Abstract
The fatty acid profiles of wild freshwater fish are poorly characterized as a human food source for several classes of fatty acids, particularly for branched chain fatty acids (BCFA), a major bioactive dietary component known to enter the US food supply primarily via dairy and beef fat. We evaluated the fatty acid content of 27 freshwater fish species captured in the northeastern US with emphasis on the BCFA and bioactive polyunsaturated fatty acids (PUFA) most associated with fish, specifically n-3 (omega-3) eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Mean BCFA content across all species was 1.0 ± 0.5% (mean ± SD) of total fatty acids in edible muscle, with rainbow smelt (Osmerus mordax) and pumpkinseed (Lepomis gibbosus) the highest at >2% BCFA. In comparison, EPA + DHA constituted 28% ± 7% of total fatty acids. Across all fish species, the major BCFA were iso-15:0, anteiso-15:0, iso-16:0, iso-17:0 and anteiso-17:0. Fish skin had significantly higher BCFA content than muscle tissues, at 1.8% ± 0.7%, but lower EPA and DHA. Total BCFA in fish skins was positively related with that in muscle (r2 = 0.6). The straight chain saturates n-15:0 and n-17:0 which have been identified previously as markers for dairy consumption were relatively high with means of 0.4% and 0.6%, respectively, and may be an underappreciated marker for seafood intake. Consuming a standardized portion, 70 g (2.5 oz), of wild freshwater fish contributes only small amounts of BCFA, 2.5–24.2 mg, to the American diet, while it adds surprisingly high amounts of EPA + DHA (107 mg to 558 mg).
Keywords: branched chain fatty acids (BCFA), fish, skin, DHA, EPA, n-3, northeastern United States
INTRODUCTION
Finfish are known as a lean source of protein and n-3 (omega-3) long chain polyunsaturated fatty acids (LCPUFA) accessible to both developed and developing countries. Global per capita consumption of fish has increased from 9.9 to 19.2 kg in the past 50 years, and has grown faster than the rate of world population expansion in the most recent decade, owing to the rapid expansion of aquaculture especially in Asian countries.1 The northeastern United States has many lakes and streams with a variety of native fish species. Common fishes utilized for food include walleye (Sander vitreus), white perch (Morone americana), yellow perch (Perca flavescens), lake trout (Salvelinus namaycush), salmon species, and channel catfish (Ictalurus punctatus) in addition to dozens of other species less commonly consumed, despite potentially being nutritious and palatable.
From a health perspective, omega-3 docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) are a main motivation for higher fish consumption. Apparent DHA/EPA deficiencies are corrected by fish consumption and are connected to lower risk of coronary heart disease in a series of prospective observational studies.2 Omega-3 LCPUFA are also linked to improved cognitive abilities in children3 and visual acuity in infants.4 The Dietary Guidelines for Americans recommends 8–12 ounces/week of seafood,5 particularly for pregnant and lactating women because placental and breast milk transfer depletes DHA starting at the beginning of pregnancy.6 The American Heart Association also recommends seafood consumption, especially for secondary cardiovascular disease prevention.
While the content and health benefits of DHA in fish are widely studied, branched chain fatty acids (BCFA) in fish are less well characterized. BCFA are mostly saturated fatty acids with a terminal propan-2-yl (isopropyl) group (iso) or butan-2-yl (sec-butyl) group (anteiso)7 (Figure S1). Those with 14 to 18 carbons in the chain are most common in the US food supply as components of dairy and beef fat.8 Reports of BCFA levels in fish are typically below 5% though they vary greatly, from low ranges of 0.3%–1.5% in fish caught near Senegal9 to a surprisingly high 40% in flathead gray mullet (Mugil cephalus) captured in a mangrove estuary.10 Chinese carp species cultured for food fish had BCFA ranging from 1.8 to 4%,11 while common carp (Cyprinus carpio) captured in Madagascar had 4–5% BCFA.12 Ongoing research shows that BCFA are active compounds with bioactivity benefits, such as development of gut microbiota,13 and antitumor14 effects.
Fish skin is considered an integral part of a food fish in many parts of the world but is less appreciated in the US. As a result, fish skin poses a waste problem in the fish processing industry in the US. However, fish skin can be nutritionally valuable in terms of healthy lipids. Fish skin has more total lipid per unit mass and a significant amount of n-3 LCPUFA.9,15 We are aware of only one report of the BCFA content of fish skin, showing about 0.3% iso-15:0 and 0.3% iso-17:0 in skins of three edible fish from the Senegalese coast.9
Our purpose was to characterize the fatty acid profile of common fish from the New York State area as a representative of the fresh waters of the northeastern US. For the first time, we emphasize the full range of food fatty acids in wild caught freshwater fish, specifically BCFA and odd-carbon-numbered as well as the much better studied omega-3 EPA and DHA. From these data, we quantify BCFA intake from fish and compare it to other diet components and shed new light on the origin of odd chain fatty acids as a biomarker of intake in humans in the context of highly cited associations with cardiovascular disease. We also compare these US fish of known origin to previous reports of very high BCFA levels from fish caught in Asia.
MATERIALS AND METHODS
Sampling.
Twenty-seven species of wild fishes were caught in Oneida Lake, Cayuga Lake, Whitney Point Reservoir, the Adirondack region, and some creeks in the states of New York and Pennsylvania. Locations and dates of capture, length, sample size, and dietary information on each of the fish species are presented in Table S1. Fish are listed from highest to lowest muscle total BCFA content, which is presented in Table 1. Fish were identified by fish biologists from Cornell University and the New York State Department of Environmental Conservation. Fish were put into a cooler packed with ice upon capture and transported to Cornell University immediately. All fish were kept at −80 °C until processing.
Table 1.
