Abstract
Cardiac sarcoidosis (CS) remains an intriguing infiltrating disorder and one of the most important forms of inflammatory cardiomyopathy. Identification of patients with CS is of extreme importance because they are at higher risk of sudden death, and heart-failure progression. And while it remains a diagnostic conundrum, a great amount of experience has been accumulated over the last decade with the advent of fluorine-18 fluorodeoxyglucose positron emission tomography and cardiac magnetic resonance with late gadolinium enhancement imaging. They have both proven to be advanced imaging techniques that provide important, and often complementary, diagnostic and prognostic information for the management of CS. However, they have also shown to have limitations, and, thus, there is a continued need for developing more specific imaging probes for identifying cardiac inflammation. The aim of the present manuscript is to provide the reader with a better understanding of the histopathology of the disease, how this potentially relates to noninvasive imaging detection, and the best strategies available for the diagnosis and management of patients with CS.
Keywords: Cardiovascular imaging, F-18 fluorodeoxyglucose, cardiac MRI, inflammation, sarcoidosis
Sarcoidosis is a complex inflammatory condition of unknown etiology resulting from the growth of abnormal inflammatory cells in the form of nodules, referred to as noncaseating granulomas. These lesions are capable of affecting any organ in the body, including the heart. According to necropsy and imaging studies, cardiac involvement appears to affect approximately 20%−25% of patients with systemic sarcoidosis in the US,1–3 although, the number may be higher in Japan.4 From a cardiovascular viewpoint, patients with cardiac sarcoidosis (CS) may remain asymptomatic or develop varying degrees of heart failure or rhythm disturbances ranging from complete heart block to sustained ventricular arrhythmias.5 In fact, sudden cardiac death is considered the leading cause of death (followed by progressive heart failure) among patients with CS.6 Consequently, multiple societies have recommended the use of implantable cardioverter defibrillators placement for primary prevention of sudden cardiac death in many of these patients.7–10 Moreover, premier imaging societies including the American Society of Nuclear Cardiology, the Society of Nuclear Medicine and Molecular Imaging, the European Association of Nuclear Medicine, and the European Association of Cardiovascular Imaging have now published consensus documents and position statements that specify how to utilize imaging in evaluating patients with known or suspected CS.11,12
However, despite significant advancements in cardiovascular imaging, the clinical diagnosis of CS remains challenging. Detection of noncaseating granulomas on endomyocardial biopsy (EMB) has a poor sensitivity (20%−30%) due to sampling error,13,14 and it can be associated with significant peri-procedure complications.15 Consequently, the diagnosis of CS relies on noninvasive imaging. Fluorine-18 fluorodeoxyglucose (FDG) with positron emission tomography (PET) and cardiac magnetic resonance (CMR) with late gadolinium enhancement (LGE) have become standard of care in the evaluation and management of patients with suspected CS, and are often used as a diagnostic alternative to EMB.16,17 This review will provide an overview of histopathologic changes that occur in CS, and will discuss how different imaging techniques can be used to detect patients with known or suspected CS. In addition, we will discuss the complementary role of CMR and PET imaging and their clinical use in diagnosis and patient management.
HISTOPATHOLOGY FINDINGS IN CS
Knowledge of the most typical histopathologic changes in CS is useful for understanding the roles of different imaging techniques available in the evaluation of these patients. In addition, an understanding of the histopathology of sarcoidosis may explain why different tests may differ with respect to diagnosing various patterns of disease activity.
The histopathology hallmark of CS is the presence of noncaseating granulomas, mostly composed of macrophages and T lymphocytes (target for radionuclide molecular imaging). In later stages of disease, patients can develop varying degrees of myocardial fibrosis, best detected by CMR imaging.
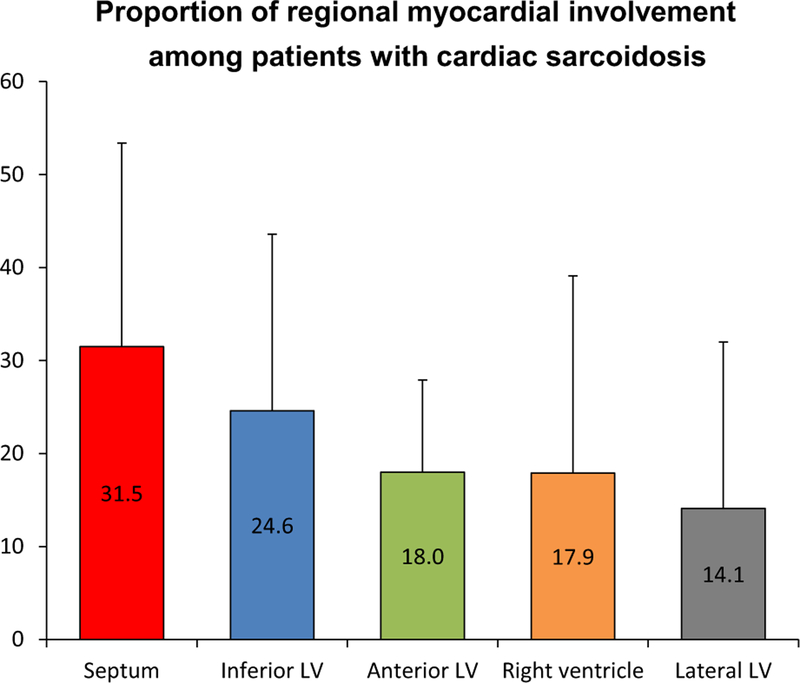
Sarcoidosis can involve any part of the heart, including the coronary vessels, pericardium, and valves. However, the myocardium is the most frequently affected cardiac structure.2,6,18 The left ventricle, and in particular the interventricular septum, is most commonly involved (Figure 1).2,6 For reasons not wholly understood, granulomas are most often seen in the basal segments and commonly involve the mid-wall and subepicardium, whereas, the sub-endocardium and distal segments are infrequently involved.6,18
Figure 1.
Distribution of regional myocardial involvement among patients with pathology-proven cardiac sarcoidosis. Caption based on one autopsy study18.
The cellular immune response of sarcoidosis in the heart is less well studied than in the lungs; however, similar to pulmonary sarcoidosis,19,20 data from post-mortem studies indicate that CS can have at least three different histologic features (Table 1).4,18 The first feature corresponds to a lymphocytic predominant infiltrate, with some interstitial edema, and few scattered epithelioid-cell granulomas or collection of histiocytes (not to confuse with giant cell myocarditis, which is associated with widespread myocyte necrosis). This is the least common type found in postmortem specimens. The identification of such activity would most likely require molecular imaging techniques, such as FDG PET. The second feature consists of predominantly well-formed granulomas, with varying degree of fibrosis, and appears to be the most frequently encountered type of CS. Both FDG PET and CMR may identify such features. The third feature is characterized by areas of replacement of the myocardium by fibrotic changes with few (if any) granulomas, and possibly some chronic interstitial lymphocytic cells. This pattern would be best detected by LGE-CMR or abnormal myocardial perfusion. Based on the available experience from systemic sarcoidosis, sarcoid tissue at any site may persist as active sarcoidosis, resolve, or progress to fibrosis.19 Unfortunately, longitudinal histological assessment of CS is lacking, which precludes ascertainment of the progression (or regression) from one feature to the other, along with their clinical implications. As such, we recommend that rather than using the term stages of disease—which implies a linear progression from one stage to the next—the term patterns of disease activity may instead be used.
