Abstract
Background.
Stress is associated with cognitive and emotional dysfunction, and increases risk for a variety of psychological disorders, including depression and posttraumatic stress disorder. Prefrontal cortex is critical for executive function and emotion regulation, is a target for stress hormones, and is implicated in many stress-influenced psychological disorders. Extinction of conditioned fear provides an excellent model system for examining how stress-induced changes in corticolimbic structure and function are related to stress-induced changes in neural function and behavior, as the neural circuitry underlying this behavior is well characterized.
Objectives.
This review examines how acute and chronic stress influence extinction and describes how stress alters the structure and function of the medial prefrontal cortex, a potential neural substrate for these effects. In addition, we identify important unanswered questions about how stress-induced change in prefrontal cortex may mediate extinction deficits and avenues for future research.
Key Findings.
A substantial body of work demonstrates deficits in extinction after either acute or chronic stress. A separate and substantial literature demonstrates stress-induced neuronal remodeling in medial prefrontal cortex, along with several key neurohormonal contributors to this remodeling, and there is substantial overlap in prefrontal mechanisms underlying extinction and the mechanisms implicated in stress-induced dysfunction of—and neuronal remodeling in—medial prefrontal cortex. However, data directly examining the contribution of changes in prefrontal structure and function to stress-induced extinction deficits is currently lacking.
Conclusions.
Understanding how stress influences extinction and its neural substrates as well as individual differences in this effect will elucidate potential avenues for novel interventions for stress-sensitive disorders characterized by deficits in extinction.
Keywords: Chronic stress, infralimbic cortex, prelimbic cortex, extinction
Stress, Psychopathology, and Extinction
Stressful experiences, which broadly can be considered to be challenges to an organism’s wellbeing, homeostasis—or, in the more modern formulation, allostasis (McEwen 2017)—can vary in their intensity, duration, and chronicity. Further, cognitive processing of potentially stressful events can vary across individuals. Thus, it is not surprising that such challenges can have a range of effects, varying from beneficial to detrimental (Sapolsky 2003). Nonetheless, excessive, chronic, or repeated stress can produce cognitive and emotional dysfunction. For instance, stressful life events play a role in precipitating episodes of major depression, and traumatic stressors can trigger posttraumatic stress disorder (Lechin et al. 1996; Turner and Lloyd 2004). Furthermore, risk for mood and anxiety disorders increases with repeated exposure to stress (Risch et al. 2009). Such chronic stressors have adverse effects on many behaviors in the absence of overt psychopathology. For instance, several studies have demonstrated stress-induced deficits on a variety of cognitive and emotion-regulation tasks, including extinction of conditioned fear, attentional set-shifting, spatial learning and recognition, and working memory in animal models (reviewed in Conrad et al. 2017; Hurtubise and Howland 2017; Maren and Holmes 2016).
A Brief Overview of Extinction Circuitry
Extinction of conditioned fear provides an excellent model system for examining how stress-induced changes in corticolimbic structure and function are related to stress-induced changes in behavior, as the neural circuitry underlying this behavior is well characterized, and involves interactions among the medial prefrontal cortex, hippocampus, and basolateral amygdala (Figure 1). During fear conditioning, an animal, typically a rat or mouse, is placed in a chamber and acquires a learned fear response to a neutral stimulus, such as a tone, that is presented in the chamber and paired with an aversive unconditioned stimulus (US), such as a footshock (Figure 2). Repeated pairings of the tone with the footshock result in a conditioned fear response to the tone, now a conditioned stimulus (CS). In rodent models, the animal’s freezing during the tone, defined as the absence of all movement except that due to breathing, is a common measure of the conditioned fear response (CR). After time for consolidation of the fear memory, the animal is then presented with the CS in the absence of the US. Repeated presentation of the CS alone results in extinction of the conditioned fear response—the animal learns that the tone no longer predicts the footshock, and no longer freezes in response to presentation of the tone (Orsini and Maren 2012; Quirk and Mueller 2007). Memory for extinction can be measured by presentation of the CS on subsequent days. High levels of freezing during the CS indicate poor retention or retrieval of the extinction memory.
Figure 1.
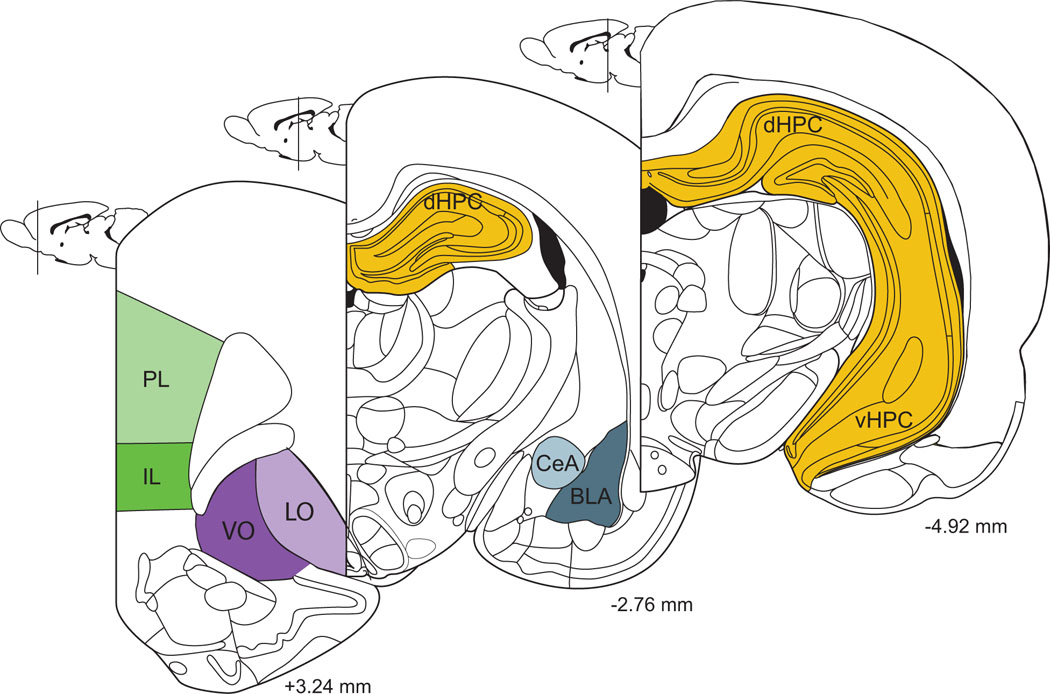
Schematic diagram of coronal sections through the forebrain, with brain regions likely to play a role in stress effects on extinction identified. Coordinates given are relative to Bregma in rat brain. PL, prelimbic; IL, infralimbic; VO, ventral orbitofrontal; LO, Lateral Orbitofrontal; dHPC, dorsal hippocampus; vHPC, ventral hippocampus; CeA, central amygdala; BLA, basolateral amygdala. Adapted from Paxinos and Watson (1998).
Figure 2.
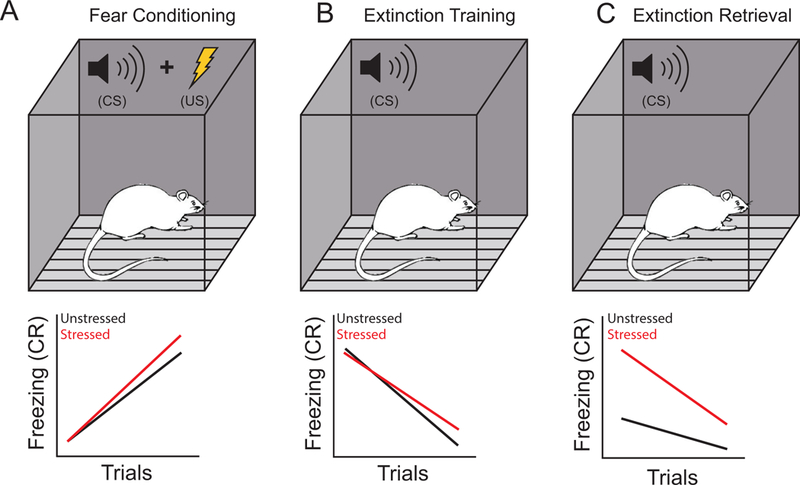
Stress effects on fear conditioning and extinction. A. Above. During fear conditioning, repeated pairings of a conditioned stimulus (CS) such as a tone with an unconditioned stimulus (US) such as a footshock come to elicit a conditioned fear response to the tone. Below. Prior chronic stress may increase the conditioned fear response (CR; often measured as freezing during tone presentation) during conditioning. This effect is more likely with more intense stressors and/or fewer habituation trials prior conditioning. B. Above. During extinction training (or acquisition), the CS is presented in the absence of the US. Below. Prior chronic stress may impair extinction acquisition; this effect varies across studies. C. Above. Extinction retrieval testing typically occurs approximately 24 hours after acquisition of extinction, and consists of CS-alone presentations. Below. Prior chronic stress produces robust impairments of extinction retrieval.
Interactions among the medial prefrontal cortex, hippocampus, and amygdala underlie fear conditioning and extinction. The basolateral amygdala is a key site of convergence for unconditioned and conditioned stimuli, a critical requirement for the neuroplasticity necessary for learning of fear conditioning (Maren et al. 2001), and the acquisition of fear conditioning is mediated by amygdaloid nuclei (reviewed in McCullough et al. 2016). The basolateral amygdala is also involved in mediating the initial acquisition of extinction, as either temporary inactivation (Akirav et al. 2006) or blockade of glutamatergic transmission (Herry et al. 2006; Sotres-Bayon et al. 2007) in basolateral amygdala prevents or attenuates extinction. Thus, the basolateral amygdala plays a critical role in the acquisition of conditioned fear.