Weight Percent for BCFA and Major PUFA of 27 Fish Species Caught in the Northeastern United States (Muscle)
| fatty acid wt % | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| iso-13:0 | ai-13:0 | iso-14:0 | iso-15:0 | ai-15:0 | iso-16:0 | iso-17:0 | ai-17:0 | iso-18:0 | ai-19:0 | iso-20:0 | total BCFA | ARA | EPA | DHA | |
| rainbow smelt | 0.03 | 0.46 | 0.20 | 0.18 | 0.29 | 0.56 | 0.38 | 0.06 | 2.17 | 5.72 | 9.97 | 26.44 | |||
| pumpkinseed | 0.01 | 0.01 | 0.15 | 0.10 | 0.16 | 0.73 | 0.68 | 0.03 | 0.19 | 2.07 | 9.91 | 8.00 | 15.41 | ||
| white sucker | 0.01 | 0.15 | 0.08 | 0.16 | 0.61 | 0.44 | 0.11 | 0.04 | 0.03 | 1.64 | 11.64 | 8.59 | 14.69 | ||
| lake trout | 0.13 | 0.05 | 0.11 | 0.65 | 0.45 | 0.12 | 0.06 | 1.58 | 5.44 | 7.25 | 24.61 | ||||
| freshwater drum | 0.04 | 0.27 | 0.05 | 0.13 | 0.39 | 0.18 | 0.06 | 0.06 | 1.19 | 16.15 | 14.11 | 4.95 | |||
| alewife | 0.01 | 0.17 | 0.08 | 0.13 | 0.44 | 0.23 | 0.06 | 0.04 | 1.17 | 5.43 | 9.79 | 15.15 | |||
| common shiner | 0.03 | 0.02 | 0.02 | 0.16 | 0.23 | 0.13 | 0.27 | 0.21 | 0.06 | 1.13 | 12.59 | 11.16 | 10.16 | ||
| white crappie | 0.07 | 0.23 | 0.04 | 0.10 | 0.47 | 0.14 | 0.07 | 1.12 | 15.15 | 5.77 | 14.65 | ||||
| walleye | 0.03 | 0.16 | 0.04 | 0.08 | 0.38 | 0.20 | 0.18 | 0.01 | 1.08 | 9.47 | 6.44 | 21.46 | |||
| channel catfish | 0.14 | 0.04 | 0.13 | 0.34 | 0.24 | 0.07 | 0.05 | 1.01 | 8.46 | 8.18 | 15.66 | ||||
| greater redhorse | 0.01 | 0.11 | 0.09 | 0.09 | 0.35 | 0.25 | 0.09 | 0.99 | 5.84 | 15.43 | 14.41 | ||||
| black crappie | 0.01 | 0.12 | 0.05 | 0.07 | 0.43 | 0.10 | 0.09 | 0.08 | 0.95 | 17.17 | 9.14 | 11.49 | |||
| smallmouth bass | 0.03 | 0.16 | 0.04 | 0.10 | 0.38 | 0.20 | 0.04 | 0.95 | 14.14 | 4.95 | 19.38 | ||||
| golden shiner | 0.03 | 0.10 | 0.03 | 0.07 | 0.55 | 0.14 | 0.91 | 10.49 | 12.40 | 16.74 | |||||
| slimy sculpin | 0.02 | 0.01 | 0.04 | 0.18 | 0.15 | 0.03 | 0.27 | 0.20 | 0.02 | 0.91 | 8.01 | 17.36 | 17.78 | ||
| brown bullhead | 0.04 | 0.10 | 0.11 | 0.06 | 0.39 | 0.12 | 0.05 | 0.88 | 14.02 | 7.77 | 16.52 | ||||
| redbreast sunfish | 0.21 | 0.04 | 0.12 | 0.26 | 0.23 | 0.87 | 10.60 | 6.20 | 18.69 | ||||||
| blacknose dace | 0.01 | 0.01 | 0.01 | 0.10 | 0.11 | 0.09 | 0.16 | 0.22 | 0.02 | 0.74 | 4.50 | 11.62 | 24.04 | ||
| rock bass | 0.10 | 0.03 | 0.07 | 0.27 | 0.11 | 0.09 | 0.04 | 0.01 | 0.72 | 7.88 | 9.69 | 24.94 | |||
| longnose dace | 0.05 | 0.09 | 0.02 | 0.08 | 0.06 | 0.07 | 0.10 | 0.18 | 0.07 | 0.71 | 3.47 | 16.8 | 22.93 | ||
| fantail darter | 0.01 | 0.02 | 0.13 | 0.09 | 0.03 | 0.20 | 0.16 | 0.02 | 0.66 | 4.58 | 20.61 | 18.23 | |||
| bowfin | 0.07 | 0.07 | 0.05 | 0.29 | 0.18 | 0.65 | 12.35 | 10.69 | 13.76 | ||||||
| chain pickerel | 0.07 | 0.08 | 0.06 | 0.28 | 0.11 | 0.61 | 11.62 | 9.54 | 29.65 | ||||||
| white perch | 0.04 | 0.15 | 0.12 | 0.03 | 0.12 | 0.08 | 0.03 | 0.58 | 13.44 | 15.09 | 16.31 | ||||
| burbot | 0.08 | 0.06 | 0.04 | 0.29 | 0.09 | 0.56 | 21.37 | 7.37 | 13.90 | ||||||
| yellow perch | 0.14 | 0.03 | 0.09 | 0.17 | 0.10 | 0.03 | 0.56 | 10.82 | 9.03 | 17.01 | |||||
| bluegill | 0.13 | 0.10 | 0.05 | 0.13 | 0.12 | 0.52 | 15.18 | 5.11 | 15.39 | ||||||
| mean CVb (%) | 92 | 54 | 81 | 24 | 42 | 36 | 28 | 24 | 68 | 53 | 151 | 19 | 13 | 13 | 14 |
ai: anteiso-methyl fatty acid. ARA: arachidonic acid. EPA: eicosapentaenoic acid. DHA: docosahexaenoic acid. Blank entries are those below our detection limit.
Mean coeffcient of variation (CV) for any specific fatty acid is obtained by first calculating CV for each species and then taking the mean of all species.
Fatty Acid Analysis.
Two hundred milligrams of fish muscle at the dorsal fin, caudal fin, and belly and 50 mg of skin were homogenized, placed into separate glass tubes, and extracted and methylated by a modified one-step hydrolysis and methylation procedure, as described previously.16 Tricosanoic acid (23:0) was added quantitatively and served as internal standard to calibrate areas to mg FA in sample after response correction (Sigma Chemical Company). Reported total fat content reflects fatty acids, without a correction for nonfatty acid lipid components. Fatty acid methyl esters (FAME) were analyzed as previously discussed.17 Briefly, a BPX-70 capillary column (25 m × 0.22 mm × 0.25 μm; SGE) with H2 as carrier gas was installed in a HP 5890 gas chromatograph with a flame ionization detector (GC-FID), which was used for quantitative analysis. A FAME mixture of equal weight (GLC462; Nu-Chek Prep, Inc.) was used to calculate response factors, and six BCFA were used as authentic reference standards (iso-14:0, anteiso-15:0, iso-16:0, anteiso-17:0, iso-18:0, and iso-20:0; Larodan Fine Chemicals AB). Table S2 presents the retention time of a sample and both standards from the same run. Concentrations of all FA are expressed as %, w/w. FAME identities were determined by chemical ionization, electron ionization (EI) mass spectrometry (MS), and BCFA structures were verified by EIMS/MS as described previously18 using a Varian Star 3400 GC coupled to a Varian Saturn 2000 ion trap MS. Briefly, in MS-2 of the rearranged molecular ion, collisional activation of iso-BCFA yields a characteristic [M – 43] ion corresponding to isopropyl cleavage and anteiso-BCFA yields two ions, [M – 29] and [M – 57], corresponding to cleavage on either side of the methyl branch. The mass spectrometry parameters are as follows. M + ions for BCFAME were isolated for fragmentation in EIMS2 mode. The ionization mode was set to “EI auto mode” using the default parameters set by the Varian Saturn software V5.5.2. Ion preparation parameters were as follows: isolation window 3.0 amu; waveform type residence; excitation storage level was calculated using a q value of 0.215; excitation amplitude was set to 0.80 V. Segment set point parameters were as follows: scan rate 1 s; count threshold 1; emission current 0.5 μA. All spectra were collected under identical instrument settings, including collision energy (excitation amplitude) and mass isolation window. In our hands, these conditions provided suitable fragment intensities across all BCFAME without the need to customize parameters.