Table 1.
Proposed histopathology features in cardiac sarcoidosis and their anticipated imaging findings
| Characteristics | Predominantly lymphocytes | Predominantly granulomas | Predominantly scar |
|---|---|---|---|
| Histopathology finding | Lymphocytes, scattered macrophage giant cells | Well-formed granulomas and varying degrees of fibrosis | Predominant fibrosis with few (if any) granulomas |
| Myocardial perfusion | Normal | Normal or abnormal | Abnormal |
| Myocardial FDG uptake | Abnormal | Abnormal | Mostly normal |
| T2-weighted CMR | Possibly abnormal | Possibly abnormal | Normal |
| Myocardial LGE | Normal | Usually abnormal | Abnormal |
CMR, cardiac magnetic resonance; LGE, late gadolinium enhancement
ECHOCARDIOGRAPHY
In patients with CS, echocardiography is useful to assess left and right ventricular sizes, functions, and coexisting valvular disease. It may also be useful to assess the indirect effects of pulmonary sarcoidosis on right ventricular geometry function and afterload. Earlier manifestations of cardiac involvement may include new diastolic dysfunction or areas of asymmetric wall thickness (suggesting edema from active inflammation) with otherwise preserved left ventricular function.21,22 More advanced phenotypes can range from global left ventricular dysfunction to scarred/thinned out segments, focal aneurysms or burnt out severe left/biventricular dysfunction.23 The latter may be indistinguishable from any other form of advanced cardiomyopathy. Unfortunately, none of these findings described above are specific to CS.24
When compared to CMR and FDG PET, echocardiography has low sensitivity for detection of CS, ranging from 25% to 65%.25–27 In a recent publication, Kouranos et al demonstrated that CMR had a substantially higher sensitivity to detect cardiac involvement when compared to echocardiography, 97% vs 27%.26 Given the above findings a negative echocardiogram should not be used to exclude cardiac involvement in patients with known extracardiac or suspected CS. Accordingly, in patients in whom further testing is needed to detect CS, FDG PET, or CMR should be considered as first-line testing options.
CARDIAC FDG PET
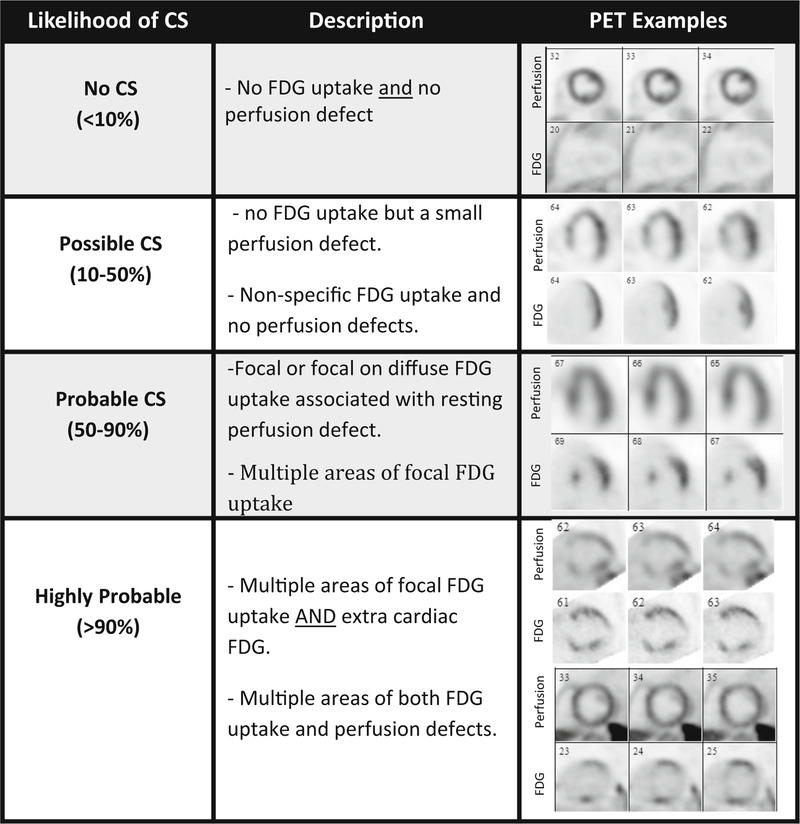
Cardiac FDG PET imaging is aimed at identifying metabolically active sarcoid lesions under the premise that granulomatous inflammatory cells are FDG-avid. The hallmark of CS on FDG PET imaging is the presence of focal or multifocal increased FDG uptake, especially when associated with perfusion defects (perfusion-metabolic mismatch; see examples in Figure 2). Patients that have no FDG uptake, but do have a resting myocardial perfusion defect, may still have cardiac involvement despite the absence of any active inflammation; a pattern often described as ‘‘burned out’’ sarcoidosis.
Figure 2.
Use of FDG PET/CT and myocardial perfusion imaging to identify various patterns of disease activity and estimate the likelihood of cardiac sarcoidosis. Adapted based on Vita et al44.
When interpreting FDG PET images, there are certain patterns that signify a higher likelihood of having CS. For instance, patients that have multiple areas of focal FDG uptake, as well as rest perfusion defects, are more likely to have CS, especially if they also have extracardiac FDG uptake in a pattern which is consistent with sarcoidosis. On the other hand, isolated FDG uptake along the lateral wall which does not correspond to any perfusion defects, and appears homogenous, is associated with a lower likelihood CS. (Figure 2)
Since its first description in the late 1990s,28 a substantial amount of data have accumulated on the clinical use of FDG PET in the evaluation and management of CS. However, owing to the lack of a gold standard, the true diagnostic performance of FDG PET (and other modalities) is not entirely known. Using the Japanese Ministry of Health and Welfare (JMHW) as the reference standard for diagnosis of CS, prior studies have reported a sensitivity of 89% and specificity of 78% for FDG PET, numbers that are actually comparable to the performance of LGE-CMR (sensitivity 75%−100% and specificity 76.9%−78%).29–31 However, the JMHW criteria have limitations, including the lack of adequate validation, and the requirement for extra-CS in the diagnostic criteria. Consequently, isolated CS a well-described clinical entity that may occur in approximately 25% of cases32 cannot be diagnosed using these clinical criteria.33 Thus, to this date, the true diagnostic accuracies of FDG PET and CMR remain incompletely elucidated.
