Abstract
RATIONALE:
Recovery from a traumatic experience requires extinction of cue-based fear responses, a process that is impaired in post-traumatic stress disorder. While studies suggest a link between fear behavioral flexibility and noradrenaline signaling, the role of specific receptors and brain regions in these effects is unclear.
OBJECTIVES:
Here we examine the role of prazosin, an α1-adrenergic receptor (α1-AR) antagonist, in auditory fear conditioning and extinction.
METHODS:
C57Bl/6N mice were subjected to auditory fear conditioning and extinction in combination with systemic (0.1-2 mg/kg) or local microinjections (3 or 6 mM) of the α1-AR antagonist prazosin into the prelimbic division of medial prefrontal cortex or basolateral amygdala. Conditioned fear and anxiety-like behaviors were compared with vehicle-injected control animals.
RESULTS:
Mice that received systemic prazosin prior to fear conditioning exhibited similar initial levels of cue-elicited freezing compared to vehicle controls on the following day. However, at all doses tested fear that was acquired during prazosin treatment was more readily extinguished, whereas anxiety-like behavior on the day of extinction was unaffected. A similar pattern of results was observed when prazosin was microinjected into the basolateral amygdala but not the prelimbic cortex. In contrast to pre-conditioning injections, prazosin administration prior to extinction had no effect on freezing.
CONCLUSIONS:
Our results indicate that α1-AR activity during aversive conditioning is dispensable for memory acquisition, but renders conditioned fear more impervious to extinction. This suggests that behavioral flexibility is constrained by noradrenaline at the time of initial learning via activation of a specific AR isoform.
Keywords: ADRA1A, terazosin, exposure therapy
Introduction.
The extinction of debilitating fear reactions triggered by discrete, contextual, and situational reminders of traumatic events is thought to be an important component of recovery from conditions like post-traumatic stress disorder (PTSD; Milad and Quirk 2012). Unfortunately, extinction efficacy varies among individuals, and diagnosis with PTSD is associated with profound extinction impairments (Jovanovic and Norrholm 2011; Maren and Holmes 2016; Zuj et al. 2016). One causal factor that has been implicated in extinction resistant fear in both rodents and humans is elevated noradrenergic signaling (Gazarini et al. 2014; Lin et al. 2016; Rodrigues et al. 2009; Soeter and Kindt 2011; 2012). Therefore, while more frequently considered within the context of hyperarousal and sleep disturbance, a positive correlation between PTSD and serum noradrenaline levels may also be relevant to the etiology of impaired extinction (Geracioti et al. 2001; Liberzon et al. 1999; Pervanidou and Chrousos 2012; Pitman 1989; Yehuda et al. 1998). However, our understanding of how noradrenaline signaling acts via specific receptors and brain regions to influence behavioral flexibility remains incomplete.
Although its synthesis is limited to relatively sparse cellular populations, noradrenaline is released throughout the brain and exerts a range of physiological effects via three distinct adrenergic receptor (AR) classes: α1, α2 and β. To date most investigations of noradrenaline in fear conditioning have focused on limbic system β-ARs, which recruit intracellular Gs signaling and are required for fear memory acquisition (Bush et al. 2010; Grillon et al. 2004). However, interest in Gq-coupled α1-ARs has gained momentum since the selective α1-AR antagonist prazosin began to show promise in alleviating recurrent nightmares and other symptoms of PTSD (George et al. 2016; Singh et al. 2016). Despite the increasing prevalence of prazosin therapy in this condition, little is known about the role of α1-ARs in fear memory regulation. Therefore, we conducted systemic and brain-region-specific pharmacological manipulations in mice to determine the effects of prazosin on cue-evoked defensive behavior after fear conditioning. We demonstrate that acquisition of fear conditioning is not affected by systemic α1-AR antagonism with prazosin. However, fear responses acquired during prazosin treatment are more susceptible to extinction. Targeted cannulation experiments indicate that the extinction-enhancing effects of prazosin are mediated by the basolateral amygdala. Our data indicate that noradrenaline acting through α1-ARs in the basolateral amygdala sets the stage for behavioral inflexibility in fear conditioning.
Methods and Methods.
Animals.
All experiments were approved in advance by the Institutional Care and use Committee of the Icahn School of Medicine at Mount Sinai. Three-week old male C57Bl/6N mice were ordered from Charles River (Strain Code 027) and allowed to acclimate in our animal facility for at least one week prior to behavior experiments or surgical procedures. Mice were housed 2-5 per cage with access to food and water ad libitum on a 12 hour light-dark cycle (lights on at 0700 hours). All experiments were carried out during the light phase.
