Abstract
Rationale.
Memories can return to a labile state and become amenable to modification by pharmacological and behavioral manipulations after retrieval. This process may reduce the impact of aversive memories and provide a promising therapeutic technique for the treatment of anxiety disorders. A growing body of evidence suggests that the mammalian neuropeptide oxytocin (OT) plays a role in the regulation of emotional memories in animals. However, the effects of OT on threat memory in humans remain largely unknown.
Objectives.
This study aimed to investigate the effects of OT administration following threat memory retrieval on subsequent memory expression in human participants.
Methods.
In a double blind, randomized, placebo-controlled, between-subject design, 61 healthy human individuals completed a three-day experiment. All participants underwent threat conditioning on day 1. On day 2, participants were randomized to receive an intranasal dose of OT (40 IU) or placebo after memory retrieval, or an intranasal dose of OT (40 IU) without retrieval. On day 3, subjects were tested for extinction and reinstatement.
Results.
On day 3, all groups showed equivalent stimulus discrimination during the early phase of extinction. However, the group that received OT following a memory reminder showed a greater decline of stimulus discrimination by the late phase of extinction relative to the two other groups.
Conclusions.
The results indicate that OT did not block reconsolidation to prevent the return of threat memory but rather interacted with post-retrieval processes to facilitate next day extinction. The study provides novel preliminary evidence for the role of OT in human threat memory.
Keywords: Oxytocin, Fear conditioning, extinction, reactivation, reconsolidation
In anxiety disorders, maladaptive threat memories are prevalent, and treatments have focused on coping with them. Until recently, it was thought that memories are relatively stable after memory consolidation, a process that occurs during a short window immediately after learning. However, recent findings demonstrate that memories can again become vulnerable to pharmacological or behavioral interference during a consolidation-like phase called reconsolidation, which may occur when a memory is retrieved (Nader et al., 2000; Agren, 2014; Lee et al., 2017). The ability to disrupt the reconsolidation of threat memory provides a promising approach to alleviate the maladaptive effects of threat memories in anxiety disorders.
A promising pharmacological candidate for therapeutic interventions regulating threat memory is oxytocin (OT). OT is a mammalian neuropeptide synthesized in the paraventricular and supraoptic nuclei of the hypothalamus and centrally released within these regions as well as limbic sites, such as the hippocampus and amygdala (Neumann, 2007; Campbell, 2008). The OT receptor is abundantly expressed in the medial prefrontal cortex (mPFC), and the central and basolateral nuclei of the amygdala (Maroun & Wagner, 2016). These regions are known for their role in regulating defensive responses and threat memory.
Evidence in rodents indicates that OT enhancement attenuates anxiety-like behaviors (Sabihi et al., 2014). The specific effects of OT on threat conditioning and extinction, however, have been somewhat complex. On the one hand, enhancing OT neurotransmission using central administration of OT in rodents prior to threat conditioning decreased threat expression and facilitated extinction. When administrating prior to extinction training, OT impaired threat extinction (Toth et al., 2012). A subsequent rodent study showed that OT can act both to reduce as well as enhance threat responses, depending on the timing and neural circuit location of OT manipulation. For example, direct application of OT into the mPFC infralimbic region (IL-mPFC) after threat memory retrieval facilitated extinction training on the next two consecutive days; whereas OT enhancement in the amygdala resulted in impaired extinction (Lahoud & Maroun., 2013). Systemic administration of OT in rodents after threat memory retrieval reduced freezing 24 hours later, indicating reconsolidation blockade (Hou et al., 2015).
In healthy human participants, OT treatment following threat conditioning and prior to immediate extinction training facilitated extinction with increased activation of the prefrontal cortex and amygdala inhibition (Eckstein et al., 2015), as well as enhanced extinction recall twenty-four hours later (Acheson et al., 2013). However, administration of OT prior to exposure therapy for arachnophobia hindered treatment response (Acheson et al., 2015). Thus, despite the growing body of research implicating OT as a promising candidate for targeting threat memory, extant evidence yields a complex pattern of results, pointing to interactions particularly with the timing and mode of learning.
To further clarify OT’s ability to modify threat memories, we examined administration of OT immediately following threat memory reactivation and examined effects on subsequent threat memory retrieval and extinction in healthy humans. Specifically, we report a double blind, placebo-controlled, between-subjects design study aimed at investigating the effect of intranasal OT on threat memory reconsolidation in 61 healthy humans. We use a three-day threat conditioning, reactivation, and extinction procedure, which has been widely used to assess various drug effects on reconsolidation across species (for review see, Kroes et al., 2016). We hypothesized that post-retrieval OT would block reconsolidation and prevent the return of threat memory. Given the mixed evidence, however, another possibility is that post-retrieval OT would facilitate extinction.
Methods and Material
Participants
The study was approved by and conducted in accordance with regulations of the Ethics committee of South China Normal University. Seventy-nine healthy participants were recruited from South China Normal University via flyers and Internet advertisements (see Table 1 for demographics). Seventy-nine participants were screened over the phone and upon arrival at the laboratory to assure they did not meet criteria for current or past psychiatric disorders; were not taking any medicine and having no allergies to specific medications; did not have a current or recent diagnosis of substance abuse or dependence, history of cardiac illness, seizure disorder, brain injury, neurologic disorder, color-blindness or history of head injury with loss of consciousness for more than 3 min. Since endogenous OT levels can fluctuate across the menstrual cycle, female participants who were pregnant, in menstrual period or currently using hormonal contraceptives during the follicular phase of the menstrual cycle (i.e., up to 10 days following onset of menstruation) would be excluded. Five subjects were excluded due to equipment malfunction or had non-measurable SCR to the shock (< 0.02). Thirteen subjects were removed from the study due to the failed acquisition (Mean CS- > CS+ in late acquisition). Therefore, 61 subjects were included in final analysis. All participants provided written informed consent and were financially compensated for their participation.
