Abstract
Mammalian target of rapamycin (mTOR) signaling has emerged as a key regulator in a wide range of cellular processes ranging from cell proliferation, immune responses, and electrolyte homeostasis. mTOR consists of two distinct protein complexes mTORC1 and mTORC2, each with distinct downstream signaling events. mTORC1 has been implicated in pathological conditions such as cancer and type 2 diabetes in humans and inhibition of this pathway with rapamycin has been shown to attenuate salt-induced hypertension in Dahl salt-sensitive (SS) rats. A number of studies have found that the mTORC2 pathway is involved in the regulation of renal tubular sodium and potassium transport but its role in hypertension has remained largely unexplored. In the present study, we therefore determined the effect of mTORC2 inhibition with compound PP242 upon salt-induced hypertension and renal injury in SS rats. We found that PP242 not only completely prevented but also reversed salt-induced hypertension and kidney injury in SS rats. PP242 exhibited potent natriuretic actions and chronic administration tended to produce a negative Na+ balance even during high salt feeding. The results indicate that mTORC2 and the related downstream associated pathways play an important role in regulation of sodium balance and arterial pressure regulation in SS rats. Therapeutic suppression of the mTORC2 pathway represents a novel pathway for the potential treatment of hypertension.
Keywords: mTORC2, PP242, Dahl S rat, salt-sensitive hypertension, renal injury
Introduction
Hypertension and the effects of dietary salt on blood pressure remain a major cause of global mortality and a primary risk factor for renal, cardiovascular and cerebrovascular disease1. In nearly 50% of hypertensive patients, blood pressure increases in response to salt (salt-sensitivity)2. This figure increases to 75% in African-American populations, who also suffer a disproportionate incidence of hypertension 2–4 with a higher incidence of end stage renal disease 5, 6. Despite extensive research, the underlying genetic and molecular mechanisms of common forms of hypertension remain largely unclear. Dahl salt-sensitive (SS) rats were utilized in the present studies since they represent a naturally occurring genetic model possessing many of the same traits observed in the salt-sensitive African American population7.
We have begun to explore the role of mammalian target of rapamycin (mTOR) pathways in hypertension. mTOR is a serine/threonine kinase in the PI3K-related kinase (PIKK) family that forms the catalytic subunit of two distinct protein complexes, known as mTORC1 and mTORC2. mTORC1 plays important role in the regulation of cell proliferation, cell growth and the immune system. It is known to be deregulated in several pathological conditions8, 9. We have recently found that inhibition of mTORC1 with rapamycin reduces salt-induced hypertension and kidney injury in SS rats10. Rapamycin did not inhibit renal mTORC2 activity in that study10 and provided no information about the participation or relevance of the mTORC2 pathway in salt-induced hypertension in SS rats.
Given evidence that the mTORC2 pathway can alter renal tubular electrolyte transport11–14 and given the absence of studies assessing its role in cardiovascular disease and hypertension, we have explored the potential role of this pathway in salt-induced hypertension in SS rat model. There are currently no pharmacological tools to selectively inhibit mTORC2 without affecting mTORC1 and the development of such compounds has been difficult given that both complexes share the same catalytic domain15. Currently, the most effective pharmacological tools to inhibit mTORC2 are ATP competitive inhibitors such as PP242, AZD8055 and Torin1 which also inhibit mTORC111. In the present study, PP242 was used to study the effect of mTORC2 inhibition in the development of salt-induced hypertension and kidney injury in SS rats. The extent of inhibition of the mTORC1 pathway by PP242 in our study was assessed by determining the phosphorylation of unique motifs related to their final downstream effector ribosomal protein S6 at S235/236. Ribosomal S6 kinase1 (S6K1), a downstream effector of mTORC1, phosphorylates ribosomal protein S6 at serine 235, 236, 240, 244 and 247 and the ratio of pS6S235/236/S6 was used as the functional marker of mTORC1 kinase activity9. Inhibition of mTORC2 was assessed by determining activity of its immediate downstream effector kinase AKT at serine 473 and the ratio of pAKTS473/AKT was used as the functional marker of mTORC2 kinase activity9. The results of the present study indicate that the mTORC2 pathway plays an important role in determining blood pressure salt-sensitivity in the commonly used SS rats.
Methods Summary
All supporting data used for this study are available within the article and its online supplementary files. Experiments were performed with male Dahl SS/JrHsdMcwi rats. PP242 (i.p.,15 mg/kg/day) or vehicle (30% PEG, 0.5% Tween 80, and 5% propylene glycol dissolved in sterile ultra-pure water) was administered daily to male SS rats (10 wk old) while fed a 0.4% NaCl diet (4 days) followed by treatment during 21 days of a high 4.0% NaCl diet. Radiotelemetry catheters and transmitters were surgically implanted for 24hrs/day recording of blood pressure and heart rate as we have described10,. Body weight was measured daily and on the final day of the 4.0% NaCl diet period, rats were placed in a metabolic cage for a 24 hr urine collection. Western blot, immunohistochemistry, sodium balance, renal interstitium infusion of PP242, renal tubular injury, and glomerular score were performed as we have described10, 16, 17 and detailed in the online- Data Supplement.
Statistical Methods
Data are presented as mean values ± standard error. A two-way analysis of variance (ANOVA) for repeated measures test was used to compare chronically recorded blood pressure measurements, Na+ balance studies, and acute renal interstitial and i.v. infusions of PP242. Student’s t-test was used to compare between the vehicle and PP242 treatments in the studies. Paired t-test was used to compare the effect of treatments on body weight of SS rats fed a 4.0% NaCl diet. p<0.05 was considered significant.
