Abstract
Rationale:
Environmental stimuli, or cues, associated with the use of drugs such as cocaine are one of the primary drivers of relapse. Thus, identifying mechanisms to reduce the motivational properties of drug cues is an important research goal.
Objectives:
The purpose of this study was to identify cellular signaling events in the nucleus accumbens (NAc) that are induced when a cocaine cue memory is either extinguished through repeated cue presentation in the absence of drug, or when the memory is reactivated and reconsolidated by a brief cue re-exposure. Signaling events specific to extinction or reconsolidation represent potential targets for pharmacotherapeutics that may enhance extinction or disrupt reconsolidation to reduce the likelihood of relapse.
Methods:
Male Sprague-Dawley rats were trained to self-administer cocaine paired with an audiovisual cue. Following a period of self-administration, the memory for the cocaine-associated cue was either extinguished, reactivated, or not manipulated (control) 15 min before sacrifice. Tissue from the NAc was subsequently analyzed using mass spectrometry based phosphoproteomics to identify cellular signaling events induced by each condition.
Results:
Extinction and reconsolidation of the cocaine cue memory produced both common and distinct changes in protein phosphorylation. Notably, there were no significant changes in protein phosphorylation that were modulated in the opposite direction by the two behavioral conditions. Comparison of NAc phosphoproteomic changes to previously identified changes in the basolateral amygdala (BLA) revealed that cue extinction increases phosphorylation at serine (S) 883 of the GABAB receptor subunit 2 and on S14 of syntaxin 1a in both regions, while no common regional signaling events were identified in the reconsolidation group.
Conclusions:
Phosphoproteomics is a useful tool for identifying signaling cascades involved in different memory processes and revealed novel potential targets for selectively targeting extinction versus reconsolidation of a cocaine cue memory. Furthermore, cross region analysis suggests that cue extinction may produce unique signaling events associated with increased inhibitory signaling.
Keywords: cocaine, proteomics, memory, extinction, reconsolidation, self-administration, phosphorylation
Introduction
Many psychiatric disorders are characterized by maladaptive emotional and/or behavioral responses to stimuli, or cues, in the environment. Substance use disorders in particular are associated with increased attention toward drug-associated cues and less attention toward natural reward cues relative to healthy subjects (Garavan et al., 2000). In addition, drug users commonly report that encountering drug-associated cues in the environment provokes craving and relapse (O’Brien et al. 1993; Childress et al. 1999; Sinha and Li 2007; Fox et al. 2007). Therefore, much clinical and preclinical research has investigated mechanisms by which the influence of these environmental cues could be reduced. One strategy is to extinguish the association between the cue and the emotional/behavioral reaction. For example, repeatedly exposing someone to cues associated with their drug use (e.g., paraphernalia, people, places) in the absence of the drug can lead to a gradual reduction in the ability of those cues to induce craving and relapse (O’Brien et al. 1990; Conklin and Tiffany 2002; Price et al. 2013). This procedure is generally referred to as exposure therapy in clinical populations and as extinction learning in preclinical models. Successful extinction learning requires repeated presentation of the cues over several trials to produce sufficient learning of the lack of association between the cues and drug reinforcement to reduce craving. However, if the amount of cue exposure is insufficient, then it is possible that the original memory will simply be reactivated and restabilized into long-term storage by a process known as memory reconsolidation (Merlo, Milton, Goozee, Theobald, & Everitt, 2014; Taylor, Olausson, Quinn, & Torregrossa, 2009; Torregrossa & Taylor, 2012). In this situation, the ability of the cues to induce craving and relapse will be either unchanged or greater due to the potential for reconsolidation-induced memory strengthening. Therefore, it is critical that any therapy aimed at enhancing extinction processes would not unintentionally enhance reconsolidation. Likewise, treatments aimed at inhibiting reconsolidation should not also have the ability to inhibit extinction. Thus, it is important to identify signaling cascades that can be targeted to selectively influence extinction or reconsolidation, or ideally, that can both enhance extinction and inhibit reconsolidation.
In order to accomplish this goal, we recently undertook an experiment using unbiased proteomics to identify the signaling cascades that are engaged by cocaine-cue extinction learning versus reconsolidation relative to controls. Specifically, we measured changes in protein phosphorylation in the basolateral amygdala (BLA) as phosphorylation is the primary mechanism by which protein activity, localization, and function are regulated and because neural activity in the BLA is critical for the expression of cue-associated memories (Fuchs, Feltenstein, & See, 2006; Huttlin et al., 2010; Rich et al., 2016). We found that several protein phosphorylation events occurred after both extinction and reconsolidation, which likely represent signaling events generally important for the stabilization of any memory (i.e., reconsolidation of the original memory and consolidation of the new extinction memory). However, we also identified several memory-process-specific signaling events, and a few phosphorylation events that were regulated in the opposite direction by cocaine cue extinction versus reconsolidation, including a newly identified phosphorylation event on calcium-calmodulin-dependent kinase IIα (CaMKII). We went on to show that inhibiting CaMKII in the BLA could both enhance extinction and disrupt reconsolidation to reduce relapse-like cocaine-seeking behavior. These data demonstrated the potential for novel targeting of each memory process independently (Rich et al., 2016).
Nonetheless, the BLA is not the only brain region implicated in the consolidation, reconsolidation, or extinction of cocaine-associated memories. For example, the nucleus accumbens (NAc), particularly the core sub-region, is critical for cue-induced reinstatement of cocaine seeking (Fuchs et al. 2004). Moreover, reconsolidation of a cocaine-associated spatial memory requires activation of the extracellular signal regulated kinase (ERK) in the NAc (Miller and Marshall 2005), and we have shown that cocaine cue extinction learning can be enhanced/made context independent via activation of NMDA receptors in the NAc (Torregrossa, Gordon, & Taylor, 2013). Consequently, it is likely that extinction-specific and reconsolidation-specific signaling events in the NAc may also regulate the strength of cocaine memories, and subsequently craving and relapse behavior. However, it is not known if the same or different signaling events regulate memory across brain regions. Therefore, in this study we performed a phosphoproteomic analysis of the NAc from rats, previously reported in Rich et al., 2016, that self-administered cocaine paired with an audiovisual cue. Comparisons were made between groups that either had the cocaine cue memory reactivated, extinguished, or was left unmanipulated to identify novel potential targets for reducing the strength of cocaine memories.
Materials and Methods
Subjects.
A total of 24 adult male Sprague-Dawley rats, weighing 275-325 g on arrival, were used in all studies. All rats were housed in pairs with ad libitum access to food and water (unless noted otherwise) on a 12 h light-dark cycle in a temperature- and humidity-controlled room. Rats were given at least 5 days to acclimate to the facility before undergoing surgical procedures. Following surgery, rats were individually housed and given at least 1 week to recover before the start of behavioral training. Rats were food-deprived 24 h prior to the start of behavioral experiments and maintained at −90% of their free-feeding body weight (−20 g of chow per day) for the duration of testing. All behavioral experiments were run during the light-cycle. Sprague-Dawley rats were obtained from Charles River (Kingston, NY) All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by Yale University’s Institutional Animal Care and Use Committee.
Surgical procedures.
Rats were implanted with indwelling jugular catheters (CamCaths, Cambridge, UK) as previously described (Rich et al., 2016). Briefly, rats were fully anesthetized with ketamine hydrochloride (87.5 mg/kg; i.m.) and xylazine hydrochloride (5 mg/kg; i.m.) and then received an analgesic (Rimadyl, 5 mg/kg; s.c.) and 5 ml of Lactated Ringer’s (s.c.) prior to surgery. Betadine and 70% ethanol were applied to all incision sites. Catheters were fed subcutaneously to the midscapular region, where they exited through a round incision. Catheters were kept patent by daily infusions of 0.1 ml of an antibiotic solution of cefazolin (10.0 mg/ml) dissolved in heparinized saline (30 USP heparin/ml).
