Abstract
A number of analyses associated with the uncontrolled manifold (UCM) hypothesis have been used recently to investigate stability of actions across populations. We explored whether some of those methods have an advantage for clinical studies because they require fewer trials to achieve consistent findings. We compared the number of trials needed for the analysis of inter-trial variance, analysis of motor equivalence, and analysis in the space of referent coordinates. Young healthy adults performed four-finger accurate force production tasks under visual feedback with the right (dominant) and left hand over three days. Three methods [analytical (M1), experimental (M2), and Cumulative Mean (M3) methods] were used to define the minimal number of trials required to reach certain statistical criteria. Two of these methods, M1 and M2, showed qualitatively similar results. Fewer trials (M1: 5–13, M2: 4–10) were needed for analysis of motor equivalence compared to inter-trial variance analysis (M1: 14–24, M2: 10–14). The third method (M3) showed no major differences among the outcome variables. The index of synergy in the inter-trial variance analysis required a very small number of trials (M1, M2: 2–4). Variables related to referent coordinates required only a few trials (under 3), whereas the synergy index in this analysis required the largest number of trials (M1: 24–34, M2: 12–16). This is the first study to quantify the number of trials needed for UCM-based methods of assessing motor coordination broadly used in clinical studies. Clinical studies can take advantage of specific recommendations based on the current data regarding the number of trials needed for each analysis thus allowing minimizing the test session duration without compromising data reliability.
Keywords: Hand, Synergy, Variance, Motor equivalence, Referent coordinate
Introduction
All natural actions involve large sets of elements such as joints, digits, and muscles. This fact has been addressed as the problem of motor redundancy (Bernstein 1967). Alternatively, within the principle of abundance (Latash 2012), the large sets of elements are viewed as a means of ensuring task-specific stability of salient performance variables. Under stability we imply ability of a variable to return to a state or trajectory after a small, transient perturbation (reviewed in Latash 2016; Latash and Huang 2015). Stability of salient performance variables is crucial for success in everyday movements given the frequent unpredictable changes in external forces and in the intrinsic body states.
Stability of performance variables during multi-finger force production hand tasks has been quantified using the framework of the uncontrolled manifold (UCM) hypothesis (Scholz and Schöner 1999) using a variety of methods. One of the methods is based on quantifying inter-trial variance within a space (UCM) where the performance variable remains unchanged (VUCM) and within its orthogonal complement (ORT), where the performance variable changes (VORT) (reviewed in Latash et al. 2002). Relative amounts of the two variance components reflect relative stability of processes within the two states because trajectories are expected to converge more (diverge less) in more stable directions compared to less stable directions. Within another method, the displacements within the UCM and ORT spaces are quantified during quick actions addressed, respectively, as motor equivalent (ME) and non-motor equivalence (nME) (Mattos et al. 2015). This method is based on the idea that a quick action is associated with a descending input into a subsystem within the central nervous system involved in ensuring stability of performance variables. This input plays the role of a perturbation and produces larger motion in less stable directions. A third method has been introduced recently (Ambike et al. 2016) based on estimating hypothetical control variables associated with referent coordinates (RC) for the involved muscle groups that translate into RC for the hand and its apparent stiffness, k (Feldman 1980, 1986, 2015). Co-varied adjustments of RC and k across trials have been related to stability of the performance variable produced by the subject (Reschechtko and Latash 2017, 2018). Although the variables computed by these three methods seem to be redundant, a recent study suggested they are complementary (de Freitas et al. 2018), with some advantage of the method of estimating ME and nME for more applied studies.
Recent analyses of performance stability in multi-element tasks in neurological patients (reviewed in Latash and Huang 2015) have demonstrated its high sensitivity to a number of neurological disorders affecting subcortical structures, including Parkinson’s disease, multisystem atrophy, and multiple sclerosis. Indices of stability also have shown sensitivity to subclinical conditions as demonstrated in studies of professional welders, a population at higher risk for parkinsonism (Lewis et al. 2016). Collecting multiple trials to obtain large sets of data points, however, may be challenging for certain populations.
Analysis of inter-trial variance, by its nature, requires collecting multiple trials. There has been only one attempt to apply a similar analysis to samples collected within a single trial during a multi-finger task (Scholz et al. 2003). This extension of the method, however, faces major statistical problems because of the lack of independence of consecutive samples and the possible non-stationarity of the performance. The {ME; nME} indices potentially can be measured in single trials, and their correlation with the {VUCM; VORT} indices has been shown in studies of postural tasks in both healthy persons (Furmanek et al. 2018) and patients with Parkinson’s disease (Falaki et al. 2017a). It is clear, however, that measuring {ME; nME} in only one trial is likely to produce unreliable estimates. The third of the aforementioned methods, analysis of {RC; k} pairs across trials, uses analysis of performance variability across actual data sets and surrogate data sets created by forming {RC; k} pairs, with RC taken from one trial and k from another trial (Müller and Sternad 2003). It also requires collecting multiple trials.
In most studies using these methods, the number of collected trials typically was on the order of two dozen. It has remained unknown how many trials actually are needed to obtain reliable estimates of the outcome variables related to action stability. The goal of this study was to explore this issue by identifying the minimum number of trials needed to obtain reliable estimates of stability-related indices within the inter-trial variance analysis, analysis of motor equivalence, and analysis in the space of referent coordinates. In particular, we explored whether some of those methods have an advantage for clinical studies because they require fewer trials to achieve reliable estimates of the main outcome variables. We assume that each of the outcome variables can be characterized by a probability density function (distribution) similar to a normal distribution characterized by a certain mean and standard deviation. The problem of estimating the mean reliably based on the smallest number (NCR) of trials is addressed using a within-a-subject design. Further, we analyze NCR across subjects to be able to generalize the findings to the population. Three different methods of analysis to estimate NCR were applied to the same dataset collected in a very simple task of steady-state constant total force production with four fingers pressing in parallel.
The task was performed by the subjects using their right and left hands on three different days. We expected the three methods of identifying NCR to be able to distinguish among the main outcome variables related to action stability. In particular, we expected smaller NCR values in the analysis of motor equivalence (ME and nME) because these indices can be measured in individual trials compared to the analysis of inter-trial variance and co-variation in the {RC; k} space (Hypothesis 1). Whereas we expected variation in NCR for different outcome variables, we hypothesized that the results would be consistent across days (Hypothesis 2). Few earlier studies also documented more stable performance in steady-state tasks by the non-dominant hand (Park et al. 2012; Parsa et al. 2016) in accordance with the dynamic dominance hypothesis (Sainburg 2005). Hence, we also explored possible differences between the dominant and non-dominant hands and hypothesized that the non-dominant hand would require fewer trials (smaller NCR) for reliable estimation of stability indices (Hypothesis 3). Testing this hypothesis is relevant to the main goals of the study because it informs researchers whether testing one hand only could be sufficient in clinical studies. In addition to its practical implications, defining the minimal number of trials needed to estimate action stability using different methods can help in designing future studies, in particular, those dealing with issues of motor learning and motor disorders.
Methods
The dataset analyzed in this study has been presented partially in a recent publication (de Freitas et al. 2018). Hence, the apparatus, experimental procedures, and the methods of analysis will be described only briefly here.
Participants
Fourteen healthy, right-handed volunteers (29.5 ± 5.6 years old; mean ± standard deviation), with no history of neurological disease or recent injury to the upper extremities, were tested. All participants gave written informed consent according to the protocol approved by the Penn State Hershey Institutional Review Board.
