Abstract
Rationale:
Many studies have found that ethanol intoxication and withdrawal impairs initial acquisition or extinction of learned behaviors. Rapid reconditioning following extinction is a form of post-extinction re-emergence of conditioned behavior that has not been studied for its interaction with ethanol intoxication or withdrawal.
Objectives:
The goals of this paper were to define the parameters that allow rapid post-extinction reacquisition of fear in mice and investigate the effect of acute ethanol withdrawal and intoxication on acquisition, extinction, and post-extinction reconditioning.
Methods:
We examined acquisition, extinction, and post-extinction reconditioning of contextual fear in male C57BL/6 mice. Acute ethanol withdrawal occurred 6 hr following a 4 g/kg injection of 20% ethanol and acute ethanol intoxication occurred 5 min following a 1.5 g/kg injection of 20% ethanol.
Results:
A weak context-shock pairing caused rapid reacquisition of conditioned freezing following moderate, but not extensive extinction. Acute ethanol intoxication impaired initial conditioning and acute ethanol withdrawal impaired rapid reacquisition after extinction, but not reconditioning or extinction itself.
Conclusions:
These findings show that rapid reconditioning occurs following moderate, but not extensive extinction in C57BL/6J mice. Additionally, acute ethanol withdrawal and intoxication may differentially affect different phases of conditioning. Results are discussed in terms of current ideas about post-extinction behavior and ethanol’s effects on memory.
Keywords: rapid reacquisition, extinction, contextual fear conditioning, acute ethanol withdrawal, acute ethanol intoxication, C57BL/6J mice
Introduction
Alcohol use disorders (AUDs) and post-traumatic stress disorder (PTSD) are highly comorbid conditions (Blanco et al., 2013) and this comorbidity tends to cause more severe symptoms in both disorders (Blanco et al., 2013; Herman, 1992; Saladin, Brady, Dansky, & Kilpatrick, 1995). There are multiple theories for why this comorbidity exists (e.g. stress, self-medication, high-risk population, etc.; Brown, Read, & Kahler, 2003; McCauley, Killeen, Gros, Brady, & Back, 2012; Stewart, 1996), yet the direct links between AUDs and PTSD are still unknown. To begin to elucidate AUD and PTSD comorbidity, it is important to understand how preclinical models of both disorders interact.
In the rodent laboratory, fear conditioning procedures are used to model some of the memory aspects of PTSD, such as the formation of a long-term fear memory and conditioned behavior to fear-related cues (Pitman, 1989; Rothbaum & Davis, 2003). Additionally, with repeated presentations of the previously fearful stimulus, the extinction of conditioned fear behavior models some aspects of exposure therapy. Many studies have found that extinction causes relatively temporary changes in behavior, with the suppressed behavior returning with time (spontaneous recovery; Pavlov, 1927; Rescorla, 2004), changes in context (renewal; Bouton & Bolles, 1979), re-exposure to the unconditioned stimulus (US; reinstatement; Rescorla & Heth, 1975), or after a weak reconditioning episode. This reconditioning episode involves the re-pairing of the CS and US and has been shown to result in rapid or slow reacquisition of fear (Bouton, 1986; Bouton, Woods, & Pineño, 2004; Leung, Bailey, Laurent, & Westbrook, 2007), suggesting that the CS-US association is suppressed but not erased by extinction. Although there are fewer studies of rapid reconditioning relative to the other unmasking procedures, rapid reacquisition of fear is of interest because it mimics the susceptibility of those with post-traumatic stress disorder (PTSD) to relapse after a mild stressor and to become hypervigilant, which are symptoms that worsen in those with comorbid AUDs (Saladin et al., 1995). Little is known about the mechanisms and circuitry of reconditioning and how ethanol intoxication or withdrawal may interact with rapid reacquisition of fear.
Ethanol acts on different cellular and molecular processes and has complicated behavioral effects. Ethanol intoxication can impair fear conditioning (Broadwater & Spear, 2013; Gould, 2003; Melia, Ryabinin, Corodimas, Wilson, & LeDoux, 1996) and extinction (Bisby et al., 2015; Broadwater & Spear, 2013; Holmes et al., 2012; Lattal, 2007). Yet, ethanol intoxication can sometimes promote fear conditioning depending on the dose and timing of ethanol administration (Brace & Pihl, 1997; Gulick & Gould, 2007), where mild intoxication can enhance fear conditioning, but a larger intoxicating dose can impair development of a similar memory. Further, ethanol has selective effects, depending on whether conditioning involves contextual or discrete cues (Gould, 2003; Kitaichi et al., 1995; Melia et al., 1996), which is thought to be caused by ethanol’s particularly strong effects on the hippocampus and hippocampus-dependent tasks, such as contextual fear conditioning (Ryabinin, Melia, Cole, Bloom, & Wilson, 1995). Additionally, ethanol can create an internal context that may signal the operation of acquisition or extinction contingencies (Cunningham, 1979a; Lattal, 2007).
Although acute alcohol intoxication has been shown to alter fear conditioning and extinction, less is known about the effects of withdrawal from alcohol on these learning processes. Withdrawal from alcohol is generally assumed to create a negative internal state that is alleviated through the negative reinforcing effects of alcohol (De Witte, Pinto, Ansseau, & Verbanck, 2003; Heilig, Egli, Crabbe, & Becker, 2010; Koob & Le Moal, 2008). Similar to intoxication, withdrawal from ethanol has been found to both enhance and impair fear conditioning and extinction (Bertotto, Bustos, Molina, & Martijena, 2006; Borlikova, Elbers, & Stephens, 2006; Quiñones-Laracuente, Hernandez-Rodriguez, Bravo-Rivera, Melendez, & Quirk, 2015; Ripley, O'Shea, & Stephens, 2003). Further, ethanol withdrawal has also been shown to have specific effects on the brain that are distinct from ethanol intoxication, in particular on learning-related brain regions such as the mPFC, amygdala, and hippocampus (reviewed in Vilpoux, Warnault, Pierrefiche, Daoust, & Naassila, 2009; White & Best, 2000).
Acute ethanol withdrawal (AEW), which is the peak of withdrawal following the first ethanol intoxication, results in both physical and psychological alterations (Karadayian & Cutrera, 2013; Karadayian, Busso, Feleder, & Cutrera, 2013), including alterations in brain activity (Kozell, Hitzemann, & Buck, 2005; Vilpoux et al., 2009) and impairments in initial contextual fear conditioning in C57BL/6J mice (Tipps, Raybuck, Buck, & Lattal, 2015). Little is known about effects of AEW on later learning, such as extinction or post-extinction re-emergence of behavior, processes which tend to be altered in patients with PTSD (Rothbaum & Davis, 2003).
The following experiments establish a basic post-extinction reconditioning protocol in mice (Experiments 1a and 1b). We then evaluate effects of AEW on extinction (Experiment 2), rapid reconditioning following extinction (Experiment 3), and initial conditioning (Experiment 4). Finally, we evaluate the effects of acute intoxication on post-extinction reconditioning relative to initial conditioning (Experiment 5). These results show that rapid reconditioning occurs following moderate but not extensive extinction, and that AEW and acute intoxication can dampen this process.
Methods
Animals and Housing
C57BL/6J male mice were purchased from Jackson Laboratory (Bar Harbor, ME) at 6 weeks of age and were housed 4 mice to an individually ventilated cage. All experimental procedures were approved by the Oregon Health & Science University Institutional Animal Use and Care Committee and were conducted in accordance with National Institutes of Health (NIH) “Principles of Laboratory Animal Care” (NIH Publication No. 86-23, revised 1985). The room temperature was constant at 22°C ± 1°C and the mice were kept on a 12h light-dark schedule (light started at 0600 and dark started at 1800). Food and water were available ad libitum and all behavioral experiments occurred from 0900 to 1400.
Acute Ethanol Withdrawal
Acute ethanol withdrawal (AEW) is defined as 6 hr following a 4 g/kg intraperitoneal (IP) injection of 20% v/v ethanol (Tipps et al., 2015). This procedure has previously caused signs of alcohol withdrawal in many inbred strains of mice (increased handling-induced convulsions), especially in withdrawal sensitive lines like DBA/2J (Metten & Crabbe, 1994). The strain used in this paper, C57BL/6J, shows low withdrawal sensitivity, but in response to AEW, B6 mice have previously shown impairments in contextual fear conditioning (Tipps et al., 2015). Animals in the control saline groups (SAL) received an IP injection of saline of a proportionate volume 6 hr prior to behavior. Animals that did not receive complete injections were removed from results and analyses. Injections were given in the morning of the light cycle 6 hr prior to behavior.
Acute Ethanol Intoxication
Acute ethanol intoxication (INTX) is defined as 5 min following 1.5 g/kg IP injection of 20% v/v ethanol. This dose has previously been used as an intoxicating dose in C57BL/6J strain (Crabbe, Cameron, Munn, Bunning, & Wahlsten, 2001) and has caused impairments of both contextual fear conditioning and extinction (Gould, 2003; Lattal, 2007).
Apparatus.
The fear conditioning room contained four Coulbourn Instruments mouse-conditioning chambers (H10-11M-TC; Allentown, PA) in sound- and light-attenuating chambers with a fan producing 70 dB of background noise. Each chamber was equipped with a circular Plexiglas arena (21.5 cm in diameter and 23 cm in height) placed on a grid floor of stainless steel rods (3.2 mm in diameter, spaced 6.4 mm apart). The grid floor was set to deliver a .35 mA scrambled shock via a 110/120 VAC 50-60 Hz computer-controlled shock generator (Coulbourn H13-15). The apparatus also contained a house light that was lit as soon as the session commenced and terminated as soon as the session was over. This was the context that was conditioned to shock. Before and between each round of behavioral testing, the grid floor, Plexiglas arena, and tray were cleaned with 95 percent ethanol.
General Contextual Fear Conditioning Procedure.
