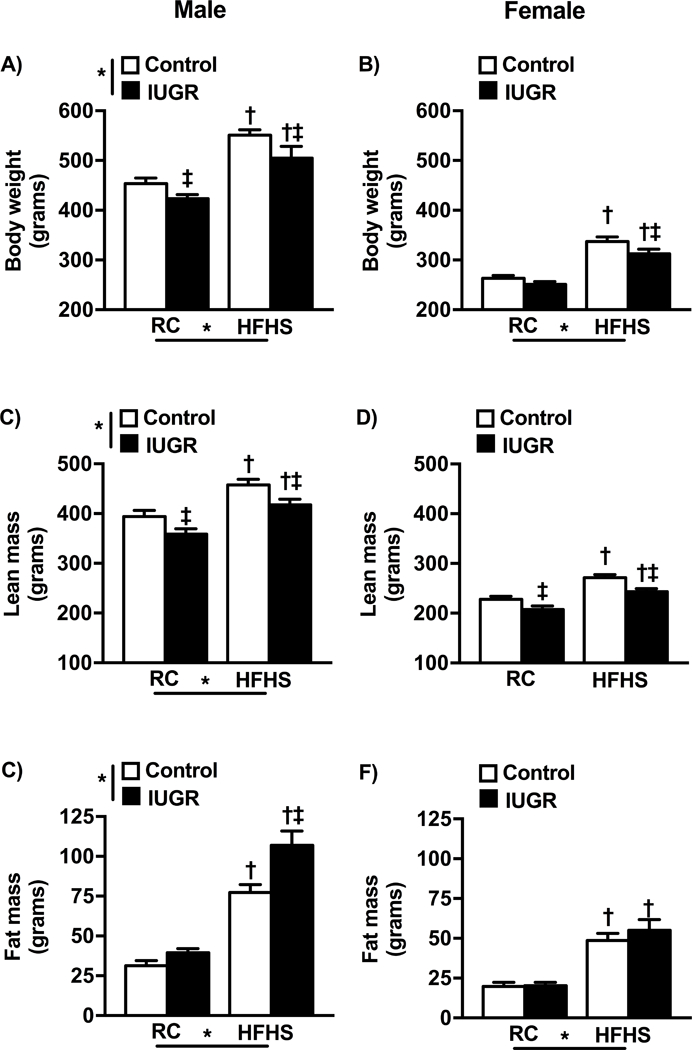
Body weight (a and b), lean body weight(c and d) and fat mass(e and f) in male and female control and growth-restricted (IUGR) offspring at 6 months of age on regular chow (RC) or an enriched diet high in fat and sugar (HFHS). *P value next to each label represents significance in the effect (P< 0.05), IUGR or HFHS, using two-way Analysis of Variance (ANOVA); †P<0.05 HFHS versus RC in similar group; ‡ P<0.05 control versus IUGR on same diet using Bonferroni post hoc test, (n= 7–12 per group). Data values represent mean±SE.
The effect of intrauterine growth restriction and an enriched post-natal diet on food intake.
Food intake was only decreased in female HFHS IUGR relative to female regular chow IUGR (Results, Supplemental Materials).
The effect of intrauterine growth restriction and an enriched post-natal diet on circulating insulin and leptin under non-fasting conditions.
Male control offspring exposed to the post-natal diet high in fat and sugar exhibited an increase in non-fasting plasma insulin levels (Fig 2a, 2b); an observation not demonstrated in male or female IUGR offspring. Plasma leptin levels were elevated in all HFHS groups (Fig 2c, 2d) although plasma leptin levels were elevated to a greater degree in male control versus male IUGR counterparts.
Figure 2.
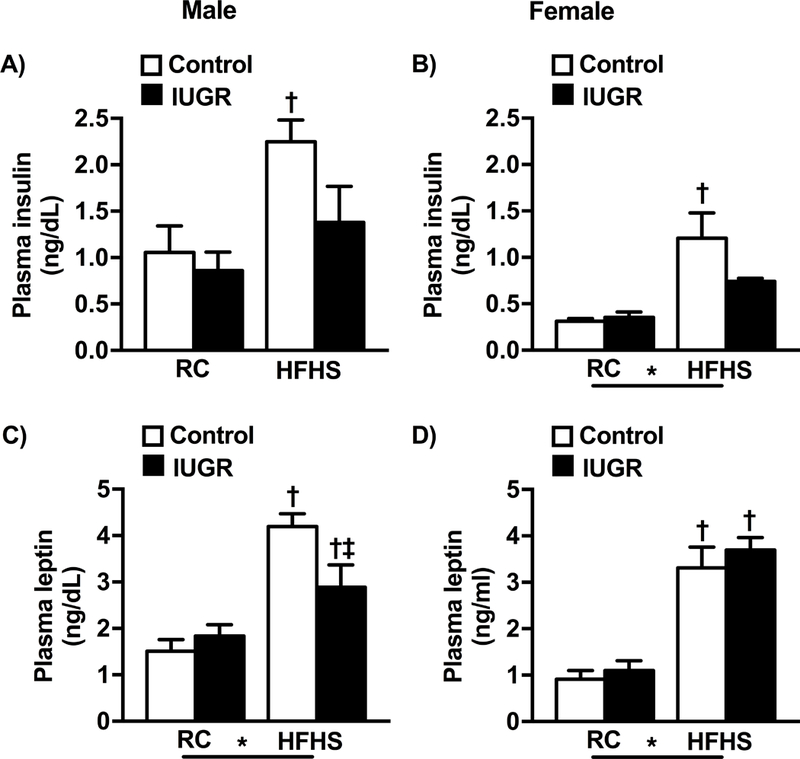
Plasma insulin (a and b) and leptin (c and d) in male and female control and growth-restricted (IUGR) offspring at 6 months of age on regular chow (RC) or an enriched diet high in fat and sugar (HFHS). *P value next to each label represents significance in the effect (P< 0.05), IUGR or HFHS, using two-way ANOVA; †P<0.05 HFHS versus RC in similar group (control or IUGR); ‡ P<0.05 control versus IUGR on same diet using Bonferroni post hoc test, (n= 4–10 per group). Data values represent mean±SE.