Surgery.
Mice were deeply anesthetized with isoflurane and mounted in a stereotaxic frame (Stoelting, Wood Dale, IL, USA) for intracranial cannula implantation. Coordinates were obtained from the mouse brain atlas (Franklin & Paxinos, 2007) with anteroposterior (AP), mediolateral (ML), and dorsoventral (DV) positions referenced from Bregma. A midline incision was made to reveal Bregma, the skull was cleaned with hydrogen peroxide, and small holes were drilled through the skull at the designated stereotaxic coordinates. Single guide cannula (PlasticsOne, Roanoke, VA, USA) were bilaterally implanted above the basolateral amygdala (mm from Bregma: AP −1.4, ML ± 3.3, DV −3.7), and bilateral guide cannula (PlasticsOne) were implanted above the prelimbic cortex (mm from Bregma: AP +1.8, ML ± 0.6, DV −1.2). Guide cannula were secured to the skull using anchor screws and dental cement. Matching dummy cannula were inserted into the guide cannula and secured with a dust cap to ensure guide cannula patency. Post-operative analgesia was provided with banamine (2.5 mg/kg). Mice recovered in their home cages for one week before behavioral testing.
After behavioral testing, cannula placement was verified by infusion of 0.2 μl fast green (0.1% in saline; Sigma-Aldrich, Burlington, MA, USA) at a rate of 0.1 μl per minute. After infusion, the internal cannula was left in place for 5 minutes. Mice were then deeply anesthetized with isoflurane prior to decapitation, and brains were rapidly removed, frozen on pulverized dry ice, and stored at −80°C. Brains were cryostat sectioned at 25 μm, mounted onto charged slides, and allowed to dry overnight before freezing at −80°C. For histology, sections were thawed, rehydrated in graded ethanol, stained with cresyl violet, dehydrated in graded ethanol, and coverslipped with non-aqueous mounting media. Histology confirmed bilateral hits for 19/23 BLA cannula and 18/24 mPFC cannula. Representative images were captured on a Revolve4 microscope (Echo, San Diego, CA, USA) at 4x magnification with an iPadPro camera. Behavioral results from mice with mistargeted cannula were excluded from statistical analysis.
Drug Administration.
Prazosin hydrochloride (Tocris; Minneapolis, MN, USA) was reconstituted in 100% DMSO stored at 4°C for no more than 1 month. Freshly prepared working concentrations of prazosin were diluted in normal saline heated to 37°C and injected/infused 1 hour prior to behavioral manipulation. For IP injections, working concentrations were prepared from a 10 mg/ml stock with 10% final DMSO concentration and 100 μl injection volume. For cannula infusion, working concentrations were prepared from a 50 mg/ml stock with 5% final DMSO concentration. A 0.2 μl volume was infused at a rate of 0.1 μl per minute through internal cannula that extended 1.0 mm below basolateral amygdala guide cannula and 0.5 mm below prelimbic cortex guide cannula. After infusion, the internal cannula was left in place for 5 minutes to allow adequate absorption of the drug.
Fear Conditioning and Extinction.
Mice were handled for 3 consecutive days prior to fear conditioning. Fear conditioning and extinction learning were conducted with an NIR Video Fear Conditioning System (MedAssociates; St. Albans, VT, USA) as previously described (Lucas et al. 2014). On the first day, mice underwent fear conditioning consisting of 6 pairings of an auditory tone (2 kHz, 80 dB, 20 s) with foot shock (1 mA, 2 s) in which the tone and foot shock co-terminated. An acclimation period of 200 s in training arena preceded the onset of cues, and pairings were separated by an 80 s inter-trial interval. The training arena was cleaned with 70% ethanol between sessions. On the second and third days, mice underwent extinction and re-extinction that consisted of 20 tone presentations in an altered context cleaned with isopropanol between sessions. Data were analyzed with VideoFreeze (MedAssociates), and freezing bouts ≥ 1 second were included in analyses.
Elevated plus maze.
For the prazosin dose response experiment, the elevated plus maze test was used to assess anxiety-like behavior 3 hours prior to extinction training on the second day. Mice were placed in the center of the elevated plus maze (Model ENV-560A, Med Associates), and exploration of the maze was tracked with Ethovision software (Noldus, Leesburg, VA, USA) over a 5 minute period. The maze was cleaned with Virox Disinfectant (Oakville, ON, USA) between sessions.