Statistical Analysis.
Fatty acid compositions were analyzed with ANOVA and paired sample t tests carried out in JMP Pro 12 software for windows. Specifically, select fatty acids common to all fish species 14:0, 15:0, 16:0, 16:1n-7, anteiso-17:0, 17:0, 18:0, 18:1n-9, 18:2n-6, 18:3n-3, 18:4n-3, 20:4n-6, 20:5n-3, 22:6n-3, and total BCFA were investigated with ANOVA to detect differences in tissue types. Three specific fatty acid classes, saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), and polyunsaturated fatty acids (PUFA), were also analyzed for the same purpose. Paired sample t tests between muscle at the dorsal fin and skin were used to verify the fatty acids that showed a significant difference between muscle and skin in the ANOVA test. Coefficient of determination (r2) and the fitted linear model were obtained in Microsoft Excel. ANOVA was performed to determine the significance of the regression. We also used ANOVA to examine the effects of sampling location, habitat type (lake or stream), and foraging guild on percent total BCFA, percent EPA, and percent DHA. We selected models using AIC19 and found that including multiple variables in models and interactions between variables provided little improvement to model suitability. ANOVA of percent BCFA, percent EPA, and percent DHA by sampling location, habitat type, and foraging guild20 were performed in R (version 3.2.2). Significance level was set at p ≤ 0.05 if not otherwise specified.
RESULTS AND DISCUSSION
Fatty Acids of Muscle Tissues in 27 Fish Species.
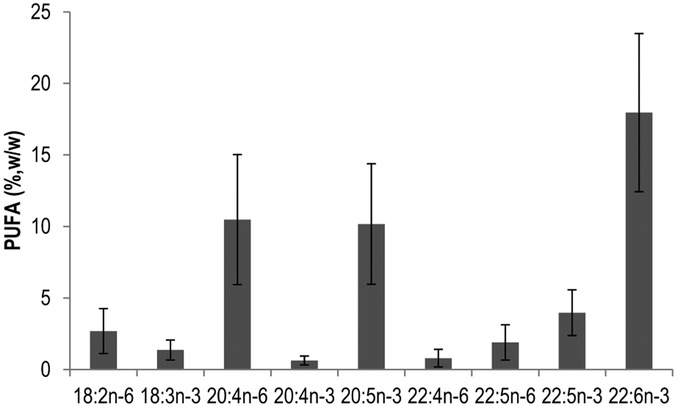
Muscle tissues at the dorsal fin of 27 fish species were analyzed for fatty acids profile. Figure 1 is a summary of the main classes of FA present in the analyzed fish. SFA comprised 31 ± 5% (mean ± SD) of total FA, with two-thirds being palmitic acid (16:0). MUFA were 17 ± 3%, and PUFA were highest at 52 ± 6%. Mean BCFA content was 1.0 ± 0.5% of total FA, or 3.2% of SFA. Figure 2 shows that arachidonic acid (ARA, 20:4n-6), EPA (20:5n-3), and DHA (22:6n-3) were the major PUFA. Total EPA + DHA had a mean of 28 ± 7%, and ARA was 10 ± 5%. Linoleic acid (18:2n-6) and linolenic acid (18:3n-3) comprised 2.7% and 1.4% of total FA separately. Eicosatetraenoic acid (20:4n-3) and adrenic acid (22:4n-6) were both lower than 1%. The isomers of docosapentaenoic acid (22:5n-6 and 22:5n-3) were 1.9% and 4.0%. Full fatty acid profiles and typical chromatograms from GC-FID and EIMS/MS are presented in Table S3 and Figure S2.
Figure 1.
Overall fatty acid (FA) composition (%, w/w; mean ± SD) of 27 fish species in the northeastern United States. FA were grouped as follows: saturated fatty acids (SFA); monounsaturated fatty acids (MUFA); polyunsaturated fatty acids (PUFA); branched chain fatty acids (BCFA).
Figure 2.
Major polyunsaturated fatty acids (PUFA) composition (>0.5%, w/w of total FA; mean ± SD) of 27 fish species in the northeastern United States.
Table 1 shows the BCFA chain length distribution and concentrations as well as concentrations of ARA, EPA, and DHA in the 27 fish species. Fish BCFA had 13–20 carbons with iso-15:0, anteiso-15:0, iso-16:0, iso-17:0, and anteiso-17:0 comprising about 90% of total BCFA by weight. iso-13, anteiso-13, iso-18:0, anteiso-19:0, and iso-20:0 were also found at low concentrations in some fish. In addition, iso-12:0 was detected at trace levels in a few samples (data not shown). Total BCFA mean values ranged from 0.5% to 2.2% of total fatty acids, with about half the species near 1%. Ten fish species that had more than 1% BCFA and rainbow smelt and pumpkinseed exceeded 2% BCFA. All BCFA followed the fragmentation pattern described in Materials and Methods.
Phytanic acid, or 3,7,11,15-tetramethylhexadecanoic acid, is a plant derived polymethyl BCFA. It is widely distributed in nature and has been reported in dairy products21 in addition to the monomethyl BCFA. More than half of fish species had phytanic acid, averaging 0.2% of total FA. Phytanic acid was found in all stream fish analyzed but was not found in most piscivorous fish living in bigger lakes. In our tabulations of BCFA we did not include phytanic acid because its post-ingestion metabolic fate, peroxisomal α-oxidation, is different than for iso and anteiso monomethyl BCFA.
Normal odd-carbon-numbered and trans fatty acids comprised another minor component of all fish. Margaric acid (n-17:0) was the highest odd straight chain FA with an overall mean of 0.6%, w/w. Together with n-15:0, they made up 1% of total FA and were consistently present in all measured fish samples. Additionally, small amounts of 13:0 and 17:1n-8 were present in some fish. Vaccenic acid (trans-11–18:1) varied and in some cases was comparable to oleic acid (cis-9–18:1) in some fish. trans-9–18:1 was detected in some fish at very low levels compared with either oleic acid or vaccenic acid.
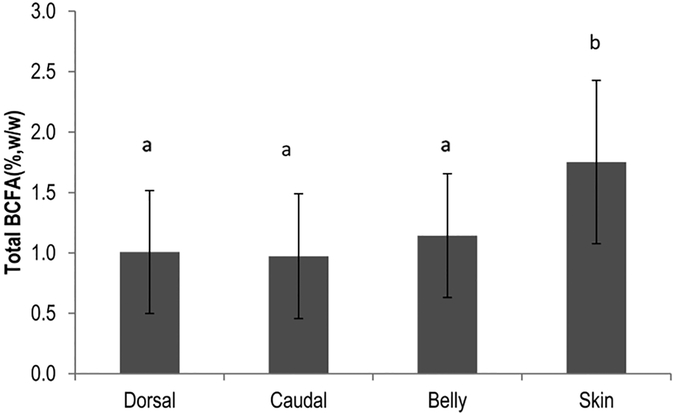
BCFA Are Higher in Fish Skins.
