For many patients with severe obesity, bariatric surgery offers the only viable option for achieving significant long-term weight loss and reducing their dependence on pharmacological treatment of type 2 diabetes (T2D). In this issue of Hypertension, Schiavon et al1 provide additional evidence from the GATEWAY trial that bariatric surgery is also an effective strategy for reducing the number of antihypertensive medications needed to achieve adequate 24-hour blood pressure (BP) control and reducing the burden of resistant hypertension in patients with moderate to severe obesity.
The pandemic of obesity and associated cardiorenal and metabolic diseases is rapidly becoming our greatest global public health threat.2 Obesity/overweight accounts for 65–75% of the risk for primary (essential) hypertension,3 the leading cause of cardiovascular (CV) mortality worldwide, and age-adjusted hypertension-related deaths have risen in parallel with increasing obesity.4 Obesity is also the most important risk factor for diabetes mellitus which interacts with hypertension to cause microvascular and macrovascular injury and ultimately damage to target organs such as the kidneys, heart and brain. Weight loss and reduced adiposity are therefore important therapeutic goals for most patients with hypertension.
Although the mainstay for treatment of obesity and associated metabolic disorders continues to be lifestyle therapy aimed at caloric restriction and increased physical activity, most people are unable to achieve and maintain adequate reductions in adiposity by diet and exercise. Several new weight loss medications have been approved for clinical use in the past few years but they are still less than optimal and not widely used by primary care physicians. Anti-obesity drugs often produce only modest weight loss, little or no reduction in BP, and some may have adverse CV effects.
Although many effective antihypertensive drugs are available, adequate BP control is still achieved in <50% of hypertensive patients and those with “resistant hypertension” are usually overweight or obese. Thus, many obese patients with hypertension are placed on regimens of ≥3 antihypertensive drugs as well as multiple drugs to control dyslipidemia, insulin resistance and hyperglycemia, inflammation and other adverse effects of excessive adiposity.
Because lifestyle therapy and anti-obesity medications are often ineffective in producing satisfactory weight loss lasting >1 year, surgical interventions are increasingly used to achieve long-term reductions in adiposity as well as to treat cardiometabolic disorders in obese patients. Bariatric surgery has been has so successful in producing >70% remission of T2D that it is often called “metabolic surgery”.5
Bariatric surgery also confers benefits for treatment of hypertension and cardiorenal diseases. In the first major meta-analysis of the impact of bariatric surgery on CV and metabolic diseases, Buchwald et al6 reported that Roux-en-Y gastric bypass (RYGB) surgery induced remission of hypertension in ~75% of the patients. Sarkhosh et al7 performed a comprehensive analysis of available databases that included human studies of laparoscopic sleeve gastrectomy between 2000 to 2011 and reported that this procedure resulted in complete resolution of hypertension in 58% of patients and that 75% experienced improvement or resolution of their hypertension.
Despite this rosy picture, concerns about long-term durability of antihypertensive effects of bariatric surgery were raised after a report from the Swedish Obesity Study which followed 627 obese control subjects and 641 obese patients who underwent RYGB, vertical banded gastroplasty, or gastric banding.8 The surgically treated group had greater weight loss and lower incidence rates of hypertriglyceridemia, hyperuricemia and diabetes than the control group but differences between the groups for incidences of hypertension and hypercholesterolemia were not significant after 10 years. Important limitations of the Swedish Obesity Study were that it was not randomized, BP was not a primary outcome, and only 34 of the surgically treated patients who completed the 10-year follow up received RYGB. Subsequent analysis of Swedish Obesity Study data included a larger number of RYGB patients and demonstrated average reductions of systolic/diastolic BP of 12.1/7.3 and 5.1/5.6 mmHg at 2 and at 10 years follow up, respectively.9
Additional large meta-analyses have consistently shown benefits of bariatric surgery on BP in obese hypertensive patients with follow ups varying from 1 to 14 years.10 Multiple studies have also shown that RYGB or sleeve gastrectomy improves outcomes in patients with heart failure or chronic kidney disease, perhaps at least partly due to improved control of hypertension.11, 12
Although bariatric surgery is increasingly used to treat obesity and its co-morbidities, the first randomized clinical trial (RCT) to prospectively assess BP effects of RYGB was the “Gastric Bypass to Treat Obese Patients with Steady Hypertension (GATEWAY)” study.13 In this trial, 100 patients with obesity and hypertension were randomized to RYGB or medical therapy (MT) alone. Patients who received RGYB were 6 times more likely to reduce the total number of antihypertensive medications by ≥30% while maintaining BP control and 51% of the patients showed complete remission of hypertension, defined as SBP/DBP <140/90 mmHg 12 months after surgery. The STAMPEDE trial (Surgical Treatment and Medications Potentially Eradicate Diabetes Efficiently Trial), a RCT of RYGB or sleeve gastrectomy in patients with T2D, also reported reductions in antihypertensive mediations 12 months after surgery and ~60% of the patients were able to stop their medications while maintaining hypertension control.14 After 5 years, only 20% of patients required >3 drugs compared to 61% before surgery.15 In contrast to the GATEWAY study, the STAMPEDE trial and most previous RCTs were conducted in patients with uncontrolled T2D and hypertension remission/improvement were secondary endpoints.
