Abstract
Glutamate is the primary excitatory neurotransmitter in neurons and glia. N-methyl-D-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), and kainate receptors are major ionotropic glutamate receptors. Glutamatergic neurotransmission is strongly linked with Ca2+ homeostasis. Research has provided ample evidence that brain aging is associated with altered glutamatergic neurotransmission and Ca2+ dysregulation. Much of the work has focused on the hippocampus, a brain region critically involved in learning and memory, which is particularly susceptible to dysfunction during senescence. The current review examines Ca2+ regulation with a focus on the NMDA receptors in the hippocampus. Integrating the knowledge of the complexity of age-related alterations in Ca2+ homeostasis and NMDA receptor-mediated glutamatergic neurotransmission will positively shape the development of highly effective therapeutics to treat brain disorders including cognitive impairment.
Keywords: Aging, calcium homeostasis, hippocampus, glutamatergic neurotransmission, N-methyl-D-aspartate receptor, synaptic function, LTP and LTD
Introduction
The hypothesized role of senescent glutamatergic synapses in age-related memory decline is founded on studies examining the mechanisms for age-related changes in calcium (Ca2+)-dependent synaptic plasticity. The current review focuses on assessing age-associated changes in Ca2+ regulation and N-methyl-D-aspartate (NMDA) receptor-mediated synaptic transmission, in the hippocampus. In particular, we focus on possible mechanisms for an age-related decline in the function of NMDA glutamate receptors. The activity of NMDA receptors is critical for synaptic plasticity, long-term potentiation (LTP), and long-term depression (LTD), in the hippocampus.
An age-related shift in synaptic plasticity
LTP is a rapid and long lasting increase in synaptic transmission in response to intense synaptic activity. The induction of LTP requires activation of postsynaptic NMDA receptors resulting in a large, yet brief, influx of Ca2+ through the NMDA receptor channel. In turn, this large rise in intracellular Ca2+ activates Ca2+ sensitive kinases. Kinase activity increases the strength of the synaptic response through phosphorylation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) glutamate receptors, which leads to insertion of additional AMPA-glutamate channels into the post-synaptic membrane [1]. Early studies demonstrated that decay of LTP increased in aged animals and correlated with forgetting, suggesting that impaired acquisition and retention of information were related to impairment in the induction and maintenance of LTP [2].
In contrast to LTP, which requires a brief but large increase in intracellular Ca2+, LTD is induced by a modest and prolonged rise in intracellular Ca2+. The small and sustained rise in Ca2+ activates Ca2+-sensitive phosphatases that decrease synaptic transmission through dephosphorylation of AMPA-glutamate receptors, resulting in their removal from the post-synaptic membrane [3]. Initial studies of LTD indicated developmental regulation, such that the ability to induce LTD in the hippocampus declined from neonatal to adult periods. Thus, the discovery that aged animals exhibit increase in susceptibility to induction of LTD was unexpected [4–7]. The increase in susceptibility to LTD was shown to contribute to the decay of LTP and the induction or magnitude of LTD correlated with increased forgetting in older animals [7–9]. The results implicate LTD as a mechanism for enhancing the decay of LTP and an increased level of forgetting observed with advanced age.
The shift in synaptic plasticity during aging, favoring LTD over LTP, likely contributes to other correlates of cognitive aging including a decrease in synaptic strength and reduced transmission through the hippocampus of older animals [8,9]. In turn, the weakening of synaptic transmission could contribute to the decrease in activation of the hippocampus of aging-memory impaired humans, recorded as a decrease in the functional magnetic resonance imaging (fMRI) blood oxygen-level dependent (BOLD) signal during learning or recall [10]. Moreover, LTD is involved in synapse removal, such that LTD may decrease synaptic connectivity and contribute to the reduction in hippocampal volume [11–13]. Together, these results point to Ca2+ dysregulation as a mechanism for senescent physiology characterized by decreased LTP, increased LTD, and decreased synaptic transmission.
Ca2+-dysregulation with advance age
Thirty years ago, observations of age-related changes in how neurons handle Ca2+ led to the formulation of the Ca2+ hypothesis of brain aging [14]. The initial Ca2+ hypothesis proposed that a small and prolonged increase in Ca2+ would over time, have toxic effects, resulting in neuronal death, similar to that observed following a large increase over a short period. Initial studies provided an evidence for a small and sustained upsurge in intracellular free Ca2+ with advance age [15]. Due to the discoveries that normal aging is not associated with a loss of neurons [16,17], the hypothesis has changed to reflect altered Ca2+-dependent physiology, including senescent synaptic function [8,18,19].
The Ca2+ ion is a central signaling molecule in numerous cellular functions including apoptosis, energy production, gene regulation, cell proliferation, membrane excitability, synaptic transmission, and plasticity. Due to the ubiquitous nature of Ca2+ signaling, Ca2+ is one of the most highly regulated ions with the concentration inside the cell maintained at a level 10,000 times lower than the concentration in the extracellular space [20–22]. Accordingly, any change in Ca2+ regulating mechanisms can result in an alteration in cell function. Age related changes have been reported for Ca2+-buffering and extrusion mechanisms [23–25]. In addition, aging hippocampal neurons exhibit a shift in the sources of Ca2+. Moreover, the shift in level of different Ca2+ sources likely results in changes in the subcellular localization of Ca2+ at the synapse, dendrite, and soma. During neural activity, intracellular Ca2+ rises at the synapse mainly due to influx of Ca2+ into the cell through NMDA receptors. In the soma and dendrites, Ca2+ signals arise due to receptor activity; however, much of the Ca2+ arises from voltage‐dependent Ca2+ channels (VDCCs) and release of Ca2+ from intercellular Ca2+ stores (ICS). In the case of aging, Ca2+ from NMDA receptors appears to decrease and that from VDCCs and ICS increases (Fig 1).
Figure 1.
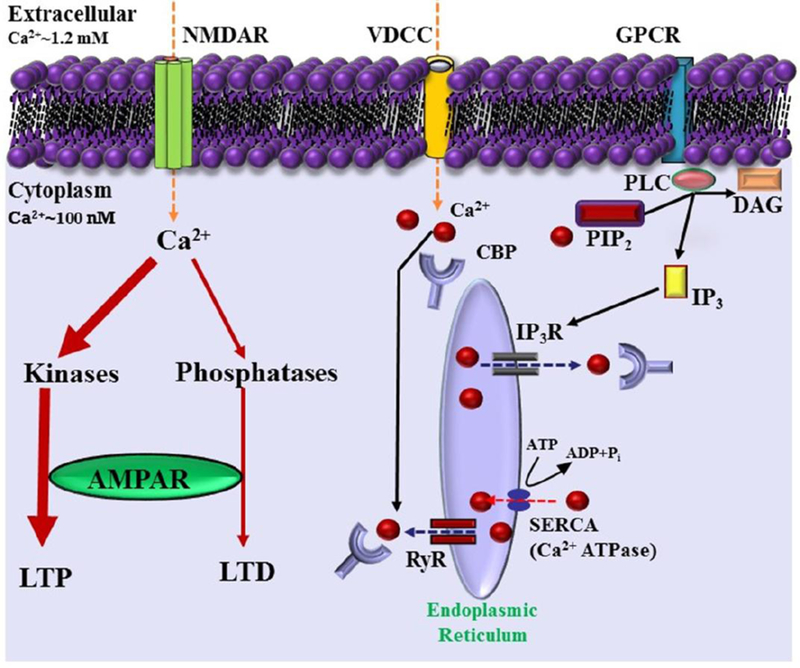
Model illustrating various Ca2+ sources including NMDA receptor (NMDAR), voltage-dependent Ca2+ channels (VDCC), and G protein-coupled receptor (GPCR). Ca2+ (red balls) influxes into the cytosol (yellow dashed arrows) through these sources in a healthy neuron. The release of Ca2+ into the cytoplasm also occurs from the intracellular Ca2+ stores through inositol (1,4,5)-trisphosphate receptor (IP3R) and ryanodine receptors (RyR) involving phospholipase C (PLC), diacylglycerol (DAG) and inositol (1,4,5)-trisphosphate (IP3). Organelles, including the endoplasmic reticulum act as a Ca2+ buffering system, releasing and sequestering Ca2+. Further, the model depicts Ca2+ buffering and extrusion pathways (red dashed arrows), involving plasma membrane Ca2+ ATPase, sarcoplasmic reticulum Ca2+ ATPases (SERCA), and various Ca2+ binding proteins (CBP).