Figure 3 shows the percent BCFA of muscle at dorsal fin, caudal fin, belly, and skin. Fish skins contain significantly more BCFA at a concentration of 1.8 ± 0.7% of total FA. Muscle at three anatomic parts of all fishes is not significantly different, indicating a homogeneous distribution of BCFA in fish muscle. Similar to fish muscle, iso-15:0, anteiso-15:0, iso-16:0, iso-17:0, and anteiso-17:0 were also the major BCFA in fish skins. Full fatty acid profiles of fish skins are presented in Table S4.
Figure 3.
Total BCFA (%, w/w; mean ± SD) for muscle tissues at dorsal fin, caudal fin, belly, and skin.
A linear regression model was used to explore the relationship of BCFA in fish skin and muscle. Total skin and muscle BCFA were strongly correlated (r2 = 0.6, p < 0.001). The significant intercept of 0.7 in Figure 4 suggests that skin incorporate BCFA preferentially when they are available at lower levels, noting also that skin BCFA was greater than muscle BCFA in all samples. For most fish studied, anteiso-17:0 was among the most abundant BCFA and was very highly correlated between skin and muscle levels (r2 = 0.9, p < 0.001).
Figure 4.
Linear regression model between fish skin total BCFA and fish muscle total BCFA (top), p < 0.001; fish skin anteiso-17:0 and fish muscle anteiso-17:0 (bottom), p < 0.001.
The percent weights of most fatty acids in fish skin were generally significantly different than in any part of fish muscle of the same fish. Fish skin had markedly higher concentrations of odd straight chain fatty acids, specifically n-15:0 and n-17:0. Fish skin had 10.7% higher monounsaturated fatty acids (MUFA) but 11.8% lower PUFA by weight than muscle (p < 0.001). Fish skin was significantly lower in EPA and DHA, at 7.3 ± 3.0% and 10.5 ± 4.4%, respectively. Fish skin has the same amount of ARA as belly tissue but slightly lower than muscle at dorsal/caudal fins. Our paired sample t test revealed no significant difference in total saturated straight fatty acids between fish muscle and fish skin, even though both odd straight chain fatty acids and BCFA were significantly higher in fish skins.
BCFA and EPA + DHA Intake from Investigated Fish Species.
While the annual world apparent consumption of fish reached 19.2 kg per capita in 2012,1 consumption in the US has always been higher than the world average. From 2009 to 2011, per capita human consumption of fish and shellfish in the US was 21.7 kg/year, corresponding to 6.6 kg (14.5 lbs) of edible seafood consumed per capita for Americans, equal to a daily intake of 18 g of seafood. The Dietary Guidelines for Americans recommends 8–12 oz of seafood per week, 32–49 g/d, about double the actual intake.22 Table 2 presents the estimated fat content of the 27 species of wild fish investigated here, along with intake of BCFA and EPA + DHA with consumption of about 70 g or a 2.5 oz standardized serving of any of these fish. Fat content ranged from 0.43% to 2.29%, indicating that all of these wild fish would be considered lean fish compared to, for instance, salmon species. Consuming one serving of these locally produced fish provides 2.5 mg to 24.2 mg of total BCFA and 107 mg to 558 mg of total EPA + DHA. This amount of BCFA would increase the estimated American BCFA daily intake of 492 mg8 by only a few milligrams, and thus add a trivial level of BCFA to the diets of those that consume dairy and beef or other ruminant foods. The greatest addition of BCFA to the diet would be from 70 g of alewife skin that could provide at most 12% fat and 207 mg of BCFA, according to our data. We found no evidence of species with BCFA above a few percent in our sampling.
Table 2.
Total Fat Content of 27 Fish Species Caught in the Northeastern United States and BCFA/EPA + DHA Intake from 70 g Fillet (Mean ± STD)
| total fat content (%)a | BCFA (mg) in 70 g fillet | EPA + DHA (mg) in 70 g fillet | |
|---|---|---|---|
| rainbow smelt | 0.74 ± 0.09 | 11.3 ± 3.2 | 189.4 ± 8.1 |
| pumpkinseed | 1.67 ± 0.48 | 24.2 ± 6.0 | 274.5 ± 100.8 |
| white sucker | 1.27 ± 0.75 | 14.6 ± 16.3 | 207.0 ± 155.1 |
| lake trout | 1.55 ± 0.25 | 17.2 ± 2.9 | 346.3 ± 61.6 |
| freshwater drum | 1.05 ± 0.25 | 8.8 ± 1.6 | 140.9 ± 34.2 |
| alewife | 1.09 ± 0.28 | 9.0 ± 1.7 | 191.3 ± 44.4 |
| Common shiner | 0.75 ±0.18 | 6.0 ± 2.7 | 112.2 ± 8.6 |
| white crappie | 1.86 ± 0.45 | 14.6 ± 2.1 | 266.4 ± 50.8 |
| walleye | 1.14 ± 0.31 | 8.6 ± 5.5 | 222.6 ± 44.9 |
| channel catfish | 1.49 ± 0.10 | 10.6 ± 1.4 | 249.2 ± 49.7 |
| greater redhorse | 1.38 ± 0.39 | 9.6 ± 4.4 | 289.9 ± 72.8 |
| black crappie | 1.10 ± 0.13 | 7.4 ± 0.9 | 159.5 ± 20.7 |
| Smallmouth bass | 0.81 ± 0.15 | 5.4 ± 2.0 | 138.2 ± 38.7 |
| golden shiner | 1.34 ± 0.35 | 8.6 ± 0.7 | 273.7 ± 82.5 |
| slimy sculpin | 0.43 ± 0.07 | 2.8 ± 1.5 | 107.0 ± 18.6 |
| brown bullhead | 1.46 ± 0.58 | 9.0 ± 2.8 | 249.4 ± 121.8 |
| redbreast sunfish | 1.48 ± 0.79 | 9.0 ± 6.7 | 258.7 ± 98.2 |
| blacknose dace | 1.08 ± 0.24 | 5.4 ± 2.8 | 271.7 ± 38.5 |
| rock bass | 2.29 ± 0.93 | 11.6 ± 5.7 | 558.4 ± 223.7 |
| longnose dace | 1.08 ± 0.17 | 5.4 ± 0.6 | 302.7 ± 52.8 |
| fantail darter | 0.53 ± 0.10 | 2.5 ± 0.4 | 144.4 ± 21.1 |
| bowfin | 1.77 ± 0.64 | 8.1 ± 2.9 | 304.6 ± 88.0 |
| chain pickerel | 1.20 ± 0.91 | 5.1 ± 4.7 | 329.6 ± 247.9 |
| white perch | 0.74 ± 0.32 | 3.0 ± 1.3 | 164.2 ± 71.6 |
| burbot | 1.83 ± 0.97 | 7.2 ± 4.5 | 273.0 ± 112.7 |
| yellow perch | 0.67 ± 0.16 | 2.6 ± 0.5 | 122.6 ± 23.1 |
| bluegill | 1.05 ± 0.38 | 3.8 ± 1.3 | 151.6 ± 47.2 |
Estimated from total fatty acids without corrections for variable non-fatty acid lipid components.