Self-administration Training.
Rats were trained to self-administer cocaine in operant conditioning chambers (MedAssociates, St. Albans, VT) using our standard procedures (Rich et al., 2016; Torregrossa & Kalivas, 2008; Torregrossa, Sanchez, & Taylor, 2010). Cocaine hydrochloride was generously provided by the National Institute on Drug Abuse, Research Triangle Park, NC, and was dissolved in sterile 0.9% saline (2 mg/ml) and filter-sterilized for self-administration. Rats were trained to self-administer cocaine during daily sessions for 1 h, on a fixed ratio 1 (FR1) schedule of reinforcement with a 10 s timeout as published (Rich et al., 2016). Responses on the active lever (counterbalanced across left and right levers) produced a cocaine infusion paired with a 10 s tone-light conditioned stimulus (CS). Pump durations were adjusted daily according to body weight in order to deliver a 1.0 mg/kg dose per infusion. Responses on the inactive lever were recorded but had no programmed consequences. Rats underwent training for at least 10 d and until they administered at least 8 infusions per day over 3 consecutive days. Two rats that did not acquire self-administration were excluded from all analyses. The program was controlled by and data were collected using MedPC (MedAssociates).
Instrumental lever extinction.
After successful acquisition of self-administration, rats underwent instrumental lever extinction for 5 days. During these daily 1 h sessions, responses on both the active and inactive levers were recorded but had no programmed consequences. Throughout lever extinction, rats received no cocaine or cocaine-associated cue reinforcement. Lever extinction was conducted to reduce the motivational value of other cues in the self-administration context, such as the levers, so that subsequent memory manipulations were specific to the discrete cue associated with cocaine infusion (Torregrossa et al., 2010).
Memory manipulations.
As previously published (Rich et al., 2016), following cocaine self-administration and instrumental lever extinction, rats were assigned to one of three memory manipulation groups (extinction, memory reactivation, or no manipulation controls) based on a matching procedure that ensured that each group had no statistical differences in their cocaine infusions acquired over days or differences in rates of lever extinction behavior. All groups were placed in a novel context (different in flooring texture, shape, and smell) for a 30 min session on 2 consecutive days and underwent their assigned memory manipulation (see below). During these sessions, rats had no opportunity for instrumental responding, i.e., the levers were retracted.
Extinction:
For cue extinction, the cocaine-associated CS was presented for 10 s, 60 times, with each presentation separated by 30 s, on each of the two days. Thus, the rats were exposed to a total of 120 CS presentations, which we have previously shown to significantly reduce cue-induced reinstatement on a subsequent test day (Torregrossa et al., 2010).
Memory Reactivation:
For cue reactivation, the CS was presented 3 times at the end of the last session on the second day, with each CS presentation separated by 1 min. Previous work from our lab has shown that 3 CS presentations are sufficient to induce memory reactivation and reconsolidation, but is not sufficient to produce extinction (Rich et al., 2016; Sanchez, Quinn, Torregrossa, & Taylor, 2010; Wan, Torregrossa, Sanchez, Nairn, & Taylor, 2014).
No Memory Manipulation Control:
The control group was placed in the operant chambers for the same amount of time as rats in the cue extinction and reactivation groups, but with no CS presentations. Thus, the time spent in the operant boxes and the type of operant box was equivalent between groups prior to sacrifice.
Tissue Collection.
Fifteen minutes following memory or control manipulations, rats were lightly anesthetized with isofluorane to minimize stress prior to euthanasia by focused microwave irradiation. Focused microwave irradiation was used to preserve the phosphorylation state of proteins. Importantly, because no group had the opportunity to make instrumental responses prior to sacrifice, differences in behavioral activity should not substantially affect levels of protein phosphorylation. The brains were immediately dissected and individual brain regions, including the NAc (primarily the core subregion) were obtained and stored at −80 C until processing.
Label-free quantitative proteomics: sample preparation.
Brain regions of interest, including the amygdala reported previously (Rich et al., 2016) and the NAc, reported here, were homogenized by sonication in a buffer containing urea (ThermoFisher, 8 M), ammonium bicarbonate (Thermofisher, 0.4 M), and protease (Pierce, at 1% of lysis buffer) and phosphatase inhibitor cocktails (Pierce, at 2.5% of lysis buffer). Samples from 2-3 rats in each experimental group were randomly pooled to create a total of 3 biological samples per group. Pooled samples were then analyzed by the Yale/NIDA Neuroproteomics Center. Note that NAc and BLA samples were processed and analyzed by mass spectrometry within 6 months of each other. Briefly, 20 μL of 45 mM DTT was added to each sample and incubated at 37 °C for 20 min to reduce Cys residues. Samples were cooled and 20 μL of 100 mM iodoacetamide (IAM) was added to each sample and incubated at room temperature in the dark for 20 min for alkylation of the reactive free sulfhydro of the reduced Cys. Dual enzymatic digestion was carried out by adding 600 μL of dH2O and 30 μL of 1 mg/mL Lys C followed by incubation at 37 °C for 4 h, with subsequent digestion by incubation with 30 μL of 1 mg/mL trypsin overnight at 37 °C. Samples were macrospin desalted and dried by speedvac. Pellets were dissolved in 50 μL of a solution containing 0.5% TFA and 50% acetonitrile. Samples were then subjected to titanium dioxide (TiO2) phosphopeptide enrichment using TopTips (Glygen, Columiba, MD). A three step conditioning of the TopTip was utilized with 1 min at 2000 RPM on a bench top centrifuge (ThermoFisher) for each step. First, the TopTip was washed with 2 × 60 μL 100% acetonitrile, then with 2 × 60 μL 0.2 M sodium phosphate (pH 7.0), and finally with 2 × 60 μL 0.5% TFA in a 50% acetonitrile solution. The acidified digest supernatants were loaded into the TopTip, and bound phosphopeptides were washed with 2 × 40 μL of a buffer containing 0.5% TFA in 50% ACN. spun at 1,000 rpm for 1 min, and then at 3,000 rpm for 2 min. Phosphopeptides were eluted from each TopTip by 3 washes with 30 μL of 28% high purity ammonium hydroxide (ThermoFisher). The eluted fraction was dried and re-dried with 2 × 30 μL water by speedvac. Enriched fractions were dissolved in 10 μL of 70% formic acid and 30 μL of 50 mM sodium phosphate. Peptide concentrations were determined by Nanodrop to load 0.3 μg/5μL of each sample.
LC/MS-MS. 5 μL of each sample was injected onto a LTQ Orbitrap XL LC-MS/MS system. Peptide separation was performed on the nanoACQUITY™ ultra-high pressure liquid chromatography (UPLC™) system (Waters, Milford, MA), using a Waters Symmetry® C18 180 μm × 20 mm trap column and a 1.7 μm, 75 μm × 250 mm nanoACQUITY™ UPLC™ column (35 °C). Trapping was done at 15 μL/min, with 99% Buffer A (0.1% formic acid in water) for 1 min. Peptide separation was performed over 120 min at a flow rate of 300 nL/min beginning with 95% Buffer A and 5% Buffer B (0.075% formic acid in acetonitrile) to 40% B from 1–9 min, to 85% B from 9-91 min, held at 85% B from 91-95 min, then returned to 5% B from 95-96 min. Two washes were made between each sample run to ensure no carry over (1. 100% acetonitrile, 2. Buffer A). The LC was in-line with an LTQ-Orbitrap XL mass spectrometer. MS was acquired in the Orbitrap using 1 microscan, and a maximum inject time of 900 ms followed by 3-6 data dependent MS/MS acquisitions in the ion trap (with precursor ion threshold of >3000). The total cycle time for both MS and MS/MS fragmentation by collision induced dissociation (CID) were first isolated with a 2 Da window followed by normalized collision energy of 35%. Dynamic exclusion was activated where former target ions were excluded for 30 sec. Three technical replicates were injected for each sample and all samples and replicates were randomized across the entire run time.