As reviewed extensively elsewhere (e.g., Maren and Holmes 2016; Tronson et al. 2012) and in this special issue, medial prefrontal cortex contributes to both expression and extinction of conditioned fear. The medial prefrontal cortex is composed of several functionally and neuroanatomically distinct subregions, including the prelimbic and infralimbic cortices, which appear to play opposing roles in the modulation of conditioned fear (Figure 2). For instance,. C-Fos expression in prelimbic cortex is increased after retrieval of the conditioned fear memory (e.g., Knapska and Maren 2009), and neural activity in prelimbic cortex is associated with freezing during extinction (Burgos-Robles et al. 2009). Temporary inactivation of prelimbic cortex during extinction disrupts conditioned fear expression (Corcoran and Quirk 2007), while stimulation of prelimbic cortex during extinction increases fear expression and slows extinction of the fear memory (Vidal-Gonzalez et al. 2006). More recent studies have used optogenetic techniques to demonstrate that silencing of projections from the prelimbic cortex to the basolateral amygdala reduces expression of recently acquired fear CRs (Do-Monte et al. 2015b). Together, these studies indicate that the prelimbic cortex is involved in the expression of conditioned fear.
On the other hand, the infralimbic cortex appears to play a role in extinction. Rats with lesions of the infralimbic cortex exhibit normal acquisition of fear conditioning and initial extinction, but deficits in the ability to retrieve extinction memory (Quirk et al. 2000). Likewise, electrical stimulation (Milad et al. 2004), pharmacological activation (Chang and Maren 2011), or optogenetic activation (Do-Monte et al. 2015a) of the infralimbic cortex during extinction training facilitates extinction retrieval. Conversely, pharmacological (Burgos-Robles et al. 2007) or optogenetic blockade (Do-Monte et al. 2015a) of activity in the infralimbic cortex impairs extinction retrieval. Further, extinction learning potentiates excitability of infralimbic neurons that project to the basolateral amygdala (Bloodgood et al. 2018), and neurons in the infralimbic cortex increase firing rates in response to the CS during extinction retrieval (Milad and Quirk 2002). Finally, chemogenetic inhibition of prefrontal projections to basolateral amygdala impairs extinction (Bloodgood et al. 2018). Taken together, such evidence suggests that the infralimbic cortex is necessary for the consolidation of extinction. Thus, there is regional specificity in the involvement of medial prefrontal cortex in the modulation of fear conditioning and extinction: prelimbic cortex facilitates fear expression while infralimbic cortex is involved in fear extinction or suppression.
Importantly, extinction appears to involve the formation of a new, “CS-no US” memory, which is highly dependent on the context in which extinction occurs. For instance, an extinguished CR will reappear, or renew, when the CS is presented in a different context than that in which the extinction learning occurred (Bouton and Ricker 1994). This context dependence is mediated by the hippocampus, and extinction learning potentiates the hippocampus-to-medial prefrontal cortex pathway (reviewed in Bouton et al. 2006).
Links Among Stress, Extinction, and Psychopathology
Variations in the ability to acquire, consolidate, and/or retrieve an extinction memory could contribute to stress-sensitive disorders such as post-traumatic stress disorder (PTSD; Holmes and Singewald 2013; Milad et al. 2009). Patients with PTSD have impairments in both the ability to extinguish an aversive CR (Orr et al. 2000; Peri et al. 2000) and later retrieval of the extinction memory (Milad et al. 2009). These extinction deficits could contribute to the persistence of traumatic memories in the absence of the trauma-inducing stimulus, a hallmark of PTSD (Holmes and Singewald 2013; Yehuda and LeDoux 2007). While PTSD is perhaps the best-known example of a disorder characterized by impaired fear extinction, other stress-sensitive disorders such as schizophrenia feature impaired fear extinction (Holt et al. 2009). Furthermore, aberrant processes relevant to extinction have been implicated in relapse to drug-seeking after abstinence has been achieved (Khoo et al. 2017). Thus, extinction could perhaps be conceptualized as an example of behavioral or cognitive flexibility, which is impaired in a variety of stress-sensitive disorders.
In this paper, we review how acute and chronic stress influence extinction in animal models, focusing on the effects of stress in adulthood. We then describe how stress alters a potential neural substrate for these effects, the medial prefrontal cortex. We conclude by identifying important unanswered questions and avenues for future research.
Effects of Chronic Stress on Extinction and its Neural Substrates
Effects of Chronic Stress on Extinction
In male rats, the acquisition of either contextual or cued fear conditioning can be enhanced with prior exposure to chronic restraint stress, with stressed males freezing more to either CS or context than unstressed males (e.g., Conrad et al. 1999b; Conrad et al. 2001; Farrell et al. 2010; Hoffman et al. 2015; Sanders et al. 2010). Chronic exposure to glucocorticoids at levels similar to those produced by chronic stress also facilitates acquisition of fear conditioning (Conrad et al. 2004).
Others have demonstrated that stress impairs extinction (see Table 1). For instance, one week of daily restraint stress impairs retrieval of extinction of conditioned fear in the absence of a significant facilitation of fear learning (Miracle et al. 2006; Wilber et al. 2011). Subsequent studies have shown that one week of unpredictable mild stress produced similarly specific changes in extinction retrieval (Garcia et al. 2008), as does a longer-term stressor (6 hr of daily restraint stress for 3 weeks; Baran et al. 2009). Application of 2 h of restraint, 20 min of forced swim, and ether exposure in rapid succession on the same day (the “Single Prolonged Stressor” model) likewise impairs extinction acquisition and retrieval 7 days later while not significantly altering fear learning (Knox et al. 2012a; Knox et al. 2012b; Knox et al. 2010). In mice, just 3 episodes of 10-min swim stress before fear conditioning attenuated the rate of extinction, though the experimental design did not allow for differentiation between extinction acquisition and extinction retrieval (Izquierdo et al. 2006). Note that in all of these studies, stress exposure occurred prior to fear conditioning. Thus, the possibility that the apparent impairment of extinction reflected a facilitation of conditioning, and thus increased resistance to extinction, cannot be completely ruled out. However, the interpretation of these results as impaired extinction is strengthened by findings that the deficit in extinction remains when either unstressed and stressed groups are matched for initial fear learning (Goswami et al. 2010; Miracle et al. 2006) or freezing during initial extinction is used as a covariate (Goswami et al. 2010; though note that this effect was in PTSD-susceptible Lewis rats). Furthermore, a study using extensive habituation trials (presentations of tone alone prior to fear conditioning; Baran et al. 2009) found deficits in extinction retrieval in restraint-stressed rats, despite similar fear memory acquisition between unstressed and stressed rats. Importantly, extinction-naïve stressed rats showed comparable freezing to tone when tested one day later. This pattern of results suggests that differences between stressed and unstressed rats during testing for extinction retrieval do indeed reflect deficits in the extinction memory. Subsequent studies have demonstrated that an acute platform stressor applied after acquisition of conditioned fear impairs extinction (Maroun et al. 2013; Schayek and Maroun 2015). While these studies eliminate the possibility of stress-induced facilitation of acquisition, the experimental design, in which stress was applied immediately after a probe for fear retrieval, leaves open the possibility that stress strengthened memory reconsolidation, thus promoting fear during extinction training. Nonetheless, taken together, these results strongly suggest that prior chronic stress impairs extinction retrieval. However, future studies might benefit from experimental designs that more clearly isolate stress effects to extinction, via interposing episodes of either acute or chronic stress between conditioning and extinction, with appropriate delays between testing for fear memory, initiating stressor exposure, and subsequent extinction trials.
Table 1.
Studies of the effects of stress on extinction of conditioned fear, indicating stressor type, chronicity, timing relative to testing of fear conditioning and extinction, and the direction of effects on fear conditioning and extinction
| Study | Species | Sex | Stressor | Duration | Timing | Effect |
|---|---|---|---|---|---|---|
| Miracle et al. 2006 | Rat | Male | 3 h daily restraint | 7 days | prior to conditioning | ↔ fc ↔ ext acq ↓ ext ret |
| Izquierdo et al. 2006 | Mouse | Male | 10 min daily swim | 3 days | prior to conditioning | ↔ fc ↔ ext acq/ret |
| Garcia et al. 2008 | Rat | Male | chronic mild | 21 days | prior to conditioning | ↔ fc ↔ ext acq ↓ ext ret |
| Baran et al. 2009 | Rat | Male | 6 h daily restraint | 21 days | prior to conditioning | ↔ fc ↔ ext acq ↓ ext ret |
| Baran et al. 2009 | Rat | Female | 6 h daily restraint | 21 days | prior to conditioning | ↓ f ret |
| Farrell et al. 2010 | Rat | Male | 3 h daily restraint | 7 days | prior to conditioning | ↑ fc ↓ext acq ↓ext ret |
| Goswami et al. 2010 | Rat | Male | 10 min predator odor | 1 day | prior to conditioning | ↔ fc ↓ext acq ↓ext ret |
| Knox et al. 2010 | Rat | Male | single prolonged | 1 day | prior to conditioning | ↔ fc ↔ ext acq ↓ ext ret |
| Wilber et al. 2011 | Rat | Male | 3 h daily restraint | 7 days | prior to conditioning | ↔ fc ↔ ext acq ↓ ext ret |
| Knox et al. 2012a | Rat | Male | single prolonged | 1 day | prior to conditioning | ↔ fc ↔ ext acq ↓ ext ret |
| Knox et al. 2012b | Rat | Male | single prolonged | 1 day | prior to conditioning | ↔ fc ↔ ext acq ↓ ext ret |
| Maroun et al. 2013 | Rat | Male | 20 min elevated platform | 1 day | after fear retention testing | fc N.A. ↓ext acq ↓ext ret |
| Hoffman et al. 2015 | Rat | Male | 6 h daily restraint | 21 days | prior to conditioning | ↑ fc ↓ recon dis |
| Schayek & Maroun 2015 | Rat | Male | 20 min elevated platform | 1 day | after fear retention testing | fc N.A. ↓ext acq ↓ext ret |
, no change;
impaired;
facilitated; fc, fear conditioning; f ret, fear retrieval; ext acq, extinction acquisition; ext ret, extinction retrieval
Effects of Chronic Stress on Medial Prefrontal Cortex
Stress-induced changes in the neural substrates of extinction are well documented. For instance, stress-induced changes in the medial prefrontal cortex appear to be responsible for the stress-induced increase in freezing during extinction memory testing, as removal of the infralimbic cortex before the chronic stressor occludes the stress-induced impairment of extinction retrieval without altering the effect of stress on initial fear learning (Farrell et al. 2010; though this study could not rule out the possibility that the occlusion might be due to effects upstream of infralimbic cortex). Further, in male rats, stress-induced alterations in fear conditioning and extinction are associated with stress-induced changes in neurophysiology. For instance, the deficit in extinction retrieval induced by 7 days of prior chronic restraint stress is accompanied by alterations in activity of infralimbic cortex neurons (Wilber et al. 2011). In prelimbic cortex, stress prevented the tone-evoked inhibition of activity seen in unstressed rats. In infralimbic cortex, neurons in unstressed rats exhibited increased firing rate in response to the CS, whereas in stressed rats, this tone-related increase in firing was absent. In addition, chronic restraint stress impaired induction of LTP in the hippocampus-to-medial prefrontal cortex pathway (Cerqueira et al. 2007a). Given that hippocampal projections to prefrontal cortex play a critical role in extinction learning (Knapska et al. 2012; Knapska and Maren 2009), this impaired plasticity could have important contributions to the stress-induced impairment of extinction. Indeed, low-frequency stimulation of the hippocampus-to-medial prefrontal cortex pathway after extinction acquisition blocks potentiation of the pathway and impairs extinction retrieval (Garcia et al. 2008). Similarly, even an acute elevated platform stressor impairs the induction of long-term potentiation in the amygdala-to-mPFC pathway (Maroun and Richter-Levin 2003), and application of the Single Prolonged Stressor model decreases functional connectivity of the ventral medial prefrontal cortex and the basolateral amygdala, as assessed with expression of the immediate early gene c-Jun during extinction retrieval (Knox et al. 2018). Taken together, these studies provide support for the notion that stress-induced alterations in the medial prefrontal cortex contribute to deficits in extinction.