Schiavon et al1 now report in this issue of Hypertension the GATEWAY sub-study in which they performed detailed analyses of the 24-hour ambulatory BP (ABPM) profile, including BP variability, non-dipping status, and resistant hypertension prevalence 12 months after RYGB or MT alone. Participants were randomized (1:1) to either RYGB combined with MT or MT alone. After 12 months, the average BMI reduction was 10.8 kg/m2 in the surgical group compared to 0.2 kg/m2 in the MT control group. The 24-hour BP profile was similar and office BP was well controlled at the same level (~123 mmHg systolic BP) in both groups. However, the RYGB groups required fewer (0–1, average =0.73) antihypertensive medications compared to the MT control group (2.5–4.0, average = 3.04) to achieve the target BP. In fact, none of the patients who received RYGB required ≥3 antihypertensive medications 12 months after surgery whereas ~15% of those receiving MT alone were classified as having resistant hypertension. Another finding that could have implications for prevention of target organ injury was the reduced ABPM variability after RYGB. Previous studies suggest that higher BP variability is associated with increased risk of CV events, over and above the effect of mean BP.16 Surprisingly, RYBP did not significantly alter nighttime non-dipping of BP despite the need for fewer anti-hypertensive medications.
In addition to being the first RCT to prospectively assess BP effects of RYGB, the GATEWAY study has other strengths including the standard protocol for BP measurements and 24-hour ABPM with assessment of BP variability and non-dipping status. Also, the average BMI before surgery was 36.9 kg/m2 and the study cohort contained patients with class I obesity (BMI 30.0–34.9), suggesting that RGYB may be useful for BP control while improving metabolic and inflammatory profiles in patients with moderate obesity. Perhaps the BP effect of surgery would be even greater in more obese subjects, but GATEWAY did not include subjects with extreme obesity (BMI >40 kg/m2). The study was not adequately powered for separate analyses of BP changes after RYGB in patients with class 1 and 2 obesity. Thus, the number of patients with BMI <35 kg/m2 studied in RCTs is still modest, and even fewer patients have been assessed with BMI <30 kg/m2 who have high levels of visceral adiposity and increased cardiometabolic risk.
GATEWAY has some limitations. Although the study is planned for 5 years of follow up, current data are from 12 months after RYGB. It would be reassuring to have much longer follow up with larger numbers of subjects to confirm durability of BP reductions after bariatric surgery. Also, GATEWAY was a small single-center, open-label trial and compliance with study medications was based on patient self-reports. Finally, there has been a rapid shift in bariatric procedures use worldwide, with sleeve gastrectomy replacing RYBG as the surgical treatment of choice for most patients with severe obesity.17 The sleeve gastrectomy procedure is technically less complex, produces fewer post-surgical complications, and may be nearly as effective in treating obesity and T2D as RYGB, although there is still a need for additional long-term outcome data, especially for remission of hypertension and cardiometabolic disorders.17
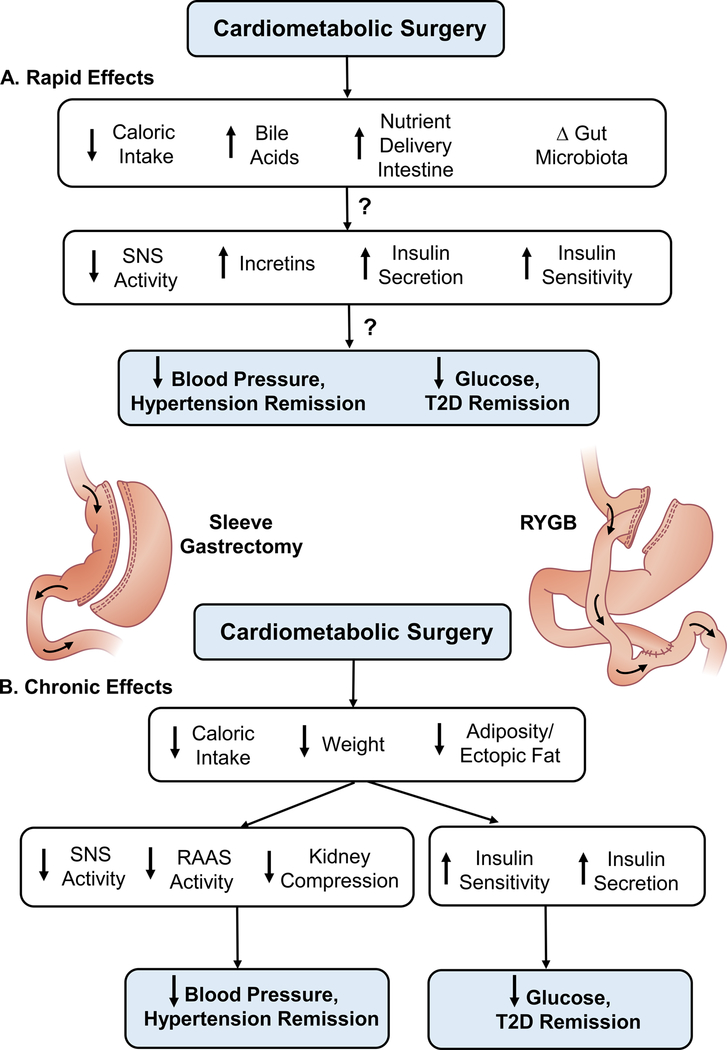