In all experiments, initial contextual fear conditioning consisted of a 12-min exposure to the context (described in the Apparatus section above) with four 2 sec, .35 mA footshocks at 2.5, 5, 9, and 11.5 min into the session. Extinction consisted of 24 min of non-shocked context exposure. Post-extinction reconditioning was a weak conditioning session consisting of 3 min session with a single unsignaled footshock (2 sec, .35 mA) delivered 2.5 min into the session. Test sessions were identical to extinction sessions (24-min nonreinforced exposure to the context). All sessions occurred at the same time of day separated by 24 hr. The level of contextual fear conditioning was assessed by sampling freezing, the absence of movement except for breathing (Fanselow & Bolles, 1979), every 8 seconds by the experimenter.
Detailed Behavioral Schedules.
Experiment 1a: Rapid Reacquisition of Contextual Fear Following Reconditioning in C57BL/6J Mice (Moderate Extinction).
The aim of this experiment was to behaviorally characterize reacquisition of contextual fear following moderate extinction. Mice were subdivided into three groups that received different behavioral treatments before a mild conditioning session: Group Recondition (RECOND), Group Context (CTX) and Group Initial Condition (COND). RECOND received strong initial conditioning followed by moderate extinction and weak reconditioning. Control groups that did not receive initial conditioning were matched in exposure to the context (Group Context; CTX), or were naive to the context (Group Initial Condition; COND), before common weak contextual fear conditioning in which all groups received a single footshock in the context. During extinction, CTX provided an indication of when RECOND had reached a behavioral floor, as C57BL/6J mice tend to freeze even without exposure to shock (Tipps et al., 2014a). This design allowed for a direct comparison of initial conditioning to reconditioning.
Phase 1: Conditioning (or Control Treatment).
In this and all subsequent experiments, all groups were moved from the vivarium to a room adjacent to the procedure room during each day of the experiment. All groups were handled in this room daily for three days prior to Phase 1. On Day 1, RECOND received a 12-min contextual fear conditioning session (described above in General Contextual Fear Conditioning Procedure). CTX received a 12min context exposure with no footshocks. COND did not receive any context exposure or shocks, but was moved into and handled in the procedure room to equate transport and handling among the groups.
Phase 2: Extinction.
On the following day both RECOND and CTX received extinction (described above). COND was handled and returned to the home cage. This treatment continued for five additional days, resulting in a total of six days of extinction (or context exposure in the case of CTX) prior to reconditioning. Six days of extinction was necessary to reach a similar average freezing between RECOND and CTX (i.e. when conditioned animals showed freezing behavior similar to that of animals that had never received conditioning), which was important before starting the next phase. An additional extinction criterion of freezing below 30 percent in the last extinction session was followed for all reconditioning studies to ensure that freezing during tests was due to reconditioning, not initial conditioning (i.e. mice freezing above 30 percent in E6 were removed from analysis). All mice met extinction criterion in Experiment 1a.
Phase 3: Reconditioning.
On Day 8, all groups (RECOND, CTX, and COND) received reconditioning as described above. For Group RECOND, this was the second experience of shock in the context and was therefore considered a reconditioning session. For both CTX and COND, this session was the first experience of shock in the context and thus was considered to be an initial conditioning session. A short session with one shock was chosen to avoid the possibility of all groups reaching a behavioral ceiling (high freezing levels) if given a session similar to Day 1 with 4 shocks, as it has been reported that reconditioning can cause rapidly reacquired behavior in one to two trials (McAllister & McAllister, 1988; 1994; 2006).
Phase 4: Test.
Day 9 was a nonreinforced test (24-min context exposure) received by all groups. This day served as a critical day to compare the behavior after reconditioning (RECOND), conditioning after context exposure (CTX), and conditioning without context pre-exposure (COND).
Experiment 1b: Massive Extinction before Reconditioning Prevents Rapid Reacquisition of Contextual Fear.
To examine the effect of massive extinction on post-extinction reacquisition, this experiment had identical groups and behavioral schedules as Experiment 1a, except that Phase 2 extinction was extended to 14 days instead of 6 days. Thus, Phase 2 (Extinction) occurred on Days 2 through 15, Phase 3 (Reconditioning) occurred on Day 16, and Phase 4 (Test) occurred on Day 17.
Experiment 2: AEW Does Not Affect Extinction of Contextual Fear.
This experiment examined the impact of AEW on extinction of contextual fear. After initial conditioning, groups received either extinction (EXT) or no extinction (NoEXT) under AEW or control saline injections (SAL). The NoEXT groups were included to examine the extinction-independent effects of AEW.
All animals received contextual fear conditioning (12-min session with four shocks) on Day 1. On the following day, half of the mice received a single 24-min extinction session 6 hr following ethanol (EXT-AEW) or saline injection (EXT-SAL). The other half received the same injections, but were returned to their homecages (NoEXT-AEW or NoEXT-SAL). On Day 3, all groups received a test session to assess the fear memory associated with the context. One EXT-SAL and one NoExt-AEW did not receive complete injections and were removed from the analysis.
Experiment 3: AEW Moderately Impairs Rapid Reacquisition of Contextual Fear.
This experiment examined the effect of AEW on rapid reacquisition of contextual fear. The behavioral schedule was identical to Experiment 1a, except on Day 8 animals were given injections to induce AEW (RECOND-AEW, CTX-AEW, and COND-AEW) or control saline injections (RECOND-SAL, CTX-SAL, and COND-SAL) 6 hr prior to Phase 3 reconditioning. Five mice did not meet extinction criterion (below 30% time spent freezing in the final extinction session) and were excluded from the analyses.
Experiment 4: AEW Does Not Affect Contextual Fear Conditioning.
This experiment examined the impact of AEW on initial conditioning of contextual fear. Mice received either strong conditioning (12 min session with four footshocks; 4 Shock groups) or weak conditioning (3 min session with one footshock; 1 Shock groups). This resulted in four groups: 4 Shock-AEW, 4 Shock-SAL, 1 Shock-AEW, and 1 Shock-SAL. The following day all animals received 24-min nonreinforced context test.
Experiment 5: Acute Ethanol Intoxication Generally Impairs Fear Learning.
This experiment examined the effect of acute ethanol intoxication on initial acquisition and post-extinction reacquisition of contextual fear. The behavioral schedule was identical to Experiment 1a, except on Day 8 animals were given intoxicating injections of ethanol (acute ethanol intoxication; RECOND-INTX, CTX-INTX, and COND-INTX) or saline control injections (RECOND-SAL, CTX-SAL, and COND-SAL) immediately prior to Phase 3.
Statistics
The main dependent variable was the percent time the mouse spent freezing. R-Studio (Boston, MA) and GraphPad Software Prism 6 (La Jolla, CA) were used to run all statistics and create figures, respectively. Freezing behavior was analyzed with analyses of variance (ANOVAs) that had main factors of Group (RECOND, COND, and CTX; Ext and NoExt; or 1 Shock and 4 Shock), Treatment (AEW or Saline), and, if a repeated measures ANOVA (RMANOVA), Time (3 min bins, Extinction sessions, or pre- and post-shock). Any failure to meet the homogeneity of variances criterion for an ANOVA (as measured by the Brown-Forsythe Levene’s test) was accounted for using a Welch correction. If significance was found for main effects or interactions, Tukey’s HSD tests (or Games-Howell for unequal variances among groups) were used for simple comparisons between groups and sessions. A priori hypotheses evaluating group differences were conducted with simple t-tests. For all statistical tests, significance was set at α = 0.05.
Results
Experiment 1a: Rapid Reacquisition of Contextual Fear Following Reconditioning in C57BL/6J Mice (Moderate Extinction).
The purpose of Experiments 1a and 1b was to characterize reacquisition following a moderate or massive amount of extinction between the initial conditioning and the subsequent reconditioning session in mice. There is evidence for both rapid (Leung et al., 2007; Napier, Macrae, & Kehoe, 1992) and slow (Bouton, 1986; Bouton et al., 2004; Leung et al., 2007) reacquisition following extinction that can depend on the amount of extinction received prior to reconditioning. Much of this work has occurred in rats, which generally show more rapid extinction to a lower asymptote compared to mice (Lattal & Maughan, 2012; Tipps, Raybuck, Buck, & Lattal, 2014a), so this work expands characterization of these behavioral effects to mice. Twenty-four male C57BL/6J mice were used in Experiment 1a and the full schedule can be seen in Figure 1A.
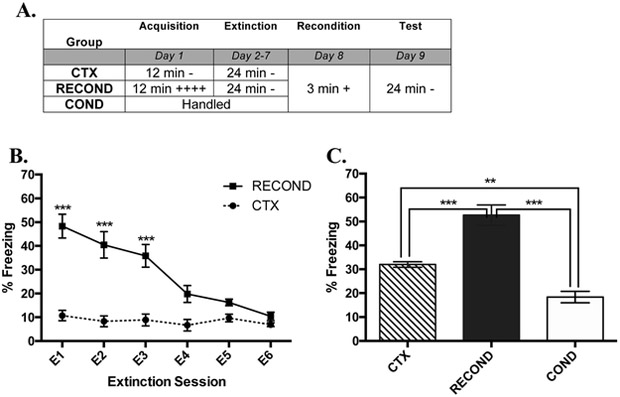
Fig. 1. Rapid Reacquisition of Contextual Fear Following Reconditioning in C57BL/6J Mice (Moderate Extinction).
(A) Overview of the design of Experiment 1a. The times listed represent the total time of exposure to the context for a given session. A plus sign indicates a single 2-s .35 mA shock and a minus sign indicates exposure to the context without shock. (B) Freezing during each extinction session (E) by mice that received fear conditioning (RECOND) or context exposure (CTX) on Day 1 (Day 2-7). (C) Mean freezing from Test (1 day after reconditioning) for Groups CTX, COND (which received no initial conditioning or extinction), and RECOND. Significance between groups is represented by *** p < .001; ** p < .01; * p < .05. RECOND, n= 8; CTX, n=8; COND, n=8. Error bars represent standard error of the mean.
Phase 1: Conditioning.
As expected, shocks during initial conditioning in the group that would subsequently receive reconditioning (Group RECOND) caused more freezing during acquisition than context exposure alone (Group CTX). A t-test comparing groups found a significant effect of Group (t(14) = 5.54, p < .001), as RECOND froze significantly more than CTX during acquisition (data not shown).
Phase 2: Extinction.
