Abstract
Objective:
The high rate of cannabis (CAN) use in emerging adults is concerning given prior research suggesting neurocognitive deficits associated with CAN use in youth. Regular CAN use downregulates endocannabinoid activity, while aerobic exercise upregulates cannabinoid receptor 1 activity and releases endocannabinoids. Here we investigate the influence of regular CAN use on neuropsychological performance, and whether aerobic fitness moderates these effects.
Method:
79 young adults (37 CAN users) aged 16–26 participated. Groups were balanced for aerobic fitness level. Exclusion criteria included: left-handedness, past-year independent Axis-I disorders, major medical/neurologic disorders, prenatal issues, or prenatal alcohol/illicit drug exposure. After three weeks of abstinence, participants completed a neuropsychological battery and a maximal oxygen consumption test (VO2 max). Multiple regressions tested whether past-year CAN use, V02 max, and CAN*V02 max interaction predicted neuropsychological performance, controlling for past-year alcohol use, cotinine, gender, and depression symptoms.
Results:
Increased CAN use was associated with decreased performance on working memory and psychomotor tasks. High aerobic fitness level was related to better performance on visual memory, verbal fluency, and sequencing ability. CAN*V02 max predicted performance of psychomotor speed, visual memory, and sequencing ability.
Conclusions:
Following monitored abstinence, increased CAN use was associated with poorer performance in working memory and psychomotor speed. Higher aerobic fitness level moderated the impact of CAN on visual memory, executive function and psychomotor speed, as more aerobically fit CAN users demonstrated better performance relative to low-fit users. Therefore, aerobic fitness may present an affordable and efficacious method to improve cognitive functioning in CAN users.
Keywords: Cannabis, Marijuana, VO2 Max, Neurocognition, Emerging adulthood, Aerobic fitness
INTRODUCTION
Cannabis (CAN) impacts the brain by interacting with the endogenous endocannabinoid (eCB) system, which includes cannabinoid receptor 1 (CB1R, primarily in CNS) and two endogenous ligands (anandamide; AEA and 2-arachidonoylglycerol, 2-AG)(Herkenham et al., 1990; Di Marzo et al., 2005; Eggan and Lewis, 2007). Regular exogenous CAN exposure downregulates the CB1Rs (Hirvonen et al., 2012) and is linked with neurocognitive deficits (see Lisdahl et al., 2014). Despite these findings, daily CAN use amongst teens and young adults remains high (approximately 6% of 12th graders and 8% of young adults; Miech et al., 2017).
Prior studies have often noted negative relationships between CAN use and neuropsychological functioning in emerging adults (for review, see Lisdahl et al., 2014; Ganzer et al., 2016). More specifically, after periods of brief (12+ hours) abstinence, findings suggest deficits in learning and memory, working memory, planning, and decision-making (Becker et al., 2017) and in executive functioning (Dahlgren et al., 2016). However, studies such as these do not account for more long-term changes in cognitive functioning even after cessation of use. Thames and colleagues (2014) found that, whether recent- (past 4-weeks) or past-use (greater than 4 weeks abstinence) and regardless of age, CAN users demonstrated decreased cognitive performance across attention, processing speed, and executive functioning, with mild recovery in the past-user group relative to the recent-user group. Following a month of abstinence, emerging adult CAN users have also been found to have slower psychomotor speed, poorer complex attention, story memory, visual memory, visuospatial functioning, cognitive flexibility, working memory and sequencing (Medina et al., 2007; Jacobus et al., 2014; Jacobus et al., 2015; Winward et al., 2014); similarly, we found poorer psychomotor speed, sustained attention, and cognitive inhibition in CAN users after a week of abstinence, with male users having greater psychomotor slowing (Lisdahl & Price, 2012). A recent meta-analysis of CAN studies of emerging adults concluded that cognitive deficits do not persist beyond 72 hours of abstinence (Scott et al., 2018). However, some studies included did not adequately control for other substance use, used non-standardized neuropsychological measures, did not exclude for psychiatric comorbidities, or did not assess for dose-dependent relationships. As these differences in study design may have obscured the relationship between the chronic effects of cannabis and cognitive functioning, further assessment of the chronic impact of cannabis is warranted with careful consideration of other variables that may be important moderators (e.g., fitness level). Given these neuropsychological findings after withdrawal and excluding for psychiatric comorbidities, there is increased interest in investigating more chronic impacts of CAN use and examining potential ameliorative tools that may reverse cannabis-related cognitive deficits.
Animal models demonstrate numerous positive effects of aerobic exercise (AE) on brain health, including improved cognition, neurogenesis, and protection of the nervous system from injury in regions including the prefrontal cortex (PFC; Cotman et al., 2007; Ding et al., 2004; van Praag et al., 1999; Vaynman et al., 2004; Stranahan et al., 2010). Pre-clinical research has shown that AE of moderate intensity resulted in greater neurogenesis compared to high-intensity interval training (Leasure & Jones, 2008) or anaerobic resistance training (Nokia et al., 2016). Notably, a series of studies have found that exercise blocks or reverses the negative effects of alcohol binge drinking on neurogenesis in the dentate gyrus and hippocampus in animals (Helfer et al., 2009; Hamilton et al., 2015; Leasure & Nixon, 2010; Crews, Nixon, & Wilkie, 2004), although to date no such research exists in CAN exposure for animals or humans. There are multiple possible mechanisms underlying the benefits of aerobic fitness, including decreased inflammatory response and oxidative stress (Sakurai et al., 2009; Radak et al., 2007), increased c-FOS expression (Sim et al., 2008; Dragunow, 1996; He, Yamada, & Nabeshima, 2002; Vann et al., 2000), increased CB1R activity in the hippocampus (Ferreira-Vieira et al., 2014) and improved catecholaminergic function in brain regions such as the PFC and limbic system (Waters et al., 2005; Dunn et al., 1996; Elam, Svensson, & Thoren, 1987; Heyes, Garnett, & Coates, 1985; Chaouloff, 1989; Dunn & Dishman, 1991). Converging lines of evidence also suggest changes in growth factors, such as brain-derived neurotrophic factor (BDNF), insulin-like growth factor-1 (IGF-1) and vascular endothelial growth factor (VEGF), may underlie AE-related neurocognitive changes (Cotman & Berchtold, 2002; Lee et al., 2006; Kim et al., 2007; Egan et al., 2003; Castren et al., 1998; Li et al., 2000; Liu et al., 2000; Whiteman et al., 2014; Mueller et al., 2015). More specific to CAN users, Koltyn and colleagues has reported that AE results in release of circulating eCB concentrations (Koltyn, Brellenthin, Cook, Sehgal, & Hillard, 2014), resulting in improved mood (Brellenthin, Crombie, Hillard, & Koltyn, 2017). Therefore, AE may help restore circulating eCB concentrations in CAN users.
Prior research has suggested that AE may improve cognitive functioning in adolescents and young adults (for review, see Herting & Chu, 2017). Aerobic fitness has been linked to better executive functioning, spatial memory, psychomotor speed, and attention in adolescents (Lee et al., 2014; Wengaard et al., 2017), and attention and working memory in young adults (Hwang et al., 2017). VO2 max (aka maximal oxygen consumption) provides a quantitative measure of one’s capability and capacity for AE (Bassett & Howley, 2000). By definition, it is the highest amount of oxygen that an individual can consume in order to make energy aerobically. It also provides a good indication of overall fitness, in that it requires unified and high-level functioning of multiple systems including the ventilaory, cardiovascular, hematologic, and muscular systems (Bassett & Howley, 2000). VO2 max increases as a result of AE, such as running, walking, biking, etc. (Bassett & Howley, 2000). Therefore, the more aerobic activity a person engages in, the higher their VO2 max will be. Other research has linked VO2 max with neurocognitive function. For example, in healthy young adults, superior VO2 max performance was related to larger entorhinal cortex volume, which was in turn associated with better memory (Whiteman et al., 2016).