Potential Prefrontal Substrates of Stress-Induced Deficits in Extinction: Morphology
The medial prefrontal cortex is a target for hormones involved in the stress response, such as corticosterone (Meaney and Aitken 1985). Stress-induced alterations in neuronal morphology are perhaps the best-documented effects of stress on prefrontal cortex (Figure 3). In male rats, chronic restraint stress produces retraction of apical dendrites of pyramidal neurons in the prelimbic cortex (Cook and Wellman 2004; Garrett and Wellman 2009; Liston et al. 2006; Martin and Wellman 2011; Radley et al. 2005; Radley et al. 2006; Radley et al. 2004), an effect that is mimicked with chronic corticosterone administration (Cerqueira et al. 2005; Cerqueira et al. 2007b; Wellman 2001). A similar pattern of stress-induced retraction is seen in apical dendritic branches of neurons within the infralimbic region of medial prefrontal cortex (Izquierdo et al. 2006; Moench et al. 2016; Shansky et al. 2009). Finally, even shorter, milder episodes of stress are sufficient to produce dendritic atrophy in medial prefrontal cortex: 10 minutes of restraint stress for 10 days (Brown et al. 2005) or 3 weeks of vehicle injection alone (Wellman 2001) reduce dendritic arborization within the medial prefrontal cortex, again with retraction occurring only in distal portions of the apical arbor. The medial prefrontal cortex appears to be particularly sensitive to even acute stress, as a single episode of forced swimming in mice (Izquierdo et al. 2006) or exposure to an elevated platform stressor in rats (Moench et al. 2016) produces reductions in apical dendritic branch length in the infralimbic cortex. Given the relationship between dendritic structure, dendritic spines and synaptic input, and neural firing rates (e.g., Rall et al. 1992; Spruston 2008), along with functional distinctions across dendritic compartments (e.g., apical versus basilar, distal tufts versus proximal trunk; Gordon et al. 2006; Han and Heinemann 2013) and differential inputs across dendritic compartments (e.g., Groenewegen 1988; Swanson and Cowan 1977; Urban-Ciecko and Barth 2016) these stress-induced structural alterations, with their specificity to the apical dendrite and their distinction spatial distribution within the apical arbor, may have important functional implications. Consistent with this notion, in stressed rats, apical dendritic branch length and number are strongly and negatively correlated with freezing during extinction retrieval (Moench et al. 2016).
Figure 3.
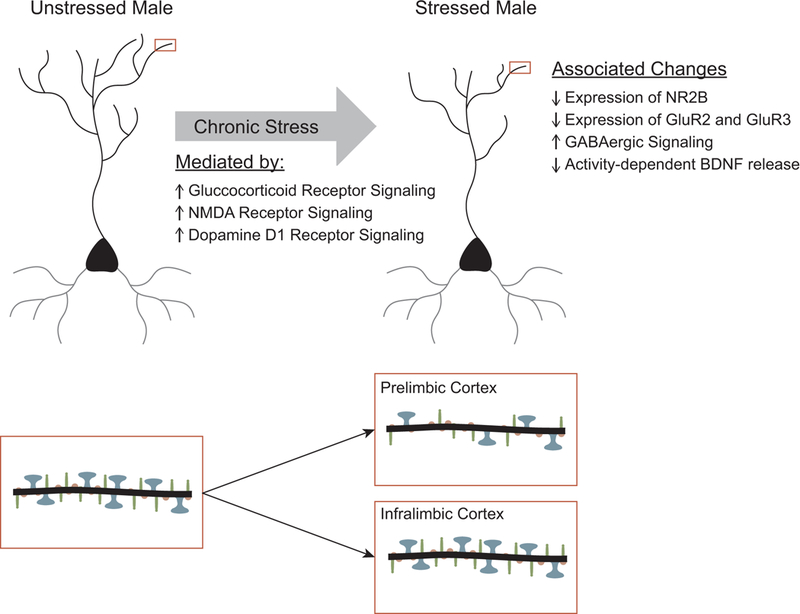
Summary of chronic stress-induced neuronal remodeling in medial prefrontal cortex. Above. Chronic stress produces retraction of apical dendrites of pyramidal neurons in both prelimbic and infralimbic cortex. Blockade of either glucocorticoid, NMDA, or D1 receptors prevents the stress-induced dendritic remodeling. Below. Chronic stressors decrease spine density in prelimbic cortex and decrease the ratio of mushroom (blue, large heads) to thin spines (red, small heads). The effects of stress on spine density in infralimbic cortex is less studied, but may be less robust.
Dendritic spines are the major sites of excitatory inputs onto prefrontal pyramidal cells; thus, stress-induced alterations in dendritic spine density could also contribute to extinction deficits. Indeed, stress- and corticosterone-induced dendritic retraction is coupled with a decrease in spine density in the anterior cingulate and prelimbic cortices (Barfield et al. 2017; Hains et al. 2009; Liu and Aghajanian 2008; Radley et al. 2006; Radley et al. 2008; Swanson et al. 2013). Importantly, this decrease in spine density correlates with impairment of working memory, a cognitive function mediated by dorsal medial prefrontal cortex, and prevention of the stress-induced spine loss via inhibition of protein kinase C prevents the working memory deficit (Hains et al. 2009).
Alternatively, variations in spine morphology are thought to reflect differences in function. For instance, larger, mushroom-type spines are perhaps more mature, less labile, so-called “memory spines,” whereas thin spines are thought to be more plastic (Segal 2017). Accordingly, numerous alterations in dendritic spine morphology after acute or chronic stress have been reported. For instance, a shift in spine morphology from large mushroom spines to smaller thin spines, and thus an overall reduction in spine volume and surface area, has been demonstrated in the dorsal medial prefrontal cortex following either three weeks of daily restraint stress (Barfield et al. 2017; Radley et al. 2008; though cf effects of chronic corticosterone administration, ) or two weeks of chronic variable stress (Radley et al. 2013).
On the other hand, the effects of stress and glucocorticoids on dendritic spines in infralimbic cortex are less clear. In mice, administration of corticosterone via drinking water at a dose that produces restraint-stress levels of corticosterone in plasma significantly reduced dendritic spine density on apical branches of deep-layer pyramidal cells in infralimbic cortex. However, chronic restraint stress (6 h/day for 3 weeks) did not significantly alter spine density in infralimbic cortex of rats (Shansky et al. 2009). These disparate results may reflect sustained high levels of corticosterone in the former study versus habituation to restraint in the later, which suggests that subregions of the medial prefrontal cortex may be differentially sensitive to stress. However, alterations in spine morphology or density accompanying sub-chronic or acute stress may nonetheless contribute to stress-induced deficits in extinction, as density of thin spines and estimates of total numbers of spines on apical terminal branches are strongly negatively correlated with freezing during extinction in rats exposed to one episode of elevated platform stress (Moench et al. 2016). Important areas for future research include understanding how subregion-specific stress-induced neuronal remodeling influences the physiology of the medial prefrontal cortex and related circuitry; and how these influences are expressed in prefrontally mediated processes such as extinction.
Potential Prefrontal Substrates of Stress-Induced Deficits in Extinction: Pharmacology
NMDA receptor-mediated glutamatergic activity in medial prefrontal cortex is critical for the consolidation of extinction. For instance, the frequency of NMDA receptor-dependent burst firing in the infralimbic cortex during extinction acquisition associates strongly with retrieval of extinction the following day (Burgos-Robles et al. 2007); and suppression of NMDA receptor-dependent bursting via infusion of an NMDA receptor antagonist into the infralimbic cortex either during extinction learning or immediately after training trials impairs later retrieval of extinction (Burgos-Robles et al. 2007; Fiorenza et al. 2012). Conversely, infusion of an NMDA receptor agonist facilitates extinction (Fiorenza et al. 2012). Finally, Vieira and colleagues (2015) used a viral construct to locally delete the obligate NR1 subunit of the NMDA receptor on CAMKIIα-expressing neurons in ventral medial prefrontal cortex to demonstrate that NMDA receptors on excitatory neurons in medial prefrontal cortex are critical for extinction. Therefore, alteration in the glutamatergic system could provide the substrate underlying stress-induced alterations in extinction.