Apparatus and Experimental Procedures
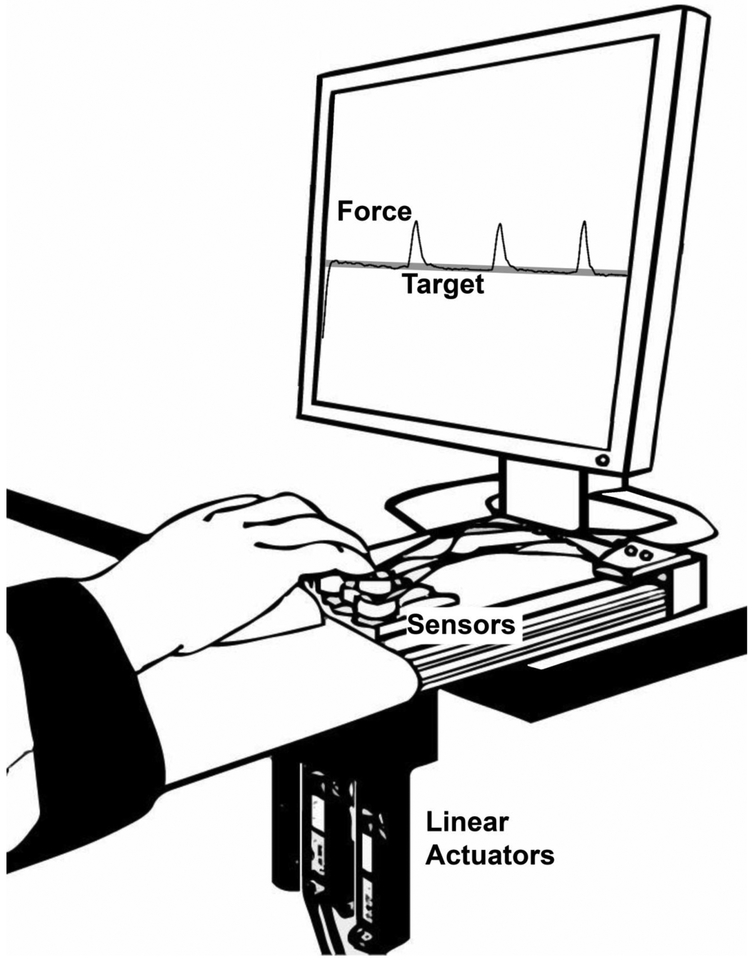
Participants were tested on three different days (two consecutive days, Day-1 and Day-2; and 7–8 days after the first day, Day-3). They sat with their forearms resting on a wooden board, parallel to the ground and placed their index (I), middle (M), ring (R), and little (L) fingers on top of the corresponding force sensors (Honeywell, Model 31, 25 LBS, Columbus, OH, USA) of the “inverse piano” (IP) device (Fig. 1A; Martin et al. 2011). The sensors were mounted on top of linear actuators (PS01–23×80; Linmot, Spruitenbach, Switzerland), which were positioned 3-cm apart from each other in the medial-lateral direction. The position of each sensor was adjusted in the anterior-posterior direction such that each finger was slightly flexed in the medial and distal phalanges when the fingertip was placed on the sensor (Fig. 1B). The linear actuators were controlled by a four-channel servo drive (Linmot E400-AT), which allowed each sensor to be lifted and lowered along its vertical axis. The surface of each sensor in contact with the tip of the finger was covered with sandpaper (100-grit) to increase friction. To avoid movement of the forearm during the tasks, a Velcro strap was placed slightly above the wrist joint to fix the forearm on the board. A customized LabVIEW routine (2014, National Instruments, Austin, TX, USA) was used to present visual feedback, control the IP, and record the force data. The force data were sampled at 200 Hz with a 16-bit resolution (NI PCI-6225, National Instruments) using a desktop computer.
Figure 1:
Experimental setup with finger position on the force sensors and depiction of the linear actuators used to generate the three ‘inverse piano’ perturbations.
First, participants performed two trials of maximal voluntary contraction (MVC) by pressing with their four fingertips as hard as they could to reach maximal force within 3–4 s. Instantaneous visual feedback of the total force (FTOT) was provided on the 19” monitor placed approximately 0.6 m away at the eye level during the whole experiment. The trial with the highest FTOT value was used to set the target force level in the trials with accurate constant force production (Figure 1).
During the main task, participants were asked to produce a constant FTOT level at 20% of the MVC for 24 s. During each trial, the four fingers were simultaneous lifted by 1 cm over 0.5 s (2 cm/s) and immediately lowered over the same distance and time on the 6th, 12th, and 18th s from the beginning of the trial, referred to as IP1, IP2, and IP3 episodes, respectively. The following instruction was provided verbally to the participants: “Please, rest your four fingers on the sensors. When you see a horizontal red line (target), press the sensors with all four fingers and match the target with the black line. The total force produced by all your fingers controls the black line. After a few seconds, you may feel the four sensors going up and down. Please, do not react to that! Do not press harder or lift your fingers off the sensors during the perturbation. Keep the same effort you were using before the perturbation. During the perturbation, the force matching is not required. As soon as the sensor movement ends, try to match the target line again as quickly as you can until the next perturbation or until the end of the trial. Three sensor up-and-down movements will happen in each trial.”
On each day, participants were given two practice trials followed by 15 to 20 trials, depending on the participant’s ability to follow the instructions. Extra trials were collected if participants showed non-monotonic force profiles during the ascending phase of the perturbation; such cases were noted by the experimenter. Rest intervals of about 1 min were given after each set of five trials and when requested to prevent fatigue. None of the participants reported fatigue.
Data Analysis
Computation of outcome variables
Data processing was performed using customized LabVIEW (2017 version) routines. The force signals were filtered with a 4th-order, zero-lag Butterworth low-pass filter with a cutoff frequency of 5 Hz. For each trial with accurate constant FTOT production, eleven variables were computed for each IP perturbation within a time interval from 0.5 s prior to the perturbation to 2 s after the perturbation. These outcome variables were related to task performance and analysis of performance stability as described later in this section. For each of the outcome variables, the main dependent variable of the study was computed: the minimal number of trials needed to obtain a reliable estimate of that outcome variable (NCR).
To assess accuracy of the task performance prior to each IP episode, the root mean square error (RMSE) and the coefficient of variation (CV) of FTOT were computed using the data starting from −500 ms prior to each IP perturbation episode until the IP initiation time.
Inter-trial analysis of variance based on the UCM hypothesis (Latash et al. 2002) was used to compute two variance indices, within the UCM and orthogonal to the UCM, VUCM and VORT (each normalized per dimension in the corresponding space), and an index of synergy ΔV, reflecting the normalized difference between VUCM and VORT: ΔV = (VUCM – VORT)/VTOT, where VTOT is total variance per dimension (for details see Appendix A1). The equation for ΔV makes its values limited by −4 (when all variance is VORT) and +1.33 (when all variance is VUCM). Hence, ΔV values were log-transformed with a modified Fisher’s z-transformation resulting in ΔVZ= 0.5 × ln[(ΔV + 4)/(1.33 − ΔV)] (cf. Park et al. 2010). The VUCM, VORT, and ΔVZ values computed for each subject and for each time sample then were averaged within a time interval of 0.5 s immediately prior to the IP perturbation initiation.
Analysis of motor equivalence involved the computation of the magnitudes of displacement in the finger force space within the UCM (motor equivalent, ME) and orthogonal to the UCM (non-motor equivalence, nME). Both indices were normalized by the square root of the dimensionality of the corresponding spaces (Mattos et al. 2011, 2015). ME and nME motions were computed between two instants of time: −0.5 s before each IP and 2 s after the end of the perturbation (details in Appendix A1). This was done for each of the three IP episodes separately. These times were selected to represent steady states prior to and after each IP (de Freitas et al. 2018).