The effect of intrauterine growth restriction and an enriched post-natal diet on systemic and renal hemodynamics.
In regular chow fed offspring at 6 months of age, mean arterial pressure (MAP) was significantly increased in male IUGR relative to male control (Fig 3a). MAP did not differ upon comparison of female IUGR to female control (Fig 3b); findings consistent with our previous reports 27,31. However, MAP was significantly elevated in all HFHS relative to regular chow same-sex counterparts but no longer differed by birth weight in male offspring (Fig 3a, 3b) suggesting that the effect of IUGR on blood pressure was diminished by exposure to a HFHS diet. GFR was reduced in male IUGR offspring (Fig 3c). The decrease in GFR in male IUGR was significantly enhanced in response to the chronic HFHS diet (Fig 4c), even when adjusted to kidney weight (Fig 3e). GFR did not differ in female IUGR on regular chow compared to same-sex control (Fig 3d); chronic exposure to a diet enriched in fat and sugar did not alter GFR in female offspring regardless of birth weight. Thus, these findings imply that a decrease in renal function in response to a HFHS diet is specific to male IUGR.
Figure 3.
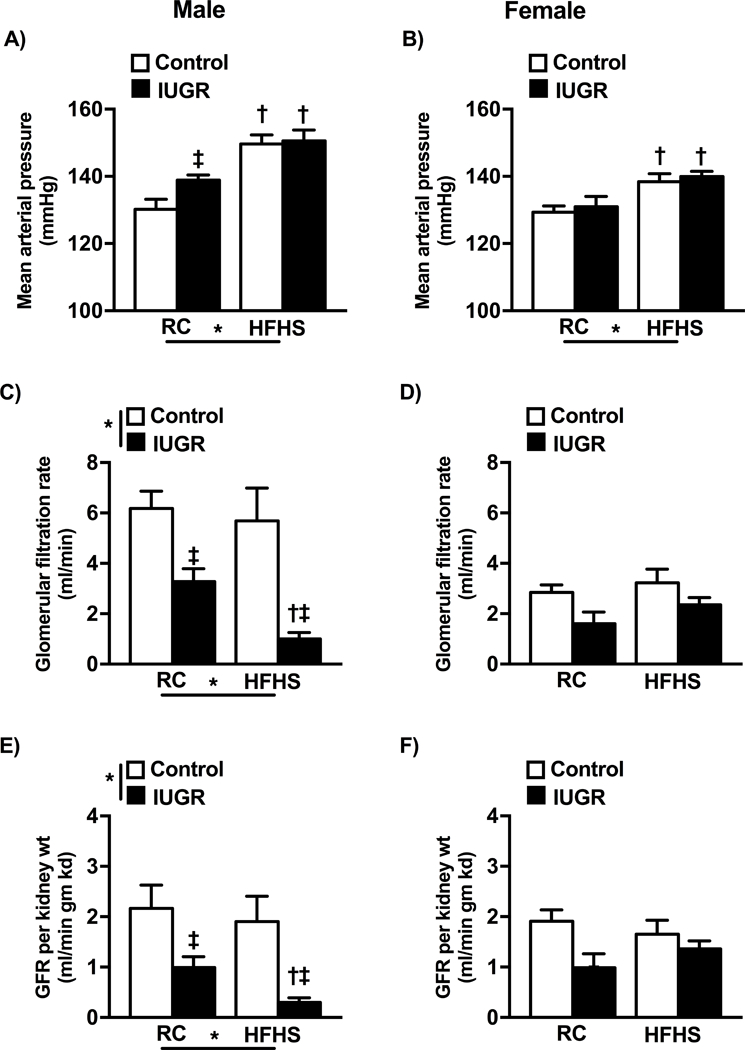
Mean arterial pressure (MAP) (a and b )and glomerular filtration rate (GFR)(c and d) and GFR/kidney weight (e and f) at 6 months of age in conscious, chronically instrumented male and female control and growth-restricted (IUGR) offspring on regular chow (RC) or an enriched diet high in fat and sugar (HFHS).*P values next to each label represent the significance in the effect (P< 0.05), IUGR or HFHS, using two-way ANOVA; †P<0.05 HFHS versus RC in similar group (control or IUGR); ‡ P<0.05 control versus IUGR on same diet using Bonferroni post hoc test, (n= 8–14 per group). Data values represent mean±SE.
Figure 4.