The NMDA Receptor
NMDA receptors represent one of the ligand-gated non-selective cation ionotropic glutamate receptors, which are present in high density within the hippocampus and play pivotal physiological and pathophysiological roles in the central nervous system [26–28]. NMDA receptors are hetero-tetrameric protein complexes composed of two classes of related subunits from seven homologous genes, GluN1, GluN2A-GluN2D, and GluN3A-GluN3B [29–36]. The majority of NMDA receptors are assemblies of two GluN1 subunits, the ubiquitously expressed and obligatory subunit, and two GluN2A-D subunits, a modulatory subunit. In addition, GluN3 subunits (GluN3A and GluN3B), without involving GluN2 subunits, can assemble with GluN1 subunits to form functional receptors [35,37–40]. Developmentally, the expression of GluN1, GluN2B, and GluN3A decreases with age compared to adulthood, while an increase in the expression of GluN2A and GluN3B is reported during development [40].
NMDA receptors, along with AMPA and kainite receptors, are critical for the rapid regulation of synaptic plasticity including LTP and LTD, which are important cellular correlates for learning and memory function [41–47]. The induction of LTP and LTD involves activation of NMDA receptors, Ca2+ entry, and differential activation of kinases/phosphatases [18]. Interestingly, recent work indicates that amyloid beta could act on GluN2B subunits, through metabotropic mechanisms, to influence phosphatase/kinase activity, influencing synaptic function and spine loss [48,49].
Physiological studies consistently indicate that NMDA receptor mediated excitatory postsynaptic potentials in the Schaffer collateral pathway of the hippocampus are reduced by approximately 50–60% in aged animals [50–57]. In turn, a decrease in NMDA receptor function is likely to influence induction of AMPA receptor mediated synaptic plasticity [48,58] (i.e. metaplasticity [59]).
Expression of NMDA receptor during aging
Alteration in expression of specific NMDA receptor subunits might be a potential mechanism for the observed decrease in the NMDA receptor function [60]. GluN1 subunit of NMDA receptor is highly expressed in the hippocampus; results demonstrate that GluN1 subunit is susceptible to aging process. A significant decrease in the expression of GluN1 protein levels with advancing age is observed in the hippocampus [51,61–65]. GluN1 mRNA expression of GluN1 subunit also declines with increasing age in the hippocampus [60,66]. In contrast, other studies report modest or no age-related decrease in GluN1 protein expression in the whole hippocampus [67,68]. These studies suggest that the GluN1 subunit of the NMDA receptor is variably susceptible to the influence of aging process.
The GluN2B subunit is highly expressed throughout the brain during early stages of development and declines at the onset of sexual maturity; GluN2A subunit-containing NMDA receptors increase across the same life span [69–74]. A shift in GluN2A and GluN2B expression in the hippocampus is thought to contribute to developmental changes in cognition and synaptic function [75]. GluN2A subunit of NMDA receptor is highly expressed in the hippocampus and other brain regions. Aging is associated with no change or a modest decrease in the expression of GluN2A mRNA expression in the hippocampus [60,76,77]. Similarly, there is some indication that expression of GluN2A protein is decreased in the hippocampus of aged animals when compared with middle aged animals [63], while other studies indicate no age-related change in the GluN2A subunit in the hippocampus [62,78,79].
Interestingly, GluN2B subunits of NMDA receptor display slower channel kinetics and greater Ca2+ conductance. These channels, by taking longer duration to close, allow more Ca2+ influx into the cell over a longer period, and are therefore thought to be more conducive to the induction of activity dependent synaptic plasticity. Additionally, upregulation of GluN2B significantly augments LTP and memory function in rodents [80,81], including aged mice [82]. Finally, studies that involve viral vector-mediated upregulation of GluN2B expression in adult hippocampus suggest that increasing the level of GluN2B expression can improve cognitive function during aging [54,79].
In contrast to GluN2A, expression of GluN2B protein [62–64, 67, 68, 78, 83, 84] and GluN2B mRNA [60, 76–78, 83, 85] is generally reported to decline in the hippocampus with advanced age. One problem is that few studies have examined the expression of both subunits in the same animal. For studies that examine the protein expression of both subunits, some studies suggest that decreased expression, mainly of GluN2B, increases the ratio of GluN2A/GluN2B protein in several brain regions [62, 78, 86]. However, other reports indicate that both subunits decline equally with advanced age [63, 67].
Modification of existing receptors
In addition, to the required binding of glutamate and postsynaptic depolarization, NMDA receptors are regulated by posttranslational modification of the receptor. In adults, the NMDA receptor synaptic responses can undergo LTP (NMDAR-LTP) and LTD (NMDAR-LTD) [87]. NMDAR-LTP depends on the activity of NMDARs, a subsequent increase in intracellular Ca2+, and kinase activity [88–90]. Activation of kinases, such as tyrosine kinase [91, 92], protein kinase C (PKC) [93, 94], protein kinase A [95], and CAMKII increases NMDA receptor mediated currents. Interestingly, aging is associated with a shift in the balance of kinase/phosphatase activity, favoring an increase in the phosphatase activity [96–98].
With advancing age, the cellular localization, basal or stimulation induced activity may be altered [53, 99]. In contrast to kinases, the activity of protein phosphatase appears to be augmented with normal aging, influencing synaptic function [96, 97, 100]. Protein phosphatases, including calcineurin and protein phosphatase 1, decrease NMDA receptor currents [92, 95, 101]. Phosphorylation state of GluN1, GluN2A or GluN2B subunits can rapidly regulate surface expression and localization of the NMDA receptors [102–105]. Inhibition of phosphatase activity increases the AMPA receptor component of synaptic [97] transmission, specifically in aged animals[97] .The increase in synaptic transmission due to phosphatase inhibition may be linked to increased susceptibility to induction of LTD and thus, represent reversal of LTD. Inhibition of phosphatase activity also increases NMDA receptor-mediated synaptic transmission in aged animals; however, the increase is relatively small relative to the decrease in the NMDA synaptic response associated with aging [53].
Recent work suggests that redox regulation of NMDA receptor function contributes to schizophrenia (Steullet et al., 2016), stressor-induced depressive-like behavior (Ibi et al., 2017), and synaptic plasticity during development [106]. In young animals, NMDA receptor function is modulated by redox state, such that under oxidizing conditions, disulfide bonds can form between cysteine residues in the NMDA receptor subunits [107–109]. Three pairs of cysteine residues are located within the N-terminal regulatory domain of the receptor (two pairs reside in GluN1 and one pair resides in GluN2A subunit) [108, 110, 111]. The formation of disulfide bonds between cysteine residues is thought to decrease the current through the NMDA receptor. In contrast to younger animals, little or no effect of oxidizing agents was observed for older animals, suggesting that cells were already in an oxidized state. In contrast, reducing conditions enhanced NMDA receptor mediated synaptic responses in hippocampus of aged animals [53, 55, 56, 112–114]. Recent results provide evidence for a link between the redox-mediated decline in NMDA receptor function and the emergence of an age-related cognitive phenotype with impairment in the rapid acquisition and retention of novel spatial information [55, 56]. These results demonstrate that the age-related decrease in NMDA receptor-mediated synaptic responses at CA3-CA1 hippocampal synapses with advanced age is related to redox state such that the reducing agent, dithiothreitol (DTT) significantly enhanced the NMDA receptor component of the synaptic response to a greater extent in cognitively impaired animals relative to unimpaired animals [55] (Fig 2).
Figure 2.
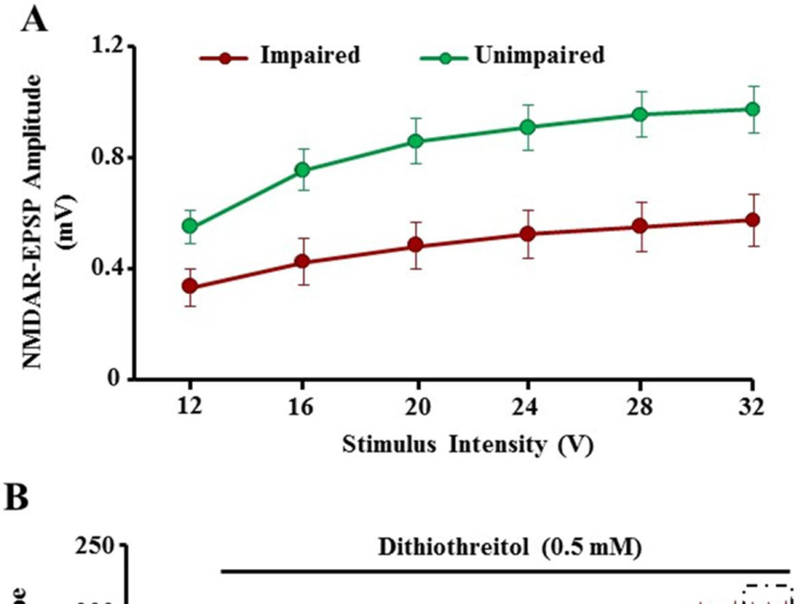
Redox environment contributes to the decline in NMDA receptor function associated with cognitive impairment. A) Input–output curves for the mean NMDA receptor EPSP (NMDAR-EPSP) amplitude evoked by increasing stimulating voltage. B) Time course of changes in the slope of NMDA receptor-mediated EPSP obtained from hippocampal slices 10 min before and 60 min after bath application of the reducing agent DTT (0.5 mM, solid line) in unimpaired (red circles) and impaired (green circles) animals. C) Bars represent the mean ± SEM change in NMDAR-EPSP slope after the application of DTT in unimpaired (red circles) and impaired (green circles) animals. The asterisk indicates a significant increase in NMDAR-EPSP slope in impaired animals when compared with unimpaired (modified from Kumar and Foster, JNS, 2013).