Results
PP242 prevented salt-induced hypertension in SS rats.
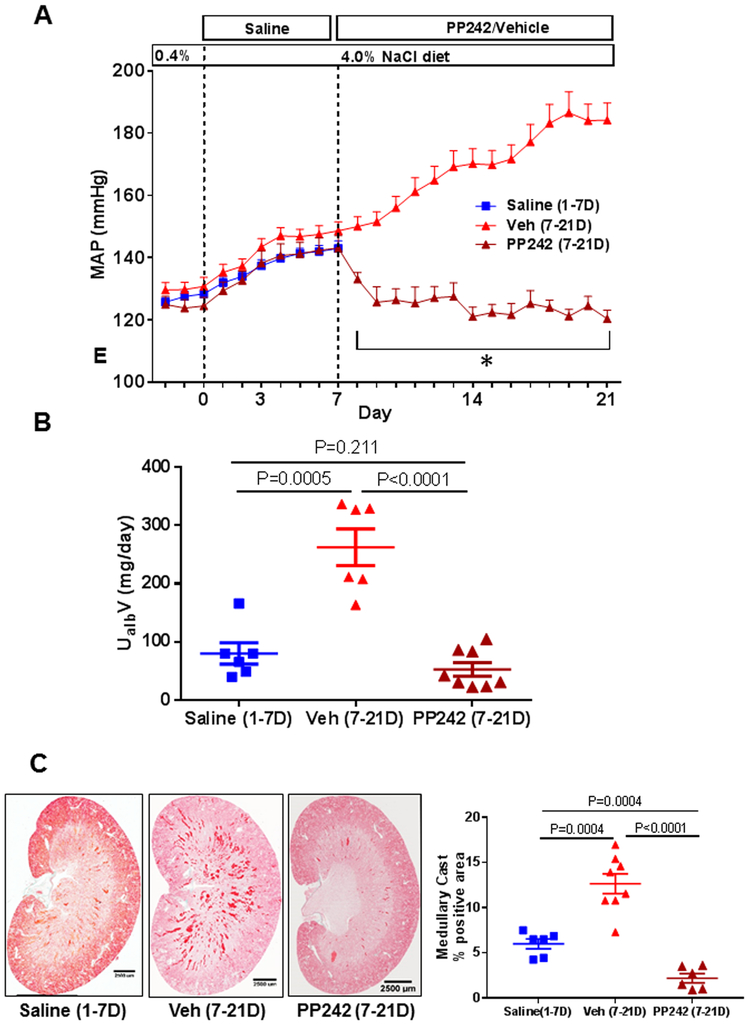
One week following recovery from implantation of radiotransmitters, control levels of continuously recorded arterial pressures were obtained over a 3 day period while rats continued to receive the control salt (0.4% NaCl) diet. Daily PP242 treatment was then begun (i.p., 15 mg/kg/day) and effects of the drug or vehicle alone were determined over the next four days prior to switching to the high salt (4.0% NaCl) diet. As shown in Figure 1A, PP242 tended to lower the mean arterial pressure (MAP) by day four of injection during the control salt period (p<0.05). When switched to the high salt diet, MAP rose progressively in the vehicle treated SS rats reaching 150 ± 2 mmHg by day 10 and 168 ± 3 mmHg by day 21. PP242 treated rats exhibited no increase of MAP after switching to the high salt diet throughout the 21 day period of recording. As shown in Figure S1A-C, PP242 nearly abolished diurnal rhythms of MAP, diastolic blood pressure (DBP) and systolic blood pressure (SBP) in SS compared to vehicle treated rats. However, diurnal heart rate (HR) rhythms remained unaffected in PP242 treated rats compared to vehicle treated rats (Figure S1D).
Figure 1.
(A) Mean arterial pressure (MAP; 24 hrs average) was measured by telemetry in rats fed a 0.4% NaCl diet for 7 days the final 4 days of which rats were treated with PP242 or vehicle prior to switching to 4.0% NaCl diet for 21 days. Open circles represent PP242 (n=7) and black circles represent vehicle (n=7) treated SS rats. * Significant difference between vehicle and PP242 treated SS rats (P<0.05) as determined using a two-way RM ANOVA; Holm-Sidak post hoc. (B) Hypertrophic index was determined by measuring the ratio of right kidney weight (gm) and total body weight (gm) of these rats.
During the study, the animals remained active and appeared healthy based on levels of alertness, appearance of their eyes, and the condition of their fur and grooming. However, PP242 treated rats did not gain body weight throughout the study (298 ± 7 g on day 0 compared to 302 + 6 g on day 21 of 4.0% NaCl diet) in contrast to vehicle treated rats which averaged a 60 g weight increase during the same period. Renal hypertrophy which is commonly observed in high salt fed SS rats10 was not found in PP242 treated rats in which a 24% lower kidney to body weight ratio was observed compared to vehicle treated rats (renal hypertrophy index; P<0.05) (Figure 1B).
PP242 treatment prevented renal injury, and immune cell infiltration.