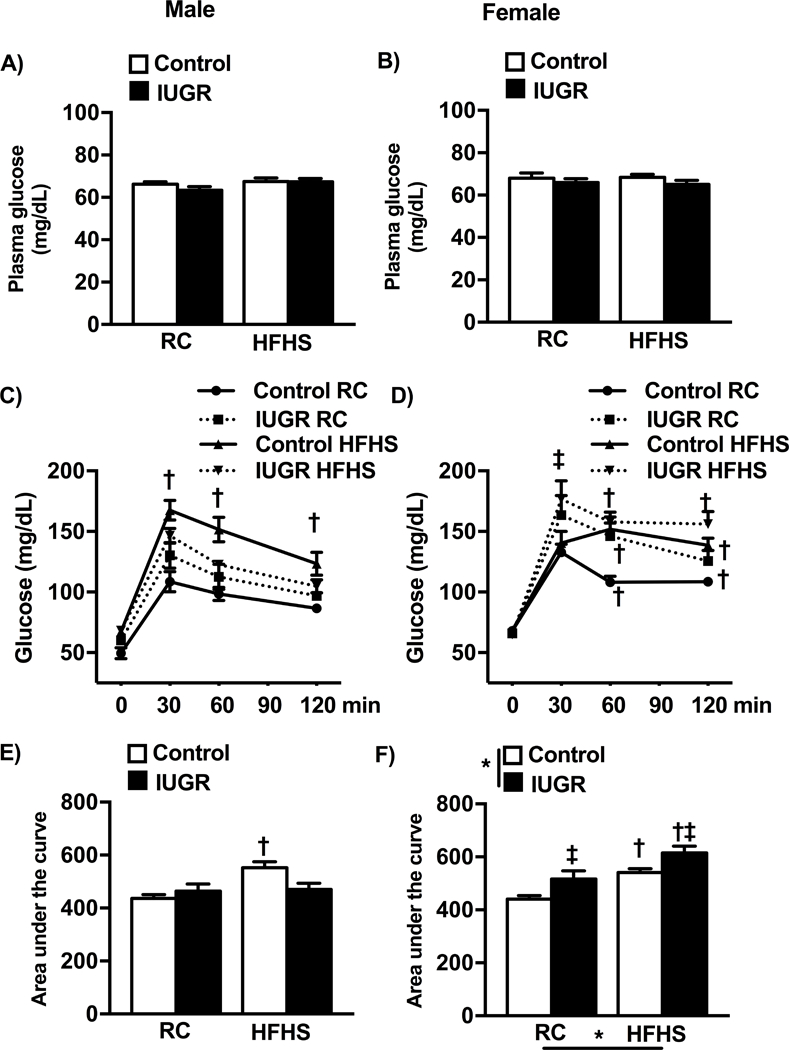
Fasting blood glucose (a and b), oral glucose tolerance test (OGTT) (c and d) and the area under the curve (AUC) calculated for the OGTT (e and f) in male and female control and growth-restricted (IUGR) offspring at 6 months of age on regular chow (RC) or an enriched diet high in fat and sugar (HFHS). *P value next to each label represents significance in the effect (P< 0.05), IUGR or HFHS, using two-way ANOVA; †P<0.05 HFHS versus RC in similar group (control or IUGR); ‡ P<0.05 control versus IUGR on same diet using Bonferroni post hoc test, (n= 8–11 per group). Data values represent mean±SE.
Metabolic consequences of intrauterine growth restriction and an enriched post-natal diet.
As previously reported, fasting blood glucose concentration at 6 months of age did not differ in offspring on regular chow32,33; no differences were observed in offspring exposed to the HFHS diet (Fig 4a, 4b). When challenged with an oral glucose load in the fasted state, only male HFHS control exhibited an impaired glucose tolerance response (Fig 4c) resulting in an increase in area under the curve (AUC) (Fig 4e). Glucose intolerance was observed in female HFHS control and female HFHS IUGR although only female HFHS IUGR retained an elevated glucose concentration at 120 minutes post challenge relative to female regular chow control counterparts (Fig 4d) resulting in a greater AUC for HFHS IUGR relative to HFHS control (Fig 4f).
The effect of intrauterine growth restriction and an enriched post-natal diet on protein expression of glucose transporters (GLUT), GLUT-2 and GLUT-4.
To investigate the pathogenesis of sex- and birthweight-specific impaired glucose tolerance, we examined the relative protein expression of glucose transporters within the peripheral tissues. GLUT-4 protein expression in adipose tissue of male HFHS control and male HFHS IUGR was significantly decreased relative to male regular chow counterparts; no significant difference was observed between female offspring regardless of diet or birth weight (Fig 5a, 5b). Similarly, only male HFHS IUGR offspring exhibited a reduction in GLUT-4 protein expression in muscle (Fig 5c, 6d). Within the pancreas, GLUT-2 protein expression did not significantly differ in male offspring regardless of birth weight or diet, but GLUT-2 was significantly reduced in female regular chow IUGR and in female HFHS control and HFHS IUGR relative to female control regular chow (Fig 6a, 6b). GLUT-2 protein expression in the liver was decreased in HFHS offspring regardless of birthweight relative to regular chow same-sex counterparts (Fig 6c, 6d).
Figure 5.