The mechanism for redox regulation of NMDA receptor function is unclear. An age-related shift in the composition of NMDA receptor subunits could render the NMDA receptor more susceptible to redox regulation. As noted above, the GluN2A subunit has unique extracellular cysteine residues, and reducing the extracellular disulfide bonds, by the addition of extracellular glutathione, increases the NMDA receptor response of diheteromeric GluN1-GluN2A receptors [115]. However, in the case of aging, addition of extracellular glutathione and other antioxidants that do not readily pass the cell membrane do not increase the NMDA receptor response in aged animals [53, 116]. Rather, intracellular application of glutathione increased the NMDA receptor response demonstrating an important role for intracellular redox state [53]. Furthermore, the DTT-mediated growth of the NMDA receptor response depends on the activity of Ca2+/calmodulin-dependent protein kinase II (CaMKII) [53]. Interestingly, NMDAR-LTP is sensitive to redox conditions, [117, 118] and is impaired in aged animals [79], suggesting that redox mechanisms may underlie an age-related decrease in NMDAR-LTP.
Modification of NMDA receptor function by agonist
In addition to binding glutamate, glycine acts as a co-agonist, binding to the GluN1 subunits. D-serine might represent another physiological co-agonist of the NMDA receptor as it can bind at the glycine-binding site [119–123]. D-serine acts as a neuronal signaling molecule leading to upregulation of NMDA receptors. In addition, D-serine is highly expressed in the brain and released from astrocytes [120, 124–127]. D-serine is required for NMDA receptor activation, and may have preferential affinity/effectiveness for NMDA receptors that contain GluN2B subunits [121]. The levels of D-serine are dramatically reduced with advanced age [128–130]. One possibility is a loss of the serine racemase enzyme, which generates D-serine from L-serine [131, 132]. A decline in serine racemase mRNA is observed in the hippocampus of aged rats [130]. The enzyme serine racemase generates D-serine from L-serine; pharmacological or viral gene delivery tools could be employed to increase endogenous levels of D-serine or serine racemase expression. Future studies to upregulate the expression of serine racemase, in order to enhance the endogenous level of D-serine, could provide another avenue to restore impaired NMDA receptor function during aging and under pathological conditions.
Influence of VDCCs and ICS on NMDA receptor function
In addition to binding of the transmitter glutamate, NMDA receptor activation requires postsynaptic depolarization to relieve the Mg2+ block of the channel. For CA1 neurons from aged animals, depolarization induced by a burst of afferent activity is reduced, due to activation of Ca2+-dependent K+ channels. The augmentation in the afterhyperpolarization (AHP) amplitude diminishes the activation of NMDA channels, further contributing to impaired synaptic plasticity [18, 19]. The enhanced AHP with age is linked to increased involvement of VDCCs and internal Ca2+ stores [112, 133–147]. Thus, changes in Ca2+ from VDCCs or ICS can act through the AHP to impair NMDA receptor function.
VDCCs are ion channels in the plasma membrane open in response to membrane depolarization and allow Ca2+ influx into the cell from the extracellular space. In hippocampal CA1 pyramidal neurons of the rat, the L-type Ca2+ currents are increased [148, 149] and an increase in the density of functional L-type VDCCs have been reported for aged animals [150]. The idea that L-channels are increased in the hippocampus during senescence is also supported by mRNA and protein expression studies indicating an increase in Cav1.3 [151–153]. Treatments to reduce the AHP permits increase activation of NMDA receptor, to shift the threshold for induction of synaptic plasticity [154, 155]. In aged rats, under L-channel blockade, the induction of LTP is facilitated for low level synaptic activation, which would not induce synaptic modification in young animals [154]. It should be noted that L-channel blockade does not completely ameliorate age-related differences. The AHP amplitude is reduced but not to the levels observed in young animals [112, 146].
In addition to Ca2+ influx from outside the cell, ICS play a major role in regulating larger Ca2+ signals [156, 157]. Organelles, including the endoplasmic reticulum, mitochondria, and lysosomes act as Ca2+ buffering systems - releasing and sequestering Ca2+ [158–164]. Thus, there are at least two possible mechanisms by which ICS can regulate Ca2+ homeostasis: 1) release of stored Ca2+ to enhance Ca2+ signals and 2) removing cytosolic Ca2+ following a large influx.
Two pathways control the release of Ca2+ from the endoplasmic reticulum, Ca2+-induced Ca2+ release (CICR) and the inositol (1,4,5)-trisphosphate (IP3) pathway activated by G protein-coupled receptors. G protein-coupled receptors activate phospholipase C to form diacylglycerol and IP3, which act on IP3 receptors (IP3Rs) to release Ca2+ from ICS. Previous studies have observed an age associated decrease in IP3Rs in several brain regions [165–168]. Despite a general decrease in the receptor, the literature suggests that a decrease in IP3 induced Ca2+ release is either limited to cortical cells [165] or no age-related change is observed [169]. The disconnect between a reduction in IP3R expression and the apparent absence of an effect of age on IP3-induced Ca2+ release may be due to increased oxidation of the IP3Rs which has been demonstrated to increase IP3R function in brain cells [170, 171]. As such, reduced expression may act as compensation for an altered redox state, in order to maintain proper IP3 signaling.
CICR is a Ca2+ amplification process that is initiated by influx of Ca2+ through membrane channels (i.e. VDCCs) (Fig 1). The intracellular Ca2+ binds ryanodine receptors (RyRs) to release additional Ca2+ into the cytosol from the endoplasmic reticulum. Accumulating evidence supports a role of altered CICR in contributing to altered physiology of normal aging. The increased involvement of RyRs does not appear to be due to increased RyR expression [167]. Rather, an age-related increase in oxidative stress and a shift in the intracellular redox state may enhance the responsiveness of RyRs to intracellular Ca2+ [112, 172–174]. The redox state influences the formation of cysteine disulfide bonds. The disulfide bonds of RyR for ICS determine Ca2+ release and the amplitude of the AHP. In the case of NMDA receptors, redox sensitive disulfide bonds are localized to NMDA receptor subunits and to molecules such as Ca2+/calmodulin-dependent protein kinase II that modify NMDA receptor function. The age-dependent specificity of oxidizing agents and DTT provide strong support for the tenet that redox stress mediates senescent physiology. Increased CICR appears to contribute to the larger AHP during aging [5, 138, 147]. As noted above, hippocampal cells exhibit increase Ca2+ from L-type Ca2+ channels, which could provide a source of Ca2+ to fill ICS and activate CICR from ICS.
The increase in CICR activates Ca2+-dependent potassium channels in the membrane, inducing larger AHP. Attenuating CICR, by blocking RyR or depletion of Ca2+ from ICS, has a greater influence in reducing the amplitude of the AHP in aged animals [112, 138], indicating an aging-specific mechanism. Moreover, attenuation of CICR promotes induction of LTP and inhibits LTD during senescence [5, 138]. Thus, the relative shift in Ca2+ sources, with reduced extracellular influx of Ca2+ from NMDA receptors and increased release of Ca2+ from ICS, underlies senescent physiology, which is characterized by enhanced amplitude of AHP, a decrease in NMDA voltage-gated channel activity, decreased synaptic transmission, and reduced synaptic plasticity.