Table 1.
Demographics characteristics by treatment group
| Reminder +OT |
Reminder +PLC |
No Reminder +OT |
|
|---|---|---|---|
| Percent female (%) | 59.09 | 57.14 | 38.89 |
| Mean age (SD) | 20.09 (2.93) | 20.52 (2.11) | 21.11 (1.97) |
| Height (SD) | 1.65 (0.06) | 1.67 (0.09) | 1.65 (0.08) |
| Weight (SD) | 64.95 (26.17) | 62.38 (21.75) | 61.35 (16.42) |
| BMI (SD) | 23.79 (9.07) | 21.97 (6.00) | 22.40 (5.63) |
OT, Oxytocin; PLC, Placebo; SD, standard deviation; BMI, Body mass index
Assignment to treatment group
On day 2, the participants were assigned to reminder+OT group, reminder+placebo group, and no reminder+OT. The participants were randomly assigned into these three groups, with only one constraint ensuring equal gender distribution across the groups. This procedure allows for evaluation of treatment effects without potential confounds from between-group differences in strength of initial conditioning.
Treatment
The OT Nasal Spray was purchased from Sichuan Meike Pharmaceutical Limited Company of China. This polypeptide drug is a colorless transparent liquid. Participants self-administered OT (the reminder+OT group and no reminder+OT group) or placebo (reminder+placebo group) intranasally by applying five 0.1-ml puffs per nostril (40 international units of OT in total) after a reminder (non-reinforeced CS+) or no reminder. This dose has been shown to increase the sensitivity of emotional recognition in healthy humans and produce no side effects (Leknes et al., 2013; MacDonald et al. 2011). The placebo spray consisted of normal saline and was administered in an identical fashion. To blind the drug treatment, OT or saline were placed in plain white bottles identified by a number between 1–9. Each participant was assigned a number, which was then used by another experimenter unaware of the correspondence between numbers and content. The identity of drug treatment was unblinded after completion of data collection.
Apparatus
Computer tasks.
A Lenovo desktop computer, 22-inch monitor and headphones were used to present the visual stimuli and the video (played during the waiting period after drug/placebo administration) to the participants. The software E-Prime 2.0 was used to program and present experimental procedures while integrating the SCRs and mild electric shocks with the threat conditioning and extinction computer tasks. We employed images of two cylinders with different colors (purple, blue) that served as CS+ and CS-. Assignment of colors to stimuli was counterbalanced across participants, and the images were identical in size and resolution.
Skin conductance (SCR) measurements.
SCR was measured using shielded Ag-AgCl electrodes, filled with standard NaCl electrolyte gel, and attached to the middle phalanges of the second and third fingers of the non-dominant hand. The electrode cables were grounded through an RF filter panel. The skin conductance signal was amplified and recorded with a BIOPAC MP36 Systems skin conductance module connected to a computer. Data were continuously recorded at a rate of 200 samples per second. An off-line analysis of the analog skin conductance waveforms was conducted with AcqKnowledge software (BIOPAC Systems).
Delivery of mild non-painful electric shocks.
The US was a mild electric shock with duration of 200ms, delivered to the wrist of the non-preferred hand using a Digitimer DG2A Constant Voltage Stimulator (Digitimer Ltd.). The stimulating bar electrode was attached to the participant’s non-dominant wrist with medical tape. Before acquisition, shock intensity levels were set manually for each individual by delivering gradually more intense shocks (with a maximum of 60V) until the subject reported that the shock level was unpleasant but not painful.
Procedure
The experiment was conducted over 3 consecutive days at approximately the same time each day. There were three experimental stages: threat conditioning on day 1, reactivation and/or drug/placebo-administration on day 2, extinction and reinstatement tests on day 3. Procedures and timeline for these three phases are depicted in Figure. 1.
Figure. 1. Schematic depiction of the experimental design.
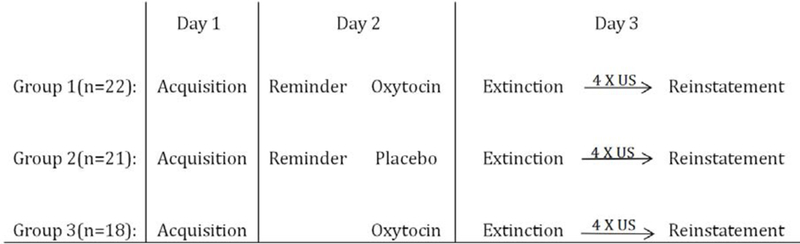
Participants were initially screened by a telephone interview. On day 1, they were asked to fill out the Symptoms Checklist 90 (SCL-90) and the State-Trait Anxiety Inventory (STAI-S and STAI-T, respectably) prior to the experiment. Then, SCR and electric shocks were tested in order to verify equipment and measurement integrity. During acquisition, the CS+ was paired with the US in a partial reinforcement schedule (43% of presentation), and the CS- was never paired with a shock. SCR was measured continuously.