Statistical Analyses.
All data were analyzed with Prism Graphpad (La Jolla, CA, USA). Normal distribution and homogeneity of variance were tested before proceeding to the appropriate parametric or non-parametric tests. Baseline freezing was analyzed with two-tailed unpaired t-tests (2 groups) or one-way ANOVA followed by Tukey’s post-test (≥ 3 groups). CS-evoked freezing during fear conditioning and extinction was analyzed with two-way repeated-measures ANOVA followed by Holm-Bonferroni post-test in the case of significant interaction. All freezing data are presented as mean ± SEM. Elevated plus maze data, which violated homogeneity of variance, was analyzed with Kruskal-Wallis and presented as box-and-whisker plots. For all tests, familywise α = 0.05.
Results.
Fear acquired during α1-AR blockade is more readily extinguished.
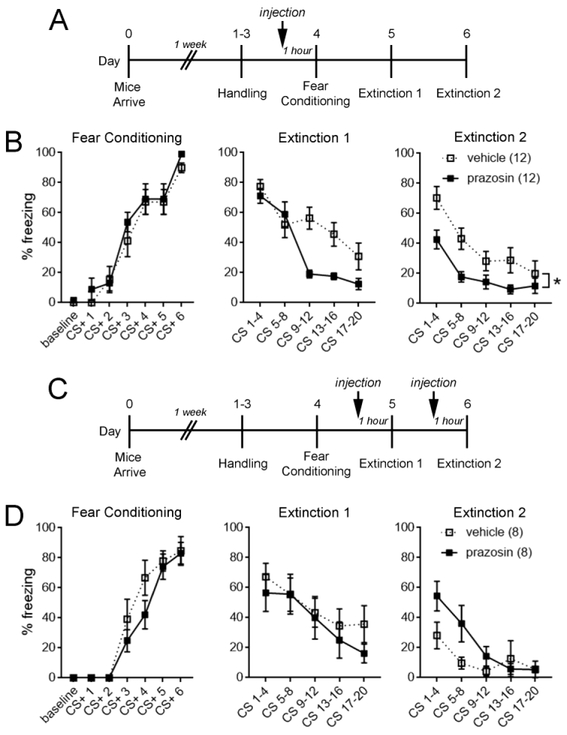
To examine effects of systemic α1-AR blockade during fear conditioning, IP injections of the selective α1-AR antagonist prazosin (2 mg/kg) or vehicle (10% DMSO) were administered to mice 1 hour prior to training (Fig. 1 A). Prazosin administration had no effect on fear memory acquisition or retrieval (Extinction 1, CS 1-4) but significantly attenuated freezing during extinction days 1 (main effect of treatment, F(1,22) = 6.21, p = 0.02) and 2 (main effect of treatment, F(1,22) = 6.19, p = 0.02; Fig. 1B). Similar results were obtained from male mice at 8-12 weeks of age (Figure S1). Because a ceiling effect might have obscured a role for α1-ARs in fear memory acquisition and retrieval, we also performed systemic prazosin administration prior to low-intensity training and found that memory retrieval was still unaffected by prazosin (Figure S2).
Figure 1. Fear acquired during α1-AR blockade is more readily extinguished.
A. Experimental timeline for B. B. Systemic injection of prazosin (2 mg/kg) prior to fear conditioning significantly reduced freezing behavior during extinction days 1 and 2 while having no effect on fear memory acquisition or retrieval (Extinction 1, CS 1–4). C. Experimental timeline for D. D. Systemic injection of prazosin prior to extinction days 1 and 2 did not reduce cue-evoked freezing behavior. Two-way repeated-measures ANOVA, * p < 0.05 for main effect of treatment. n/group indicated in parentheses in legend. CS, conditioned stimulus.
Due to the short half-life of prazosin (2-3 hours), enhanced extinction in prazosin-treated mice is unlikely to be accounted for by the carryover of unmetabolized drug. However, to evaluate the direct effect of α1-AR blockade on extinction learning, prazosin (2 mg/kg) or vehicle (10% DMSO) was administered to mice 1 hour prior to each extinction session (Figure 1C). Contrary to prazosin treatment during fear acquisition, prazosin administered during extinction learning had no effect on freezing (main effect of treatment: Extinction 1, F(1,14) = 0.40, p = 0.53; Extinction 2, F(1,14) = 2.34, p = 0.15; Fig. 1D). These data suggest that prazosin indirectly facilitates extinction by interfering with a process that occurs during initial fear acquisition.
