Abstract
Mucopolysaccharidosis Type VII (MPS7, also called β-glucuronidase deficiency or Sly syndrome; MIM 253220) is an extremely rare autosomal recessive lysosomal storage disease, caused by mutations in the GUSB gene. β-glucuronidase (GUSB) is a lysosomal hydrolase involved in the stepwise degradation of glucuronic acid-containing glycosaminoglycans (GAGs). Patients affected with MPS VII are not able to completely degrade glucuronic acid-containing GAGs, including chondroitin 4-sulfate, chondroitin 6-sulfate, dermatan sulfate, and heparan sulfate. The accumulation of these GAGs in lysosomes of various tissues leads to cellular and organ dysfunctions. Characteristic features of MPS VII include short stature, macrocephaly, hirsutism, coarse facies, hearing loss, cloudy cornea, short neck, valvular cardiac defects, hepatosplenomegaly, and dysostosis multiplex. Oral manifestations in patients affected with MPS VII have never been reported. Oral manifestations observed in three patients consist of wide root canal spaces, taurodontism, hyperplastic dental follicles, malposition of unerupted permanent molars, and failure of tooth eruption with malformed roots. The unusual skeletal features of the patients include maxillary hypoplasia, hypoplastic midface, long mandibular length, mandibular prognathism, hypoplastic and aplastic mandibular condyles, absence of the dens of the second cervical vertebra, and erosion of the cortex of the lower border of mandibles. Dogs affected with MPS VII had anterior and posterior open bite, maxillary hypoplasia, premolar crowding, and mandibular prognathism. Unlike patients with MPS VII, the dogs had unremarkable mandibular condyles. This is the first report of oral manifestations in patients affected with MPS VII.
Keywords: MPS VII, dental anomalies, tooth eruption, hypoplastic mandibular condyle, dental follicle, taurodontism, unerupted teeth
INTRODUCTION
Mucopolysaccharidosis Type VII (MPS VII), also called β-glucuronidase deficiency or Sly syndrome (MIM 253220), is a rare type of mucopolysaccharidoses caused by mutations in the GUSB gene, which encodes β-glucuronoside glucuronosyl hydrolase (GUSB, EC 3.2.1.31; MIM *61149). GUSB is a lysosomal hydrolase involved in the stepwise degradation of glucuronic acid-containing glycosaminoglycans (GAGs) [Sly et al., 1973; Tomatsu et al., 2009]. Patients affected with MPS VII are not able to completely degrade glucuronic acid-containing GAGs, including chondroitin 4-sulfate, chondroitin 6-sulfate, dermatan sulfate, and heparan sulfate. The accumulation of these GAGs in lysosomes in various tissues leads to cascades of cellular and organ dysfunction. MPS VII involves multiple organ systems and clinical severity covers a broad range, from the most severe phenotype of nonimmune hydrops to adult onset in patients with normal intelligence. Here we report for the first time the oral manifestations in patients affected with MPS VII.
MATERIALS AND METHODS
All procedures performed were in accordance with the ethical standards of the Human Experimentation Committee of the Faculty of Dentistry, Chiang Mai University and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent for including the patients in the study was obtained from their parents. Animal studies were conducted with the approval of the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Clinical report
Patient 1
Patient 1 was a product of gravida 1, and prenatal ultrasound detected hydrops. She was born at 32 wk, apgar 9/10. Both parents were of Thai descent with no consanguinity. At birth, the patient had clubfeet and mild hepatomegaly. Global developmental delay was first noted at 8 months of age. At age 1 year, echocardiography showed moderate secundum atrial septal defect; no treatment was recommended. Computer tomography of the brain showed left ventriculomegaly without definite focal lesions. Patient 1 was referred for genetic consultation at 17 months of age. The findings at that time included macrocephaly, hypertelorism, mild coarse facial features, obstructive sleep apnea, umbilical hernia, left hip dysplasia, bilateral clubfeet, and global developmental delay (Fig. 1A–E). Surgical repairs to correct the umbilical hernia and inguinal hernia were performed at age 4 years old. Declining mobility and loss of ability to walk independently were noted at around 5 years old. At that time, she had lower extremity muscle atrophy, hyperreflexia, and bilateral positive clonus.
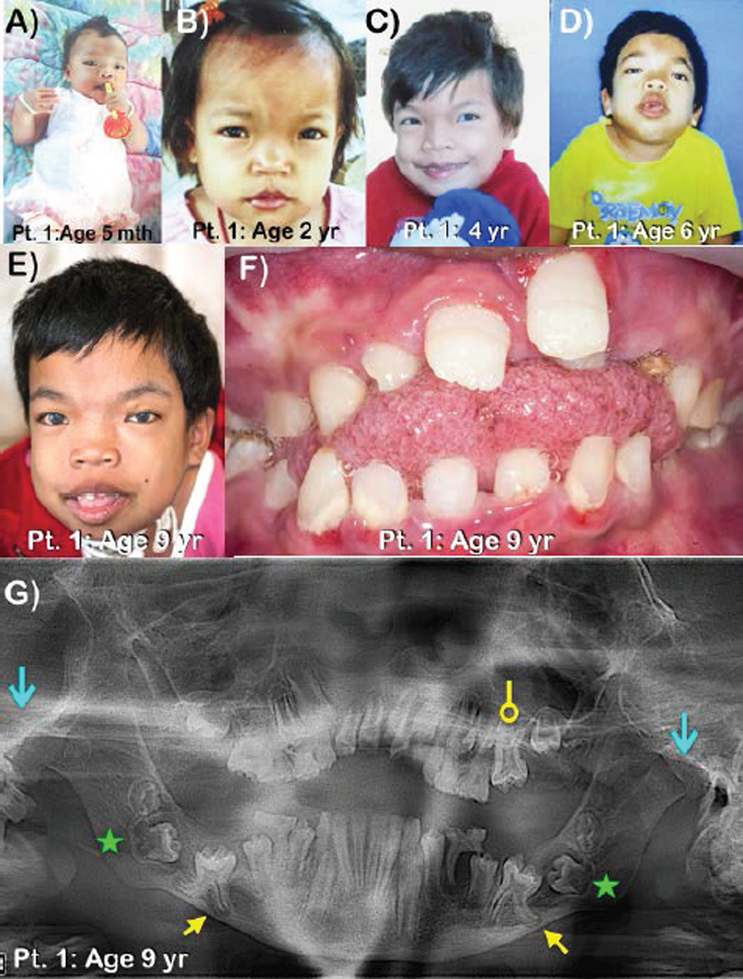
Figure 1.