As can be seen in Figure 1B, freezing extinguished over the course of six extinction sessions in Group RECOND to similar levels to Group CTX, which was not fear conditioned. The two-way RMANOVA revealed a significant main effect of Group (F(1,14) = 50.29, p < .001), of Extinction session (F(5,70) = 21.42, p < .001), and a significant Group X Extinction session interaction (F(5,70) =16.50, p < .001).
Tukey’s post hoc analyses of the Group X Extinction Session interaction demonstrated that Group RECOND had significantly greater freezing compared to CTX in E1 (q (14) = 9.74, p < .001), E2 (q (14) = 7.59, p < .001), and E3 (q (14) = 7.08, P < .001). This shows that three extinction sessions reduced RECOND fear behavior to CTX levels and six sessions caused the group to be visually indistinguishable (Figure 1B), which ensured that the next phase of the experiment would begin from a common point of behavior.
Phase 3: Reconditioning.
To assess if reconditioning led to immediate differences in freezing, the average percent freezing for the 30 sec immediately before (pre) and after (post) shock (Time) of each Group (RECOND, COND, and CTX) was compared during the brief reconditioning session in a RMANOVA (data not shown). There was a significant effect of Group (F2,21)= 7.73. p < .01), but not Time (F (1,21) = .559, p = .463) or a Time X Group interaction (F (2,21) = .036, p = .964). The main effect of Group was driven by RECOND freezing significantly more than COND (q (21) = 3.09, p < .05) and CTX (q (21) = 2.91, p < .05).
Phase 4: Test.
A Welch-corrected one-way ANOVA was used to analyze the data from Test 1 administered 24 hr following reconditioning (Figure 1C). The ANOVA showed a significant main effect of Group (F (2,11.571) =25.71, p < .001). Games-Howell post hoc revealed that the main effect of group was driven by significantly more freezing by RECOND than both COND (P < .001) and CTX (P < .001), which shows that expression of rapid reacquisition of contextual fear occurs after reconditioning with moderate extinction. We also found that Group CTX showed more freezing behavior than COND (P < .001), which suggests that moderate context pre-exposure possibly enhances acquisition (Rudy & O’Reilly, 1999).
Experiment 1b: Massive Extinction before Reconditioning Prevents Rapid Reacquisition of Contextual Fear.
This experiment examined how extensive extinction affected reacquisition of fear to a context. Previously, massive extinction prevented rapid reconditioning and had an inhibitory effect on reestablishment of contextual fear in rats (Leung et al. 2007). Twenty-four naive male C57BL/6J mice were used this this experiment (3 groups, n=8/group). This experiment was identical to Experiment 1a with the exception of an extended number of days of extinction sessions for Phase 2, extinction (behavioral timeline in Figure 2A).
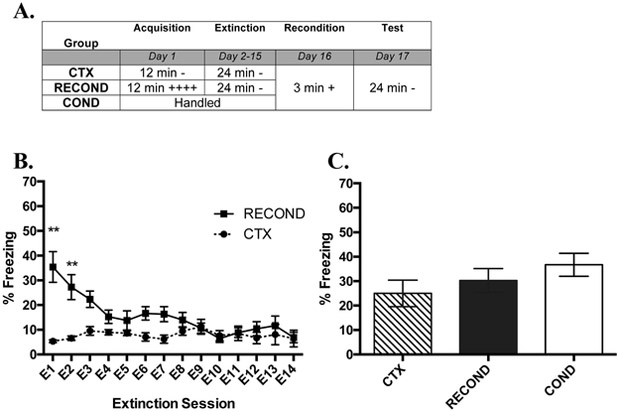
Fig. 2. Massive Extinction before Reconditioning Prevents Rapid Reacquisition of Contextual Fear Conditioning.
(A) Overview of the design of Experiment 1b. The times listed represent the total time of exposure to the context for a given session. A plus sign indicates a single .35 mA shock and a minus sign indicates exposure to the context without shock. (B) Freezing during each extinction session (E) by mice that received fear conditioning (RECOND) or context exposure (CTX) on Day 1 (Day 2-15). (C) Mean freezing from Test (1 day after reconditioning) for each group. Significance between groups is represented by *** p < .001; ** p < .01; * p < .05. RECOND, n= 8; CTX, n=8; COND, n=8. Error bars represent standard error of the mean.
Phase 1: Conditioning.
A t-test showed that RECOND had significantly larger average percent freezing than CTX on Day 1 (t((7,22) =5.31, p < .01; data not shown) and that conditioning led to greater within-session acquisition of freezing behavior relative to nonreinforced context exposure.
Phase 2: Extinction.
In Figure 2B, RECOND and CTX showed similar levels of freezing after the first few days of extinction. A two-way RMANOVA showed a significant effect of Extinction session (F (13,182) =6.48, p < .001), of Group (F (1,14) =7.01, p < .05), and a significant Group X Extinction session interaction (F (13,182) =8.53, p < .001).
The significant interaction was driven by the larger amount of freezing displayed by animals in Group RECOND compared to group CTX in E1 (q (14) = 6.81, p < .01) and E2 (q (14) = 5.70, p < .01), but not later sessions, showing a reduction in fear behavior in Group RECOND during massive extinction.
Phase 3: Reconditioning.
During reconditioning, groups did not differ in their freezing in response to weak conditioning parameters (data not shown). A RMANOVA comparing average percent freezing during 30 sec pre- and post-shock across groups (Time X Group) showed a significant main effect of Time (F (1,21) = 6.27, p < .05), but not a significant main effect of Group (F (2,21) = 1.55, p = .236) or Time X Group interaction (F (2,21) = .674, p = .520). This suggests that reconditioning did not lead to within session differences in reacquisition of freezing behavior, but freezing did increase in all groups following shock.
Phase 4: Test.
Twenty-four hr following weak reconditioning, all three groups were exposed to the context for 24 min and average freezing behavior was analyzed using a one-way ANOVA (Figure 2C). The ANOVA did not show a significant main effect of Group (F (2,21) =1.38, p =.274). Further, no significant difference in groups was detected when comparing the first 6 min of Test 1 (F (2,21) =2.46, p =. 109; data not shown). This result shows that massive extinction prevents the rapid reacquisition effect seen in Experiment 1a.
Experiment 2: AEW Does Not Affect Extinction of Contextual Fear.
Chronic forms of withdrawal impair extinction of conditioned fear behavior (Ripley et al., 2003), but the effects of initial withdrawal from ethanol (i.e. AEW) on extinction are unknown. Thirty-two naive male C57BL/6J mice were used in this experiment (4 groups, n=8/group) and the full behavioral schedule can be seen in Figure 3A.
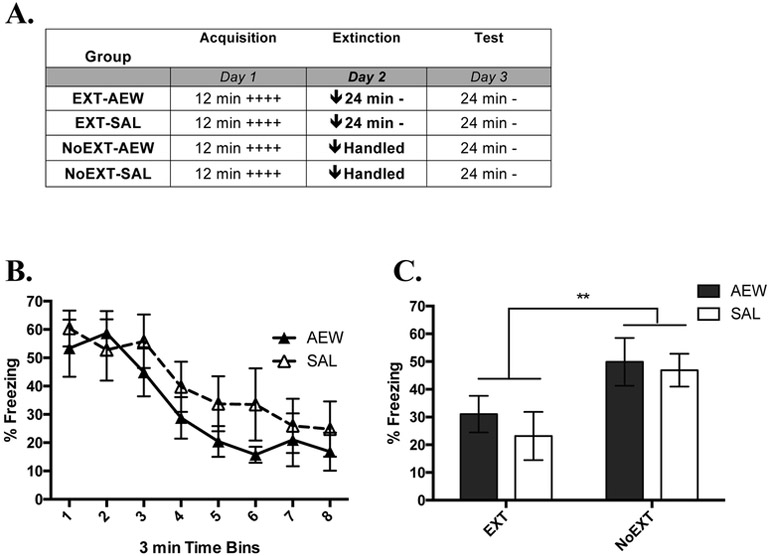
Fig. 3. AEW Does Not Affect Extinction of Contextual Fear.
(A) Overview of the design of Experiment 2. The times listed represent the total time of exposure to the context for a given session. A plus sign indicates a single .35 mA shock and a minus sign indicates exposure to the context without shock. A downward arrow indicates the administration of ethanol or saline prior to the session. (B) Mean percent freezing during the extinction session (Day 2) in which mice were 6 hr post acute ethanol injection (EXT-AEW) or saline injection (EXT-SAL). (C) Mean percent freezing during the test session (Day 3) of all groups; EXT-AEW, n = 8; EXT-SAL, n = 7; NoEXT-AEW, n = 7; NoEXT-SAL, n = 8; **, p < .01. Error bars represent standard error of the mean.
Conditioning.
Prior to AEW or extinction treatment on Day 2, we determined group assignments by balancing the average percent freezing displayed by each animal during conditioning. A two-way ANOVA, comparing percent freezing during acquisition across future Treatment (AEW or Saline) and future Extinction (Ext or NoExt) did not find a significant main effect of Treatment (F (1,26) = 0.008, p = 0.931; data not shown), Extinction (F (1,26) = 0.040, p = 0.843; data not shown) or Treatment x Extinction interaction (F (1,26) = 0.086, p = 0.772; data not shown), confirming groups were balanced before manipulation the next day.
Extinction During AEW.
Figure 3B shows the course of extinction. An ANOVA found a significant main effect of Time (F (1,13)) = 29.60, p < .001), which suggests that extinction successfully lowered the percent time freezing within a session. The ANOVA did not find a significant main effect of Treatment on extinction (F (1,13) = 0.917, p = 0.356) or a significant Time X Treatment interaction (F (1,13) = 0.208, p = 0.656), suggesting that AEW did not alter the expression of freezing during initial retrieval or extinction.
Test Following Extinction During AEW.
In the test comparing the retention of extinction (Figure 3C), a two-way ANOVA comparing Extinction training (EXT or NoEXT) and Treatment (AEW or Saline) found a significant main effect of Extinction training (F (1,26) = 7.97, p < .01), but not of Treatment (F (1,26)) = 0.541, p = 0.468) or an Extinction x Treatment interaction (F (1,26)) = 0.109, p = 0.744), suggesting that acute ethanol withdrawal affected neither the development nor the expression of extinction 24 hr later.