FDG provides a unique role for assessing the response to anti-inflammatory therapy (Figures 3 and 4),34 and risk stratification. Osborne and colleagues observed that in 23 patients with serial FDG PET scans, a reduction in intensity and extent of myocardial inflammation by FDG PET was associated with a significant improvement in left ventricular ejection fraction. Furthermore, Blankstein and colleagues evaluated 117 patients with known or suspected CS and showed that the presence of focal FDG uptake on cardiac PET identified patients at higher risk of death or ventricular tachycardia, even after adjusting for ejection fraction and other clinical factors.35 Similarly, development of complete heart block in patients with sarcoidosis has been strongly associated with the presence of focal FDG uptake in the interventricular septum, and unlike subjects without myocardial inflammation on PET, heart block has the potential to recover in some patients with coexisting myocardial inflammation after steroid therapy.36
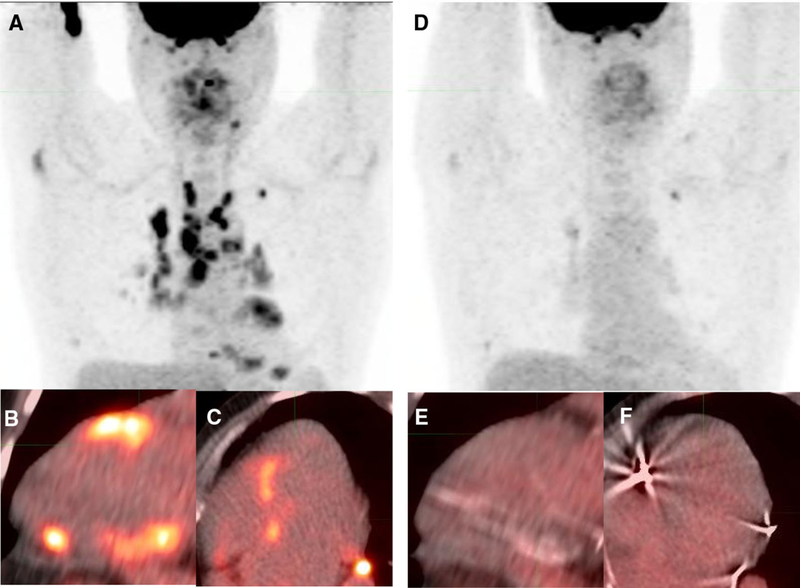
Figure 3.
Clinical utility of FDG PET/CT for treatment response monitoring. Baseline whole body (A) and cardiac (B-C) FDG PET/CT images demonstrate extensive thoracic and cardiac inflammation in a patient with pulmonary sarcoidosis presenting with intermittent heart block. Following corticosteroid therapy, 5 months later, there is resolution of FDG resolution on whole body (D) and cardiac (E-F) FDG PET/CT.
Figure 4.
57-y/o male with pulmonary and ocular sarcoid presented with palpitations, which were attributed to NSVT. CMR showed small areas of focal mid myocardial LGE in the mid-inferior and mid-lateral wall (panel A, solid arrows). Subsequently, cardiac FDG PET identified active myocardial inflammation along the lateral wall (arrow in multiple panels). The latter responded to treatment with steroids, although a small amount of residual pulmonary FDG uptake persisted. (see panels B vs C for comparison of FDG uptake). The top two rows represent imaging prior to treatment; the bottom two rows represent imaging after treatment.
FDG has a number of limitations, the most important being that healthy myocardial cells can utilize glucose as their energy source, thereby compromising distinction between physiologic and pathologic FDG uptake. To circumvent this limitation, several strategies to suppress FDG uptake by normal myocardium have been described, including prolonged fasting, dietary switch to a lipid-rich/carbohydrate-deprived diet 24 hours before the exam, and use of intravenous heparin prior to FDG injection. Unfortunately, even after strict adhesion to these techniques, at least 10%−15% of FDG PET remain nondiagnostic due to incomplete FDG suppression,37 thus making the distinction of pathologic from physiologic FDG uptake not possible in some cases. In addition, ischemic (hibernating) myocardium,38 and the failing heart39 can cause glucose upregulation from mechanisms other than inflammation, which may potentially yield further false positive scans. Furthermore, myocardial inflammation may be seen in other forms of dilated cardiomyopathy as well, and its presence has been recently associated with adverse myocardial remodeling and disease progression.40 Finally, the radiation exposure from a typical cardiac FDG PET/CT protocol, including a limited whole body FDG PET (skull-base through mid-thighs), is not trivial, however; with the development of three-dimensional acquisition and advent of more sensitive PET systems, it is expected that radiation exposure will continue to decrease substantially in the years to come.41
CARDIAC MAGNETIC RESONANCE IMAGING
The use of CMR in CS is based on identifying myocardial LGE in a typical distribution. Gadolinium is a biologically inert contrast agent that after intravenous administration remains in areas of expanded extracellular space (e.g., most often fibrosis, but in some cases marked inflammation) thus allowing for its visualization on delayed images (usually 10 minutes after injection).42 While the presence of myocardial LGE is common in a number of nonischemic cardiomyopathies, there are certain patterns of myocardial involvement that are considered typical for sarcoidosis.43,44
From a diagnostic perspective, the sensitivity (75%−100%) and specificity (76.9%−78%) of LGE-CMR have been reportedly comparable with FDG PET,29–31 but these comparisons have used a suboptimal reference standard, as discussed above. However, CMR has a number of potential advantages over FDG PET that deserve consideration. First, the higher spatial resolution of CMR compared with PET allows for visualization of subcentimeter lesions as well as the distinction between subepicardium, mid-myocardium, and subendocardium involvement, features which are potentially helpful to distinguish among various alternative diagnoses. This is relevant as sarcoid heart disease can consist of both microscopic and macroscopic lesions.18 Yet, the vast majority of clinically relevant sarcoid-related lesions are macroscopic in the form of either granulomatous nodules and/or scar formation. In fact, the presence of LGE appears to be the strongest predictor for mortality and sustained ventricular arrhythmias among individuals with suspected CS, and has a very high negative predictive value for adverse outcomes in general and ventricular arrhythmic events in particular.17,45 In a recent meta-analysis that included 694 patients with suspected sarcoidosis from 7 different studies, Hulten and colleagues observed that ventricular arrhythmias occurred only in patients with myocardial LGE, and the annualized incidence of all-cause mortality was significantly higher in patients with LGE (3.1%) than without LGE (0.6%; P = .04).46
Another important advantage of CMR is the significantly low number of nondiagnostic scans, and the fact that no dietary preparation is required prior to testing. Obviously, CMR is not exempt from technical issues, including gating and respiratory motion artifacts,47 which can affect the diagnostic quality of CMR, to the point of rendering it nondiagnostic in rare cases. Nevertheless, there is a higher nondiagnostic rate of PET due to incomplete FDG suppression, and thus CMR is often considered the most suitable initial test for evaluating patients with suspected CS.48
On the other hand, CMR is usually contraindicated in patients with implantable cardiac devices, and administration of gadolinium is also contraindicated in patients with advanced renal dysfunction.49 Another limitation is that unlike FDG, CMR cannot be reliably used to assess response to therapy.34 However, in this respect, there are emerging data showing that the use of precontrast quantitative T2-weighted CMR imaging (T2-mapping), a well-established marker of tissue edema in acute myocardial infarction and myocarditis, may have the potential to serve as a marker of disease activity. A recent study showed that among patients with suspected CS, myocardial T2 signal was significantly higher in patients with electrocardiographic abnormalities and arrhythmias compared to those without dysrhythmias.50 The same group also observed that compared to baseline (70.0 ± 5.5 ms), T2 signal decreased significantly after 4 months of immunosuppressant therapy (59.2 ± 6.1 ms; P = .017).51 While this preliminary data is intriguing, additional studies are needed to further define the role of T2-weighted imaging in detection of disease activity, especially since tissue edema has not been yet demonstrated to be a typical feature of sarcoidosis.
