Abstract
This analysis compares self-reports of product use with objective measures of non-adherence—quarterly plasma dapivirine levels and monthly residual dapivirine (DPV) levels in used rings — in MTN-020/ASPIRE, a phase 3 trial of a monthly DPV vaginal ring among women aged 18–45 years in Malawi, South Africa, Uganda and Zimbabwe. For participants on active product (N=1211) we assessed self-reported monthly nonadherence, as measured by 1) whether the ring was ever out, and out for ≥12 hours in the previous month and, 2) by a self-rating scale assessing ability to keep the vaginal ring inserted, and compared the self-reports to two biomarkers of non-use separately and as a composite measure. For this analysis, a plasma DPV value ≤95 pg/ml and residual ring ≥ 23.5 mg were used to classify non-adherence (i.e. the ring never being in the vagina the previous month.) Compared to self-reports, non-adherence was found to be substantially higher for the composite measure as well as its two components, an indication that ring removal was likely underreported in the trial. The discrepancy between the self-report measure of ring outage and the composite indicator was greater for those aged 18–21 than for those older, evidence that younger women are more likely to underreport nonadherence. Despite underreporting of non-adherence, self-reports of the ring never being out were significant in predicting the composite objective measure. Furthermore, the association between the self-rating scale and the objective measure was in the expected direction and significant, although 11% of those 18–21 and 7% of those 22+ who rated their ability to keep the ring inserted as good, very good or excellent in the four weeks prior to exit were considered non-adherent according to the objective measure. This analysis indicates that while self-reports are significantly associated with objective measures of adherence in the ASPIRE trial, they were inflated — more so by those younger — and therefore may have limited utility identifying those who have challenges using products as directed.
Introduction
Women in sub-Saharan Africa continue to bear a disproportionate burden of HIV infections1. Given limited options to protect themselves against HIV, considerable effort has been made in developing and testing vaginal microbicides. A well-documented challenge facing microbicide trials, however, is participants’ reluctance to disclose nonuse of study products. In comparison to more objective measures of adherence, results from several trials indicate inflated estimates of product use based on self-reports whether in face-to-face interviews or computer assisted interviews designed to be more discrete2. For example, in the VOICE trial — a phase 2B placebo controlled randomized study involving daily dosing with oral tenofovir disoproxil fumarate, oral tenofovir-emtricitabine, or 1% tenofovir vaginal gel in over 5000 women in South Africa, Uganda and Zimbabwe — women overwhelmingly reported that they regularly used product. Based on questions on product use in the prior week asked at monthly follow-up visits, the mean proportion of doses reported to be taken was 90%. Adherence reports from audio-computer assisted self-interviewing were almost as high. Yet in a sample of nearly 650 participants in the three active arms of the trial, the drug was detected in 30% or less of plasma samples3.
Self-reported behavioral data, if accurate, can provide useful information to support or explain trial results4. However, when participants over-report adherence this compromises researchers’ ability to assess safety and efficacy of products, to identify and address the challenges facing participants and to develop a clear understanding of product acceptability and trial experiences. In a qualitative ancillary study, “VOICE-D”, conducted in several VOICE sites, women were only willing to acknowledge non-use of study product after provision of their plasma tenofovir pharmacokinetic (PK) results retrospectively from quarterly samples tested in the trial. Indeed, after being presented with their plasma PK results, participants in the VOICE-D study recommended that realtime product adherence monitoring and feedback should be implemented in future trials in order to encourage use as well as greater honesty in self-reports5. This unwillingness or hesitancy to admit to non-use of product is likely for a variety of reasons including embarrassment or distress about disappointing study staff and concern that disclosure of non-use may jeopardize ongoing trial participation6.
The goal of this analysis is to compare self-reports of non-use of the ring assessed via face-to-face interviews and audio computer assisted self-interviewing (ACASI) in the MTN-020 ASPIRE trial — a phase 3 randomized, double-blind, placebo-controlled trial of a monthly vaginal ring containing the antiretroviral (ARV) drug dapivirine (DPV) — with two objective biomarkers of non-adherence. One of the potential advantages of a vaginal ring over coitally dependent or daily use products such as those investigated in VOICE is that it is longer-acting and just requires the user to leave the product in place over the course of the month to be adherent78. The ASPIRE trial found that the DPV ring reduced the risk of HIV infection overall by 27%. Protection was observed among those over 21 (56%) but not among those 18–21, due in part to lower adherence — as measured by the presence of drug in plasma as well as residual drug in used rings — among the younger women9.
In this paper we attempt to answer two questions: First, is there an association between self-reports of ring non-use and more objective measures of non-adherence in the ASPIRE trial? Second, based on objective measures of ring non-use, did non-adherent participants under-report ring non-use?