Experiment 3: AEW Moderately Impairs Rapid Reacquisition of Contextual Fear.
Experiment 3 aimed to examine the effect of AEW on rapid reacquisition relative to its effects on initial acquisition. Rapid reacquisition of contextual fear could be impaired by AEW as AEW previously impaired initial acquisition of contextual fear (Tipps et al., 2015) and both are processes of promoting fear memory formation. However, despite identical parameters during the conditioning/reconditioning session (Phase 3), AEW may affect one process more than the other due to the potentially larger prediction error that may occur with initial conditioning (Mackintosh, 1975; Rescorla & Wagner, 1972). Forty-eight male C57BL/6J mice were used in Experiment 3 (6 groups, n=8/group). The full behavioral schedule can be seen in Figure 4A.
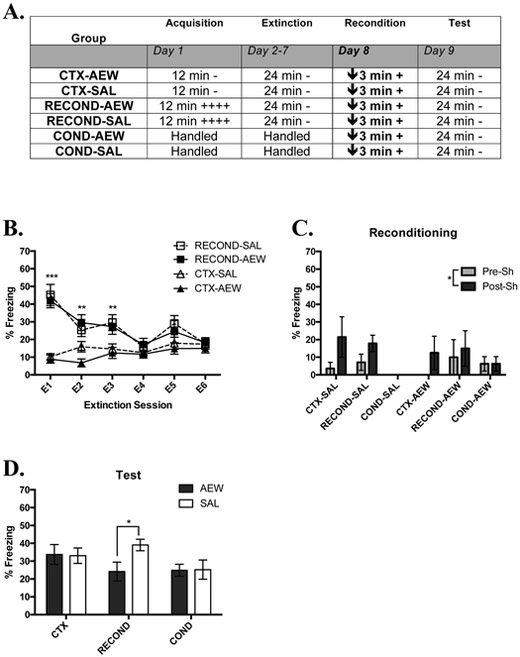
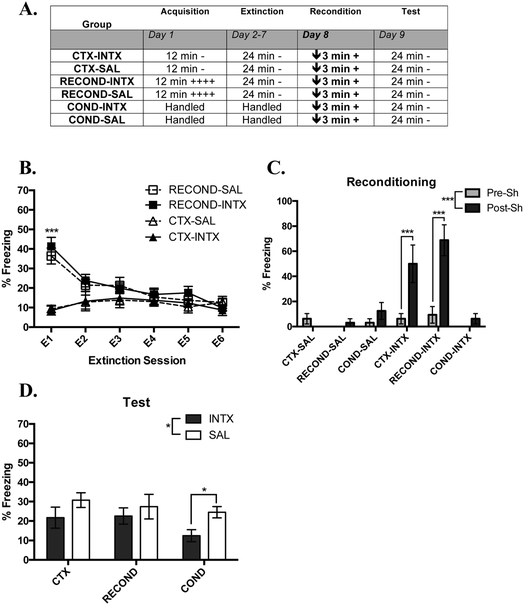
Fig. 4. AEW Moderately Impairs Rapid Reacquisition of Contextual Fear.
(A) Overview of the design of Experiment 3. The times listed represent the total time of exposure to the context for a given session. A plus sign indicates a single .35 mA shock and a minus sign indicates exposure to the context without shock. A downward arrow indicates the administration of ethanol or saline prior to the session. (B) Mean percent freezing during extinction sessions (Days 2-7) of RECOND and CTX groups. (C) Mean percent Freezing of all groups during the 30 sec pre and post shock during Phase 3 under AEW or SAL (Day 8). (D) Mean percent freezing of all groups during the test session (Day 9) 24 hrs following reconditioning under AEW or SAL. The RECOND-SAL vs COND-SAL difference wasp =.054. CTX-AEW, n = 8; CTX-SAL, n = 7; RECOND-AEW, n = 5; RECOND-SAL, n = 7; COND-AEW, n = 8; COND-SAL, n = 8; *, p < .05 **, p < .01; ***, p < .001. Error bars represent the standard error of the mean.
Phase 1: Conditioning.
To compare differences in freezing due to conditioning, a two-way ANOVA comparing average percent freezing across Group (RECOND and CTX) and future Treatment (AEW or SAL) found that there was a significant effect of Group (F (1, 23) = 39.42,p < .001; data not shown), but not Treatment (F (1, 23) = 0.385, p = .541; data not shown) or a Treatment x Group Interaction (F (1, 23) = 0.038, p = .846). The significant Group effect was driven by RECOND responding significantly more to the context-shock pairings than CTX to nonreinforced context exposure. The lack of a significant Treatment effect or interaction shows that treatments were balanced before extinction.
Phase 2: Extinction.
To compare extinction between groups with differing conditioning experiences, a three-way RMANOVA compared the between-subjects measures of Group (RECOND and CTX) and future Treatment (AEW or Saline) and the within-subjects factor of Extinction session (Extinction sessions 1 through 6; Figure 4B). The three-way RMANOVA found a significant main effect of Group (F (1,23) =28.57, p < .001), Extinction Session (F (5,115) =9.28, p < .001) and a Group X Extinction Session interaction (F(5, 115) = 22.24, p < .001), but not significant effect of Treatment (F (1,23) = .631, p = .435) or any other interaction.
The significant Group X Extinction Session effect was again driven by RECOND groups freezing more than CTX groups in E1 (q (25) = 13.03,p < .001), E2 (q (25) = 6.61,p < .01), and E3 (q (24) = 5.91,p < .01) as revealed by post-hoc analyses, but not in later extinction sessions when groups converged. Unsurprisingly, this showed that groups initially differed in freezing due to their different behavioral treatments; i.e. conditioning lead to significantly more freezing than mere context exposure. It also showed that by three extinction sessions groups were statistically indistinguishable. However, after 6 sessions groups were visually similar in freezing and balanced across treatments before receiving different treatments following extinction (Figure 4B).
Phase 3: Reconditioning During AEW.
To assess the effects of AEW directly during reconditioning, a three-way RMANOVA comparing the freezing to context in the 30 sec pre- and post-shock (Time) among Group (RECOND, COND, and CTX) and Treatment (AEW or Saline) was run. There was a significant main effect of Time (F(1, 37) = 4.57, p < .05; Figure 4C). There was also a trending main effect of Group (F (2, 37) = 2.72,p = .078), driven by a trend in higher freezing in RECOND relative to COND (q (40) = 1.89, p = .081). There was no significant main effect of Treatment (F (1, 37) = .00, p = 1.00), suggesting that AEW did not alter the ability of animals to freeze during reconditioning. Additionally, there were not any significant interactions.
Phase 4: Test Following Reconditioning During AEW.
Figure 4D shows the mean freezing of all groups in the test assessing the strength of acquisition or reacquisition of contextual fear conditioning under AEW or control conditions 24 hr before. In a two-way ANOVA comparing Group (RECOND, CTX, and COND) and Treatment (AEW or saline), there were not significant effects of Group (F (2,37) = 2.10, p = .136), Treatment (F (1,37) = 1.14, p = .292), or Group X Treatment interaction (F (2,37)) = 1.55, p = .225). Given the results from experiments above and from Tipps et al. (2015), there were three a priori hypotheses tested with unpaired t-tests.
In Tipps et al. (2015), AEW impaired contextual fear conditioning and thus we expected that COND-AEW would show significantly impaired fear memory in the test session compared to COND-SAL. However, there was no significant difference between groups (t (14) = .062, p = .952, Figure 4D). This indicates that AEW may not always impair contextual fear conditioning, in particular when brief context exposure is paired with 1 shock. We also hypothesized that rapid reacquisition following reconditioning would occur in saline groups. An unpaired t-test comparing RECOND-SAL and COND-SAL was trending on significance (t(13) = 2.11, p = .054; Fig 4D), which suggests that there was a mild rapid reacquisition of fear behavior in reconditioned animals when compared to conditioned animals. The final a priori hypothesis was that AEW would impair the rapid reacquisition of fear because previous work suggests that AEW impairs initial conditioning (Tipps et al., 2015). An unpaired t-test comparing RECOND-AEW and RECOND-SAL was significant (t (10) = 2.56, p < .05; Figure 4C) with animals that were reconditioned while experiencing AEW showing significantly less retention of conditioned freezing compared to animals reconditioned under SAL.
Experiment 4: AEW Does Not Affect Contextual Fear Conditioning.
Due to the lack of acquisition impairment by AEW in the last experiment, the goal of Experiment 4 was to examine if initial acquisition must be strong to be affected by AEW, as previously seen in Tipps (2015). Thirty-two male C57BL/6J mice were used (4 groups, n=8/group). The full behavioral schedule is in Figure 5A.
Fig. 5. AEW Does Not Affect Contextual Fear Conditioning.
(A) Overview of the design of Experiment 4. The times listed represent the total time of exposure to the context for a given session. A plus sign indicates a single .35 mA shock and a minus sign indicates exposure to the context without shock. A downward arrow indicates the administration of ethanol or saline prior to the session. (B) Mean percent freezing during strong (4 Shock) or weak (1 Shock) conditioning (Day 1) in which animals were 6 hr post acute ethanol injection (AEW) or saline injection (SAL). (C) Mean percent freezing during the test session (Day 2) 24 hrs following strong conditioning. 1 Shock- AEW, n = 8; 1 Shock-SAL, n = 8; 4 Shock-AEW, n = 8; 4 Shock-SAL, n = 8. ***, p < .001. Error bars represent the standard error of the mean.
Conditioning During AEW.
To assess if AEW impaired freezing response during conditioning, a two-way ANOVA comparing the freezing during acquisition between Group (1 Shock or 4 Shocks) and Treatment (AEW or Saline) was run. We found a significant main effect of Group (F (1,28) = 88.42, p < .001; Figure 5B), but no significant effect of Treatment (F (1,28)) = .09, p = .77). This demonstrates that 4 shocks resulted in a stronger within-conditioning-session freezing response compared to a shorter, 1 shock conditioning session, but there was no effect of AEW on freezing during conditioning.
Test Following Acquisition During AEW.