PP242 treated rats displayed a complete protection from the renal injury compared to vehicle treated rats assessed at the end of the 21 day period of the high salt diet. As shown in Figure 2A, PP242 treated rats exhibited very low levels of medullary tubular casts averaging only 2% positive cast area versus 12% positive area in vehicle treated rats. Similarly, albumin excretion (UalbV) and protein excretion rates (UprotV) were significantly lower in PP242 treated rats averaging 33 ± 7 mg/day compared to 256 ± 37 mg/day in vehicle treated and 84 ± 10 mg/day compared to 351 ± 44 mg/day in vehicle treated SS rats, respectively (Figure 2B&C). Further evidence of the protective effects of PP242 treatment are seen in Figure 2D-F which compares the glomerular injury scores, inflammatory CD3 (T-lymphocyte) and CD68 (macrophage) labeled cells. PP242 treatment significantly protected glomerular injury in SS rats compared to vehicle treated SS rats (Figure 2D). As seen in Figure 2E&F, CD3+ and CD68+ cells/mm2 were significantly reduced in the renal medulla in PP242 treated rats compared to vehicle treated SS rats. Similarly, PP242 tretament reduced both CD3 and CD68 labeled cells in the renal cortex compared with vehicle treated rats (Figures S2A-B).
Figure 2:
(A) Outer medullary tubular injury and representative images of kidney depict the protein cast in vehicle or PP242 treated rats. (B&C) Summary of albumin (UalbV) excretion rate and protein excretion (UprotV) rate. (D) Quantification of glomerular injury and representative images of cortical glomerulus from vehicle or PP242 treated rats are shown. (E&F) Quantification of T lymphocyte (CD3+) and macrophage (CD68+) measured by cells per mm2 of kidneys of these rats. CD3+ cells/mm2 and CD68+ cells/mm2 in the renal medulla and representative kidney sections illustrating the immunohistochemical localization of T cells and macrophage cells in the renal medulla are shown.
Ability of PP242 to reverse early stage salt-induced hypertension in SS rats.
To determine if PP242 could also reverse salt-induced hypertension, SS rats were fed a 4.0% NaCl diet for 7 days to produce hypertension and then randomly divided into two groups; one treated daily with PP242 and the other with vehicle over the following 14 days (days 7–21) of 4.0% NaCl diet. A third group of SS rats was treated daily with an i.p. saline injection during the first 7 days of the 4.0% NaCl diet at which point the experiment was stopped and kidney collected for assessment of the effects of the high salt diet and associated hypertension on renal injury prior to PP242 treatment. As seen in Figure 3A, MAP initially rose equally in each of the three groups of rats, reaching approximately 143 mmHg by day 7 of 4.0% NaCl. PP242 (i.p.,15 mg/kg/day) resulted in complete normalization of MAP by the second day of treatment which was sustained throughout the remaining 12 days of therapy despite the maintenance of the 4.0% NaCl diet. The MAP of vehicle treated rats continued to rise throughout the 4.0% NaCl challenge, averaging 184 ± 10 mmHg by day 21. The body weight of the vehicle treated rats increased from 324 ± 14 to 360 ± 14 g during this same period. In contrast the PP242 treated rats which showed no change of body weight averaging 311 ± 26 g during the control period and 308 ± 21 g at the end of the 21 day study.
Figure 3:
(A) Mean arterial pressure (MAP; 24 hrs average) was measured by telemetry in rats fed a 4.0% NaCl diet for 7 days treated with saline (blue square, n=6) and then treated with PP242 (brown triangle, n=7) or vehicle (red triangle, n=7) for 14 days. * Significant difference between vehicle and PP242 treated SS rats (p<0.05) as determined using a two-way RM ANOVA; Holm-Sidak post hoc. (B) Showing albumin excretion rate (C) Medullary protein cast and representative images of kidney depicting protein cast in saline/vehicle/PP242 treated rats.
In addition to reducing MAP, PP242 treatment resulted in an almost complete reversal of renal injury. As shown in Figure 3B, Urine albumin excretion rate determined in the group of SS rats treated with saline for 7-days of the 4.0% NaCl diet averaged 80 ±18 mg/day, a value higher than those determined in the PP242 treated rats after 21-days of high salt (52 ±12 mg/day). Similarly, the histological analysis (Figure 3C) showed that PP242 treatment significantly reduced medullary protein casts to levels less than those observed after 7-days of 4.0% NaCl and well below those of the vehicle treated rats.
Indices of renal inflammation were also reduced by PP242 treatment as seen by the significant reduction of infiltrated T lymphocytes assessed by CD3/mm2 (Figure S3A). After 14 days of PP242 treatment, T-lymphocytes were reduced from levels of 277± 15 observed after 7 days of 4.0% NaCl diet to levels of 45 ± 4 CD3/mm2 (Figure S3A). Consistent with these lymphocyte counts the tissue protein level of CD3 as determined by Western blot analysis was significantly reduced (P<0.0001) in the medullary tissue (Figure S3B) and in the renal cortex (Figure S3C&D). All indices of renal injury were significantly higher in the vehicle treated rats than both the PP242 and saline treated rats. Taken together, these results show that PP242 is a potent antihypertensive drug which not only prevents but also reverses salt-induced hypertension. The reversal of hypertension was associated with significant reductions in both renal injury and renal inflammation.
PP242 produced natriuresis acutely and chronically.