COMBINED USE OF CMR AND FDG PET
There is growing evidence supporting the combined use of CMR and FDG PET imaging for enhancing both the diagnostic and prognostic performance of evaluating patients with suspected CS (Figure 5).44,52,53 Supporting the complementary value of this multimodality approach, Vita and colleagues recently categorized the likelihood of CS in 107 patients (Table 2) as follows: (1) no (< 10%); (2) possible (10%−50%); (3) probable (50%−90%); or (4) highly probable (> 90%). A final adjudicated diagnosis (including imaging, clinical data, and pathology) was ascertained by consensus and used as Reference.44 In total, 85% had LGE on CMR, whereas 76% had abnormal FDG on PET. Among those with LGE, 66% had abnormal FDG uptake, supporting the notion that LGE cannot be reliably used to identify patients who may benefit from anti-inflammatory therapies. When added to CMR results, PET findings were used to reclassify 45% of patients as having a higher or lower likelihood of CS, 80% of them correctly reclassified based on the final adjudicated diagnosis.
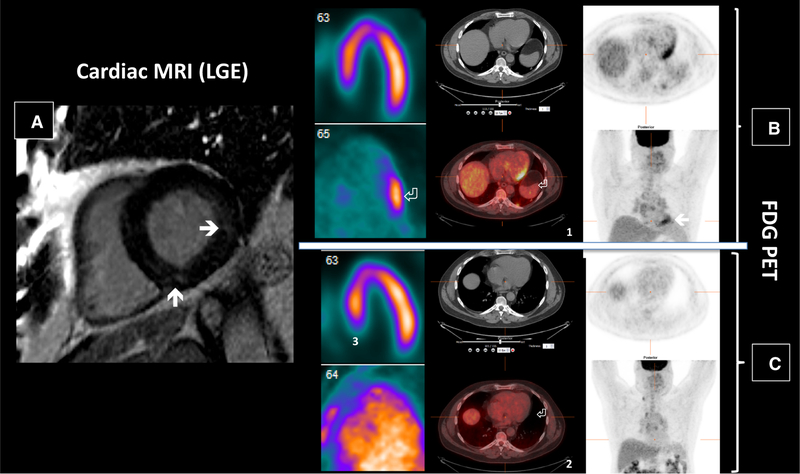
Figure 5.
65 y/o male with known pulmonary and parotid sarcoidosis presented with unexplained syncope. CMR (panel A) revealing focal subepicardial LGE in the apical anterior and antero-septal wall (solid arrows), while FDG PET (panels B and C) demonstrated a large amount of left (white arrow) and right (arrowhead) ventricular myocardial inflammation.
Table 2.
Defining likelihood of cardiac sarcoidosis
| Category | Probability of CS (%) | Potential clinical implications |
|---|---|---|
| No cardiac sarcoidosis | < 10 | No further evaluation or treatment. |
| Possible cardiac sarcoidosis | < 50 | Likelihood of CS low enough that treatment not recommended; further evaluation could be considered in the future in selected cases depending on clinical scenario. |
| Probable cardiac sarcoidosis | 50–90 | Likelihood of CS is sufficiently high to consider treatment. Further diagnostic testing could be considered, if it will impact patient management. |
| Highly probable sarcoidosis | > 90 | Likelihood of CS is high. Treatment generally recommended, even if biopsy is not available or is negative. Further diagnostic testing not required. |
Treatment refers to either immunosuppressive therapies (when inflammation is present) or to use of ICD for prevention of SCD, especially if other indications for such therapies is present
In another study, Dweck and colleagues also showed the importance of combining PET and CMR. The authors prospectively investigated 25 patients with clinical suspicion of CS on a hybrid PET-MR system.52 They observed that eight patients had neither characteristic sarcoid LGE nor increased FDG uptake, and the diagnosis of CS was then unlikely. In contrast, eight out of nine patients with characteristic sarcoid LGE pattern also had focally increased myocardial FDG uptake matching the location of LGE. This group was consistent with active CS, whereas, the subject without FDG uptake (who had known extra-CS) was felt to have inactive CS. The remaining eight patients demonstrated increased myocardial FDG uptake without LGE. Patients with matching LGE and focal FDG uptake tended to have relatively little variation in myocardial FDG activity between 10 and 70 minutes, whereas, patients with diffuse and focal on diffuse FDG uptake had a clear stepwise increment of myocardial FDG activity over time, starting at 10 minutes and extending possibly beyond 70 minutes, strongly suggesting that, in the absence of LGE, these latter patterns most likely represent physiologic (from incomplete suppression) rather than pathologic FDG uptake. Nevertheless, it is important to acknowledge the fact that focal and focal on diffuse FDG uptake without accompanying LGE may still represent early CS in a small proportion of patients with high pretest probability (e.g., heart block in a patient with known sarcoidosis).54
In addition to its complementary diagnostic value, the classification of groups by PET and CMR appears to provide different risk profiles as well. This was suggested in another study where 56 patients with suspected CS were sequentially evaluated with PET/CT and MRI systems, and retrospectively followed for 2.6 years (IQR 1.2–4.1) for the occurrence of major events.53 The main findings were that the risk of all-cause death and ventricular arrhythmic events (n = 16/56) was similarly elevated between LGE-positive/FDG-positive (n = 7/20, HR 10.1 [95% CI 1.2–84]) and LGE-positive/FDG-negative (n = 8/16, HR 13.3 [1.7–107]) individuals, in referenced to patients with absent LGE and FDG, whom had the best outcomes (n = 1/20). Of note, FDG-positive/LGE-negative patients were not documented in this study, which was most likely the result of excluding cases showing diffuse and focal on diffuse myocardial FDG uptake from the study. In addition, a number of LGE-positive/FDG-negative patients had neither typical LGE pattern nor clinical history of sarcoidosis; thus, it is possible that some of these cases may represent cardiomyopathies other than sarcoidosis. While limited in size, this study, together with the findings of Vita et al,44 suggests that CMR may have a larger contribution than FDG PET when assessing prognosis.