Patient 1 at ages A) 5 months B) 2 years C) 4 years D) 6 years and E) 9 years. Note thick eyebrows and bilateral cloudy cornea. F) Frontal view of teeth at ages 9 years. Large anterior open bite and macroglossia are noted. G) Panoramic radiograph of patient 1 at age 9 years. Note generalized wide root canal spaces of the permanent teeth, large anterior open bite, taurodontism of the maxillary left first permanent molar (circle-ended arrow), and malposition of unerupted mandibular permanent molars. Unerupted first permanent molars have thin enamel and very prominent cusps. Roots of the unerupted first permanent molars are unusually diverged. The lower border of the mandible at the tips of the roots of the unerupted first permanent molars is eroded (yellow arrows). Unerupted second permanent molars with hypoplastic dental follicles are indicated (green stars). Mandibular condyles are severely hypoplastic (blue line arrows).
Radiographic examination showed skeletal abnormalities and spinal cord compression, consistent with dysostosis multiplex. An MRI of the spine showed basilar impression with compression of the cervicomedullary junction. Increased kyphotic angulation at the cervicothoracic junction was evident with associated narrowing of the central spinal canal and spinal cord compression from the C7-T2 levels. Platyspondyly of the cervical spine and hook-liked and anterior beak protrusion of the thoracolumbar spine were observed. At this point, a clinical diagnosis of MPS was made. Neurosurgery was recommended for spinal cord decompression, but the parents declined this treatment. At age 8.5 years, the patient had a respiratory tract infection and cardiac arrest. She received CPR, was intubated and subsequently received a tracheostomy. Since then, she had multiple respiratory tract infections requiring ICU admission.
At age 9 years, developmental evaluation revealed delay in all milestones, with her status around the 4 year old level. Her weight was 17 kg (< 3rd centile), and head circumference was 56 cm (>98th centile). Due to severe contraction of hips and knees, neither length nor height could be measured. Clinical findings consisted of coarse facies, hypertelorism, bilateral cloudy cornea, short neck, pectus carinatum, hepatosplenomegaly, and flexion contractures of the elbows (Fig. 1E). She could not sit straight as a result of kyphosis and severe hip dysplasia and was wheel chair bound. Echocardiography showed a malformed aortic valve, and hyperechoic aortic valve annulus and aortic root.
Oral examination at age 9 years showed mixed dentition with large anterior open bite, macroglossia, and multiple dental caries of deciduous teeth (Fig. 1F). Panoramic radiograph showed large anterior open bite, generalized wide root canal spaces, taurodontism of the maxillary left first permanent molar, and malposition of unerupted mandibular permanent molars. The developing mandibular second permanent molars had hyperplastic dental follicles (cystic-like dental follicles). The unerupted first permanent molars had prominent cusps. The roots of the unerupted first permanent molar appeared unusually diverged and seemed to erode the adjacent cortex of the lower border of the mandible. Severely hypoplastic mandibular condyles were observed (Fig. 1G).
Diagnosis was established at age 6.5 years by molecular analysis. At age 9 years, â-glucuronidase analysis showed low activity, 4.45 nmol/hr/mg, 14% of expected enzyme activity (31.4–224 nmol/hr/mg). Mutation analysis of GUSB showed the homozygous base substitution c.1856C>T; corresponding to the amino acid substitution p.Ala619Val. The deduced amino acid change is predicted to be disease-causing by Mutation Taster and SIFT. The parents and the younger brother were heterozygous for the mutation. Allele frequency according to ExAC is 0.00000823. This mutation has been reported to be pathogenic in Japanese patients [Tomatsu et al., 1990, 1991; Yamada at al., 1998; Yamada et al., 2011].
Patient 2
The patient is a female child born to third degree consanguineous parents of Indian origin (Fig. 2A). The couple had two miscarriages and an intrauterine death of the fetus at 7 months of gestation due to severe non-immune hydrops. The clinical findings of this patient were reported when she was 6 months old [Nampoothiri et al., 2008].