Conclusion
Ideas about the role of senescent glutamatergic synapses in contributing to the age-related cognitive impairment are based on studies delineating the mechanisms for age-associated changes in Ca2+-dependent synaptic plasticity. Specifically, aging associated alterations in Ca2+ regulation modify NMDA glutamate receptor mediated synaptic transmission including impaired LTP and enhanced LTD. In addition to altered synaptic plasticity, alterations in NMDA receptor subunit expression profile, molecular and biochemical modulatory mechanisms, and alterations in Ca2+ sources provide impetus for altering the NMDA receptor-mediated synaptic transmission. These altered senescent glutamatergic synaptic plasticity mechanisms contribute to cognitive aging. Due to the critical importance of NMDA glutamate receptors in synaptic transmission and cognitive function, a selective upregulation of NMDA receptor function may provide an avenue for treating age-associated cognitive deficits. Clearly, future research will need to delineate the contributions of several mechanisms in optimizing specific subunit contribution and influence of upregulation in mediating cognition. Thus, it will be imperative for future research to determine whether enhancing or inhibiting NMDA receptor function by upregulating or downregulating different subunits expression configurations will be beneficial in preserving cognitive domain and promoting successful cognitive aging.
Acknowledgements
Supported by National Institute of Aging grants R37AG036800, RO11049711, RO1037984, and RO1052258 and the Evelyn F. McKnight Brain Research Foundation. This work was partially supported by the University of Florida Claude D. Pepper Older Americans Independence Center (P30-AG028740).
References
- 1.Nicoll RA, Malenka RC (1999) Expression mechanisms underlying NMDA receptor-dependent long-term potentiation. Annals of the New York Academy of Sciences 868:515–525 [DOI] [PubMed] [Google Scholar]
- 2.Barnes CA, McNaughton BL (1985) An age comparison of the rates of acquisition and forgetting of spatial information in relation to long-term enhancement of hippocampal synapses. Behavioral neuroscience 99:1040–1048. [DOI] [PubMed] [Google Scholar]
- 3.Lee HK, Kameyama K, Huganir RL, Bear MF (1998) NMDA induces long-term synaptic depression and dephosphorylation of the GluR1 subunit of AMPA receptors in hippocampus. Neuron 21:1151–1162 [DOI] [PubMed] [Google Scholar]
- 4.Kumar A (2010) Carbachol-induced long-term synaptic depression is enhanced during senescence at hippocampal CA3-CA1 synapses. Journal of neurophysiology 104:607–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar A, Foster TC (2005) Intracellular calcium stores contribute to increased susceptibility to LTD induction during aging. Brain research 1031:125–128 [DOI] [PubMed] [Google Scholar]
- 6.Kumar A, Foster TC (2007) Shift in Induction Mechanisms Underlies an Age-Dependent Increase in DHPG-Induced Synaptic Depression at CA3 CA1 Synapses. Journal of neurophysiology 98:2729–2736 [DOI] [PubMed] [Google Scholar]
- 7.Norris CM, Korol DL, Foster TC (1996) Increased susceptibility to induction of long-term depression and long- term potentiation reversal during aging. The Journal of neuroscience : the official journal of the Society for Neuroscience 16:5382–5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foster TC (1999) Involvement of hippocampal synaptic plasticity in age-related memory decline. Brain Res Rev 30:236–249 [DOI] [PubMed] [Google Scholar]
- 9.Foster TC, Kumar A (2007) Susceptibility to induction of long-term depression is associated with impaired memory in aged Fischer 344 rats. Neurobiology of learning and memory 87:522–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Febo M, Foster TC (2016) Preclinical Magnetic Resonance Imaging and Spectroscopy Studies of Memory, Aging, and Cognitive Decline. Front Aging Neurosci 8:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Q, Homma KJ, Poo MM (2004) Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron 44:749–757 [DOI] [PubMed] [Google Scholar]
- 12.Foster TC (2006) Biological markers of age-related memory deficits: treatment of senescent physiology. CNS Drugs 20:153–166 [DOI] [PubMed] [Google Scholar]
- 13.Shinoda Y, Kamikubo Y, Egashira Y, Tominaga-Yoshino K, Ogura A (2005) Repetition of mGluR-dependent LTD causes slowly developing persistent reduction in synaptic strength accompanied by synapse elimination. Brain research 1042:99–107 [DOI] [PubMed] [Google Scholar]
- 14.Khachaturian ZS (1989) Calcium, membranes, aging, and Alzheimer's disease. Introduction and overview. Annals of the New York Academy of Sciences 568:1–4 [DOI] [PubMed] [Google Scholar]
- 15.Michaelis ML, Johe K, Kitos TE (1984) Age-dependent alterations in synaptic membrane systems for Ca2+ regulation. Mech Ageing Dev 25:215–225 [DOI] [PubMed] [Google Scholar]
- 16.Rapp PR, Gallagher M (1996) Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proceedings of the National Academy of Sciences of the United States of America 93:9926–9930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.West MJ (1993) Regionally specific loss of neurons in the aging human hippocampus. Neurobiology of aging 14:287–293 [DOI] [PubMed] [Google Scholar]
- 18.Foster TC (2012) Dissecting the age-related decline on spatial learning and memory tasks in rodent models: N-methyl-D-aspartate receptors and voltage-dependent Ca(2)(+) channels in senescent synaptic plasticity. Prog Neurobiol 96:283–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foster TC, Norris CM (1997) Age-associated changes in Ca2+-dependent processes: relation to hippocampal synaptic plasticity. Hippocampus 7:602–612 [DOI] [PubMed] [Google Scholar]
- 20.Berridge MJ, Lipp P, Bootman MD (2000) The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1:11–21 [DOI] [PubMed] [Google Scholar]
- 21.Orrenius S, Zhivotovsky B, Nicotera P (2003) Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol 4:552–565 [DOI] [PubMed] [Google Scholar]
- 22.Rizzuto R (2001) Intracellular Ca(2+) pools in neuronal signalling. Curr Opin Neurobiol 11:306–311 [DOI] [PubMed] [Google Scholar]
- 23.Verkhratsky A, Toescu EC (1998) Calcium and neuronal ageing. Trends Neurosci 21:2–7 [DOI] [PubMed] [Google Scholar]
- 24.Brewer LD, Porter NM, Kerr DS, Landfield PW, Thibault O (2006) Chronic 1alpha,25-(OH)2 vitamin D3 treatment reduces Ca2+ -mediated hippocampal biomarkers of aging. Cell calcium 40:277–286 [DOI] [PubMed] [Google Scholar]
- 25.Kumar A, Bodhinathan K, Foster TC (2009) Susceptibility to Calcium Dysregulation during Brain Aging. Front Aging Neurosci 1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cotman C, Monaghan D (1989) Multiple excitatory amino acid receptor regulation of intracellular Ca2+. Implications for aging and Alzheimer's disease. Annals of the New York Academy of Sciences 568:138–148 [DOI] [PubMed] [Google Scholar]
- 27.Cotman CW, Geddes JW, Bridges RJ, Monaghan DT (1989) N-methyl-D-aspartate receptors and Alzheimer's disease. Neurobiology of aging 10:603–605; discussion 618–620 [DOI] [PubMed] [Google Scholar]
- 28.Morris RG (2013) NMDA receptors and memory encoding. Neuropharmacology 74:32–40 [DOI] [PubMed] [Google Scholar]
- 29.Dingledine R, Borges K, Bowie D, Traynelis SF (1999) The glutamate receptor ion channels. Pharmacological reviews 51:7–61 [PubMed] [Google Scholar]
- 30.Cull-Candy S, Brickley S, Farrant M (2001) NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol 11:327–335 [DOI] [PubMed] [Google Scholar]
- 31.Kutsuwada T, Kashiwabuchi N, Mori H, Sakimura K, Kushiya E, Araki K, Meguro H, Masaki H, Kumanishi T, Arakawa M, et al. (1992) Molecular diversity of the NMDA receptor channel. Nature 358:36–41 [DOI] [PubMed] [Google Scholar]
- 32.Meguro H, Mori H, Araki K, Kushiya E, Kutsuwada T, Yamazaki M, Kumanishi T, Arakawa M, Sakimura K, Mishina M (1992) Functional characterization of a heteromeric NMDA receptor channel expressed from cloned cDNAs. Nature 357:70–74 [DOI] [PubMed] [Google Scholar]
- 33.Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH (1992) Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science 256:1217–1221 [DOI] [PubMed] [Google Scholar]
- 34.Moriyoshi K, Masu M, Ishii T, Shigemoto R, Mizuno N, Nakanishi S (1991) Molecular cloning and characterization of the rat NMDA receptor. Nature 354:31–37 [DOI] [PubMed] [Google Scholar]