Low doses of prazosin accelerate extinction without impacting anxiety.
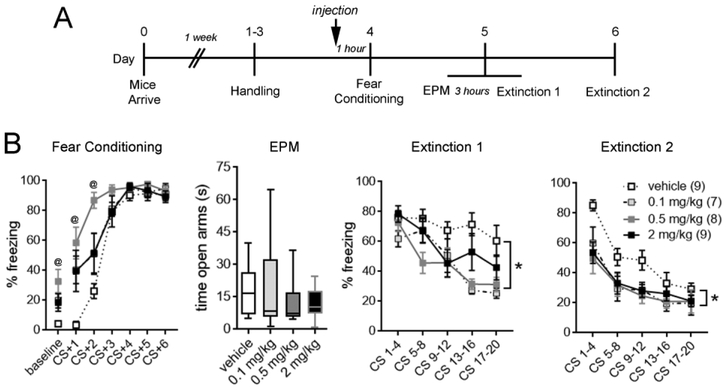
In humans, the therapeutic dose of prazosin ranges from 3-20 mg per day (George et al. 2016; Singh et al. 2016). To determine the dose response range of prazosin in facilitating extinction, IP injections of 0.1, 0.5, or 2 mg/kg prazosin or vehicle (10% DMSO) were administered 1 hour prior to fear conditioning (Fig. 2A). Consistent with a sedative effect of α1-AR blockade, mice that were administered prazosin exhibited increased immobility during baseline (F(3,29) = 4.72, p = 0.008) and the first two tone-shock pairings, but not subsequent trials, of fear conditioning (main effect of treatment, F(3,29) = 4.95, p = 0.007; treatment x trial interaction, F(15,145) = 4.20, p < 0.0001). To determine whether enhanced extinction learning after prazosin administration could be attributable to an altered anxiety state (Fig. 1B), anxiety-like behavior was assessed with the elevated plus maze 3 hours prior to extinction day 1. Prazosin administration during fear acquisition did not affect time spent in the open arms of the elevated plus maze at any dose (p = 0.81), indicating that differences in anxiety states could not have accounted for enhanced extinction in prazosin-treated animals. At all tested doses, prazosin attenuated freezing during extinction days 1 (main effect of treatment, F(3,29) = 4.88, p = 0.007) and 2 (main effect of treatment, F(3,29) = 3.62, p = 0.02; Fig. 2B). These results suggest that prazosin doses within the human therapeutic range (0.1 mg/kg) are sufficient to render fear memories more susceptible to extinction.
Figure 2. Low doses of prazosin accelerate extinction without impacting anxiety.
A. Experimental timeline. B. Systemic administration of prazosin prior to fear conditioning enhanced freezing during baseline and the first and second, but not subsequent trials of tone-shock pairings. Baseline, one-way ANOVA followed by Tukey’s post-test. CS-evoked freezing, two-way repeated-measures ANOVA, treatment x trial interaction followed by Holm-Bonferroni post-test. @ < 0.05 vehicle versus prazosin groups. Prazosin did not affect time spent in the open arms of an elevated plus maze (EPM; Kruskal-Wallis, p = 0.81) or freezing during fear memory retrieval (Extinction 1, CS 1-4) at any dose. However, prazosin at doses as low as 0.1 mg/kg significantly attenuated freezing during extinction days 1 and 2. Two-way repeated-measures ANOVA, * p < 0.05 for main effect of treatment. n/group indicated in parentheses in legend. CS, conditioned stimulus.
α1-AR blockade in the basolateral amygdala recapitulates the fear-attenuating properties of systemic prazosin.