The relationship between aerobic fitness and CAN use has been understudied. A longitudinal study (Henchoz et al., 2014) had young adult males self-report their frequency of exercise and complete a substance use screen. They found fewer cannabis use disorder (CUD) diagnoses and better mental health outcomes in those who maintained regular exercise habits, and lower prevalence of CUD in those who adopted new exercise routines. Another study of adult non-treatment seeking individuals with CUD found reduced cannabis use and craving following two weeks of AE (Buchowski et al., 2011). Whether aerobic fitness level may moderate cognitive functioning in CAN users have not been previously studied. To date, no one has reported whether aerobic fitness moderates measures of cognitive function in CAN-using youth.
Here we aim to investigate the potential moderating effect of aerobic fitness level on neuropsychological outcomes in regular CAN users. We predict that following three weeks of monitored abstinence, greater past year CAN use would be associated with greater cognitive deficits in a dose-dependent manner, especially on psychomotor speed, complex attention, and executive function tasks (Lisdahl et al., 2012; Medina et al., 2007). We also predict that greater aerobic fitness would be associated with better neuropsychological performance. Finally, we hypothesize that CAN use would interact with aerobic fitness level, such that individuals with higher levels of past-year CAN use and lower VO2 max would have worse neuropsychological performance relative to individuals with higher VO2 max and greater levels of past-year cannabis use, and controls.
METHODS
Participants.
Seventy-nine participants (37 CAN users, 42 controls) were recruited through local newspaper advertisements and fliers placed around Milwaukee, Wisconsin, for the larger parent study (PI: Lisdahl, R01 DA030354). Efforts were made to balance for gender and physical activity levels across substance use groups. Individuals were considered healthy controls if they had smoked less than 5 joints (or their equivalent) in the past year and no more than 20 joints in their lifetime. CAN users had smoked at least 52 times in the past year (approximately weekly users). Further, each participant was classified as being high-fit if they were above the 50th percentile in VO2 max performance, and low-fit if they were below the 50th percentile by age and gender (Pescatello, 2014). Inclusion criteria included being a fluent English speaker between 16 and 26 years old. Exclusion criteria for all participants included: being left handed, MRI contraindications, past year co-morbid independent Axis-I disorders, major medical or neurologic disorders, prenatal issues (e.g., gestation <35 weeks) or prenatal alcohol (>4 drinks/day or >7 drinks/week) or illicit drugs (>10 uses), or excessive illicit drug use in lifetime (>50 uses of any drug category except nicotine, alcohol, or CAN). Participants confirmed three weeks of abstinence from all alcohol and drug use (other than tobacco) through self-report and drug toxicology screen. The University of Wisconsin Milwaukee and Medical College of Wisconsin IRBs approved all aspects of this study.
Procedure.
Screening.
Eligibility criteria were established through a two-step screening process. First, participants called in to a study line after seeing fliers around the community advertising for active and sedentary participants. After receiving oral consent from the participant, or oral consent from the parent/guardian and oral assent from the participant (if under 18), they completed a 5–10 minute phone screen. Each parent and participant were screened separately to answer initial eligibility question (including age, ethnicity, MRI contraindications, and yes/no questions regarding psychiatric and substance use history). Next, written consent/assent (if under 18) was received via mail and a 45-minute detailed phone or in –person screen was scheduled. Participants then completed lifetime history of substance use via the Customary Drinking and Drug Use Record (CDDR) (Brown et al., 1998; Stewart & Brown, 1995). Participants and their parents separately completed youth psychiatric history though the Mini International Psychiatric Interview (MINI) or MINI-Kid (if under 18) (Sheehan et al., 1998). All participants and their parents were compensated $20 for their time during the phone screen. If eligible, participants were scheduled for study sessions. If ineligible, participants were not informed of the specific reason for ineligibility to protect study integrity.
Study Sessions.
Five sessions were scheduled over the course of 3.5–4 weeks for all eligible participants. The initial three sessions each occurred one week apart. They consisted of a mini-neuropsychological battery to assess impact of CAN withdrawal symptoms on cognitive function (data not examined here), psychological questionnaires, and urinalysis and drug patch analysis to ensure abstinence. The fourth session (data presented here) occurred one week after the third session and consisted of a 3-hour fully neuropsychological battery and a VO2 max testing session. The final and fifth session occurred within 24–48 hours of the fourth session and consisted of MRI scanning. For the present study, only data from the fourth session, consisting of the neuropsychological battery and VO2 max, are included, to ensure no potential withdrawal symptoms impact findings (Budney et al., 2004).
Verifying Abstinence.
Abstinence was evaluated at each study session to ensure participants remained abstinent from all alcohol and drugs (other than nicotine) for the duration of the study. The ACCUTEST SplitCup 10 Panel drug test was used to measure amphetamines, barbiturates, benzodiazepines, cocaine, ecstasy, methadone, methamphetamines, opiates, PCP, and THC. Urine samples were also tested using NicAlert to test cotinine level, a metabolite of nicotine. Participants also wore PharmChek Drugs of Abuse Patches which continuously monitor sweat toxicology for the presence of cocaine, benzoylecgonine, heroin, 6MAM, morphine, codeine, amphetamines, methamphetamine, THC, and phencyclidine. At the start of each session, participants also underwent breathalyzer screens. If positive for THC at Sessions 1–3, participants were considered eligible to remain in the study if their THC level, as measured by the PharmChek patch, went down over time. If positive for any drug or having a breath alcohol concentration greater than .000 at the start of Session 4 (neuropsychology battery and VO2 maximum testing) or Session 5 (MRI scan), participants were ineligible for study participation.
Measures
Psychiatric Disorder Screening.
Participants and their parents separately completed youth psychiatric history though the Mini International Psychiatric Interview (MINI) (for 18 and older) or MINI-Kid (if under 18) (Sheehan et al., 1998).
Substance Use.
Lifetime Use.
Customary Drinking and Drug Use Record (CDDR) (Brown et al., 1998; Stewart & Brown, 1995) was used to measure frequency/quantity of lifetime usage, age of first use, age of regular use, and symptoms of substance use disorder for alcohol, nicotine and cannabis. Past Year Use. A modified version of the Timeline Follow-Back (TLFB; Sobell & Sobell, 1992; Lisdahl & Price, 2012) was used to measure past 365 days of CAN (joints), alcohol (standard drinks), and other drug use in standard units. For CAN, participants reported method and amount used (e.g., concentrates, hits, joints, etc.) and this was converted to a standard unit of joints. After being cued to memories, holidays, and personally significant events, participants recalled frequency and amount of drug use on each day or, if unable to recall day-by-day information, weekly averages. Last date of use was also captured to measure length of abstinence from substances other than tobacco.
Mood.
Participants completed the 21-item Beck Depression Inventory—2nd Edition to measure current depressive symptoms from the past 2 weeks (BDI; Beck, 1996).
Neuropsychological Battery.
Cognitive tasks and variables included the following:
Select subtests from the Delis-Kaplan Executive Functioning Scale (D-KEFS; Delis, Kaplan, & Kramer 2001) were used. Specifically: Color-Word Interference, which consisted of subtests with measuring reading of words, then colors, then two tasks which required inhibiting aspects of reading/naming (e.g., naming color without reading the word). Variables assessed included total reading time in seconds for word reading, color naming, color-word interference, and color-word interference with switching; Verbal Fluency, wherein the participant had 60 seconds to list as many words as they could that started with a specific letter (variable assessed: FAS total correct); and Trail Making Tests (TMT), which consisted of various connect-the-dot tasks designed to measure simple attention, psychomotor speed, information processing speed, and cognitive flexibility (variables included time to complete in seconds for each of the five conditions).