Methods
Trial Population, Trial Design and Follow-up
The ASPIRE trial [ClinicalTrials.gov number NCT01617096] was conducted from August 2012-through June 2015 at 15 sites among 2,629 women 18–45 years of age in Malawi, South Africa, Uganda and Zimbabwe. Women were randomized 1:1 to either a silicone elastomer vaginal matrix ring containing 25mg of dapivirine or a placebo vaginal ring. Participants were taught to insert and remove the ring and were instructed to wear it for an entire month. At monthly follow-up visits the ring used in the prior month was collected and women were provided with a new ring. The median follow-up was 1.6 years (interquartile range, 1.1 – 2.3) with over 35% of participants contributing more than 2 years of follow-up.
Objective Measures of Adherence
The ASPIRE trial included two approaches to assessing adherence in DPV vaginal rings, one a point measure that provides an indication of adherence at a particular moment in time and the other, a cumulative measure, that provides a measure of adherence from insertion until the ring is removed10. Plasma samples (point measure), collected quarterly, were tested for the presence of dapivirine with a validated ultra-performance liquid chromatography-tandem mass spectrometry assay. Residual dapivirine levels in used rings (cumulative measure) were assessed monthly beginning 12 months after study initiation with acetone extraction and high-pressure liquid chromatography. Two different measures of non-adherence were defined in the primary analysis. Women were considered non-adherent if: 1) the plasma DPV level was ≤95 pg per milliliter, and 2) the returned ring contained ≥23.5 mg of dapivirine9. Note that the residual DPV level is a measure of how much drug was left over in the ring; it is not standardized for days of use.
Self-reports of product use
At monthly visits women were asked about product use via a face-to-face interview recorded on a CRF that included two questions:
How many times in the past month has the participant had the vaginal ring out, in total?
How many of these times was the vaginal ring out for more than 12 hours continuously?
At the month-three quarterly visit and at the product use end visit (PUEV) women were also asked the following question via ACASI: “Please rate your ability, over the past 4 weeks, to keep the vaginal ring inserted as instructed”. This question was adapted from a single item self-report adherence measure developed to assess medication adherence in HIV treatment studies and has been found to have good predictive validity for clinical outcomes including viral load and CD4 cell count. The question has the advantage of being easily implemented and was designed to reduce the over reporting of adherence, particularly the over reporting of perfect adherence. The measure has been reported to have a lower “ceiling effect” than other self-reported indicators of adherence11. We limited the analysis to the responses from PUEV and compared them to the objective measures for the prior month because we didn’t have residual ring information for many women at the month 3 visit since these data were not collected in the first year of the trial.
Details of the trial design, sample description, procedures, and primary findings are provided elsewhere9.
Analysis
Given that biomarkers of adherence rely on detection of drug, the sample is, by necessity, limited to active arm participants. For ring outage, the unit of analysis is the study quarterly visit with women contributing multiple visits. We constructed dichotomous measures of self-reported product use based on the responses to the two questions listed above:
ring ever out vs. ring never out;
ring ever out>12 hours vs ring never out >12 hours.
For the self-rating question, we restricted the analysis to the product use end visit and thus the unit of analysis is the woman.
We constructed a composite dichotomous measure of non-adherence based on the two objective measures and the definition used in the primary analysis of the ASPIRE trial. For this analysis, a plasma value ≤95 pg/ml and residual ring ≥23.5 mg signifies some degree of non-adherence. On its own, the plasma level only indicates whether the ring was used in the previous eight hours. The ring residual identifies those who, in all likelihood, never used the ring in the prior four weeks. Visits were excluded from the analysis if:
the participant was on product hold or had no access to the ring;
self-report, plasma concentration or residual ring data were missing;
the follow-up visit was 32 days or more since the last visit because of a concern with a drop off in the level of dapivirine in plasma for those who had the ring in for more than a month.
Note that we did not exclude visits with very short follow-up times; only 7% of visits were <21 days apart from the prior visit. When the analyses were run without the visits, the results were similar.
The availability of data for this analysis depends on when the participant enrolled during the nearly 3 year’s duration of the trial. Women who enrolled in August 2012 when the first site was activated would not have residual ring data until after the first year of their participation. For nearly 45% of the sample, data are first available at the 3-month visit. On average, each woman contributed data from approximately 5 quarterly follow-up visits. Some ring outage analyses were also conducted with visits that included self-report and dapivirine plasma concentrations but no residual ring data, for which there is a larger sample, as participants who were enrolled during the first year of the ASPIRE trial implementation can be included. Given the ASPIRE trial demonstrated a protective effect of the ring among women older than 21 but not among those 18–21, we disaggregate analyses by age group.