- 35.Laube B, Kuhse J, Betz H (1998) Evidence for a tetrameric structure of recombinant NMDA receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience 18:2954–2961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar A (2015) NMDA Receptor Function During Senescence: Implication on Cognitive Performance. Frontiers in neuroscience 9:473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Hallaq RA, Jarabek BR, Fu Z, Vicini S, Wolfe BB, Yasuda RP (2002) Association of NR3A with the N-methyl-D-aspartate receptor NR1 and NR2 subunits. Molecular pharmacology 62:1119–1127 [DOI] [PubMed] [Google Scholar]
- 38.Schuler T, Mesic I, Madry C, Bartholomaus I, Laube B (2008) Formation of NR1/NR2 and NR1/NR3 heterodimers constitutes the initial step in N-methyl-D-aspartate receptor assembly. The Journal of biological chemistry 283:37–46 [DOI] [PubMed] [Google Scholar]
- 39.Sucher NJ, Akbarian S, Chi CL, Leclerc CL, Awobuluyi M, Deitcher DL, Wu MK, Yuan JP, Jones EG, Lipton SA (1995) Developmental and regional expression pattern of a novel NMDA receptor-like subunit (NMDAR-L) in the rodent brain. The Journal of neuroscience : the official journal of the Society for Neuroscience 15:6509–6520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Low CM, Wee KS (2010) New insights into the not-so-new NR3 subunits of N-methyl-D-aspartate receptor: localization, structure, and function. Molecular pharmacology 78:1–11 [DOI] [PubMed] [Google Scholar]
- 41.Collingridge G (1987) Synaptic plasticity. The role of NMDA receptors in learning and memory. Nature 330:604–605 [DOI] [PubMed] [Google Scholar]
- 42.Morris RG, Anderson E, Lynch GS, Baudry M (1986) Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature 319:774–776 [DOI] [PubMed] [Google Scholar]
- 43.Morris RG (1989) Synaptic plasticity and learning: selective impairment of learning rats and blockade of long-term potentiation in vivo by the N-methyl-D-aspartate receptor antagonist AP5. The Journal of neuroscience : the official journal of the Society for Neuroscience 9:3040–3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lisman JE, Fellous JM, Wang XJ (1998) A role for NMDA-receptor channels in working memory. Nature neuroscience 1:273–275 [DOI] [PubMed] [Google Scholar]
- 45.Mondadori C, Weiskrantz L (1993) NMDA receptor blockers facilitate and impair learning via different mechanisms. Behav Neural Biol 60:205–210 [DOI] [PubMed] [Google Scholar]
- 46.Mondadori C, Weiskrantz L, Buerki H, Petschke F, Fagg GE (1989) NMDA receptor antagonists can enhance or impair learning performance in animals. Exp Brain Res 75:449–456 [DOI] [PubMed] [Google Scholar]
- 47.Martin SJ, Grimwood PD, Morris RG (2000) Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci 23:649–711 [DOI] [PubMed] [Google Scholar]
- 48.Dore K, Stein IS, Brock JA, Castillo PE, Zito K, Sjostrom PJ (2017) Unconventional NMDA Receptor Signaling. The Journal of neuroscience : the official journal of the Society for Neuroscience 37:10800–10807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Foster TC, Kyritsopoulos C, Kumar A (2017) Central role for NMDA receptors in redox mediated impairment of synaptic function during aging and Alzheimer's disease. Behavioural brain research 322:223–232 [DOI] [PubMed] [Google Scholar]
- 50.Barnes CA, Rao G, Shen J (1997) Age-related decrease in the N-methyl-D-aspartateR-mediated excitatory postsynaptic potential in hippocampal region CA1. Neurobiology of aging 18:445–452. [DOI] [PubMed] [Google Scholar]
- 51.Eckles-Smith K, Clayton D, Bickford P, Browning MD (2000) Caloric restriction prevents age-related deficits in LTP and in NMDA receptor expression. Brain research Molecular brain research 78:154–162 [DOI] [PubMed] [Google Scholar]
- 52.Billard JM, Rouaud E (2007) Deficit of NMDA receptor activation in CA1 hippocampal area of aged rats is rescued by D-cycloserine. The European journal of neuroscience 25:2260–2268 [DOI] [PubMed] [Google Scholar]
- 53.Bodhinathan K, Kumar A, Foster TC (2010) Intracellular Redox State Alters NMDA Receptor Response during Aging through Ca2+/Calmodulin-Dependent Protein Kinase II. The Journal of neuroscience : the official journal of the Society for Neuroscience 30:1914–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brim BL, Haskell R, Awedikian R, Ellinwood NM, Jin L, Kumar A, Foster TC, Magnusson K (2013) Memory in aged mice is rescued by enhanced expression of the GluN2B subunit of the NMDA receptor. Behavioural brain research 322:223–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kumar A, Foster TC (2013) Linking redox regulation of NMDAR synaptic function to cognitive decline during aging. The Journal of neuroscience : the official journal of the Society for Neuroscience 33:15710–15715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee WH, Kumar A, Rani A, Foster TC (2014) Role of antioxidant enzymes in redox regulation of N-methyl-D-aspartate receptor function and memory in middle-aged rats. Neurobiology of aging 35:1459–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kumar A, Rani A, Scheinert RB, Ormerod BK, Foster TC (2018) Nonsteroidal anti-inflammatory drug, indomethacin improves spatial memory and NMDA receptor function in aged animals. Neurobiology of aging 70:184–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zorumski CF, Izumi Y (2012) NMDA receptors and metaplasticity: mechanisms and possible roles in neuropsychiatric disorders. Neurosci Biobehav Rev 36:989–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abraham WC (2008) Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci 9:387. [DOI] [PubMed] [Google Scholar]
- 60.Magnusson KR (2000) Declines in mRNA expression of different subunits may account for differential effects of aging on agonist and antagonist binding to the NMDA receptor. The Journal of neuroscience : the official journal of the Society for Neuroscience 20:1666–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu F, Day M, Muniz LC, Bitran D, Arias R, Revilla-Sanchez R, Grauer S, Zhang G, Kelley C, Pulito V, Sung A, Mervis RF, Navarra R, Hirst WD, Reinhart PH, Marquis KL, Moss SJ, Pangalos MN, Brandon NJ (2008) Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nature neuroscience 11:334–343 [DOI] [PubMed] [Google Scholar]
- 62.Mesches MH, Gemma C, Veng LM, Allgeier C, Young DA, Browning MD, Bickford PC (2004) Sulindac improves memory and increases NMDA receptor subunits in aged Fischer 344 rats. Neurobiology of aging 25:315–324 [DOI] [PubMed] [Google Scholar]
- 63.Magnusson KR, Nelson SE, Young AB (2002) Age-related changes in the protein expression of subunits of the NMDA receptor. Brain research Molecular brain research 99:40–45 [DOI] [PubMed] [Google Scholar]
- 64.Coultrap SJ, Bickford PC, Browning MD (2008) Blueberry-enriched diet ameliorates age-related declines in NMDA receptor-dependent LTP. Age 30:263–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Newton IG, Forbes ME, Linville MC, Pang H, Tucker EW, Riddle DR, Brunso-Bechtold JK (2008) Effects of aging and caloric restriction on dentate gyrus synapses and glutamate receptor subunits. Neurobiology of aging 29:1308–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Adams MM, Morrison JH, Gore AC (2001) N-methyl-D-aspartate receptor mRNA levels change during reproductive senescence in the hippocampus of female rats. Exp Neurol 170:171–179 [DOI] [PubMed] [Google Scholar]
- 67.Sonntag WE, Bennett SA, Khan AS, Thornton PL, Xu X, Ingram RL, Brunso-Bechtold JK (2000) Age and insulin-like growth factor-1 modulate N-methyl-D-aspartate receptor subtype expression in rats. Brain Res Bull 51:331–338 [DOI] [PubMed] [Google Scholar]
- 68.Zhao X, Rosenke R, Kronemann D, Brim B, Das SR, Dunah AW, Magnusson KR (2009) The effects of aging on N-methyl-d-aspartate receptor subunits in the synaptic membrane and relationships to long-term spatial memory. Neuroscience 162:933–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu XB, Murray KD, Jones EG (2004) Switching of NMDA receptor 2A and 2B subunits at thalamic and cortical synapses during early postnatal development. The Journal of neuroscience : the official journal of the Society for Neuroscience 24:8885–8895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH (1994) Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12:529–540 [DOI] [PubMed] [Google Scholar]