Unlike dairy products, which contain similar percent of BCFA regardless of total fat content,8 various fish species exhibit a wide range of BCFA and total fat content. For example, rainbow smelt has a relatively low fat content while still providing a high amount of BCFA; on the other hand rock bass is relatively low in percent BCFA but will provide consumers with similar amount of BCFA with much higher % mass of total fat and EPA + DHA. This complicates nutritional assessment of local wild fish consumption, but gives consumers more flexibility in terms of species preference, availability, economic affordability, and nutritional requirements.
BCFA levels of 27 fish species in northeastern United States ranged from 0.5%–2.2%, w/w of total FA, comparable with a few studies,9,11,23 lower than others,12,24 but well below the observed 40% in flathead gray mullet reported elsewhere.10 The high percent BCFA in the mullet could be due to its mangrove habitat and dependence on microbe-rich detritus for food. Five percent BCFA was reported in holothurians (e.g., sea cucumbers), which are considered a delicacy in Asian cuisines.25 Holothurians feed on detritus as well and are consistent with the hypothesis that detritus feeding has a major impact on fish BCFA level. Detritus and marine sediments are enriched with BCFA due to the microbial activity, yielding similar chain length BCFA (C14–18) as are found in fish muscle.26 Our results included more kinds of BCFA than most studies and aligned with results from Lei et al.,11 except they identified a small amount of iso-8:0 in one species and iso-10:0 in another but no iso-20:0 in Asian carps.
Some reports are available on the BCFA content of marine fish. Ackman presented 1.7% and 1.0% BCFA of total saturated fatty acids (SFA) in Atlantic herring (Clupea harengus) and Atlantic cod (Gadus morhua), respectively.27 A relatively high percent of BCFA, from 2.9 to 7.8%, w/w, of total FA, were found in various marine fishes including yellowfin tuna (Thunnus albacares).24b Cosper and Ackman also reported a high percent of BCFA in mummichog (Fundulus heteroclitus) and Atlantic silverside (Menidia menidia) at 6.3% and 2.8%, respectively.24a
Remarkably, EPA and DHA were from about 5% to 20% and 5% to 30% of total FA, respectively. Total EPA and DHA comprised 28% of total FA on average, indicating that freshwater fish are generally very rich in n-3 PUFA, compared with about 20% and 10% EPA + DHA in salmons fed fish oil and palm oil/rapeseed oil, respectively.28 Chain pickerel (Esox niger) had the highest DHA of 30% and EPA of 10% while white sucker (Catostomus commersonii) had 15% DHA and 9% EPA in our study. These values are very close to those of a previous study, which found that a related pickerel species had 27% DHA and 10% EPA and white sucker had 17% DHA and 6% EPA.29
Habitat and ecological factors, such as temperature and foraging mode, respectively, are known to influence fish fatty acid composition.30 In the present work, we found that location, habitat, and foraging guild were associated with EPA but not DHA or BCFA (Table S5). Fish from streams had significantly greater EPA (14.5% ± 4.2%) than fish from lakes (8.8% ± 2.8%), and invertivores had greater EPA (13.7% ± 4.6%) than fish consuming other fish (piscivores) or with mixed diets (8.9% ± 2.8%). Higher EPA in stream invertivores likely originates from diatoms that have high EPA content31 and dominate algal assemblages in the streams we sampled. These data indicate that stream fish in general may be especially good sources of EPA.
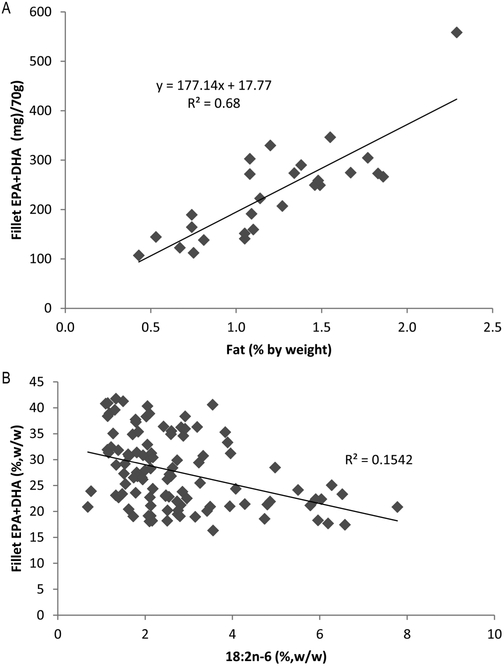
Our study complements a 2014 study that surveyed 76 species of commercially available finfish in six regions of the United States.32 Our study identified the amount of minor BCFA, normal odd-carbon-numbered fatty acids, and major PUFA across wild freshwater fish species around New York State while the previous study reported major fatty acids in wild or cultured, marine or freshwater fish. Channel catfish is the top farmed species in the United States, reported to have 8.0% fat and 31 mg of EPA + DHA in 70 g fillets.32 The wild channel catfish investigated by us had 1.5% fat and 249 mg of EPA + DHA in 70 g fillets. Importantly, wild channel catfish is generally leaner but contains much higher levels of health related omega-3 fatty acids than farmed channel catfish. Most of the wild overlapping species in the two studies shared similar content of EPA + DHA, apart from white perch and lake trout, which had lower EPA and DHA in our study. Total fat content, breeding status of the fish, and age may all explain differences. DHA and EPA levels in 70 g of fillet were strongly and directly correlated with total fat content (Figure 5A, r2 = 0.68). For example, white crappie (Pomoxis annularis) was not among those that had the highest fat percent EPA + DHA, but it was a good source in terms of unit mass of fillet. Total fat content of our fish was relatively low, from 0.4 to 2.3%, even in the trout species. It is generally accepted that wild fish have a much lower total fat than their farmed counterparts.33
Figure 5.
(A) Positive relationship between mass of EPA + DHA in 70 g fillet and percent fat content, p < 0.001. (B) Negative relationship between total EPA + DHA and linoleic acid (18:2n-6), p < 0.001.