We first present descriptive data characterizing the analysis sample. We then present measures of adherence based on self-reports of ring outage and the biomarkers. We compare the self-report data with the biological data and analyze biomarkers of adherence among participants who report the ring never being out. We then examine whether younger women are more likely to report non-adherence using generalized estimating equation (GEE) logistic regression with a logit link, independent correlation structure and robust standard errors to account for the within participant correlation across study visits. We also compare the self-rating scale of adherence for younger and older women and present results from a multinomial regression model with the self-rating of adherence as the dependent variable and age as an independent variable to determine if younger women’s assessment of adherence differed from older women We then investigate whether the reasons given by participants for the ring being out differ by age among those reporting non-adherence. Finally, we estimate multivariable models of ring non-adherence using GEE logistic regression with a logit link, independent correlation structure and robust standard errors to determine if self-reports are significantly associated with non-use as determined by the more objective measure of adherence. We also examine the percentage classified non-adherent based on the objective measure for categories of the self-rating scale and estimate logistic regression models with the objective measure of non-adherence at the product use end visit as the outcome variable and the self-rating variable and age as the independent variables. To account for participant differences, in addition to age, country and days between visits are also controlled for in the multivariate models.
The ASPIRE protocol was approved annually by the Institutional Review Boards and ethics committees at each of the study sites and was overseen by the regulatory infrastructure of the U.S. National Institute of Allergy and Infectious Diseases of the National Institutes of Health and the Microbicide Trials Network. All participants provided written informed consent.
Results
The availability of data for this analysis depends on when the participant enrolled during the nearly 3 year’s duration of the trial. Women who enrolled in August 2012 when the first site was activated would not have residual ring data until after the first year of their participation. For nearly 45% of the sample, data are first available at the 3-month visit. On average, each woman contributed data from approximately 5 quarterly follow-up visits.
The analysis sample consists of 1211 active arm participants out of the 1313 total active arm participants enrolled in the ASPIRE trial. Table 1 provides background data on the characteristics of ASPIRE participants at baseline for whom there was self-report, plasma concentration and residual ring data. About one-fifth of the sample was under age 22, and about two-fifths were married. Virtually all report a primary sex partner in the prior three months. The analysis sample is very similar to the total active arm participants.
Table 1:
Characteristics of participants at baseline with self-report, plasma concentration and residual ring data
| Mean (95% CI) or % (n) N=1211 | |
|---|---|
| Age | 27.2 (26.9, 27.6) |
| Age group | |
| 18–21 | 20.3 (246) |
| ≥ 22 | 79.7 (965) |
| Married | 41.5 (503) |
| Primary sex partner in past 3 months | 99.6 (1206) |
| Number of other sex partners in past 3 months | |
| None | 83.6 (1013) |
| 1 | 12.0 (145) |
| 2 | 2.3 (28) |
| >2 | 2.1 (25) |
| Country | |
| Malawi | 10.6% |
| South Africa | 52.6% (637) |
| Uganda | 10.3% (125) |
| Zimbabwe | 26.5% (321) |
| Timing of first visit with self-report, plasma and residual ring data | |
| 3 months | 44.8 (542) |
| 6 months | 12.8 (155) |
| 9 months | 14.7 (178) |
| 12 months | 21.2 (257) |
| Other* | 6.5 (79) |
| Timing of last visit with self-report, plasma and residual ring data | |
| > 24 months | 37.2% (451) |
| 18–24 months | 30.4% (368) |
| 12-<18 months | 24.9% (301) |
| < 12 months | 7.5% (91) |
Visit >12 months (n=67) or non-quarterly visit <12 months (n=12)
We first present measures of ring outage separately by age group with the unit of analysis being the follow-up visit (Table 2). We present both self-report measures and disaggregate the composite biological measure into its two components. Column A includes data from visits with self-report, plasma and residual ring data whereas Column B includes data from visits with just plasma and self-report data. While there is a difference by age in the measures, what is more striking is that non-adherence is substantially higher for the composite objective measure as well as its two components compared to the self- report measures. For example, while women reported the ring ever out in 4.1% of visits (with self-report, plasma and residual ring data), at 18.7% of visits the composite objective measure indicated participants were not adherent.