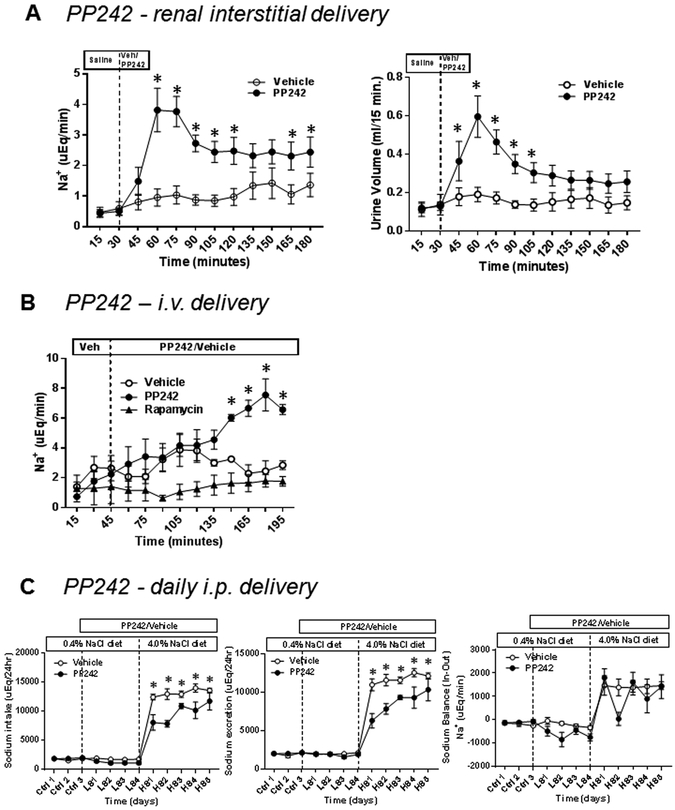
The effects of PP242 upon renal electrolyte excretion were studied first in anesthetized rats in which the compound was delivered both intrarenally into the outer medullary interstitial space (at the medullary-cortical junction), and by intravenous infusion. As summarized in Figure 4A, acute renal interstitial (r.i.) infusion of PP242 (5 mg/kg; 20 min) in anesthetized SS rats produced nearly a 4-fold increase of Na+ excretion (P<0.05) and a 3-fold increase of urine output (P<0.05) compared to rats receiving vehicle. The peak natriuretic response occurred 30 minutes after the initiation of PP242 administration. In contrast, K+ excretion was not significanly altered by PP242 compared to vehicle (Figure S4).
Figure 4:
(A) Anesthesized SS rats were infused renal intersititially with PP242 at the dose of 5mg/kg (black circle, n=6) or vehicle (open circle, n=6), and urine was collected at 15 minute intervals. The average mean value Na+ excretion rate and urine volume determined in these rats. (B) Na+ excretion rate of i.v. infusion of PP242 with dose 10 ug/kg/min (black circle, n=3) or rapamycin (10 ug/kg/min; black triangle, n=4) or vehicle (open circle, n=3) was determined in SS rats. (C) Sodium balance in chronic administration of PP242 i.p. 15mg/kg/day (black circle, n=5) or vehicle (open circle, n=6) to SS rats. Rats were fed a gel diet delivering a fixed sodium intake per day. In the control period rats were not receiving PP242 or vehicle. Rats were treated with PP242 or vehicle while maintaining at 0.4% NaCl diet for 4 days before switching to 4% NaCl diet for 5 days. * P<0.05 vs Vehicle.
Intravenous (i.v.) infusion of PP242 (10 μg/kg/min) over a 3 hour period resulted in an approximately 4-fold increase of Na+ excretion, although the response occured gradually over the infusion period (Figure 4B). In contrast, rapamycin (10 μg/kg/min) had no effect on Na+ excretion the duration of the study (Figure 4B)
Figure 4C summarizes the effects of PP242 upon the daily intake and excretion of Na+ and the calculated Na+ balance in conscious SS rats. While rats continued to receive a a 0.4% NaCl diet PP242 (15 mg/day i.p.) or vehicle treatment was begun. After four days, rats were switched to a high (4% NaCl) liquid diet for five days. Na+ intake was determined based on volume of liquid diet consumed. As summarized in Figure 4C, switching to the 4.0% NaCl diet caused a significant increase in Na+ intake in both groups of rats, although intake was blunted in PP242 treated rats. This reduction of liquid food intake in PP242 treated rats then tended to increase on days 3–5. Given the reduction of Na+ intake, Na+ excretion levels were lower in the PP242 rats than the vehicle treated rats. The calculated Na+ balance nevertheless tended to be negative in the PP242 treated rats and albeit not significant is consistent with the acute natriuretic effects of this compound shown in Figure 4A&C.
The effects of PP242 on mTORC1/C2 in the prevention of salt-induced hypertension.