Freezing behavior in the test following strong or weak conditioning under AEW or saline is displayed in Figure 5C. A two-way ANOVA comparing Group (1 Shock or 4 Shocks) and Treatment (AEW or Saline) found a significant main effect of Group (F (1,28) = 17.11, p < .001), but not Treatment (F (1,28) = .091, p = .765) or Group X Treatment interaction (F (1,28 = 1.07, p = .310). This demonstrates that freezing at test increased with an increase in shock number, but neither weak nor strong conditioning was affected by AEW.
Experiment 5: Acute Ethanol Intoxication Generally Impairs Fear Learning.
This experiment examined the effect of acute ethanol intoxication on acquisition and rapid post-extinction reacquisition of contextual fear. We hypothesized that intoxication would impair acquisition (as seen in similar behavioral preparations in Gould, 2003) and rapid reacquisition, as both involve context-shock associations. For Experiment 5, 48 male C57BL/6J mice were used (6 groups, n=8/group). Refer to Figure 6A for the full schedule.
Fig. 6. Acute Ethanol Intoxication Generally Impairs Fear Learning.
(A) Overview of the design of Experiment 5. The times listed represent the total time of exposure to the context for a given session. A plus sign indicates a single .35 mA shock and a minus sign indicates exposure to the context without shock. A downward arrow indicates the administration of ethanol or saline prior to the session. (B) Mean percent freezing during extinction sessions (Days 2-7) of RECOND and CTX groups. (C) Mean percent freezing for 30 sec pre and post shock of all groups during Phase 3 (reconditioning) under acute intoxication (INTX) or SAL (Day 8). Further significant main effects and interactions are detailed in the text. (D) Mean percent freezing of all groups during the test session (Day 9) 24 hrs following reconditioning under acute intoxication (INTX) or SAL. The RECOND-AEW vs COND-AEW difference was p = .071. CTX-AEW, n = 8; CTX-SAL, n = 8; RECOND-AEW, n = 8; RECOND-SAL, n = 8; COND-AEW, n = 8; COND-SAL, n = 8; *, p < .05 **, p < .01; ***, p < .001. Error bars represent the standard error of the mean.
Phase 1: Conditioning.
Prior to extinction, percent freezing during acquisition was compared across groups and future treatment. A two-way ANOVA comparing Groups (RECOND vs CTX) and Treatment (INTX vs. SAL) found a significant effect of Group (F (1,28) = 53.44, p < .001; data not shown), but did not show significant effects of future Treatment (F (1,28) = .042, p = .834) nor Group x Treatment interaction (F (1,28) = .398, p = .533). This suggests that conditioning caused significantly more freezing than context exposure (RECOND > CTX), but that the groups were balanced across treatments.
Phase 2: Extinction.
Extinction of RECOND and CTX was analyzed in a three-way RMANOVA (Figure 6B), which found a significant main effect of Group (RECOND > CTX; F (1,28) =12.57,p < .01), Extinction Session (F (5,140) = 17.09, p < .001) and a Group X Extinction Session interaction (F (5,140) = 24.73,p < .001), but not significant effect of Treatment (F (1,28) = .060, p = .808).
The significant Group X Extinction Session effect was again driven by RECOND groups freezing more than CTX groups in E1 (q (30) = 12.54, p < .001) as revealed by post-hoc analyses, but not in later extinction sessions when groups converge. This demonstrates that groups had statistically similar freezing by extinction session 2, but extinction extended to 6 sessions to maintain procedural similarity to previous experiments.
Phase 3: Reconditioning During Acute Intoxication.
To assess the effects of acute intoxication directly on reconditioning, a three-way RMANOVA comparing the freezing in the 30 sec pre- and post-shock (Time) among Groups (RECOND, COND, and CTX) and Treatment (INTX or Saline) was run (Figure 6C). There was a significant main effect of Group (F (2, 42) =4.12, p < .05) driven by RECOND freezing significantly more than COND (q (45) = 4.37, p < .01). There were also significant main effects of Time (F (1, 42) =34.10, p < .001) and Treatment (F (1, 42) =19.92, p < .001). In addition to main effects, there was a significant Group X Treatment interaction (F (2, 42) = 8.40, p < .001), Group X Time interaction (F (2, 42) = 4.21, p < .05), Treatment X Time interaction (F (1,42) = 27.13, p < .001), and Group X Treatment X Time three-way interaction (F (2, 42) = 8.15, p < .01).
The three-way interaction was driven by RECOND-INTX showing significantly more post-shock freezing than RECOND-SAL (q (42) = 9.74, p < .001) and CTX-INTX showing significantly more post-shock freezing than CTX-SAL (q (42) =6.85, p < .001), whereas COND-INTX did not freeze significantly more post-shock than COND-SAL (q (42) = .00, p = .999). Additionally, the RECOND and CTX treatment differences were not reliable before shock (all q (42) < 0.5, p > .98). The interaction was also driven by RECOND-INTX (q (42) =9.92, p < .001) and CTX-INTX (q (42) =5.78,p < .001) freezing more following shock than before shock, whereas others group did not significantly increase their freezing following shock. In the 30 sec post-shock, there were additional significant differences: RECOND-INTX froze more than COND-INTX (q (42) = 8.97, p < .001), COND-SAL (q (42) = 9.57, p < .001) and CTX-SAL (q (42) = 10.04, p < .001); and CTX-INTX froze more than COND-INTX (q (42) =4.93,p < .001), COND-SAL (q (42) =4.81, p < .001), and RECOND-SAL (q (42) =7.38, p < .001).
These interaction effects show that intoxication might facilitate short-term rapid reacquisition during reconditioning and acquisition during conditioning of a pre-exposed context.
Phase 4: Test Following Reconditioning.
In the test following reconditioning (Figure 6D), a two-way ANOVA comparing Group (RECOND, CTX, and COND) and Treatment (Intoxication or saline) found a main significant effect of Treatment (F(1,42) = 5.72, p < .05), but not of Group (F (2,42)) = 1.74, p = .187) or Group X Treatment interaction (F (2,42) = .339, p = .715). The significant main effect of Treatment was driven by the general dampening effect of intoxication during reconditioning on freezing behavior in the subsequent test day.
Many examples in the literature show that intoxication impairs initial contextual fear conditioning (Gould, 2003; Kitaichi et al., 1995), thus we predicted decreased freezing in COND and CTX groups who were intoxicated during conditioning (Day 8, Reconditioning day for RECOND). An unpaired t-test revealed significantly lower freezing behavior by COND-INTX when compared to COND-SAL (t (14) = 2.88, p < .05; Figure 6D), but a non-significant difference between CTX-INTX and CTX-SAL (t (14) = 1.38, p = .191), suggesting that context pre-exposure may prevent impairment of conditioning by intoxication. We also hypothesized that if initial conditioning and reconditioning can both be affected by AEW under certain conditions, they may be similarly impacted by intoxication. Thus, we examined the difference in RECOND-INTX and RECOND-SAL in the test and found there was no significant difference in their freezing behavior (t (14) = .639, p = .533). We also hypothesized that we would replicate the rapid reacquisition effect in the non-intoxicated animals, but surprisingly when comparing RECOND-SAL and COND-SAL there are no significant differences in freezing behavior (t (14) = .411, p = .687). There was however a trending difference between RECOND and COND groups within the intoxication condition (t (14) = 1.96, p = .071; Figure 6D).
General Discussion
These experiments demonstrate that conditioned contextual fear can be rapidly reacquired following moderate extinction in mice. Thus, rapid reconditioning appears to involve the unmasking of a context-shock association that is suppressed by moderate amounts of extinction. Although the rapid reacquisition effect was eliminated after massive extinction, we found no evidence that extended extinction caused a retardation of reacquisition after extinction, suggesting that extended extinction did not increase inhibition (Pavlov, 1927) or cause slow reacquisition (Bouton, 1986). Further, these experiments demonstrate the acute ethanol intoxication and AEW have different effects on contextual fear. Reacquisition after moderate extinction was mildly impaired by AEW, whereas rapid reacquisition was dampened, but not impaired, by ethanol intoxication.
The major finding from the first set of studies was that post-extinction reconditioning caused rapid reacquisition of contextual fear following moderate, but not massive extinction. In Experiment 1a, conditioning with a single footshock within the context after extinction led to the rapid reacquisition of contextual fear in mice. The finding of rapid reacquisition has been seen in the literature previously (Bouton et al., 2004; Leung et al., 2007; Napier et al., 1992) and this work serves to extends these findings in contextual fear in mice, in which previously reconditioning had never been directly compared to initial conditioning, but instead was used to test extinction strength (Bolkan & Lattal, 2014).
In Experiment 1a, enhanced reacquisition of fear following a mild footshock showed that extinction to a behavioral floor (freezing levels equal to a group that received context exposure in the absence of conditioning) does not remove the memory of the initial conditioning, but rather that reconditioning might access the original conditioning memory and strengthen its expression. Similar findings have been shown by a variety of post-extinction phenomena (Bouton & Bolles, 1979; Pavlov, 1927; Rescorla, 2004; Rescorla & Heth, 1975), and our findings reinforce the utility of rapid reacquisition as another tool for studying the re-emergence of behavior following extinction.
The reconditioned mice in Experiment 1a displayed enhanced fear behavior after a mild footshock compared not only to a group conditioned and exposed to the context for the first time, but also to a group that received initial conditioning following equal context exposure (CTX). This suggests that the enhanced fear expression of the reconditioned mice was not due to longer context exposure, but was caused by previous experiences of footshock in the context. Similar findings of rapid reacquisition have been found before in contextual fear reconditioning after moderate extinction (Leung, Bailey, Laurent, & Westbrook, 2007). Experiment 1a also found, however, that context pre-exposure promoted acquisition of initial fear, which has been reported previously (Rudy & O'Reilly, 1999). The finding that reconditioning is larger than the effect of context pre-exposure further demonstrates that the increased freezing in the reconditioning group is not simply due to their previous exposure to the context.
In Experiment 1b, mice that received massive extinction, or extinction well beyond when behavior was reduced to their behavioral floor (14 extinction sessions), did not show rapid reacquisition following reconditioning, suggesting that rapid reconditioning may be limited to conditions in which moderate amounts of extinction occur. However, slow reacquisition did not occur following extensive extinction, as shown in previous reports (Bouton & Swartzentruber, 1989; Leung et al., 2007). The absence of an impaired reacquisition effect following extensive extinction could be due in part to the relatively high levels of freezing we observe in mice at asymptote after extinction (e.g., Lattal & Maughan, 2012). Further, it is possible that an impairment in reacquisition would be revealed with additional context-shock pairings. The conditions that lead to rapid and slowed reacquisition following extinction of contextual fear will require further research.