Linoleic and linolenic acids are naturally very low in these lean wild fish, comprising only about 5% of total FA on average. Linoleic acid derived from seeds oils and grain based aquaculture feeds results in much greater tissue levels, and suppresses accumulation of omega-3 EPA and DHA.34 Some low EPA + DHA fish such as common shiner (Luxilus cornutus) and bluegill (Lepomis macrochirus) had higher linoleic and linolenic acids. Although there was only a weak negative association (r2 = 0.15, p < 0.001) of linoleic acid and total EPA + DHA, only when linoleic acid was between 1 and 4% of total FA would EPA + DHA level exceed 30%, w/w. On the other hand, when linoleic acid level was >4%, EPA + DHA level was consistently low (Figure 5B). This effect was manifest when farmed channel catfish and wild channel catfish were compared. Farmed channel catfish had 12.3% linoleic acid and 0.5% EPA + DHA, w/w, in the data of Cladis et al.32 In stark contrast, wild channel catfish analyzed in our study had 2.6% linoleic acid and 23.8% EPA + DHA, w/w. The results point to production of channel catfish with a dramatically different nutrient profile than wild catfish. The mean linolenic acid level in our study was about half of linoleic acid, and these two fatty acids were correlated (r2 = 0.45, p < 0.001), consistent with their common terrestrial plant origin.
n-15:0 and n-17:0 were correlated at r2 = 0.57 (p < 0.001), suggesting a common origin of odd numbered straight FA in fish or a tightly controlled elongation process from n-15:0 to n-17:0. It is generally accepted that n-15:0 and n-17:0 are biomarkers for bovine milk intake and n-15:0, at about 0.9–1.2% of total FA, is higher than n-17:0 in milk.35 In contrast, n-17:0 was higher in our analyzed fish samples than n-15:0, in line with other reports.9,11,36 Our mean level of n-17:0 was at 0.59%, w/w. A widely cited meta-analysis of intake and circulating fatty acids association with cardiovascular disease detected n-17:0 as associated with reduced risk, along with EPA, DHA, and ARA. The authors speculated that margaric acid was a biomarker of milk and dairy fat.2 Our data suggest that n-17:0 may be an overlooked biomarker for seafood intake, from both freshwater and marine species as shown by others.9,36b,c Figure 6 presents the level of n-15:0 and n-17:0 in popular seafood in the US and Asia, plotted together with n-15:0 and n-17:0 concentrations in bovine milk. The figure demonstrates that both seafood and milk are sources of odd chain fatty acids. Moreover, the dominance of n-17:0 over n-15:0 in seafood indicates that n-17:0 may be a more specific biomarker for seafood consumption. In contrast, n-15:0 is richer in milk and may be a better indicator for milk and dairy intake.
Figure 6.
n-15:0 and n-17:0 in US freshwater fish in the present study compared to literature data for several fishes popular in the US and Asian and US cow’s milk. Seafood is richer in n-17:0 in all seafood species, while milk is richer in n-15:0. “Tuna” is four common tuna species reported by Roubal;36c “Salmon” is four salmon species reported by Gruger et al.;36b “Sardinella spp.” includes two species reported by Njinkoue et al.;9 “Asian carp” is four common carp species reported by Lei et al.;11 “shrimp” includes three species reported by Bottino et al.;36a “Milk” is US retail milk reported by O’Donnell-Megaro et al.35
Dairy BCFA mainly originate from bacterial fermentation in the rumen.37 Dietary intake or synthesis in oil producing glands analogous to human sebaceous and meibomian glands is more plausible as a mechanism for fish BCFA accumulation. Besides some being piscivorous, fish eat a wide range of aquatic organisms including phytoplankton, zooplankton, macroalgae, and invertebrate and its larvae. Some phytoplankton contain similar BCFA as in fish but at a higher concentration, 3–6%,38 as do various algae,39 mollusks,27,38,40 and shrimps.10,40b,d,41
In conclusion, mean BCFA content was 1.0 ± 0.5% of total FA in muscle of freshwater fish common to the northeastern United States. Fish skin had 1.8 ± 0.7% BCFA of total FA, and linear regression showed that total BCFA content in fish skin is highly correlated with BCFA concentration in fish muscle. Since a serving of fish is about 70 g (2.5 oz), consuming a serving of locally captured fish in the northeastern United States would provide 2.5–24.2 mg of BCFA and 107–558 mg of total EPA + DHA. Because the concentration of BCFA in fish is similar to that in ruminant foods, higher consumption of fish could contribute significant amounts of BCFA. Freshwater fish could be a major source of EPA + DHA in the human diet. Finally n-17:0 was at surprisingly high levels and may be a previously unappreciated biomarker for fish consumption.
Supplementary Material
ACKNOWLEDGMENTS
The contents of this work are solely the responsibility of the author and do not necessarily represent the official views of the NCCAM, ODS, or the National Institutes of Health. We thank an anonymous manuscript referee for helpful comments.
Funding
This work was supported by NIH Grant R01 AT007003 from the National Center for Complementary and Alternative Medicine (NCCAM) and the Office of Dietary Supplements (ODS).
ABBREVIATIONS USED
- ARA
arachidonic acid
- BCFA
branched chain fatty acids
- DHA
docosahexaenoic acid
- EI
electron ionization
- EPA
eicosapentaenoic acid
- FAME
fatty acid methyl esters
- LCPUFA
long chain polyunsaturated fatty acids
- MS
mass spectrometry
- MUFA
monounsaturated fatty acids
- PUFA
polyunsaturated fatty acids
- SFA
saturated fatty acids
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI:10.1021/acs.jafc.6b03491.
Fatty acid profiles for all fish muscle and skin as well as branched chain, sampling details, statistical tables and typical branched chain retention time, chromatograms and structures are shown (PDF)
Notes
The authors declare no competing financial interest.