California Verbal Learning Test-II (CVLT-II; Delis, Kramer, Kaplan, & Ober, 2000) is a 16-item verbal learning and recall measure with five learning trials, a distractor list, and both a brief and long delay. Variables assessed in this study included initial learning (trial 1), total learning (total trials 1–5), free recall (long delay free recall), and recognition (hits).
Rey Complex Figure Task (RCFT; Meyers & Meyers, 1995) is a figure which a participant first copies then later has to recall from memory. Variables assessed include visuospatial skills (direct copy score) and visual recall (immediate recall, long delay recall).
Penn Emotional Faces Memory Task of the Penn’s Computerized Neurocognitive Battery (PennCNP; Gur et al., 2010) measures immediate recognition of faces following a learning trial and was assessed by facial memory total correct.
The Weschler Adult Intelligence Scale, Third Edition (WAIS-III; Wechsler, 1997) Letter-Number Sequencing (LNS) subtest was used to measure working memory through total raw sequencing score.
Conner’s Continuous Performance Task, 2nd Edition (CPT-II; Conners, 2000) is a task designed to assess sustained attention. Number of omission errors and number of commission errors were used in the present analyses.
Iowa Gambling Task (IGT) is a measure of decision-making through trying to maximize money in a gambling card task earned and examined here by total net money (won-borrowed; Bechara, Damasio, Damasio, & Anderson, 1994).
To estimate intelligence and quality of education (Manly, Jacobs, & Touradji, 2002), the Wide Range Achievement Test-4th edition (WRAT-4) word reading subtest was used, with normed scores calculated. This test was only used for group comparisons in selection of covariates to ensure groups did not differ in premorbid IQ.
Neurocognitive variables that were not normally distributed (RCFT direct copy; CVLT-II long delay free recall; D-KEFS Trails Condition 4; CPT-II Omission errors) were log-transformed and used in all analyses. All raw scores were used, unless otherwise noted (i.e., WRAT-4 Word Reading). Measurement of Effort. An embedded effort measure from the CVLT-II, the Forced Choice trial, was assessed to ensure proper engagement by participants. No participants were below cut-off scores.
Body Size and Composition.
Body height and mass were measured using standard procedures (Pescatello, 2014). In addition, a Tanita SC-331S Body Composition Monitor (Tanita, Arlington Heights, IL) was used to measure additional fitness characteristics, such as weight, BMI, and fat percentage.
Aerobic Fitness (VO2 maximum).
Participants were asked to refrain from food and caffeine for 4 hours prior to the exercise tests. Prior to each exercise test, the metabolic measurement system, ParvoMedics TrueOne 2400 (ParvoMedics, Salt Lake City, UT) was calibrated according to the manufacturer’s instructions using a 3 Liter syringe for the pneumotachometer, and a two-point calibration for the gas analyzers (room air and a certified gas 4.08% CO2, 115.98% O2, balance N2). Participants were fitted with the rubber mouthpiece connected to a Hans Rudolf 2700 series two-way nonrebreathing valve (Kansas City, MO), noseclip, and heart rate strap (Polar Wearlink 31, Finland) for the collection of expired gases and measurement of heart rate. Participants completed a maximal incremental exercise test on a treadmill (Full Vision Inc., TMX425C Trackmaster, Newton, KS) following the Bruce Protocol until volitional fatigue. Expired gases were measured continuously using a ParvoMedics TrueOne 2400 metabolic measurement system (ParvoMedics, Salt Lake City, UT); this has been shown to be a valid measure of expired gases at rest and during increasing intensities of activity (Bassett et al., 2001). Trained exercise physiology research assistants, supervised by AS, utilized Howley and colleagues (1995) criteria to determine attainment of VO2 max. Metabolic data were averaged over 1 minute and exported into a spreadsheet for analysis.
Data analysis
To determine selection of covariates, two groups were formed: a CAN user group with at least an average of one joint per week over the past year, and a control group with less than 5 joints (or equivalent) in the past year and no more than 20 in their lifetime. Continuous variables reflecting total past year CAN use (not groups) were used in the primary analyses. In addition, participants were again divided into a high (at or above 50th percentile) or low (below 50th percentile) fitness group, based on normed performance on VO2 max (Pescatello, 2014), though again continuous variables, not group, were used in analyses. Between-group differences on demographic variables by both CAN group and fitness group were measured with analyses of variance (ANOVAs) and χ2 tests. Controlling for potential confounds (i.e., past year alcohol use, cotinine level, BDI-II score, gender, and BMI), multiple regressions were run to examine whether past year cannabis use or VO2 max performance independently related to cognitive functioning after the required abstinence period. The potential interactive effect of past-year CAN use and VO2 max performance was also assessed as a second block in the regression; for ease of presentation, the interaction term used is CAN*VO2, though total past year joints and actual VO2 max performance, not groups, were used to calculate the interaction. Twenty-two separate multiple regressions were run, with covariates and independent variables assessed in the first block of the regression, and the interaction variable added in the second block. DFBetas were examined to rule out outliers. Notably, results remain consistent regardless of whether or not length of abstinence is included as a covariate. No correction for multiple comparisons was employed due to power limitations.
RESULTS
Demographic, Drug Use and Fitness Differences According to Drug Group
Demographics.
Substance use groups did not differ significantly on age [F(1,77)=.96, p=.33], education [F(1,77)=.87, p=.35], reading level (from the WRAT-4) [F(1,77)=.94, p=.34], race [χ2=5.81, p=.44], or ethnicity [χ2=3.00, p=.22]. They differed by gender [χ2=3.98, p=.05] and depression symptoms as measured by the BDI-II [F(1,77)=8.89, p=.004] (these are included as covariates in all analyses). See Table 1.
Table 1.
Demographics, Substance Use, and Fitness Characteristics by Substance Group.
| Controls (n=42) % or M (SD) Range |
CAN (n=37) % or M (SD) Range |
|
|---|---|---|
| Age (y) | 20.86 (2.70) 16–25 |
21.41 (2.19) 17–26 |
| Education (y) | 14.26 (2.35) 9–19 |
13.84 (1.55) 11–18 |
| Reading Score (WRAT-IV) | 106.64 (10.20) 87–133 |
104.14 (12.78) 72–133 |
| *BDI-II Total | 2.71 (3.12) 0–10 | 5.32 (4.60) 0– 19 |
| Gender (% female) | 55% | 32% |
| % Caucasian | 69% | 59% |
| % Not Hispanic/Latino/a | 88% | 78% |
| *Past Year Cannabis Use (joints) | .38 (1.12) 0–5 |
427.73 (432.36) 53–2306 |
| *Lifetime Cannabis Use (joints) | 2.32 (4.89) 0–20 |
1162.49 (1341.16) 101– 6000 |
|
*Past Year Alcohol Use (standard drinks) |
103.90 (168.22) 0–698.50 |
318.37 (282.73) 0–1120.50 |
| *Cotinine Level | 1.10 (.58) 0–3 |
2.08 (1.77) 0–6 |
| Length of Abstinence (days) | 46.48 (26.68) 12–388 |
25.24 (7.96) 11–49 |
| VO2 Max Performance (ml/kg/min) | 41.25 (10.27) 20.80–62.90 |
43.98 (9.05) 25.80–62.80 |
| Weight (lb) | 148.85 (22.05) 108.8–194.8 |
149.91 (27.81) 103.4–213.4 |
| Height (inches) | 66.80 (3.60) 60.50–76.50 |
67.05 (3.99) 59.0–75.5 |
| BMI (kg/m2) | 23.54 (3.80) 17.4–39.1 |
23.54 (4.24) 16.4–33.6 |
| Body fat (%) | 22.13 (10.09) 6.4–42.1 |
18.67 (8.47) 3.6–40.7 |
Notes: M = mean; SD = standard deviation.
p<.05; length of abstinence measures last use of any substance other than tobacco; length of abstinence data was missing from 9 controls as they denied any history of substance use
Fitness Characteristics.