- 71.Laurie DJ, Bartke I, Schoepfer R, Naujoks K, Seeburg PH (1997) Regional, developmental and interspecies expression of the four NMDAR2 subunits, examined using monoclonal antibodies. Brain research Molecular brain research 51:23–32 [DOI] [PubMed] [Google Scholar]
- 72.Laurie DJ, Seeburg PH (1994) Regional and developmental heterogeneity in splicing of the rat brain NMDAR1 mRNA. The Journal of neuroscience : the official journal of the Society for Neuroscience 14:3180–3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Law AJ, Weickert CS, Webster MJ, Herman MM, Kleinman JE, Harrison PJ (2003) Changes in NMDA receptor subunit mRNAs and cyclophilin mRNA during development of the human hippocampus. Annals of the New York Academy of Sciences 1003:426–430 [DOI] [PubMed] [Google Scholar]
- 74.Law AJ, Weickert CS, Webster MJ, Herman MM, Kleinman JE, Harrison PJ (2003) Expression of NMDA receptor NR1, NR2A and NR2B subunit mRNAs during development of the human hippocampal formation. The European journal of neuroscience 18:1197–1205 [DOI] [PubMed] [Google Scholar]
- 75.Dumas TC (2005) Developmental regulation of cognitive abilities: modified composition of a molecular switch turns on associative learning. Prog Neurobiol 76:189–211 [DOI] [PubMed] [Google Scholar]
- 76.Magnusson KR (2001) Influence of diet restriction on NMDA receptor subunits and learning during aging. Neurobiology of aging 22:613–627 [DOI] [PubMed] [Google Scholar]
- 77.Magnusson KR, Kresge D, Supon J (2006) Differential effects of aging on NMDA receptors in the intermediate versus the dorsal hippocampus. Neurobiology of aging 27:324–333 [DOI] [PubMed] [Google Scholar]
- 78.Clayton DA, Browning MD (2001) Deficits in the expression of the NR2B subunit in the hippocampus of aged Fisher 344 rats. Neurobiology of aging 22:165–168 [DOI] [PubMed] [Google Scholar]
- 79.Clayton DA, Mesches MH, Alvarez E, Bickford PC, Browning MD (2002) A hippocampal NR2B deficit can mimic age-related changes in long-term potentiation and spatial learning in the Fischer 344 rat. The Journal of neuroscience : the official journal of the Society for Neuroscience 22:3628–3637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cui Y, Jin J, Zhang X, Xu H, Yang L, Du D, Zeng Q, Tsien JZ, Yu H, Cao X (2011) Forebrain NR2B overexpression facilitating the prefrontal cortex long-term potentiation and enhancing working memory function in mice. PloS one 6:e20312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ (1999) Genetic enhancement of learning and memory in mice. Nature 401:63–69 [DOI] [PubMed] [Google Scholar]
- 82.Cao X, Cui Z, Feng R, Tang YP, Qin Z, Mei B, Tsien JZ (2007) Maintenance of superior learning and memory function in NR2B transgenic mice during ageing. The European journal of neuroscience 25:1815–1822 [DOI] [PubMed] [Google Scholar]
- 83.Dyall SC, Michael GJ, Whelpton R, Scott AG, Michael-Titus AT (2007) Dietary enrichment with omega-3 polyunsaturated fatty acids reverses age-related decreases in the GluR2 and NR2B glutamate receptor subunits in rat forebrain. Neurobiology of aging 28:424–439 [DOI] [PubMed] [Google Scholar]
- 84.Liu P, Smith PF, Darlington CL (2008) Glutamate receptor subunits expression in memory-associated brain structures: regional variations and effects of aging. Synapse 62:834–841 [DOI] [PubMed] [Google Scholar]
- 85.Marquez Loza A, Elias V, Wong CP, Ho E, Bermudez M, Magnusson KR (2017) Effects of ibuprofen on cognition and NMDA receptor subunit expression across aging. Neuroscience 344:276–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Magnusson KR, Scruggs B, Zhao X, Hammersmark R (2007) Age-related declines in a two-day reference memory task are associated with changes in NMDA receptor subunits in mice. BMC neuroscience 8:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hunt DL, Castillo PE (2012) Synaptic plasticity of NMDA receptors: mechanisms and functional implications. Curr Opin Neurobiol 22:496–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aniksztejn L, Ben-Ari Y (1995) Expression of LTP by AMPA and/or NMDA receptors is determined by the extent of NMDA receptors activation during the tetanus. J Neurophysiol 74:2349–2357 [DOI] [PubMed] [Google Scholar]
- 89.Li HB, Jackson MF, Yang K, Trepanier C, Salter MW, Orser BA, Macdonald JF (2011) Plasticity of synaptic GluN receptors is required for the Src-dependent induction of long-term potentiation at CA3-CA1 synapses. Hippocampus 21:1053–1061 [DOI] [PubMed] [Google Scholar]
- 90.Peng Y, Zhao J, Gu QH, Chen RQ, Xu Z, Yan JZ, Wang SH, Liu SY, Chen Z, Lu W (2010) Distinct trafficking and expression mechanisms underlie LTP and LTD of NMDA receptor-mediated synaptic responses. Hippocampus 20:646–658 [DOI] [PubMed] [Google Scholar]
- 91.Heidinger V, Manzerra P, Wang XQ, Strasser U, Yu SP, Choi DW, Behrens MM (2002) Metabotropic glutamate receptor 1-induced upregulation of NMDA receptor current: mediation through the Pyk2/Src-family kinase pathway in cortical neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience 22:5452–5461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang LY, Orser BA, Brautigan DL, MacDonald JF (1994) Regulation of NMDA receptors in cultured hippocampal neurons by protein phosphatases 1 and 2A. Nature 369:230–232 [DOI] [PubMed] [Google Scholar]
- 93.Ben-Ari Y, Aniksztejn L, Bregestovski P (1992) Protein kinase C modulation of NMDA currents: an important link for LTP induction. Trends Neurosci 15:333–339 [DOI] [PubMed] [Google Scholar]
- 94.Chen L, Huang LY (1992) Protein kinase C reduces Mg2+ block of NMDA-receptor channels as a mechanism of modulation. Nature 356:521–523 [DOI] [PubMed] [Google Scholar]
- 95.Raman IM, Tong G, Jahr CE (1996) Beta-adrenergic regulation of synaptic NMDA receptors by cAMP-dependent protein kinase. Neuron 16:415–421 [DOI] [PubMed] [Google Scholar]
- 96.Foster TC, Sharrow KM, Masse JR, Norris CM, Kumar A (2001) Calcineurin links Ca2+ dysregulation with brain aging. The Journal of neuroscience : the official journal of the Society for Neuroscience 21:4066–4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Norris CM, Halpain S, Foster TC (1998) Alterations in the balance of protein kinase/phosphatase activities parallel reduced synaptic strength during aging. Journal of neurophysiology 80:1567–1570 [DOI] [PubMed] [Google Scholar]
- 98.Foster TC (2007) Calcium homeostasis and modulation of synaptic plasticity in the aged brain. Aging cell 6:319–325 [DOI] [PubMed] [Google Scholar]
- 99.Foster TC (2004) Age-related changes in synaptic phosphorylation and dephosphorylation. In: Mattson M (ed) Protein Phosphorylation in Aging and Age-Related Disease Elsevier, Amsterdam, pp 133–152 [Google Scholar]
- 100.Mulkey RM, Endo S, Shenolikar S, Malenka RC (1994) Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature 369:486–488 [DOI] [PubMed] [Google Scholar]
- 101.Lieberman DN, Mody I (1994) Regulation of NMDA channel function by endogenous Ca(2+)-dependent phosphatase. Nature 369:235–239 [DOI] [PubMed] [Google Scholar]
- 102.Chung HJ, Huang YH, Lau LF, Huganir RL (2004) Regulation of the NMDA receptor complex and trafficking by activity-dependent phosphorylation of the NR2B subunit PDZ ligand. The Journal of neuroscience : the official journal of the Society for Neuroscience 24:10248–10259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gardoni F, Schrama LH, Kamal A, Gispen WH, Cattabeni F, Di Luca M (2001) Hippocampal synaptic plasticity involves competition between Ca2+/calmodulin-dependent protein kinase II and postsynaptic density 95 for binding to the NR2A subunit of the NMDA receptor. The Journal of neuroscience : the official journal of the Society for Neuroscience 21:1501–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hallett PJ, Spoelgen R, Hyman BT, Standaert DG, Dunah AW (2006) Dopamine D1 activation potentiates striatal NMDA receptors by tyrosine phosphorylation-dependent subunit trafficking. The Journal of neuroscience : the official journal of the Society for Neuroscience 26:4690–4700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lin Y, Jover-Mengual T, Wong J, Bennett MV, Zukin RS (2006) PSD-95 and PKC converge in regulating NMDA receptor trafficking and gating. Proceedings of the National Academy of Sciences of the United States of America 103:19902–19907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Francis-Oliveira J, Vilar Higa GS, Mendonca Munhoz Dati L, Carvalho Shieh I, De Pasquale R (2018) Metaplasticity in the Visual Cortex: Crosstalk Between Visual Experience and Reactive Oxygen Species. The Journal of neuroscience : the official journal of the Society for Neuroscience 38:5649–5665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aizenman E, Hartnett KA, Reynolds IJ (1990) Oxygen free radicals regulate NMDA receptor function via a redox modulatory site. Neuron 5:841–846 [DOI] [PubMed] [Google Scholar]