Data analyses.
Behavioral Data
Rats were assigned to memory manipulation groups using a matching procedure to ensure no statistical differences in cocaine self-administration or instrumental extinction parameters between groups. After dividing the rats into the three groups, behavioral data was analyzed using a Two-way repeated measures ANOVA, with day of training as the repeated measure, within-subject factor, and to-be memory manipulation group as the between group factor. Significance was set at p=0.05.
Proteomics Data
Chromatographic/spectral alignment, feature extraction, data filtering, and statistical analysis was carried out using Nonlinear Dynamics Progenesis LC-MS software (www.nonlinear.com). Raw data files were imported into the program and detected mass spectral features were aligned based on retention time of the detected m/z peaks based on a randomly selected reference run. All other runs were automatically aligned to the reference run to minimize retention time variability between runs. No adjustments were necessary in the m/z dimension due to high mass accuracy of the spectrometer (typically < 3 ppm). All runs were selected for detection with an automatic detection limit. Features within retention time ranges of 0-5 min were filtered out, as were features with charge state greater than +6 or singly charged peptides (as no MS/MS fragmentations were taken for these charge states during data collection) for reduction of false positive peptide assignments. A normalization factor was then calculated for each run to account for differences in sample load between injections. The experimental design grouped multiple injections from each condition. Stringent conditions were set in MASCOT to filter out low scoring identified peptides by imposing a confidence probability score (p) of < 0.05. Additionally, a positively identified protein that was quantified contained at least two unique identified tryptic peptides. The filtered MS/MS spectral features along with their precursor spectra were exported in the form of an .mgf file (Mascot generic file) for database searching using the Mascot algorithm (Hirosawa et al. 1993). The data was searched against the Uniprot (Rattus norvegicus) database. The confidence level was set to 95% within the MASCOT search engine for peptides assigned hits based on randomness. MS/MS analysis was based on the use of trypsin and the following variable modifications: carbamidomethyl (Cys), Oxidation (Met), Phospho (Ser, Thr, Tyr). Other search parameters included peptide mass tolerance of ± 15 ppm, fragment mass tolerance of ± 0.5 Da, and maximum missed cleavages of 3. A decoy search (based on the reverse sequence search) was performed to estimate False Discovery Rate (FDR), with setting of acceptable protein ID having FDR of 2%. Using the Mascot database search algorithm, a protein is considered identified when Mascot lists it as significant (bold red) and more than 2 unique peptides match the same protein.
The Mascot significance score match is based on a MOWSE score and relies on multiple matches to more than one peptide from the same protein. The Mascot search results were exported to an .xml file using a significance cutoff of < 0.05, and ion score cutoff of 28, and a requirement of at least one bold (first time any match to the spectrum has appeared in the report) and red (top scoring peptide match for this spectrum) peptide. The .xml file was then imported into the Progenesis LCMS software, where search hits were assigned to corresponding detected features, identified as described above.
Once proteins and protein modifications for each peptide were determined, the intensity value for each sample was log2 transformed and averaged within each group. When the same modification was identified on a protein as separate peptides (due to charge state or cleavage differences), the average intensity value of that peptide was determined for each sample, so that one value of modified peptide abundance could be compared across groups. The three groups were compared by ANOVA and post-hoc t-tests compared each group to each other. Given that the purpose of this study was to discover potential new targets for manipulating cocaine-associated memories and to better understand similarities and differences between signaling associated with extinction and reconsolidation processes in the NAc, we present data where statistical significance from the ANOVA was corrected for multiple comparisons using the Benjamini-Hochberg procedure with a 10% false discovery rate (Benjamini and Hochberg 1995). Individual t-tests were also performed to identify memory manipulation specific signaling patterns, and results with p-values <0.1 are shown. Self-organized heat maps of phosphorylation and sample of peptides with ANOVA p-values less than 0.1 were prepared in Cluster 3.0 and Treeview. Values were median centered by protein and subject, then normalized by phosphorylation and sample. Un-centered correlation was used as the similarity metric and the clustering method was centroid linkage.
Results
Cocaine self-administration and instrumental extinction
Figure 1a illustrates the timeline of experimental procedures. The brain tissue analyzed in the present study was obtained from rats whose behavior prior to tissue collection was previously published (Rich et al., 2016). Here we modified these data as the samples that were pooled to create the three memory conditions was slightly different from the prior published study due to sample loss. Nevertheless, there were no significant differences in the amount of cocaine self-administered (Fig. 1b) or in the rate of instrumental extinction (Fig. 1c) between rats that would subsequently be assigned to control, reactivation, and extinction groups. Analysis of the self-administration data indicated a significant effect of day of training [F(9,189) = 3.05, p=0.002], indicating that the rats increased infusions earned over days, but there was no effect of the group to which they would be assigned [F(2,21) = 1.04, p=0.37], nor an interaction [F(18,189) = 1.07, p=0.39], indicating that the effects of the memory manipulations were not likely due to differences in prior cocaine self-administration. Similarly, there were no differences between the to be assigned memory manipulation groups on instrumental lever performance [F(2,21) = 0.63, p=0.54] or interaction [F(8,84) = 0.48, p=0.87] with day of training, though there was a significant effect of day [F(4,84) = 35.32, p<0.0001]. These results indicate successful cocaine self-administration and subsequent extinction of lever pressing.
Figure 1. Experimental Timeline and Behavioral Results.
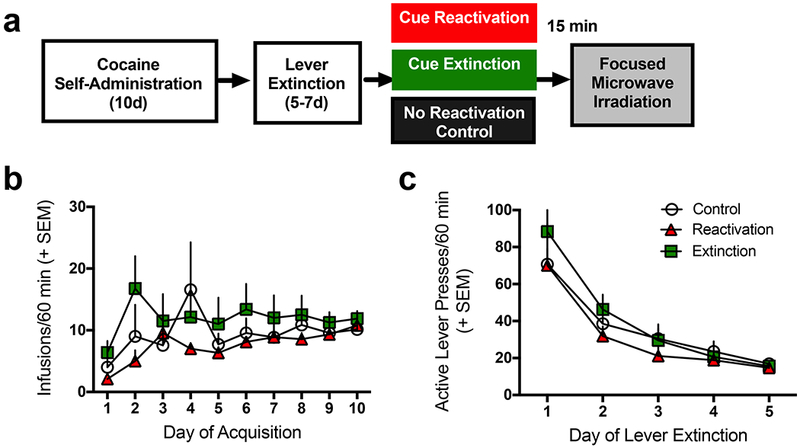
(a) Timeline of the experimental procedures. (b) Comparison of cocaine infusions earned during self-administration between groups that would later be assigned to the cocaine cue memory reactivation (n=9), extinction (n=8), or no manipulation control groups (n=7). No significant differences were observed between groups. (c) Comparison of active lever presses during instrumental extinction between groups later to be assigned to memory conditions listed in (b). No significant differences in extinction learning were observed between groups.
Phosphoproteomic analysis
The phosphoproteomics analysis quantified 1022 unique phosphorylation events on 372 unique proteins (indicative of multiple phosphorylation events on some proteins). A complete list of all phosphopeptides identified in this study can be found in Supplementary Table 1.
First, we performed a cluster analysis across all the phosphopeptides identified as significantly different in any of the three memory conditions to determine if unique protein phosphorylation signatures would be observed based on memory state (Fig. 2 & Fig. S1). We expected that extinction and reconsolidation associated phosphorylation patterns would result in distinct clusters, and that the extinction condition might be the most different given that cue extinction weakens cocaine-seeking behavior, while cocaine seeking would be high in both the control and reconsolidation conditions (Rich et al., 2016; Torregrossa et al., 2010). However, the cluster analysis suggests that both the reconsolidation and extinction groups are more similar to each other than either is to the control group, with a small number of phosphopeptides differentiating the extinction and reconsolidation conditions (left side of Fig. 2).