Consistent with this hypothesis, stress-induced changes in neuronal morphology and function are accompanied by pronounced alterations in the prefrontal glutamatergic system. For instance, two weeks of exposure to stress levels of corticosterone administered via drinking water markedly decreased expression of the NR2B subunit of the NMDA receptor and the GluR2 and 3 subunits of the AMPA receptor in ventral medial prefrontal cortex (comprised of infralimbic cortex and ventral portions of prelimbic cortex), and reduced expression was associated with impaired extinction of contextual fear (Gourley et al. 2009). Likewise, seven days of daily restraint or variable stress altered expression of the NR1, NR2A, and NR2B subunits of the NMDA receptor and the GluR1 and GluR1 subunits of the AMPA receptor in adolescent (1-month-old) rats, and impaired AMPA- and NMDA-receptor mediated neuronal excitability in medial prefrontal cortex (Wei et al. 2014; Yuen et al. 2012). Similar stress-induced decreases in glutamatergic receptor expression and transmission have been reported in medial prefrontal cortex of adult rats and mice (Jett et al. 2017; Shepard and Coutellier 2018). Given the well-documented role for NMDA receptor-dependent activity in prefrontal cortex in extinction (Burgos-Robles et al. 2007; Santini et al. 2001; Vieira et al. 2015), it is likely that this stress-induced dysfunction of the prefrontal glutamatergic system contributes to the stress-induced impairment of extinction.
Interestingly, changes in the glutamatergic system may be driven in part by stress-induced increases in GABAergic inhibition of pyramidal cells. Using patch-clamp recordings, Herman and colleagues (McKlveen et al. 2016) recently demonstrated that 2 weeks of chronic variable stress (rotation on a platform orbital shaker, warm swim, cold swim, cold exposure, brief hypoxia, overnight social isolation, overnight social crowding) produced increased miniature inhibitory postsynaptic currents in pyramidal neurons in infralimbic cortex, an effect that was blocked by the application of a GABAA receptor antagonist. This increased inhibition may have been due to increased GABAergic innervation of the pyramidal cells, as the number of Gad65-positive puncta on CAMKII-positive cells was markedly increased in rats subjected to chronic variable stress (McKlveen et al. 2016).
Potential Mechanisms of Chronic Stress-Induced Alterations in mPFC and Extinction Deficits
Surprisingly little is known about the mechanisms underlying the chronic stress-induced alterations that lead to extinction deficits. To our knowledge, no studies to date have attempted to prevent stress-induced extinction deficits via manipulations during the application of the stressor. On the other hand, a growing body of literature has begun to elucidate the mechanisms underlying stress-induced changes structure and function in medial prefrontal cortex, providing potential clues to the origins of the stress-induced extinction deficit. For instance, systemic administration of the glucocorticoid receptor blocker RU38486 during restraint prevents the apical dendritic retraction and spine loss resulting from 10 days of daily restraint (Liu and Aghajanian 2008). This is consistent with the finding that, in vitro, glucocorticoid receptors mediate the stress-induced decreases in functional glutamatergic receptors and impairment of glutamatergic transmission in medial prefrontal cortex (Yuen et al. 2012). However, it is not known whether, in vivo, glucocorticoid receptors contribute to stress-induced dendritic retraction via direct actions in prefrontal cortex, or by modulation of inputs to prefrontal cortex.
In addition, systemic administration of a competitive NMDA receptor blocker during daily restraint stress also prevents stress-induced dendritic retraction in medial prefrontal cortex (Martin and Wellman 2011). Again, this could be due either to a direct effect in mPFC or alteration of inputs to mPFC. Although unfortunately the aforementioned study did not assess spine density, a small literature implicates NMDARs and AMPA receptors (AMPARs) in chronic stress-induced loss of spines. For instance, a single dose of either the NMDAR blocker ketamine or the specific NMDAR 2B blocker Ro25–6981 following 21 days of chronic unpredictable mild stress (including cold, disruption of light-dark cycle, crowding, shaking, and exposure to an aversive odor) rescues spines, particularly in layer V of PL (Li et al. 2011). This effect involved activation of the mTOR (mammalian target of rapamaycin) signaling pathway, as the beneficial effect of NMDAR blockade was prevented by administration of rapamycin. Additionally, NMDAR- and AMPAR-excitatory post-synaptic currents (EPSCs) are reduced following 5 or 7 days, but not 1 or 3 days, of repeated behavioral stressors (Li et al. 2011; Yuen et al. 2012). Further examination of the mechanisms underlying decreased activity at these glutamatergic receptors demonstrated that the reduction in EPSC amplitude is dependent on ubiquitin/proteasome degradation of GluR1 and NR1 subunits (Yuen et al. 2012). Given the profound and multifaceted influence of glucocorticoids and glucocorticoid receptors on glutamatergic transmission, including both classical and nongenomic effects on glutamate release and clearance as well as NMDAR and AMPAR responses (reviewed in Popoli et al. 2012), it is interesting to speculate that glutamatergic receptors on dendritic spines may be the final common pathway by which stress-induced increases in glucocorticoids alter both dendritic morphology in mPFC and prefrontally mediated behaviors such as extinction.
Consistent with this notion, modulators of glutamatergic activity, such as dopaminergic signaling and brain derived neurotrophic factor (BDNF), are critical for extinction learning and have also been implicated in stress-induced alterations in medial prefrontal cortex. For instance, dopamine modulates NMDA receptor-mediated currents via D1 receptors (reviewed in Tritsch and Sabatini 2012). Administration of SCH23390, which blocks the dopaminergic D1 family of receptors, either systemically (Ball et al. 2018) or directly into the infralimbic cortex (Fiorenza et al. 2012; Hikind and Maroun 2008), impairs extinction, whereas infusion of a D1-family agonist facilitates extinction (Fiorenza et al. 2012). There are interactions between glucocorticoids and D1 receptors (reviewed in Sinclair et al. 2014). Thus, it is not surprising that intra-mPFC administration of the D1 blocker SCH23390 prevents stress-induced dendritic retraction (Lin et al. 2015) and attenuates stress-facilitated, priming-induced drug seeking after extinction (Ball et al. 2018).
BDNF signaling in the ventral hippocampus-infralimbic cortex pathway is both necessary and sufficient for the extinction of fear memories, as BDNF infusion into the infralimbic but not prelimbic cortex can either facilitate or even induce extinction (Rosas-Vidal et al. 2014), and infusion of an antibody to BDNF into infralimbic but not prelimbic cortex impairs extinction (Rosas-Vidal et al. 2014). BDNF infusion into the hippocampus increases BDNF release in infralimbic cortex (McGinty et al. 2010), increases the firing rate of IL neurons (Rosas-Vidal et al. 2014), and facilitates extinction in poor learners (Peters et al. 2010), suggesting that projections from hippocampus to IL are critical for extinction. Given that chronic stress reduces BDNF expression in hippocampus (Lakshminarasimhan and Chattarji 2012), it is possible that this reduction leads to reduced BDNF release in IL, resulting in decreased synaptic plasticity, and impaired extinction. Indeed, BDNF appears to play a key role in synaptic remodeling in hippocampus (An et al. 2008) and amygdala (Govindarajan et al. 2006) following stress. However, evidence for its role in mPFC is mixed, with some reports of stress-induced downregulation of BDNF mRNA (reviewed in Calabrese et al. 2009), and other studies failing to find stress-induced changes (Chiba et al. 2012; Lin et al. 2009). On the other hand, infusion of BDNF into infralimbic cortex can reverse some of the behavioral effects of stress (Graybeal et al. 2011). Further, Yu and colleagues (2012) demonstrated that 7 days of chronic restraint stress reduced spine density in mPFC of Val66Met knock-in mice, in which activity-dependent release of BDNF is reduced, compared to stressed wild type (WT) mice. Interestingly, although the behavioral and neuroendocrine profile of these knock-in mice was comparable to that of WT prior to stress exposure, spine density was not different in unstressed WT versus knock-in mice. Nonetheless, stress-induced increases in plasma corticosterone and adrenocorticotropic hormone (ACTH) were greater in knock-in mice relative to WT. Thus, low basal levels of BDNF are not sufficient to decrease spine density, but could contribute to the development of a heightened response to stress, leading to downstream effects on spinogenesis. This finding illustrates the importance of considering gene × environment interactions in the pathophysiology of stress-sensitive disorders. In addition, administration of ketamine, an NMDA antagonist, did not ameliorate the effects of BDNF deficiency in Val66Met knock-in mice, but does ameliorate stress-induced prefrontal spine loss in WT mice and rats, indicating that BDNF plays a crucial role in synaptogenesis in mPFC. (Liu and colleagues 2012) suggest a mechanism by which glucocorticoids, NMDARs, and BDNF interact to mediate synaptogenesis: 1) ketamine transiently increases the presynaptic release of glutamate, leading to a burst of action potentials at the synapse; 2) this bursting increases AMPAR stimulation, facilitating the release of BDNF; 3) BDNF binds to its receptor (TrkB), activating mTOR, which has the downstream effect of the translation of synaptic proteins, thus leading to synaptogenesis (Liu et al. 2012). Therefore, if BDNF is down-regulated as in Val66Met knock-in mice or by chronic stress, the downstream events of ketamine administration or natural rises in glutamate will be blocked, resulting in either synaptic stasis or potentially, synaptic pruning in cases where excess levels of glucocorticoids are present (see Figure 4). This synaptic pruning in turn could contribute to stress-induced deficits in extinction.
Figure 4.