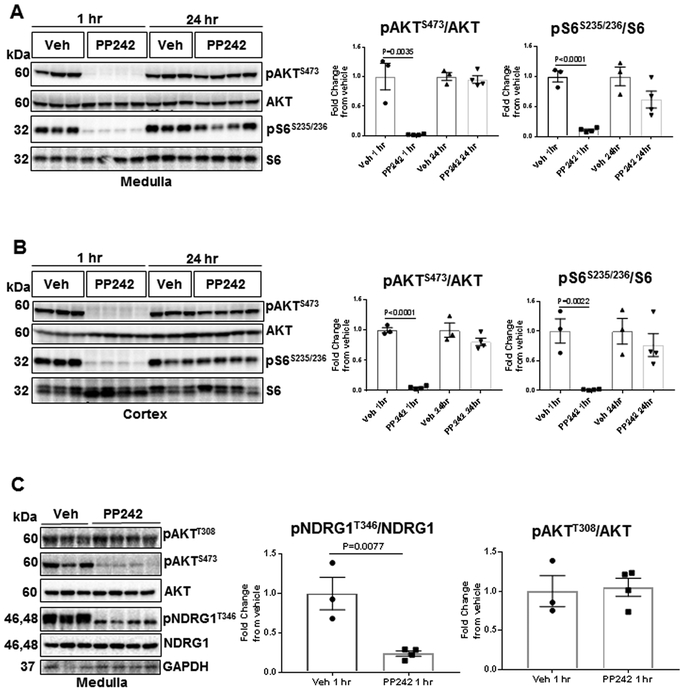
On day 21 of the high salt diet period the kidneys were removed for Western blot analysis of cortical and medullary tissue, 24 hr after the last PP242 injection. As seen in Figure 5A, pAKTS473/AKT expression in PP242 treated rats was not significantly changed in the renal medulla but tended to be suppressed in the renal cortex (Figure 5B; P=0.06). However, pS6S235/236/S6 was significantly reduced in both regions of the kidney (Figure 5C&D; P<0.05).
Figure 5:
(A-D) Western blots of pAKTS473 and pS6S235/236 were normalized with total endogenous AKT and S6 proteins, respectively. Graphs represent the intensity (A.U.) of pAKTS473/AKT or pS6S235/236/S6 in the cortex and medulla of SS rats treated with PP242 (open circle, n=6) or vehicle (black circle, n=6). Corresponding representative immunoblots are shown below each graph. Student’s t-test was used to compare PP242 and vehicle treatment.
PP242 inhibited short-term mTORC2 and SGK1 kinase activities in SS rats.
Studies in mice found that PP242 has short half-life and did not sustain the suppression of mTORC1/C2 past 4 hours11. To estimate PP242’s duration of drug action in SS rats, a comparison was made of the one hour effects versus 24 hour effects on inhibition of AKT and S6 phosphorylation after 15 mg/kg i.p. injection of PP242. As summarized in Figure 6A&B, phosphorylation of both AKT and S6 were strongly reduced in both cortical and medullary tissue one hour following treatment. At 24 hours, the inhibitory effect of PP242 on phosphorylation of AKT and S6 was no longer apparent in either the renal cortex or medulla.
Figure 6:
. (A&B) SS rats were injected with 15 mg/kg PP242 or vehicle and kidneys were harvested after 1 hr and 24 hr of treatment, respectively. Western blots of pAKTS473 and pS6S235/236 were normalized with total endogenous AKT and S6 proteins, respectively. (C) To determine inhibitory effect of PP242 on surrogate marker of SGK1 (pNDRG1T346/NDRG1), kidneys were excised 1 hr post-IP injection. Medullary tissue was used to asses pAKTT308, pAKTS473 and pNDRG1T346 as well as AKT, NDRG1 and GAPDH expression by Western blot analysis. In all panel graphs represent the fold change of pAKTS473/AKT or pS6S235/236/S6 or pNDRG1T346/NDRG1 or pAKTT308/AKT from vehicle in the cortex or medulla of these rats. Student’s t-test was used to compare PP242 and vehicle treatment.
Studies were also carried out to assess whether PP242 at the dose used in the present study actually resulted in a reduction of the mTORC2-SGK1 pathway as anticipated from published studies11. Since the antibodies available for SGK1 lacked appropriate sensitivity and specificity, effective antibodies for pNDRG1T346 and NDRG1 were used and measured pNDRG1T346/NDRG1 which is considered an acceptable surrogate marker of SGK1 activity18. As shown in Figure 6C, 1 hour following i.p. injection of PP242 (15 mg/kg) to SS rats, pNDRG1T346 expression was reduced in medulla. pAKTT308, which is upstream of mTORC2, was not reduced. These results indicate that the dose of PP242 used in the present studies was effective in reducing activity of SGK1 in the kidney which is a pathway related to tubular sodium transport.
Discussion.
Many of the underlying mechanisms responsible for naturally occurring forms of hypertension and salt-sensitivity remain obscure. Among the many physiological pathways that have been examined in efforts to elucidate underlying mechanisms responsible for naturally occurring forms of hypertension, the mTOR signaling pathways have been given little attention in this field. Our focus on the mTORC2 pathway in the present study was motivated by evidence that activation of this kinase have been found to regulate renal tubular sodium transporters including ENaC, NCC, and NKCC211–13, 19–21. It was logical therefore to ask whether this pathway could be involved in sodium homeostasis and salt-sensitive forms of hypertension since the role of mTORC2 in the regulation of cardiovascular function and more specifically in hypertension had not yet been elucidated.
Potent anti-hypertensive actions of chronic PP242 treatment.
The present results show that the combined inhibition of mTORC1 and mTORC2 with PP242 completely prevented salt-induced hypertension in SS rats. The antihypertensive effects were far more striking than was observed in our previous study with mTORC1 inhibition alone with rapamycin10. Furthermore, PP242 was also able to completely reverse both hypertension and renal injury when administered early in the development of the disease.
It is not yet possible to selectively inhibit only the mTORC2 pathway. Essential components of mTORC2 including Protor1/2, SGK1, and Rictor have been targeted in mouse models although the consequences on cardiovascular function and hypertension were not evaluated 11, 13, 18–22. One study evaluated the effects of a tissue specific Rictor knockout mouse (in the mouse brain and adipose tissue) and found only a 5 mmHg rise of blood pressure and reduced nocturnal dipping23.