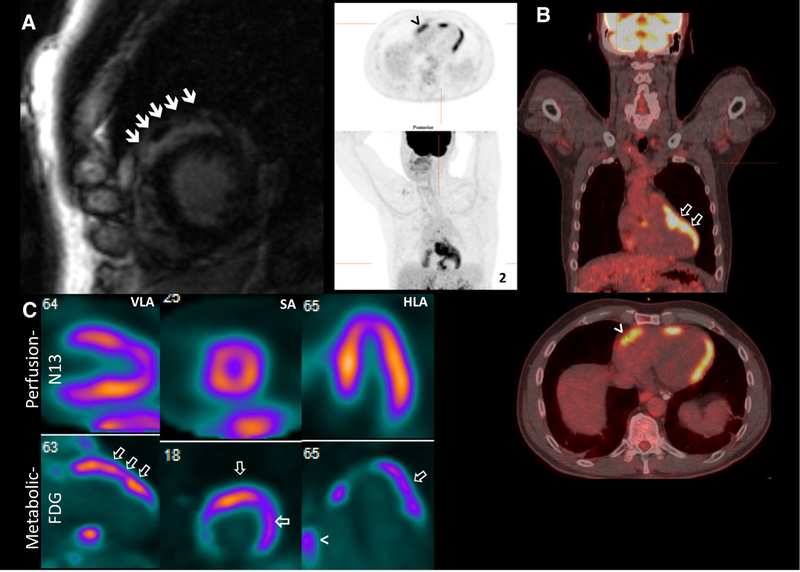
Finally, with the advent of integrated PET-MR scanners, simultaneous acquisition of FDG PET and LGE-CMR is nowadays a reality. However, these systems remain very costly and limited to only a few centers in the world. Consequently, the vast majority of combined evaluations (at least in the near and intermediate future) will continue to be performed sequentially on stand-alone MRI and PET/CT scanners. A proposed diagnostic algorithm taking advantage of the information provided by CMR and FDG PET/CT is presented in Figure 6.
Figure 6.
Proposed algorithm for the evaluation of patients with suspected cardiac sarcoidosis.
FUTURE NUCLEAR TECHNIQUES FOR IMAGING INFLAMMATION
Despite its widespread use, FDG lacks specificity and is, thus, not an ideal tracer for the detection of myocardial inflammation. As a result, there is an ongoing effort to identify new potential molecular targets for the identification of CS, which unite at least the following two characteristics: (1) exhibiting minimal myocardial uptake under basal conditions so that differentiation of pathologic vs physiologic uptake can be facilitated, and (2) being a sensitive marker of inflammation. It is worth mentioning that the development of sarcoidosis-specific radiotracers seems a challenging task, especially since the condition itself remains a diagnosis of exclusion on histology, and since there is no adequate diagnostic reference standard for validating new tracers. Nevertheless, aside from their diagnostic capabilities, new tracers should also aid in assessing response to therapy.
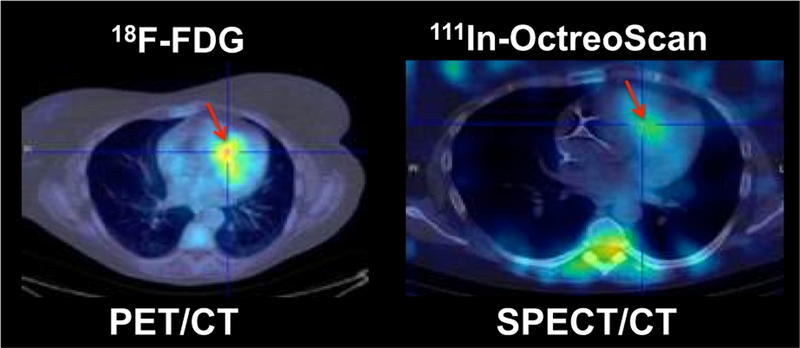
When considering future targets for imaging inflammation, activated macrophages in sarcoidosis have been shown to overexpress the somatostatin receptor subtype 2 (sstr-2).55,56 Indium-111 (In-111) penteotride (OctreoScan), and the PET agents, gallium-68 (Ga-68) DOTATOC, Ga-68 DOTATATE, and Ga-68 DOTA-NOC are radiopharmaceuticals that bind preferentially to sstr-2, and have an advantageous biodistribution for cardiac imaging as they lack cardiac uptake under baseline conditions.57,58 Although originally developed for the detection of neuroendocrine tumors, somatostatin receptor-targeted (SSTR) scintigraphy has been shown to be of potential diagnostic value in patients with sarcoidosis localized to the lung, mediastinum, hilar lymph nodes,59–62 and most recently, the heart (Figure 7).63–66 In one small series among patients with myocarditis, including CS, Ga-68 DOTATOC was compared to LGE-CMR, and a close spatial relation between SSTR uptake and LGE was observed.63 In a different study, Ga-68 DOTANOC was performed within 7 days from FDG PET in 19 patients with suspected CS. The JMHW criteria were used as the reference, and three patients were deemed as definitely having CS. The authors reported that FDG was rated as inconclusive in 11 out of 19 patients, whereas no DOTANOC scan was considered inconclusive. Similarly, FDG was positive in 1 out of 3 patients with CS, and negative/inconclusive in 14 out of 16 patients without sarcoidosis. In contrast, they found that Ga-68 DOTANOC was positive in 3 out of 3 patients with sarcoidosis and negative in 16 out of 16 patients without the condition. The authors concluded that SSTR PET imaging might carry a higher diagnostic accuracy than FDG PET. However, this study size is small, and the number of inconclusive scans (58%) was exceedingly high compared with current standards, and therefore, these data, although promising, should be taken with caution and interpreted as preliminary.
Figure 7.
Patient with cardiac sarcoidosis showing evidence of inflammation in the anterior septum (red arrow) by FDG PET/CT as well as by OctreoScan SPECT/CT.
Imaging cell proliferation is another appealing molecular target. F-18 3′-fluoro-3′-deoxythymidine (FLT) is a radiotracer that accumulates in high-turnover cells and has found important clinical applications in tumor proliferation imaging. After administration, FLT is taken up by cells and phosphorylated by thymidine kinase 1 (TK), leading to intracellular trapping. Thus, FLT is considered a marker of cellular TK activity, an enzyme closely related to cellular proliferation.67 Myocardial FLT uptake is low in normal hearts.