The stability of FTOT also was quantified within the space of hypothetical control variables, {RC; k} (Ambike et al. 2016; Reschechtko and Latash 2017), where RC is the referent coordinate and k is the apparent stiffness. This analysis views the hand as a single effector controlled by the {RC; k} pair. {RC; k} pairs were calculated using an interval of 300 ms of the ascending phase of the sensors’ displacement for each of the IP perturbations. The 300 ms interval started 50 ms after the onset of the ascending phase to remove the data set that did not include reflex-mediated changes in force. The last part of the ascending phase (150 ms) was not used to avoid possible effects of the voluntary corrections on the hand response.
The analysis of {RC; k} synergies involved several steps. First, for each IP perturbation, linear regression analysis was run between FTOT (i.e., the sum of the forces produced by each finger) and hand vertical coordinate data (displacement of the hand produced by the movement of the sensors) over the time interval of 300 ms. Only data with R2 > 0.9 in this regression analysis were accepted (approximately 90% of all IP episodes). These data allowed estimating RC as the X-axis intercept of the regression line and k as the slope of the line. A single RC and a single k value were computed per IP, totaling 45–60 sets per subject.
Two more outcome variables were computed within this analysis, coefficient of determination (R2) and an index of inter-trial co-variation in the {RC; k} space, RSD. Assuming a fixed value of FTOT (i.e., 10% of MVC), the equation FTOT = k(AC – RC) defines a hyperbolic relation between RC and k for the hand. AC stands for the actual hand vertical coordinate at steady state; it was set at AC = 0 for convenience and consistency with earlier studies (Ambike et al. 2016; Reschechtko and Latash 2017). Note that this choice has no effect on any of the computed variables. Therefore, FTOT = −k*C. The {RC; k} data over several IP episodes (see later for details about the specific number of such episodes) were used to compute the coefficient of determination (R2) for the hyperbolic regression. Finally, the method of surrogate data analysis (Muller and Sternad 2003) was used to estimate inter-trial co-variation between RC and k since the UCM in the two-dimensional {RC; k} space corresponding to a fixed FTOT value was hyperbolic and the variance analysis could not be employed. Surrogate data sets were created by forming 1000 {RC; k} pairs with RC taken from one trial and k from another trial; this procedure effectively eliminated possible co-variation between RC and k. Each surrogate {RC; k} pair was used to compute FTOT,SUR. Standard deviations of FTOT over an actual dataset and over the matching surrogate data set (n=1000) were computed, and then the ratio between the two was computed: RSD = SD(FTOT,SUR)/SD(FTOT,ACT). Note that RSD > 1 means that the original data set contained an inter-trial co-variation between RC and k that helped to reduce the variability of FTOT (Ambike et al. 2016).
Estimation of the required number of trials
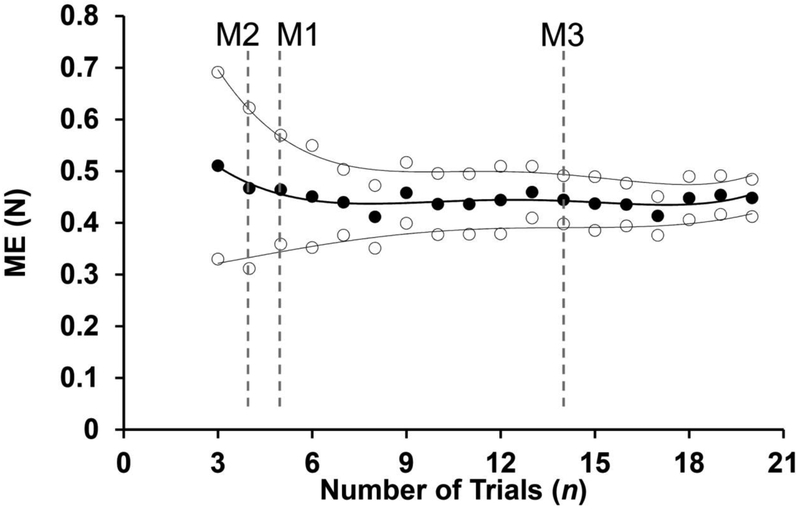
In this section, we will refer to each IP episode as a trial. Therefore, the data set for each subject had from 45–60 trials. We used three different methods to define the critical number of trials (NCR) required to achieve reliable estimates of each of the outcome variables: Analytical Method (M1); Mean Deviation Method (M2) and Cumulative Mean Method (M3). The analysis was run based on a range of a minimum NCR equal to 3 and a maximum equal to 20. For each participant, the analysis was run by using a specific number of trials (n = 3, 4, 5 … 20) selected randomly from the original data set to compute the outcome variables. For the first two methods, the analyses were performed 20 times (referred as casts), using different trials selected from the data subsets each time. This was done, in particular, to ensure close to normal distributions (cf. the Central Limit Theorem). The mean and standard deviation (SD) for each outcome variable over the 20 casts then were calculated. The averages (±SD) of ME computed across the 20 casts as a function of the number of trials (n) are presented in Figure 2 for a representative participant. The average ME values were close to 0.5 independent of n. The SD, however, dropped when n increased. These data were used further to compute NCR using the Analytical Method and Mean Deviation Method. For the Cumulative Mean Method (M3), the trials were selected in the same order they were performed. Therefore, only the data from the first 20 trials (IP episodes) were used in M3.
Figure 2:
ME values averaged across 20 casts (black circles) for the right hand on the first day of evaluation for a representative participant. The white circles represent the averaged ME ± standard deviation. The gray dashed lines represent the NCR computed using M1, M2, and M3.
Analytical Method (M1).
For this method only, the mean () and SDn obtained using n = 20 trials were used. We assumed that a data set with 20 trials provided a good estimate of the probability density function based on many earlier studies that performed analyses of synergies using 15–25 trials (e.g., Ambike et al. 2016; Jo et al. 2016, 2017; Mattos et al. 2011, 2015; Scholz et al. 2003). The coefficient of variation (CV20) was defined as the ratio between SD20 and for each outcome variable. NCR was defined within this method as the smallest n, which allowed estimating the mean observed for n = 20 within a ±10% error margin with a probability of 99%. We chose this value as reflecting high confidence in the computed outcome variable. Assuming a normal distribution, NCR ≥ (m*CV20/0.1)2, where m = 2.65. For the example in Figure 2, NCR = 5 indicating that at least 5 trials had to be used to estimate the mean of ME for this participant.
Mean Deviation Method (M2).
NCR was defined as the smallest n when the mean, , of an outcome variable was within ±10% of that variable value computed for n = 20 [NCR: ], where is the average across n trials and is the outcome variable averaged across 20 trials. Note that this is an experimental method that is similar in the assumed criterion to analytical M1. Using this method, NCR = 4 for the example in Figure 2.
Cumulative Mean Method (M3).
For this method, NCR was defined based on the sequential averaging analysis described earlier (Hamill and McNiven 1990; James et al. 2007). Two major steps were required before defining NCR. Firstly, the outcome variables were computed using the data set with different number of trials following the order in which they were performed by the participants. Specifically, we selected the first three trials (IP perturbations) and calculated all outcome variables using these data (n = 3). Next, we added the data from the fourth trial and calculated the outcomes variables again (n = 4). We kept including new trials in the order they were recorded and computing the outcome variables until we reached 20 trials (n = 20). For each outcome variable, we also calculated the average value from n=3 to n = 20 () and its respective standard deviation, SD (). Next, the cumulative mean (CM) for a specific outcome variable (x) was computed as CMn= ∑x3:n./(n-2)]. For example, CM4 was the average of the specific outcome variable calculated with 3 and with 4 trials. CM5 was the average of the outcome calculated with 3, 4, and 5 trials. Finally, NCR was defined as the n when its CM value entered the range of of SD(X3…20) for the first time. For the data presented in Figure 2, M3 produced NCR =14.
