Abstract
There are multiple prognostic indicators for Diffuse large B-cell lymphoma (DLBCL) including; the International Prognostic Index (IPI), and gene expression profiling (GEP) to classify the disease into germinal center B-cell and activated B-cell subtypes, the latter harboring inferior prognosis. More recently, tumor-associated-macrophages (TAM) and lymphocyte-to-monocyte ratio (LMR) were found to have prognostic implications in DLBCL. However, consensus is yet to be reached in terms of the significance of each. In this study, we evaluated the prognostic value of tumor-associated-macrophages (TAM) as assessed by CD163 or CD68 positivity by IHC on tissue biopsies and lymphocyte-to-monocyte ratio (LMR) was calculated from peripheral blood differential, with focus on the inclusion of rituximab as a treatment modality.
The number of CD68-positive cells in the tumor microenvironment did not exhibit significant prognostic value, whereas higher number of CD163-positive cells was associated with inferior OS in patients treated with chemotherapy alone. This effect was no longer evident in patients treated with rituximab containing chemo-immunotherapy. On the other hand, the prognostic significance of LMR on survival was more persistent regardless of treatment. There was no association between LMR and the number of CD163-positive cells. Our results suggest that LMR is the more easily and widely available prognostic marker in this era of chemo-immunotherapy. Our finding supports previous literature that the effect of TAM can vary according to treatment. Interaction between rituximab and TAM warrant further scientific investigation for mechanistic insights into targeted therapeutics.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma (NHL), and accounts for approximately 30% of NHL in the western population.(1, 2) It is a clinically, immunophenotypically, and genetically heterogeneous disease. The backbone of treatment has been combination chemotherapy with cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) and addition of rituximab to the regimen (R-CHOP) has significantly improved the prognosis of the disease.(3-6)
Various prognostic systems incorporating clinical and pathological features have been explored, with the international prognostic index (IPI) being the most widely used to date.(7) Gene expression profiling (GEP) has been successful in classifying the disease into germinal center B-cell (GCB) and activated B-cell (ABC) subtypes, with the latter harboring an inferior prognosis.(8, 9) Targeted agents have been developed that shows improved efficacy in ABC-DLBCL patients, allowing this classification to be used to differentiate treatment strategies.(10) However, the translation of GEP into a more widely available immunohistochemistry (IHC) based classification has resulted in conflicting results.(11-14)
Tumor microenvironment has been known to play an important role in tumor progression and response to treatment both in solid tumors and lymphomas.(15-18) Of the various cellular components of the microenvironment, macrophages have been known to exhibit tumor promoting functions including angiogenesis, tumor cell invasion, migration, metastasis, and suppression of anti-tumor immunity, and the infiltration of tumor associated macrophages (TAMs) or enrichment of TAM-associated gene signatures have been shown to have prognostic implication in various tumors including Hodgkin lymphoma and follicular lymphoma.(15, 19-21) However, its significance in DLBCL has thus far been controversial mainly due to difference in the method used to evaluate macrophages as well as types of treatment given.(22-24)
Another known prognostic marker in DLBCL is the peripheral blood monocyte and lymphocyte count, and the lymphocyte-to-monocyte ratio (LMR).(25, 26) Peripheral blood monocytes together with tissue resident macrophages are the sources of TAM, however, the relationship between peripheral blood monocytes and TAM and their prognostic significance in DLBCL has not been fully elucidated. =In this study, we assessed the prognostic significance of LMR and TAM in DLBCL in light of using rituximab as a treatment modality.