Figure 2. Cluster Analysis of Cocaine Memory-Associated Phosphoproteome.
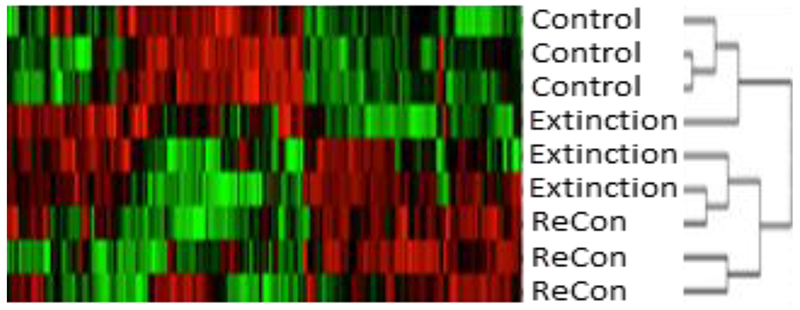
Heat map of cluster analysis with each sample from Control, Extinction, and Reconsolidation (Recon) groups shown on the left of the map, and individual phosphopeptides in each column. Red indicates where phosphopeptide abundance is greater than other groups and green indicates where abundance is less than other groups. The pattern of the heat map suggests that samples from the Extinction and Reconsolidation groups have a more similar phosphoproteome than the control group.
Next, we identified the specific phosphorylation events that were significantly regulated by both the extinction and reconsolidation of a cocaine cue memory relative to controls. We found 10 unique phosphorylation events regulated in the same direction with a cut-off of p<0.016 (Benjamini-Hochberg corrected p-value) from the ANOVA. Table 1 gives the protein and gene name, the peptide identified and its phosphorylation state (changes in oxidation and carbamidomethylation are not shown), the p-value from the ANOVA, estimated fold change of the extinction and reconsolidation groups from the control group and the associated p-value from individual t-tests. A fold change greater than 1 is an increase in phosphopeptide abundance, while a value less than 1 represents a decrease in phosphopeptide abundance relative to the comparison group. All phosphorylation events that were regulated by both extinction and reconsolidation changed in the same direction relative to the control group, such that both reconsolidation and extinction of the cocaine-cue memory produced either increases or decreases in the abundance of the phosphopeptide. Thus, several signaling events in the NAc correspond to both the reconsolidation of the original cocaine-cue memory and the consolidation of the extinction of that memory, supporting the hypothesis that extinction training involves new learning and that memory consolidation and reconsolidation can invoke the activity of similar pathways. Note, two peptides listed in Table 1 are starred to indicate that the extinction and reconsolidation groups also significantly differed from each other in addition to being different from the control group. In other words, changes in phosphorylation of microtubule-associated protein 2 (Map2) and SRC kinase signaling inhibitor 1 were significantly greater in the extinction condition relative to the reconsolidation condition, while both groups increased significantly compared with controls. Figure 3 illustrates examples of the group differences in phosphopeptide abundance for 4 of the proteins in Table 1: Map2 (Fig. 3a), alpha-adducin (Fig. 3b), cell cycle exit and neuronal differentiation protein (Cend1; Fig. 3c), and receptor-type tyrosine-protein phosphatase-like N (Ptprn; Fig. 3d). All of these phosphopeptides were significantly altered in both the extinction and reconsolidation conditions (see Table 1 for statistics).
Table 1. Extinction and Reconsolidation Regulated Signaling.
Phosphopeptides found to be regulated by both extinction and reconsolidation relative to controls. The Table gives the protein name with the gene name in parentheses and the phosphopeptide from that protein identified by mass spectrometry including the site of modification denoted by a “p” prior to the modified amino acid. The additional columns show the p-value from the ANOVA and the fold change and p-value from the pair-wise comparison between the extinction (Ext) group relative to control and the reconsolidation (Recon) group relative to control. Fold changes greater than 1 represent increases in phosphopeptide abundance, while decreases are less than 1. Astericks (*) indicate where the extinction and reconsolidation groups were also significantly different from each other.
| Protein (gene name) | Peptide + Modification(s) | ANOVA | Fold Change Ext vs Control | p-value | Fold Change Recon vs Control | p-value |
|---|---|---|---|---|---|---|
| Microtubule-associated protein 2 (Map2) | VAIIRpTPPK | 0.00003 | 1.48* | 0.041 | 1.15* | 0.032 |
| Microtubule-associated protein 1B (Map1b) | TTKpTPEDGGYSCEITEK | 0.00005 | 0.68 | 0.068 | 0.41 | 0.079 |
| Leucine-rich repeat-containing protein 7 (Lrrc7) | LETpTPTTpSPLPERK | 0.0002 | 2.08 | 0.006 | 1.76 | 0.055 |
| Alpha-adducin (Add1) | SPPDQSAVPNpTPPSpTPVK | 0.0005 | 1.63 | 0.014 | 1.66 | 0.026 |
| Connector enhancer of kinase suppressor of ras 2 (Cnksr2) | GSEpSPNSFLDQEYRK | 0.001 | 4.12 | 0.031 | 2.6 | 0.031 |
| Receptor-type tyrosine-protein phosphatase-like N (Ptprn) | AEDpSSEGHEEEVLGGHGEK | 0.002 | 0.4 | 0.012 | 0.35 | 0.055 |
| Beta-arrestin-1 (Arrb1) | DDKDEEDDGTGpSPHLNNR | 0.012 | 1.56 | 0.067 | 1.55 | 0.051 |
| Rho GTPase-activating protein 35 (Arhgap35) | TSFSVGpSDDELGPIR | 0.013 | 0.61 | 0.069 | 0.69 | 0.005 |
| Cell cycle exit and neuronal differentiation protein (Cend1) | ADPVLLNNHSNLKPAPTVPAAPSpSPDTTSEPK | 0.015 | 0.35 | 0.015 | 0.4 | 0.027 |
| SRC kinase signaling inhibitor 1 (Srcin1) | RGpSDELTVPR | 0.015 | 1.52* | 0.002 | 1.25* | 0.02 |
Figure 3. Representative Phosphoproteins Regulated by Both Cocaine Memory Reactivation and Extinction.
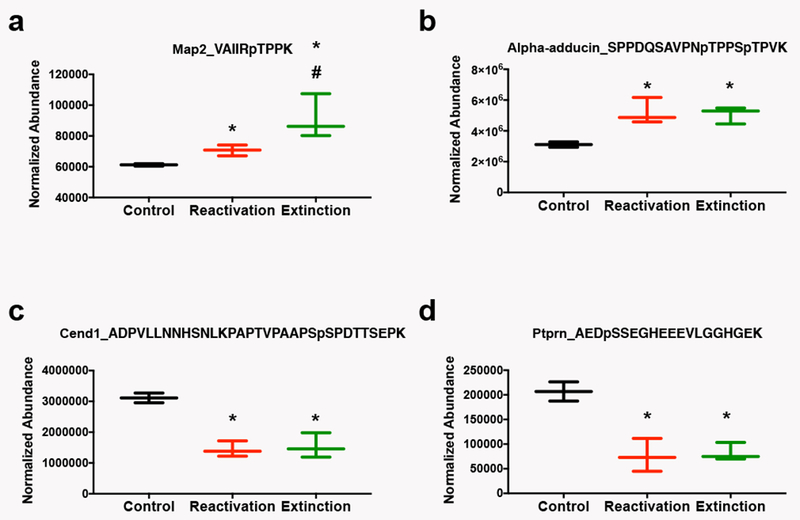
Data are displayed as box and whisker plots showing the minimum to maximum levels of expression of phosphopeptides. The peptide and modifications identified are shown at the top of each graph. a) Microtubule associated protein 2 (Map2). b) Alpha-adducin. c) Cell cycle exit and neuronal differentiation protein (Cend1). d) Receptor-type tyrosine-protein phosphatase-like N (Ptprn). *p<0.05 relative to control; #p<0.05 relative to reactivation by t-test.