On day 2, reactivation and drug administration were conducted. Participants were randomly assigned into 3 experimental groups, in reminder+OT group and reminder+placebo group, participants were connected to the SCR and shock electrodes as described for threat acquisition on Day 1; and presented with one unreinforced CS+ (the reminder). OT or placebo administration immediately followed reactivation. Participants in the no reminder+OT group self-administered the OT in the waiting area without the reminder. Then, all participants watched a neutral (Planet travel guides for Uranus and Neptune) video for 60min after drug administration. Before leaving, we gave participants a side effects checklist and asked them to fill it out and bring it back on day 3.
On Day 3, On Day 3, participants were asked to rate their shock expectancy before the experiment, by asking “How much do you expect to receive a shock today?”; they were instructed to choose their option from “1 (unlikely)” to “5 (very likely)”. Then, the extinction and reinstatement tests were conducted. All participants returned to the lab and were fitted with the SCR and shock electrodes as described for threat acquisition on Day 1. In order to measure the return of conditioned defensive responses, participants saw 8 presentations of each stimulus type (CS+, CS-) in a random order without the US. Reinstatement immediately followed, during which participants received four unsignaled mild electric shocks (without stimuli presentation) to reinstate remaining conditioned defensive responses. This was followed by a re-extinction session where participants again saw non-reinforced presentations of the CS+ and CS- (8 trials each). SCR was measured continuously.
Data analysis
Demographics and questionnaires data were analyzed by using one-way ANOVA, follow up t-tests were used to assess specific difference. Analysis of SCR was conducted on the responses during each session, broken down into blocks of two trials each (Figure S1). Repeated measures ANOVA was used to examine the overall effects of stimulus, block and treatment group, and any interactions between the factors. Follow up t-tests were used to assess specific difference. Analyses were conducted using SPSS version 18 (IBM, Armonk, NY) and alpha was set at P < 0.05.
Results
Demographics
There were no significant gender, age and BMI differences between treatment groups (Table1). In initial analyses, gender was entered as a factor, and BMI and age as covariates, and were then dropped from the models as none had a significant effect.
Questionnaires
No group differences were found between groups on STAI-S, STAI-T, SCL-90 or subscales of SCL-90 (Table 2). Analysis of shock expectancy prior to the experimental session on day 3 (Table 2) using one-way ANOVA showed significant group difference (F(2,58) = 3.84, P < 0.05); follow up t-tests confirmed significantly higher shock expectancy in no reminder+OT group compared to the reminder+OT group (t(38) = −2.69, P < 0.05), and the reminder+PLC group (t(37) = 2.17, P < 0.05).
Table 2.
Means for questionnaires by treatment group
| Reminder +OT |
Reminder + PLC |
No Reminder + OT |
|
|---|---|---|---|
| STAIa | |||
| State | 37.36 (10.51) | 36.67 (7.84) | 33.39 (6.00) |
| Trait | 42.55 (9.02) | 41.38 (9.12) | 38.56 (6.02) |
| SCL-90b | |||
| Somatization | 13.59 (1.92) | 14.05 (3.51) | 12.78 (1.06) |
| Obsessive-Compulsive | 15.82 (5.03) | 16.19 (6.49) | 14.00 (3.41) |
| Interpersonal Sensitivity | 12.95 (4.83) | 12.95 (4.33) | 11.28 (2.52) |
| Depression | 18.14 (6.24) | 18.62 (7.70) | 15.50 (2.68) |
| Anxiety | 12.36 (2.85) | 12.90 (3.55) | 11.00 (1.24) |
| Hostility | 7.55 (2.11) | 8.14 (2.85) | 6.39 (0.78) |
| Phobic Anxiety | 8.77 (2.49) | 8.57 (2.11) | 7.61 (1.33) |
| Paranoid Ideation | 7.64 (2.32) | 7.57 (2.77) | 6.67 (1.19) |
| Psychoticism | 12.91 (3.87) | 13.86 (6.22) | 11.33 (1.88) |
| Other | 8.59 (1.89) | 9.33 (3.31) | 8.44 (2.28) |
| Shock expectancy | 3.54(1.10) | 3.71(1.10) | 4.44(0.98) |
Note. Standard deviations in parentheses
STAI- State Trait Anxiety Inventory
SCL-90-Symptoms Checklist 90 OT, Oxytocin; PLC, Placebo
Learned defensive responses
Day 1 - Conditioned threat acquisition
To assure equivalent and significant acquisition across the randomly assigned groups (Figure 2), we conducted ANOVA with factors of group (reminder+OT, reminder+PLC, no reminder+OT) x block (1,2,3,4) x stimulus (CS+, CS-). There was no evidence for group differences as indicated by the non-significant main effect of group (F(2,58) = 2.21, P > 0.05, partial η2 = 0.07), group x block interaction (F(6,174) = 1.48, P > 0.05, partial η2 = 0.05), group x stimulus interaction (F(2,58) = 0.80, P > 0.05, partial η2 = 0.03), or group x block x stimulus interaction (F(6,174) = 1.17, P > 0.05, partial η2 = 0.04). SCR responses were higher to the CS+ compared to the CS- and this differential responding increased from early blocks to late blocks as indicated by a main effect of block (F(3,174) = 22.77, P < 0.001, partial η2 = 0.28) and stimulus (F(1,58) = 59.53, P < 0.001, partial η2 = 0.51), and also a block x stimulus interaction (F(3,174) = 12.70, P < 0.001, partial η2 = 0.18).
Figure 2. Stimulus discrimination during threat acquisition.