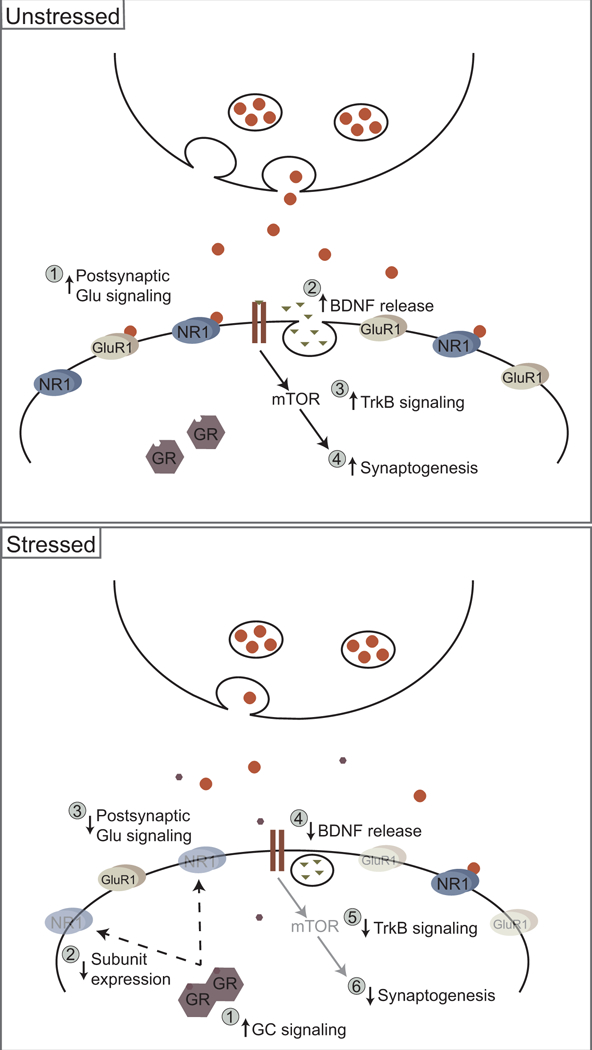
Possible neurochemical/molecular pathways for stress-induced synaptic remodeling in medial prefrontal cortex. Above. In an unstressed animal, increases in postsynaptic glutamatergic signaling (1) are associated with BDNF release (2); BDNF’s activity at its TrkB receptor (3) results in increased synaptogenesis via an mTOR-dependent pathway (4). Below. During chronic stress, increased glucocorticoid signaling (1) results in loss of functional NMDA and AMPA receptors (2), which decreases postsynaptic glutamatergic signaling (3). Concurrent reductions in BDNF release (4) in medial prefrontal cortex decreases TrkB signaling (5), resulting in decreased synaptogenesis (6).
Unanswered Questions and Future Directions
Circuit-Level Considerations
Several stress-related disorders, including anxiety (Weniger et al. 2006), depression (Fossati et al. 2004), PTSD (Chen et al. 2006), and schizophrenia (Suzuki et al. 2005), have been associated with changes in the volume of the prefrontal cortex, amygdala, and hippocampus, implicating these regions as important targets for investigating stress effects in the brain. In fact, all three structures are rich in corticosteroid receptors and are involved in the regulation of hypothalamic-pituitary-adrenal axis activity (Bhatnagar et al. 2004; Dayas et al. 1999; Ulrich-Lai and Herman 2009). Further, these brain regions are directly and indirectly interconnected (Ishikawa and Nakamura 2003; 2006; McDonald et al. 1996; Vertes 2006), allowing for reciprocal regulation of activity (Bruchey et al. 2007; Knapska et al. 2012; Knapska and Maren 2009; Likhtik et al. 2008; Orsini et al. 2011; Quirk et al. 2003; Rosenkranz and Grace 2001; Sotres-Bayon et al. 2004). Indeed, interactions among the medial prefrontal cortex, hippocampus, and amygdala are critical to appropriate fear conditioning and extinction (Knapska et al. 2012). Yet chronic stress has very different effects across corticolimbic structures. For instance, whereas chronic stress via a variety of manipulations results in apical dendritic retraction in medial prefrontal cortex, changes in orbitofrontal cortex are quite different: the chronic restraint procedure (6 h/21 days) that produces dendritic retraction in anterior cingulate, prelimbic, and infralimbic cortex increases dendritic material in the lateral orbitofrontal cortex in rats (Liston et al. 2006), as does three weeks of chronic unpredictable stress (Dias-Ferreira et al. 2009). Interestingly, this dendritic growth can occur quite rapidly, after just one exposure to four hours of restraint (Godar et al. 2015). Despite this stress-induced dendritic outgrowth, chronic administration of stress levels of corticosterone produces significant and long-lasting reductions in spine densities in orbitofrontal cortex. This persistent spine loss is associated with depression-like behaviors. Interestingly, administration of lower doses of glucocorticoids produced increases in spine densities in orbitofrontal cortex, and prevention of this glucocorticoid-induced spine proliferation via knockdown of p190rhogap—a protein associated with spine stabilization—resulted in induction of depression-like behaviors (Gourley et al. 2013). Given the reciprocal connections and considerable overlap in targets of medial prefrontal cortex and orbitofrontal cortex, along with recent evidence that some functions of orbitofrontal cortex may parallel those of infralimbic cortex—including playing a role in certain kinds of extinction (Gourley and Taylor 2016), the differential effects of stress on these prefrontal regions could profoundly impact the function of the neural circuitry underlying extinction.
Likewise, chronic stress may also produce a very different pattern of changes in the basolateral amygdala compared to the medial prefrontal cortex. Chattarji and colleagues found that as little as ten days of immobilization stress (2 h per day) increased both length and number of dendritic branches of pyramidal and stellate neurons in the basolateral amygdala in rats (Govindarajan et al. 2006; Mitra et al. 2005; Vyas et al. 2006; Vyas et al. 2002; Vyas et al. 2004). Similar changes in dendritic length are seen after chronic restraint stress in mice (6 h/day for 21 days; Johnson et al. 2009). These changes in dendritic morphology are paralleled by increases in spine density in both rats and mice (Govindarajan et al. 2006; Mitra et al. 2005; Vyas et al. 2006), an effect that is associated with increased freezing during extinction retrieval (Maroun et al. 2013). Interestingly, whereas stress-induced changes in dendritic morphology in medial prefrontal cortex has been demonstrated to be reversible within seven days (Moench and Wellman 2017), the effects of stress on amygdaloid morphology may be more persistent. Vyas and colleagues (Vyas et al. 2004) demonstrated that the dendritic hypertrophy in basolateral amygdala resulting from chronic immobilization (2 h/day, 10 days) persists up to 21 days after the cessation of stress. Likewise, stellate and pyramidal neurons in the basolateral amygdala neurons undergo dendritic hypertrophy in as little as one day after traumatic brain injury (which may perhaps be considered a physiological stressor), which persists at least four weeks later, despite lack of neuronal loss in this structure (Hoffman et al. 2017). Note, however, that the effect of stress on the basolateral amygdala may depend on intensity or modality of the stressor: others have found that daily restraint stress (6 h/day, 10 days) resulted in significant debranching and retraction of pyramidal neurons in basolateral amygdala (Grillo et al. 2015), as did acute elevated platform stress (Maroun et al. 2013). Potential stress-induced alterations in dendritic length and branch number have not been assessed across multiple subregions of the amygdala. However, stress-induced changes in dendritic spine density have been shown to vary across amygdaloid nuclei. For instance, in the medial amygdaloid nucleus of mice, chronic restraint stress (6 h/day for 3 weeks) decreased spine density on stellate neurons, whereas the same manipulation increased spine density in basolateral amygdala (Bennur et al. 2007).
Finally, chronic stress-induced alterations in the morphology of hippocampal neurons—most prominently, pyramidal neurons area CA3—have been extensively documented (e.g., Christian et al. 2011; Conrad et al. 1999a; Magariños and McEwen 1995; Magariños et al. 1996). However, morphology of hippocampal neurons may be less sensitive to stress than is either the medial prefrontal cortex or the basolateral amygdala. For instance, in a parametric study, McLaughlin et al (2007) found that rats exposed to 6 h of daily restraint for three weeks demonstrated pronounced retraction of CA3 apical dendrites, whereas 6 hours of daily restraint for 10 days did not. The intensity or type of the daily stressor may also be critical: 2 h of restraint per day for either 10 days or three weeks also failed to produce CA3 dendritic retraction (McLaughlin et al. 2007), whereas three weeks of social defeat (Kole et al. 2004) or 10-day immobilization stressor resulted in dendritic retraction in CA3 (Christian et al. 2011).
Delineating the differential contributions of these corticolimbic structures to stress-induced alterations in behaviors mediated by them is critical. Studies employing temporary inactivation of specific corticolimbic structures during stress or subsequent behavioral testing; administering, for instance glucocorticoid receptor blockers into specific corticolimbic structures; or conditional, viral, or chemogenetic inactivation of specific genes or gene products within these structures could tease apart the contribution of each of these interconnected structures to both stress-induced dendritic alterations and their contributions to stress-induced changes in behaviors mediated by these structures. Ultimately, it is likely that stress-induced deficits in extinction are an emergent property of the differential changes in activity in the circuit as a whole. Studies that simultaneously assess stress-induced changes in the activity of critical nodes in the extinction circuit during extinction and extinction retrieval are critically needed to shed light on this issue.
Sex Differences in Stress Effects on Extinction and its Neural Substrates.
Despite dramatic sex differences in the rates and expression of stress-related psychological disorders (Cover et al. 2014), the vast majority of the research on the neurobiological mechanisms underlying stress effects on emotional behavior has focused on males, as have the preceding sections of this review. Investigations of the mechanisms underlying potential stress-induced plasticity of corticolimbic structures in females may provide the groundwork necessary to develop sex-specific treatment for stress-related psychopathology. A handful of studies has begun to address this issue, and has demonstrated that indeed stress has very different effects on the structure and function of corticolimbic brain regions involved in extinction. For example, while chronic stress impairs extinction in male rats, similar deficits are not observed in female rats (Baran et al. 2009; Hoffman et al. 2010). Further, whereas male rats exhibit dendritic retraction in mPFC following stress, female rats either exhibit no dendritic changes or may even exhibit dendritic hypertrophy (Garrett and Wellman 2009; Moench and Wellman 2017; Shansky et al. 2010). Finally, two weeks of unpredictable mild stress differentially impacts dorsal medial prefrontal cortex in female mice, producing more pronounced increases in parvalbumin expression (Shepard et al. 2016) and increases in phosphorylated ERK on parvalbumin-positive cells, likely due to increased glutamatergic input onto these cells (Shepard and Coutellier 2018). Systematic investigation of the phenomenology, functional implications, and mechanisms underlying such sex-dependent effects of stress is critical for developing sex-specific treatments for stress-sensitive psychopathologies.