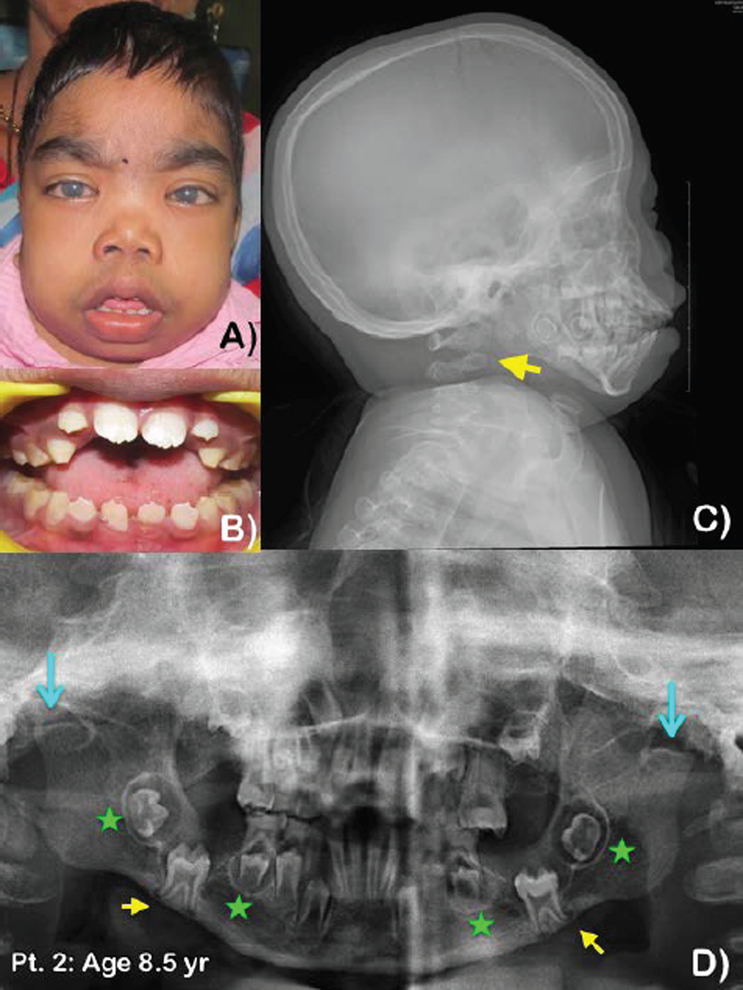
Figure 2. Patient 2 at age 8.5 years.
A) Bilateral cloudy cornea, short neck, hirsutism, and incompetent lip. B) Crowding of teeth with anterior open bite and macroglossia. Pathologic attrition of primary teeth. C) Lateral cephalograph of patient 2 at age 8.5 years. Note thick cranium and absence of the dens of the second cervical spine (arrow). D) Panoramic radiograph of patient 2 at ages 8.5 years. Note generalized wide root canal spaces of the permanent teeth. Anterior open bite, and malposition of unerupted mandibular first permanent molars. Roots of the first permanent molars are slightly diverged. The cortex of the lower border of the mandible near the apices of the molar roots was eroded (yellow arrows). Note hyperplastic dental follicles of the mandibular second permanent molars and the mandibular second premolar (green stars), severely hypoplastic right mandibular condyle, and aplastic left mandibular condyle (blue line arrows).
At age 6 months she presented with a history of poor weight gain, breathlessness, and dysmorphic features. Echocardiography demonstrated thickened mitral valve leaflets with prolapse of the posterior mitral leaflet resulting in severe mitral regurgitation. She had bilateral corneal clouding, hypertelorism, flat nasal bridge, epicanthic folds, a long philtrum and thin upper lip, low-set ears with normally shaped pinnae, a small anterior fontanel, and prominent hypertrichosis (Fig. 2A). She had a hepatomegaly of 6 cm and splenomegaly of 4 cm. Radiographic examination revealed broad ribs, ovoid vertebrae, and mild beaking of the second lumbar vertebra. Pelvis radiograph showed marked hypoplasia of the inferior portions of the ilia, with slanted acetabulae. Her blood β-glucuronidase level was 6.4 nmol/hr/mg, which is only 1.8% of the expected level (350–500 nmol/hr/mg), confirming the diagnosis of MPS VII. Patient 2 was positive for GUSB mutation, which will be published elsewhere. Surgical repair of the mitral valve was not contemplated. Evaluation at 8.5 years showed severe motor and mental regression. Ability to communicate and mobility were very limited.
Oral examination at age 8.5 years showed early mixed dentition with anterior open bite, macroglossia, and pathologic attrition of primary molars, most likely from bruxism (Fig. 2B). Panoramic radiograph showed a large anterior open bite and malposition of the unerupted mandibular first permanent molars. The root apices of the first permanent molars appeared slightly diverged and seemed to erode the adjacent cortex of the lower border of the mandible. Hyperplastic dental follicles of the mandibular second permanent molars and the mandibular second premolars were noted. The heads of the mandibular condyles were severely hypoplastic on the right and aplastic on the left (Fig. 2D). Lateral cephalograph showed thick cranium and absence of the dens of the second cervical vertebra (Fig. 2C).
Patient 3
The patient was born at term with weight 3400 g (50th centile) to a non consanguineous couple. He was hospitalized for 4 months after birth due to aspiration pneumonia, recurrent pneumonia and severe gastroesophageal reflux, and required fundoplication and gastrostomy before he went home. He had mildly delayed development. He sat with support at 10 months, walked at 2 years and talked at 2.5 years of age. He was diagnosed with MPS VII at age 4 years. Physical examination at that time revealed a head circumference of 53 cm (>97th centile), height of 97.5 cm (25th centile), and weight of 16 kg (50th centile). Coarse face, frontal bossing, bilateral cloudy cornea, and claw hands were also observed. He also had otitis media with effusion, which required myringotomy. His blood β-glucuronidase activity was 2.88 nmol/mg prot/hr (by National Taiwan University Hospital, normal range 110.3+/−32.4 nmol/mg prot/hr). At age 15 years old, he had severe adenotonsillar hypertrophy and severe obstructive sleep apnea requiring BiPAP. His echocardiography showed mild thickening of the aortic valve and moderate aortic regurgitation. An MRI showed fracture at the posterior superior aspect of the vertebral body of L5 with diffuse bulging disc compromised thecal sac.