Materials and Methods
Patients
Patients newly diagnosed as DLBCL at Memorial Sloan Kettering Cancer Center (MSKCC) between 1990 and 2014 were evaluated for biospecimen availability. Cases were excluded if they had a history of low-grade B-cell lymphoma, Hodgkin lymphoma, AIDS/HIV infection, primary central nervous system DLBCL, and post-transplant lymphoproliferative disorder. A total of 142 patients were included in the study. Relevant clinical information including age, gender, stage, serum lactate dehydrogenase (LDH) levels, international prognostic index (IPI), monocyte and lymphocyte count at diagnosis of DLBCL, type of treatment and survival were collected from the medical record. Lymphocyte-to-monocyte ratio (LMR) was calculated as absolute lymphocyte count (ALC)/absolute monocyte count (AMC). The study was performed under approval from the institutional review board of MSKCC.
Immunophenotypic Analysis
Tissue microarrays (TMA) were constructed from formalin-fixed, paraffin-embedded tissue with cores in triplicate. CD163 and CD68 expression were evaluated by IHC performed on TMAs using anti-CD163 (10D6, Vector Laboratories, Burlingame, CA, USA) and anti-CD68 (KP1, Dako, Glostrup, Denmark) antibody performed on automated platform (Ventana Medical Systems Inc, Tucson, AZ, USA) according to manufacturer protocols. Stained slides for CD163 and CD68 were scanned using the Aperio system (Leica Biosystems, Nussloch, Germany), and the number of CD163 or CD68 positive nucleated cells at magnification of 20x were manually counted. The cases were classified into four categories according to the number of CD163-positive nucleated cells: score 0: 0-50 cells; score 1: 51-100 cells; score 2: 101-150 cells; score 3: >150 cells per high-powered field, 40X objective (Figure 1). The number of CD163 or CD68-positive cells was also analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA). We also assessed for BCL2 and MYC expression by IHC using anti-BCL2 (124, Dako, Glostrup, Denmark) and anti-MYC (Y69, Abcam, Cambridge, UK) antibodies on TMAs. BCL2 and MYC were scored for positivity among the tumor cells at 10% increments. The cell-of-origin (COO) classification was determined according to the Hans algorithm.(11)
Figure 1.
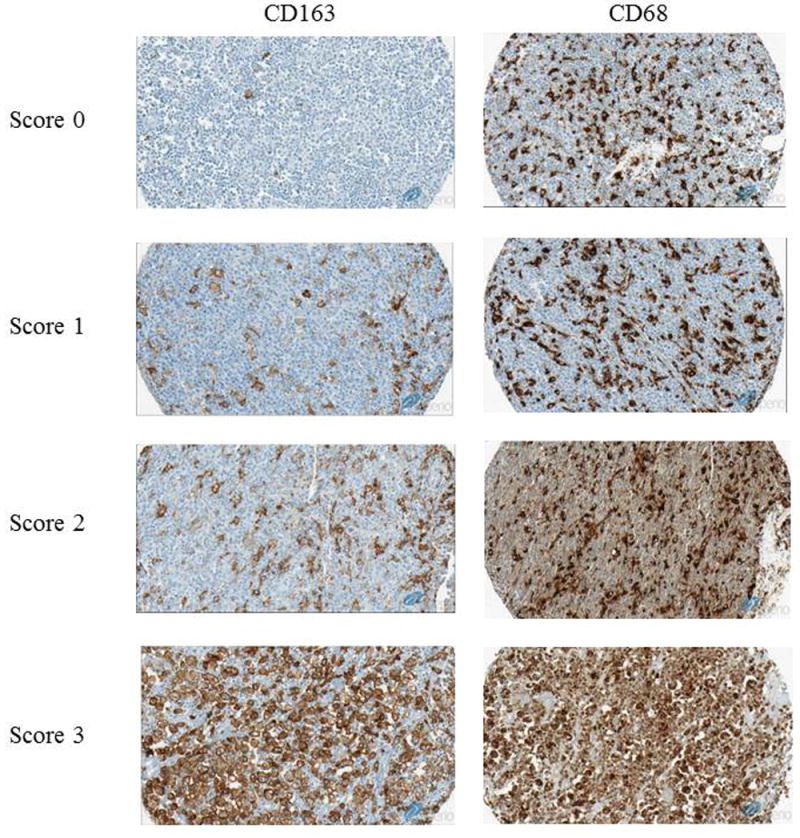
Representative stains for CD163 and CD68. Left Column: Representative stains of CD163 scanned in Aperio at x20 magnification. Score 0: 0-50 cells, score 1: 51-100 cells, score 2:101-150 cells, score 3: >150 cells. Right Column: Corresponding CD68 stains of the cases stained for CD163. CD68 tended to stain more cells compared to CD163.