NCR values for each of the outcome variables were defined using each of the three methods for the left and right hands performing the tasks on each of the three testing days.
Statistical Analyses
The statistical analyses were run in the IBM SPSS statistics package (version 25). The Shapiro-Wilks test was run to check the normality of the data distribution before further analysis. First, we ran three repeated measures (RM) analyses of variance (ANOVA) with the factors, Day (three levels, Day-1, Day-2, and Day-3), Hand (two levels, right and left), and Variable (nine levels, RMSE, CV, VUCM, VORT, ΔVZ, ME, nME, Fisher Z R2 and RSD) on the corresponding NCR computed for each outcome variable and for each method separately (M1, M2, and M3). This analysis showed the ability of each method to distinguish among the outcome variables and tested our hypothesis related to possible changes in NCR across days (Hypothesis-2) and also the hypothesis that smaller NCR may be found for the non-dominant hand (Hypothesis-3). Only the methods able to distinguish among the outcome variables consistently (in this study, M1 and M2) were used in further analyses to test Hypothesis-1 (i.e., that fewer trials would be needed in the analysis of motor equivalence).
A two-way RM-ANOVA was performed on NCR averaged across days and hands with the factors Method (M1 vs. M2) and Variable (nine levels). Two outcome variables, RC and k, were not used in this analysis as they showed NCR ≤ 3 (floor effect) across days and hands. To test Hypothesis-1, two RM-ANOVA, for M1 and M2, were run on NCR for the outcome variables of the motor equivalence and variance structure analyses. In case of sphericity violations, the Greenhouse–Geisser correction was used. Pairwise contrasts with Bonferroni corrections were performed to explore significant effects. For all statistical analyses, α = 0.05.
Results
The first two methods, M1 and M2, produced NCR values that varied within a broad range across outcome variables. The third method, M3, however, showed very similar NCR values across outcome variables (on average, about 14 trials). Overall, all three methods produced NCR values that were consistent across the three testing days and showed no major differences between the two hands (see Table 1a in Appendix A2 for the results of statistical analyses related to the Hand and Day factors).
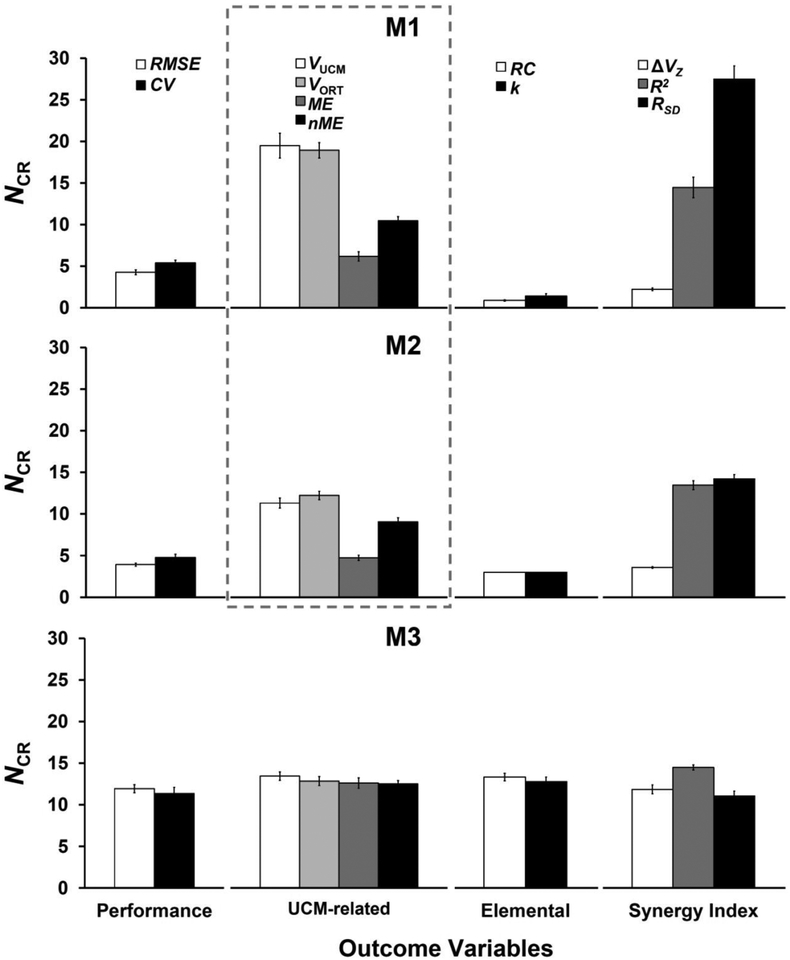
These results are illustrated in Figure 3, which shows the NCR values averaged across the three testing days and two hands. The highest values were seen for variables related to the analysis of hypothetical control variables, RC and k (R2 and RSD), and the UCM-based inter-trial variance analysis (VUCM and VORT). Significantly smaller NCR values were seen for the analysis of motor equivalence (ME and nME). Even smaller values were observed for outcome variables describing accuracy of performance (RMSE and CV); they varied between 4 and 5. Very small values were found for the elemental variables involved in the analysis of synergies in the space of hypothetical control variables (RC and k). In fact, those values were close to unity for M1 (analytical) and at the lowest possible value, n = 3, for M2, demonstrating a floor effect. Variables reflecting strength of a multi-finger synergy stabilizing total force showed the smallest NCR values for the analysis of inter-trial variance (ΔVZ) and the largest values for analysis of synergies at the {RC; k} level (RSD).
Figure 3:
NCR values averaged across participants, two hands, and three days computed using M1, M2, and M3 for all outcome variables. Note the qualitatively similar patterns for M1 and M2. In contrast, only small differences are seen across outcome variables for M3. Data are presented in four clusters of outcome variables: performance, UCM-based analyses (inter-trial variance and motor equivalence), elemental variables of referent coordinates, and synergy indices. The dashed box represents the comparison between M1 and M2 related to our Hypothesis-1.
The patterns across outcome variables for M1 and M2 were qualitatively similar, whereas the NCR magnitudes were consistently higher for M1. The data for each outcome variable of each hand and day are presented in Table 1 for M1, Table 2 for M2, and Table 3 for M3. The three-way RM-ANOVAs confirmed a significant main effect of Variable for M1 and M2 [M1: F(2.6,28.3) = 82.57; p < 0.001; M2: F(8,88) = 93.93; p < 0.001]. In this analysis, RC and k data were excluded because of the mentioned floor effect. The differences across the variables for M3 [F(8,88) = 3.47; p < 0.01] were relatively small (see the bottom panel of Figure 3), and these differences also varied across the evaluation day as indicated by the Variable × Day interaction [F(16,176) = 1.71; p < 0.05]. In particular, NCR for R2 differed from that for RMSE and VORT on the first day and from nME and RSD on the third day of evaluation. These quantitatively small differences are unrelated to any of our hypotheses; hence we view them as spurious.