The second major finding from these studies was the selective impairment of rapid reacquisition, but not initial acquisition or extinction of contextual fear by AEW. Experiment 3 demonstrated that post-extinction reconditioning caused moderate rapid reacquisition of fear that was reduced by AEW. We did not observe an effect on initial conditioning, as was seen previously in our lab (Tipps et al., 2015). Conditioning had relatively weak parameters (3 min context exposure and a single .35 mA shock) compared to Tipps et al. (2015). Thus, Experiment 4 compared the effects of AEW on strong (4 shock-context pairings) and weak (1 shock-context pairing) contextual fear conditioning and found that AEW impaired neither weak nor strong conditioning. It is important to note that the conditioning parameters used in Experiment 3 and 4 were not identical to Tipps et al. (2015), in which they used two context-shock pairings (2 pairings separated by a 90-second inter-trial interval in a 6.5-minute session). It is possible that AEW only impairs acquisition of contextual fear under certain circumstances, which is consistent with findings that ethanol intoxication’s effects on conditioning depend on administration, dose, and timing (Gulick & Gould, 2007).
Another complication is that initial acquisition and reacquisition may rely on brain regions or mechanisms that may be differentially affected by acute withdrawal. Studies have found that other processes, such as short-term and long-term memory (Bekinschtein et al., 2007; McGaugh & Dawson, 1971; Yeh, Lin, & Gean, 2004) and conditioning- and retrieval-induced plasticity (Alberini, 2005; Hertzen, 2005), while relying on similar neural mechanisms, have a distinct patterns of molecular processes. Further, the acquisition of initial extinction requires activity in the BLA, while re-extinction does not (Laurent, Marchand, & Westbrook, 2008), so it is entirely likely that acquisition and reacquisition of contextual fear could recruit a different set of brain regions that are selectively susceptible to the effects of AEW.
In Experiment 2, acute withdrawal had no effect on the development or expression of extinction when tested 24 hr later. These results differ from the effects of acute intoxication, which has been shown to impair extinction learning (Bisby et al., 2015; Holmes et al., 2012; Lattal, 2007). This suggests that, like acquisition and reacquisition, intoxication and AEW may act on different mechanisms and neural targets, particularly on those involved in extinction learning. This hypothesis is supported by the pattern of neural activity during AEW. For example, AEW induces an increase in activity in the prelimbic cortex of the mPFC (Kozell et al., 2005), a region implicated in the formation and expression of fear conditioning memories (Peters, Kalivas, & Quirk, 2009; I. Vidal-Gonzalez, Vidal-Gonzalez, Rauch, & Quirk, 2006). But the impact of AEW on IL, which is important for extinction (Laurent & Westbrook, 2009), is unknown. The molecular studies in conjunction with our behavioral results suggest that AEW may have a distinct pattern of neural activity that may preferentially affect circuits associated with fear-promoting learning.
The final major finding of these studies was the general impairment of conditioned freezing by acute ethanol intoxication. In Experiment 5, acute intoxication caused a general impairment in freezing behavior and impaired conditioning, as in previous studies (Gould, 2003; Gulick & Gould, 2007; Kitaichi et al., 1995; Melia et al., 1996; Stragier et al., 2015). This impairment was not observed in mice that received context pre-exposure or in mice that received extinction prior to reconditioning. This suggests that a history of exposure to a context may overcome some of the impairing effects of acute intoxication.
During acute intoxication there was a general increase in freezing immediately after shock was paired with context, but the increased freezing did not persist into a long-term memory difference during the test. Conversely, animals in AEW did not differ in post-shock freezing during acquisition or reacquisition (Experiment 3 and 4) or in the freezing response to the context during extinction (Experiment 1). This suggests that ethanol intoxication, but not withdrawal, may increase the short-term consequences of shock. However, work has shown that ethanol withdrawal, not intoxication, can cause increased sensitivity to stressors and tactile sensitivity (Rassnick, Koob, & Geyer, 1992; Smith, Hostetler, Heinricher, & Ryabinin, 2016).
The somewhat selective impact of ethanol intoxication on acquisition and AEW on reacquisition provides additional evidence that acquisition and reacquisition rely on different neurobiology. There is also a potential circuit-based explanation for ethanol’s disparate effects on specific phases of fear memory. Hippocampal place cells, for example, show an initial reduction in neural activity in response to both acute and chronic ethanol intoxication (White & Best, 2000) and intoxication-induced reductions in hippocampal activity have been shown to interfere with contextual fear conditioning (Melia et al., 1996). Conversely, ethanol withdrawal causes increased hippocampal activity (Kozell et al., 2005; Matsumoto, Leah, Shanley, & Wilce, 1993). Perhaps acute ethanol intoxication’s reduction in hippocampal activity causes impairments of conditioning and extinction (Lattal, 2007), as both rely on hippocampal activity (Peters et al., 2009; Rudy & O'Reilly, 1999; Stafford, Raybuck, Ryabinin, & Lattal, 2012). AEW, which increases hippocampal activity (Kozell et al., 2005), impaired reacquisition, but left initial acquisition and extinction intact. This hypothesis will have to be studied in more depth, but the biphasic response of the hippocampus, and likely other brain regions, to ethanol administration could explain the memory-specific impairments of acute ethanol intoxication and withdrawal.
Several factors need to be considered when evaluating the effects of ethanol intoxication and withdrawal on conditioning, extinction, and reconditioning. First, the strain of mouse used here, C57BL/6J, shows robust contextual fear conditioning relative to other strains, but resists the effects of alcohol withdrawal (Metten & Crabbe, 1994). Even so, AEW does cause a moderate impairment of rapid reacquisition of fear following reconditioning in B6 mice, which suggests that impairments can occur, even in a withdrawal resistant strain. Moreover, strains of mice that are more susceptible to withdrawal have also been shown to display poor conditioning and spontaneous recovery of contextual fear, such as the DBA/2J strain (Balogh, Radcliffe, Logue, & Wehner, 2002; Lattal & Maughan, 2012; Tipps et al., 2014), which complicates their use in the study of effects of ethanol and AEW on fear conditioning.
Second, multiple studies have shown that ethanol intoxication can operate as an internal stimuli that can also be paired with a learning contingency (Cunningham, 1979b; 1979a; Lattal, 2007). Although not evaluated here, it is possible that AEW created an internal stimulus that signaled the operation of conditioning, extinction, or reconditioning contingencies. The stimulus properties of AEW should be a point of future study, as perhaps rapid reacquisition might show state-dependent expression.
Third, rapid reacquisition was shown in alcohol naïve mice in Experiment 1a and more mildly in Experiment 3, but not Experiment 1b and 5, which shows that rapid reacquisition is achieved only under certain conditions (Bouton, 2002; Leung et al., 2007). Similar results are seen with spontaneous recovery, which does not always occur following a delay in extinction (Rescorla, 2004). The lack of rapid reacquisition in Experiment 1b is likely due to the massive extinction prior to reconditioning, but Experiment 5 used the same moderate extinction parameters that resulted in rapid reacquisition in Experiments 1a and 3. There are key differences in Experiment 5 that could block rapid reacquisition. Foremost, the timing of administration of saline injection immediately (Exp. 5), as opposed to 6 hrs (Exp. 3), prior to reconditioning could have prevented rapid reacquisition of fear behavior via stress, which has been known to impair learning (Kim & Diamond, 2002). Additionally, extinction behavior and response to reconditioning shock differed greatly in Experiment 5. The extinction curve of reconditioning animals was much steeper than seen in previous studies and by the second extinction session the reconditioning animals were statistically equivalent to conditioning-naive CTX animals. Perhaps the reconditioning animals were over-extinguished and this blocked rapid reacquisition as in Experiment 1b. Similarly, treatment differences during reconditioning could have led to an altered rapid reacquisition effect in the retention test. In Experiment 5, the alcohol naive animals did not show rapid reacquisition, but intoxicated mice showed a trending rapid reacquisition effect during reconditioning.
These factors may interact with how testing is arranged in different experiments. We used a common assessment procedure to evaluate the effects of reconditioning compared to initial conditioning. If one were to measure changes in freezing from the end of extinction to the post-reconditioning test, then we would appear to observe more robust reconditioning. Another comparison that is popular particularly in the early reacquisition literature is to look at rate of initial acquisition vs. rate of reacquisition, which is difficult in our approach that uses a single reacquisition trial. We have previously found that within-subject changes from acquisition to test may lead to very different interpretations compared to between-group common test comparisons (Stafford & Lattal, 2009; Tipps et al., 2014a). Our approach could be more sensitive to changes in baseline behavior that may fluctuate from experiment to experiment or session to session (Bouton, 1986).
Finally, contextual fear conditioning and rapid reacquisition of fear do not encapsulate the complexities of PTSD, but rather model the formation of a fear memory that can be preferentially enhanced by a mild re-pairing of context and fear. Similarly, withdrawal following a single exposure to ethanol does not model AUD, such that chronic ethanol withdrawal can actually cause different effects relative AEW. Accordingly, many brain regions that show activation following repeated or chronic ethanol withdrawal, (Borlikova et al., 2006; Olive et al., 2001), do not show activation following initial withdrawal or AEW (Borlikova et al., 2006; Kozell et al., 2005; Vilpoux et al., 2009). Therefore, we cannot expect that a single experience of ethanol withdrawal and a formation of a relatively mild fear memory will mimic the human pattern of AUD-PTSD comorbidity,
However, rapid reacquisition could provide a behavioral correlate for which to better understand the behavior and neurobiology of persistent fear memory and the susceptibility of fear behavior reemergence to a mild reminder, such as is seen in PTSD. Therefore, we can draw insight into how initial withdrawal from ethanol use and fear memory might interact to alter disorder progression and likelihood of comorbidity. Critically, we gain insight into the circuitry of later learning events (reconditioning), which appear to be differential affected by ethanol intoxication and withdrawal relative to initial conditioning and extinction.