Substance use groups did not differ significantly in weight [F(1,76)=.04, p=.85], body fat percentage [F(1,76)=2.63, p=.11], BMI [F(1,76)=.00, p=.99], height [F(1,77)=.08, p=.78], waist circumference [F(1,76)=.99, p=.32], or VO2 max [F(77)=1.55, p=.22].
Drug Use Patterns.
CAN use groups differed significantly in drug use patterns, including past year CAN use [F(1,77)=41.11, p<.001], lifetime cannabis use [F(1,77)=31.48, p<.001], cotinine level [F(1,77)=11.65, p=.001], and past year alcohol use [F(1,77)=17.26, p<.001]. The latter two are covariates in all analyses.
Demographic, Drug Use and Fitness Differences According to Aerobic Fitness Group
Demographics.
Fitness groups did not differ significantly by age [F(1,77)=.30, p=.59], education [F(1,77)=.02, p=.89], reading level (from the WRAT-4) [F(1,77)=2.84, p=.10], depression symptoms as measured by the BDI-II [F(1,77)=.16, p=.69], race [χ2=6.87, p=.33], or ethnicity [χ2=3.54, p=.17]. They differed by gender [χ2=11.67, p=.001], which was included as a covariate for all multiple regression analyses. See Table 2.
Table 2.
Demographics, Substance Use, and Fitness Characteristics by Fitness Group.
| Low-Fit (n=35) % or M (SD) Range |
High-Fit (n=44) % or M (SD) Range |
|
|---|---|---|
| Age (y) | 20.94 (2.65) 16–26 |
21.25 (2.35) 16–25 |
| Education (y) | 14.03 (2.13) 9–18 |
14.09 (1.94) 10–19 |
| Reading Score (WRAT-IV) | 103.06 (11.54) 72–133 |
107.39 (11.18) 90–133 |
| BDI-II Total | 4.14 (4.77) 0–19 |
3.77 (3.47) 0–13 |
| *Gender (% female) | 66% | 27% |
| % Caucasian | 51% | 75% |
| % Not Hispanic/Latino/a | 77% | 89% |
| Past Year Cannabis Use (joints) | 209.96 (453.17) 0–2306 |
193.03 (278.46) 0–1394 |
| Lifetime Cannabis Use (joints) | 292.07 (453.17) 0–1668 |
747.43 (1350.06) 0–6000 |
| *Past Year Alcohol Use (standard drinks) | 125.65 (195.17) 0–800 |
266.95 (275.27) 0–1120.50 |
| *Cotinine Level | 1.17 (.75) 0–3 |
1.86 (1.65) 0–6 |
| Length of Abstinence (days) | 36.90 (32.14) 20–197 |
34.10 (57.45) 11–388 |
| *VO2 Max Performance (ml/kg/min) | 33.83 (5.45) 20.80–43.80 |
49.44 (6.21) 39.20–62.90 |
| Weight (lb) | 148.46 (26.82) 103.4–213.4 |
150.05 (23.16) 108.2–190.8 |
| *Height (inches) | 65.04 (2.76) 59–71 |
68.41 (3.81) 61–76.5 |
| *BMI (kg/m2) | 24.75 (4.60) 17.4–33.9 |
22.56 (3.13) 16.4–33.6 |
| *Body fat (%) | 25.82 (8.92) 6.4–42.1 |
16.23 (7.62) 3.6–36.8 |
Notes: M = mean; SD = standard deviation.
p<.05; length of abstinence measures last use of any substance other than tobacco; length of abstinence data was missing from 3 high-fit and 6 low-fit participants as they denied any history of substance use
Drug Use Patterns.
Fitness groups differed by cotinine level [F(1,77)=5.28, p=.02] and past year alcohol use [F(1,77)=6.58, p=.01] (high-fit youth used more alcohol and had used nicotine more recently), but not past year CAN use [F(1,77)=.04, p=.84] or lifetime CAN use [F(1,77)=3.57, p=.06]. In addition, in individuals who had used CAN, fitness groups did not differ by age of first use [χ2=4.23, p=.94] or regular use [χ2=6.85, p=.55].
Fitness Characteristics.
Fitness groups did not differ significantly in weight [F(1,76)=.08, p=.78], but as expected they did differ by fat percentage [F(1,76)=26.20, p<.001], BMI [F(1,76)=6.23, p=.02], height [F(1,77)=19.40, p<.001], and VO2 max performance [F(77)=136.89, p<.001]. BMI was included as a covariate to control for potential impact of body composition on cognition.
Primary Outcomes
All results are on cognitive performance at a single time point following a 3-week period of abstinence.
CAN Results.
Greater past year CAN use was significantly associated with decreased LNS performance [beta=−.39, t=−2.89, p=.005, f2=.12] and slower time to complete TMT motor sequencing task [beta=.31, t=2.35, p=.02, f2=.08].
VO2 Max Results.
Better VO2 Max performance significantly related to better verbal fluency [beta=.32, t=2.16, p=.03, f2=.07], TMT motor sequencing [beta=−.31, t=−2.26, p=.03, f2=.07], and facial memory recognition [beta=.45, t=3.14, p=.003, f2=.14].
CAN*VO2 Results.
CAN interacted with VO2 max to predict CVLT Trial 1, TMT switching, TMT motor sequencing, and RCFT delayed recall. CAN interacted with VO2 Max in association with CVLT Trial 1 performance [beta=−.25, t=−1.94, p=.05, f2=.06], with more fit controls having better initial learning, while cannabis users’ performance remained the same regardless of fitness level. On TMT switching [beta=−.29, t=−2.37, p=.02, f2=.08], individuals with higher past-year CAN use and higher VO2 max and individuals with less (or no) cannabis use and lower VO2 max exhibited faster performance than either low-fit users or high-fit controls (see Figure 1). On TMT motor sequencing [beta=−.32, t=−2.86, p=.006, f2=.12], individuals who used more CAN in the past year and had better on VO2 max performed more quickly than either individuals with high past year CAN use and low VO2 max or controls. On RCFT Delayed Recall [beta=.26, t=2.02, p=.05, f2=.06], individuals with more past year CAN use and with higher VO2 max attained the highest raw score (see Figure 2).
Figure 1.
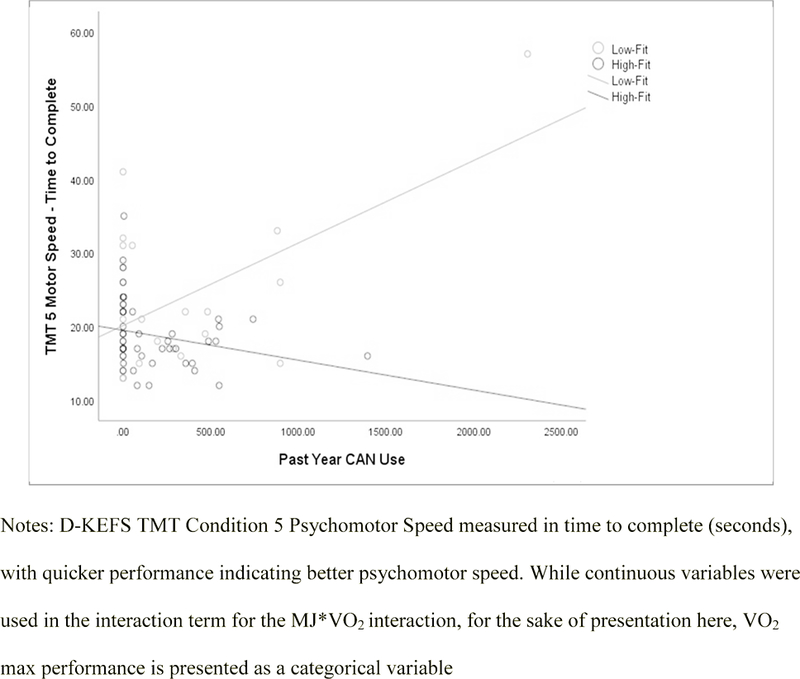
TMT Condition 5 (Motor Speed) by CAN and VO2 Max.