Closing Thoughts
A growing body of evidence demonstrates that prior stress—either chronic or acute—increases freezing during extinction retrieval. However, whether this effect reflects a strengthening of fear acquisition and memory or weakening of extinction acquisition or memory awaits careful testing. Likewise, while several studies have identified potential stress-induced alterations in mPFC structure and function that may underlie alterations in extinction, much work remains to be done, both at the level of neurochemical mechanisms and localization of these mechanisms to specific structures, to test the hypotheses that these changes mediate stress-induced deficits in extinction. Ultimately, a full understanding of the neural mechanisms underlying stress-induced impairments in extinction will require investigations at the circuit level of analysis, to account for differential and opposing effects of stress on the neural circuitry underlying extinction. Finally, investigation of the neural basis for individual differences, such as sex differences in vulnerability to stress-induced alterations in extinction, will be instrumental in identifying avenues for novel interventions for stress-sensitive disorders characterized by deficits in extinction.
Acknowledgement of funding
This work was supported in part by National Institute of Health Award Number T32MH103213 and National Institute of Health Award Number T32HD049336.
Footnotes
Conflict of Interest Statement
Cara L. Wellman and Kelly M. Moench declare that they have no conflict of interest.
References
- Akirav I, Raizel H, Maroun M (2006) Enhancement of conditioned fear extinction by infusion of the GABA(A) agonist muscimol into the rat prefrontal cortex and amygdala. Eur J Neurosci 23: 758–64. [DOI] [PubMed] [Google Scholar]
- An JJ, Gharami K, Liao G-Y, Woo NH, Lau AG, Vanevski F, Torre ER, Jones KR, Feng Y, Lu B, Xu B (2008) Distinct role of long 3’ UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell 134: 175–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball KT, Stone E, Best O, Collins T, Edson H, Hagan E, Nardini S, Neuciler P, Smolinsky M, Tosh L, Woodlen K (2018) Chronic restraint stress during withdrawal increases vulnerability to drug priming-induced cocaine seeking via a dopamine D1-like receptor-mediated mechanism. Drug Alcohol Depen 187: 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran SE, Armstrong CE, Niren DC, Hanna JJ, Conrad CD (2009) Chronic stress and sex differences on the recall of fear conditioning and extinction. Neurobiol Learn Mem 91: 323–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barfield ET, Gerber KJ, Zimmermann KS, Ressler KJ, Parsons RG, Gourley SL (2017) Regulation of actions and habits by ventral hippocampal trkB and adolescent corticosteroid exposure. PLoS Biol 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennur S, Shankaranarayana Rao BS, Pawlak R, Strickland S, McEwen BS, Chattarji S (2007) Stress-induced spine loss in the medial amygdala is mediated by tissue-plasminogen activator. Neuroscience 144: 8–16. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C, Denski K (2004) Regulation of chronic stress-induced changes in hypothalamic-pituitary-adrenal activity by the basolateral amygdala. Ann N Y Acad Sci 1032: 315–9. [DOI] [PubMed] [Google Scholar]
- Bloodgood DW, Sugam JA, Holmes A, Kash TL (2018) Fear extinction requires infralimbic cortex projections to the basolateral amygdala. Translational Psychiatry 8: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Ricker ST (1994) Renewal of extinguished responding in a second context. Animal Learning Behav 22: 317–324. [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S (2006) Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatr 60: 352–360. [DOI] [PubMed] [Google Scholar]
- Brown S, Henning S, Wellman CL (2005) Short-term, mild stress alters dendritic morphology in rat medial prefrontal cortex. Cerebral Cortex 15: 1714–1722. [DOI] [PubMed] [Google Scholar]
- Bruchey AK, Shumake J, Gonzalez-Lima F (2007) Network model of fear extinction and renewal functional pathways. Neuroscience 145: 423–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ (2009) Sustained Conditioned Responses in Prelimbic Prefrontal Neurons Are Correlated with Fear Expression and Extinction Failure. The Journal of Neuroscience 29: 8474–8482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ (2007) Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron 53: 871–880. [DOI] [PubMed] [Google Scholar]
- Calabrese F, Molteni R, Racagni G, Riva MA (2009) Neuronal plasticity: A link between stress and mood disorders. Psychoneuroendocrinol 34, Supplement 1: S208–S216. [DOI] [PubMed] [Google Scholar]
- Cerqueira JJ, Mailliet F, Almeida OFX, Jay TM, Sousa N (2007a) The prefrontal cortex as a key target of the maladaptive response to stress. J Neurosci 27: 2781–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira JJ, Pego JM, Taipa R, Bessa JM, Almeida OF, Sousa N (2005) Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J Neurosci 25: 7792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira JJ, Taipa R, Uylings HB, Almeida OF, Sousa N (2007b) Specific configuration of dendritic degeneration in pyramidal neurons of the medial prefrontal cortex induced by differing corticosteroid regimens. Cerebral Cortex 17: 1998–2006. [DOI] [PubMed] [Google Scholar]
- Chang CH, Maren S (2011) Medial prefrontal cortex activation facilitates re-extinction of fear in rats. Learn Memory 18: 221–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Xia W, Li L, Liu J, He Z, Zhang Z, Yan L, Zhang J, Hu D (2006) Gray matter density reduction in the insula in fire survivors with posttraumatic stress disorder: a voxel-based morphometric study. Psychiatry Res 146: 65–72. [DOI] [PubMed] [Google Scholar]
- Chiba S, Numakawa T, Ninomiya M, Richards MC, Wakabayashi C, Kunugi H (2012) Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Progress in Neuro-psychopharmacology & Biological Psychiatry 39: 112–119. [DOI] [PubMed] [Google Scholar]
- Christian KM, Miracle AD, Wellman CL, Nakazawa K (2011) Chronic stress-induced hippocampal dendritic retraction requires CA3 NMDA receptors. Neuroscience 174: 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, LeDoux JE, Magarinos AM, McEwen BS (1999a) Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci 113: 902–913. [DOI] [PubMed] [Google Scholar]
- Conrad CD, LeDoux JE, Magarinos AM, McEwen BS (1999b) Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci 113: 902–13. [DOI] [PubMed] [Google Scholar]
- Conrad CD, MacMillan DD 2nd, Tsekhanov S, Wright RL, Baran SE, Fuchs RA (2004) Influence of chronic corticosterone and glucocorticoid receptor antagonism in the amygdala on fear conditioning. Neurobiology of Learning and Memory 81: 185–99. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Mauldin-Jourdain ML, Hobbs RJ (2001) Metyrapone reveals that previous chronic stress differentially impairs hippocampal-dependent memory. Stress 4: 305–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Ortiz JB, Judd JM (2017) Chronic stress and hippocampal dendritic complexity: Methodological and functional considerations. Physiol Behav 178: 66–81. [DOI] [PubMed] [Google Scholar]
- Cook SC, Wellman CL (2004) Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol 60: 236–248. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ (2007) Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci 27: 840–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover KK, Maeng LY, Lebrón-Milad K, Milad MR (2014) Mechanisms of estradiol in fear circuitry: implications for sex differences in psychopathology. Translational Psychiatry 4: e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Day TA (1999) Neuroendocrine responses to an emotional stressor: evidence for involvement of the medial but not the central amygdala. Eur J Neurosci 11: 2312–22. [DOI] [PubMed] [Google Scholar]
- Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, Costa RM, Sousa N (2009) Chronic stress causes frontostriatal reorganization and affects decision-making. Science 325: 621–625. [DOI] [PubMed] [Google Scholar]
- Do-Monte FH, Manzano-Nieves G, Quiñones-Laracuente K, Ramos-Medina L, Quirk GJ (2015a) Revisiting the Role of Infralimbic Cortex in Fear Extinction with Optogenetics. J Neurosci 35: 3607–3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do-Monte FH, Quinones-Laracuente K, Quirk GJ (2015b) A temporal shift in the circuits mediating retrieval of fear memory. Nature 519: 460-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell MR, Sayed JA, Underwood AR, Wellman CL (2010) Lesion of infralimbic cortex occludes stress effects on retrieval of extinction but not fear conditioning. Neurobiol Learn Mem 94: 240–6. [DOI] [PubMed] [Google Scholar]
- Fiorenza NG, Rosa J, Izquierdo I, Myskiw JC (2012) Modulation of the extinction of two different fear-motivated tasks in three distinct brain areas. Behav Brain Res 232: 210–216. [DOI] [PubMed] [Google Scholar]
- Fossati P, Radtchenko A, Boyer P (2004) Neuroplasticity: from MRI to depressive symptoms. Eur Neuropsychopharmacol 14 Suppl 5: S503–10. [DOI] [PubMed] [Google Scholar]
- Garcia R, Spennato G, Nilsson-Todd L, Moreau J-L, Deschaux O (2008) Hippocampal low-frequency stimulation and chronic mild stress similarly disrupt fear extinction memory in rats. Neurobiol Learn Mem 89: 560–6. [DOI] [PubMed] [Google Scholar]
- Garrett JE, Wellman CL (2009) Chronic stress effects on dendritic morphology in medial prefrontal cortex: sex differences and estrogen dependence. Neuroscience 162: 195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godar SC, Bortolato M, Richards SE, Li FG, Chen K, Wellman CL, Shih JC (2015) Monoamine oxidase A is required for rapid dendritic remodeling in response to stress. Int J Neuropsychopharm 18: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon U, Polsky A, Schiller J (2006) Plasticity compartments in basal dendrites of neocortical pyramidal neurons. J Neurosci 26: 12717–12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami S, Cascardi M, Rodriguez-Sierra OE, Duvarci S, Pare D (2010) Impact of predatory threat on fear extinction in Lewis rats. Learn Mem 17: 494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Kedves AT, Olausson P, Taylor JR (2009) A history of corticosterone exposure regulates fear extinction and cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology 34: 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Swanson AM, Koleske AJ (2013) Corticosteroid-Induced Neural Remodeling Predicts Behavioral Vulnerability and Resilience. J Neurosci 33: 3107–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Taylor JR (2016) Going and stopping: dichotomies in behavioral control by the prefrontal cortex. Nature Neurosci 19: 656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan A, Rao BS, Nair D, Trinh M, Mawjee N, Tonegawa S, Chattarji S (2006) Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. PNAS 103: 13208–13213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybeal C, Feyder M, Schulman E, Saksida LM, Bussey TJ, Brigman JL, Holmes A (2011) Paradoxical reversal learning enhancement by stress or prefrontal cortical damage: rescue with BDNF. Nature Neurosci 14: 1507–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo CA, Risher M, Macht VA, Bumgardner AL, Hang A, Gabriel C, Mocaër E, Piroli GG, Fadel JR, Reagan LP (2015) Repeated restraint stress-induced atrophy of glutamatergic pyramidal neurons and decreases in glutamatergic efflux in the rat amygdala are prevented by the antidepressant agomelatine. Neuroscience 284: 430–443. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ (1988) Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal-prefrontal topography. Neuroscience 24: 379–431. [DOI] [PubMed] [Google Scholar]