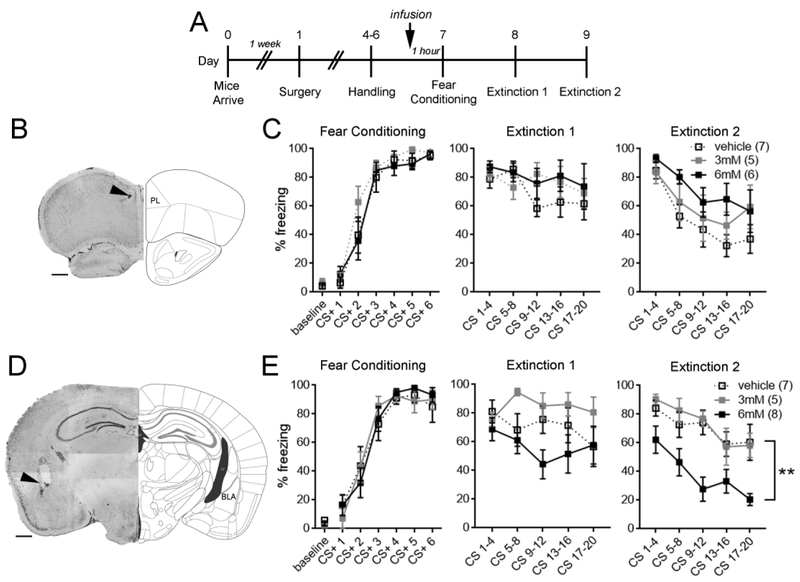
Since prazosin could have poor blood-brain barrier permeability (Taylor et al. 1977), it is possible that above effects of α1-AR blockade are attributable to peripheral rather than central mechanisms. To examine this possibility, guide cannula were chronically implanted in either the prelimbic cortex or the basolateral amygdala (Fig. S3), brain structures in which noradrenaline signaling has been previously implicated in fear conditioning (Bush et al. 2010; Fitzgerald et al. 2015). Prazosin (3 mM or 6 mM) or vehicle (5% DMSO) was bilaterally infused into the prelimbic cortex or basolateral amygdala 1 hour prior to fear conditioning (Fig. 3A). While α1-AR blockade in the prelimbic cortex had no effect on fear conditioning or extinction day 1 (Fig. 3B-C), 6 mM prazosin infused into the basolateral amygdala led to enhancement of extinction day 2 (main effect of treatment; F(2,17) = 8.93, p = 0.002; Fig. 3D-E). These results suggest that the basolateral amygdala is a critical locus of α1-AR-mediated effects.
Figure 3. α1-AR blockade in the basolateral amygdala is sufficient to increase susceptibility to extinction.
A. Experimental timeline. B. Representative image of cannula placement in the prelimbic (PL) cortex. C. Prazosin infusion into the prelimbic cortex did not affect fear conditioning or extinction. D. Representative image of cannula placement in the basolateral amygdala (BLA). E. Basolateral amygdala prazosin infusion at 6mM significantly attenuated freezing during extinction day 2 while having no impact on fear memory acquisition or retrieval (Extinction 1, CS 1-4). Two-way repeated-measures ANOVA, ** p < 0.005 for main effect of treatment. n/group indicated in parentheses in legend. CS, conditioned stimulus. Scale bars = 500 μM.
Discussion.
The noradrenergic substrates that regulate fear behavioral flexibility represent potentially valuable therapeutic targets. Here we found that the rate of extinction learning in mice is constrained by α1-AR activity. However, the time frame when α1-AR blockade influenced the outcome of extinction training was not during extinction itself but instead during initial fear conditioning. Our results further indicate that the basolateral amygdala, but not the prelimbic cortex, is an important locus for these effects. This implies that the conditions for extinction impairment can to some extent be formed during a traumatic event as a result of noradrenaline release in the amygdala. Because our study did not include female mice, however, it will be important for future studies to examine whether these effects are sex-dependent.
In contrast to the β-AR antagonist propranolol (Bush et al. 2010), and similar to the α1-AR antagonist terazosin (Lazzaro et al. 2010), prazosin did not impede fear acquisition, since there were no group differences in freezing after high (Fig. 1,2) or low-intensity training (Fig. S2). However, contrary to our findings, Lazzaro and colleagues observed increased tone-evoked freezing following fear conditioning that coincided with systemic and amygdala-specific terazosin injections in rats (Lazzaro et al. 2010). A number of factors could have contributed to this discrepancy, including species differences (rats versus mice), pharmacological differences between terazosin and prazosin, or dose selection. Because this study did not examine extinction, we are unable to evaluate whether terazosin treatments might nevertheless improve extinction in rats despite initially higher freezing levels during memory retrieval.
Intriguingly, facilitation of extinction by prazosin is highly reminiscent of the relief by immediate post-training propranolol injections of the so-called immediate extinction deficit, a phenomenon in which the extinction of newly-acquired fear memory (less than 1 day old) is profoundly impaired (Fitzgerald et al. 2015; Giustino et al. 2017). Maren and colleagues demonstrated that this deficit is rescued by systemic as well as amygdala-specific propranolol injections (Giustino et al. 2017). Despite these similarities, a critical difference in the outcome of propranolol and prazosin treatments is that the benefits of propranolol appear to be limited to the initial hours after fear conditioning, as propranol administration prior to delayed extinction impairs extinction learning (Fitzgerald et al. 2015). When viewed in this light, the circuit changes underlying more persistent effects of α1-ARs might represent more useful targets for alleviating extinction impairments in PTSD patients, who do not typically seek treatment in the immediate aftermath of trauma.