REFERENCES
- (1).FAO. The State of World Fisheries and Aquaculture 2014; Rome, 2014; p 3. [Google Scholar]
- (2).Chowdhury R; Warnakula S; Kunutsor S; Crowe F; Ward HA; Johnson L; Franco OH; Butterworth AS; Forouhi NG; Thompson SG; Khaw KT; Mozaffarian D; Danesh J; Di Angelantonio E Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann. Intern. Med 2014, 160 (6), 398–406. [DOI] [PubMed] [Google Scholar]
- (3).(a) Jiao J; Li Q; Chu J; Zeng W; Yang M; Zhu S Effect of n-3 PUFA supplementation on cognitive function throughout the life span from infancy to old age: a systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr 2014, 100 (6), 1422–36. [DOI] [PubMed] [Google Scholar]; (b) Joffre C; Nadjar A; Lebbadi M; Calon F; Laye S n-3 LCPUFA improves cognition: the young, the old and the sick. Prostaglandins, Leukotrienes Essent. Fatty Acids 2014, 91 (1–2), 1–20. [DOI] [PubMed] [Google Scholar]; (c) Zhang Y; Chen J; Qiu J; Li Y; Wang J; Jiao J Intakes of fish and polyunsaturated fatty acids and mild-to-severe cognitive impairment risks: a dose-response meta-analysis of 21 cohort studies. Am. J. Clin. Nutr 2016, 103, 330–340. [DOI] [PubMed] [Google Scholar]
- (4).Uauy RD; Birch DG; Birch EE; Tyson JE; Hoffman DR Effect of dietary omega-3 fatty acids on retinal function of very-low-birth-weight neonates. Pediatr. Res 1990, 28 (5), 485–92. [DOI] [PubMed] [Google Scholar]
- (5).U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans, 8th ed.; 2015. [Google Scholar]
- (6).(a) Brenna JT; Varamini B; Jensen RG; Diersen-Schade DA; Boettcher JA; Arterburn LM Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am. J. Clin. Nutr 2007, 85 (6), 1457–1464. [DOI] [PubMed] [Google Scholar]; (b) Holman RT; Johnson SB; Ogburn PL Deficiency of essential fatty acids and membrane fluidity during pregnancy and lactation. Proc. Natl. Acad. Sci. U. S. A 1991, 88 (11), 4835–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Favre HA; Powell WH Nomenclature of organic chemistry: IUPAC recommendations and preferred names 2013; Royal Society of Chemistry: Cambridge, U.K., 2013. [Google Scholar]
- (8).Ran-Ressler RR; Bae S; Lawrence P; Wang DH; Brenna JT Branched-chain fatty acid content of foods and estimated intake in the USA. Br. J. Nutr 2014, 112 (4), 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Njinkoué J-M; Barnathan G; Miralles J; Gaydou E-M; Samb A Lipids and fatty acids in muscle, liver and skin of three edible fish from the Senegalese coast: Sardinella maderensis, Sardinella aurita and Cephalopholis taeniops. Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol 2002, 131 (3), 395–402. [DOI] [PubMed] [Google Scholar]
- (10).Alikunhi NM; Narayanasamy R; Kandasamy K Fatty acids in an estuarine mangrove ecosystem. Rev. Biol. Trop 2010, 58 (2), 577–587. [DOI] [PubMed] [Google Scholar]
- (11).Lei L; Li J; Luo T; Fan YW; Zhang B; Ye J; Ye H; Sun Y; Deng ZY Predictable effects of dietary lipid sources on the fatty acids compositions of four 1-year-old wild freshwater fish from Poyang Lake. J. Agric. Food Chem 2013, 61 (1), 210–8. [DOI] [PubMed] [Google Scholar]
- (12).Rasoarahona JR; Barnathan G; Bianchini JP; Gaydou EM Annual evolution of fatty acid profile from muscle lipids of the common carp (Cyprinus carpio) in Madagascar inland waters. J. Agric. Food Chem 2004, 52 (24), 7339–44. [DOI] [PubMed] [Google Scholar]
- (13).Ran-Ressler RR; Khailova L; Arganbright KM; Adkins-Rieck CK; Jouni ZE; Koren O; Ley RE; Brenna JT; Dvorak B Branched chain fatty acids reduce the incidence of necrotizing enterocolitis and alter gastrointestinal microbial ecology in a neonatal rat model. PLoS One 2011, 6 (12), e29032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).(a) Lin T; Yin X; Cai Q; Fan X; Xu K; Huang L; Luo J; Zheng J; Huang J 13-Methyltetradecanoic acid induces mitochondrial-mediated apoptosis in human bladder cancer cells. Urol Oncol 2012, 30 (3), 339–45. [DOI] [PubMed] [Google Scholar]; (b) Wongtangtintharn S; Oku H; Iwasaki H; Inafuku M; Toda T; Yanagita T Incorporation of branched-chain fatty acid into cellular lipids and caspase-independent apoptosis in human breast cancer cell line, SKBR-3. Lipids Health Dis 2005, 4, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Yang P; Collin P; Madden T; Chan D; Sweeney-Gotsch B; McConkey D; Newman RA Inhibition of proliferation of PC3 cells by the branched-chain fatty acid, 12-methyltetradecanoic acid, is associated with inhibition of 5-lipoxygenase. Prostate 2003, 55 (4), 281–91. [DOI] [PubMed] [Google Scholar]
- (15).(a) Sağglık S; Alpaslan M; Gezgin T;Çetintürkc K; Tekinay A; Güven KC Fatty acid composition of wild and cultivated gilthead seabream (Sparus aurata) and sea bass (Dicentrarchus labrax). Eur. J. Lipid Sci. Technol 2003, 105 (2), 104–107. [Google Scholar]; (b) Sahena F; Zaidul I; Jinap S; Yazid A; Khatib A; Norulaini N Fatty acid compositions of fish oil extracted from different parts of Indian mackerel (Rastrelliger kanagurta) using various techniques of supercritical CO 2 extraction. Food Chem. 2010, 120 (3), 879–885. [Google Scholar]
- (16).(a) Garces R; Mancha M One-step lipid extraction and fatty acid methyl esters preparation from fresh plant tissues. Anal. Biochem 1993, 211 (1), 139–43. [DOI] [PubMed] [Google Scholar]; (b) Zhou Y; Nijland M; Miller M; Ford S; Nathanielsz PW; Brenna JT The influence of maternal early to mid-gestation nutrient restriction on long chain polyunsaturated fatty acids in fetal sheep. Lipids 2008, 43 (6), 525–31. [DOI] [PubMed] [Google Scholar]
- (17).Ran-Ressler RR; Sim D; O’Donnell-Megaro AM; Bauman DE; Barbano DM; Brenna JT Branched chain fatty acid content of United States retail cow’s milk and implications for dietary intake. Lipids 2011, 46 (7), 569–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Ran-Ressler RR; Lawrence P; Brenna JT Structural characterization of saturated branched chain fatty acid methyl esters by collisional dissociation of molecular ions generated by electron ionization. J. Lipid Res 2012, 53 (1), 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Burnham KP; Anderson DR Model selection and multimodel inference: a practical information-theoretic approach, 2nd ed.; Springer: New York, 2002. [Google Scholar]
- (20).Simberloff D; Dayan T The guild concept and the structure of ecological communities. Annu. Rev. Ecol. Syst 1991, 22, 115–143. [Google Scholar]
- (21).(a) Lough AK The chemistry and biochemistry of phytanic, pristanic and related acids. Prog. Chem. Fats Other Lipids 1975, 14 (1), 1–48. [DOI] [PubMed] [Google Scholar]; (b) Vlaeminck B; Lourenco M; Bruinenberg M; Demeyer D; Fievez V Odd and branched chain fatty acids in rumen contents and milk of dairy cows fed forages from semi-natural grasslands. Commun. Agric. Appl. Biol. Sci 2004, 69 (2), 337–340. [PubMed] [Google Scholar]
- (22).National Marine Fisheries Service. Fisheries of the United States 2013: Current Fishery Statistics; http://www.st.nmfs.noaa.gov/Assets/commercial/fus/fus13/FUS2013.pdf (accessed May 25).