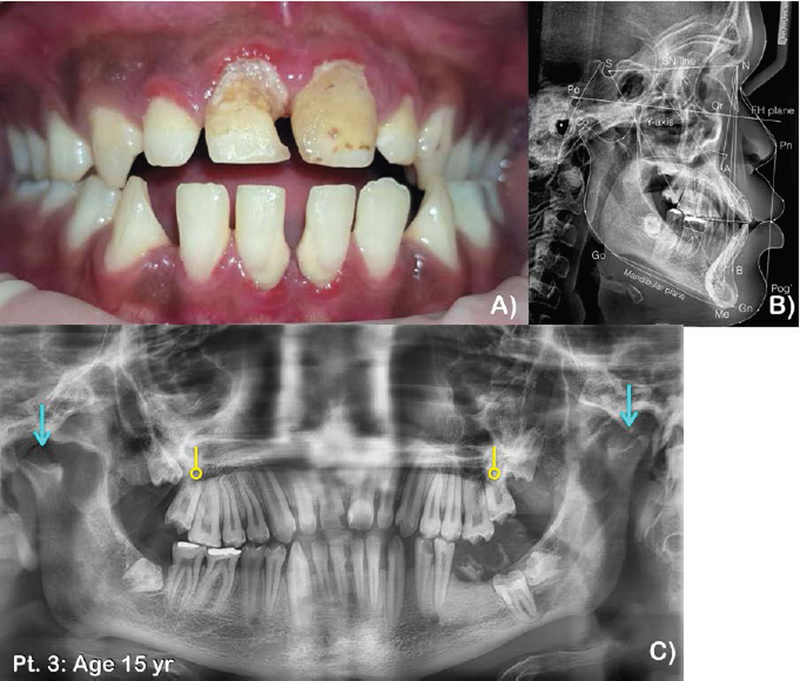
Oral manifestations at age 15 years consisted of permanent dentition with anterior open bite. Multiple dental decay and severe gingivitis were observed. The maxillary permanent molars and mandibular left second mandibular molar had not erupted (Fig. 3A). Panoramic and lateral cephalometric radiographs showed unerupted third permanent molars and maxillary second permanents. Generalized wide root canal spaces and taurodontism of the maxillary second permanent molars were observed. Mandibular condyles were severely hypoplastic (Fig. 3B,C).
Figure 3. Patient 3 at age 15 years.
A) Frontal view of teeth. Anterior open bite and posterior crossbite. Multiple dental caries and gingivitis are noted. B) Lateral cephalograph of patient 3 at age 15 years. Note maxillary hypoplasia, hypoplastic midface, anterior open bite, hypoplastic mandibular condyle, and unerupted molars. C) Panoramic radiograph of patient 3 at age 15 years. Permanent dentition with anterior open bite. Note generalized wide root canal spaces of the permanent teeth, taurodontism of the maxillary second permanent molars (circle-ended arrows), anterior open bite, malposition of unerupted maxillary and mandibular permanent molars, and retained roots of the mandibular left first permanent molar. Mandibular condyles are severely hypoplastic (blue line arrows).
Mutation analysis of GUSB showed compound heterozygous mutations of c.647G>A (p.Arg216Gln), c.1270C>T (p.His 424Tyr). The amino acid change p.Arg216Gln is predicted as disease-causing or damaging by Mutation Taster, SIFT, and PolyPhen-2. SIFT predicted the amino acid change p.His 424Tyr to be damaging. Both variants are not found in ExAC, gnomAD, and 1000 Genome.
Oral manifestations in dogs affected with MPS VII
Materials and methods
With Institutional Animal Care and Use Committee approval, MPS VII dogs were raised at the School of Veterinary Medicine at the University of Pennsylvania under NIH and USDA guidelines for the care and use of animals in research. As in humans, MPS VII in dogs is inherited as an autosomal recessive trait. MPS VII dogs have a mutation in the GUSB gene resulting in a single amino acid substitution that leads to production of enzyme with little or no activity. The animals were from a breeding colony originally established from a single heterozygous animal [Haskin et al 1984]. MPS VII-affected and heterozygous control animals (n = 3 each) were euthanized at 6 months-of-age via intravenous injection of pentobarbital (80 mg/kg), and skulls collected postmortem. Skulls were imaged by cone beam computed tomography (CBCT; 3D Accuitomo 170; Morita Corporation, Osaka, Japan). InVesalius (Renato Archer Information Technology Center; Campinas, Brazil) was used to generate three-dimensional surface reconstructions (Fig. 4A) and ImageJ (National Institutes of Health; Bethesda, USA) was used to generate lateral and coronal projections (Fig. 4B–C).