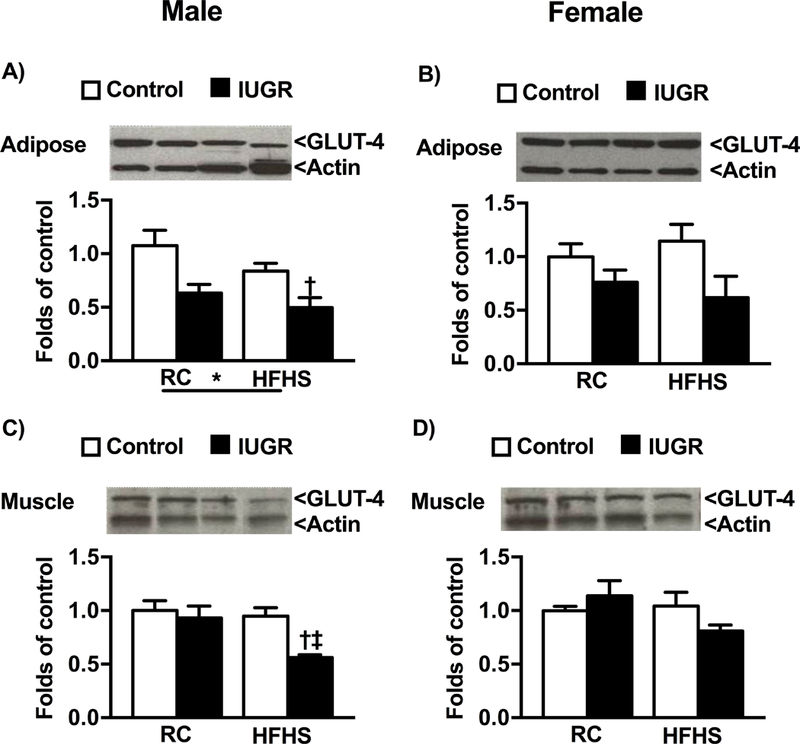
Protein expression of glucose receptor transporters-4 (GLUT-4) in male and female control and growth-restricted (IUGR) offspring at 6 months of age on regular chow (RC) or high fat and sugar (HFHS) in adipose (a and b) and muscle (c and d). *P value next to each label represents significance in the effect (P< 0.05), IUGR or HFHS, using two-way ANOVA; †P<0.05 HFHS versus RC in similar group (control or IUGR); ‡ P<0.05 control versus IUGR on same diet using Bonferroni post hoc test, (n= 4–6 per group). Data values represent mean±SE.
Figure 6.
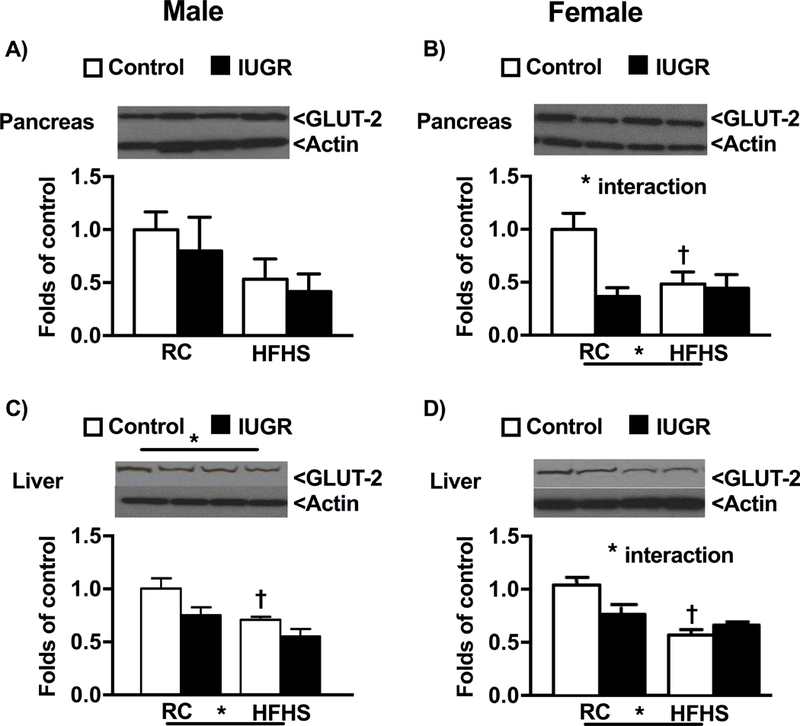
Protein expression of glucose receptor transporters-2 (GLUT-2) in male and female control and growth-restricted (IUGR) offspring at 6 months of age on regular chow (RC) or high fat and sugar (HFHS) in pancreas (a and b) and liver (c and d).*P value next to each label represents significance in the effect (P< 0.05), IUGR or HFHS, using two-way ANOVA; †P<0.05 HFHS versus RC in similar group (control or IUGR); ‡ P<0.05 control versus IUGR on same diet using Bonferroni post hoc test, (n= 4–6 per group). Data values represent mean±SE.
DISCUSSION
The major findings from this study include the following: In response to an enriched high fat and high sugar postnatal diet, blood pressure was increased in control and IUGR offspring regardless of sex. GFR was only reduced in male IUGR; an enriched postnatal diet exacerbated this decrease. Male HFHS control offspring demonstrated impaired glucose homeostasis whereas glucose intolerance was exacerbated in female HFHS IUGR offspring.
IUGR induced via a model of placental insufficiency in rat offspring maintained on regular chow after weaning programs a sex difference in blood pressure by 3 months of age; blood pressure is elevated in male IUGR relative to male control, whereas blood pressure does not differ in female IUGR compared to female age-matched control.27 In the current study the sex-specific programming of increased blood pressure in IUGR offspring remained apparent in regular chow-fed groups at 6 months of age as previously reported.30,31 However, chronic post-natal exposure to a diet rich in fat and sugar was associated with an increase in blood pressure in all groups regardless of sex or birth weight relative to their same-sex regular chow counterparts. In humans, promotion of accelerated weight gain induced by enriched formula in infants born beyond 37 weeks’ gestation, but with a birth weight below the 10th percentile for sex and gestational age, increases blood pressure indicating that promotion of faster weight gain following slow fetal growth has an adverse effect on later cardiovascular health.34 Findings from the current study implicate that the effect of accelerated early growth on later blood pressure is not limited to offspring born IUGR. The mechanisms that mediate the increase in blood pressure following early-accelerated growth and increased adiposity are not clear, but may involve leptin. Hyperleptinemia plays a key role in obesity-related hypertension via activation of the sympathetic nerves.35,36 Moreover, the kidneys play a key role in obesity-induced hypertension with the renal sympathetic nerves providing a mechanistic link.37 Chronic exposure to a diet enriched in fat and sugar was associated with a significant increase in circulating leptin, total fat mass and visceral fat at 6 months of age in all groups. We previously reported that bilateral renal denervation abolishes the increase in blood pressure in male IUGR offspring on regular chow implicating a role for the renal nerves.38,39 Yet, leptin was not elevated in male IUGR offspring on regular chow. Moreover, fat mass or adiposity did not differ in male regular chow fed IUGR offspring relative to regular chow male control. Collectively, these findings suggest activation of the renal sympathetic nerves may contribute to increased blood pressure in male offspring on the enriched diet regardless of birth weight. However, other factors in addition to the renal nerves may contribute to the exacerbated increase in blood pressure in male IUGR on enriched chow versus regular chow-fed IUGR offspring.