Figure 2.
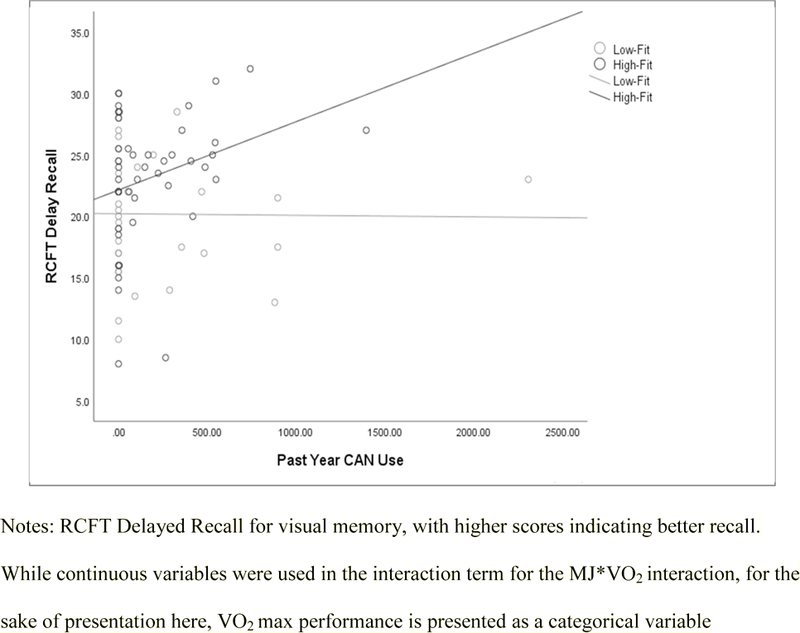
RCFT Delayed Recall by CAN and VO2 Max.
Covariate Results.
Alcohol.
Past year alcohol was related to increased DKEFS FAS total correct raw score [beta=.32, t=2.65, p=.01, f2=.10], quicker DKEFS inhibition/switching Stroop completion [beta=−.27, t=−2.13, p=.04, f2=.07], and fewer commissions in CPT [beta=−.32, t=−2.56, p=.01, f2=.10].
Gender.
Females performed better than males on Penn CNP facial memory recognition [beta=.44, t=3.19, p=.002, f2=.15].
DISCUSSION
The present study aimed to investigate the impact of adolescent and emerging adult CAN use on neuropsychological functioning following 3-weeks of monitored abstinence, and to assess whether aerobic fitness level moderated the impact of CAN on cognitive function. We found that past year CAN use, in a dose-dependent fashion, was associated with poorer working memory and psychomotor speed following a monitored three-week abstinence period. In addition, we found that the novel interaction between cannabis use and aerobic fitness level was associated with performance on working memory, sequencing ability, psychomotor speed, and visual memory. In general, CAN users who had higher aerobic fitness level performed better on these cognitive tasks than CAN users who had lower aerobic fitness level.
Consistent with prior findings (Thames et al., 2014; Medina et al., 2007; Lisdahl & Price, 2012), we found a significant relationship between increased CAN use and poorer attention/working memory and psychomotor speed, even after a monitored abstinence period of three weeks. Inconsistent with prior research (Solowij et al., 2011; Becker et al., 2014; Medina et al., 2007; Fried et al., 2005; Jacobus et al., 2015), we did not find a significant relationship between past year CAN use and memory in the present study. However, we previously did not find a relationship between verbal memory and CAN in a separate sample (Lisdahl & Price, 2012) and attributed this finding to the likely recovery of verbal memory function (Hanson et al., 2010) and hippocampal volume (Yucel et al., 2016) within the first couple weeks of abstinence from CAN.
We also found that CAN use and aerobic fitness level interacted to predict performance on neuropsychological tasks measuring psychomotor speed, visual memory, and sequencing ability. No other studies to date have examined these relationships. More specifically, when looking at the CAN users, high-fit CAN users performed better than low-fit CAN users on tests of sequencing ability, psychomotor speed, and visual memory. Other groups have found reduced symptoms of CUD and reduced craving in CAN users who exercise (Henchoz et al., 2014; Buchowski et al., 2011). The underlying mechanism for this finding is likely multi-factorial. Engaging in aerobic activity, which improves overall aerobic fitness, may counteract the downregulation of regular CAN use on CB1R (Hirvonen et al., 2012; Ferreira-Vieira et al., 2014), especially in frontal and parietal cortical regions. Engaging in AE may also increase circulating eCB levels (Koltyn et al., 2014), which are linked with cognitive function (Egerton, Allison, Brett, & Pratt, 2006; Lee & Gorzalka, 2012). AE also results in release of neurotrophic growth factors such as brain-derived neurotrophic factor (BDNF), insulin-like growth factor-1 (IGF-1) and vascular endothelial growth factor (VEGF) (Cotman & Berchtold, 2002; Lee et al., 2006; Kim et al., 2007; Egan et al., 2003; Castren et al., 1998; Li et al., 2000; Liu et al., 2000; Whiteman et al., 2014; Mueller et al., 2015), improved catecholaminergic (dopamine, norepinephrine, and epinephrine) function (Waters et al., 2005; Dunn et al., 1996; Elam, Svensson, & Thoren, 1987; Heyes, Garnett, & Coates, 1985; Chaouloff, 1989; Dunn & Dishman, 1991), increased c-FOS expression (Sim et al., 2008; Dragunow, 1996; He, Yamada, & Nabeshima, 2002; Vann et al., 2000), decreased inflammatory response and oxidative stress (Sakurai et al., 2009; Radak et al., 2007), and increased CB1R activity in the hippocampus (Ferreira-Vieira et al., 2014).
Another surprising result was the lack of relationship between alcohol use and neuropsychological outcomes, and occasional positive relationships. One reason for this may be the present study’s assessment of total use, rather than patterns of use that may be more neurotoxic (e.g., frequency, heavy episodic or binge drinking). Others have found age of onset of use (Nguyen-Louie et al., 2017), frequency (Nguyen-Louie et al., 2015), or binge drinking patterns (Nguyen-Louie et al., 2016) to be related to decrements in neuropsychological functioning, none of which were specifically investigated here. Alternatively, the socially facilitative nature of initial alcohol experimentation (see Varlinskaya & Spear, 2015) may lead to better social and, by extension, neuropsychological outcomes, as has been indicated across the lifespan (see Kang, Boss, & Clowtis, 2016).
Given the present results, aerobic fitness may be an exciting and low-cost means of intervening to improve psychological and cognitive functioning in CAN users, as has been suggested by our group (Lisdahl, Gilbart, Shollenbarger & Wright, 2013) and others (Brellenthin & Koltyn, 2016). Augmentation of existing cognitive behavioral and motivational interviewing interventions for substance using youth with AE may boost youth’s ability to process, manipulate, and retain information, leading to superior outcomes. There is also potential opportunity to utilize AE as a prevention technique, as improving youth’s executive function, processing speed and memory may reduce risk for substance use initiation.