In a rat model of DOCA-salt induced hypertension, increased levels of PI3K/Akt/mTOR were found in the kidney suggesting participation of the mTORC2 pathway24, but we have found no other hypertension related studies related to this pathway in mice or rats. Nor have cardiovascular or blood pressure effects of rapamycin or PP242 been evaluated in human subjects.
The degree of protection from hypertension that we have observed in SS rats with PP242 treatment exceeded what we and others have previously observed with antioxidant therapies25, 26, inhibition of the renin-angiotensin system27, or renal denervation28. The only comparable response was that which we observed with chronic renal medullary infusion of L-arginine into the remaining kidney of SS rats29. It is also notable that PP242 was also capable of completely reversing hypertension, renal injury, and immune cell infiltration when administered in the early stages of hypertension.
Natriuretic actions of PP242 contribute importantly to antihypertensive actions.
A number of studies that have found that the mTORC2 pathway is involved in the regulation of renal tubular sodium11, 12 and potassium transport13. Activated mTORC2 physically associates and phosphorylates serum-and glucocorticoid-induced protein kinase 1 (SGK1) at serine 422. This then provides a docking site for phosphatidylinositol-3 kinase (PDK1) which phosphorylates threonine at 256 within the activation loop of SGK1 for its full activation12. SGK1 phosphorylation of NEDD4–2 which binds, internalizes and degrades ENaC thereby modifying the actions of aldosterone in the cortical collecting ducts resulting in natriuresis30,31. The data is less consistent regarding K+ transport and kalliuresis11, 12, 20. Mice lacking mTORC2 activity in the distal tubule (Rictorfl/fl Ksp-Cre) exhibited reduced ROMK activity and developed hyperkalemia on a high K+ diet. This occurred despite a 10-fold increase in serum aldosterone levels with only a mild impairment of ENaC activity observed13. Our present observations in rats with PP242 treatment did not find kalliuresis which suggests that in SS rats the natriuretic effects of inhibition of SGK1 upon Na+ transport probably predominates over K+ channel effects and plays the dominant role in reducing blood pressure salt-sensitivity.
In the present study, PP242 tended to progressively reduce MAP even during the low salt control period which become most apparent on day four of treatment prior to switching to 4.0% high salt feeding. A nearly 3-fold increase of Na+ excretion with significant increases in the urine volume were observed with either intravenous or intrarenal administration of PP242. These natiruretic responses are similar to those observed by others in mice following intraperitoneal PP242 injections, although no signficant increases of urine volume were found 11. The robust natriuretic responses observed with PP242 treatment but not with the specific inhibition of mTORC1 with rapamycin indicates that the mTORC2 pathway plays an important role in salt-induced hypertension in SS rat. The data also indicate that the immediate natriuretic responses in responses to i.p. PP242 are relatively short in duration since when renal tissue expression of pAKTS473 and pS6S235/236 was determined one hour following administration of the drug, both pAKTS473 and pS6S235/236 were clearly reduced (Figure 6A&B). When analyzed 24 hours after i.p. PP242 administration expression of pAKTS473 and pS6S235/236 were no longer remained suppressed. Such short-term inhibitory responses are known to occur with commonly used diruretic agents such as furosemide which has a circulating half-life of only 2 hours but provides a sustained reduction of arterial pressure throughout the day. This because a potent natriuretic compound immediately reduces the extracellular fluid volume and many hours may be required to replete the system of sodium and water with normal eating and drinking.
PP242 contributes to reduction of renal inflammatory responses in SS rats.
To the extent that we could determine, PP242 totally prevented glomerular injury and infiltration of T-cell (CD3) and macrophage (CD68) cells in SS rats fed a high salt diet for 21 days. Lymphocyte infiltration was reduced to a greater extent than was previously observed with rapamycin treatment10. This was probably attributable to the control of arterial pressure since as we have previously shown by servo-control studies in SS rats, renal perfusion pressure is a major determinant of lymphocyte infiltration32. With chronic rapamycin treatment, hypertension was only partially blunted in SS rats10 and only partial reduction of immune cell infiltration although rapamycin is well recognized to reduce immune and inflammatory responses33. We have also shown that chronic rapamycin treatment reduced tissue phosphorylation of S6 in the kidneys of high salt fed SS rats10. It therefore appears that dual mechanisms are responsible for protection from renal injury with PP242 treatment, the reduction of renal perfusion pressure and a parallel inhibition of mTORC1 mediated inflammatory responses.
Conclusions.
It is evident from these studies that the dual inhibition of mTORC1 and mTORC2 with PP242 strongly protected SS rats from salt-induced hypertension and renal injury. The extent to which the antihypertensive effects of PP242 treatment were due to inhibition of mTORC1 versus mTORC2 could not be directly assessed given the absence of a specific mTORC2 inhibitor. However, comparisons of the antihypertensive effects that we have observed with rapamycin treatment alone compared to those of PP242 suggest that inhibition of mTORC2 alone was solely responsible for actions. PP242 acutely administered in the dose used in our chronic study produced an immediate and potent natriuretic response which was not observed with rapamycin. Sodium balance tended to remain negative despite a very high level salt in the diet. We and others have found that rapamycin alone produces an increase of arterial pressure and appears to blunt the hypertensive effects in SS rats fed a high salt diet largely by reducing renal inflammation and fibrotic injury10 whereas PP242 completely abolished these phenotypes in SS rats.
Perspective.