To the best of our knowledge, the effect of a postnatal enriched diet on renal function in offspring exposed to an adverse fetal environment such as undernutrition, placental insufficiency or IUGR is not yet reported. We previously reported that GFR is not reduced at 3 months of age in male IUGR offspring maintained on regular chow after weaning.27 However, in the present study, GFR and GFR/kidney weight were reduced in male regular chow IUGR offspring by 6 months of age. Furthermore, exposure to a diet enriched in fat and sugar was associated with a greater decline in GFR and GFR/kidney weight in male IUGR, but the HFHS diet had no effect on GFR or GFR/kidney weight in male control offspring. The differential effect of the HFHS diet on renal function in male offspring may result from the lack of renal reserve programmed in response to IUGR. Male offspring exposed to maternal dietary protein restriction during gestation have a slight but non-significant decrease in GFR associated with a reduction in nephron number. GFR normalized to kidney weight is reduced whereas glomerular volume is increased in this model of maternal protein restriction suggesting adaptive responses to the reduction in nephron number contribute to increased risk for renal injury.24 A reduction in nephron number is a common finding in many experimental models of developmental insult and in individuals born low birth weight.19,40 A reduction in glomerular number is reported in male offspring in other models of placental insufficiency induced via bilateral ligation of the uterine vessels at day 18 of gestation41 or bilateral uterine artery ligation at day 19 of gestation.42 However, kidney weight in IUGR offspring relative to same-sex controls did not differ in this study. Thus, whether IUGR is associated with a reduction in nephron number in this model requires further investigation.
GFR was not significantly altered in female IUGR offspring exposed to the enriched diet relative to their regular chow counterparts. Thus, the HFHS diet resulted in a sex-specific reduction in GFR in IUGR rats. We previously reported that the reduction in GFR in response to acute ANG II is greater in male, but not female IUGR offspring relative to same-sex control acute ANG II counterparts.28 Male IUGR offspring also demonstrate a greater reduction in effective renal plasma flow and an enhanced increase in renal vascular resistance in response to acute ANG II not observed in female IUGR littermates relative to control counterparts suggesting that the renal response to the vasoactive factor ANG II is sex-specific.28,29 Gamba et al report the age-related decline in GFR is enhanced by 9 months of age in Wistar rats on a lipid enriched diet43 suggesting that exposure to an enriched diet up to 9 months of age is associated with a reduction in GFR in normal birth weight rats. Our study suggests that a diet high in fat and sugar serves as a second hit to reduce renal function in male IUGR offspring as early as 6 months of age. Roza et al. report that 8 weeks of high fat intake is associated with no change in renal function in normal birth weight female Wistar rats in young adulthood.44 Our study proposes that female rats maintain renal function following a longer exposure to an enriched high fat and high sugar diet regardless of birth weight. This sex-specific outcome mimics that observed previously by our laboratory, that renal function including GFR in male IUGR is hypersensitive to a secondary insult not observed in female IUGR littermates.28,29 Future studies are needed to determine if indicators of renal injury including proteinuria differ and whether exposure to an enriched diet leads to greater renal risk in female IUGR offspring with age.
Studies investigating the effect of a mismatch of pre- and post-natal nutrition on body weight, weight gain, body composition, and metabolic abnormalities with an emphasis on sex-specific outcomes are limited.22,25,45 In the current study, the effects of a chronic post-natal enriched diet on metabolic health were sex-specific and not limited to animals exposed to the in utero insult of placental insufficiency. Glucose tolerance was impaired in male HFHS control offspring. Yet, the HFHS diet had no effect on glucose tolerance in the male IUGR offspring despite an increase in body weight relative to control counterparts that was also associated with less lean mass and a greater total fat mass. We previously reported that male IUGR offspring on regular chow develop glucose intolerance by 12 months of age relative to age-matched male control.33 However, this occurs without the development of an increase in body weight, visceral fat or total fat mass, or a loss in lean mass compared to control offspring. Collectively, these studies suggest that male IUGR offspring are resistant to glucose intolerance at 6 months of age despite the challenge of a post-natal diet high in fat and sugar. Yet, based on our previous study, IUGR induced via placental insufficiency eventually results in the development of impaired glucose homeostasis in later life in male offspring.33 We previously reported that female IUGR offspring maintained on regular chow exhibit glucose intolerance as early at 6 months of age relative to female control offspring.32 In the current study, AUC for the oral glucose intolerance test (OGTT) was increased in female HFHS IUGR relative to regular chow IUGR offspring at 6 months of age; AUC was also greater in female HFHS IUGR compared to female HFHS control. Thus, IUGR induced via placental insufficiency programs the development of impaired glucose homeostasis in female IUGR offspring that is exacerbated by a diet rich in fat and sugar.