Experimental data has shown that granulomatous inflammatory cells, including macrophages, epithelioid, and multinucleated cells, can exhibit both low-turnover and high-turnover behaviors within the same lymph node.68 In preclinical studies, FLT uptake has been shown to be comparable to that of FDG in rat models of granulomatous disease,69 and in recent studies FLT has been shown to accumulate in both extracardiac70 and CS.71,72 Additional studies are required to further evaluate the clinical utility of these novel probes for myocardial inflammation detection.
CONCLUSIONS/FUTURE DIRECTIONS
Despite significant recent developments, the diagnosis of CS remains challenging. Nevertheless, FDG PET and LGE-CMR have both proven to be advanced imaging techniques that provide important, and often complementary, diagnostic and prognostic information for the management of CS. As a result, current algorithms for diagnosing and treating individuals with suspected sarcoidosis should incorporate both CMR and FDG PET and identify subgroups in whom both tests may be needed (e.g., those in whom any one test is inconclusive, or the diagnosis is uncertain). Such an approach could aid in estimating the likelihood of CS and identify those who are most likely to benefit from immunosuppressive therapies. At the same time, more studies are needed relating how imaging findings could be used to enhance the type, duration, and intensity of immunosuppressive therapies. In addition, there is a need for developing more specific imaging probes, as well as serum biomarkers, for identifying cardiac inflammation.
Acknowledgments
This work was supported in part by a grant from the National Institutes of Health (1T32HL094301, Dr. Bravo).
Abbreviations
- CS
Cardiac sarcoidosis
- CMR
Cardiac magnetic resonance imaging
- FDG
Fluorine-18 fluorodeoxyglucose
- PET
Positron emission tomography
- LGE
Late gadolinium enhancement
Footnotes
Disclosure
The authors have no conflict of interest to disclose.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s12350-018-01488-9) contains supplementary material, which is available to authorized users.
References
- 1.Longcope WT, Freiman DG. A study of sarcoidosis; based on a combined investigation of 160 cases including 30 autopsies from The Johns Hopkins Hospital and Massachusetts General Hospital. Medicine (Baltim) 1952;31:1–132. [PubMed] [Google Scholar]
- 2.Silverman KJ, Hutchins GM, Bulkley BH. Cardiac sarcoid: A clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation 1978;58:1204–11. [DOI] [PubMed] [Google Scholar]
- 3.Sharma OP, Maheshwari A, Thaker K. Myocardial sarcoidosis. Chest 1993;103:253–8. [DOI] [PubMed] [Google Scholar]
- 4.Matsui Y, Iwai K, Tachibana T, et al. Clinicopathological study of fatal myocardial sarcoidosis. Ann NY Acad Sci 1976;278:455–69. [DOI] [PubMed] [Google Scholar]
- 5.Pierre-Louis B, Prasad A, Frishman WH. Cardiac manifestations of sarcoidosis and therapeutic options. Cardiol Rev 2009;17:153–8. [DOI] [PubMed] [Google Scholar]
- 6.Roberts WC, McAllister HA Jr, Ferrans VJ. Sarcoidosis of the heart. A clinicopathologic study of 35 necropsy patients (group 1) and review of 78 previously described necropsy patients (group 11). Am J Med 1977;63:86–108. [DOI] [PubMed] [Google Scholar]
- 7.Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): Developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation 2008;117:e350–408. [DOI] [PubMed] [Google Scholar]
- 8.Kron J, Sauer W, Schuller J, et al. Efficacy and safety of implantable cardiac defibrillators for treatment of ventricular arrhythmias in patients with cardiac sarcoidosis. Europace Eur Pacing Arrhythm Card Electrophysiol J Work Groups Card Pacing Arrhythm Card Cell Electrophysiol Eur Soc Cardiol 2013;15:347–54. [DOI] [PubMed] [Google Scholar]
- 9.Hiramitsu S, Morimoto S, Uemura A, et al. National survey on status of steroid therapy for cardiac sarcoidosis in Japan. Sarcoidosis Vasc Diffus Lung Dis 2005;22:210–3. [PubMed] [Google Scholar]
- 10.Birnie DH, Sauer WH, Bogun F, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm Off J Heart Rhythm Soc 2014;11:1305–23. [DOI] [PubMed] [Google Scholar]
- 11.Chareonthaitawee P, Beanlands RS, Chen W, et al. Joint SNMMI-ASNC expert consensus document on the role of (18)F-FDG PET/ CT in cardiac sarcoid detection and therapy monitoring. J Nucl Cardiol Off Publ Am Soc Nucl Cardiol 2017;24:1741–58. [DOI] [PubMed] [Google Scholar]
- 12.Writing Group, Document Reading Group, EACVI Reviewers. A joint procedural position statement on imaging in cardiac sarcoidosis: From the Cardiovascular and Inflammation and Infection Committees of the European Association of Nuclear Medicine, the European Association of Cardiovascular Imaging, and the American Society of Nuclear Cardiology. Eur Heart J Cardiovasc Imaging 2017;18:1073–89. [DOI] [PubMed] [Google Scholar]
- 13.Uemura A, Morimoto S, Hiramitsu S, Kato Y, Ito T, Hishida H. Histologic diagnostic rate of cardiac sarcoidosis: Evaluation of endomyocardial biopsies. Am Heart J 1999;138:299–302. [DOI] [PubMed] [Google Scholar]
- 14.Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med 2007;357:2153–65. [DOI] [PubMed] [Google Scholar]
- 15.Deckers JW, Hare JM, Baughman KL. Complications of transvenous right ventricular endomyocardial biopsy in adult patients with cardiomyopathy: A seven-year survey of 546 consecutive diagnostic procedures in a tertiary referral center. J Am Coll Cardiol 1992;19:43–7. [DOI] [PubMed] [Google Scholar]
- 16.Okumura W, Iwasaki T, Toyama T, et al. Usefulness of fasting 18F-FDG PET in identification of cardiac sarcoidosis. J Nucl Med Off Publ Soc Nucl Med 2004;45:1989–98. [PubMed] [Google Scholar]
- 17.Greulich S, Deluigi CC, Gloekler S, et al. CMR imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC Cardiovasc Imaging 2013;6:501–11. [DOI] [PubMed] [Google Scholar]
- 18.Tavora F, Cresswell N, Li L, Ripple M, Solomon C, Burke A. Comparison of necropsy findings in patients with sarcoidosis dying suddenly from cardiac sarcoidosis versus dying suddenly from other causes. Am J Cardiol 2009;104:571–7. [DOI] [PubMed] [Google Scholar]