One conclusion that is clear from these experiments and the work of others is that the study of the interaction of alcohol and memory is complicated with mixed results (see Tipps, et al., 2014b). For example, alcohol intoxication does not always impair conditioning, but can actually enhance depending on the dose (Gulick & Gould, 2007). Also, repeated or acute withdrawal has been shown to impair contextual fear conditioning by several labs, including our own (Ripley et al., 2003; Stephens, Brown, Duka, & Ripley, 2001; Tipps et al., 2015), but others have found that contextual fear conditioning is unaffected (Borlikova et al., 2006) or even enhanced (Bertotto et al., 2006) by withdrawal. Any number of factors including species and levels of performance in the control group could drive these differences. Thus, this paper serves as a call for a systematic assessment of the conditions under which ethanol impairs (or enhances) memory so that we can better understand the effects of alcohol on memory in general and improve both behavioral and pharmacological treatments of those comorbid for PTSD and AUD.
Acknowledgments
Funding support was provided by National Institute on Drug Abuse (NIDA) grant R01DA025922 (KML), National Institute on Alcohol Abuse and Alcoholism grant T32AA007468 (ARW), and Department of Defense grant W81XWH-12-2-0048 (KML).
Footnotes
Conflict of Interest Statement
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Alberini CM (2005). Mechanisms of memory stabilization: are consolidation and reconsolidation similar or distinct processes? Trends in Neurosciences, 28(1), 51–56. 10.1016/j.tins.2004.11.001 [DOI] [PubMed] [Google Scholar]
- Balogh SA, Radcliffe RA, Logue SF, & Wehner JM (2002). Contextual and cued fear conditioning in C57BL/6J and DBA/2J mice: context discrimination and the effects of retention interval. Behavioral Neuroscience, 116(6), 947–957. [DOI] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Igaz LM, Bevilaqua LRM, Izquierdo I, & Medina JH (2007). Persistence of Long-Term Memory Storage Requires a Late Protein Synthesis- and BDNF-Dependent Phase in the Hippocampus. Neuron, 55(2), 261–277. 10.1016/j.neuron.2006.11.025 [DOI] [PubMed] [Google Scholar]
- Bertotto SE, Bustos SG, Molina VA, & Martijena ID (2006). Influence of ethanol withdrawal on fear memory: Effect of d-cycloserine. Neuroscience, 142(4), 979–990. 10.1016/j.neuroscience.2006.07.013 [DOI] [PubMed] [Google Scholar]
- Bisby JA, King JA, Sulpizio V, Degeilh F, Valerie Curran H, & Burgess N (2015). Extinction learning is slower, weaker and less context specific after alcohol. Neurobiology of Learning and Memory, 125, 55–62. 10.1016/j.nlm.2015.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco C, Xu Y, Brady K, Pérez-Fuentes G, Okuda M, & Wang S (2013). Comorbidity of posttraumatic stress disorder with alcohol dependence among US adults: results from National Epidemiological Survey on Alcohol and Related Conditions. Drug and Alcohol Dependence, 132(3), 630–638. 10.1016/j.drugalcdep.2013.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolkan SS, & Lattal KM (2014). Opposing effects of D-cycloserine on fear despite a common extinction duration: interactions between brain regions and behavior. Neurobiology of Learning and Memory, 113, 25–34. 10.1016/j.nlm.2013.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlikova GG, Elbers NA, & Stephens DN (2006). Repeated withdrawal from ethanol spares contextual fear conditioning and spatial learning but impairs negative patterning and induces over-responding: evidence for effect on frontal cortical but not hippocampal function? European Journal of Neuroscience, 24(1), 205–216. http://doi.org/10.llll/j.1460-9568.2006.04901.x [DOI] [PubMed] [Google Scholar]
- Bouton ME (1986). Slow reacquisition following the extinction of conditioned suppression. Learning and Motivation, 17(1), 1–15. 10.1016/0023-9690(86)90017-2 [DOI] [Google Scholar]
- Bouton ME (2002). Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biological Psychiatry, 52(10), 976–986. [DOI] [PubMed] [Google Scholar]
- Bouton ME, & Bolles RC (1979). Role of Conditioned Contextual Stimuli in Reinstatement of Extinguished Fear. Journal of Experimental Psychology. Animal Behavior Processes, 5(4), 368–378. 10.3758/BF03199629 [DOI] [PubMed] [Google Scholar]
- Bouton ME, & Swartzentruber D (1989). Slow reacquisition following extinction: Context, encoding, and retrieval mechanisms. Journal of Experimental Psychology. Animal Behavior Processes, 75(1), 43–53. 10.1037/0097-7403.15.L43 [DOI] [Google Scholar]
- Bouton ME, Woods AM, & Pineño O (2004). Occasional reinforced trials during extinction can slow the rate of rapid reacquisition. Learning and Motivation, 35(4), 371–390. 10.1016/j.lmot.2004.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwater M, & Spear LP (2013). Consequences of ethanol exposure on cued and contextual fear conditioning and extinction differ depending on timing of exposure during adolescence or adulthood. Behavioural Brain Research, 256, 10–19. 10.1016/j.bbr.2013.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PJ, Read JP, & Kahler CW (2003). Comorbid posttraumatic stress disorder and substance use disorders: Treatment outcomes and the role of coping, (pp. 171–188). Washington: American Psychological Association, 10.1037/10460-009 [DOI] [Google Scholar]
- Bruce KR, & Pihl RO (1997). Forget “drinking to forget”: enhanced consolidation of emotionally charged memory by alcohol. Experimental and Clinical Psychopharmacology, 5(3), 242–250. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Cameron AJ, Munn E, Bunning M, & Wahlsten D (2001). Overview of Mouse Assays of Ethanol Intoxication (pp. 9.26.1–9.26.19). Hoboken, NJ, USA: John Wiley & Sons, Inc; 10.1002/0471142301.ns0926s42 [DOI] [PubMed] [Google Scholar]
- Cunningham CL (1979a). Alcohol as a cue for extinction: State dependency produced by conditioned inhibition. Animal Learning & Behavior, 7(1), 45–52. 10.3758/BF03209656 [DOI] [Google Scholar]
- Cunningham CL (1979b). Flavor and location aversions produced by ethanol. Behavioral and Neural Biology, 27(3), 362–367. [DOI] [PubMed] [Google Scholar]
- De Witte P, Pinto E, Ansseau M, & Verbanck P (2003). Alcohol and withdrawal: from animal research to clinical issues. Neuroscience & Biohehavioral Reviews, 27(3), 189–197. 10.1016/S0149-7634(03)00030-7 [DOI] [PubMed] [Google Scholar]
- Fanselow MS, & Bolles RC (1979). Naloxone and shock-elicited freezing in the rat. Journal of Comparative and Physiological Psychology, 93(4), 736–744. [DOI] [PubMed] [Google Scholar]
- Gould TJ (2003). Ethanol disrupts fear conditioning in C57BL/6 mice. Journal of Psychopharmacology, 77(1), 77–81. 10.1177/0269881103017001702 [DOI] [PubMed] [Google Scholar]
- Gulick D, & Gould TJ (2007). Acute Ethanol Has Biphasic Effects on Short- and Long-Term Memory in Both Foreground and Background Contextual Fear Conditioning in C57BL/6 Mice. Alcoholism, Clinical and Experimental Research, 31(9), 1528–1537. http://doi.org/10.1111/j.1530-0277.2007.00458.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, & Becker HC (2010). REVIEW: Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addiction Biology, 15(2), 169–184. 10.1111/j.1369-1600.2009.00194.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JL (1992). Complex PTSD: A syndrome in survivors of prolonged and repeated trauma. Journal of Traumatic Stress, 5(3), 377–391. 10.1002/jts.2490050305 [DOI] [Google Scholar]
- Hertzen, von LSJ (2005). Memory Reconsolidation Engages Only a Subset of Immediate-Early Genes Induced during Consolidation. The Journal of Neuroscience, 25(8), 1935–1942. 10.1523/JNEUROSCI.4707-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Fitzgerald PJ, MacPherson KP, DeBrouse L, Colacicco G, Flynn SM, et al. (2012). Chronic alcohol remodels prefrontal neurons and disrupts NMDAR-mediated fear extinction encoding. Nature Neuroscience, 75(10), 1359–1361. 10.1038/nn.3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadayian AG, & Cutrera RA (2013). Alcohol hangover: type and time-extension of motor function impairments. Behavioural Brain Research, 247, 165–173. 10.1016/j.bbr.2013.03.037 [DOI] [PubMed] [Google Scholar]
- Karadayian AG, Busso MJ, Feleder C, & Cutrera RA (2013). Alterations in affective behavior during the time course of alcohol hangover. Behavioural Brain Research, 253, 128–138. 10.1016/j.bbr.2013.07.011 [DOI] [PubMed] [Google Scholar]
- Killcross AS, Kieman MJ, Dwyer D, & Westbrook RF (1998). Loss of latent inhibition of contextual conditioning following non-reinforced context exposure in rats. The Quarterly Journal of Experimental Psychology. B, Comparative and Physiological Psychology, 57(1), 75–90. 10.1080/713932668 [DOI] [PubMed] [Google Scholar]
- Kim JJ, & Diamond DM (2002). The stressed hippocampus, synaptic plasticity and lost memories. Nature Reviews. Neuroscience, 3(6), 453–462. [DOI] [PubMed] [Google Scholar]
- Kitaichi K, Minami Y, Amano M, Yamada K, Hasegawa T, & Nabeshima T (1995). The attenuation of suppression of motility by triazolam in the conditioned fear stress task is exacerbated by ethanol in mice. Life Sciences, 57(8), 743–753. [DOI] [PubMed] [Google Scholar]
- Koob GF, & Le Moal M (2008). Neurobiological mechanisms for opponent motivational processes in addiction. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 353(1507), 3113–3123. 10.1098/rstb.2008.0094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozell LB, Hitzemann R, & Buck KJ (2005). Acute alcohol withdrawal is associated with c-Fos expression in the basal ganglia and associated circuitry: C57BL/6J and DBA/2J inbred mouse strain analyses. Alcoholism, Clinical and Experimental Research, 29(11), 1939–1948. [DOI] [PubMed] [Google Scholar]