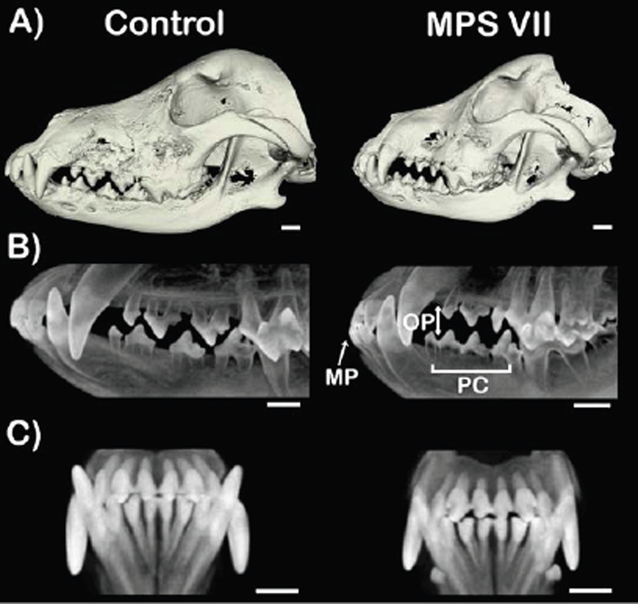
Figure 4. Representative cone beam computed tomography imaging of skulls from control (heterozygous) and MPS VII-affected dogs.
A) Three-dimensional surface reconstructions. Note mandibular prognathism and lateral open bite. Mandibular condyle of the affected dog is unremarkable. B) Lateral projections showing mandibular prognathism (MP), premolar crowding (PC) and posterior open bite (OP) of an MPS VII-affected dog. C) Coronal projections. Scale bar = 10 mm. Note anterior open bite.
RESULTS
From CBCT imaging, of the three MPS VII-affected animals examined, two exhibited mandibular prognathism and premolar crowding, while one of these animals also exhibited anterior and posterior open bite (Fig. 4B,C).
Mutant protein model
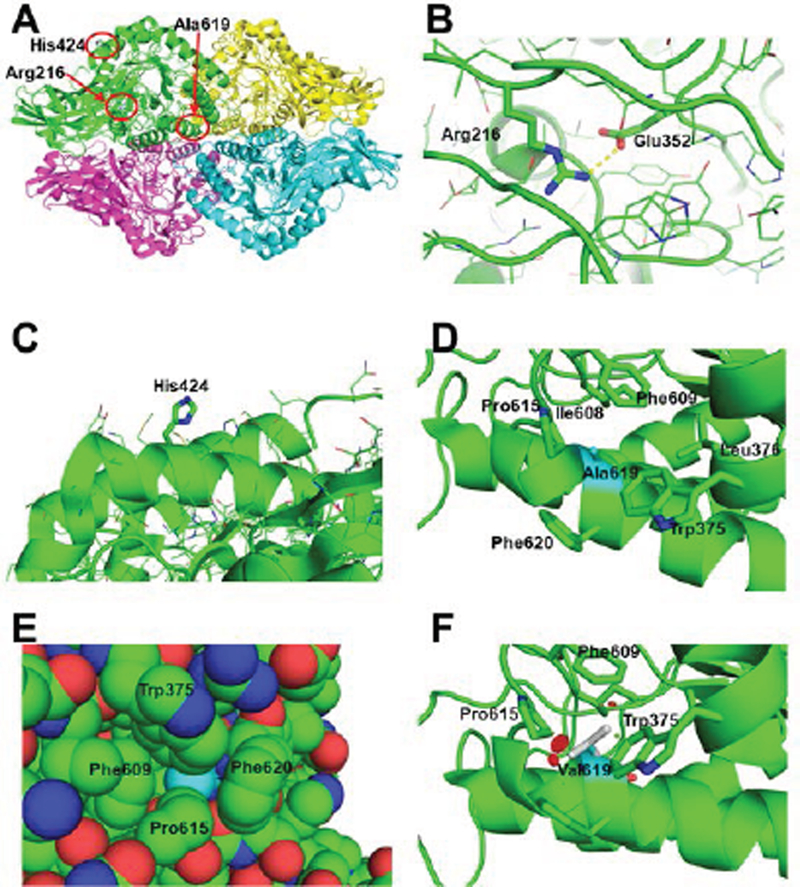
Ala619 is packed tightly between Trp375, Leu376, Ile608, Phe609, Pro615, and Phe620 on the side of the (β/α)8 barrel catalytic domain of human GUS [Jain et al., 1996; Hassan et al., 2013] (Fig. 5A,D,E). Addition of two methyl groups in the side chain of Val (compared to Ala) will create clashes with the surrounding residues, which will require rearrangement of the packing and destabilize the structure (Fig. 5F). The part of the structure including Ala619 interacts closely with two other subunits, so destabilization of this region may destabilize the native tetramer structure, which is required for the activity of the enzyme [Hassan et al., 2013].
Figure 5. Structural basis for the GUSB defects.
A) The quaternary structure of human GUSB (PDB: 3HN3, Hassan et al., 2013), showing the regions of the Arg216Gln, His424Tyr and Ala619Val mutations in subunit A (green) highlighted by the red circles. B) Close up of the interaction of the Arg216 residue with Glu352 (illustrated as thicker sticks). The yellow dotted line represents a hydrogen bond (2.8 Å). The sidechains of other amino acids in the region are shown as line drawings. C) Zoom of the surface area of His424 (thick sticks) with the side chains of surrounding residues shown in line drawings. D) Close-up of the area of Ala619 (in cyan carbons, while other residues are with green carbons) in human GUSB. The neighboring residues producing the hydrophobic pocket containing Ala619 are shown with stick sidechains, including Trp375, Leu376, Ile608, Phe609, Pro615, and Phe620. E) van der Waals space filling representation of Ala619 (cyan) and surrounding residues, showing it is tightly packed inside the structure. F) One of three possible rotomers of the mutated Val619, showing clashes with surrounding residues (red disks), in this case Pro615, the main chain of the α-helix including Val619 and Phe609. The other two rotomers also clashed with other surrounding residues (not shown).