- (23).Ould El Kebir MV; Barnathan G; Siau Y; Miralles J; Gaydou EM Fatty acid distribution in muscle, liver, and gonads of rays (Dasyatis marmorata, Rhinobatos cemiculus, and Rhinoptera marginata) from the East Tropical Atlantic Ocean. J. Agric. Food Chem 2003, 51 (7), 1942–7. [DOI] [PubMed] [Google Scholar]
- (24).(a) Cosper CI; Ackman RG Occurrence of cis-9,10-methylenehexadecanoic and cis-9, 10-methyleneoctadecanoic acids in the lipids of immature and mature Fundulus heteroclitus (L.), and in roe. Comp Biochem Physiol B 1983, 75 (4), 649–54. [DOI] [PubMed] [Google Scholar]; (b) Dhaneesh KV; Noushad KM; Kumar TTA Nutritional evaluation of commercially important fish species of Lakshadweep archipelago, India. PLoS One 2012, 7 (9), e45439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Lewis R Fatty acid composition of some marine animals from various depths. J. Fish. Res. Board Can 1967, 24 (5), 1101–1115. [Google Scholar]
- (26).Leo RF; Parker PL Branched-chain Fatty acids in sediments. Science 1966, 152 (3722), 649–50. [DOI] [PubMed] [Google Scholar]
- (27).Ackman RG; Sipos JC Isolation of the saturated fatty acids of some marine lipids with particular reference to normal odd-numbered fatty acids and branched-chain fatty acids. Comp. Biochem. Physiol 1965, 15 (4), 445–56. [DOI] [PubMed] [Google Scholar]
- (28).(a) Bell JG; Henderson RJ; Tocher DR; McGhee F; Dick JR; Porter A; Smullen RP; Sargent JR Substituting fish oil with crude palm oil in the diet of Atlantic salmon (Salmo salar) affects muscle fatty acid composition and hepatic fatty acid metabolism. J. Nutr 2002, 132 (2), 222–230. [DOI] [PubMed] [Google Scholar]; (b) Bell JG; McEvoy J; Tocher DR; McGhee F; Campbell PJ; Sargent JR Replacement of fish oil with rapeseed oil in diets of Atlantic salmon (Salmo salar) affects tissue lipid compositions and hepatocyte fatty acid metabolism. J. Nutr 2001, 131 (5), 1535–1543. [DOI] [PubMed] [Google Scholar]
- (29).Moghadasian MH; Moghadasian P; Le K; Hydamaka A; Zahradka P Lipid Analyses of Four Types of Fish From Manitoba Lakes. E Chronicon Nutr. 2015, 1 (2), 41–48. [Google Scholar]
- (30).Ahlgren G; Blomqvist P; Boberg M; Gustafsson IB Fatty acid content of the dorsal muscle—an indicator of fat quality in freshwater fish. J. Fish Biol 1994, 45 (1), 131–157. [Google Scholar]
- (31).Twining CW; Brenna JT; Hairston NG; Flecker AS Highly unsaturated fatty acids in nature: what we know and what we need to learn. Oikos 2016, 125 (6), 749–760. [Google Scholar]
- (32).Cladis DP; Kleiner AC; Freiser HH; Santerre CR Fatty acid profiles of commercially available finfish fillets in the United States. Lipids 2014, 49 (10), 1005–18. [DOI] [PubMed] [Google Scholar]
- (33).(a) Ackman RG; Takeuchi T Comparison of fatty acids and lipids of smolting hatchery-fed and wild Atlantic salmon Salmo salar. Lipids 1986, 21 (2), 117–20. [DOI] [PubMed] [Google Scholar]; (b) Karapanagiotidis IT; Bell MV; Little DC; Yakupitiyage A; Rakshit SK Polyunsaturated fatty acid content of wild and farmed tilapias in Thailand: effect of aquaculture practices and implications for human nutrition. J. Agric. Food Chem 2006, 54 (12), 4304–10. [DOI] [PubMed] [Google Scholar]
- (34).Gibson RA; Muhlhausler B; Makrides M Conversion of linoleic acid and alpha-linolenic acid to long-chain polyunsaturated fatty acids (LCPUFAs), with a focus on pregnancy, lactation and the first 2 years of life. Matern. Child Nutr 2011, 7 (Suppl. 2), 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).O’Donnell-Megaro AM; Barbano DM; Bauman DE Survey of the fatty acid composition of retail milk in the United States including regional and seasonal variations. J. Dairy Sci 2011, 94 (1), 59–65. [DOI] [PubMed] [Google Scholar]
- (36).(a) Bottino NR; Gennity J; Lilly ML; Simmons E; Finne G Seasonal and nutritional effects on the fatty acids of three species of shrimp, Penaeus setiferus, P. aztecus and P. duorarum. Aquaculture 1980, 19 (2), 139–148. [Google Scholar]; (b) Gruger EH; Nelson R; Stansby ME Fatty acid composition of oils from 21 species of marine fish, freshwater fish and shellfish. J. Am. Oil Chem. Soc 1964, 41 (10), 662–667. [Google Scholar]; (c) Roubal WT Tuna fatty acids: II. Investigation of the composition of raw and processed domestic tuna. J. Am. Oil Chem. Soc 1963, 40 (6), 215–218. [Google Scholar]
- (37).Keeney M; Katz I; Allison M On the probable origin of some milk fat acids in rumen microbial lipids. J. Am. Oil Chem. Soc 1962, 39 (4), 198–201. [Google Scholar]
- (38).Budge S; Parrish C; Mckenzie C Fatty acid composition of phytoplankton, settling particulate matter and sediments at a sheltered bivalve aquaculture site. Mar. Chem 2001, 76 (4), 285–303. [Google Scholar]
- (39).Heiba HI; Al-Easa HS; Rizk AF Fatty acid composition of twelve algae from the coastal zones of Qatar. Plant Foods Hum. Nutr 1997, 51 (1), 27–34. [DOI] [PubMed] [Google Scholar]
- (40).(a) Meziane T; Tsuchiya M Fatty acids as tracers of organic matter in the sediment and food web of a mangrove/intertidal flat ecosystem, Okinawa, Japan. Mar. Ecol.: Prog. Ser 2000, 200, 49–57. [Google Scholar]; (b) Perry G; Volkman J; Johns R; Bavor H Fatty acids of bacterial origin in contemporary marine sediments. Geochim. Cosmochim. Acta 1979, 43 (11), 1715–1725. [Google Scholar]; (c) Zhukova NV; Kharlamenko VI; Svetashev VI; Rodionov IA Fatty acids as markers of bacterial symbionts of marine bivalve molluscs. J. Exp. Mar. Biol. Ecol 1992, 162 (2), 253–263. [Google Scholar]; (d) Calado R; Rosa R; Morais S; Nunes ML; Narciso L Growth, survival, lipid and fatty acid profile of juvenile monaco shrimp Lysmata seticaudata fed on different diets. Aquacult. Res 2005, 36 (5), 493–504. [Google Scholar]
- (41).(a) Calado R; Figueiredo J; Rosa R; Nunes M; Narciso L Effects of temperature, density, and diet on development, survival, settlement synchronism, and fatty acid profile of the ornamental shrimp Lysmata seticaudata. Aquaculture 2005, 245 (1), 221–237. [Google Scholar]; (b) Fricke H; Gercken G; Schreiber W; Oehlenschlager J Lipid, sterol and fatty acid composition of Antarctic krill (Euphausia superba Dana). Lipids 1984, 19 (11), 821–827. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.