What cellular mechanisms might account for extinction resistance following α1-AR stimulation? Aversive foot shocks trigger a massive release of noradrenaline into the basolateral amygdala (Quirarte et al. 1998), which plays an important role in both the acquisition and extinction of fear memory (Rodrigues et al. 2009). Therefore, a clear possibility is that α1-ARs support persistent changes in excitability or synaptic signaling that interfere with subsequent extinction-related plasticity. These changes may ultimately impair the reorganization of ensemble activity during extinction and the recruitment of extinction-correlated conditioned stimulus responses (Grewe et al. 2017; Herry et al. 2008). Although noradrenaline acts on both α- and β-ARs in the basolateral amygdala, these receptors elicit distinct intracellular responses and may selectively modulate specific cell types. Stimulation of α1-ARs increases inhibitory synaptic transmission in several brain regions including the basolateral amygdala (Braga et al. 2004; Han et al. 2002; Hillman et al. 2009; Kaneko et al. 2008; Luo et al. 2015; Skelly et al. 2017). Interestingly, this effect is reportedly lost in slice preparations of the basolateral amygdala following fear conditioning (Skelly et al. 2017). This suggests that molecular events linked to prior learning, and perhaps directly arising from α1-AR signaling, can occlude the modulation of inhibitory neuronal populations by α1-ARs. Given the involvement of amygdala GABAergic plasticity in fear (Lucas et al. 2016) and extinction (Davis et al. 2017; Trouche et al. 2013) learning, these effects may provide a clue as to the mechanism by which pre-training prazosin treatments can improve behavioral flexibility.
Long relied on for the treatment of hypertension, prazosin has more recently gained favor as an “off-label” therapy for PTSD-related nightmares, sleep disturbance, and hyperarousal (George et al. 2016; Singh et al. 2016). While treatment for PTSD is typically initiated long after the experience of stress, our results suggest that prazosin may also confer therapeutic benefits when used as a prophylactic against the development of extinction-resistant fear during initial trauma or re-traumatization. It is also possible that α1-AR activity contributes to an ongoing dysregulation of other aspects of fear learning in these psychiatric populations. For example, in a recent study of normal human subjects we demonstrated that therapeutic doses of prazosin improve the learned discrimination of safe and threatening visual stimuli in a fear conditioning paradigm (Homan et al. 2017). Other work indicates that prazosin can disrupt the reconsolidation of cued fear memories in rats (Do Monte et al., 2013). Future investigations of α1-AR signaling might therefore help establish novel applications for prazosin as well as identify new mechanistic bases for intervention after trauma.
Supplementary Material
A. Experimental timeline. B. Systemic injection of prazosin (1 mg/kg) prior to fear conditioning significantly reduced freezing behavior during extinction day 2 while having no effect on fear memory acquisition or retrieval (Extinction 1, CS 1-4). Two-way repeated-measures ANOVA, p < 0.05 for main effect of treatment. n/group indicated in parentheses in legend. CS, conditioned stimulus.
A. Experimental timeline. B. Systemic injection of prazosin (2 mg/kg) prior to low-intensity fear conditioning (3 tone-shock pairing) did not affect fear memory retrieval. n/group indicated in parentheses in legend.
A. Prelimbic cortex cannula placements. B. Basolateral amygdala cannula placements. Taxonomy according to The Mouse Brain in Stereotaxic Coordinates, 3rd Edition, by Franklin & Paxinos (2007).
Acknowledgments.
We would like to thank Stephen Salton, Matthew Shapiro, Paul Kenny, Anne Shaefer, Schahram Akbarian, Zhenyu Yue, and Glenn Cruse for use of equipment, and Wei-Jye Lin, Kirstie Cummings, Molly Heyer, and Philip Avigan for technical advice and assistance. This work was funded by NIH grant MH105414 (R.L.C.) and seed funds from NC State University (E.K.L).