There are limitations to consider. While relationships between CAN use, fitness, and neuropsychological functioning were found, causality cannot be established due to the cross-sectional nature of this study; longitudinal studies, such as the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org/), are needed to clarify directionality of relationships. Recent research has indicated that CAN users included in studies such as this are not representative of the “typical” CAN user (e.g., lack of psychiatric co-morbidity, lower frequency of use; Rosen et al., 2018); therefore, these results may not generalize and/or may not reveal the true extent of relationships between CAN use and cognition in higher-risk groups. Even in domains where deficits were found, these were modest effects well below the typical 1.5 standard deviations associated with clinical significance; the real-world functional implications about such relative declines are lesser known, although CAN users have reported lower academic achievement (Fergusson & Boden, 2008; Horwood et al., 2010; Suerken et al., 2016), reduced salaries (Fergusson & Boden, 2008) and reduced life satisfaction (Fergusson & Boden, 2008; Grevensteine & Kroninger-Jungaberle, 2015). Another limitation is that, while participants were encouraged to exercise as long as possible, only 80% reached their true VO2 max as defined by Howley and colleagues (1995) and therefore their predicted performance is used in lieu of maximum performance. In addition, while VO2 max is the gold standard in assessing cardiorespiratory health and is typically highly correlated with recent aerobic activity, it does not indicate exact levels of physical activity or sedentary behaviors Other factors also influence VO2 maximum performance (gender, genetics, age, body composition, etc.). In this sample, adiposity (body fat composition) was too highly correlated with VO2 max (r’s > .65) to be included in the same regressions due to multicollinearity; although gender and age were controlled for in the regressions. Larger samples may want to consider examining the unique influences of adiposity, levels of physical activity, and aerobic fitness on neurocognition in cannabis users.
Taken together, our results provide further evidence of increased CAN use being related to poorer working memory, sequencing ability and psychomotor speed- even after three weeks of monitored abstinence. Most notably, our results also suggest aerobic fitness may moderate these effects, such that individuals with higher VO2 max and more past year CAN use may have better neuropsychological performance than low-fit users. AE, therefore, may be a promising ameliorative cognitive intervention for regular CAN users, though future research is needed to assess causality and effectiveness of such an approach.
Acknowledgements:
This study was supported by funding from NIDA (R01 DA030354; P.I.: Lisdahl, K.M.) and manuscript preparation was supported by funding by the National Institutes of Health (U01DA041025; P.I.: Lisdahl, K.M.). The funding sources had no further role in the study design, data collection, analysis, interpretation, the writing of the report, or in the decision to submit the article for publication.
Footnotes
The authors have no conflicts of interest to declare.
References
- Bassett DR, Howley ET, Thompson DL, King GA, Strath SJ, McLaughlin JE, & Parr BB (2001). Validity of inspiratory and expiratory methods of measuring gas exchange with a computerized system. Journal of Applied Physiology, 91(1), 218–224. [DOI] [PubMed] [Google Scholar]
- Bassett DR & Howley ET (2000). Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc, 32(1), 70–84. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, & Anderson SW (1994). Insensitivity to future consequences following damage to human prefrontal cortex. Cognition, 50(1–3), 7–15. [DOI] [PubMed] [Google Scholar]
- Beck AT. (1996). Beck Depression Inventory-2nd edition Psychological Corporation; New York: New York. [Google Scholar]
- Becker MP, Collins PF, & Luciana M (2014). Neurocognition in college-aged daily marijuana users. Journal of Clinical and Experimental Neuropsychology, 36(4), 379–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker MP, Collins PF, Schultz A, Urosevic S, Schmaling B, & Luciana M (2017). Longitudinal changes in cognition in young adult cannabis users. J Clin Exp neuropsychol, epub ahead of print. [DOI] [PMC free article] [PubMed]
- Brellenthin AG & Koltyn KF (2016). Exercise as an adjunctive treatment for cannabis use disorder. Am J Drug Alcohol Abuse, 42(5), 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brellenthin AG, Crombie KM, Hillard CJ, & Koltyn KF (2017). Endocannabinoid and mood responses to exercise in adults with varying activity levels. Med Sci Sport Exerc, 49(8), 1688–1696. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, & Vik PW (1998). Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. J Stud Alcohol, 59, 427–438. [DOI] [PubMed] [Google Scholar]
- Buchowski MS, Meade NN, Charboneau E, Park S, Dietrich MS, Cowan RL, & Martin PR (2011). Aerobic exercise training reduces cannabis craving and use in non-treatment seeking cannabis-dependent adults. PLoS One, 6(3), e17465. doi: 10.1371/journal.pone.0017465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, & Vandrey R (2004). Review of the validity and significance of cannabis withdrawal syndrome. The American Journal of Psychiatry, 161(11), 1967–1977. [DOI] [PubMed] [Google Scholar]
- Castrén E, Berninger B, Leingärtner A & Lindholm D (1998). Regulation of brain-derived neurotrophic factor mRNA levels in hippocampus by neuronal activity. Prog Brain Res, 117, 57–64. [DOI] [PubMed] [Google Scholar]
- Chaouloff F (1989). Physical exercise and brain monoamines: a review. Acta Physiol Scand, 137, 1–13. [DOI] [PubMed] [Google Scholar]
- Conners CK (2000). Conners’ Continuous Performance Test user’s manual Toronto, Canada: Multi-Health Systems. [Google Scholar]
- Cotman CW & Berchtold NC (2002). Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci, 25, 295–301. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC & Christie LA (2007). Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci, 30, 464–472. [DOI] [PubMed] [Google Scholar]
- Crane NA, Schuster RM, Mermelstein RJ, & Gonzalez R (2015). Neuropsychological sex differences associated with age of initiated use among young adult cannabis users. J Clin Exp Neuropsychol, 37(4), 389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Nixon K & Wilkie ME (2004). Exercise reverses ethanol inhibition of neural stem cell proliferation. Alcohol, 33, 63–71. [DOI] [PubMed] [Google Scholar]
- Dahlgren MK, Sagar KA, Racine MT, Dreman MW, & Gruber SA (2016). Marijuana use predicts cognitive performance on tasks of executive function. J Stud Alcohol Drugs, 77(2), 298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Li J, Luan X, Ding YH, Lai W, Rafols JA… Diaz FG (2004). Exercise pre-conditioning reduces brain damage in ischemic rats that may be associated with regional angiogenesis and cellular overexpression of neurotrophin. Neuroscience, 124, 583–591. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, & Kramer JH (2001a). Delis-Kaplan Executive Function System (D-KEFS) San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Delis DC Kramer JH, Kaplan E, & Ober BA (2000). California Verbal Learning Test – second edition Adult version. Manual Psychological Corporation, San Antonio, TX. [Google Scholar]
- Di Marzo V, De Petrocellis L, & Bisogno T (2005). The biosynthesis, fate, and pharmacological properties of endocannabinoids. Handb Exp Pharmacol, 168, 147–185. [DOI] [PubMed] [Google Scholar]
- Dragunow M (1996). A role for immediate-early transcription factors in learning and memory. Behav Genet, 26, 293–299. [DOI] [PubMed] [Google Scholar]
- Dunn AL & Dishman RK (1991). Exercise and the neurobiology of depression. Exerc Sport Sci Rev, 19, 41–98. [PubMed] [Google Scholar]