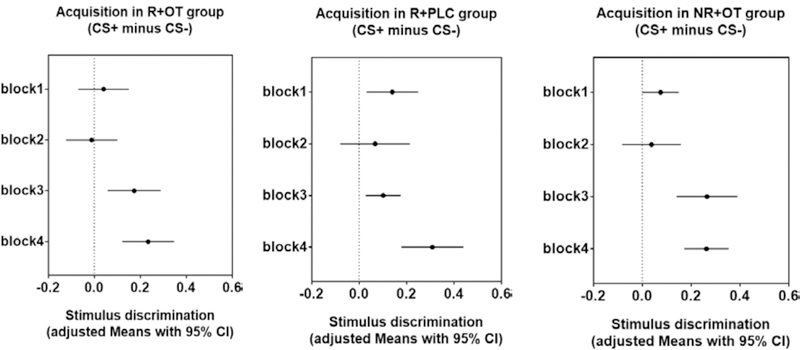
Means with 95% confidence intervals in each group during four blocks of acquisition. Confidence intervals that do not cross the vertical dashed line at zero indicate that the corresponding contrast is different from zero and thus statistically significant. The results show successful and similar acquisition in all groups.
Follow up t-tests confirmed significantly higher responding to the CS+ vs. CS- in all groups during the last two blocks of acquisition (block3: reminder+OT group, P < 0.01; reminder+placebo group, P < 0.01; no reminder+OT group, P < 0.001; block4: reminder+OT group, P < 0.001; reminder+placebo group, P < 0.001; no reminder +OT group, P < 0.001). These results confirm sufficient and similar acquisition across all groups.
Day 2 - Threat memory reactivation
Independent samples t-tests confirmed similar reactivation between the reminder+OT and reminder+placebo groups (t(41) = 1.36, P > 0.05)
Day3 - Threat extinction
To assess the overall effect of post-retrieval OT administration on next day extinction (Figure 3), we examined conditioned defensive responses to the stimuli during the four blocks of extinction. Repeated measures ANOVA was conducted with between-subjects factor of group (3 treatment groups), and within-subjects factors of block (block1, block2, block3, block4) and stimulus (CS+, CS-), which yielded a significant 3-way interaction (F(6,174) = 2.22, P < 0.05, partial η2 = 0.07).
Figure 3. Stimulus discrimination during threat extinction.
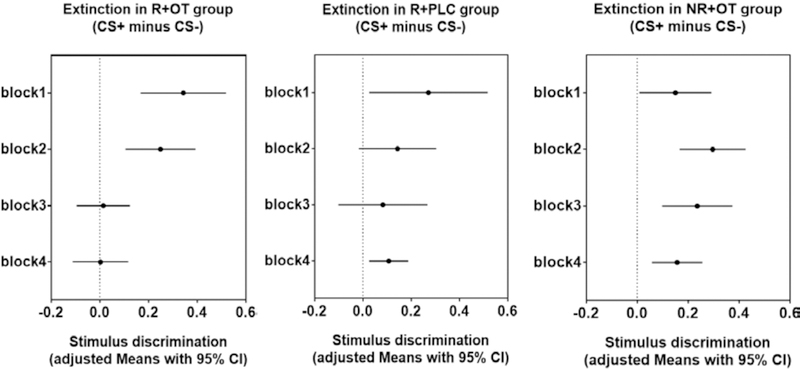
Means with 95% confidence intervals in each group during four blocks of extinction. Confidence intervals that do not cross the vertical dashed line at zero indicate that the corresponding contrast is different from zero and thus statistically significant. A significant 3-way interaction of group x block x stimulus and follow-up t-tests confirmed memory recovery in all groups at the beginning of day 3 extinction (24 hours after drug administration), but only the group that had post-retrieval administration of OT showed successful and adequate extinction by the end of extinction session.
Follow up t-tests indicated that threat memory recovery was evident in all groups: all groups showed significant stimulus discrimination at the first block of extinction (reminder+OT group, t(21) = 4.06, P < 0.01; reminder+placebo group, t(20) = 2.31, P < 0.05; no reminder+OT group, t(17) = 2.24, P < 0.05). However, only reminder+OT group showed no evidence for stimulus discrimination at the last block of extinction (reminder+OT group, t(21) = 0.05, P > 0.05; reminder+placebo group, t(20) = 2.76, P < 0.05; no reminder+OT group, t(17) = 3.35, P < 0.01]. These results indicate that post-retrieval administration of OT facilitate extinction after twenty-four hours by allowing successful extinction, whereas other treatments rendered the fear less amenable to attenuation.
The effect of post-reactivation OT administration on extinction may be exhibited not only by lack of stimulus discrimination by the end of the extinction session, but also by a significant reduction in discrimination (CS+ minus CS-) from early to late phase of extinction (Figure 4). To assess the degree of reduction in stimulus discrimination during the four blocks of extinction, we conducted paired sample t-tests to compare stimulus discrimination between blocks during extinction for each group. The analysis confirmed that only the reminder+OT group showed significant and gradual reduction in stimulus discrimination from early to late blocks: the discrimination in block 1 was significantly higher than in block 3 (t(21) = 3.15, P < 0.01) and block 4 (t(21) = 3.48, P < 0.01). Furthermore, discrimination in block 2 was significantly higher than in block 3 (t(21) = 2.58, P < 0.05) and block 4 (t(21) = 2.97, P < 0.01). The no reminder+OT group maintained similar stimulus discrimination across all blocks on extinction (all P’s > 0.05); and despite the reduction from block 1 to block 3 (t(20) = 2.25, P < 0.05), the reminder+placebo group maintained significant discrimination during last block of extinction (Figure 3, middle panel).