- 108.Choi Y, Chen HV, Lipton SA (2001) Three pairs of cysteine residues mediate both redox and zn2+ modulation of the nmda receptor. The Journal of neuroscience : the official journal of the Society for Neuroscience 21:392–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sullivan JM, Traynelis SF, Chen HS, Escobar W, Heinemann SF, Lipton SA (1994) Identification of two cysteine residues that are required for redox modulation of the NMDA subtype of glutamate receptor. Neuron 13:929–936 [DOI] [PubMed] [Google Scholar]
- 110.Choi YB, Lipton SA (2000) Redox modulation of the NMDA receptor. Cell Mol Life Sci 57:1535–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lipton SA, Choi YB, Takahashi H, Zhang D, Li W, Godzik A, Bankston LA (2002) Cysteine regulation of protein function--as exemplified by NMDA-receptor modulation. Trends Neurosci 25:474–480 [DOI] [PubMed] [Google Scholar]
- 112.Bodhinathan K, Kumar A, Foster TC (2010) Redox sensitive calcium stores underlie enhanced after hyperpolarization of aged neurons: role for ryanodine receptor mediated calcium signaling. Journal of neurophysiology 104:2586–2593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Haxaire C, Turpin FR, Potier B, Kervern M, Sinet PM, Barbanel G, Mothet JP, Dutar P, Billard JM (2012) Reversal of age-related oxidative stress prevents hippocampal synaptic plasticity deficits by protecting d-serine-dependent NMDA receptor activation. Aging cell [DOI] [PubMed] [Google Scholar]
- 114.Robillard JM, Gordon GR, Choi HB, Christie BR, MacVicar BA (2011) Glutathione restores the mechanism of synaptic plasticity in aged mice to that of the adult. PloS one 6:e20676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kohr G, Eckardt S, Luddens H, Monyer H, Seeburg PH (1994) NMDA receptor channels: subunit-specific potentiation by reducing agents. Neuron 12:1031–1040 [DOI] [PubMed] [Google Scholar]
- 116.Yang YJ, Wu PF, Long LH, Yu DF, Wu WN, Hu ZL, Fu H, Xie N, Jin Y, Ni L, Wang JZ, Wang F, Chen JG (2010) Reversal of aging-associated hippocampal synaptic plasticity deficits by reductants via regulation of thiol redox and NMDA receptor function. Aging cell 9:709–721 [DOI] [PubMed] [Google Scholar]
- 117.Gozlan H, Chinestra P, Diabira D, Ben-Ari Y (1994) NMDA redox site modulates long-term potentiation of NMDA but not of AMPA receptors. Eur J Pharmacol 262:R3–4 [DOI] [PubMed] [Google Scholar]
- 118.Bernard CL, Hirsch JC, Khazipov R, Ben-Ari Y, Gozlan H (1997) Redox modulation of synaptic responses and plasticity in rat CA1 hippocampal neurons. Exp Brain Res 113:343–352 [DOI] [PubMed] [Google Scholar]
- 119.Hood WF, Compton RP, Monahan JB (1989) D-cycloserine: a ligand for the N-methyl-D-aspartate coupled glycine receptor has partial agonist characteristics. Neurosci Lett 98:91–95 [DOI] [PubMed] [Google Scholar]
- 120.Mothet JP, Parent AT, Wolosker H, Brady RO Jr., Linden DJ, Ferris CD, Rogawski MA, Snyder SH (2000) D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proceedings of the National Academy of Sciences of the United States of America 97:4926–4931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Priestley T, Laughton P, Myers J, Le Bourdelles B, Kerby J, Whiting PJ (1995) Pharmacological properties of recombinant human N-methyl-D-aspartate receptors comprising NR1a/NR2A and NR1a/NR2B subunit assemblies expressed in permanently transfected mouse fibroblast cells. Molecular pharmacology 48:841–848 [PubMed] [Google Scholar]
- 122.Labrie V, Roder JC (2010) The involvement of the NMDA receptor D-serine/glycine site in the pathophysiology and treatment of schizophrenia. Neuroscience and biobehavioral reviews 34:351–372 [DOI] [PubMed] [Google Scholar]
- 123.Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, Oliet SH (2006) Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell 125:775–784 [DOI] [PubMed] [Google Scholar]
- 124.Nishikawa T (2005) Metabolism and functional roles of endogenous D-serine in mammalian brains. Biological & pharmaceutical bulletin 28:1561–1565 [DOI] [PubMed] [Google Scholar]
- 125.Schell MJ, Molliver ME, Snyder SH (1995) D-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proceedings of the National Academy of Sciences of the United States of America 92:3948–3952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.De Miranda J, Panizzutti R, Foltyn VN, Wolosker H (2002) Cofactors of serine racemase that physiologically stimulate the synthesis of the N-methyl-D-aspartate (NMDA) receptor coagonist D-serine. Proceedings of the National Academy of Sciences of the United States of America 99:14542–14547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fossat P, Turpin FR, Sacchi S, Dulong J, Shi T, Rivet JM, Sweedler JV, Pollegioni L, Millan MJ, Oliet SH, Mothet JP (2012) Glial D-serine gates NMDA receptors at excitatory synapses in prefrontal cortex. Cerebral cortex 22:595–606 [DOI] [PubMed] [Google Scholar]
- 128.Junjaud G, Rouaud E, Turpin F, Mothet JP, Billard JM (2006) Age-related effects of the neuromodulator D-serine on neurotransmission and synaptic potentiation in the CA1 hippocampal area of the rat. Journal of neurochemistry 98:1159–1166 [DOI] [PubMed] [Google Scholar]
- 129.Mothet JP, Rouaud E, Sinet PM, Potier B, Jouvenceau A, Dutar P, Videau C, Epelbaum J, Billard JM (2006) A critical role for the glial-derived neuromodulator D-serine in the age-related deficits of cellular mechanisms of learning and memory. Aging cell 5:267–274 [DOI] [PubMed] [Google Scholar]
- 130.Turpin FR, Potier B, Dulong JR, Sinet PM, Alliot J, Oliet SH, Dutar P, Epelbaum J, Mothet JP, Billard JM (2011) Reduced serine racemase expression contributes to age-related deficits in hippocampal cognitive function. Neurobiology of aging 32:1495–1504 [DOI] [PubMed] [Google Scholar]
- 131.Wolosker H, Blackshaw S, Snyder SH (1999) Serine racemase: a glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proceedings of the National Academy of Sciences of the United States of America 96:13409–13414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wolosker H, Sheth KN, Takahashi M, Mothet JP, Brady RO Jr., Ferris CD, Snyder SH (1999) Purification of serine racemase: biosynthesis of the neuromodulator D-serine. Proceedings of the National Academy of Sciences of the United States of America 96:721–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Landfield PW, Pitler TA (1984) Prolonged Ca2+-dependent afterhyperpolarizations in hippocampal neurons of aged rats. Science 226:1089–1092 [DOI] [PubMed] [Google Scholar]
- 134.Kerr DS, Campbell LW, Hao SY, Landfield PW (1989) Corticosteroid modulation of hippocampal potentials: increased effect with aging. Science 245:1505–1509 [DOI] [PubMed] [Google Scholar]
- 135.Pitler TA, Landfield PW (1990) Aging-related prolongation of calcium spike duration in rat hippocampal slice neurons. Brain research 508:1–6 [DOI] [PubMed] [Google Scholar]
- 136.Gong LW, Gao TM, Huang H, Zhou KX, Tong Z (2002) ATP modulation of large conductance Ca(2+)-activated K(+) channels via a functionally associated protein kinase A in CA1 pyramidal neurons from rat hippocampus. Brain research 951:130–134 [DOI] [PubMed] [Google Scholar]
- 137.Hsu KS, Huang CC, Liang YC, Wu HM, Chen YL, Lo SW, Ho WC (2002) Alterations in the balance of protein kinase and phosphatase activities and age-related impairments of synaptic transmission and long-term potentiation. Hippocampus 12:787–802 [DOI] [PubMed] [Google Scholar]
- 138.Kumar A, Foster TC (2004) Enhanced long-term potentiation during aging is masked by processes involving intracellular calcium stores. Journal of neurophysiology 91:2437–2444 [DOI] [PubMed] [Google Scholar]