To investigate a potential mechanism that contributes to sex-specific altered glucose homeostasis in response to a post-natal diet high in fat and sugar, we examined tissue specific expression of glucose transporters, a family of proteins that tightly regulate glucose. GLUT-4 mediates glucose uptake in response to insulin in adipose tissue.46 Expression of adipose GLUT-4 is downregulated in obesity46 and a decrease in adipose GLUT-4 is reported in low birth weight men.47 In this study protein expression of adipose GLUT-4 was decreased in male HFHS control and male HFHS IUGR offspring; however, only male HFHS control offspring demonstrated glucose intolerance. Adipose GLUT-4 protein expression was not altered in female offspring regardless of birth weight or diet. Therefore, the effect of an enriched diet on glucose tolerance in male or female offspring may not be related to alterations in adipose GLUT-4. GLUT-2, a glucose sensor, is required for glucose-stimulated insulin secretion in the pancreas. Protein expression of GLUT-2 was decreased in the pancreas of female offspring that exhibited an impaired OGTT. In male offspring, pancreatic GLUT-2 protein expression was not altered regardless of birth weight or postnatal diet. Thus, these findings implicate a role for impaired glucose-stimulated insulin release as a contributor to altered glucose homeostasis in female control and IUGR offspring, but not male control offspring. A reduction in GLUT-2 in the liver also leads to impaired glucose-stimulated insulin secretion. Protein expression of GLUT-2 in the liver was reduced in male and female control and IUGR offspring that demonstrated an impaired glucose tolerance test. Yet, liver GLUT-2 was also reduced in male regular chow and male HFHS IUGR offspring that maintained glucose tolerance suggesting that protein expression of liver GLUT-2 was dysregulated in male offspring. Skeletal muscle is responsible for approximately 80% of all glucose uptake by peripheral tissues. Muscle GLUT-4 is reduced in low birth weight men.48,49 However, in this study protein expression of GLUT-4 was reduced in muscle tissue of male HFHS IUGR offspring that did not demonstrate an impaired OGTT. Muscle GLUT-4 was decreased only in female groups that exhibited an impaired OGTT. There are many isoforms of GLUT proteins and they exhibit tissue and functional differences.50 Impaired glucose transport is linked to insulin resistance and other components of the metabolic syndrome. Although protein expression of GLUT proteins may not reflect functional activity, further investigation is beyond the scope of this study. Clearly, further investigation is needed to determine the exact mechanisms that contribute to sex- and diet-specific impaired glucose homeostasis in control and IUGR offspring.
Perspectives
Our laboratory has extensively characterized this model of IUGR and reports that the mechanisms of increased blood pressure in male IUGR closely mimic the etiology of increased blood pressure in low birth weight individuals (as reviewed in 51). This study expanded our investigation to determine whether a greater mismatch of pre- versus post-natal nutrition would further impair the developmental programming of chronic disease. Our findings demonstrated that IUGR programs sex-specific dysregulation in renal function in offspring exposed to a diet enriched in fat and sugar. GFR was reduced in male IUGR offspring exposed to the enriched diet, but renal function was retained in male control and female offspring that exhibited a HFHS diet-induced increase in blood pressure. Glucose intolerance in female IUGR offspring was exacerbated by the HFHS diet but male IUGR offspring were resistant. Findings from this study indicate that sex matters in the capability of an animal to adapt to a secondary hit in a manner that may be organ and system dependent. Clearly, more studies are needed to determine the mechanisms that contribute to, and the life-long effect, of a diet rich in fat and sugar on sex-specific renal and metabolic health in offspring exposed to a developmental insult.
Supplementary Material
Novelty and Significance.
What is new?
Fetal growth restriction followed by chronic dietary excess of fat and sugar increased blood pressure in control and IUGR offspring regardless of sex. Yet, GFR was only reduced in male IUGR.
Unlike male control, male IUGR offspring were resistant to impaired glucose homeostasis regardless of diet; however, glucose intolerance was exacerbated by exposure to a chronic high fat, high sugar diet in female IUGR.
What is relevant?
A mismatch of prenatal versus postnatal nutrition heightens the risk for chronic disease in later life; however, sensitivity to excess fat and sugar is not IUGR dependent.
Susceptibility of renal and metabolic consequences in IUGR offspring exposed a diet high in fat and sugar is sex-specific.