Table 1:
NCR defined by the analytical model (M1).
| LEFT HAND | RIGHT HAND | |||||
|---|---|---|---|---|---|---|
| Day1 | Day2 | Day3 | Day1 | Day2 | Day3 | |
| RMSE | 3.5 ± 0.3 | 4.1 ± 0.5 | 4.7 ± 0.8 | 5.2 ± 0.5 | 4.4 ± 0.5 | 3.8 ± 0.4 |
| CV | 4.1 ± 0.4 | 5.3 ± 0.7 | 4.8 ± 0.7 | 7.3 ± 1.0 | 6.0 ± 0.8 | 5.0 ± 0.7 |
| VUCM | 18.0 ± 2.4 | 24.5 ± 4.3 | 20.2 ± 2.7 | 23.6 ± 3.0 | 16.7 ± 2.2 | 14.0 ± 1.7 |
| VORT | 15.1 ± 1.6 | 18.3 ± 1.9 | 18.1 ± 2.5 | 23.9 ± 2.4 | 19.8 ± 2.3 | 18.4 ± 1.7 |
| ΔVZ | 2.3 ± 0.5 | 2.0 ± 0.2 | 2.8 ± 0.4 | 2.7 ± 0.3 | 2.0 ± 0.2 | 1.6 ± 0.2 |
| ME | 7.4 ± 1.0 | 5.1 ± 0.6 | 5.0 ± 0.5 | 6.7 ± 0.8 | 6.6 ± 1.5 | 6.3 ± 0.9 |
| nME | 13.5 ± 1.3 | 9.6 ± 1.3 | 11.4 ± 0.9 | 10.5 ± 1.0 | 9.6 ± 1.2 | 8.3 ± 0.9 |
| RC | 1.0 ± 0.2 | 0.9 ± 0.2 | 0.8 ± 0.1 | 1.1 ± 0.2 | 0.8 ± 0.1 | 0.7 ± 0.2 |
| k | 1.0 ± 0.2 | 3.8 ± 1.4 | 1.3 ± 0.4 | 1.1 ± 0.3 | 0.8 ± 0.1 | 0.6 ± 0.1 |
| R2 | 20.1 ± 4.2 | 10.8 ± 2.6 | 8.9 ± 1.7 | 18.2 ± 2.8 | 19.7 ± 4.1 | 9.0 ± 1.5 |
| RSD | 34.0 ± 5.6 | 23.9 ± 3.1 | 25.3 ± 4.4 | 25.2 ± 3.1 | 26.2 ± 3.2 | 30.2 ± 4.0 |
RMSE, root mean square error; CV, coefficient of variation; VUCM, variance within the uncontrolled manifold space; VORT, variance within the space orthogonal to the UCM; ΔVZ, synergy index;ME, motor equivalence; nME, non-motor equivalence; RC, referent coordinate; k, apparent stiffness; R2= coefficient of determination of the hyperbolic regression; RSD = SD(FTOT,SUR)/SD(FTOT,ACT).
Table 2:
NCR defined by (M2).
| LEFT HAND | RIGHT HAND | |||||
|---|---|---|---|---|---|---|
| Day1 | Day2 | Day3 | Day1 | Day2 | Day3 | |
| RMSE | 4.6 ± 0.7 | 3.2 ± 0.1 | 3.9 ± 0.3 | 4.4 ± 0.6 | 4.1 ± 0.4 | 3.2 ± 0.1 |
| CV | 5.4 ± 1.0 | 5.3 ± 1.0 | 5.0 ± 0.8 | 4.8 ± 0.5 | 4.1 ± 0.4 | 4.2 ± 0.5 |
| VUCM | 11.0 ± 1.1 | 13.8 ± 1.5 | 9.9 ± 1.5 | 11.3 ± 1.5 | 12.1 ± 1.7 | 9.8 ± 1.3 |
| VORT | 11.7 ± 0.9 | 11.0 ± 1.6 | 10.4 ± 1.6 | 12.7 ± 1.3 | 13.4 ± 1.3 | 14.2 ± 1.2 |
| ΔVZ | 4.0 ± 0.3 | 3.9 ± 0.3 | 3.6 ± 0.2 | 3.1 ± 0.1 | 3.5 ± 0.3 | 3.3 ± 0.1 |
| ME | 6.1 ± 1.0 | 3.9 ± 0.4 | 5.0 ± 0.7 | 4.5 ± 0.5 | 4.3 ± 0.5 | 4.6 ± 0.6 |
| nME | 9.9 ± 1.4 | 10.2 ± 1.4 | 10.0 ± 1.3 | 8.2 ± 1.3 | 8.5 ± 1.3 | 7.5 ± 1.0 |
| RC | 3.0 ± 0.0 | 3.0 ± 0.0 | 3.0 ± 0.0 | 3.0 ± 0.0 | 3.0 ± 0.0 | 3.0 ± 0.0 |
| k | 3.0 ± 0.0 | 3.0 ± 0.0 | 3.0 ± 0.0 | 3.0 ± 0.0 | 3.0 ± 0.0 | 3.0 ± 0.0 |
| R2 | 13.8± 1.1 | 12.8 ± 1.0 | 12.0 ± 1.0 | 14.5 ± 1.1 | 14.3 ± 1.3 | 13.4 ± 1.3 |
| RSD | 15.2 ± 1.1 | 13.6 ± 1.4 | 11.6 ± 1.5 | 15.5 ± 1.3 | 13.6 ± 1.2 | 15.9 ± 0.8 |
RMSE, root mean square error; CV, coefficient of variation; VUCM, variance within the uncontrolled manifold space; VORT, variance within the space orthogonal to the UCM; ΔVZ, synergy index; ME, motor equivalence; nME, non-motor equivalence; RC, referent coordinate; k, apparent stiffness; R2= coefficient of determination of the hyperbolic regression; RSD= SD(FTOT,SUR)/SD(FTOT,ACT).
Table 3:
NCR defined by cumulative mean (M3).
| LEFT HAND | RIGHT HAND | |||||
|---|---|---|---|---|---|---|
| Day1 | Day2 | Day3 | Day1 | Day2 | Day3 | |
| RMSE | 13.5 ± 1.2 | 13.3 ± 1.5 | 10.9 ± 1.4 | 9.5 ± 1.4 | 12.4 ± 1.5 | 12.1 ± 1.0 |
| CV | 11.1 ± 1.7 | 11.9 ± 1.3 | 11.7 ± 1.2 | 12.4 ± 1.4 | 10.9 ± 1.3 | 10.1 ± 1.5 |
| VUCM | 14.6 ± 1.2 | 14.6 ± 1.2 | 12.1 ± 1.2 | 11.2 ± 1.3 | 13.6 ± 1.4 | 14.6 ± 1.2 |
| VORT | 12.6 ± 1.2 | 14.1 ± 1.1 | 13.5 ± 1.4 | 11.2 ± 1.3 | 13.4 ± 1.2 | 12.2 ± 1.2 |
| ΔVZ | 14.5 ± 1.3 | 13.9 ± 1.1 | 10.4 ± 1.4 | 11.1 ± 1.1 | 11.1 ± 1.3 | 10.0 ± 1.6 |
| ME | 12.6 ± 1.3 | 12.7 ± 1.2 | 14.4 ± 0.9 | 14.7 ± 1.0 | 10.3 ± 1.2 | 11.0 ± 1.7 |
| nME | 13.5 ± 1.0 | 11.3 ± 1.4 | 11.8 ± 1.1 | 13.3 ± 0.9 | 14.1 ± 1.1 | 11.2 ± 1.3 |
| RC | 14.2 ± 1.5 | 15.3 ± 0.6 | 12.3 ± 1.2 | 13.0 ± 1.5 | 13.1 ± 1.1 | 12.0 ± 1.3 |
| k | 13.8 ± 1.7 | 14.8 ± 0.6 | 12.3 ± 1.4 | 13.9 ± 1.4 | 10.8 ± 1.6 | 11.1 ± 1.4 |
| R2 | 14.9 ± 0.6 | 13.2 ± 0.8 | 14.8 ± 0.6 | 15.7 ± 0.4 | 14.0 ± 1.3 | 14.3 ± 0.6 |
| RSD | 12.8 ± 1.5 | 12.2 ± 1.4 | 8.5 ± 1.0 | 12.2 ± 1.5 | 11.8 ± 1.2 | 9.3 ± 1.2 |
RMSE, root mean square error; CV, coefficient of variation; VUCM, variance within the uncontrolled manifold space; VORT, variance within the space orthogonal to the UCM; ΔVZ, synergy index; ME, motor equivalence; nME, non-motor equivalence; RC, referent coordinate; k, apparent stiffness; R2= coefficient of determination of the hyperbolic regression; RSD= SD(FTOT,SUR)/SD(FTOT,ACT).