Table 2:
Percentage of participants classified as non-adherent by age at baseline, aggregated over all visits
| Total | Age 18–21 | Age ≥22 | ||||
|---|---|---|---|---|---|---|
| A | B | A | B | A | B | |
| Ring ever out (self-report) | 4.1% | 5.5% | 5.5% | 7.7% | 3.8% | 4.9% |
| Ring ever out more than 12 hours (self-report) | 1.4% | 2.5% | 1.5% | 3.6% | 1.3% | 2.2% |
| Plasma ≤ 95 pg/ml | 11.4% | 14.2% | 14.7% | 17.9% | 10.5% | 13.3% |
| Residual drug ≥ 23.5 mg | 15.2% | 19.3% | 14.2% | |||
| Plasma ≤ 95 pg/ml or residual drug ≥ 23.5 mg |
18.7% | 23.8% | 17.4% | |||
| (# of visits) | (6245) | (7631) | (1208) | (1517) | (5037) | (6114) |
A: Visits with self-report, plasma and residual ring data. B: Visits with self-report and plasma data
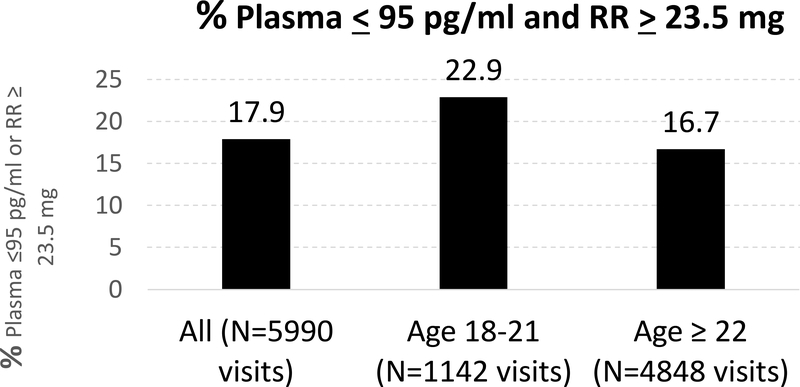
The self-report and objective indicators for each participant were also compared. For 17.9% of visits where participants report the ring never being out, the composite objective measure of adherence, which indicates non-use, is incompatible with the selfreports (Figure 1). If the self-reports of the ring never being out were accurate, we would expect percentages close to zero. Using the comprehensive measure of adherence as the gold standard we also calculated sensitivity and specificity of the self-report data and Cohen’s kappa statistic. While sensitivity is very high, 96.3% for ring never out (N=5540 visits) and 99.0% for ring never out more than 12 hours (N=5695 visits), specificity is low, 8.9% for ring never out (N=44 visits) and 5.9% for ring never out more than 12 hours (N=29 visits). If women were adherent they were very likely to never report the ring being out but if women were non-adherent they were also very likely to never report the ring out. In addition, the kappa for both self-reported measures is small (−0.01) indicating that this measure of self-reported behavior is not a good predictor of objective adherence.
Figure 1:
Biological measure of non-adherence among participants who report ring never out, aggregated over all visits
The discrepancy between the self-report measure and the composite indicator is greater for those aged 18–21 than for those 22 and older, evidence that younger women are more likely to underreport non-adherence. The results comparing the self-reports with the objective measure using the responses to the question about the ring being out more than 12 hours are virtually the same as those for the ring ever being out. For 18.0% of visits (N=6160) where participants report the ring never being out for more than 12 hours, the objective measure is incompatible with the self- reports; the percentages are 23.2% (N=1190) for those 18–21 and 16.8% (N=4970) for those 22+ (data not shown in table).
Next, we considered whether younger women are more likely to report removing the ring both for visits with plasma, self-report and residual ring data and for visits with plasma and self-report data for which the sample is larger (Table 3). In unadjusted models, women aged 18–21 were significantly more likely to report the ring ever out than women aged 22 and older, odds ratio (OR) =1.61 (95% CI. 1.26, 2.07) for the larger sample of visits and OR=1.48 (95% CI 1.09, 2.02) for the smaller sample limited to those with selfreport, plasma and residual drug data. For the “ring ever out more than 12 hours”, age is only significant for the unadjusted model with the larger sample. However, for both samples and for both measures of ring outage, once country is controlled, age becomes insignificant.
Table 3:
The association between age and self-reports of the ring being out*
| Visits with plasma and self-report data: | ||||
| Outcome: Selfreport | OR (95% CI) age < 22 versus 18–21 | p-value | aOR (95% CI) age <22 versus 18–21** | p-value** |
| Ring ever out | 1.61 (1.26, 2.07) | <0.001 | 1.28 (0.99, 1.66) |
0.06 |
| Ring ever out > 12 hours | 1.69 (1.20, 2.39) | 0.003 | 1.34 (0.94, 1.91) |
0.1 |
| Visits with plasma, self-report, and residual ring data: | ||||
| Outcome: Selfreport | OR (95% CI) for age < 22 versus 18–21 | p-value | aOR (95% CI) for age < 22 versus 1821** |
p-value** |
| Ring ever out | 1.48 (1.09, 2.02) |
0.01 | 1.20 (0.86, 1.68) |
0.3 |
| Ring ever out > 12 hours | 1.12 (0.65, 1.93) |
0.7 | 0.90 (0.52, 1.58) |
0.7 |
From GEE analyses with logit link, independent correlation structure and robust standard errors
Controlling for country
Comparison between responses to the adherence self-rating question asking about ability to keep the vaginal ring inserted as instructed at the product use end visit differed slightly by age group. While those 18–21 were less likely to answer “excellent” they were also slightly less likely to report fair, poor or very poor. The largest difference is in a middle category, with nearly 35% of the younger group reporting “good compared to 24% of the older group (Table 4). The lowest three categories of the self-rating scale were collapsed into one group because very few participants assessed their ability as fair, poor and very poor. The difference between age groups in self-rating is only significant for the good category (Table 5).