The Arg216 residue is highly conserved from human to Escherichia coli GUS proteins. In the structure, it is found in a loop region between the á-helical and â-sheet domains, and forms a salt bridge hydrogen bond (2.8 Å) with Glu352 (Fig. 5A,B). Thus, the Arg216Gln mutation, which would remove this interaction, is likely to be highly destabilizing to the protein structure (Fig. 5A,B). In contrast, the His424 residue is not conserved and is projects from a surface á-helix into the surrounding solution with little interaction with other residues. The His424Tyr mutant could conceivably create a hydrophobic patch on the surface of the protein that could cause abnormal protein-protein interactions, or the nucleotide change may promote alternative splicing to generate nonproductive transcripts (Fig. 5A,C).
DISCUSSION
Abnormal tissues and organs in the MPS patients are the result of massive accumulation of GAGs. This accumulation directly obstructs cellular functions or interferes with important genetic signaling pathways crucial for cellular functions and hematopoietic stem-cell growth and differentiation [Gupta et al., 1998; Oussoren et al., 2011]. GAG accumulation in lysosomes also interferes with the function of lysosomes as end-station of the endocytic and autophagocytic transport pathways [Oussoren et al., 2011]. GAG accumulation disrupts every system in the body, including bone, cartilage, and tooth development, because GAGs have crucial roles in regulatory functions involving the FGF, BMP, TGF-β, and WNT signaling pathways in tooth, endochondral, and membranous bone ossification [van der Eerden et al., 2003; Pan et al., 2005; Coulson-Thomas, 2016]. Lysosomal GAG storage in chondrocytes disrupts the growth and maintenance of cartilage [Peck et al., 2015]. Its accumulation in osteoblasts, osteocytes, and osteoclasts leads to poor bone remodeling [Oussoren et al 2011]. All lines of evidence show that a number of factors contribute to abnormal bone remodeling and tooth development in MPS patients [Wilson et al., 2009; Wilson and Brömme, 2010].
We report oral manifestations in three patients and three dogs affected with MPSVII. The dental findings found in our patients consist of hyperplastic dental follicles, malposition of unerupted molars, failure of tooth eruption with malformed roots, wide root canal spaces, and taurodontism. The skeletal findings include maxillary hypoplasia, hypoplastic midface, mandibular prognathism, long mandibular length, anterior open bite, hypoplastic and aplastic mandibular condyles, absence of the dens of the second cervical vertebra, and erosion of the cortex of the lower border of the mandibles. The oral findings found in our patients are similar to those of patients with other types of MPS, especially MPS VI [Kayserili and Kantaputra, 2012; Kantaputra et al., 2014a, 2014b].
In MPS VII mice, the cells most affected were those with highest macromolecular turnover, such as presecretory ameloblasts, odontoblasts, and periodontal ligament fibroblasts. Abnormal mineralization of enamel and dentin was also observed in mice affected with MPS VII [Gritli-Linde et al., 1995], but not in our patients or dogs with MPS VII. The large dental follicles are likely to be filled with GAGs, which may result in malposition and failure of tooth eruption [Roberts et al., 1984]. However, we cannot explain why only mandibular molars are affected. In a number of MPS patients, the mandibular first permanent molars failed to erupt and appeared to sit with diverged roots on the lower border of the mandibles. The important anatomical structures near the unerupted permanent molars are the mandibular canal and inferior alveolar nerve. It is hypothesized that the inferior alveolar nerve in the mandibular canal physically obstructs the eruption of the developing mandibular first permanent molars. The proximity of the growing molar root apices and the mandibular cortex may result in diverged or deformed molar roots and abnormal membranous bone remodeling, seen as eroded mandibular cortex in our patients and patients with other types of MPS [Kayserili and Kantaputra, 2012; Kantaputra et al., 2014a, 2014b]. Interestingly, in patient 2, the thick cranium, which is composed of membranous bones, probably was the result of aberrant membranous ossification and bone remodeling [Alliston 2010].
The hypoplastic or aplastic mandibular condyles found in our patients is a very common finding in MPS patients. This is likely the result of GAG accumulation in the condylar cartilage, because GAG accumulation results in increased inflammation and chondrocyte apoptosis [Simonaro et al., 2001]. The aplasia of the dens or the odontoid process, a continuation upward of the cartilaginous mass of the second cervical spine, observed in patient 2 is most likely a result of abnormal endochondral ossification [Morishita and Petty, 2011]. This anomaly makes the patient predisposed to atlanto-axial instability, and thus spinal cord compression and neurological complications, most commonly spastic tetraparesis [Pizzutillo et al., 1989; White 2011]. Generalized wide root canal spaces in all three patients and taurodontism in patients 1 and 3 suggest that alteration of root formation might be a primary effect of GUSB mutations or a secondary effect from the accumulation of GAGs.