- 19.Thomson AD. The pathology of sarcoidosis. Postgrad Med J 1958;34:248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poulter LW. Immune aspects of sarcoidosis. Postgrad Med J 1988;64:536–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nihoyannopoulos P, Dawson D. Restrictive cardiomyopathies. Eur J Echocardiogr 2009;10:iii23–33. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal A, Sulemanjee NZ, Cheema O, Downey FX, Tajik AJ. Cardiac sarcoid: A chameleon masquerading as hypertrophic cardiomyopathy and dilated cardiomyopathy in the same patient. Echocardiography 2014;31:E138–41. [DOI] [PubMed] [Google Scholar]
- 23.Adlan AM, Prasad SK, Varnava AM. Sarcoidosis presenting as dilated cardiomyopathy. Heart 2011;97:1896. [DOI] [PubMed] [Google Scholar]
- 24.Patel AR, Klein MR, Chandra S, et al. Myocardial damage in patients with sarcoidosis and preserved left ventricular systolic function: An observational study. Eur J Heart Fail 2011;13:1231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freeman AM, Curran-Everett D, Weinberger HD, et al. Predictors of cardiac sarcoidosis using commonly available cardiac studies. Am J Cardiol 2013;112:280–5. [DOI] [PubMed] [Google Scholar]
- 26.Kouranos V, Tzelepis GE, Rapti A, et al. Complementary role of CMR to conventional screening in the diagnosis and prognosis of cardiac sarcoidosis. JACC Cardiovasc Imaging 2017;10:1437–47. [DOI] [PubMed] [Google Scholar]
- 27.Mehta D, Lubitz SA, Frankel Z, et al. Cardiac involvement in patients with sarcoidosis: Diagnostic and prognostic value of outpatient testing. Chest 2008;133:1426–35. [DOI] [PubMed] [Google Scholar]
- 28.Okumura W, Iwasaki T, Ueda T, et al. Usefulness of 18F-FDG PET for diagnosis of cardiac sarcoidosis. Kaku Igaku 1999;36:341–8. [PubMed] [Google Scholar]
- 29.Ohira H, Tsujino I, Ishimaru S, et al. Myocardial imaging with 18F-fluoro-2-deoxyglucose positron emission tomography and magnetic resonance imaging in sarcoidosis. Eur J Nucl Med Mol Imaging 2008;35:933–41. [DOI] [PubMed] [Google Scholar]
- 30.Smedema JP, Snoep G, van Kroonenburgh MP, et al. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol 2005;45:1683–90. [DOI] [PubMed] [Google Scholar]
- 31.Youssef G, Leung E, Mylonas I, et al. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: A systematic review and metaanalysis including the Ontario experience. J Nucl Med Off Publ Soc Nucl Med 2012;53:241–8. [DOI] [PubMed] [Google Scholar]
- 32.Okada DR, Bravo PE, Vita T, et al. Isolated cardiac sarcoidosis: A focused review of an under-recognized entity. J Nucl Cardiol Off Publ Am Soc Nucl Cardiol 2016. 10.1007/s12350-016-0658-1. [DOI] [PMC free article] [PubMed]
- 33.Sperry BW, Oldan J, Hachamovitch R, Tamarappoo BK. Insights into biopsy-proven cardiac sarcoidosis in patients with heart failure. J Heart Lung Transplant Off Publ Int Soc Heart Transplant 2015. 10.1016/j.healun.2015.12.005. [DOI] [PMC free article] [PubMed]
- 34.Osborne MT, Hulten EA, Singh A, et al. Reduction in (1)(8)F-fluorodeoxyglucose uptake on serial cardiac positron emission tomography is associated with improved left ventricular ejection fraction in patients with cardiac sarcoidosis. J Nucl Cardiol Off Publ Am Soc Nucl Cardiol 2014;21:166–74. [DOI] [PubMed] [Google Scholar]
- 35.Blankstein R, Osborne M, Naya M, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol 2014;63:329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orii M, Hirata K, Tanimoto T, et al. Comparison of cardiac MRI and 18F-FDG positron emission tomography manifestations and regional response to corticosteroid therapy in newly diagnosed cardiac sarcoidosis with complete heart block. Heart Rhythm Off J Heart Rhythm Soc 2015;12:2477–85. [DOI] [PubMed] [Google Scholar]
- 37.Osborne MT, Hulten EA, Murthy VL, et al. Patient preparation for cardiac fluorine-18 fluorodeoxyglucose positron emission tomography imaging of inflammation. J Nucl Cardiol Off Publ Am Soc Nucl Cardiol 2016. 10.1007/s12350-016-0502-7. [DOI] [PMC free article] [PubMed]
- 38.Sibille L, Chambert B, Collombier L, Kotzki PO, Boudousq V. False positive 18F-FDG PET/CT in cardiac sarcoidosis. J Mol Biol Mol Imaging 2015;2:1020. [Google Scholar]
- 39.Mielniczuk LM, Birnie D, Ziadi MC, et al. Relation between right ventricular function and increased right ventricular [18F]fluorodeoxyglucose accumulation in patients with heart failure. Circ Cardiovasc Imaging 2011;4:59–66. [DOI] [PubMed] [Google Scholar]
- 40.Nakayama T, Sugano Y, Yokokawa T, et al. Clinical impact of the presence of macrophages in endomyocardial biopsies of patients with dilated cardiomyopathy. Eur J Heart Fail 2017;19:490–8. [DOI] [PubMed] [Google Scholar]
- 41.Huang B, Law MW, Khong PL. Whole-body PET/CT scanning: Estimation of radiation dose and cancer risk. Radiology 2009;251:166–74. [DOI] [PubMed] [Google Scholar]
- 42.Kim RJ, Chen EL, Lima JA, Judd RM. Myocardial Gd-DTPA kinetics determine MRI contrast enhancement and reflect the extent and severity of myocardial injury after acute reperfused infarction. Circulation 1996;94:3318–26. [DOI] [PubMed] [Google Scholar]
- 43.Moraes GL, Higgins CB, Ordovas KG. Delayed enhancement magnetic resonance imaging in nonischemic myocardial disease. J Thorac Imaging 2013;28:84–92; quiz 93–5. [DOI] [PubMed] [Google Scholar]
- 44.Vita T, Okada DR, Veillet-Chowdhury M, et al. Complementary value of cardiac magnetic resonance imaging and positron emission tomography/computed tomography in the assessment of cardiac sarcoidosis. Circ Cardiovasc Imaging 2018;11:e007030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel MR, Cawley PJ, Heitner JF, et al. Detection of myocardial damage in patients with sarcoidosis. Circulation 2009;120:1969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hulten E, Agarwal V, Cahill M, et al. Presence of late gadolinium enhancement by cardiac magnetic resonance among patients with suspected cardiac sarcoidosis is associated with adverse cardiovascular prognosis: A systematic review and meta-analysis. Circ Cardiovasc Imaging 2016;9:e005001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferreira PF, Gatehouse PD, Mohiaddin RH, Firmin DN. Cardiovascular magnetic resonance artefacts. J Cardiovasc Magn Reson 2013;15:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blankstein R, Waller AH. Evaluation of known or suspected cardiac sarcoidosis. Circ Cardiovasc Imaging 2016;9:e000867. [DOI] [PubMed] [Google Scholar]