Figure. 4. The reduction of stimulus discrimination from block 1 to block 4 during extinction.
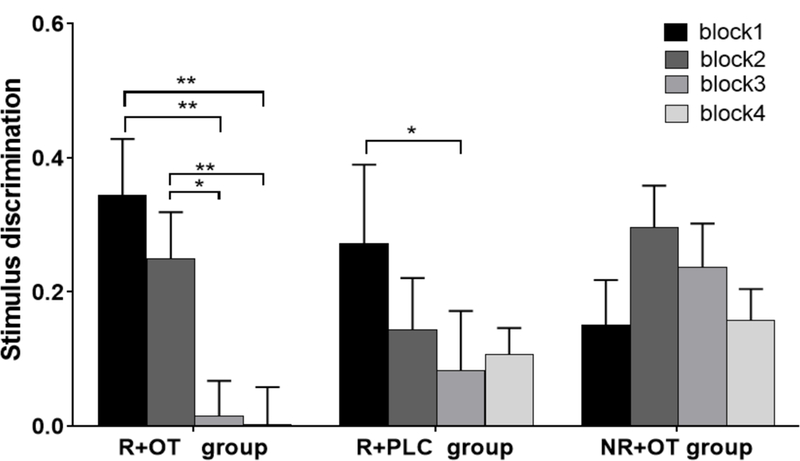
Paired sample t-tests confirmed significant and gradual reduction of stimulus discrimination (CS+ minus CS-) in the reminder+OT group (R+OT), but not in the reminder+placebo group (R+PLC) or the no reminder+OT group (NR+OT). *P < 0.05, ** P < 0.01. Error bars represent standard errors.
Finally, we calculated a threat extinction index (stimulus discrimination in first minus last block), which was the highest in the reminder+OT group (Figure 5), and significantly larger than the no reminder+OT group (t(38) = 2.58, P < 0.05). These results further indicate that post-retrieval OT administration facilitated extinction training.
Figure 5. Threat extinction index by treatment group.
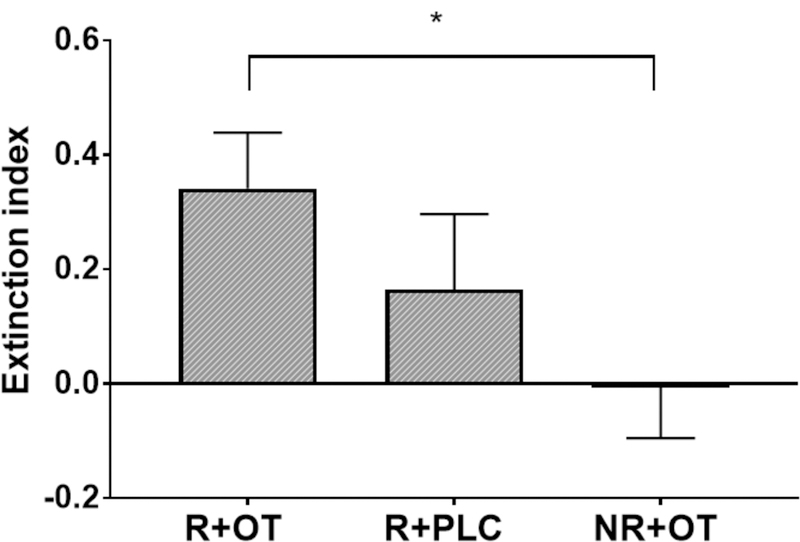
Independent sample t-tests confirmed significantly higher extinction index (stimulus discrimination in first minus last block of extinction) in reminder+OT group (R+OT) compared to the no reminder+OT group (NR+OT). *P < 0.05, Error bars represent standard errors.
Day3 – Threat reinstatement
Following the extinction session on day 3, the participants were exposed to 4 unsignaled shocks followed by a re-extinction session (reinstatement, Figure 6). Repeated measures ANOVA with between-subjects factor of group (3 treatment groups), and within-subjects factors of block (block1, block2, block3, block4) and stimulus (CS+, CS-) yielded no significant effect (F(6,174) = 1.711, P = 0.121). Reinstatement effects following extinction are typically weak and limited to the first few trials (e.g., Kindt et al., 2009; Homan et al., 2017; Hu et al., 2018;).
Figure 6. Stimulus discrimination in reinstatement.
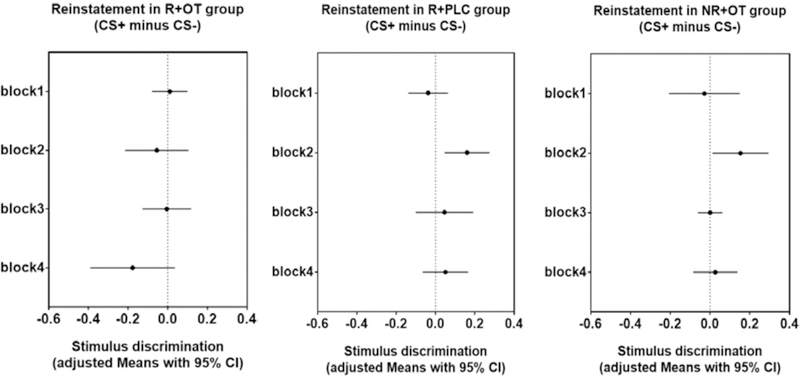
Means with 95% confidence intervals in each group of four blocks of reinstatement. Confidence intervals that do not cross the vertical dashed line at zero indicate that the corresponding contrast is different from zero and thus statistically significant. One-way ANOVA and paired sample t-tests confirmed that both reminder+placebo group (R+PLC) and no reminder+OT group (NR+OT) showed significant stimulus discrimination in the second block of reinstatement, and they are both significantly higher than reminder+OT group (R+OT).