Statistical Analysis
Overall survival (OS) was calculated from the date of diagnosis to the date of death or last follow-up, and progression-free survival (PFS) was calculated from the date of diagnosis to the date of confirmed disease progression, death or last follow-up, whichever occurred earliest. Survival was estimated using the Kaplan-Meier method and compared using either the log-rank test or by Cox-regression analysis. Due to varying cut-off value for CD163 positivity in the literature, two different cut-off values were explored in our dataset, score 0 vs 1-3, and score 0-2 vs 3 to assess for prognostic significance in dichotomized groups.(24, 27) A pre-defined cut-off value of LMR>2.1 was used to determine its prognostic significance according to published literature.(28)
Associations of two continuous variables were assessed using Spearman’s rank correlation coefficient and that for categorical variables were assessed using Fisher’s exact test or chi-square test.
All analysis was performed using SAS 9.4.
Results
Patient and Disease Characteristics
The characteristics of the patients included in the study are summarized in Table 1. The median follow-up of the entire cohort was 5.4 years. Of the 142 patients included in the study, nine (6.3%) patients did not receive any treatment, and were excluded from the survival analysis. 89 (62.7%) patients received rituximab containing combination chemotherapy, and 44 (31.0%) patients received chemotherapy without rituximab. The details of the type of treatment combined with or without rituximab are detailed in Table 1. The majority of the patients received CHOP-based chemotherapy. Patients treated with rituximab combined therapy were found to have a lower AMC at the time of diagnosis, and were more likely to be of germinal center B-cell (GCB) subtype by COO classification, however, other clinical characteristics were similar between two groups (Table 1).
Table 1.
Baseline characteristics of patients according to treatment type
| Overall | Rituximab (+) | Rituximab (-) | ||
|---|---|---|---|---|
| Number of patients | 142 | 89 | 44 | |
|
| ||||
| Median Follow-up (yrs) [range] | 5.44 [0.02-22.6] | 5.45 [0.20-12.4] | 6.67 [0.03-22.6] | |
|
| ||||
| Median Age (yrs) [range] | 62.5 [18-83] | 64 [19-83] | 59.5 [18-80] | |
|
| ||||
| ALC (/mm3) [range] | 1,270 [170-4,810] (n=136) | 1,210 [270-3,100] | 1,320 [170-4,810] (n=40) | |
|
| ||||
| AMC (/mm3) [range] | 380 [40-1,640] (n=136) | 360 [40-1,110] | 510 [40-1,050] (n=40) | |
|
| ||||
| LMR [range] | 3.14 [0.46-14.1] (n=136) | 3.25 [0.67-14.1] | 2.89 [0.46-8.88] (n = 40) | |