Based on the results of GATEWAY and previous observational studies, attention can now be focused on mechanisms by which bariatric surgery causes remission of hypertension as well as diabetes. Some of the favorable cardiometabolic effects of bariatric surgery may occur too rapidly to be directly linked to reductions in adiposity.5 Early in the postoperative period, even before significant weight loss, many patients show decreases in BP and plasma glucose. Whether BP reductions are caused mainly by decreased caloric intake which, in turn, attenuates neurohormonal mechanisms that contribute to obesity hypertension3 or via other pathways originating in the gastrointestinal (GI) tract is unclear.5 Remission of diabetes within days after bariatric surgery has been suggested to be mediated by changes in the gut microbiota, increased bile acids, increased incretins, vagal afferent stimulation, accelerated entry of food and undigested nutrients to the small intestine which may alter various GI hormones (e.g. peptide YY, GLP-1), and other factors (Figure 1).5 The importance of these mechanisms for BP regulation after bariatric surgery, however, is unclear and supporting evidence is sparse. Although it is clear that bariatric surgery can elicit reductions in BP and glucose before substantial changes in adiposity or body weight occur, there is still considerable uncertainty about whether the rapid or long-term antidiabetic and BP effects of bariatric surgery are independent of decreased caloric intake. Further studies to elucidate these mechanisms represent an important research priority.
Figure 1.
Rapid and chronic effects of bariatric (“cardiometabolic”) surgery to cause remission of hypertension and type 2 diabetes (T2D) in patients with obesity and some mechanisms that have been proposed. Abbreviations: SNS, sympathetic nervous system; RAAS, renin-angiotensin-aldosterone system; RYGB, Roux-en-Y gastric bypass; T2D, type 2 diabetes mellitus.
The study by Schiavon and colleagues adds to growing evidence that bariatric surgery can reduce hypertension as well as other risk factors for CV and renal disease. Observational studies as well as RCTs of bariatric surgery have shown promising long-term reductions of CV and kidney disease risk factors. Available RCTs, however, do not permit comparison of conventional therapies and various types of bariatric surgery, including the most commonly performed sleeve gastrectomy procedure, in different stages of obesity/metabolic disease severity. Although larger RCTs are needed to further demonstrate long-term benefits, there may be enough clinical and mechanistic evidence to support inclusion of “cardiometabolic” surgery for people with moderate obesity and hypertension as well as for those who have severe, morbid obesity and T2D.
There are many remaining questions. Does duration of obesity influence the BP response to bariatric surgery? Are the BP lowering effects of bariatric surgery blunted by longstanding obesity and hypertension which may cause injury to target organs, especially the kidneys? Can bariatric surgery in obese individuals prevent development of resistant hypertension and target organ injury if conducted early enough (e.g. in adolescence)? With the rapid increase in bariatric surgeries worldwide we should have the answers to these questions in the next several years.
Acknowledgments
SOURCES OF FUNDING
The authors’ research was supported by grants from the National Heart, Lung, and Blood Institute (P01 HL51971), the National Institute of General Medical Sciences (P20 GM104357 and U54 GM115428), and the National Institute of Diabetes and Digestive and Kidney Diseases (1K08DK099415–01A1) of the National Institutes of Health.