Nevertheless, further exploratory analysis using paired sample t-tests revealed that the reminder+OT group, who did not show stimulus discrimination in late extinction blocks, continued to exhibit no-evidence of stimulus discrimination through all blocks of re-extinction (all P’s > 0.05). However, both reminder+placebo and no reminder+OT groups showed significant stimulus discrimination in second block of reinstatement (t(20) = 2.93, P < 0.01, t(17) = 2.30, P < 0.05, respectively). One-way ANOVA confirmed both were significantly higher than stimulus discrimination during the respective second block of the reminder+OT group (F(2,58) = 3.43, P < 0.05, partial η2 = 0.11).
Discussion
The present study reports a first examination of the effects of post-retrieval administration of OT on the expression of threat memory in healthy human individuals. In contrast to some findings in rodents (Hou et al., 2015) but consistent with others (Lahoud & Maroun., 2013), the current results indicate that post-retrieval administration of OT did not block reconsolidation but rather facilitated extinction the next day, compared to post-retrieval administration of placebo or administration of OT without reactivation. Furthermore, we found that the post-retrieval OT group continued to exhibit lack of stimulus discrimination throughout reinstatement relative to other two groups, consistent with studies showing that if extinction learning were less effective, defensive reactions should recover during this phase (Vervliet et al., 2013). These results are consistent with evidence in animals and humans showing that administration of OT facilitates extinction (Toth et al., 2012; Acheson et al., 2013; Lahoud & Maroun, 2013; Eckstein et al., 2015).
There is evidence to suggest that OT could get into the brain via the intranasal route across species. For example, intranasal administration of a high dose of OT (10 µg) in mice activated Fos expression at the paraventricular nucleus, the area postrema, and the dorsal motor nucleus of the vagus (Maejima et al., 2015). In macaques, 48 IU OT (~10 µg/kg body weight) administered intranasally either with a spray or a nebulizer induced CSF OT level increase from ~35 to ~90 pg/mL after 40 minutes (Dal Monte et al., 2014). A study in humans (Striepens et al., 2013) using combined blood and cerebrospinal fluid (CSF) sampling in subjects receiving either 24 IU of OT (n = 11) or placebo (n = 4) have shown that OT levels significantly increased in both plasma and CSF. Specifically, OT plasma concentrations peaked at 15 min after intranasal administration and decreased after 75 min, and CSF concentrations took up to 75 min to reach a significant level. These findings indicate that intranasally administered OT increases concentrations of the peptide in the brain as well as blood across species, including humans.
Within the threat learning field, a human neuroimaging study showed that when OT (24 IU) was administered intranasally after threat conditioning in humans, prefrontal cortex fMRI signals to conditioned threat increased in the early phase of extinction, and evoked an unspecific inhibition of amygdalar response throughout extinction (Eckstein et al., 2015). In line with this result, rodent studies showed that injection of OT into IL-mPFC following threat memory retrieval facilitated extinction (Lahoud & Maroun, 2013). OT promotes threat extinction by inducing long-lasting LTP of excitatory postsynaptic currents in IL-mPFC brain slices (Ninan, 2011), indicating that OT increases activity-dependent strengthening of glutamtergic synapses in the IL-mPFC. As previous studies have demonstrated that potentiation of synaptic transmission in the IL-mPFC underlies extinction memory (Vouimba & Maroun, 2011; Burgos-Robles et al., 2007; Herry & Garcia., 2002), the OT-mediated facilitation of LTP in the IL-mPFC might serve as the neural basis of its extinction enhancing effect.
The present findings are inconsistent with a recent rodent study showing that post-retrieval OT blocked reconsolidation (Hou et al., 2015). A possible explanation of the discrepancy might relate to differences in dose and timing of OT administration. The effects of OT on anxiety and memory in animals are dose-dependent and time-dependent (Peters et al., 2014; Chini et al., 2014). In the current study, we have only tested a single dose of intranasal OT in a single time-point. Nevertheless, that fact the extinction facilitation was observed only when OT was preceded by a memory reminder and did not act when administered on its own, suggests that at least a partial interaction with reconsolidation processes occurred to modify the memory rendering it more susceptible to extinction. OT therefore remains a possible candidate for blocking reconsolidation, but further studies are needed to fully assess this effect, which may require a higher-dose and repeated use.
Our observation that OT administration after retrieval facilitated the extinction of a consolidated threat memory may explain some controversial findings on OT effects on exposure therapy in clinical populations. A study that investigated OT facilitation of exposure therapy for social phobia failed to show an impact on symptoms (Guastella et al., 2009). Another recent study examining arachnophobia patients also indicate that administration of intranasal OT prior to exposure therapy impeded responses as measured by self-report questionnaires (Acheson et al., 2015). These results are inconsistent with previous evidence from healthy humans demonstrating a facilitating effect of OT on extinction (Acheson et al., 2013; Eckstein et al., 2015). These latter results were obtained by conducting threat acquisition and extinction on the same day before threat acquisition was consolidated into long-term memory. By contrast, the aversive memories of clinically anxious patients are typically old and well consolidated. Moreover, spider phobia and other real-life memories in anxiety patients may at least in part reflect an innate fear as opposed to an entirely learned threat response acquired through associative learning. These characteristics of age, strength and quality of memory may influence susceptibility to OT manipulations, at least in certain doses and mode of administration.