While both extinction and reconsolidation produced several common changes in protein signaling, we also observed a number of phosphorylation events selectively regulated either by extinction or reconsolidation of the cocaine cue memory, as defined by events that were significantly different by t-test (p<0.1) from both the control group and the other memory manipulation group (Table 2). We identified 12 phosphopeptides in the extinction condition and 4 phosphopeptides in the reconsolidation condition that met these criteria. Only the fold change from the control group is shown in the Table. Figure 4 illustrates examples of 2 of the extinction-specific phosphoproteins: GABAB receptor subunit 2 (Fig. 4a) and microtubule associated protein 1a (Map1a; Fig. 4b), and two of the reconsolidation-specific phosphoproteins: Protein bassoon (Fig. 4c) and brain acid soluble protein (Basp1; Fig. 4d; See Table 2 for statistics).
Table 2. Memory Condition Specific Signaling.
Phosphopeptides found to be regulated selectively by extinction or reconsolidation relative to controls. The Table gives the protein name with the gene name in parentheses and the phosphopeptide from that protein identified by mass spectrometry including the site of modification denoted by a “p” prior to the modified amino acid. The additional columns show the uncorrected p-value from the ANOVA and the fold change and p-value from the pair-wise comparison between the extinction (Ext) group or reconsolidation (Recon) groups relative to controls (Con), and the p-value when comparing Ext and Recon groups to each other. Fold changes greater than 1 represent increases in phosphopeptide abundance, while decreases are less than 1. Shaded rows represent proteins that did not meet statistical significance after correcting for multiple comparisons by ANOVA, but showed significant differences by t-test.
| Cue Extinction Specific Protein Signaling | Ext vs. Con | Ext vs. Recon | |||
|---|---|---|---|---|---|
| Protein (gene name) | Peptide + Modification(s) | ANOVA | Fold Change from Con | p-value | p-value |
| Serine/threonine-protein kinase DCLK1 (Dclk1) | SPSPSPTpSPGSLRK | 0.0008 | 1.9 | 0.014 | 0.075 |
| Synaptotagmin-1 (Syt1) | DQALKDDDAETGLpTDGEEK | 0.0008 | 1.64 | 0.056 | 0.06 |
| Microtubule-associated protein 1B (Map1b) | DVSDERLpSPAK | 0.001 | 1.33 | 0.028 | 0.061 |
| Actin-related protein 2/3 complex subunit 5-like protein (Arpc5l) | AFHAALRNpSPINTK | 0.002 | 1.67 | 0.035 | 0.066 |
| Gamma-aminobutyric acid type B receptor subunit 2 (Gabbr2) | DPIEDINpSPEHIQR | 0.003 | 1.35 | 0.034 | 0.007 |
| Microtubule-associated protein 1A (Map1a) | CLpSPDDSTVK | 0.004 | 1.81 | 0.039 | 0.048 |
| Heat shock protein HSP 90-alpha(Hsp90aa1) | ESDDKPEIEDVGpSDEEEEEKK | 0.006 | 1.32 | 0.078 | 0.081 |
| Sodium channel protein type 2 subunit alpha (Scn2a) | GKEDEGpTPIKEDIITDK | 0.008 | 1.16 | 0.034 | 0.045 |
| Caskin-1 (Caskin1) | KVPLPGPGpSPEVK | 0.009 | 1.24 | 0.018 | 0.076 |
| Clathrin coat assembly protein AP180 (Snap91) | KPGNNEGSGAPpSPLSK | 0.0299 | 1.46 | 0.059 | 0.094 |
| Syntaxin-1A (Stx1a) | TAKDpSDDDDDVTVTVDRDR | 0.031 | 1.61 | 0.0045 | 0.083 |
| Phytanoyl-CoA hydroxylase-interacting protein-like (Phyhipl) | LDHALpSpSPSpSPCEEIK | 0.039 | 0.61 | 0.019 | 0.036 |
| Reconsolidation Specific Protein Signaling | Recon vs. Con | Recon vs. Ext | |||
| Protein (gene name) | Peptide + Modification(s) | ANOVA | Fold Change from Con | p-value | p-value |
| Protein bassoon (Bsn) | SLSDPKPLpSPTAEESAK | 6.76E-08 | 0.37 | 0.007 | 0.037 |
| Brain acid soluble protein (Basp1) | AEPEKpSEGAAEEQPEPAPAPEQEAAAPGPAAGGEAPK | 0.00001 | 0.59 | 0.048 | 0.031 |
| Microtubule-associated protein 1B (Map1b) | TPGDFNYAYQKPESTTEpSPDEEDYDYESHEK | 0.077 | 0.69 | 0.0008 | 0.009 |
Figure 4. Representative Phosphoproteins Regulated Selectively by Cocaine Memory Reactivation or Extinction.
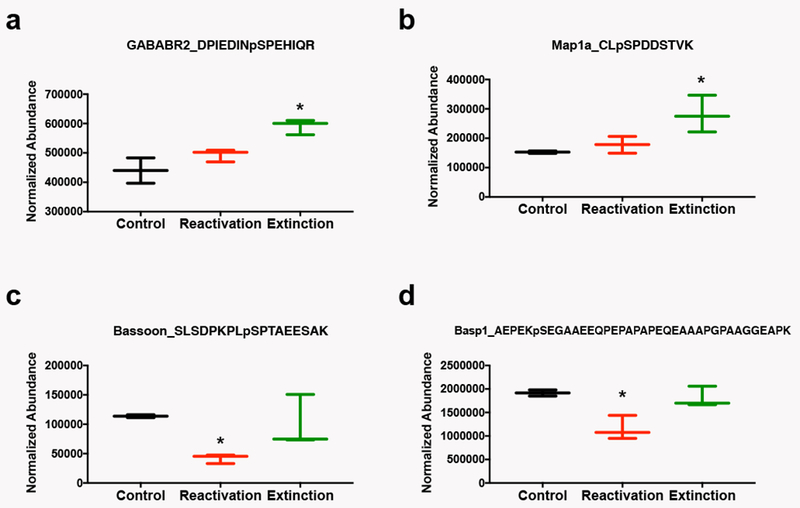
Data are displayed as box and whisker plots showing the minimum to maximum levels of expression of phosphopeptides. The peptide and modifications identified are shown at the top of each graph. a) GABAb receptor subunit 2 (GABABR2) is selectively regulated by extinction. b) Microtubule associated protein 1a (Map1a) is selectively regulated by extinction. c) Protein bassoon is selectively regulated by memory reactivation. d) Brain acid soluble protein (Basp1) is selectively regulated by memory reactivation. *p<0.05 relative to all other groups by t-test.
Comparison to memory-induced phosphorylation in the BLA
We previously published a phosphoproteomic analysis of the BLA from the same experimental animals presented here. Thus, one of the goals of the present analysis was to determine if there were any protein signaling events that occurred after extinction or reconsolidation of a cocaine cue memory in both the BLA and NAc even though these brain regions were analyzed separately. The quantitative analysis of the BLA tissue was only conducted on a subset of proteins identified in the discovery phase, thus there are more phosphopeptides in the NAc analysis. Therefore, the lack of identification of the same protein may simply be due to differences in the way the analyses were conducted, and not to a true lack of common activation. Nevertheless, we did observe changes in some of the same proteins in both regions. Overall, we identified 40 unique phosphopeptides significantly regulated (p<0.1) by cue extinction in the BLA and 28 in the NAc. Of these, 10 of the proteins identified as different in the NAc were also different in the BLA; however, the site modified by phosphorylation was different between the brain regions for 7 of these proteins, with 3 instances of the same phosphorylation event occurring in both brain regions after cue extinction (Table 3, note for some proteins multiple phosphopeptides for the same protein were identified in one of the brain regions and these are shown in pairs in the Table). These 3 phosphorylation events were at serine (S) 883 of the GABAB receptor subunit 2 (GABABR2), S14 of syntaxin1a, and S1258 of caskin-1. The direction of change was the same for GABABR2 and syntaxin1a (bolded in Table 3), both being an increase in phosphorylation, while the direction of change was opposite between the BLA and NAc for caskin-1 phosphorylation (see discussion for potential implications of the changes in phosphorylation).