Table 4:
Percentage distribution of the self-rating scale of ring adherence at the product use exit visit
| Total | Age 18–21 | Age ≥22 | |
|---|---|---|---|
| Excellent | 50.5% (499) | 46.1% (82) | 51.4% (417) |
| Very good | 19.0% (188) | 16.3% (29) | 19.6% (159) |
| Good | 25.9% (256) | 34.8% (62) | 23.9% (194) |
| Fair | 3.7% (37) | 2.8% (5) | 3.9% (32) |
| Poor | 0.3% (3) | 0% (0) | 0.4% (3) |
| Very poor | 0.6% (6) | 0% (0) | 0.7% (6) |
| N | 989 | 178 | 811 |
Visits with self-rating scale, plasma and residual ring data
Table 5:
The association between age and self-rating of adherence*
| Outcome: Self-rating | OR (95% CI) for age < 22 versus 18–21 | p-value | aOR (95% CI) for age < 22 versus 18–21** | p-value** |
|---|---|---|---|---|
| Self-rating of adherence | ||||
| Excellent | 1.0 | 0.7 | 1.0 | 0.7 |
| Very good | 0.93 (0.59, 1.47) | 0.01 | 0.90 (0.55, 1.46) | 0.002 |
| Good | 1.63 (1.12, 2.36) | 0.3 | 1.90 (1.28, 2.84) | 0.1 |
| Fair/Poor/Very poor | 0.62 (0.24, 1.62) | 0.46 (0.18, 1.24) | ||
Visits with plasma, self-report, and residual ring data
From multinomial regression
Controlling for country
We also investigated whether the reasons given for the ring being out differed by age. We grouped the reasons into 4 categories:
physical/hygienic (e.g. discomfort, menses, cleaning the ring or vagina, ring placement)
study related procedures (e.g. removed for clinical procedures, missed visit) social/sexual (e.g. partner/family objections, didn’t want the partner to know) ring came out on its own
Study related reasons were reported most often, followed by physical/hygienic, social/sexual and then ring came out on its own for both age groups. The reasons provided by women who reported the ring being out did not differ significantly by age in either unadjusted models, or models adjusted for country (Table 6).
Table 6:
Reasons for the ring being removed among those who report the ring out
| Reason | 18–21 n=117 | ≥22 n=301 | OR (95% CI)* | pvalue* | aOR (95% CI)** | pvalue** |
|---|---|---|---|---|---|---|
| Physical/hygienic | 17.1% (20) | 22.9% (69) | 0.69 (0.39, 1.23) | 0.7 | 0.73 (0.41, 1.32) | 0.3 |
| Study related/procedural | 36.8% (43) | 30.9% (93) | 1.30 (0.79, 2.13) | 0.3 | 1.27 (0.77, 2.11) | 0.3 |
| Social/sexual | 13.7% (16) | 14.0% (42) | 0.98 (0.50, 1.91) | 1.0 | 0.96 (0.47, 1.94) | 0.9 |
| Came out on its own | 8.5% (10) | 8.0% (24) | 1.08 (0.50, 2.34) | 0.8 | 0.65 (0.29, 1.48) | 0.3 |
From GEE analyses with logit link, independent correlation structure and robust errors (visits with plasma and self-report data)
Controlling for country
Table 7 provides an overview of the association between non-adherence and five different self-reported indicators of ring outage —ever out, out for more than 12 hours, number of times out, number of times out for more than 12 hours and longest number of days out. While self-reports of non-adherence are higher in non-adherent visits compared to adherent visits, as defined by the objective measure, for all five indicators, they are clearly underreported. For example, in 91.1% of non-adherent visits, participants report the ring never being out.