A rich body of literature about the ‘mechanistic target of rapamycin’ (mTOR) emerged since the discovery of these kinases in 2002 and it is well recognized that mTOR is a critical effector of cell cycle events/metabolism/growth/proliferation and signaling pathways commonly deregulated in human cancers. Several studies have found that mTOR pathways affect kidney growth, vesicle trafficking, podocyte homeostasis and tubular Na+ and K+ transport, all of which could potentially affect arterial pressure. The present study examined the role of mTOR (mTORC2) in sodium homeostasis and explored the therapeutic inhibition of these pathways on the development of salt-sensitive hypertension. The broad significance of the present study is as follows: First, inhibtion of mTORC2 with PP242 not only abolished but was able to completely reverse salt-induced hypertension in SS rats. Second, PP242 exhibited potent natriuretic and anti-inflammatory actions in SS rats. Together these data suggest that targetting mTORC2 signalling may provide a novel therapeutic target for hypertension, which has the advantage of simulataneously modulating several pathways which contribute to increased pressure, namely sodium homeostasis, renal inflammtion and renal injury. Studies will now be required to reveal the upstream regulator of mTORC2 in response to high salt diet feeding and test the ability of PP242 in other forms of hypertension.
Supplementary Material
Novelty and Significance.
-
What is new?
The first study to recognize that the mTORC2 pathway plays an important role in blood pressure salt-sensitivity in SS rats.
Systemic inhibition of mTORC1/2 with PP242 prevented and reversed the salt-induced hypertension and kidney injury in SS rats.
PP242 exhibited potent natriuretic and anti-inflammatory actions in SS rats.
-
What is relevant
It is apparent from this study that mTORC2 signaling contribute importantly to blood pressure salt-sensitivity and renal injury thereby justifying further exploration of the role of this pathways in other forms of experimental and human hypertension. .
Chronic treatment of PP242 compound not only prevented but was also able to reverse salt-induced hypertension and kidney injury in a clinically relevant model of hypertension.
-
Summary
The potent natriuretic actions of PP242 is a result of mTORC2 inhibition which appear to largely account for determining blood pressure salt-sensitivity aided by anti-inflammatory actions upon the inhibition of mTORC1 pathway in SS rats. Combined inhibition of these pathways represents a new avenue in the search for novel therapuetic targets for salt-sensitive hypertension in humans.
Acknowledgments
Source of Funding.
This work was supported by National Institiute of Health, National Institute of Heart, Lung and Blood grant HL-116264 (A.W. Cowley, Jr). LE was supported by an AHA Scientist Development Grant (17SDG33660574).
Footnotes
Disclosures.
The authors report no conflicts.
References
- 1.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: Analysis of worldwide data. Lancet. 2005;365:217–223 [DOI] [PubMed] [Google Scholar]
- 2.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27:481–490 [DOI] [PubMed] [Google Scholar]
- 3.Aviv A, Hollenberg NK, Weder A. Urinary potassium excretion and sodium sensitivity in blacks. Hypertension. 2004;43:707–713 [DOI] [PubMed] [Google Scholar]
- 4.Sullivan JM, Prewitt RL, Ratts TE. Sodium sensitivity in normotensive and borderline hypertensive humans. Am J Med Sci. 1988;295:370–377 [DOI] [PubMed] [Google Scholar]
- 5.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Stamler J. End-stage renal disease in african-american and white men. 16-year mrfit findings. JAMA. 1997;277:1293–1298 [PubMed] [Google Scholar]
- 6.Whittle JC, Whelton PK, Seidler AJ, Klag MJ. Does racial variation in risk factors explain black-white differences in the incidence of hypertensive end-stage renal disease? Arch. Intern. Med. 1991;151:1359–1364 [PubMed] [Google Scholar]
- 7.Kotchen TA, Cowley AW Jr., Frohlich ED. Salt in health and disease--a delicate balance. N Engl J Med. 2013;368:1229–1237 [DOI] [PubMed] [Google Scholar]
- 8.Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mtor pathway. Curr Opin Cell Biol. 2005;17:596–603 [DOI] [PubMed] [Google Scholar]
- 9.Saxton RA, Sabatini DM. Mtor signaling in growth, metabolism, and disease. Cell. 2017;168:960–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar V, Wollner C, Kurth T, Bukowy JD, Cowley AW Jr. Inhibition of mammalian target of rapamycin complex 1 attenuates salt-induced hypertension and kidney injury in dahl salt-sensitive rats. Hypertension. 2017;70:813–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gleason CE, Frindt G, Cheng CJ, Ng M, Kidwai A, Rashmi P, Lang F, Baum M, Palmer LG, Pearce D. Mtorc2 regulates renal tubule sodium uptake by promoting enac activity. J Clin Invest. 2015;125:117–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu M, Wang J, Jones KT, Ives HE, Feldman ME, Yao LJ, Shokat KM, Ashrafi K, Pearce D. Mtor complex-2 activates enac by phosphorylating sgk1. J Am Soc Nephrol. 