For the first two methods, but not for M3, there was also a significant Variable × Hand interaction (M1: F(3.3,36.7) = 3.4; p < 0.05; and, M2: F(4.1,44.7) = 4.1; p < 0.01). NCR was higher for VORT when the task was performed with the right hand compared to the left hand for both M1 and M2. A similar hand effect was observed for CV using M1. These effects, however, were due to the differences in Day 1 only as revealed by the significant three-way interaction Variable × Hand × Day for M1 (F(16,176)= 2.37; p < 0.01). Two outcome variables, ΔVZ and nME, showed significant differences between the two hands for M2, with greater NCR values for the left hand compared to the right hand.
Because M1 and M2 showed consistent differences across outcome variables (see Figure 3), a two-way RM-ANOVA, Method × Variable, was performed on NCR averaged across hands and days (see Table 2a in Appendix A2). Overall, NCR was greater for M1 compared to M2 as revealed by the significant effect of Method in the RM-ANOVA [F(1,11) = 108.87; p < 0.001]. There was also a Variable effect [F(3.7,41.3) = 128.03; p < 0.001]. Whereas the patterns of NCR were similar between the two methods, there was a Variable × Method interaction [F(3.6,40.1) = 34.72; p < 0.001] reflecting larger NCR for VUCM, VORT, ME, and RSD for M1 compared to M2, whereas NCR for ΔVZ was smaller for M1 than M2.
To test Hypothesis-1, we explored NCR using the motor equivalence (ME and nME) and inter-trial variance (VUCM and VORT) analyses. We found consistently lower NCR values for the analysis of motor equivalence (see data inside of the dashed rectangle in Figure 3). These differences were confirmed by the main effect of Analysis in the RM-ANOVAs, Analysis × Space (see Table 3a in Appendix A2) run on NCR for M1 [F(1,13) = 226.31; p < 0.001] and M2 [F(1,13) = 194.14; p < 0.001]. The effect of Space was significant only for M2 [F(1,13) = 17.53; p = 0.001]. There was also a significant Analysis × Space interaction for both M1 [F(1,13) = 8.27; p < 0.05] and M2 [F(1,13) = 13.87; p < 0.01]. The interaction reflected the fact that nME differed from ME, whereas there was no difference between VUCM and VORT.
Discussion
In the current study, we used three methods to determine the fewest number of trials that can be sufficient to generate stable indices of motor performance during a very simple hand task. Two of the methods, M1 and M2, showed significantly different results for groups of outcome variables associated with different analyses of force stability. The third method, M3, showed similar NCR values across all variables and analyses. Hence, we cannot recommend this method because of its lack of sensitivity. It is unable to distinguish among outcome variables with different coefficients of variation and suggests that similar numbers of trials are necessary to reach a criterion of reliability.
The results from M1 and M2 were qualitatively similar. In particular, both methods confirmed Hypothesis-1 by showing that the pair of main outcome variables in analysis of motor equivalence (ME and nME) require fewer trials to obtain reliable estimates compared to the pair of variables in the inter-trial variance analysis (VUCM and VORT). Our Hypothesis-2 also was confirmed, as the minimal number of trials was similar across days for all outcome variables. Hypothesis-3, however, obtained only weak support. Indeed, only one of the main outcome variables, namely VORT, showed a significant difference between the right and left hands (larger NCR for the right hand), and even for this variable the difference was present only during testing on the first day. This result is in the direction predicted based on earlier studies (Park et al. 2012; Parsa et al. 2016) and the dynamic dominance hypothesis (Sainburg, 2005), which all predict higher stability indices for the non-dominant (left) hand.
Methods of analysis of action stability
According to the UCM hypothesis (Scholz and Schöner 1999), the central nervous system ensures task-specific stability of salient performance variables reflecting contributions of multiple elements. In other words, the abundant space of elemental variables contributing to the task is expected to show relatively low stability in directions that lead to no changes in the salient variable (within the UCM for that variable) and high stability in directions that lead to changes in that variable (orthogonal to the UCM, ORT). The most direct method to test stability is to applya brief perturbation and quantify the system’s trajectory. This method, however, cannot be applied easily to biological systems because they react to perturbations and change their parameters related to stability during the measurement process (reviewed in Latash 2016, 2017). Hence, indirect methods of estimating stability within UCM and ORT have been developed.
One of these methods is based on the idea that each trial starts from a somewhat different initial state. As a result, trajectories are expected to diverge in unstable directions of the abundant space of elements and converge in stable directions leading to differences in inter-trial variance indices quantified for the UCM and ORT (VUCM and VORT). The two indices sometimes are reduced to a single synergy index ΔV as described in the Methods.
Another method is based on the idea that a brief perturbation or a brief corrective actionis expected to lead to a system’s deviation primarily along unstable directions (along the UCM, motor equivalent, with no effect on the performance variable) compared to more stable directions (along the ORT, non-motor equivalent, changing the performance variable). These deviations, properly quantified, have been referred to as ME and nME indices (Mattos et al. 2011, 2015).
The third method used in our study is qualitatively different. It considers the hand as a single effector and quantifies stability in the abundant space of hypothetical control variables associated with referent coordinates (RCs) for the agonist and antagonist muscle groups (Latash 2010; Feldman 2015). Changes in the pair of referent coordinates may be viewed as leading to changes in the hand RC and its apparent stiffness k, reflecting the reciprocal and co-activation commands (Feldman 1980). All three methods assume that the central nervous system is able to channel the unavoidable variability of neural processes (cf. signal-dependent noise, Harris and Wolpert 1998) into spaces that do not affect salient performance variables.
Only a few studies have compared outcome variables across the mentioned methods. In particular, two studies used cyclical, whole-body voluntary sway tasks and quantified the {ME; nME} and {VUCM; VORT} pairs across cycles (Falaki et al. 2017a; Furmanek et al. 2018). The studies documented significant correlations between ME and and between nME and , as expected from statistical considerations (Leone et al. 1961). Two studies of multi-finger force production quantified ME and nME between two steady-state time intervals separated by a voluntary quick force pulse (Cuadra et al. 2018) or an IP perturbation (de Freitas et al. 2018). In both studies, the correlation between ME and was confirmed, whereas nME did not correlate with when the quick force pulse was performed. It is possible that force corrections after the pulse led to a different distribution along ORT but preserved the distribution along UCM. Finally, a recent study showed that removing visual feedback during accurate hand force production is associated with a drop in synergy indices computed using inter-trial variance analysis (ΔV) and the analysis within the {RC; k} space (Reschechtko and Latash 2017). Whereas these studies are insufficient to claim that different sets of outcome variables are redundant, they suggest that collecting one set may be sufficient to get indices of performance stability, at least for some tasks.
In our study, we focused on differences in the “costs” for different outcome variables in terms of the number of trials that are needed, NCR. Whereas the results speak in favor of analysis of motor equivalence compared to analysis of inter-trial variance (lower NCR for ME and nME), comparison with the third group of variables, RC and k, is less trivial. On the one hand, NCR for RC and k was much smaller than for any other outcome variable. However, by themselves, these variables do not reflect stability of performance. Computation of indices related to stability, R2 and RSD, yielded a very different picture: much larger NCR values compared to the other two methods. This discrepancy is discussed further in the next section.