Table 7:
The association between self-reports of the ring being out and the objective measure of non-adherence (plasma ≤95 pg/ml and residual ring >23.5 mg)
| Self-Reported Measure | Non-adherent visits % (n) or mean (95% CI) n=494 | Adherent visits % (n) or mean (95% CI) n=5751 |
|---|---|---|
| Ring never out | 91.1% (450) | 96.3% (5540) |
| Ring never out >12 hours | 94.1% (465) | 99.0% (5695) |
| Number of times ring out | 0.13 (0.07, 0.19) | 0.05 (0.04, 0.06) |
| Number of times ring out >12 hours | 0.09 (0.02, 0.15) | 0.009 (0.007, 0.013) |
| Longest number of days ring out | 1.15 (0.55, 1.74) | 0.08 (0.04, 0.12) |
Self-reports of the ring never being out were significant for all five indicators in unadjusted GEE models predicting non-use with the objective measure of ring nonadherence; age was nearly significant (Table 8). However, for each self-reported indicator, the effect of age was reduced considerably and was insignificant in the adjusted models. That is, with self-report included in the models, age was not a significant predictor of the biological measure of non-adherence. Finally, we investigated whether the proportion of women classified as non-users based on the composite measure in the four weeks prior to exit, varied for categories of the self-rating scale reported at the product use end visit. For both age groups, small proportions of those who rated themselves as excellent, very good and good — 11.0% of those 18–21 (ranging from 6.5% −13.8%) and 7.0% of those 22+ (ranging from 4.3% - 11.9%) — were considered non-users based on the objective measure (Table 8). In addition, non-use as assessed by the objective measure varied significantly and in the expected direction for categories of the scale. As was the case for models with self-report of ring outage, age was not significant in the model with the self-rating scale (Table 9).
Table 8:
The association between self-reports of the ring being out, age and the objective measure of non-adherence (plasma ≤95 pg/ml and residual ring ≥23.5 mg): Results of GEE analyses*
| Variable | OR (95% CI) | p-value | aOR (95% CI)** | p-value** |
|---|---|---|---|---|
| Ring never out | 0.39 (0.15, 0.30) | <0.001 | 0.45 (0.31, 0.66) | <0.001 |
| Age <22 | 1.44 (0.99, 2.10) | 0.06 | 1.19 (0.80, 1.75) | 0.4 |
| Variable | OR (95% CI) | p-value | aOR (95% CI)** | p-value** |
| Ring never out >12 hours | 0.16 (0.12, 0.25) | <0.001 | 0.18 (0.11, 0.28) | <0.001 |
| Age <22 | 1.44 (0.99, 2.10) | 0.06 | 1.20 (0.81, 1.78) | 0.4 |
| Variable | OR (95% CI) | p-value | aOR (95% CI)** | p-value** |
| Number of times ring out | 1.27 (1.10, 1.47) | 0.001 | 1.23 (1.08, 1.39) | 0.001 |
| Age <22 | 1.44 (0.99, 2.10) | 0.06 | 1.20 (0.82, 1.78) | 0.4 |
| Variable | OR (95% CI) | p-value | aOR (95% CI)** | p-value** |
| Number of times ring out >12 hours | 5.75 (3.55, 9.30) | <0.001 | 5.15 (3.14, 8.46) | <0.001 |
| Age <22 | 1.44 (0.99, 2.10) | 0.06 | 1.20 (0.81, 1.78) | 0.4 |
| Variable | OR (95% CI) | p-value | aOR (95% CI)** | p-value** |
| Longest number of days ring out | 1.09 (1.04, 1.14) | <0.001 | 1.09 (1.04, 1.13) | <0.001 |
| Age <22 | 1.44 (0.99, 2.10) | 0.06 | 1.20 (0.81, 1.78) | 0.4 |
From GEE analyses with logit link, independent correlation structure and robust errors (visits with plasma, self-report and residual ring data).
The models include the covariates in the table, as well as time between clinic visits and country.