Craniofacial abnormalities in MPS VII dogs (juvenile and adult) as assessed using plain radiographs have been reported previously to include shorter and wider skulls, and hypoplastic condyles and dental enamel [Sheridan et al., 1994]. Here, high-resolution CBCT imaging of 6-month-old MPS VII dogs identified craniofacial anomalies including maxillary hypoplasia, mandibular prognathism, premolar crowding, and anterior and posterior open bite. This is the first time that CBCT was used to study craniofacial structures of dogs with MPS VII. Dogs with MPS VII evidently had milder manifestations than those of humans. Unlike those of humans, dogs with MPS VII did not have dental anomalies. Abnormalities of craniofacial structures in dogs with MPS VII, including mandibular prognathism, open bite, maxillary hypoplasia, and premolar crowding, appeared to be the effects of abnormal ossification of endochondral and membranous bones. Open bite and premolar crowding are secondary manifestations of the abnormal maxilla and mandible.
Primary dentition of our patients and other previously reported patients with all types of MPS were unremarkable. In addition, the hypoplastic mandibular condyles, which are the most common craniofacial anomaly of patients with all types of MPS, were normal in our dogs with MPS VII. We hypothesize that the formation of human primary teeth and teeth and mandibular condyles in dogs are likely to have completed before the massive GAG accumulation has its adverse effects.
CONCLUSION
This is the first report of oral manifestations in patients affected with MPS VII. These include hyperplastic dental follicles, malposition of unerupted molars, failure of tooth eruption with malformed roots, wide root canal spaces, and taurodontism. The skeletal findings include maxillary hypoplasia, hypoplastic midface, mandibular prognathism, long mandibular length, hypoplastic and aplastic mandibular condyles, absence of the dens of the second cervical vertebra, and erosion of the cortex of the lower border of the mandibles. These findings are also found in patients affected with other types of MPS, which suggests that accumulation of any type of GAG has similar detrimental effects on development of teeth, mandibular condyles, and bone remodeling. Dogs had milder manifestations, which is probably due to the early complete formation of teeth and mandibular condyles in dogs.
Acknowledgments
We thank our patients and their families for their kind cooperation and for allowing us to use their medical and dental information for the benefit of others. This work was supported by the Center of Excellence in Medical Genetics Research, Chiang Mai University; the Thailand Research Fund (TRF); Dental Association of Thailand; the Faculty of Dentistry, Chiang Mai University; and the National Institutes of Health (R01AR071975, R01DK054481, R03AR065142 and P40OD010939). The authors acknowledge the assistance of Yian Kai Lau and Marianne Contino with CBCT imaging, and the staff at the University of Pennsylvania School of Veterinary Medicine for animal care.
Footnotes
Conflict of interest: All authors declare no conflict of interest.
REFERENCES
- Alliston T 2010. Chondroitin sulfate and growth factor signaling in the skeleton: possible links to MPS VI. J Pediatr Rehabil Med 3:129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson-Thomas VJ. 2016. The role of heparan sulphate in development: the ectodermal story. Int J Exp Pathol 97:213–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritli-Linde A, Linde A, Goldberg M. 1995. Morphological alterations in dental and periodontal tissues in murine mucopolysaccharidosis type VII. Calcif Tissue Int 57:178–184. [DOI] [PubMed] [Google Scholar]
- Gupta P, Oegema TR Jr, Brazil JJ, Dudek AZ, Slungaard A, Verfaillie CM. 1998. Structurally specific heparan sulfates support primitive human hematopoiesis by formation of a multimolecular stem cell niche. Blood 92:4641–4651. [PubMed] [Google Scholar]
- Haskins ME, Desnick RJ, Di Ferrante N, Jezyk PF, Patterson DF. 1984. Beta-glucuronidase deficiency in a dog: a model of human mucopolysaccharidosis VII. Pediatr Res 18: 980–984. [DOI] [PubMed] [Google Scholar]
- Hassan MI, Waheed A, Grubb JH, Klei HE, Korolev S, Sly WS. 2013. High resolution crystal structure of human β-glucuronidase reveals structural basis of lysosome targeting. PLoS ONE 8: e79687. doi: 10.1371/journal.pone.0079687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Drendel WB, Chen ZW, Mathews FS, Sly WS, Grubb JH. 1996. Structure of human beta-glucuronidase reveals candidate lysosomal targeting and active-site motifs. Nat Struct Biol 3:375–381. [DOI] [PubMed] [Google Scholar]
- Kantaputra PN, Kayserili H, Güven Y, Kantaputra W, Balci MC, Tanpaiboon P, Uttarilli A, Dalal A. 2014a. Oral Manifestations of 17 Patients Affected with Mucopolysaccharidosis Type VI. J Inherit Metab Dis 37:263–268. [DOI] [PubMed] [Google Scholar]