Acknowledgments
SOURCES OF FUNDING
This work was supported by the National Institutes of Health Grant (NIH) HL074927 and the American Heart Grant (AHA) GRNT19900004 to B. T. Alexander. Additional funding was provided by NIH P20GM104357, P20GM121334 and HL51971. S. Intapad was supported by funding from the AHA 12POST11980021 and 16SDG27770041. J. Dasinger received support from a NIH T32HL105324 and from the AHA PRE24700010. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Bray GA, Popkin BM. Calorie-sweetened beverages and fructose: what have we learned 10 years later. Pediatr Obes 2013;8:242–248. [DOI] [PubMed] [Google Scholar]
- 2.Sharf RJ, deBoer MD. Sugar-sweetened beverages and children’s health. Ann Rev Pub Health 2016;37:273–293. [DOI] [PubMed] [Google Scholar]
- 3.Morgan RE. Does consumption of high-fructose corn syrup beverages cause obesity in children? Pediatr Obes 2013;8(4):249–54. [DOI] [PubMed] [Google Scholar]
- 4.Rebholz CM, Grams ME, Steffen LM, Crews DC, Anderson CA, Bassano LA, Coresh J, Appel LJ. Diet soda consumption and risk of incident end stage renal disease. Clin J Am Soc Nephrol 2017;12(1):79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanhope KL. Sugar consumption, metabolic disease and obesity: The state of the controversy. Crit Rev Clin Lab Sci 2016;53(1):52–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bidwell AJ. Chronic Fructose Ingestion as a Major Health Concern: Is a Sedentary Lifestyle Making It Worse? A Review. Nutrients 2017; 9(6): E549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Odermatt A The Western –style diet: a major risk factor for impaired kidney function and chronic disease. Am J Physiol Renal Physiol 2011;301:F919–F931. [DOI] [PubMed] [Google Scholar]
- 8.Karalius VP, Shoham DA. Dietary sugar and artificial sweetener intake and chronic kidney disease: a review. Adv Chronic Kidney Dis 2013;20(2):157–64. [DOI] [PubMed] [Google Scholar]
- 9.Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O’Keefe JH, Brand-Miller J. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr 2005;81:341–354. [DOI] [PubMed] [Google Scholar]
- 10.Temple NJ. Fat, sugar, whole grains and heart disease: 50 years of confusion. Nutrients 2018;10(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sacks FM, Lichtenstein AH, Wu JHY, Appel LJ, Creager MA, Kris-Etherton PM, Miller M, Rimm EB, Rudel LL, Robinson JG, Stone NJ, Van Horn LV; American Heart Association. Dietary Fats and Cardiovascular Disease: A Presidential Advisory From the American Heart Association. Circulation 2017;18;136(3):e1–e23. [DOI] [PubMed] [Google Scholar]
- 12.Rehm CD, Peñalvo JL, Afshin A, Mozaffarian D. Dietary Intake Among US Adults, 1999–2012. JAMA 2016;315(23):2542–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen XK, Wen SW, Fleming N, Demissie K, Rhoads GG, Walker M. Teenage pregnancy and adverse birth outcomes: a large population based retrospective cohort study. Int J Epidemiol 2007;36(2):368–373. [DOI] [PubMed] [Google Scholar]
- 14.Collins JW Jr, Wambach J, David RJ, Rankin KM. Women’s lifelong exposure to neighborhood poverty and low birth weight: a population-based study. Matern Child Health J 2009; 3(3):326–333. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton BE, Martin JA, Osterman MJ, Curtin SC, Mathews TJ. Births: final data for 2014. Natl Vital Stat Rep 2015;64:1–64. [PubMed] [Google Scholar]
- 16.Meyer JD, Warren N, Reisine S. Racial and ethnic disparities in low birth weight delivery associated with maternal occupational characteristics. Am J Ind Med 2010;53(2):153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lurbe E, Torro I, Rodríguez C, Alvarez V, Redón J. Birth weight influences blood pressure values and variability in children and adolescents. Hypertension 2001;38(3):389–393. [DOI] [PubMed] [Google Scholar]
- 18.Zhao H, Song A, Zhang Y, Zhen Y, Song G, Ma H. The association between birth weight and the risk of type 2 diabetes mellitus: a systematic review and meta-analysis. Endocr J 2018. doi: 10.1507/endocrj.EJ18-0072. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Luyckx VA, Perico N, Somaschini M, Manfellotto D, Valensise H, Cetin I, Simeoni U, Allegaert K, Vikse BE, Steegers EA, Adu D, Montini G, Remuzzi G, Brenner BM; writing group of the Low Birth Weight and Nephron Number Working Group. A developmental approach to the prevention of hypertension and kidney disease: a report from the Low Birth Weight and Nephron Number Working Group. Lancet 390(10092), pp.424–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Godfrey KM, Inskip HM, Hanson MA. The long-term effects of prenatal development on growth and metabolism. Semin Reprod Med 2011;29(3):257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Remacle C, Bieswal F, Bol V, Reusens B. Developmental programming of adult obesity and cardiovascular disease in rodents by maternal nutrition imbalance. Am J Clin Nutr 2011;94:1846S–1852S. [DOI] [PubMed] [Google Scholar]
- 22.Desai M, Babu J, Ross MG. Programmed metabolic syndrome: prenatal undernutrition and postweaning overnutrition. Am J Physiol Regul Integr Comp Physiol 2007;29:R2306–R2314. [DOI] [PubMed] [Google Scholar]
- 23.Whitaker KW, Totoki K, Reyes TM. Metabolic adaptations to early life protein restriction differ by offspring sex and post-weaning diet in the mouse. Nutr Metab Cardiovasc Dis 2012;22(12):1067–7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr Res 2001;49(4):460–467. [DOI] [PubMed] [Google Scholar]
- 25.Zambrano E, Bautista CJ, Deás M, Martínez-Samayoa PM, González-Zamorano M, Ledesma H, Morales J, Larrea F, Nathanielsz PW. A low maternal protein diet during pregnancy and lactation has sex- and window of exposure-specific effects on offspring growth and food intake, glucose metabolism and serum leptin in the rat. J Physiol 2006;571:221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henriksen T, Clausen T. The fetal origins hypothesis: placental insufficiency and inheritance versus maternal malnutrition in well-nourished populations. Acta Obstet Gynecol Scand 2002;81(2):112–114. [DOI] [PubMed] [Google Scholar]