|
| ||||
| Median CD163 positive cells [range] | 76 [0-494] (n=103) | 81 [1-494] (n=61) | 66 [0-459] (n=33) | |
|
| ||||
| Median CD68 positive cells [range] | 153 [14-430] (n=78) | 171 [14-430] (n=44) | 138 [33 - 409] (n=28) | |
|
| ||||
| Gender | Male | 74 (56%) | 48 (54%) | 26 (59%) |
| Female | 58 (44%) | 41 (46%) | 18 (41%) | |
|
| ||||
| IPI | Low | 42 (30%) | 29 (33%) | 12 (27%) |
| Low-intermediate | 40 (28%) | 25 (28%) | 14 (32%) | |
| High-intermediate | 28 (20%) | 18 (20%) | 8 (18%) | |
| High | 22 (15%) | 12 (13%) | 8 (18%) | |
| Missing | 10 (7%) | 5 (6%) | 2 (5%) | |
|
| ||||
| Stage | 1 | 34 (24%) | 20 (22%) | 12 (27%) |
| 2 | 25 (18%) | 17 (19%) | 8 (18%) | |
| 3 | 24 (17%) | 19 (21%) | 4 (9%) | |
| 4 | 56 (39%) | 33 (37%) | 19 (43%) | |
| Missing | 3 (2%) | 0 (0%) | 1 (2%) | |
|
| ||||
| Cell-of-origin | GCB | 66 (46%) | 47 (53%) | 12 (27%) |
| Non-GCB | 59 (42%) | 30 (34%) | 27 (61%) | |
| Missing | 17 (12%) | 12 (13%) | 5 (11%) | |
|
| ||||
| Chemotherapy regimen | CHOP/EPOCH | 102 (72%) | 76 (85%) | 26 (59%) |
| CHOP-ICE | 10 (7%) | 10 (11%) | 0 (0%) | |
| NHL-15 | 11 (8%) | 0 (0%) | 11 (25%) | |
| Other | 9 (6%) | 3 (3%) | 6 (14%) | |
| Details Unknown | 1 (1%) | 0 (0%) | 1 (2%) | |
| Untreated | 9 (6%) | 0 (0%) | 0 (0%) | |
|
| ||||
| CD163 score | 0 | 33 (23%) | 20 (22%) | 11 (25%) |
| 1 | 28 (20%) | 13 (15%) | 10 (23%) | |
| 2 | 9 (6%) | 3 (3%) | 5 (11%) | |
| 3 | 33 (23%) | 25 (28%) | 7 (16%) | |
| Missing | 39 (27%) | 28 (31%) | 11 (25%) | |
ALC: absolute lymphocyte count, AMC: absolute monocyte count, LMR: lymphocyte-to-monocyte ratio, GCB: Germinal center B-cell
CD163, CD68 staining and survival
CD163 and CD68 staining was successful and evaluable in 103 and 78 patients, respectively. The median number of CD163-positive cells was 76 (range: 0 – 494) and that of CD68-positive cells was 153 (range: 14 - 430). The manual count and computerized count using ImageJ had a high concordance with a correlation coefficient of 0.97 (p<0.0001) and 0.98 (p<0.0001) for CD163 and CD68, respectively (Figure 2 A, B). The number of CD163-positive cells and CD68-positive cells had a weak positive correlation with a correlation coefficient of 0.48 (p<0.0001) (Figure 2C).
Figure 2.
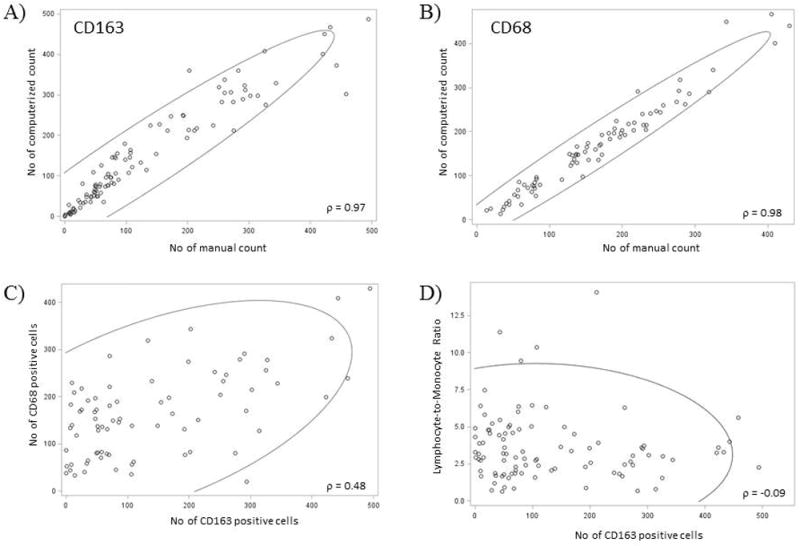
Correlation plot for manual and computerized count for CD163 and CD68 (A &B). Both CD163 and CD68 had a high correlation between manual and automated count. CD163 and CD68 had a weak but significant correlation (C). There was no association between CD163 count and LMR (D).