We used three rather different methods to estimate NCR. Method M1 is purely theoretical based and assumes perfectly normal distributions for each outcome variable. This assumption commonly is made in behavioral studies, but it frequently is violated. For example, many of the main outcome variables are non-negative, which violates the assumption of normality. Note that normality tests still may not be violated because the computed values may be far from the zone of major normality violations. As a result, our method M1 produces an estimate based on a questionable assumption. The other two methods are based on actual data. Method M2 was designed to provide a qualitative match to M1 (reaching a similar criterion), and it did produce patterns of NCR across outcome variables that were qualitatively similar to those for M1 (Figure 3). The third method, M3, was selected because it had been used earlier (Hamill and McNiven 1990; James et al. 2007). It showed little sensitivity across the outcome variables despite the fact that they showed broadly varying values of the coefficient of variation estimated over 20 trials.
Outcome variables and synergy indices
All three methods, analysis of inter-trial variance, analysis of motor equivalence, and analysis in the space of RCs, produce pairs of variables that have been reduced to a single “index of stability” (sometimes referred to as “index of synergy”). There are advantages and disadvantages in reducing pairs of outcome variables to single indices (reviewed in Latash et al. 2010). On the one hand, having a single index simplifies interpretation of findings. On the other hand, some potentially important information is inevitably lost. For example, a drop in the synergy index ΔV may be a sign of inaccurate performance (higher VORT) or more stereotypical performance (smaller VUCM), or both.
In our study, analysis of variance components, VUCM and VORT, produced relatively large values of NCR, whereas ΔVZ showed much lower NCR values, lower than those for analysis of motor equivalence. If one is interested only in the synergy index value, then analysis of inter-trial variance has an advantage compared to analysis of motor equivalence. If the goal is to identify causes for a change in ΔV, however, the necessary NCR becomes large, maybe prohibitively large for clinical studies (see later).
Note that two of our main analyses produced very different patterns of NCR estimated for individual outcome variables and computed indices. Whereas inter-trial variance analysis showed much lower NCR values for the synergy index, the analysis of RCs showed very small NCR values for the individual outcome variables (RC and k) and very high NCR values for the synergy index RSD. The latter observation is consistent with an earlier study reporting high intra-class correlations for RC and k and low intra-class correlations for RSD (de Freitas et al. 2018). Therefore, studying stability of performance in spaces of hypothetical control variables, such as RCs, is very attractive, although the currently available methods do not produce reliable indices that could be used easily in applied studies.
We purposefully did not reduce ME and nME to a single index for a number of reasons. First, the magnitude of nME is task-specific since it depends on changes in the performance variable during the instructed action. In contrast, ME has no effect on the performance variable and may be expected to be similar across tasks that require different changes in the performance variable (this has not been explored to our knowledge). Dividing ME by nME would produce task-specific values that might be hard to interpret. Second, in our study, the subject was required to return to the initial state, so nME was expected to be low. In a large number of trials, nME was indeed very close to zero because the subject returned close to the initial steady-state performance. Dividing ME by this value would produce highly varying and large values. Third, there is a problem with the reliability of ME/nME (related to the first two points). In our previous paper (de Freitas et al. 2018) on the reliability of a variety of indices used in the analysis of synergies, data on that index were not presented. We re-processed the data used in that previous study and the results showed that ME/nME had poor reliability, with ICC values ranging from 0.18 to 0.61.
Implications for clinical and motor learning studies
One of the main motivations for this study has been the recent clinical application of the UCM-based methods of action stability analysis (reviewed in Latash and Huang 2015). In particular, several studies have documented significant changes in indices of performance-stabilizing synergies across clinical conditions such as Parkinson’s disease (Park et al. 2012; Jo et al. 2015), multiple sclerosis (Jo et al. 2017), cortical stroke (Jo et al. 2016), and multi-system atrophy (Park et al. 2013). These results have suggested specificity of synergy changes in subcortical disorders, whereas cortical stroke led to relatively preserved synergies, even when performance was visibly affected. Changes in synergy indices also have been reported in subclinical populations such as professional welders who are at higher risk for movement disorders associated with basal ganglia dysfunction (Lewis et al. 2016). Moreover, synergy indices are sensitive to both drug therapy and deep brain stimulation in Parkinson’s patients (Park et al. 2013; Falaki et al. 2017a, 2018).
Whereas quantifying motor synergies is a promising tool for clinical studies, it has not been used broadly. One of the reasons is the necessity of collecting multiple trials for the inter-trial analysis of variance, which may not be possible for some clinical populations because of mental and physical fatigue. Collecting multiple trials also may be unattractive for large clinical studies because of the involved time commitment.
One solution is to use cyclical tasks, which allow collecting multiple cycles within a relatively short time (e.g., Falaki et al. 2017b). There are two potential problems with this strategy, however. First, the neural control of cyclical and discrete actions may be different (Hogan and Sternad 2007), and generalization of findings from cyclical tasks to more typical everyday discrete actions is questionable. Second, in some clinical conditions, the most sensitive indices are related to anticipatory synergy adjustments (ASAs; Park et al. 2012; Jo et al. 2015; Falaki et al. 2017a), defined as an attenuation of a synergy in preparation to an action that requires a quick change in the corresponding salient performance variable (Olafsdottir et al. 2005). Note that ASAs can be observed and quantified only in preparation to a discrete action, not in cyclical actions.
Overall, our results suggest that a small number of trials, approximately 4 trials, is likely to be sufficient for studies that are interested only in the synergy index, such as ΔVZ. If stability within the UCM is of interest, however, ME has an advantage compared to VUCM given that the former requires about 4–13 trials whereas the latter needs 10–24 trials for reliable assessment. In studies of referent coordinates, reliable estimates of RC and k may be based on a few trials, whereas estimates of their co-variation stabilizing performance may require close to 20 trials.
Of course, these estimates are valid only for the task of multi-finger force production by healthy subjects. According to the analytical method (M1), NCR is proportional to CV2 for the outcome variable as estimated based on a large number of trials, e.g., ≥20 trials. Thus, patients who are likely to show larger variance of the main outcome variables will require larger numbers of trials to satisfy the same criterion. Studies of postural synergies use muscle modes (muscle groups with proportional scaling of activation levels, Krishnamoorthy et al. 2003) as elemental variables and given the typically high noise-to-signal level of EMG signals, those studies may require correspondingly more trials. We would like to emphasize, however, that our study offers an approach that can be adjusted easily to various variables, tasks, and populations.
The necessity of collecting many trials also may represent a problem for studies regarding the effects of motor learning on indices of action stability. Indeed, effects of motor learning may be fast and seen over a few trials. In such cases, collecting many trials to provide a single estimate of an outcome variable is likely to obscure the learning effects. This problem has been addressed partially by using multiple pseudo-cyclical actions within a trial (Wu et al. 2012, 2013). Still, that approach required collecting multiple trials to perform the inter-trial variance analysis. Using indices of motor equivalence instead may allow a reduction in the number of trials and reveal the effects of learning in more detail.
Concluding comments
The study has a number of limitations. In particular, testing only young, healthy adults in only one task performed by only one set of effectors limits our ability to generalize the results. On the other hand, such a generalization should not be too complicated as long as estimates of variability of the outcome variables are available. Our statistical methods were based on a few assumptions, for example the assumption of normality of distributions of the outcome variables. For some variables, even those that did not violate the normality test, this assumption is obviously false. For example, many of the variables are by definition non-negative (such as variance, displacement magnitude, and apparent stiffness). The quantitative differences between M1 and M2 (even though the qualitative results were similar) could be a consequence of such subtle deviations from normal distributions. Further research is warranted to determine the generalizability of these measures to other tasks, which may have important clinical applications.