- 139.Tombaugh GC, Rowe WB, Rose GM (2005) The slow afterhyperpolarization in hippocampal CA1 neurons covaries with spatial learning ability in aged Fisher 344 rats. The Journal of neuroscience : the official journal of the Society for Neuroscience 25:2609–2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kumar A, Foster TC (2002) 17beta-Estradiol benzoate decreases the AHP amplitude in CA1 pyramidal neurons. Journal of neurophysiology 88:621–626 [DOI] [PubMed] [Google Scholar]
- 141.Disterhoft JF, Moyer JR Jr., Thompson LT, Kowalska M (1993) Functional aspects of calcium-channel modulation. Clin Neuropharmacol 16 Suppl 1:S12–24 [DOI] [PubMed] [Google Scholar]
- 142.Disterhoft JF, Thompson LT, Moyer JR, Mogul DJ (1996) Calcium-dependent afterhyperpolarization and learning in young and aging hippocampus. Life Sci 59:413–420 [DOI] [PubMed] [Google Scholar]
- 143.Moyer JR Jr., Thompson LT, Black JP, Disterhoft JF (1992) Nimodipine increases excitability of rabbit CA1 pyramidal neurons in an age- and concentration-dependent manner. Journal of neurophysiology 68:2100–2109 [DOI] [PubMed] [Google Scholar]
- 144.Power JM, Oh MM, Disterhoft JF (2001) Metrifonate decreases sI(AHP) in CA1 pyramidal neurons in vitro. Journal of neurophysiology 85:319–322 [DOI] [PubMed] [Google Scholar]
- 145.Moyer JR Jr., Power JM, Thompson LT, Disterhoft JF (2000) Increased excitability of aged rabbit CA1 neurons after trace eyeblink conditioning. The Journal of neuroscience : the official journal of the Society for Neuroscience 20:5476–5482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Power JM, Wu WW, Sametsky E, Oh MM, Disterhoft JF (2002) Age-related enhancement of the slow outward calcium-activated potassium current in hippocampal CA1 pyramidal neurons in vitro. The Journal of neuroscience : the official journal of the Society for Neuroscience 22:7234–7243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gant JC, Sama MM, Landfield PW, Thibault O (2006) Early and simultaneous emergence of multiple hippocampal biomarkers of aging is mediated by Ca2+-induced Ca2+ release. The Journal of neuroscience : the official journal of the Society for Neuroscience 26:3482–3490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Campbell LW, Hao SY, Thibault O, Blalock EM, Landfield PW (1996) Aging changes in voltage-gated calcium currents in hippocampal CA1 neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience 16:6286–6295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Brewer LD, Dowling AL, Curran-Rauhut MA, Landfield PW, Porter NM, Blalock EM (2009) Estradiol reverses a calcium-related biomarker of brain aging in female rats. The Journal of neuroscience : the official journal of the Society for Neuroscience 29:6058–6067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Thibault O, Landfield PW (1996) Increase in single L-type calcium channels in hippocampal neurons during aging. Science 272:1017–1020 [DOI] [PubMed] [Google Scholar]
- 151.Herman JP, Chen KC, Booze R, Landfield PW (1998) Up-regulation of alpha1D Ca2+ channel subunit mRNA expression in the hippocampus of aged F344 rats. Neurobiology of aging 19:581–587 [DOI] [PubMed] [Google Scholar]
- 152.Veng LM, Mesches MH, Browning MD (2003) Age-related working memory impairment is correlated with increases in the L-type calcium channel protein alpha1D (Cav1.3) in area CA1 of the hippocampus and both are ameliorated by chronic nimodipine treatment. Brain research Molecular brain research 110:193–202 [DOI] [PubMed] [Google Scholar]
- 153.Chen KC, Blalock EM, Thibault O, Kaminker P, Landfield PW (2000) Expression of alpha 1D subunit mRNA is correlated with L-type Ca2+ channel activity in single neurons of hippocampal "zipper" slices. Proceedings of the National Academy of Sciences of the United States of America 97:4357–4362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Norris CM, Halpain S, Foster TC (1998) Reversal of age-related alterations in synaptic plasticity by blockade of L-type Ca2+ channels. The Journal of neuroscience : the official journal of the Society for Neuroscience 18:3171–3179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shankar S, Teyler TJ, Robbins N (1998) Aging differentially alters forms of long-term potentiation in rat hippocampal area CA1. Journal of neurophysiology 79:334–341 [DOI] [PubMed] [Google Scholar]
- 156.Ly CV, Verstreken P (2006) Mitochondria at the synapse. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry 12:291–299 [DOI] [PubMed] [Google Scholar]
- 157.Mattson MP, LaFerla FM, Chan SL, Leissring MA, Shepel PN, Geiger JD (2000) Calcium signaling in the ER: its role in neuronal plasticity and neurodegenerative disorders. Trends Neurosci 23:222–229 [DOI] [PubMed] [Google Scholar]
- 158.Murchison D, Griffith WH (2007) Calcium buffering systems and calcium signaling in aged rat basal forebrain neurons. Aging cell 6:297–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Toescu EC, Verkhratsky A (2004) Ca2+ and mitochondria as substrates for deficits in synaptic plasticity in normal brain ageing. J Cell Mol Med 8:181–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Duchen MR (2000) Mitochondria and calcium: from cell signalling to cell death. The Journal of physiology 529 Pt 1:57–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nicholls DG, Budd SL (2000) Mitochondria and neuronal survival. Physiol Rev 80:315–360 [DOI] [PubMed] [Google Scholar]
- 162.Solovyova N, Veselovsky N, Toescu EC, Verkhratsky A (2002) Ca(2+) dynamics in the lumen of the endoplasmic reticulum in sensory neurons: direct visualization of Ca(2+)-induced Ca(2+) release triggered by physiological Ca(2+) entry. EMBO J 21:622–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.McGuinness L, Bardo SJ, Emptage NJ (2007) The lysosome or lysosome-related organelle may serve as a Ca2+ store in the boutons of hippocampal pyramidal cells. Neuropharmacology 52:126–135 [DOI] [PubMed] [Google Scholar]
- 164.Toescu EC, Myronova N, Verkhratsky A (2000) Age-related structural and functional changes of brain mitochondria. Cell calcium 28:329–338 [DOI] [PubMed] [Google Scholar]
- 165.Burnett DM, Daniell LC, Zahniser NR (1990) Decreased efficacy of inositol 1,4,5-trisphosphate to elicit calcium mobilization from cerebrocortical microsomes of aged rats. Molecular pharmacology 37:566–571 [PubMed] [Google Scholar]
- 166.Igwe OJ, Ning L (1993) Inositol 1,4,5-trisphosphate arm of the phosphatidylinositide signal transduction pathway in the rat cerebellum during aging. Neurosci Lett 164:167–170 [DOI] [PubMed] [Google Scholar]
- 167.Martini A, Battaini F, Govoni S, Volpe P (1994) Inositol 1,4,5-trisphosphate receptor and ryanodine receptor in the aging brain of Wistar rats. Neurobiology of aging 15:203–206 [DOI] [PubMed] [Google Scholar]
- 168.Simonyi A, Xia J, Igbavboa U, Wood WG, Sun GY (1998) Age differences in the expression of metabotropic glutamate receptor 1 and inositol 1,4,5-trisphosphate receptor in mouse cerebellum. Neurosci Lett 244:29–32 [DOI] [PubMed] [Google Scholar]
- 169.Stutzmann GE, Smith I, Caccamo A, Oddo S, Laferla FM, Parker I (2006) Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer's disease mice. The Journal of neuroscience : the official journal of the Society for Neuroscience 26:5180–5189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Long LH, Liu J, Liu RL, Wang F, Hu ZL, Xie N, Fu H, Chen JG (2009) Differential effects of methionine and cysteine oxidation on [Ca2+] i in cultured hippocampal neurons. Cell Mol Neurobiol 29:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Peuchen S, Duchen MR, Clark JB (1996) Energy metabolism of adult astrocytes in vitro. Neuroscience 71:855–870 [DOI] [PubMed] [Google Scholar]
- 172.Bull R, Finkelstein JP, Humeres A, Behrens MI, Hidalgo C (2007) Effects of ATP, Mg2+, and redox agents on the Ca2+ dependence of RyR channels from rat brain cortex. Am J Physiol Cell Physiol 293:C162–171 [DOI] [PubMed] [Google Scholar]
- 173.Gokulrangan G, Zaidi A, Michaelis ML, Schoneich C (2007) Proteomic analysis of protein nitration in rat cerebellum: effect of biological aging. Journal of neurochemistry 100:1494–1504 [DOI] [PubMed] [Google Scholar]
- 174.Hidalgo C, Bull R, Behrens MI, Donoso P (2004) Redox regulation of RyR-mediated Ca2+ release in muscle and neurons. Biol Res 37:539–552 [DOI] [PubMed] [Google Scholar]