Lastly, since explicit measures of expectancy might interact with implicit physiological measurements (Warren et al., 2014), we focused on assessing physiological threat responses and not subjective fear; future studies could specifically assess the cognitive impact of OT on subjective fear memory.
In summary, we found that OT did not block reconsolidation to prevent the return of threat memory but rather interacted with post-retrieval processes to facilitate next day extinction. The study provides novel preliminary evidence for the role of OT in human threat memory. The results suggest that OT may be used as an adjunctive treatment to exposure-based therapy by administering it following memory retrieval to facilitate subsequent exposure sessions.
Supplementary Material
Acknowledgments
Funding was provided by NIMH 105535 R01 grant and a Klingenstein-Simons Fellowship Award in the Neurosciences to D.S.; a grant from the National Natural Science Foundation of China (31871170) to C.L.; and funding from the PhD Research Startup Foundation of Guangdong province to J.H. The authors thank Xiaoting Chen for assistance with data collection, and Xifu Zheng and Li Yang for helpful discussions.
Footnotes
Conflict of interest statement: On behalf of all authors, the corresponding author states that there is no conflict of interest.
Competing financial interests
All authors report no conflicts of interest
Reference
- Acheson D, Feifel D, de Wilde S, McKinney R, Lohr J, & Risbrough V (2013). The effect of intranasal oxytocin treatment on conditioned fear extinction and recall in a healthy human sample. Psychopharmacology (Berl), 229(1), 199–208. doi: 10.1007/s00213-013-3099-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson DT, Feifel D, Kamenski M, McKinney R, & Risbrough VB (2015). Intranasal oxytocin administration prior to exposure therapy for arachnophobia impedes treatment response. Depress Anxiety, 32(6), 400–407. doi: 10.1002/da.22362 [DOI] [PubMed] [Google Scholar]
- Agren T (2014). Human reconsolidation: a reactivation and update. Brain Res Bull, 105, 70–82. doi: 10.1016/j.brainresbull.2013.12.010 [DOI] [PubMed] [Google Scholar]
- Brunet A, Orr SP, Tremblay J, Robertson K, Nader K, & Pitman RK (2008). Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J Psychiatr Res, 42(6), 503–506. doi: 10.1016/j.jpsychires.2007.05.006 [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, & Quirk GJ (2007). Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron, 53(6), 871–880. doi: 10.1016/j.neuron.2007.02.021 [DOI] [PubMed] [Google Scholar]
- Campbell A (2008). Attachment, aggression and affiliation: the role of oxytocin in female social behavior. Biol Psychol, 77(1), 1–10. doi: 10.1016/j.biopsycho.2007.09.001 [DOI] [PubMed] [Google Scholar]
- Chini B, Leonzino M, Braida D, & Sala M (2014). Learning about oxytocin: pharmacologic and behavioral issues. Biol Psychiatry, 76(5), 360–366. doi: 10.1016/j.biopsych.2013.08.029 [DOI] [PubMed] [Google Scholar]
- Dal MO, Noble PL, Turchi J, Cummins A, & Averbeck BB (2014). Csf and blood oxytocin concentration changes following intranasal delivery in macaque. Plos One, 9(8), e103677. doi: 10.1371/journal.pone.0103677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein M, Becker B, Scheele D, Scholz C, Preckel K, Schlaepfer TE, … Hurlemann R (2015). Oxytocin facilitates the extinction of conditioned fear in humans. Biol Psychiatry, 78(3), 194–202. doi: 10.1016/j.biopsych.2014.10.015 [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Howard AL, Dadds MR, Mitchell P, & Carson DS (2009). A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder. Psychoneuroendocrinology, 34(6), 917–923. doi: 10.1016/j.psyneuen.2009.01.005 [DOI] [PubMed] [Google Scholar]
- Haaker J, Golkar A, Hermans D, & Lonsdorf TB (2014). A review on human reinstatement studies: an overview and methodological challenges. Learn Mem, 21(9), 424–440. doi: 10.1101/lm.036053.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, & Garcia R (2002). Prefrontal cortex long-term potentiation, but not long-term depression, is associated with the maintenance of extinction of learned fear in mice. J Neurosci, 22(2), 577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homan P, Lin Q, Murrough J, Soleimani L, Bach D, Clem R, & Schiller D (2017). Prazosin during threat discrimination boosts memory of the safe stimulus. Learn Mem 24, 597–601. doi: 10.1101/lm.045898.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Zhao L, Zhang G, & Ding L (2015). Effects of oxytocin on the fear memory reconsolidation. Neurosci Lett, 594, 1–5. doi: 10.1016/j.neulet.2015.03.030 [DOI] [PubMed] [Google Scholar]
- Hu J, Wang W, Homan P, Wang P, Zheng X, & Schiller D (2018). Reminder duration determines threat memory modification in humans. Scientific Reports, 8(1), 8848 10.1038/s41598-018-27252-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt M, Soeter M, & Vervliet B (2009). Beyond extinction: erasing human fear responses and preventing the return of fear. Nat Neurosci, 12(3), 256–258. doi: 10.1038/nn.2271 [DOI] [PubMed] [Google Scholar]