References
- Braga MF, Aroniadou-Anderjaska V, Manion ST, Hough CJ, Li H (2004) Stress impairs alpha(1A) adrenoceptor-mediated noradrenergic facilitation of GABAergic transmission in the basolateral amygdala. Neuropsychopharmacology 29: 45–58. [DOI] [PubMed] [Google Scholar]
- Bush DE, Caparosa EM, Gekker A, Ledoux J (2010) Beta-adrenergic receptors in the lateral nucleus of the amygdala contribute to the acquisition but not the consolidation of auditory fear conditioning. Front Behav Neurosci 4: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis P, Zaki Y, Maguire J, Reijmers LG (2017) Cellular and oscillatory substrates of fear extinction learning. Nat Neurosci 20: 1624–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Monte FH, Souza RR, Wong TT, de Padua Carobrez A (2013). Systemic or intra-prelimbic cortex infusion of prazosin impaired fear memory reconsolidation. Behav Brain Res 244: 137–41. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PJ, Giustino TF, Seemann JR, Maren S (2015) Noradrenergic blockade stabilizes prefrontal activity and enables fear extinction under stress. Proc Natl Acad Sci U S A 112: E3729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazarini L, Stern CA, Piornedo RR, Takahashi RN, Bertoglio LJ (2014) PTSD-like memory generated through enhanced noradrenergic activity is mitigated by a dual step pharmacological intervention targeting its reconsolidation. Int J Neuropsychopharmacol 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George KC, Kebejian L, Ruth LJ, Miller CW, Himelhoch S (2016) Meta-analysis of the efficacy and safety of prazosin versus placebo for the treatment of nightmares and sleep disturbances in adults with posttraumatic stress disorder. J Trauma Dissociation 17: 494–510. [DOI] [PubMed] [Google Scholar]
- Geracioti TD Jr., Baker DG, Ekhator NN, West SA, Hill KK, Bruce AB, Schmidt D, Rounds-Kugler B, Yehuda R, Keck PE Jr., Kasckow JW (2001) CSF norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry 158: 1227–30. [DOI] [PubMed] [Google Scholar]
- Giustino TF, Seemann JR, Acca GM, Goode TD, Fitzgerald PJ, Maren S (2017) beta-Adrenoceptor Blockade in the Basolateral Amygdala, But Not the Medial Prefrontal Cortex, Rescues the Immediate Extinction Deficit. Neuropsychopharmacology 42: 2537–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewe BF, Grundemann J, Kitch LJ, Lecoq JA, Parker JG, Marshall JD, Larkin MC, Jercog PE, Grenier F, Li JZ, Luthi A, Schnitzer MJ (2017) Neural ensemble dynamics underlying a long-term associative memory. Nature 543: 670–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Cordova J, Morgan CA, Charney DS, Davis M (2004) Effects of the beta-blocker propranolol on cued and contextual fear conditioning in humans. Psychopharmacology (Berl) 175: 342–52. [DOI] [PubMed] [Google Scholar]
- Han SK, Chong W, Li LH, Lee IS, Murase K, Ryu PD (2002) Noradrenaline excites and inhibits GABAergic transmission in parvocellular neurons of rat hypothalamic paraventricular nucleus. J Neurophysiol 87: 2287–96. [DOI] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A (2008) Switching on and off fear by distinct neuronal circuits. Nature 454: 600–6. [DOI] [PubMed] [Google Scholar]
- Hillman KL, Lei S, Doze VA, Porter JE (2009) Alpha-1A adrenergic receptor activation increases inhibitory tone in CA1 hippocampus. Epilepsy Res 84: 97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homan P, Lin Q, Murrough JW, Soleimani L, Bach DR, Clem RL, Schiller D (2017) Prazosin during threat discrimination boosts memory of the safe stimulus. Learn Mem 24: 597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD (2011) Neural mechanisms of impaired fear inhibition in posttraumatic stress disorder. Front Behav Neurosci 5: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko K, Tamamaki N, Owada H, Kakizaki T, Kume N, Totsuka M, Yamamoto T, Yawo H, Yagi T, Obata K, Yanagawa Y (2008) Noradrenergic excitation of a subpopulation of GABAergic cells in the basolateral amygdala via both activation of nonselective cationic conductance and suppression of resting K+ conductance: a study using glutamate decarboxylase 67-green fluorescent protein knock-in mice. Neuroscience 157: 781–97. [DOI] [PubMed] [Google Scholar]