- Dunn AL, Reigle TG, Youngstedt SD, Armstrong RB & Dishman RK (1996). Brain norepinephrine and metabolites after treadmill training and wheel running in rats. Med Sci Sports Exerc, 28, 204–209. [DOI] [PubMed] [Google Scholar]
- Egan MF, et al. (2003) The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell, 112, 257–269. [DOI] [PubMed] [Google Scholar]
- Eggan SM & Lewis DA (2007). Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: A regional and laminar analysis. Cereb Cortex, 17(1), 175–191. [DOI] [PubMed] [Google Scholar]
- Egerton A, Allison C, Brett RR, & Pratt JA (2006). Cannabinoids and prefrontal cortical function: Insights from preclinical studies. Neurosvi Biobehav Rev, 30(5), 680–695. [DOI] [PubMed] [Google Scholar]
- Elam M, Svensson TH & Thorén P (1987). Brain monoamine metabolism is altered in rats following spontaneous, long-distance running. Acta Physiol Scand, 130, 313–316. [DOI] [PubMed] [Google Scholar]
- Fergusson DM & Boden JM (2008). Cannabis use and later life outcomes. Addictoin, 103(6), 969–976. [DOI] [PubMed] [Google Scholar]
- Ferreira-Vieira TH, Bastos CP, Pereira GS, Moreira FA, & Massensini AR (2014). A role for the endocannabinoid system in exercise-induced spatial memory enhancement in mice. Hippocampus, 24(1), 79–88. [DOI] [PubMed] [Google Scholar]
- Fried PA, Watkinson B, & Gray R (2005). Neurocognitive consequences of marihuana--a comparison with pre-drug performance. Neurotoxicol Teratol, 27(2), 231–239. doi: 10.1016/j.ntt.2004.11.003 [DOI] [PubMed] [Google Scholar]
- Ganzer F, Bröning S, Kraft S, Sack PM, & Thomasius R (2016). Weighing the Evidence: A Systematic Review on Long-Term Neurocognitive Effects of Cannabis Use in Abstinent Adolescents and Adults. Neuropsychol Rev, 26, 186–222 [DOI] [PubMed] [Google Scholar]
- Grevenstein D & Kroninger-Jungaberle H (2015). Two patterns of cannabis use among adolescents: Results of a 10-year prospective study using a growth mixture model. Subst Abus, 36(1), 85–89. [DOI] [PubMed] [Google Scholar]
- Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB… Gur RE (2010). A cognitive neuroscience based computerized battery for efficient measurement of individual differences: Standardization and initial construct validation. J. Neurosci Methods, 187(2), 254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KL, Winward JL, Schweinsburg AD, Medina KL, Brown SA, & Tapert SF (2010). Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict Behav, 35(11), 970–976. doi: 10.1016/j.addbeh.2010.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton GF, Criss KJ & Klintsova AY (2015). Voluntary exercise partially reverses neonatal alcohol-induced deficits in mPFC layer II/III dendritic morphology of male adolescent rats. Synapse, 69, 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henchoz Y, Dupuis M, Deline S, Studer J, Baggio S, N’Goran AA, Daeppen JB, & Gmel G (2014). Associations of physical acitivity and sport and exercise with at-risk substance use in young men: A longitudinal study. Prev. Med, 64, 27–31. [DOI] [PubMed] [Google Scholar]
- He J, Yamada K & Nabeshima T (2002). A role of Fos expression in the CA3 region of the hippocampus in spatial memory formation in rats. Neuropsychopharmacology, 26, 259–268. [DOI] [PubMed] [Google Scholar]
- Helfer JL, Goodlett CR, Greenough WT & Klintsova AY (2009). The effects of exercise on adolescent hippocampal neurogenesis in a rat model of binge alcohol exposure during the brain growth spurt. Brain Res, 1294, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, & Rice KC (1990. Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA, 87(5), 1932–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM & Chu X (2017). Exercise, cognition, and the adolescent brain. Birth Defects Res, 109(20), 1672–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyes MP, Garnett ES & Coates G (1985). Central dopaminergic activity influences rats ability to exercise. Life Sci, 36, 671–677. [DOI] [PubMed] [Google Scholar]
- Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C … Innis RB (2012). Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry, 17(6), 642–649. doi: 10.1038/mp.2011.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howley ET, Bassett DR, Welch HG (1995). Criteria for maximal oxygen uptake: Review and commentary. Med Sci Sports Exerc, 27(9), 1292–301. [PubMed] [Google Scholar]
- Horwood LJ, Fergusson DM, Hayatbakhsh MR, Najman JM, Coffey C, Patton GC… Hutchinson DM (2010). Cannabis use and education achievement: Findings from three Australasian cohort studies. Drug Alcohol Depend, 110(3), 247–253. [DOI] [PubMed] [Google Scholar]
- Hwang J, Castelli DM, Gonzalez-Lima F (2017). The positive cognitive impact of aerobic fitness is associated with peripheral inflammatory and brain-derived neurotrophic biomarkers in young adults. Physiol. Behav, 179, 75–89. [DOI] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Infante MA, Castro N, Brumback T, Meruelo AD, & Tapert SF (2015). Neuropsychological performance in adolescent marijuana users with co-occurring alcohol use: A three-year longitudinal study. Neuropsychology, 29(6), 829–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Sorg SF, Nguyen-Louie TT, & Tapert SF (2014). Cortical thickness and neurocognition in adolescent marijuana and alcohol users following 28 days of monitored abstinence. J Stud Alcohol Drugs, 5(5), 729–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DH, Boss K, & Clowtis L (2016). Social support and cognition: Early childhood versus older adulthood. West J Nurs Res, 38(12). 1639–1659. [DOI] [PubMed] [Google Scholar]
- Kim H, Lee SH, Kim SS, Yoo JH & Kim CJ (2007). The influence of maternal treadmill running during pregnancy on short-term memory and hippocampal cell survival in rat pups. Int J Dev Neurosci, 25, 243–249. [DOI] [PubMed] [Google Scholar]
- Koltyn KF, Brellenthin AG, Cook DB, Sehgal N, & Hillard C (2014). Mechanisms of exercise-induced hypoalgesia. J Pain, 15(12), 1294–1304. doi: 10.1016/j.jpain.2014.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leasure JL & Jones M (2008). Forced and voluntary exercise differentially affect brain and behavior. Neuroscience, 156, 456–465. [DOI] [PubMed] [Google Scholar]
- Leasure JL & Nixon K (2010). Exercise neuroprotection in a rat model of binge alcohol consumption. Alcohol Clin Exp Res, 34, 404–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TT & Gorzalka BB (2012). Timing is everything: Evidence for a role of corticolimbic endocannabinoids in modulating hypothalamic-pituitary-adrenal axis activity across development periods. Neuroscience, 204, 17–30. [DOI] [PubMed] [Google Scholar]
- Lee TM, Wong ML, Lau BW, Lee JC, Yau SY, & So KF (2014). Aerobic exercise interacts with neurotrophic factors to predict cognitive functioning in adolescents. Psychoneuroendocrinology, 39, 214–224. [DOI] [PubMed] [Google Scholar]
- Lee HH, Kim H, Lee JW, Kim YS, Yang HY, Chang HL… Kim CJ (2006). Maternal swimming during pregnancy enhances short-term memory and neurogenesis in the hippocampus of rat pups. Brain Dev, 28, 147–154. [DOI] [PubMed] [Google Scholar]
- Li XC, Jarvis ED, Alvarez-Borda B, Lim DA & Nottebohm F (2000). A relationship between behavior, neurotrophin expression, and new neuron survival. Proc Natl Acad Sci U S A, 97, 8584–8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl KM, & Price JS (2012). Increased marijuana use and gender predict poorer cognitive functioning in adolescents and emerging adults. J Int Neuropsychol Soc, 18(4), 678–688. doi: 10.1017/S1355617712000276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl KM, Gilbart ER, Shollenbarger S, & Wright N (2013). Dare to Delay?: The impacts of adolescent alcohol and marijuana use onset on cognition, brain structure and function. Invited review for Special Topic issue Brain Reward and Stress Systems in Addiction. Frontiers in Psychiatry; 4:53 PMID: 23847550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl KM, Wright NE, Kirchner-Medina C, Maple KE, & Shollenbarger S (2014). Considering cannabis: The effects of regular cannabis use on neurocognition in adolescents and young adults. Curr Addict Rep, 1(2), 144–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Diorio J, Day JC, Francis DD & Meaney MJ (2000). Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci, 3, 799–806. [DOI] [PubMed] [Google Scholar]