CD163 positivity as a continuous variable had a significant association with OS in patients treated without rituximab (HR=1.005, p=0.014), but not in patients treated with rituximab containing regimen (HR=0.999, p=0.66). On the other hand, CD68 positivity had no association with prognosis in either patient population (HR=0.999, p=0.87 for non-rituximab containing regimen and HR=0.992, p=0.28 for rituximab containing regimen). We assessed the prognostic significance of CD163 score 0 vs 1-3 and score 0-2 vs 3 in accordance to published literature with slight modification, to identify group of patients with prognostic significance using CD163-positive cells in the microenvironment. Patients with CD163 score of 3 (>150 cells) had a significantly worse OS (5 year OS of 53.6% vs 88.5%, p=0.022) in patients who received treatment without rituximab (Figure 3 A, B). Neither CD163 expression nor CD68 expression was significantly associated with PFS in our patient cohort (Figure 3 C, D, Table 2).
Figure 3.
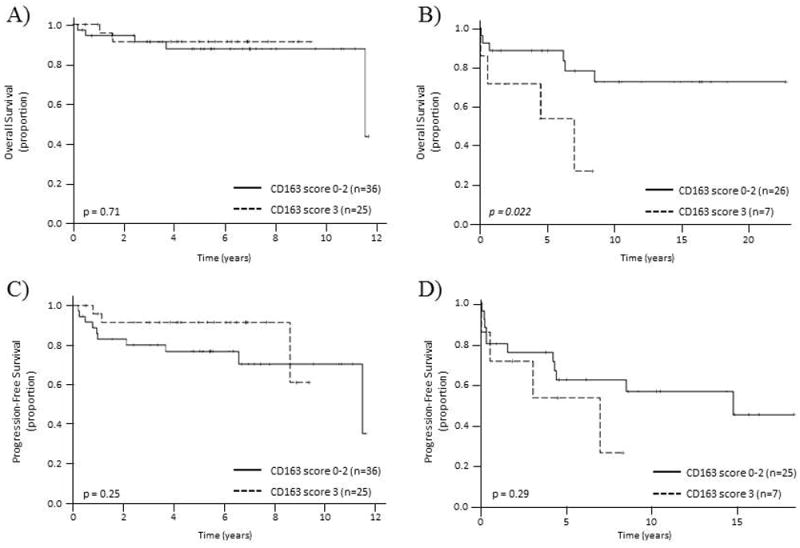
Overall survival (A & B) and progression-free survival (C & D) according to CD163 score. A) &C) Overall survival and progression-free survival of patients treated with rituximab containing regimen, B) & D) Overall survival and progression-free survival of patients treated without rituximab containing regimen.
Table 2.