Table 3. Proteins Regulated by Extinction in both the NAc and BLA.
Proteins found to be regulated by cue extinction in both the NAc and BLA. The Table gives the protein name with the gene name in parentheses and the phosphopeptide from that protein identified by mass spectrometry in the NAc and the BLA (from left to right), including the site of modification denoted by a “p” prior to the modified amino acid. The additional columns show the fold change of that phosphopeptide in the the extinction group relative to controls (Con) in the NAc and BLA (from left to right). Fold changes greater than 1 represent increases in phosphopeptide abundance, while decreases are less than 1. For two proteins (SRC kinase signaling inhibitor 1 and stathmin 1), two different phosphopeptides were identified in the BLA, but only one in the NAc.
| Protein (gene name) | Phosphopeptide NAc | Phosphopeptide BLA | NAc Fold Change from Con | BLA Fold Change from Con |
|---|---|---|---|---|
| Gamma-aminobutyric acid type B receptor subunit 2 (Gabbr2) | DPIEDINpSPEHIQR | DPIEDINpSPEHIQR | 1.35 | 1.23 |
| Syntaxin-1A (Stx1a) | TAKDpSDDDDDVTVTVDRDR | TAKDpSDDDDDVTVTVDRDR | 1.61 | 1.19 |
| Caskin-1 (Caskin1) | KVPLPGPGpSPEVK | KVPLPGPGpSPEVK | 1.24 | 0.77 |
| Receptor-type tyrosine-protein phosphatase-like N (Ptprn) | AEDpSSEGHEEEVLGGHGEK | LPEEGGSpSRAEDSpSEGHEEEVLGGHGEK | 0.4 | 1.27 |
| Sodium channel protein type 2 subunit alpha (Scn2a) | GKEDEGpTPIKEDIITDK | RFSpSPHQpSLLSIR | 1.16 | 1.12 |
| SRC kinase signaling inhibitor 1 (Srcin1) | RGpSDELTVPR | DSGSSSVFAEpSPGGK | 1.52 | 1.14 |
| RGpSDELTVPR | RFpSNVGLVHTSER | 1.52 | 0.77 | |
| Stathmin (Stmn1) | DLpSLEEIQK | ESVPEFPLpSPPK | 1.34 | 0.92 |
| DLpSLEEIQK | RASpGQAFELILpSPR | 1.34 | 1.26 | |
| Protein bassoon (Bsn) | pSLSDPKPLpSPTAEESAK | SPQVLYpSPVpSPLSPHR | 1.64 | 1.25 |
| Misshapen-like kinase 1 (Mink1) | LDSpSPVLSPGNK | SDSVLPASHGHLPQAGpSLER | 1.76 | 1.13 |
| SH3 and multiple ankyrin repeat domains protein 3 (Shank3) | SApSDINLK | SRpSPpSPpSPLPSPSPGSGPSAGPR | 2.49 | 1.21 |
We performed a similar analysis to compare reconsolidation regulated phosphopeptides across brain regions. Overall, we identified 46 unique phosphopeptides significantly regulated (p<0.1) by cocaine cue memory reconsolidation in the BLA and 21 in the NAc. Of these, 5 proteins were found to be regulated in both brain regions (with multiple phosphopeptides identified for some proteins). However, none of the phosphopeptides identified were the same between brain regions (Table 4). Given that the function of many phosphorylation events are unknown, it is still possible that some proteins were regulated in a similar manner (i.e., activated or inhibited) across brain regions during reconsolidation, but via different phosphorylation mechanisms.
Table 4. Proteins Regulated by Reconsolidation in both the NAc and BLA.
Proteins found to be regulated by reconsolidation in both the NAc and BLA. The Table gives the protein name with the gene name in parentheses and the phosphopeptide from that protein identified by mass spectrometry in the NAc and the BLA (from left to right), including the site of modification denoted by a “p” prior to the modified amino acid. The additional columns show the fold change of that phosphopeptide in the the reconsolidation group relative to controls (Con) in the NAc and BLA (from left to right). Fold changes greater than 1 represent increases in phosphopeptide abundance, while decreases are less than 1. For two proteins (Basoon and SRC kinase signaling inhibitor 1), multiple phosphopeptides were identified in one of the two brain regions.
| Protein (gene name) | Phosphopeptide NAc | Phosphopeptide BLA | NAc Fold Change from Con | BLA Fold Change from Con |
|---|---|---|---|---|
| Protein bassoon (Bsn) | pSLSDPKPLpSPTAEESAK | SPQVLYpSPVpSPLSPHR | 1.64 | 1.29 |
| SLSDPKPLpSPTAEESAK | SPQVLYpSPVpSPLSPHR | 0.37 | 1.29 | |
| SRC kinase signaling inhibitor 1 (Srcin1) | RGpSDELTVPR | RFpSNVGLVHTSER | 1.25 | 0.64 |
| RGpSDELTVPR | DSGSSSVFAEpSPGGK | 1.25 | 1.21 | |
| RGpSDELTVPR | KAEpSEELEIQKPQVK | 1.25 | 0.89 | |
| Stathmin (Stmn1) | DLpSLEEIQK | RASpGQAFELILpSPR | 1.33 | 1.54 |
| Misshapen-like kinase 1 (Mink1) | LDSpSPVLSPGNK | SDSVLPASHGHLPQAGpSLER | 1.36 | 1.1 |
| SH3 and multiple ankyrin repeat domains protein 3 (Shank3) | SApSDINLK | SRpSPpSPpSPLPSPSPGSGPSAGPR | 1.89 | 1.52 |
Discussion
In this study, we used phosphoproteomics to identify cellular signaling events in the NAc that are induced when a memory for an audiovisual cue associated with cocaine self-administration either undergoes extinction or is reactivated and reconsolidated. Overall, we found that both the extinction and reactivation of a cocaine cue memory leads to many of the same cellular signaling events as indicated by similar changes in the phosphorylation of a number of proteins. These results are consistent with studies indicating that extinction training involves new learning followed by memory consolidation (rather than forgetting) (Orsini and Maren 2012; Bouton et al. 2012; Maren 2014), and that consolidation and reconsolidation share similar cellular signaling events (Eisenberg & Dudai, 2004; Lee, Milton, & Everitt, 2006; Rich et al., 2016; Tronson & Taylor, 2007).
However, we also observed several additional changes in protein phosphorylation that were selective to the extinction training or reconsolidation condition. We observed more extinction-specific phosphorylation changes relative to reconsolidation, which may suggest that extinction training, while involving memory consolidation processes, also produces unique synaptic adaptations that lead to the suppression of cue-induced cocaine seeking (Rich et al., 2016). In addition, the relatively few selective signaling events identified in NAc compared to BLA during cocaine cue memory reactivation/reconsolidation may indicate that the NAc is less involved in the reconsolidation of memories associated with cocaine self-administration relative to other brain regions. Indeed, the majority of studies investigating the reconsolidation of discrete drug cues have focused on the BLA rather than the NAc (Fuchs, Bell, Ramirez, Eaddy, & Su, 2009; Hellemans, Everitt, & Lee, 2006; Lee, Di Ciano, Thomas, & Everitt, 2005; Merlo, Milton, Goozee, Theobald, & Everitt, 2014; Sanchez et al., 2010; Wells et al., 2013). Moreover, there is some evidence that the NAc is less involved in memories involving instrumental associations with cocaine (Théberge et al. 2010; Wells et al. 2013).