Table 9:
Percentage (n) of non-adherent women (plasma ≤95 pg/ml and residual ring ≥23.5 mg ) by self-rating categories (exact Binomial 95% CIs)
| Total | 18–21 | ≥22 | |
|---|---|---|---|
| Excellent | 5.8% (29) (3.9%, 8.2%) | 13.4% (11) (6.9%, 22.7%) | 4.3% (18) (2.6%, 6.7%) |
| Very good | 9.0% (17) (9.0%, 14.1%) | 13.8% (4) (3.9%, 31.7%) | 8.2% (13) (4.4%, 13.6%) |
| Good | 10.5% (27) (7.0%, 14.9%) | 6.5% (4) (1.8%, 15.7%) | 11.9% (23) (7.7%, 17.3%) |
| Very poor/poor/fair | 19.6% (9) (9.4%, 33.9%) | 20.0% (1) (0.5%, 71.6%) | 19.5% (8) (8.8%, 34.9%) |
Discussion
In this paper, we sought to answer two questions: 1) whether there was an association between self-reports of ring use and more objective measures of adherence in the ASPIRE trial and 2) whether non-adherent participants in ASPIRE over-reported ring use, according to our composite objective measure of adherence. The answer to both questions is yes. As has been the case in other microbicide and PrEP trials where selfreports of adherence were inflated 12,2,13–15, ring removal was underreported among participants who were not adherent according to the objective measures. However, the difference between ASPIRE and several prior trials, particularly VOICE, is that the discrepancy between self-reports and objective measures is smaller in ASPIRE, likely because the level of adherence is higher. At 17.9% of visits where women reported that the ring was never out —22.9% of visits among those aged 18–21 and 16.7% of visits among those 22 and older — plasma or residual ring levels indicated very low or no use at all during the month. Younger women were significantly more likely to report the ring out and also were more likely to underreport non-adherence, but age was not associated with plasma DPV level or residual DPV in the ring in models with self-report of ring removal or in models with the self-rating scale of ability to use at the product use end visit. Age was not significant in multivariable models predicting the composite objective measure, suggesting it was removal of the ring that likely accounted, at least in part, for the difference in the objective measure of adherence.
That younger women were more likely to underreport non-adherence is consistent with findings from numerous analyses of survey data on sexual behavior in sub-Sharan Africa, which observed considerable misreporting among young people16–20 . That social desirability bias is higher and adherence lower among younger women, particularly the unmarried, is to be expected given that they face greater challenges in navigating their sexual and reproductive lives than their older counterparts.
Few participants (slightly under 5%) classified themselves at the product use end visit as fair, poor or very poor in response to a question asking themselves to rate their ability to keep the ring inserted in the prior four weeks. Nonetheless, that approximately 20% of this group are categorized as non-users according to the objective measure and presumably more were intermittent users, suggests this scale could be utilized to easily and cheaply identify those who are likely not adherent in future open label studies where drug testing for routine monitoring is prohibitively expensive.
This study has several limitations. We started out with the assumption that self-report measures are flawed, but it is also important to acknowledge that the objective measures are imprecise. While the mean plasma elimination half-life for DPV is longer than for other ARVs, an ASPIRE participant could have inserted the ring shortly before her clinic visit and appear to be adherent when in fact she only used the ring for a few days or hours prior to the visit, what is often referred to as the “white coat effect10.” As for the residual drug analysis, given the variability in drug level in rings and the fact that only a small amount of the ARV is released and may vary among individuals, it has not yet been determined whether there is a threshold that reflects adequate use for protection; the cutoffs designated here, which were used in the primary analysis for the ASPIRE trial, distinguish only between some and no ring use9. Finally, although responses to self-rating adherence questions have been found to have predictive validity in HIV treatment trials, the question asking participants to rate their ability to keep the vaginal ring inserted as instructed is subject to varying interpretations such that the same level of adherence might be assessed differently by different participants.
Despite these limitations, and the discrepancies between the self-report and objective measures, our analyses are consistent with the observation that differences in behavior contributed to the lack of HIV-1 protection in younger women in ASPIRE.
Conclusion
While the results from the ASPIRE trial demonstrated that a vaginal ring containing DPV is safe and provides protection against HIV9, adherence and the reporting about product use remain ongoing challenges for microbicide trials among women in sub-Saharan Africa. The expectation is that real-time drug monitoring and feedback, which is a component of the counseling in HOPE (MTN 025) — the ongoing open label study following the ASPIRE trial — will improve both adherence and reporting about challenges to product use and provide additional insights about the acceptability of a vaginal ring for African women.
Table 10:
The association between self-rating of adherence, age and the objective measure of non-adherence (plasma ≤95 pg/ml and residual ring ≥23.5 mg) at the product use exit visit*
| Variable | OR (95% CI) | p-value | aOR (95% CI)** | p-value |
|---|---|---|---|---|
| Self-rating of adherence | ||||
| Excellent | 1.0 | 0.1 | 1.0 | 0.2 |
| Very good | 1.61 (0.86, 3.01) | 0.02 | 1.57 (0.84, 2.94) | 0.02 |
| Good | 1.91 (1.11, 3.30) | 0.001 | 1.98 (1.13, 3.46) | 0.002 |
| Fair/Poor/Very poor | 3.94 (1.73, 8.95) | 3.72 (1.61, 8.58) | ||
| Age <22 | 1.62 (1.04, 2.51) | 0.03 | 1.22 (0.70, 2.14) | 0.5 |
From logistic regression visits with plasma, self-report and residual ring data).
The models include the covariates in the table, as well as country.
Acknowledgements:
The Microbicide Trials Network is funded by the National Institute of Allergy and Infectious Diseases (UM1AI068633, UM1AI068615, UM1AI106707), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the U.S. National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank the study participants as well as the ASPIRE Study team members who implemented the trial.