- Manly JJ, Jacobs DM, & Touradji P (2002). Reading level attenuates differences in neuropsychological test performance between African American and White elders. Journal of the International Neuropsychological Society, 8(3):341–348. [DOI] [PubMed] [Google Scholar]
- Miech RA, Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE, & Patrick ME (2017). Monitoring the Future national survey results on drug use, 1975–2014: Volume I Secondary school students. Institute for Social Research; Ann Arbor. [Google Scholar]
- Meyers JE & Meyers KR (1995). Rey complex figure test and recognition trial: Professional manual PAR, Inc. [Google Scholar]
- Medina KL, Hanson K, Schweinsburg AD, Cohen-Zion M, Nagel BJ, & Tapert SF (2007). Neuropsychological functioning in adolescent marijuana users: Subtle deficits detectable after 30 days of abstinence. Journal of the International Neuropsychological Society, 13(5), 807–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller K, Moller HE, Horstmann A, Busse F, Lepsien J, Bluher M… Pleger B (2015). Physical exercise in overweight to obese individuals induces metabolic- and neurotrophic-related structural brain plasticity. Front Hum Neurosci, 9, 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nokia MS, Lensu S, Ahtiainen JP, Johansson PP, Koch LG, Britton SL, & Kainulainen H (2016). Physical exercise increases adult hippocampal neurogenesis in male rats provided it is aerobic and sustained. J Physiol, 594, 1855–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Louie TT, Tracas A, Squeglia LM, Matt GE, Eberson-Shumate S, & Tapert SF (2016). Learning and memory in adolescent moderate, binge, and extreme-binge drinkers. Alcohol Clin Exp Res, 40(9), 1895–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Louie TT, Castro N, Matt GE, Squeglia LM, Brumback T, & Tapert SF (2015). Effect of emerging alcohol and marijuana use behaviors on adolescents’ neuropsychological functioning over four years. J Stud Alcohol Drugs, 76(5), 738–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Louie TT, Matt GE, Jacobus J, Li I, Cota C, Castro N, & Tapert SF (2017). Earlier alcohol use onset predicts poorer neuropsychological functioning in young adults. Alcohol Clin Exp Res, 41(12), 2082–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pescatello LS (2014). ACSM’s guidelines for exercise testing and prescription 9th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins Health. [Google Scholar]
- Radak Z, Kumagai S, Taylor AW, Naito H & Goto S (2007). Effects of exercise on brain function: role of free radicals. Appl Physiol Nutr Metab, 32, 942–946. [DOI] [PubMed] [Google Scholar]
- Rosen AS, Sodos LM, Hirst RB, Vaughn D, & Lorkiewicz SA (2018). Cream of the Crop: Clinical representativeness of eligible and ineligible cannabis users in research. Subst Use Misuse, ePub ahead of print. [DOI] [PubMed]
- Sakurai T, Izawa T, Kizaki T, Ogasawara JE, Shirato K, Imaizumi K… Ohno H (2009). Exercise training decreases expression of inflammation-related adipokines through reduction of oxidative stress in rat white adipose tissue. Biochem Biophys Res Commun, 379, 605–609. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E… Dunbar GC (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry, 59 Suppl 20, 22–33. [PubMed] [Google Scholar]
- Sim YJ, Kim S, Shin MS, Chang HK, Shin MC, et al. (2008). Effect of postnatal treadmill exercise on c-Fos expression in the hippocampus of rat pups born from the alcohol-intoxicated mothers. Brain Dev, 30, 118–125. [DOI] [PubMed] [Google Scholar]
- Smirmaul BPC, Bertucci DR, & Teixeira IP (2013). Is the VO2 max that we measure really maximal? Front Physiol, 4, 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC & Sobell MB (1992). Timeline Follow-back: A technique for assessing self-reported ethanol consumption. In Allen J & Litten RZ (Eds.), Measuring Alcohol Consumption: Psychosocial and Biological Methods (pp. 41–72). Totowa, NJ: Humana Press [Google Scholar]
- Solowij N, Jones KA, Rozman ME, Davis SM, Ciarrochi J, Heaven PC… Yücel M (2011). Verbal learning and memory in adolescent cannabis users, alcohol users and non-users. Psychopharmacology (Berlin), 216(1), 131–44. [DOI] [PubMed] [Google Scholar]
- Stewart DG & Brown SA (1995). Withdrawal and dependency symptoms among adolescent alcohol and drug abusers. Addiction, 90, 627–635. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, et al. (2010). Hippocampal gene expression patterns underlying the enhancement of memory by running in aged mice. Neurobiol Aging, 31, 1937–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suerken CK, Reboussin BA, Egan KL, Sutfin EL, Wagoner KG, Spangler J, & Wolfson M (2016). Marijuana use trajectories and academic outcomes among college students. Drug Alcohol Depend, 162, 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames AD, Arbid N, & Sayegh P (2014). Cannabis use and neurocognitive functioning in a non-clinical sample of users. Addict Behav, 39(5), 994–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ & Gage FH (1999). Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A, 96, 13427–13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann SD, Brown MW, Erichsen JT & Aggleton JP (2000). Fos imaging reveals differential patterns of hippocampal and parahippocampal subfield activation in rats in response to different spatial memory tests. J Neurosci, 20, 2711–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI & Spear LP (2015). Social consequences of ethanol: Impact of age, stress, and prior history of ethanol exposure. Physiol Behav, 148, 145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Ying Z & Gomez-Pinilla F (2004). Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci, 20, 2580–2590. [DOI] [PubMed] [Google Scholar]
- Waters RP, Emerson AJ, Watt MJ, Forster GL, Swallow JG, & Summers CH (2005). Stress induces rapid changes in central catecholaminergic activity in Anolis carolinensis: restraint and forced physical activity. Brain Res Bull, 67, 210–218. [DOI] [PubMed] [Google Scholar]
- Wechsler D (1997). WAIS-III Administration and scoring manual The Psychological Corporation, San Antonio, TX. [Google Scholar]
- Whiteman AS, Young DE, He X, Chen TC, Wagenaar RC, Stern CE, & Schon K (2014). Interaction between serum BDNF and aerobic fitness predicts recognition memory in healthy young adults. Behav Brain Res, 259, 302–312. doi: 10.1016/j.bbr.2013.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengaard E, Kristoffersen M, Harris A, & Gundersen H (2017). Cardiorespiratory fitness is associated with selective attention in healthy male high-school students. Front Hum Neurosci, 11, 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman AS, Young DE, Budson AE, Stern CE, & Schon K (2016). Entorhinal volume, aerobic fitness, and recognition memory in healthy young adults: A voxel-based morephometry study. Neuroimage, 126, 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winward JL, Hanson KL, Tapert SF, Brown SA (2014). Heavy alcohol use, marijuana use, and concomitant use by adolescents are associated with unique and shared cognitive decrements. J Int Neuropsychol SOc, 20(8), 784–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel M, Lorenzetti V, Suo C, Zalesky A, Fornito A, Takagi MJ… Solowij N (2016). Hippocampal harms, protection, and recovery following regular cannabis use. Transl Psychiatry, 6, e710. [DOI] [PMC free article] [PubMed] [Google Scholar]