Supplementary Material
Acknowledgments
The study was supported in part by NIH grants NS082151 and NS095873.
References
- Ambike S, Mattos D, Zatsiorsky VM, Latash ML (2016) Synergies in the space of control variables within the equilibrium-point hypothesis. Neurosci 315: 150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein NA (1967) The Co-ordination and Regulation of Movements. Pergamon Press, Oxford [Google Scholar]
- Cuadra C, Bartsch A, Tiemann P, Reschechtko S, Latash ML (2018) Multi-finger synergies and the muscular apparatus of the hand. Exp Brain Res, 236(5), 1383–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Freitas PB, Freitas SMSF, Lewis MM, Huang X, Latash ML (2018) Stability of steady hand force production explored across spaces and methods of analysis. Exp Brain Res, 10.1007/s00221-018-5238-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falaki A, Huang X, Lewis MM, Latash ML (2017a) Dopaminergic modulation of multi-muscle synergies in postural tasks performed by patients with Parkinson’s disease. J Electromyogr Kinesiol, 33, 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falaki A, Huang X, Lewis MM, Latash ML (2017b) Motor equivalence and structure of variance: Multi-muscle postural synergies in Parkinson’s disease. Exp Brain Res 235: 2243–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falaki A, Jo HJ, Lewis MM, O’Connell B, De Jesus S, McInerney J, Huang X, Latash ML (2018). Systemic effects of deep brain stimulation on synergic control in Parkinson’s disease. Clin Neurophysiol, 129(6), 1320–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman AG (1980) Superposition of motor programs. I. Rhythmic forearm movements in man. Neurosci 5: 81–90 [DOI] [PubMed] [Google Scholar]
- Feldman AG (1986) Once more on the equilibrium-point hypothesis (λ-model) for motor control. J Mot Behav 18: 17–54. [DOI] [PubMed] [Google Scholar]
- Feldman AG (2015) Referent control of action and perception: Challenging conventional theories in behavioral science. Springer, NY. [Google Scholar]
- Furmanek M, Solnik S, Piscitelli D, Rasouli O, Falaki A, Latash ML (2018) Synergies and motor equivalence in voluntary sway tasks: The effects of visual and mechanical constraints. J Mot Behav. 10.1080/00222895.2017.1367642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill J, McNiven SL (1990) Reliability of selected ground reaction force parameters during walking. Hum Mov Sci, 9(2), 117–131. [Google Scholar]
- Harris CM, Wolpert DM (1998) Signal-dependent noise determines motor planning. Nature 394(6695): 780–784. [DOI] [PubMed] [Google Scholar]
- Hogan N, Sternad D (2007) On rhythmic and discrete movements: reflections, definitions and implications for motor control. Exp Brain Res, 181(1), 13–30. [DOI] [PubMed] [Google Scholar]
- James CR, Herman JA, Dufek JS, Bates BT (2007). Number of trials necessary to achieve performance stability of selected ground reaction force variables during landing. J Sport Sci Med, 6(1), 126–134. [PMC free article] [PubMed] [Google Scholar]
- Jo HJ, Maenza C, Good DC, Huang X, Park J, Sainburg RL, Latash ML (2016). Effects of unilateral stroke on multi-finger synergies and their feed-forward adjustments. Neurosci, 319: 194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo HJ, Mattos D, Lucassen EB, Huang X, Latash ML (2017) Changes in multidigit synergies and their feed-forward adjustments in multiple sclerosis. J Motor Beh 49: 218–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo HJ, Park J, Lewis MM, Huang X, Latash ML (2015) Prehension synergies and hand function in early-stage Parkinson’s disease. Exp Brain Res 233: 425–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latash ML (2010) Motor synergies and the equilibrium-point hypothesis. Motor Control 14: 294–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latash ML (2012) The bliss (not the problem) of motor abundance (not redundancy). Exp Brain Res 217: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latash ML (2016) Towards physics of neural processes and behavior. Neurosci Biobehav Rev 69: 136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latash ML (2017) Biological movement and laws of physics. Motor Control 21: 327–344. [DOI] [PubMed] [Google Scholar]
- Latash ML, Huang X (2015) Neural control of movement stability: Lessons from studies of neurological patients. Neurosci 301: 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latash ML, Scholz JP, Schöner G (2002) Motor control strategies revealed in the structure of motor variability. Exerc Sport Sci Rev 30: 26–31. [DOI] [PubMed] [Google Scholar]
- Leone FC, Nottingham RB, Nelson LS (1961) The folded normal distribution. Technometrics 3: 543–550. [Google Scholar]
- Lewis MM, Lee EY, Jo HJ, Du G, Park J, Flynn MR, Kong L, Latash ML, Huang X (2016) Synergy as a new and sensitive marker of basal ganglia dysfunction: a study of asymptomatic welders. Neurotoxicology 56: 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JR, Budgeon MK, Zatsiorsky VM, Latash ML (2011) Stabilization of the total force in multi-finger pressing tasks studied with the ‘inverse piano’ technique. Hum Mov Sci 30: 446–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattos D, Latash ML, Park E, Kuhl J, Scholz JP (2011) Unpredictable elbow joint perturbation during reaching results in multijoint motor equivalence. J Neurophysiol 106: 1424–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattos D, Schöner G, Zatsiorsky VM, Latash ML (2015) Motor equivalence during accurate multi-finger force production. Exp Brain Res 233: 487–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H, Sternad D (2003) A randomization method for the calculation of covariation in multiple nonlinear relations: illustrated with the example of goal-directed movements. Biol Cybern 89: 22–33. [DOI] [PubMed] [Google Scholar]
- Olafsdottir H, Yoshida N, Zatsiorsky VM, Latash ML (2005). Anticipatory covariation of finger forces during self-paced and reaction time force production. Neurosc Lett, 381(1–2), 92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Wu YH, Lewis MM, Huang X, Latash ML (2012) Changes in multifinger interaction and coordination in Parkinson’s disease. J Neurophysiol, 108: 915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Lewis MM, Huang X, Latash ML (2013) Effects of olivo-ponto-cerebellar atrophy (OPCA) on finger interaction and coordination. Clin Neurophysiol 124: 991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsa B, O’Shea DJ, Zatsiorsky VM, Latash ML (2016) On the nature of unintentional action: A study of force/moment drifts during multi-finger tasks. J Neurophysiol 116: 698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reschechtko S, Latash ML (2017) Stability of hand force production: I. Hand level control variables and multi-finger synergies. J Neurophysiol 118: 3152–3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reschechtko S, Latash ML (2018) Stability of hand force production: II. Ascending and descending synergies. J Neurophysiol 120: 1045–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL. Handedness: differential specializations for control of trajectory and position. Exerc Sport Sci Rev 33: 206–213, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz JP, Kang N, Patterson D, Latash ML (2003) Uncontrolled manifold analysis of single trials during multi-finger force production by persons with and without Down syndrome. Exp Brain Res 153: 45–58. [DOI] [PubMed] [Google Scholar]
- Scholz JP, Schöner G (1999). The uncontrolled manifold concept: Identifying control variables for a functional task. Exp Brain Res 126: 289–306. [DOI] [PubMed] [Google Scholar]
- Wu Y-H, Pazin N, Zatsiorsky VM, Latash ML (2012) Practicing elements vs. practicing coordination: Changes in the structure of variance. J Mot Behav 44: 471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y-H, Pazin N, Zatsiorsky VM, Latash ML (2013) Improving finger coordination in young and elderly persons. Exp Brain Res 226: 273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.