Footnotes
Compliance with Ethical Standards:
Conflict of Interest: The authors have no conflicts of interest to declare.
Ethical approval: The ASPIRE trial protocol was approved by the ethics review committee at each site.
Informed consent: All participants provided written informed consent.
References
- 1.Kharsany AB, Karim QA. HIV infection and AIDS in Sub-Saharan Africa: current status, challenges and opportunities. The open AIDS journal. 2016;10:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Straten A, Montgomery ET, Hartmann M, Minnis A. Methodological lessons from clinical trials and the future of microbicide research. Curr. HIV/AIDS Rep 2012;10(1):89–102. [DOI] [PubMed] [Google Scholar]
- 3.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N. Engl. J. Med February 5 2015;372(6):509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tolley EE, Harrison PF, Goetghebeur E, et al. Adherence and its measurement in phase 2/3 microbicide trials. AIDS Behav. Oct 2010;14(5):1124–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Straten A, Montgomery ET, Musara P, et al. Disclosure of pharmacokinetic drug results to understand nonadherence. AIDS. 23 October 2015;29(16):2161–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montgomery ET, Mensch B, Musara P, et al. Misreporting of product adherence in the MTN-003/VOICE trial for HIV prevention in Africa: Participants’ explanations for dishonesty. AIDS Behav. 2017;21(2):481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thurman AR, Clark MR, Hurlburt JA, Doncel GF. Intravaginal rings as delivery systems for microbicides and multipurpose prevention technologies. International Journal of Women’s Health. October/21 2013;5:695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nel A, Haazen W, Nuttall J, Romano J, Rosenberg Z, van Niekerk N. A safety and pharmacokinetic trial assessing delivery of dapivirine from a vaginal ring in healthy women. AIDS. 2014;28(10):1479–1487. [DOI] [PubMed] [Google Scholar]
- 9.Baeten JM, Palanee-Phillips T, Brown ER, et al. Use of a Vaginal Ring Containing Dapivirine for HIV-1 Prevention in Women. N. Engl. J. Med 2016;375(22):2121–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stalter RM, Moench TR, MacQueen KM, Tolley EE, Owen DH. Biomarkers and biometric measures of adherence to use of ARV-based vaginal rings. J. Int. AIDS Soc 2016;19(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldman BJ, Fredericksen RJ, Crane PK, et al. Evaluation of the single-item selfrating adherence scale for use in routine clinical care of people living with HIV. AIDS Behav. January 1 2013;17(1):307–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Straten A, Brown ER, Marrazzo JM, et al. Divergent adherence estimates with pharmacokinetic and behavioural measures in the MTN-003 (VOICE) study. Vol 192016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corneli AL, McKenna K, Perry B, et al. The science of being a study participant: FEM-PrEP participants’ explanations for overreporting adherence to the study pills and for the whereabouts of unused pills. J. Acquir. Immune Defic. Syndr April 15 2015;68(5):578–584. [DOI] [PubMed] [Google Scholar]
- 14.Minnis AM, et al. Adherence and acceptability in MTN 001: A randomized crossover trial of daily oral and topical tenofovir for HIV prevention in women. AIDS Behav. 2012;Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mensch BS, Brown ER, Liu K, et al. Reporting of adherence in the VOICE trial: did disclosure of product nonuse increase at the termination visit? AIDS Behav. 2016;20(11):2654–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mensch BS, Hewett PC, Erulkar AS. The reporting of sensitive behavior by adolescents: A methodological experiment in Kenya. Demography. 2003;40(2):247–268. [DOI] [PubMed] [Google Scholar]
- 17.Kelly CA, Hewett PC, Mensch BS, et al. Using biomarkers to assess the validity of sexual behavior reporting across interview modes among young women in Kampala, Uganda. Stud. Fam. Plann 2014;45(1):43–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nnko S, Boerma JT, Urassa M, Mwaluko G, Zaba B. Secretive females or swaggering males? An assessment of the quality of sexual partnership reporting in rural Tanzania. Soc. Sci. Med 2004;59(2):299–310. [DOI] [PubMed] [Google Scholar]
- 19.Plummer ML, Ross DA, Wight D, et al. “A bit more truthful”: The validity of adolescent sexual behavior data collected in rural northern Tanzania using five methods. Sex. Transm. Infect 2004;80((suppl II)):ii49–ii56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaba B, Pisani E, Slaymaker E, Boerma JT. Age at first sex: understanding recent trends in African demographic surveys. Sex. Transm. Infect 2004;80(suppl 2):ii28–ii35. [DOI] [PMC free article] [PubMed] [Google Scholar]