- Kantaputra PN, Kayserili H, Guven Y, Kantaputra W, Balci MC, Tanpaiboon P, Tananuvat N, Uttarilli A, Dalal A. 2014b. Clinical manifestations of 17 patients affected with mucopolysaccharidosis type VI and 8 novel ARSB mutations. Am J Med Genet Part A 164A:1443–1453. [DOI] [PubMed] [Google Scholar]
- Kayserili H, Kantaputra PN. 2012. Multiple supernumerary molars, anterior open bite, and large ear lobules in mucopolysaccharidosis Type VI patient. Am J Med Genet Part A 158A:1798–1800. [DOI] [PubMed] [Google Scholar]
- Morishita K, Petty RE. 2011. Musculoskeletal manifestations of mucopolysaccharidoses. Rheumatology (Oxford) 50 Suppl 5:v19–25. [DOI] [PubMed] [Google Scholar]
- Nampoothiri S, Kappanayil M, Hiran KR, Sunitha V. 2008. Sly Disease: Mucopolysaccharidosis Type VII. Indian Pediatr 45:859–861. [PubMed] [Google Scholar]
- Oussoren E, Brands MM, Ruijter GJ, der Ploeg AT, Reuser AJ. 2011. Bone, joint and tooth development in mucopolysaccharidoses: relevance to therapeutic options. Biochim Biophys Acta 1812:1542–1556. [DOI] [PubMed] [Google Scholar]
- Pan C, Nelson MS, Reyes M, Koodie L, Brazil JJ, Stephenson EJ, Zhao RC, Peters C, Selleck SB, Stringer SE, Gupta P. 2005. Functional abnormalities of heparan sulfate in mucopolysaccharidosis-I are associated with defective biologic activity of FGF-2 on human multipotent progenitor cells. Blood 106:1956–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck SH, O’Donnell PJ, Kang J, Shore EM, Pacifici M, Dodge GR, Haskins ME, Malhotra NR, Smith LJ. 2015. Delayed Hypertrophic Differentiation of Epiphyseal Chondrocytes Contributes to Failed Bone Formation in Mucopolysaccharidosis VII Dogs. Molecular Genetics and Metabolism 116:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzutillo PD 1, Osterkamp JA, Scott CI Jr, Lee MS. 1989. Atlantoaxial instability in mucopolysaccharidosis type VII. J Pediatr Orthop 9:76–78. [DOI] [PubMed] [Google Scholar]
- Roberts MW, Barton NW, Constantopoulos G, Butler DP, Donahue AH. 1984. Occurrence of multiple dentigerous cysts in a patient with the Maroteaux-Lamy syndrome (mucopolysaccharidosis, type VI). Oral Surg Oral Med Oral Pathol 58:169–175. [DOI] [PubMed] [Google Scholar]
- Simonaro CM, Haskins ME, Schuchman EH. 2001. Articular chondrocytes from animals with a dermatan sulfate storage disease undergo a high rate of apoptosis and release nitric oxide and inflammatory cytokines: a possible mechanism underlying degenerative joint disease in the mucopolysaccharidoses. Lab Invest 81:1319–1328. [DOI] [PubMed] [Google Scholar]
- Sheridan O, Wortman J, Harvey C, Hayden J, Haskins M 1994. Craniofacial abnormalities in animal models of mucopolysaccharidoses I, VI, and VII. J Craniofac Genet Dev Biol 14: 7–15. [PubMed] [Google Scholar]
- Sly WS, Quinton BA, McAlister WH, Rimoin DL. 1973. Beta-glucuronidase deficiency: report of clinical, radiologic, and biochemical features of a new mucopolysaccharidosis. J Pediatr 82:249–257. [DOI] [PubMed] [Google Scholar]
- Tomatsu S, Fukuda S, Sukegawa K, Ikedo Y, Yamada S, Yamada Y, Sasaki T, Okamoto H, Kuwahara T, Yamaguchi S, Kiman T, Shintaku H, Isshiki G, Orii T.1991. Mucopolysaccharidosis type VII: characterization of mutations and molecular heterogeneity. Am J Hum Genet 48:89–96. [PMC free article] [PubMed] [Google Scholar]
- Tomatsu S, Montano AM, Dung VC, Grubb JH, Sly WS. 2009. Mutations and polymorphisms in GUSB gene in mucopolysaccharidosis VII (Sly Syndrome). Hum Mutat 30:511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomatsu S, Sukegawa K, Ikedo Y, Fukuda S, Yamada Y, Sasaki T, Okamoto H, Kuwabara T, Orii T. 1990. Molecular basis of mucopolysaccharidosis type VII: replacement of Ala619 in beta-glucuronidase with Val. Gene 89:283–287. [DOI] [PubMed] [Google Scholar]
- van der Eerden BC, Karperien M, Wit JM. 2003. Systemic and local regulation of the growth plate. Endocr Rev 24:782–801. [DOI] [PubMed] [Google Scholar]
- White KK. 2011. Orthopaedic aspects of mucopolysaccharidoses. Rheumatology (Oxford) 50 Suppl 5:v26–33. [DOI] [PubMed] [Google Scholar]
- Wilson S, Hashamiyan S, Clarke L, Saftig P, Mort J, Dejica VM, Brömme D. 2009. Glycosaminoglycan-mediated loss of cathepsin K collagenolytic activity in MPS I contributes to osteoclast and growth plate abnormalities. Am J Pathol 175:2053–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S, Brömme D. 2010. Potential role of cathepsin K in the pathophysiology of mucopolysaccharidoses. J Pediatr Rehabil Med 3:139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Kato K, Sukegawa K, Tomatsu S, Fukuda S, Emura S, Kojima S, Matsuyama T, Sly WS, Kondo N, Orii T. 1998. Treatment of MPS VII (Sly disease) by allogeneic BMT in a female with homozygous Ala619Val mutation. Bone Marrow Transplant 21:629–634. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Kato K, Sukegawa K, Tomatsu S, Fukuda S, Emura S, Kojima S, Matsuyama T, Sly WS, Kondo N, Orii T. 2011. Large proteoglycan complexes and disturbed collagen architecture in the corneal extracellular matrix of mucopolysaccharidosis type VII (Sly syndrome). Invest Ophthalmol Vis Sci 52:6720–6728. [DOI] [PubMed] [Google Scholar]