- Kroes MCW, Schiller D, LeDoux JE, & Phelps EA (2016). Translational Approaches Targeting Reconsolidation. Current Topics in Behavioral Neurosciences, 28, 197–230. 10.1007/7854_2015_5008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahoud N, & Maroun M (2013). Oxytocinergic manipulations in corticolimbic circuit differentially affect fear acquisition and extinction. Psychoneuroendocrinology, 38(10), 2184–2195. doi: 10.1016/j.psyneuen.2013.04.006 [DOI] [PubMed] [Google Scholar]
- Lee JLC, Nader K, & Schiller D (2017). An Update on Memory Reconsolidation Updating. Trends Cogn Sci, 21(7), 531–545. doi: 10.1016/j.tics.2017.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leknes S, Wessberg J, Ellingsen DM, Chelnokova O, Olausson H, & Laeng B (2013). Oxytocin enhances pupil dilation and sensitivity to ‘hidden’ emotional expressions. Soc Cogn Affect Neurosci, 8(7), 741–749. doi: 10.1093/scan/nss062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald E, Dadds MR, Brennan JL, Williams K, Levy F, & Cauchi AJ (2011). A review of safety, side-effects and subjective reactions to intranasal oxytocin in human research. Psychoneuroendocrinology, 36(8), 1114–1126. doi: 10.1016/j.psyneuen.2011.02.015 [DOI] [PubMed] [Google Scholar]
- Maejima Y, Rita RS, Santoso P, Aoyama M, Hiraoka Y, & Nishimori K, et al. (2015). Nasal oxytocin administration reduces food intake without affecting locomotor activity and glycemia with c-fos induction in limited brain areas. Neuroendocrinology, 101(1), 35–44. doi: 10.1159/000371636 [DOI] [PubMed] [Google Scholar]
- Maroun M, & Wagner S (2016). Oxytocin and Memory of Emotional Stimuli: Some Dance to Remember, Some Dance to Forget. Biol Psychiatry, 79(3), 203–212. doi: 10.1016/j.biopsych.2015.07.016 [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, & Le Doux JE (2000). Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature, 406(6797), 722. [DOI] [PubMed] [Google Scholar]
- Neumann ID (2007). Stimuli and consequences of dendritic release of oxytocin within the brain. Biochem Soc Trans, 35(Pt 5), 1252–1257. doi: 10.1042/bst0351252 [DOI] [PubMed] [Google Scholar]
- Ninan I (2011). Oxytocin suppresses basal glutamatergic transmission but facilitates activity-dependent synaptic potentiation in the medial prefrontal cortex. J Neurochem, 119(2), 324–331. doi: 10.1111/j.1471-4159.2011.07430.x [DOI] [PubMed] [Google Scholar]
- Peters S, Slattery DA, Uschold-Schmidt N, Reber SO, & Neumann ID (2014). Dose-dependent effects of chronic central infusion of oxytocin on anxiety, oxytocin receptor binding and stress-related parameters in mice. Psychoneuroendocrinology, 42, 225–236. doi: 10.1016/j.psyneuen.2014.01.021 [DOI] [PubMed] [Google Scholar]
- Sabihi S, Durosko NE, Dong SM, & Leuner B (2014). Oxytocin in the prelimbic medial prefrontal cortex reduces anxiety-like behavior in female and male rats. Psychoneuroendocrinology, 45, 31–42. doi: 10.1016/j.psyneuen.2014.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, & Phelps EA (2011). Does reconsolidation occur in humans? Front Behav Neurosci, 5, 24. doi: 10.3389/fnbeh.2011.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeter M, & Kindt M (2010). Dissociating response systems: erasing fear from memory. Neurobiol Learn Mem, 94(1), 30–41. doi: 10.1016/j.nlm.2010.03.004 [DOI] [PubMed] [Google Scholar]
- Steenen SA, van Wijk AJ, van der Heijden GJ, van Westrhenen R, de Lange J, & de Jongh A (2016). Propranolol for the treatment of anxiety disorders: Systematic review and meta-analysis. J Psychopharmacol, 30(2), 128–139. doi: 10.1177/0269881115612236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striepens N, Kendrick KM, Hanking V, Landgraf R, Wüllner U, & Maier W, et al. (2013). Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Scientific Reports, 3(6163), 3440. doi: 10.1038/srep03440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth I, Neumann ID, & Slattery DA (2012). Central administration of oxytocin receptor ligands affects cued fear extinction in rats and mice in a timepoint-dependent manner. Psychopharmacology (Berl), 223(2), 149–158. [DOI] [PubMed] [Google Scholar]
- Vervliet B, Craske MG, & Hermans D (2013). Fear extinction and relapse: state of the art. Annu Rev Clin Psychol, 9, 215–248. doi: 10.1146/annurev-clinpsy-050212-185542 [DOI] [PubMed] [Google Scholar]
- Vouimba RM, & Maroun M (2011). Learning-induced changes in mPFC-BLA connections after fear conditioning, extinction, and reinstatement of fear. Neuropsychopharmacology, 36(11), 2276–2285. doi: 10.1038/npp.2011.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walum H, Waldman ID, & Young LJ (2016). Statistical and Methodological Considerations for the Interpretation of Intranasal Oxytocin Studies. Biol Psychiatry, 79(3), 251–257. doi: 10.1016/j.biopsych.2015.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren VT, Anderson KM, Kwon C, Bosshardt L, Jovanovic T, Bradley B, & Norrholm SD (2014). Human fear extinction and return of fear using reconsolidation update mechanisms: the contribution of on-line expectancy ratings. Neurobiol Learn Mem, 113, 165–173. doi: 10.1016/j.nlm.2013.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