- Hains AB, Vu MAT, Maciejewski PK, van Dyck CH, Gottron M, Arnsten AFT (2009) Inhibition of protein kinase C signaling protects prefrontal cortex dendritic spines and cognition from the effects of chronic stress. PNAS 106: 17957–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han EB, Heinemann SF (2013) Distal Dendritic Inputs Control Neuronal Activity by Heterosynaptic Potentiation of Proximal Inputs. J Neurosci 33: 1314–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Trifilieff P, Micheau J, Luthi A, Mons N (2006) Extinction of auditory fear conditioning requires MAPK/ERK activation in the basolateral amygdala. Eur J Neurosci 24: 261–9. [DOI] [PubMed] [Google Scholar]
- Hikind M, Maroun M (2008) Microinfusion of the D1 receptor antagonist, SCH23390 into the IL but not the BLA impairs consolidation of extinction of auditory fear conditioning. Neurobiol Learn Mem 90: 217–222. [DOI] [PubMed] [Google Scholar]
- Hoffman AN, Armstrong CE, Hanna JJ, Conrad CD (2010) Chronic stress, cyclic 17β-estradiol, and daily handling influences on fear conditioning in the female rat. Neurobiol Learn Mem 94: 422–433. [DOI] [PubMed] [Google Scholar]
- Hoffman AN, Paode PR, May HG, Ortiz JB, Kemmou S, Lifshitz J, Conrad CD, Thomas TC (2017) Early and Persistent Dendritic Hypertrophy in the Basolateral Amygdala following Experimental Diffuse Traumatic Brain Injury. J Neurotrauma 34: 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AN, Parga A, Paode PR, Watterson LR, Nikulina EM, Hammer RP Jr, Conrad CD (2015) Chronic stress enhanced fear memories are associated with increased amygdala zif268 mRNA expression and are resistant to reconsolidation. Neurobiol Learn Mem 120: 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Singewald N (2013) Individual differences in recovery from traumatic fear. Trends in Neurosciences 36: 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt DJ, Lebron-Milad K, Milad MR, Rauch SL, Pitman RK, Orr SP, Cassidy BS, Walsh JP, Goff DC (2009) Extinction memory is impaired in schizophrenia. Biol Psychiatr 65: 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtubise JL, Howland JG (2017) Effects of stress on behavioral flexibility in rodents. Neuroscience 345: 176–192. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Nakamura S (2003) Convergence and Interaction of Hippocampal and Amygdalar Projections within the Prefrontal Cortex in the Rat. J Neurosci 23: 9987–9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa A, Nakamura S (2006) Ventral Hippocampal Neurons Project Axons Simultaneously to the Medial Prefrontal Cortex and Amygdala in the Rat. Journal of Neurophysiology 96: 2134–2138. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A (2006) Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci 26: 5733–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jett JD, Bulin SE, Hatherall LC, McCartney CM, Morilak DA (2017) Deficits in cognitive flexibility induced by chronic unpredictable stress are associated with impaired glutamate neurotransmission in the rat medial prefrontal cortex. Neuroscience 346: 284–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SA, Wang JF, Sun X, McEwen BS, Chattarji S, Young LT (2009) Lithium treatment prevents stress-induced dendritic remodeling in the rodent amygdala. Neuroscience 163: 34–39. [DOI] [PubMed] [Google Scholar]
- Khoo SYS, Gibson GD, Prasad AA, McNally GP (2017) How contexts promote and prevent relapse to drug seeking. Genes Brain Behav 16: 185–204. [DOI] [PubMed] [Google Scholar]
- Knapska E, Macias M, Mikosz M, Nowak A, Owczarek D, Wawrzyniak M, Pieprzyk M, Cymerman IA, Werka T, Sheng M, Maren S, Jaworski J, Kaczmarek L (2012) Functional anatomy of neural circuits regulating fear and extinction. PNAS 109: 17093–17098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapska E, Maren S (2009) Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learn Mem 16: 486–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D, George SA, Fitzpatrick CJ, Rabinak CA, Maren S, Liberzon I (2012a) Single prolonged stress disrupts retention of extinguished fear in rats. Learn Mem 19: 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D, Nault T, Henderson C, Liberzon I (2012b) Glucocorticoid receptors and extinction retention deficits in the single prolonged stress model. Neuroscience 223: 163–73. [DOI] [PubMed] [Google Scholar]
- Knox D, Perrine SA, George SA, Galloway MP, Liberzon I (2010) Single prolonged stress decreases glutamate, glutamine, and creatine concentrations in the rat medial prefrontal cortex. Neuroscience Letters 480: 16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D, Stanfield BR, Staib JM, David NP, DePietro T, Chamness M, Schneider EK, Keller SM, Lawless C (2018) Using c-Jun to identify fear extinction learning-specific patterns of neural activity that are affected by single prolonged stress. Behav Brain Res 341: 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole MHP, Costoli T, Koolhaas JM, Fuchs E (2004) Bidirectional shift in the cornu ammonis 3 pyramidal dendritic organization following brief stress. Neuroscience 125: 337–347. [DOI] [PubMed] [Google Scholar]
- Lakshminarasimhan H, Chattarji S (2012) Stress leads to contrasting effects on the levels of brain derived neurotrophic factor in the hippocampus and amygdala. PloS one 7: e30481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechin F, Van der Dijs B, Benaim M (1996) Stress versus depression. Prog Neuropsychopharmacol Biol Psychiatry 20: 899–950. [DOI] [PubMed] [Google Scholar]
- Li N, Liu R-J, Dwyer JM, Banasr M, Lee B, Son H, Li X- Y, Aghajanian G, Duman RS (2011) Glutamate N-methyl-D-aspartate Receptor Antagonists Rapidly Reverse Behavioral and Synaptic Deficits Caused by Chronic Stress Exposure. Biol Psychiatr 59: 754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D (2008) Amygdala intercalated neurons are required for expression of fear extinction. Nature 454: 642–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin GL, Borders CB, Lundewall LJ, Wellman CL(2015) D1 receptors regulate dendritic morphology in normal and stressed prelimbic cortex. Psychoneuroendocrinol 51: 101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Ter Horst GJ, Wichmann R, Bakker P, Liu AH, Li XJ, Westenbroek C (2009) Sex differences in the effects of acute and chronic stress and recovery after long-term stress on stress-related brain regions of rats. Cereb Cortex 19: 1978–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS (2006) Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci 26: 7870–7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R-J, Aghajanian GK (2008) Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: Role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci USA 105: 359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R-J, Lee FS, Li X- Y, Bambico F, Duman RS, Aghajanian GK (2012) Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatr 71: 996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS (1995) Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience 69: 89–98. [DOI] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS, Flugge G, Fuchs E (1996) Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci 16: 3534–3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Holmes A (2016) Stress and fear extinction. Neuropsychopharmacology Reviews 41: 58–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Yap SA, Goosens KA (2001) The amygdala is essential for the development of neuronal plasticity in the medial geniculate nucleus during auditory fear conditioning in rats. J Neurosci 21: RC135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroun M, Ioannides PJ, Bergman KL, Kavushansky A, Holmes A, Wellman CL (2013) Fear extinction deficits following acute stress associate with increased spine density and dendritic retraction in basolateral amygdala neurons. Eur J Neurosci 38: 2611–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroun M, Richter-Levin G (2003) Exposure to acute stress blocks the induction of long-term potentiation of the amygdala-prefrontal cortex pathway in vivo. J Neurosci 23: 4406–4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KP, Wellman CL (2011) NMDA Receptor Blockade Alters Stress-Induced Dendritic Remodeling in Medial Prefrontal Cortex. Cereb Cortex 21: 2366–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough KM, Morrison FG, Ressler KJ (2016) Bridging the Gap: Towards a cell-type specific understanding of neural circuits underlying fear behaviors. Neurobiol Learn Mem 135: 27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Guo L (1996) Projections of the medial and lateral prefrontal cortices to the amygdala: A Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience 71: 55–75. [DOI] [PubMed] [Google Scholar]
- McEwen BS (2017) Allostasis and the epigenetics of brain and body health over the life course: The brain on stress. JAMA Psychiatry 74: 551–552. [DOI] [PubMed] [Google Scholar]
- McGinty JF, Whitfield TW, Berglind WJ (2010) Brain-derived neurotrophic factor and cocaine addiction. Brain Res 1314: 183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKlveen JM, Morano RL, Fitzgerald M, Zoubovsky S, Cassella SN, Scheimann JR, Ghosal S, Mahbod P, Packard BA, Myers B, Baccei ML, Herman JP (2016) Chronic Stress Increases Prefrontal Inhibition: A Mechanism for Stress-Induced Prefrontal Dysfunction. Biol Psychiatr 80: 754–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, Gomez JL, Baran SE, Conrad CD (2007) The effects of chronic stress on hippocampal morphology and function: An evaluation of chronic restraint paradigms. Brain Res 1161: 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH (1985) [3H]Dexamethasone binding in rat frontal cortex. Brain Res 328: 176–80. [DOI] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL (2009) Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatr 66: 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ (2002) Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420: 70–74. [DOI] [PubMed] [Google Scholar]