A second goal of the present study was to identify protein phosphorylation events that are regulated in the opposite direction by the extinction versus reconsolidation of a cocaine cue memory with the hope that a single target could be identified for weakening drug memories. We previously found a few opposing signaling events in the BLA (Rich et al., 2016); however, in this study we found no examples of protein phosphorylation events that were even marginally significantly regulated in the opposite direction by the two memory conditions. Thus, the NAc may not be a region that differentially encodes the current motivational value of drug-associated cues, but rather receives this information from upstream sources. Consistent with this hypothesis, we have found electrophysiological evidence that cue extinction reduces synaptic strength in the BLA, while cue memory reactivation tends to increase synaptic strength (Rich, Huang, & Torregrossa, 2018, in review). Thus, the BLA may more directly encode the motivational value of cues and this information is projected to the NAc in order to guide behavior.
Given the lack of opposing cellular signaling events for extinction and reconsolidation conditions in the NAc, we decided to determine if any phosphorylation events were common across both NAc and BLA in either the extinction or reconsolidation condition, as events that occur across multiple brain regions might be better targets for the development of a systemically administered pharmacotherapeutic agent. We found that only a few proteins were regulated in both regions in the reconsolidation condition, and in none of these proteins did we identify the same phosphorylation event across brain regions. In the extinction condition, on the other hand, we did observe two phosphoproteins that were upregulated in both the NAc and BLA relative to controls. The first of these proteins, GABABR2, is particularly interesting because phosphorylation at S883 of this protein was not only upregulated by extinction, it was also significantly decreased during reconsolidation in the BLA (Rich et al. 2016). Therefore, modulation of GABABR signaling has the potential to not only enhance extinction, but also disrupt reconsolidation. Many sites on GABABR2 are known to regulate receptor function and localization; however, the effect of S883 phosphorylation, to our knowledge, is currently unknown (Nørskov-Lauritsen and Bräuner-Osborne 2015). A nearby site, S892, is known to be phosphorylated by PKA leading to stabilization of the receptor in the membrane (Couve et al. 2002). However, whether or not phosphorylation at S883 could also increase GABABR signaling has not been investigated. Nevertheless, conceptually, it may make sense that cue extinction training could lead to increased inhibitory signaling in both the BLA and NAc to reduce the motivational value of the cue and to reduce the likelihood of producing drug seeking actions in response to the cue. Future experiments will need to further explore this possibility, particularly considering the availability of GABABR modulating agents for clinical use.
The other phosphorylation event found to be upregulated after cue extinction in both the NAc and BLA was on S14 of syntaxin la. Syntaxins are located presynaptically and are part of the SNARE complex that regulates vesicle exocytosis and neurotransmitter release (Rizo 2018). Interestingly, phosphorylation of S14 is thought to be mediated by casein kinase 2 (CK2), and has been found to result in decreased glutamate release in vitro (Gil et al. 2011). Thus, the increase in pS14 of syntaxin la may indicate that cue extinction training activates CK2 in both the NAc and BLA to inhibit glutamate release. Therefore, cue extinction training appears to not only lead to memory consolidation-associated signaling events that also occur in the reconsolidation condition, but additionally to phosphorylation events that would be predicted to inhibit cellular activity. These unique neural adaptations induced by cue extinction may promote the inhibition of cue-induced drug seeking. Future studies should investigate these possibilities more directly.
In addition to the two proteins discussed above, several other phosphorylation events were identified that were selective to either the cue extinction or reconsolidation condition. It is beyond the scope of this discussion to describe all of these events here, but it is possible that targeting some of these proteins and/or the kinases and phosphatases that regulate these signaling events could lead to novel adjunctive pharmacotherapeutics to enhance the efficacy of extinction training or disrupt reconsolidation. These results, however, should be interpreted with caution as the present study does have several limitations. For example, only one timepoint after cue extinction/memory reactivation was evaluated (15 min), which provides a single snapshot of the signaling events that occur during these memory processes and likely misses other events that occur on different timescales. Furthermore, there is no way to know the exact memory process or motivational state of the animal at the time of sacrifice. For example, it is possible that with 3 cue presentations the memory is retrieved, but not destabilized or even that “early extinction” is occurring. However, we do have several reasons to believe that 3 cue presentations destabilize memories and induces reconsolidation processes, as we have repeatedly observed pharmacological disruption of reconsolidation using this approach (Sanchez et al. 2010; Wan et al. 2014; Rich et al. 2016), and we do not observe behavioral or electrophysiological correlates of extinction after 3 cue presentations (Rich et al. 2018, in review). Nevertheless, a comparison of phosphoproteins regulated after reconsolidation disruption and subsequent memory retrieval could verify which signaling events are reconsolidation specific. Likewise, in the extinction group, it is possible that both extinction memory consolidation and reconsolidation are occurring given that extinction occurs over two days. However, in other studies we find that 120 cue presentations produces much more robust extinction learning than 60 cues, both behaviorally and electrophysiologically (Rich et al. 2016, 2018, in review), suggesting that an extinction learning process is still ongoing at the time of sacrifice in the present study.
Another limitation is that the study has relatively low power, and thus statistical conclusions are difficult to make. The purpose of this study was to carry out an unbiased, discovery-based method to identify candidate molecules for further investigation, not to definitely identify the entire phosphoproteome of extinction vs. reconsolidation in the NAc. Thus, the lack of identification of a phosphoprotein should not be interpreted as an absence of regulation, but that either a statistical difference could not be observed with the limited sample size, or that the phosphoprotein was not detected by the mass spectrometer. There are several reasons why expected protein phosphorylation changes may not be observed. First, the total homogenate from the NAc was analyzed, which could lead to the inability to detect cell type or cell compartment specific signaling events. Secondly, low abundance proteins, though potentially important regulators of plasticity, are less likely to be identified. Finally, the methodology is most robust for detection of serine and threonine phosphorylation, making it less likely that changes in tyrosine phosphorylation will be observed. Nevertheless, the protein phosphorylation events that were identified are potentially important regulators of cocaine cue memories and should be further explored in future studies.
In conclusion, a phosphoproteomic, discovery-based approach, was able to identify novel protein phosphorylation events associated specifically with the extinction or reconsolidation of a cocaine cue memory. The protein signaling events identified represent potential targets for the development of novel therapeutics that could be used in conjunction with either cue exposure therapy or a reconsolidation-based therapy to reduce the likelihood of cue motivated relapse.
Supplementary Material
Acknowledgements:
This work was supported by USPHS grants DA042029 (M.M.T.), K01DA031745 (M.M.T.), DA018343 (A.C.N., T.T.L.), and DA015222 (J.R.T.).