- Lazzaro SC, Hou M, Cunha C, LeDoux JE, Cain CK (2010) Antagonism of lateral amygdala alpha1-adrenergic receptors facilitates fear conditioning and long-term potentiation. Learn Mem 17: 489–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Abelson JL, Flagel SB, Raz J, Young EA (1999) Neuroendocrine and psychophysiologic responses in PTSD: a symptom provocation study. Neuropsychopharmacology 21: 40–50. [DOI] [PubMed] [Google Scholar]
- Lin CC, Tung CS, Lin PH, Huang CL, Liu YP (2016) Traumatic stress causes distinctive effects on fear circuit catecholamines and the fear extinction profile in a rodent model of posttraumatic stress disorder. Eur Neuropsychopharmacol 26: 1484–1495. [DOI] [PubMed] [Google Scholar]
- Lucas EK, Jegarl A, Morishita H, Clem RL (2016) Multimodal and site-specific plasticity of amygdala parvalbumin interneurons after fear learning. Neuron 91: 629–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo F, Tang H, Cheng ZY (2015) Stimulation of alpha1-adrenoceptors facilitates GABAergic transmission onto pyramidal neurons in the medial prefrontal cortex. Neuroscience 300: 63–74. [DOI] [PubMed] [Google Scholar]
- Maren S, Holmes A (2016) Stress and Fear Extinction. Neuropsychopharmacology 41: 58–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ (2012) Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol 63: 129–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervanidou P, Chrousos GP (2012) Posttraumatic stress disorder in children and adolescents: neuroendocrine perspectives. Sci Signal 5: pt6. [DOI] [PubMed] [Google Scholar]
- Pitman RK (1989) Post-traumatic stress disorder, hormones, and memory. Biol Psychiatry 26: 221–3. [DOI] [PubMed] [Google Scholar]
- Quirarte GL, Galvez R, Roozendaal B, McGaugh JL (1998) Norepinephrine release in the amygdala in response to footshock and opioid peptidergic drugs. Brain Res 808: 134–40. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, LeDoux JE, Sapolsky RM (2009) The influence of stress hormones on fear circuitry. Annu Rev Neurosci 32: 289–313. [DOI] [PubMed] [Google Scholar]
- Singh B, Hughes AJ, Mehta G, Erwin PJ, Parsaik AK (2016) Efficacy of Prazosin in Posttraumatic Stress Disorder: A Systematic Review and Meta-Analysis. Prim Care Companion CNS Disord 18. [DOI] [PubMed] [Google Scholar]
- Skelly MJ, Ariwodola OJ, Weiner JL (2017) Fear conditioning selectively disrupts noradrenergic facilitation of GABAergic inhibition in the basolateral amygdala. Neuropharmacology 113: 231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeter M, Kindt M (2011) Noradrenergic enhancement of associative fear memory in humans. Neurobiol Learn Mem 96: 263–71. [DOI] [PubMed] [Google Scholar]
- Soeter M, Kindt M (2012) Stimulation of the noradrenergic system during memory formation impairs extinction learning but not the disruption of reconsolidation. Neuropsychopharmacology 37: 1204–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Twomey TM, von Wittenau MS (1977) The metabolic fate of prazosin. Xenobiotica 7: 357–64. [DOI] [PubMed] [Google Scholar]
- Trouche S, Sasaki JM, Tu T, Reijmers LG (2013) Fear extinction causes target-specific remodeling of perisomatic inhibitory synapses. Neuron 80: 1054–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, McFarlane AC, Shalev AY (1998) Predicting the development of posttraumatic stress disorder from the acute response to a traumatic event. Biol Psychiatry 44: 1305–13. [DOI] [PubMed] [Google Scholar]
- Zuj DV, Palmer MA, Lommen MJ, Felmingham KL (2016) The centrality of fear extinction in linking risk factors to PTSD: A narrative review. Neurosci Biobehav Rev 69: 15–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. Experimental timeline. B. Systemic injection of prazosin (1 mg/kg) prior to fear conditioning significantly reduced freezing behavior during extinction day 2 while having no effect on fear memory acquisition or retrieval (Extinction 1, CS 1-4). Two-way repeated-measures ANOVA, p < 0.05 for main effect of treatment. n/group indicated in parentheses in legend. CS, conditioned stimulus.
A. Experimental timeline. B. Systemic injection of prazosin (2 mg/kg) prior to low-intensity fear conditioning (3 tone-shock pairing) did not affect fear memory retrieval. n/group indicated in parentheses in legend.
A. Prelimbic cortex cannula placements. B. Basolateral amygdala cannula placements. Taxonomy according to The Mouse Brain in Stereotaxic Coordinates, 3rd Edition, by Franklin & Paxinos (2007).