- Milad MR, Vidal-Gonzalez I, Quirk GJ (2004) Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behavioral neuroscience 118: 389–94. [DOI] [PubMed] [Google Scholar]
- Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL (2006) Chronic stress impairs recall of extinction of conditioned fear. Neurobiol Learn Mem 85: 213–218. [DOI] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S (2005) Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. PNAS 102: 9371–9376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moench KM, Maroun M, Kavushansky A, Wellman C (2016) Alterations in neuronal morphology in infralimbic cortex predict resistance to fear extinction following acute stress. Neurobiology of Stress 3: 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moench KM, Wellman CL (2017) Differential dendritic remodeling in prelimbic cortex of male and female rats during recovery from chronic stress. Neuroscience 357: 145–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK (2000) De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J Abnorm Psychol 109: 290–8. [PubMed] [Google Scholar]
- Orsini CA, Kim JH, Knapska E, Maren S (2011) Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. J Neurosci 31: 17269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Maren S (2012) Neural and cellular mechanisms of fear and extinction memory formation. Neuroscience and biobehavioral reviews 36: 1773–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates, 4 edn Academic Press, New York [Google Scholar]
- Peri T, Ben-Shakhar G, Orr SP, Shalev AY (2000) Psychophysiologic assessment of aversive conditioning in posttraumatic stress disorder. Biol Psychiat 47: 512–9. [DOI] [PubMed] [Google Scholar]
- Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ (2010) Induction of fear extinction with hippocampal-infralimbic BDNF. Science 328: 1288–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoli M, Yan Z, McEwen BS, Sanacora G (2012) The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci 13: 22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D (2003) Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci 23: 8800–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D (2007) Neural Mechanisms of Extinction Learning and Retrieval. Neuropsychopharmacology 33: 56–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JK, Lebron K (2000) The role of ventromedial prefrontal cortex in the recovery of extinguished fear. The Journal of Neuroscience 20: 6225–6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Anderson RM, Hamilton BA, Alcock JA, Romig-Martin SA (2013) Chronic stress-induced alterations of dendritic spine subtypes predict functional decrements in an hypothalamo-pituitary-adrenal-inhibitory prefrontal circuit. J Neurosci 33: 14379–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Janssen WGM, Hof PR, McEwen BS, Morrison JH (2005) Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp Neurol 196: 199–203. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WGM, Liston C, Hof PR, McEwen BS, Morrison JH (2006) Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex 16: 313–20. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, Morrison JH, Wearne SL, Hof PR (2008) Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol 507: 1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH (2004) Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience 125: 1–6. [DOI] [PubMed] [Google Scholar]
- Rall W, Burke RE, Holmes WR, Jack JJ, Redman SJ, Segev I (1992) Matching dendritic neuron models of experimental data. Physiol Rev 72: S159–S186. [DOI] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas KR (2009) Interaction Between the Serotonin Transporter Gene (5-HTTLPR), Stressful Life Events, and Risk of Depression A Meta-analysis. Jama-J Am Med Assoc 301: 2462–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Vidal LE, Do-Monte FH, Sotres-Bayon F, Quirk GJ (2014) Hippocampal-Prefrontal BDNF and Memory for Fear Extinction. Neuropsychopharmacology 39: 2161–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA (2001) Dopamine attenuates prefrontal cortical suppression of sensory inputs to the basolateral amygdala of rats. J Neurosci 21: 4090–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders MJ, Stevens S, Boeh H (2010) Stress enhancement of fear learning in mice is dependent upon stressor type: Effects of sex and ovarian hormones. Neurobiology of Learning and Memory 94: 254–62. [DOI] [PubMed] [Google Scholar]
- Santini E, Muller RU, Quirk GJ (2001) Consolidation of extinction learning involves transfer from NMDA-independent to NMDA-dependent memory. J Neurosci 21: 9009–9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM (2003) Stress and plasticity in the limbic system. Neurochem Res 28: 1735–1742. [DOI] [PubMed] [Google Scholar]
- Schayek R, Maroun M (2015) Differences in Stress-Induced Changes in Extinction and Prefrontal Plasticity in Postweanling and Adult Animals. Biol Psychiatr 78: 159–166. [DOI] [PubMed] [Google Scholar]
- Segal M (2017) Dendritic spines: Morphological building blocks of memory. Neurobiol Learn Mem 138: 3–9. [DOI] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, Lou W, McEwen BS, Morrison JH (2010) Estrogen promotes stress sensitivity in a prefrontal cortex–amygdala pathway. Cereb Cortex 20: 2560–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, McEwen BS, Morrison JH (2009) Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cereb Cortex 19: 2479–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard R, Coutellier L (2018) Changes in the Prefrontal Glutamatergic and Parvalbumin Systems of Mice Exposed to Unpredictable Chronic Stress. Molecular neurobiology 55: 2591–2602. [DOI] [PubMed] [Google Scholar]
- Shepard R, Page CE, Coutellier L (2016) Sensitivity of the prefrontal GABAergic system to chronic stress in male and female mice: Relevance for sex differences in stress-related disorders. Neuroscience 332: 1–12. [DOI] [PubMed] [Google Scholar]
- Sinclair D, Purves-Tyson T, Allen K, Weickert C (2014) Impacts of stress and sex hormones on dopamine neurotransmission in the adolescent brain. Psychopharmacol 231: 1581–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE (2007) Acquisition of fear extinction requires activation of NR2B-containing NMDA receptors in the lateral amygdala. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 32: 1929–40. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DEA, LeDoux JE (2004) Emotional Perseveration: An Update on Prefrontal-Amygdala Interactions in Fear Extinction. Learn Memory 11: 525–535. [DOI] [PubMed] [Google Scholar]
- Spruston N (2008) Pyramidal neurons: dendritic structure and synaptic integration. Nature reviews Neuroscience 9: 206–21. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Nohara S, Hagino H, Takahashi T, Kawasaki Y, Yamashita I, Watanabe N, Seto H, Kurachi M (2005) Prefrontal abnormalities in patients with simple schizophrenia: structural and functional brain-imaging studies in five cases. Psychiatry Res 140: 157–71. [DOI] [PubMed] [Google Scholar]
- Swanson AM, Shapiro LP, Whyte AJ, Gourley SL (2013) Glucocorticoid receptor regulation of action selection and prefrontal cortical dendritic spines. Communicative & Integrative Biology 6: e26068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM (1977) An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol 172: 49–84. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Sabatini BL (2012) Dopaminergic Modulation of Synaptic Transmission in Cortex and Striatum. Neuron 76: 33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronson NC, Corcoran KA, Jovasevic V, Radulovic J (2012) Fear conditioning and extinction: emotional states encoded by distinct signaling pathways. Trends in Neurosciences 35: 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RJ, Lloyd DA (2004) Stress burden and the lifetime incidence of psychiatric disorder in young adults: racial and ethnic contrasts. Archives of general psychiatry 61: 481–8. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP (2009) Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 10: 397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban-Ciecko J, Barth AL (2016) Somatostatin-expressing neurons in cortical networks. Nat Rev Neurosci 17: 401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP (2006) Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience 142: 1–20. [DOI] [PubMed] [Google Scholar]
- Vidal-Gonzalez I, Vidal-Gonzalez Bn, Rauch SL, Quirk GJ (2006) Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Memory 13: 728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira PA, Corches A, Lovelace JW, Westbrook KB, Mendoza M, Korzus E (2015) Prefrontal NMDA receptors expressed in excitatory neurons control fear discrimination and fear extinction. Neurobiol Learn Mem 119: 52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Jadhav S, Chattarji S (2006) Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience 143: 387–393. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S (2002) Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 22: 6810–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Pillai AG, Chattarji S (2004) Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience 128: 667–673. [DOI] [PubMed] [Google Scholar]
- Wei J, Yuen EY, Liu W, Li X, Zhong P, Karatsoreos IN, McEwen BS, Yan Z (2014) Estrogen protects against the detrimental effects of repeated stress on glutamatergic transmission and cognition. Mol Psychiatry 19: 588–598. [DOI] [PubMed] [Google Scholar]
- Wellman CL (2001) Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. Journal of Neurobiology 49: 245–53. [DOI] [PubMed] [Google Scholar]
- Weniger G, Lange C, Irle E (2006) Abnormal size of the amygdala predicts impaired emotional memory in major depressive disorder. J Affect Disord 94: 219–29. [DOI] [PubMed] [Google Scholar]
- Wilber AA, Walker AG, Southwood CJ, Farrell MR, Lin GL, Rebec GV, Wellman CL (2011) Chronic stress alters neural activity in medial prefrontal cortex during retrieval of extinction. Neuroscience 174: 115–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, LeDoux J (2007) Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron 56: 19–32. [DOI] [PubMed] [Google Scholar]
- Yu H, Wang D-D, Wang Y, Liu T, Lee FS, Chen Z- Y (2012) Variant brain-derived neurotrophic factor Val66Met polymorphism alters vulnerability to stress and response to antidepressants. J Neurosci 32: 4092–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z (2012) Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron 73: 962–977. [DOI] [PMC free article] [PubMed] [Google Scholar]