Footnotes
Conflicts of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Benjamini Y, Hochberg Y (1995) Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 57:289–300 [Google Scholar]
- Bouton ME, Winterbauer NE, Todd TP (2012) Relapse processes after the extinction of instrumental learning: renewal, resurgence, and reacquisition. Behav Processes 90:130–41. doi: 10.1016/j.beproc.2012.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, et al. (1999) Limbic activation during cue-induced cocaine craving. Am J Psychiatry 156:11–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST (2002) Applying extinction research and theory to cue-exposure addiction treatments. Addiction 97:155–67 [DOI] [PubMed] [Google Scholar]
- Couve A, Thomas P, Calver AR, et al. (2002) Cyclic AMP-dependent protein kinase phosphorylation facilitates GABA(B) receptor-effector coupling. Nat Neurosci 5:415–24. doi: 10.1038/nn833 [DOI] [PubMed] [Google Scholar]
- Eisenberg M, Dudai Y (2004) Reconsolidation of fresh, remote, and extinguished fear memory in medaka: old fears don’t die. Eur J Neurosci 20:3397–3403. doi: 10.1111/j.1460-9568.2004.03818.x [DOI] [PubMed] [Google Scholar]
- Fox HC, Bergquist KL, Hong K-I, Sinha R (2007) Stress-Induced and Alcohol Cue-Induced Craving in Recently Abstinent Alcohol-Dependent Individuals. Alcohol Clin Exp Res 31:395–403. doi: 10.1111/j.1530-0277.2006.00320.x [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Bell GH, Ramirez DR, et al. (2009) Basolateral amygdala involvement in memory reconsolidation processes that facilitate drug context-induced cocaine seeking. Eur J Neurosci 30:889–900. doi: 10.1111/j.1460-9568.2009.06888.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE (2004) Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 176:459–65. doi: 10.1007/s00213-004-1895-6 [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Feltenstein MW, See RE (2006) The role of the basolateral amygdala in stimulus-reward memory and extinction memory consolidation and in subsequent conditioned cued reinstatement of cocaine seeking. Eur J Neurosci 23:2809–2813. doi: 10.1111/j.1460-9568.2006.04806.x [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, et al. (2000) Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry 157:1789–98 [DOI] [PubMed] [Google Scholar]
- Gil C, Falqués A, Sarró E, et al. (2011) Protein kinase CK2 associates to lipid rafts and its pharmacological inhibition enhances neurotransmitter release. FEBS Lett 585:414–420. doi: 10.1016/j.febslet.2010.12.029 [DOI] [PubMed] [Google Scholar]
- Hellemans KGC, Everitt BJ, Lee JLC (2006) Disrupting Reconsolidation of Conditioned Withdrawal Memories in the Basolateral Amygdala Reduces Suppression of Heroin Seeking in Rats. J Neurosci 26:12694–12699. doi: 10.1523/JNEUROSCI.3101-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirosawa M, Hoshida M, Ishikawa M, Toya T (1993) MASCOT: multiple alignment system for protein sequences based on three-way dynamic programming. Comput Appl Biosci 9:161–7 [DOI] [PubMed] [Google Scholar]
- Huttlin EL, Jedrychowski MP, Elias JE, et al. (2010) A tissue-specific atlas of mouse protein phosphorylation and expression. Cell 143:1174–89. doi: 10.1016/j.cell.2010.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JLC, Di Ciano P, Thomas KL, Everitt BJ (2005) Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron 47:795–801. doi: 10.1016/j.neuron.2005.08.007 [DOI] [PubMed] [Google Scholar]
- Lee JLC, Milton AL, Everitt BJ (2006) Reconsolidation and extinction of conditioned fear: inhibition and potentiation. J Neurosci 26:10051–6. doi: 10.1523/JNEUROSCI.2466-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S (2014) Out with the old and in with the new: Synaptic mechanisms of extinction in the amygdala. Brain Res. doi: 10.1016/j.brainres.2014.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo E, Milton AL, Goozee ZY, et al. (2014a) Reconsolidation and Extinction Are Dissociable and Mutually Exclusive Processes: Behavioral and Molecular Evidence. J Neurosci 34:2422–2431. doi: 10.1523/JNEUROSCI.4001-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo E, Milton AL, Goozée ZY, et al. (2014b) Reconsolidation and extinction are dissociable and mutually exclusive processes: behavioral and molecular evidence. J Neurosci 34:2422–31. doi: 10.1523/JNEUROSCI.4001-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Marshall JF (2005) Molecular substrates for retrieval and reconsolidation of cocaineassociated contextual memory. Neuron 47:873–84. doi: 10.1016/j.neuron.2005.08.006 [DOI] [PubMed] [Google Scholar]
- Nørskov-Lauritsen L, Bräuner-Osborne H (2015) Role of post-translational modifications on structure, function and pharmacology of class C G protein-coupled receptors. Eur J Pharmacol 763:233–240. doi: 10.1016/j.ejphar.2015.05.015 [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, McLellan AT, Ehrman R (1993) Developing treatments that address classical conditioning. NIDA Res Monogr 135:71–91 [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, McLellan T, Ehrman R (1990) Integrating systemic cue exposure with standard treatment in recovering drug dependent patients. Addict Behav 15:355–65 [DOI] [PubMed] [Google Scholar]
- Orsini CA, Maren S (2012) Neural and cellular mechanisms of fear and extinction memory formation. Neurosci Biobehav Rev 36:1773–802. doi: 10.1016/j.neubiorev.2011.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price KL, Baker NL, McRae-Clark AL, et al. (2013) A randomized, placebo-controlled laboratory study of the effects of d-cycloserine on craving in cocaine-dependent individuals. Psychopharmacology (Berl) 226:739–746. doi: 10.1007/s00213-011-2592-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich MT, Abbott TB, Chung L, et al. (2016) Phosphoproteomic analysis reveals a novel mechanism of CaMKIIα regulation inversely induced by cocaine memory extinction versus reconsolidation. J Neurosci 36:. doi: 10.1523/JNEUROSCI.1108-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich MT, Huang YH, Torregrossa MM (2018) Plasticity at Thalamo-Amygdala Synapses Regulates Cocaine-Cue Memory Formation and Extinction. SSRN Electron J. doi: 10.2139/ssrn.3205405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizo J (2018) Mechanism of neurotransmitter release coming into focus. Protein Sci. doi: 10.1002/pro.3445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez H, Quinn JJ, Torregrossa MM, Taylor JR (2010) Reconsolidation of a cocaineassociated stimulus requires amygdalar protein kinase A. J Neurosci 30:4401–7. doi: 10.1523/JNEUROSCI.3149-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Li CSR (2007) Imaging stress- and cue-induced drug and alcohol craving: Association with relapse and clinical implications. Drug Alcohol Rev. 26:25–31 [DOI] [PubMed] [Google Scholar]
- Taylor JR, Olausson P, Quinn JJ, Torregrossa MM (2009) Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharmacology 56 Suppl 1:186–95. doi: 10.1016/j.neuropharm.2008.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théberge FRM, Milton AL, Belin D, et al. (2010) The basolateral amygdala and nucleus accumbens core mediate dissociable aspects of drug memory reconsolidation. Learn Mem 17:444–53. doi: 10.1101/lm.1757410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa MM, Kalivas PW (2008) Neurotensin in the ventral pallidum increases extracellular gamma-aminobutyric acid and differentially affects cue- and cocaine-primed reinstatement. J Pharmacol Exp Ther 325:556–66. doi: 10.1124/jpet.107.130310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa MMM, Gordon J, Taylor JRR (2013) Double Dissociation between the Anterior Cingulate Cortex and Nucleus Accumbens Core in Encoding the Context versus the Content of Pavlovian Cocaine Cue Extinction. J Neurosci 33:8370–8377. doi: 10.1523/JNEUROSCI.0489-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa MMM, Sanchez H, Taylor JRR (2010) D-Cycloserine Reduces the Context Specificity of Pavlovian Extinction of Cocaine Cues through Actions in the Nucleus Accumbens. J Neurosci 30:10526–10533. doi: 10.1523/JNEUROSCI.2523-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa MMM, Taylor JRJR (2012) Learning to forget: manipulating extinction and reconsolidation processes to treat addiction. Psychopharmacology (Berl) 226:. doi: 10.1007/s00213-012-2750-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR (2007) Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci 8:262–75. doi: 10.1038/nrn2090 [DOI] [PubMed] [Google Scholar]
- Wan X, Torregrossa MMM, Sanchez H, et al. (2014) Activation of exchange protein activated by cAMP in the rat basolateral amygdala impairs reconsolidation of a memory associated with self-administered cocaine. PLoS One 9:e107359. doi: 10.1371/journal.pone.0107359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells AM, Arguello AA, Xie X, et al. (2013) Extracellular signal-regulated kinase in the basolateral amygdala, but not the nucleus accumbens core, is critical for context-responsecocaine memory reconsolidation in rats. Neuropsychopharmacology 38:753–62. doi: 10.1038/npp.2012.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Childress AR, Jagannathan K, et al. (2013) Neural responses to subliminally presented cannabis and other emotionally evocative cues in cannabis-dependent individuals. Psychopharmacology (Berl). doi: 10.1007/s00213-013-3342-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