- 27.Alexander BT. Placental insufficiency leads to development of hypertension in growth restricted offspring. Hypertension 2003;41(3):457–462. [DOI] [PubMed] [Google Scholar]
- 28.Ojeda NB, Royals TP, Black JT, Dasinger JH, Johnson JM, Alexander BT. Enhanced sensitivity to acute angiotensin II is testosterone dependent in adult male growth-restricted offspring. Am J Physiol Regul Integr Comp Physiol 2010; 298:1421–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ojeda NB, Intapad S, Royals TP, Black JT, Dasinger JH, Tull FL, Alexander BT. Hypersensitivity to acute angiotensin II in female growth-restricted offspring is exacerbated by ovariectomy. Am J Physiol Regul Integr Comp Physiol 2011;301(4):R1199–R1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dasinger JH, Intapad S, Backstrom MA, Carter AJ, Alexander BT. Intrauterine growth restriction programs accelerated age-related increased cardiovascular risk in male offspring. Am J Physiol Renal Physiol 2016;311(2):F312–F319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Intapad S, Tull FL, Brown AD, Dasinger JH, Ojeda NB, Fahling JM, Alexander BT. Renal denervation abolishes the age-dependent increase in blood pressure in female intrauterine growth-restricted rats at 12 months of age. Hypertension 2013;61(4):828–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Intapad S, Dasinger JH, Brown AD, Fahling JM, Esters J, Alexander BT. Glucose intolerance develops prior to increased adiposity and accelerated cessation of estrous cyclicity in female growth-restricted rats. Pediatr Res 2016;Volume:79:Pages:962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Intapad S, Dasinger JH, Fahling JM, Backstrom MA, Alexander BT. Testosterone is protective against impaired glucose metabolism in male intrauterine growth-restricted offspring. PLoS One 2017;16;12(11):e0187843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singhal A, Cole TJ, Fewtrell M, Kennedy K, Stephenson T, Elias-Jones A, Lucas A. Promotion of faster weight gain in infants born small for gestational age: is there an adverse effect on later blood pressure? Circulation 2007;115(2):213–220. [DOI] [PubMed] [Google Scholar]
- 35.Rahmouni K Leptin-Induced Sympathetic Nerve Activation: Signaling Mechanisms and Cardiovascular Consequences in Obesity. Curr Hypertens Rev 2010;6(2):104–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, Smith G, Stec DE. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem 2010. 4;285(23):17271–17276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kassab S, Kato T, Wilkins FC, Chen R, Hall JE, Granger JP. Renal denervation attenuates the sodium retention and hypertension associated with obesity. Hypertension 1995;25:893–897. [DOI] [PubMed] [Google Scholar]
- 38.Alexander BT, Hendon AE, Ferril G, Dwyer TM. Renal denervation abolishes hypertension in low birth weight offspring from pregnant rats with reduced uterine perfusion. Hypertension 2005, 45(2):754–758. [DOI] [PubMed] [Google Scholar]
- 39.Ojeda NB, Johnson WR, Dwyer TM, Alexander BT. Early renal denervation prevents development of hypertension in growth-restricted offspring. Clin Exp Pharmacol Physiol 2007;34:1212–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hughson M, Farris AB 3rd, Douglas-Denton R, Hoy WE, Bertram JF. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int 2003;63(6):2113–2122. [DOI] [PubMed] [Google Scholar]
- 41.Wlodek ME, Westcott K, Siebel AL, Owens JA, Moritz KM. Growth restriction before or after birth reduces nephron number and increases blood pressure in male rats. Kidney Int 2008;74(2):187–95. [DOI] [PubMed] [Google Scholar]
- 42.Pham TD, MacLennan NK, Chiu CT, Laksana GS, Hsu JL, Lane RH. Uteroplacental insufficiency increases apoptosis and alters p53 gene methylation in the full-term IUGR rat kidney. Am J Physiol Regul Integr Comp Physiol 2003;285(5):R962–70. [DOI] [PubMed] [Google Scholar]
- 43.Gamba CV, Caraviello AZ, Matsushita A, Alves GM, da Silva LN, Gomes GN, Gil FZ. Effects of dietary lipids on renal function of aged rats. Braz J Med Biol Res 2001;34(2):265–269. [DOI] [PubMed] [Google Scholar]
- 44.Roza NA, Possignolo LF, Palanch AC, Gontijo JA. Effect of long-term high-fat diet intake on peripheral insulin sensibility, blood pressure, and renal function in female rats. Food Nutr Res 2016;60:28536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krechowec SO, Vickers M, Gertler A, Breier BH. Prenatal influences on leptin sensitivity and susceptibility to diet-induced obesity. J Endocrinol 2006;189(2):355–363 [DOI] [PubMed] [Google Scholar]
- 46.Shepherd PR, Kahn BB. Glucose transporters and insulin action: implications for insulin resistance and diabetes mellitus. N Engl J Med 1999;341:248.–. [DOI] [PubMed] [Google Scholar]
- 47.Ozanne SE, Jensen CB, Tingey KJ, et al. Decreased protein levels of key insulin signaling molecules in adipose tissue from young men with a low birthweight: potential link to increased risk of diabetes? Diabetologia 2006;49:2993–2999. [DOI] [PubMed] [Google Scholar]
- 48.Vaag A, Jensen CB, Poulsen P, Brøns C, Pilgaard K, Grunnet L, Vielwerth S, Alibegovic A. Metabolic aspects of insulin resistance in individuals born small for gestational age. Horm Res 2006;65(3):137–43. [DOI] [PubMed] [Google Scholar]
- 49.Ozanne SE, Jensen CB, Tingey KJ, Storgaard H, Madsbad S, Vaag AA. Low birthweight is associated with specific changes in muscle insulin-signalling protein expression. Diabetologia 2005;48:547–542 [DOI] [PubMed] [Google Scholar]
- 50.Lacombe VA. Expression and regulation of facilitative glucose transporters in equine insulin-sensitive tissue: from physiology to pathology. ISRN Vet Sci 4;2014:409547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dasinger JH, Alexander BT. Gender differences in developmental programming of cardiovascular diseases. Clin Sci (Lond) 2016;130 (Part 5), Pages: 337–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon acceptance, we will make all data underlying the findings described in our manuscript fully available without restriction in compliance with Hypertension’s policy.