- 49.Bernstein EJ, Schmidt-Lauber C, Kay J. Nephrogenic systemic fibrosis: A systemic fibrosing disease resulting from gadolinium exposure. Best Pract Res Clin Rheumatol 2012;26:489–503. [DOI] [PubMed] [Google Scholar]
- 50.Crouser ED, Ono C, Tran T, He X, Raman SV. Improved detection of cardiac sarcoidosis using magnetic resonance with myocardial T2 mapping. Am J Respir Crit Care Med 2014;189:109–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crouser ED, Ruden E, Julian MW, Raman SV. Resolution of abnormal cardiac MRI T2 signal following immune suppression for cardiac sarcoidosis. J Investig Med 2016;64:1148–50. [DOI] [PubMed] [Google Scholar]
- 52.Dweck MR, Abgral R, Trivieri MG, et al. Hybrid magnetic resonance imaging and positron emission tomography with fluorodeoxyglucose to diagnose active cardiac sarcoidosis. JACC Cardiovasc Imaging 2017. 10.1016/j.jcmg.2017.02.021. [DOI] [PMC free article] [PubMed]
- 53.Bravo PE, Raghu G, Rosenthal DG, et al. Risk assessment of patients with clinical manifestations of cardiac sarcoidosis with positron emission tomography and magnetic resonance imaging. Int J Cardiol 2017;241:457–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ohira H, Birnie DH, Pena E, et al. Comparison of (18)F-fluorodeoxyglucose positron emission tomography (FDG PET) and cardiac magnetic resonance (CMR) in corticosteroid-naive patients with conduction system disease due to cardiac sarcoidosis. Eur J Nucl Med Mol Imaging 2016;43:259–69. [DOI] [PubMed] [Google Scholar]
- 55.van Hagen PM. Somatostatin receptor expression in clinical immunology. Metabolism 1996;45:86–7. [DOI] [PubMed] [Google Scholar]
- 56.ten Bokum AM, Hofland LJ, de Jong G, et al. Immunohistochemical localization of somatostatin receptor sst2A in sarcoid granulomas. Eur J Clin Investig 1999;29:630–6. [DOI] [PubMed] [Google Scholar]
- 57.Balon HR, Goldsmith SJ, Siegel BA, et al. Procedure guideline for somatostatin receptor scintigraphy with (111)In-pentetreotide. J Nucl Med Off Publ Soc Nucl Med 2001;42:1134–8. [PubMed] [Google Scholar]
- 58.Bombardieri E, Ambrosini V, Aktolun C, et al. 111In-pentetreotide scintigraphy: Procedure guidelines for tumour imaging. Eur J Nucl Med Mol Imaging 2010;37:1441–8. [DOI] [PubMed] [Google Scholar]
- 59.Kwekkeboom DJ, Krenning EP, Kho GS, Breeman WA, Van Hagen PM. Somatostatin receptor imaging in patients with sarcoidosis. Eur J Nucl Med 1998;25:1284–92. [DOI] [PubMed] [Google Scholar]
- 60.Lebtahi R, Crestani B, Belmatoug N, et al. Somatostatin receptor scintigraphy and gallium scintigraphy in patients with sarcoidosis. J Nucl Med Off Publ Soc Nucl Med 2001;42:21–6. [PubMed] [Google Scholar]
- 61.Vanhagen PM, Krenning EP, Reubi JC, et al. Somatostatin analogue scintigraphy in granulomatous diseases. Eur J Nucl Med 1994;21:497–502. [DOI] [PubMed] [Google Scholar]
- 62.Nobashi T, Nakamoto Y, Kubo T, et al. The utility of PET/CT with 68Ga-DOTATOC in sarcoidosis: Comparison with 67Gascintigraphy. Ann Nucl Med 2016. 10.1007/s12149-016-1095-6. [DOI] [PubMed]
- 63.Lapa C, Reiter T, Li X, et al. Imaging of myocardial inflammation with somatostatin receptor based PET/CT—A comparison to cardiac MRI. Int J Cardiol 2015;194:44–9. [DOI] [PubMed] [Google Scholar]
- 64.Reiter T, Werner RA, Bauer WR, Lapa C. Detection of cardiac sarcoidosis by macrophage-directed somatostatin receptor 2-based positron emission tomography/computed tomography. Eur Heart J 2015;36:2404. [DOI] [PubMed] [Google Scholar]
- 65.Gormsen LC, Haraldsen A, Kramer S, Dias AH, Kim WY, Borghammer P. A dual tracer (68)Ga-DOTANOC PET/CT and (18)F-FDG PET/CT pilot study for detection of cardiac sarcoidosis. EJNMMI Res 2016;6:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smedema JP, van Kroonenburgh MJ, Snoep G, Backes W, Gorgels AP. Images in cardiovascular medicine. Cardiac sarcoidosis in a patient with hypertrophic cardiomyopathy demonstrated by magnetic resonance imaging and single photon emission computed tomography dual-isotope scintigraphy. Circulation 2004;110:e529–31. [DOI] [PubMed] [Google Scholar]
- 67.Shields AF, Grierson JR, Dohmen BM, et al. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med 1998;4:1334–6. [DOI] [PubMed] [Google Scholar]
- 68.van der Gaag RD, van Maarsseveen AC, Broekhuizen-Davies JM, Stam J. Application of in vitro techniques to determine proliferation in human sarcoid lymph nodes. J Pathol 1983;139:239–45. [DOI] [PubMed] [Google Scholar]
- 69.Zhao S, Kuge Y, Kohanawa M, et al. Usefulness of 11C-methionine for differentiating tumors from granulomas in experimental rat models: A comparison with 18F-FDG and 18F-FLT. J Nucl Med Off Publ Soc Nucl Med 2008;49:135–41. [DOI] [PubMed] [Google Scholar]
- 70.Kim SK, Im HJ, Kim W, Kim TS, Hwangbo B, Kim HJ. F-18 fluorodeoxyglucose and F-18 fluorothymidine positron emission tomography/computed tomography imaging in a case of neurosarcoidosis. Clin Nucl Med 2010;35:67–70. [DOI] [PubMed] [Google Scholar]
- 71.Norikane T, Yamamoto Y, Maeda Y, Noma T, Nishiyama Y. 18F-FLT PET imaging in a patient with sarcoidosis with cardiac involvement. Clin Nucl Med 2015;40:433–4. [DOI] [PubMed] [Google Scholar]
- 72.Norikane T, Yamamoto Y, Maeda Y, Noma T, Dobashi H, Nishiyama Y. Comparative evaluation of (18)F-FLT and (18)F-FDG for detecting cardiac and extra-cardiac thoracic involvement in patients with newly diagnosed sarcoidosis. EJNMMI Res 2017;7:69. [DOI] [PMC free article] [PubMed] [Google Scholar]