Footnotes
DISCLOSURES
None
REFERENCES
- 1.Schiavon CA, Ikeoka D, Santucci EV, Santos RN, Damiani LP, Oliveira JD, Torreglosa CR, Bersch-Ferreira AC, T M, Barros S, Halpern H, Monteiro FLJ, Cohen RV, Noujaim PM, de Souza MG, Amodeo C, Bortolotto L, Berwanger O, Cavalcanti AB and drager LF. The effects of bariatric surgery versus medical therapy on the 24-h ambulatory blood pressure and the prevalence of resistant hypertension: The GATEWAY Randomized Clinical Trial. Hypertension 2018:In press. [Google Scholar]
- 2.Gregg EW and Shaw JE. Global Health Effects of Overweight and Obesity. N Engl J Med. 2017;377:80–81. [DOI] [PubMed] [Google Scholar]
- 3.Hall JE, do Carmo JM, da Silva AA, Wang Z and Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116:991–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kung HC and Xu J. Hypertension-related Mortality in the United States, 2000–2013. NCHS Data Brief. 2015:1–8. [PubMed] [Google Scholar]
- 5.Pareek M, Schauer PR, Kaplan LM, Leiter LA, Rubino F and Bhatt DL. Metabolic Surgery: Weight Loss, Diabetes, and Beyond. J Am Coll Cardiol. 2018;71:670–687. [DOI] [PubMed] [Google Scholar]
- 6.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K and Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37. [DOI] [PubMed] [Google Scholar]
- 7.Sarkhosh K, Birch DW, Shi X, Gill RS and Karmali S. The impact of sleeve gastrectomy on hypertension: a systematic review. Obes Surg. 2012;22:832–7. [DOI] [PubMed] [Google Scholar]
- 8.Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjostrom CD, Sullivan M, Wedel H and Swedish Obese Subjects Study Scientific G. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–93. [DOI] [PubMed] [Google Scholar]
- 9.Hallersund P, Sjostrom L, Olbers T, Lonroth H, Jacobson P, Wallenius V, Naslund I, Carlsson LM and Fandriks L. Gastric bypass surgery is followed by lowered blood pressure and increased diuresis - long term results from the Swedish Obese Subjects (SOS) study. PLoS One. 2012;7:e49696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Owen JG, Yazdi F and Reisin E. Bariatric Surgery and Hypertension. Am J Hypertens. 2017;31:11–17. [DOI] [PubMed] [Google Scholar]
- 11.Sundstrom J, Bruze G, Ottosson J, Marcus C, Naslund I and Neovius M. Weight Loss and Heart Failure: A Nationwide Study of Gastric Bypass Surgery Versus Intensive Lifestyle Treatment. Circulation. 2017;135:1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedman AN, Wahed AS, Wang J, Courcoulas AP, Dakin G, Hinojosa MW, Kimmel PL, Mitchell JE, Pomp A, Pories WJ, Purnell JQ, le Roux C, Spaniolas K, Steffen KJ, Thirlby R and Wolfe B. Effect of Bariatric Surgery on CKD Risk. J Am Soc Nephrol. 2018;29:1289–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schiavon CA, Bersch-Ferreira AC, Santucci EV, Oliveira JD, Torreglosa CR, Bueno PT, Frayha JC, Santos RN, Damiani LP, Noujaim PM, Halpern H, Monteiro FLJ, Cohen RV, Uchoa CH, de Souza MG, Amodeo C, Bortolotto L, Ikeoka D, Drager LF, Cavalcanti AB and Berwanger O. Effects of Bariatric Surgery in Obese Patients With Hypertension: The GATEWAY Randomized Trial (Gastric Bypass to Treat Obese Patients With Steady Hypertension). Circulation. 2018;137:1132–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE and Bhatt DL. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, Navaneethan SD, Singh RP, Pothier CE, Nissen SE, Kashyap SR and Investigators S. Bariatric Surgery versus Intensive Medical Therapy for Diabetes - 5-Year Outcomes. N Engl J Med. 2017;376:641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehlum MH, Liestol K, Kjeldsen SE, Julius S, Hua TA, Rothwell PM, Mancia G, Parati G, Weber MA and Berge E. Blood pressure variability and risk of cardiovascular events and death in patients with hypertension and different baseline risks. Eur Heart J. 2018;39:2243–2251. [DOI] [PubMed] [Google Scholar]
- 17.Arterburn D and Gupta A. Comparing the Outcomes of Sleeve Gastrectomy and Roux-en-Y Gastric Bypass for Severe Obesity. JAMA. 2018;319:235–237. [DOI] [PubMed] [Google Scholar]