2010;21:811–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grahammer F, Nesterov V, Ahmed A, Steinhardt F, Sandner L, Arnold F, Cordts T, Negrea S, Bertog M, Ruegg MA, Hall MN, Walz G, Korbmacher C, Artunc F, Huber TB. Mtorc2 critically regulates renal potassium handling. J Clin Invest. 2016;126:1773–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sengupta S, Lorente-Rodriguez A, Earnest S, Stippec S, Guo X, Trudgian DC, Mirzaei H, Cobb MH. Regulation of osr1 and the sodium, potassium, two chloride cotransporter by convergent signals. Proc Natl Acad Sci U S A. 2013;110:18826–18831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu K, Toral-Barza L, Shi C, Zhang WG, Lucas J, Shor B, Kim J, Verheijen J, Curran K, Malwitz DJ, Cole DC, Ellingboe J, Ayral-Kaloustian S, Mansour TS, Gibbons JJ, Abraham RT, Nowak P, Zask A. Biochemical, cellular, and in vivo activity of novel atp-competitive and selective inhibitors of the mammalian target of rapamycin. Cancer Res. 2009;69:6232–6240 [DOI] [PubMed] [Google Scholar]
- 16.Evans LC, Ivy JR, Wyrwoll C, McNairn JA, Menzies RI, Christensen TH, Al-Dujaili EA, Kenyon CJ, Mullins JJ, Seckl JR, Holmes MC, Bailey MA. Conditional deletion of hsd11b2 in the brain causes salt appetite and hypertension. Circulation. 2016;133:1360–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor NE, Cowley AW Jr. Effect of renal medullary h2o2 on salt-induced hypertension and renal injury. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1573–1579 [DOI] [PubMed] [Google Scholar]
- 18.Pearce LR, Sommer EM, Sakamoto K, Wullschleger S, Alessi DR. Protor-1 is required for efficient mtorc2-mediated activation of sgk1 in the kidney. Biochem. J. 2011;436:169–179 [DOI] [PubMed] [Google Scholar]
- 19.Fantus D, Rogers NM, Grahammer F, Huber TB, Thomson AW. Roles of mtor complexes in the kidney: Implications for renal disease and transplantation. Nat Rev Nephrol. 2016;12:587–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faresse N, Lagnaz D, Debonneville A, Ismailji A, Maillard M, Fejes-Toth G, Naray-Fejes-Toth A, Staub O. Inducible kidney-specific sgk1 knockout mice show a salt-losing phenotype. Am J Physiol Renal Physiol. 2012;302:F977–985 [DOI] [PubMed] [Google Scholar]
- 21.Lang F, Pearce D. Regulation of the epithelial na+ channel by the mtorc2/sgk1 pathway. Nephrol Dial Transplant. 2016;31:200–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zschiedrich S, Bork T, Liang W, Wanner N, Eulenbruch K, Munder S, Hartleben B, Kretz O, Gerber S, Simons M, Viau A, Burtin M, Wei C, Reiser J, Herbach N, Rastaldi MP, Cohen CD, Tharaux PL, Terzi F, Walz G, Godel M, Huber TB. Targeting mtor signaling can prevent the progression of fsgs. J. Am. Soc. Nephrol. 2017;28:2144–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dragert K, Bhattacharya I, Pellegrini G, Seebeck P, Azzi A, Brown SA, Georgiopoulou S, Held U, Blyszczuk P, Arras M, Humar R, Hall MN, Battegay E, Haas E. Deletion of rictor in brain and fat alters peripheral clock gene expression and increases blood pressure. Hypertension. 2015;66:332–339 [DOI] [PubMed] [Google Scholar]
- 24.Ma SK, Choi JS, Joo SY, Kim HY, Kim CS, Bae EH, Lee JU, Kim SW. Activation of the renal pi3k/akt/mtor signaling pathway in a doca-salt model of hypertension. Chonnam Med J. 2012;48:150–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: Nadph oxidases as therapeutic targets. Nat Rev Drug Discov. 2011;10:453–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chockalingam A, Campbell NR, Fodor JG. Worldwide epidemic of hypertension. Can. J. Cardiol. 2006;22:553–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tojo A, Onozato ML, Kobayashi N, Goto A, Matsuoka H, Fujita T. Angiotensin ii and oxidative stress in dahl salt-sensitive rat with heart failure. Hypertension. 2002;40:834–839 [DOI] [PubMed] [Google Scholar]
- 28.Peleli M, Flacker P, Zhuge Z, Gomez C, Wheelock CE, Persson AEG, Carlstrom M. Renal denervation attenuates hypertension and renal dysfunction in a model of cardiovascular and renal disease, which is associated with reduced nadph and xanthine oxidase activity. Redox biology. 2017;13:522–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyata N, Zou AP, Mattson DL, Cowley AW Jr. Renal medullary interstitial infusion of l-arginine prevents hypertension in dahl salt-sensitive rats. Am. J. Physiol. 1998;275:R1667–1673 [DOI] [PubMed] [Google Scholar]
- 30.Mistry AC, Wynne BM, Yu L, Tomilin V, Yue Q, Zhou Y, Al-Khalili O, Mallick R, Cai H, Alli AA, Ko B, Mattheyses A, Bao HF, Pochynyuk O, Theilig F, Eaton DC, Hoover RS. The sodium chloride cotransporter (ncc) and epithelial sodium channel (enac) associate. Biochem. J. 2016;473:3237–3252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Martinez JM, Alessi DR. Mtor complex 2 (mtorc2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (sgk1). Biochem. J. 2008;416:375–385 [DOI] [PubMed] [Google Scholar]
- 32.Evans LC, Petrova G, Kurth T, Yang C, Bukowy JD, Mattson DL, Cowley AW Jr. Increased perfusion pressure drives renal t-cell infiltration in the dahl salt-sensitive rat. Hypertension. 2017;70:543–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J, Kim SG, Blenis J. Rapamycin: One drug, many effects. Cell Metab. 2014;19:373–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.