- Lattal KM (2007). Effects of ethanol on encoding, consolidation, and expression of extinction following contextual fear conditioning. Behavioral Neuroscience, 121(6), 1280–1292. 10.1037/0735-7044.12L6.1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattal KM, & Maughan DK (2012). A parametric analysis of factors affecting acquisition and extinction of contextual fear in C57BL/6 and DBA/2 mice. Behavioural Processes, 90(1), 49–57. 10.1016/j.beproc.2012.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent V, & Westbrook RF (2009). Inactivation of the infralimbic but not the prelimbic cortex impairs consolidation and retrieval of fear extinction., 16(9), 520–529. 10.1101/lm.1474609 [DOI] [PubMed] [Google Scholar]
- Laurent V, Marchand AR, & Westbrook RF (2008). The basolateral amygdala is necessary for learning but not relearning extinction of context conditioned fear., 15(5), 304–314. 10.1101/lm.928208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung HT, Bailey GK, Laurent V, & Westbrook RF (2007). Rapid reacquisition of fear to a completely extinguished context is replaced by transient impairment with additional extinction training. Journal of Experimental Psychology. Animal Behavior Processes, 33(3), 299–313. 10.1037/0097-7403.33.3.299 [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ (1975). A theory of attention: Variations in the associability of stimuli with reinforcement. Psychological Review, 82(4), 276–298. 10.1037/h0076778 [DOI] [Google Scholar]
- Matsumoto I, Leah J, Shanley B, & Wilce P (1993). Immediate Early Gene Expression in the Rat Brain during Ethanol Withdrawal. Molecular and Cellular Neurosciences, 4(6), 485–491. 10.1006/mcne.1993.1060 [DOI] [PubMed] [Google Scholar]
- McAllister DE, & McAllister WR (1994). Extinction and Reconditioning of Classically Conditioned Fear before and after Instrumental Learning: Effects of Depth of Fear Extinction. Learning and Motivation, 25(4), 339–367. 10.1006/lmot.1994.1018 [DOI] [Google Scholar]
- McAllister WR, & McAllister DE (1988). Reconditioning of extinguished fear after a one-year delay. Bulletin of the Psychonomic Society, 26(5), 463–466. 10.3758/BF03334914 [DOI] [Google Scholar]
- McAllister WR, & McAllister DE (2006). Recovery of conditioned fear by a single postextinction shock: effect of similarity of shock contexts and of time following extinction. Learning & Behavior, 34(1), 44–49. [DOI] [PubMed] [Google Scholar]
- McCauley JL, Killeen T, Gros DF, Brady KT, & Back SE (2012). Posttraumatic Stress Disorder and Co-Occurring Substance Use Disorders: Advances in Assessment and Treatment. Clinical Psychology: Science and Practice, 19(2), 283–304. http://doi.org/10.1111/cpsp.12006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL, & Dawson RG (1971). Modification of memory storage processes. Behavioral Science, 16(1), 45–63. 10.1002/bs.3830160105 [DOI] [PubMed] [Google Scholar]
- Melia KR, Ryabinin AE, Corodimas KP, Wilson MC, & LeDoux JE (1996). Hippocampal-dependent learning and experience-dependent activation of the hippocampus are preferentially disrupted by ethanol. Neuroscience, 74(2), 313–322. [DOI] [PubMed] [Google Scholar]
- Metten P, & Crabbe JC (1994). Common genetic determinants of severity of acute withdrawal from ethanol, pentobarbital and diazepam in inbred mice. Behavioural Pharmacology, 5(4 And 5), 533–547. [DOI] [PubMed] [Google Scholar]
- Napier RM, Macrae M, & Kehoe EJ (1992). Rapid reaquisition in conditioning of the rabbit's nictitating membrane response. Journal of Experimental Psychology. Animal Behavior Processes, 18(2), 182–192. [DOI] [PubMed] [Google Scholar]
- Olive MF, Mehmert KK, Nannini MA, Camarini R, Messing RO, & Hodge CW (2001). Reduced ethanol withdrawal severity and altered withdrawal-induced c-fos expression in various brain regions of mice lacking protein kinase C-epsilon. Neuroscience, 703(1), 171–179. [DOI] [PubMed] [Google Scholar]
- Pavlov PI (1927). Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex. Annals of neurosciences. Oxford Univ. Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, & Quirk GJ (2009). Extinction circuits for fear and addiction overlap in prefrontal cortex., 16(5), 279–288. 10.1101/lm.1041309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman RK (1989). Post-traumatic stress disorder, hormones, and memory. Biological Psychiatry, 26(3), 221–223. 10.1016/0006-3223(89)90033-4 [DOI] [PubMed] [Google Scholar]
- Quiñones-Laracuente K, Hemández-Rodriguez MY, Bravo-Rivera C, Melendez RI, & Quirk GJ (2015). The effect of repeated exposure to ethanol on pre-existing fear memories in rats. Psychopharmacology, 252(19), 3615–3622. 10.1007/s00213-015-4016-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassnick S, Koob GF, & Geyer MA (1992). Responding to acoustic startle during chronic ethanol intoxication and withdrawal. - PubMed -NCBI. Psychopharmacology, 106(3), 351–358. 10.1007/BF02245417 [DOI] [PubMed] [Google Scholar]
- Rescorla RA (2004). Spontaneous recovery., 11(5), 501–509. 10.1101/lm.77504 [DOI] [PubMed] [Google Scholar]
- Rescorla RA, & Heth CD (1975). Reinstatement of fear to an extinguished conditioned stimulus. Journal of Experimental Psychology. Animal Behavior Processes, 7(1), 88–96. [PubMed] [Google Scholar]
- Rescorla RA, & Wagner AR (1972). A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and non reinforcement In Black AH & Prokasy WF (Eds.), Classical conditioning II current research and theory (pp. 64–99). New York: pdfs.semanticscholar.org. [Google Scholar]
- Ripley TL, O'Shea M, & Stephens DN (2003). Repeated withdrawal from ethanol impairs acquisition but not expression of conditioned fear. European Journal of Neuroscience, 18(2), 441–448. 10.1046/j.1460-9568.2003.02759.x [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, & Davis M (2003). Applying Learning Principles to the Treatment of Post-Trauma Reactions. Annals of the New York Academy of Sciences, 1008(1), 112–121. 10.1196/annals.1301.012 [DOI] [PubMed] [Google Scholar]
- Rudy JW, & O'Reilly RC (1999). Contextual fear conditioning, conjunctive representations, pattern completion, and the hippocampus. Behavioral Neuroscience, 113(5), 867–880. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Melia KR, Cole M, Bloom FE, & Wilson MC (1995). Alcohol selectively attenuates stress-induced c-fos expression in rat hippocampus. The Journal of Neuroscience, 75(1), 721–730. 10.1523/JNEUROSCI.15-01-00721. 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saladin ME, Brady KT, Dansky BS, & Kilpatrick DG (1995). Understanding comorbidity between PTSD and substance use disorders: two preliminary investigations. Addictive Behaviors, 20(5), 643–655. [DOI] [PubMed] [Google Scholar]
- Smith ML, Hostetler CM, Heinricher MM, & Ryabinin AE (2016). Social transfer of pain in mice. Science Advances, 2(10), el600855–el600855. 10.1126/sciadv.1600855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford JM, & Lattal KM (2009). Direct comparisons of the size and persistence of anisomycin-induced consolidation and reconsolidation deficits., 16(8), 494–503. 10.1101/lm.1452209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford JM, Raybuck JD, Ryabinin AE, & Lattal KM (2012). Increasing Histone Acetylation in the Hippocampus-Infralimbic Network Enhances Fear Extinction. Biological Psychiatry, 72(1), 25–33. 10.1016/j.biopsych.2011.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens DN, Brown G, Duka T, & Ripley TL (2001). Impaired fear conditioning but enhanced seizure sensitivity in rats given repeated experience of withdrawal from alcohol. European Journal of Neuroscience, 74(12), 2023–2031. 10.1046/j.0953-816x.2001.01824.x [DOI] [PubMed] [Google Scholar]
- Stewart SH (1996). Alcohol abuse in individuals exposed to trauma: a critical review. Psychological Bulletin, 120(1), 83–112. [DOI] [PubMed] [Google Scholar]
- Stragier E, Martin V, Davenas E, Poilbout C, Mongeau R, Corradetti R, & Lanfumey L (2015). Brain plasticity and cognitive functions after ethanol consumption in C57BL/6J mice. Translational Psychiaty, 5(12), e696–e696. 10.1038/tp.2015.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipps ME, Raybuck JD, Buck KJ, & Lattal KM (2014a). Delay and trace fear conditioning in C57BL/6 and DBA/2 mice: issues of measurement and performance., 21(8), 380–393. 10.1101/lm.035261.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipps ME, Raybuck JD, & Lattal KM (2014b). Substance abuse, memory, and post-traumatic stress disorder. Neurobiology of learning and memory, 112, 87–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipps ME, Raybuck JD, Buck KJ, & Lattal KM (2015). Acute ethanol withdrawal impairs contextual learning and enhances cued learning. Alcoholism, Clinical and Experimental Research, 39(2), 282–290. http://doi.org/10.1111/acer.12614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, & Quirk GJ (2006). Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear., 13(6), 728–733. 10.1101/lm.306106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilpoux C, Wamault V, Pierrefiche O, Daoust M, &Naassila M (2009). Ethanol-Sensitive Brain Regions in Rat and Mouse: A Cartographic Review, Using Immediate Early Gene Expression. Alcoholism, Clinical and Experimental Research, 33(6), 945–969. http://doi.org/10.1111/j.1530-0277.2009.00916.x [DOI] [PubMed] [Google Scholar]
- White AM, & Best PJ (2000). Effects of ethanol on hippocampal place-cell and interneuron activity. Brain Research, 876(1-2), 154–165. [DOI] [PubMed] [Google Scholar]
- Yeh S-H, Lin C-H, & Gean P-W (2004). Acetylation of nuclear factor-kappaB in rat amygdala improves long-term but not short-term retention of fear memory. Molecular Pharmacology, 65(5), 1286–1292. 10.1124/mol.65.5.1286 [DOI] [PubMed] [Google Scholar]