Factors associated with survival
| OS | PFS | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Rituximab (+) | Rituximab (-) | Rituximab (+) | Rituximab (-) | |||||
|
| ||||||||
| HR | p-value | HR | p-value | HR | p-value | HR | p-value | |
| CD163 | 0.999 | 0.66 | 1.005 | 0.014 | 0.997 | 0.28 | 1.003 | 0.13 |
| CD68 | 0.992 | 0.28 | 0.999 | 0.87 | 0.995 | 0.28 | 0.996 | 0.23 |
| LMR | 0.77 | 0.15 | 0.66 | 0.066 | 0.803 | 0.096 | 0.681 | 0.033 |
| ALC | 0.782 | 0.58 | 0.215 | 0.14 | 0.757 | 0.41 | 0.369 | 0.098 |
| AMC | 3.003 | 0.32 | 0.514 | 0.52 | 2.002 | 0.43 | 0.612 | 0.49 |
| Age | 1.017 | 0.41 | 1.057 | 0.018 | 1.004 | 0.78 | 1.032 | 0.051 |
|
| ||||||||
| Gender | - | 0.48 | - | 0.86 | - | 0.42 | - | 0.83 |
| IPI | - | 0.78 | - | 0.53 | - | 0.32 | - | 0.034 |
| Stage | - | 0.16 | - | 0.024 | - | 0.11 | - | 0.005 |
| COO | - | 0.84 | - | 0.04 | - | 0.14 | - | 0.59 |
| MYC ≥40% | - | 0.47 | - | 0.0001 | - | 0.75 | - | 0.036 |
| BCL2 ≥70% | - | 0.26 | - | 0.98 | - | 0.091 | - | 0.18 |
LMR, CD163 expression and survival
Data on peripheral blood monocyte and lymphocyte count at the time of DLBCL diagnosis was available for 136 patients. The median ALC was 1,270/mm3, and the median AMC was 380/mm3 leading to a median LMR of 3.14 in the entire population. Patients treated with rituximab containing chemotherapy had a lower AMC in this patient cohort, however, there was no difference in the LMR between patients treated with or without rituximab.
There was no association between number of CD163-positive cells in the microenvironment and the peripheral blood LMR assessed either as a continuous variable (ρ = -0.09, p=0.63) (Figure 2D) or as a categorical variable (p=0.91).
We evaluated the prognostic significance of low LMR (LMR≤2.1) on OS, and found that lower LMR was predictive of decreased survival (5 year OS of 73.9% vs 91.7%, p=0.048) in patients treated with rituximab combined regimen, and the same trend was seen (5 year OS of 75.0% vs 86.3%, p= 0.075) in patients treated without rituximab (Figure 4 A, B). There was also a significant difference in PFS according to LMR in patients treated without rituximab (5yr PFS of 43.8% vs 71.2%, p=0.041) but not in those treated with rituximab containing regimen (Figure 4 C, D).
Figure 4.
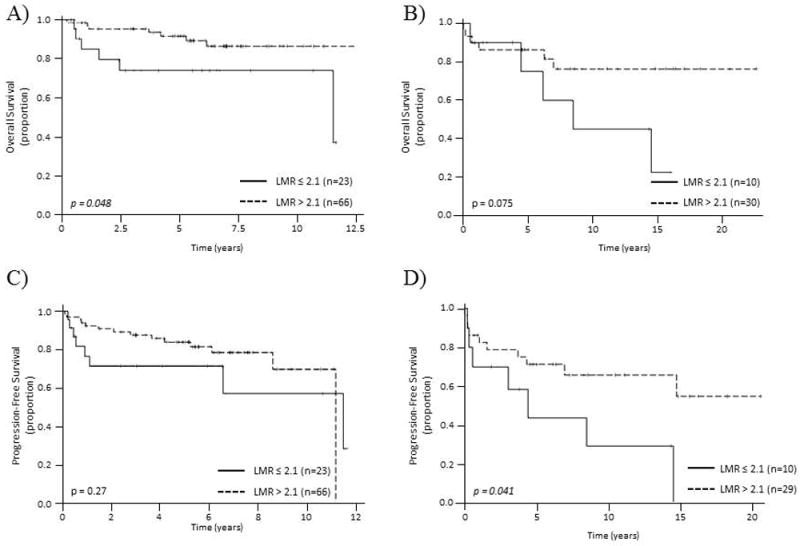
Overall survival (A&B) and progression-free survival (C&D) according to LMR. The prognostic significance of LMR>2.1 was evaluated by log-rank test according to type of treatment received. A) &C) Overall survival and progression-free survival of patients treated with rituximab containing regimen, B) & D) Overall survival and progression-free survival of patients treated without rituximab containing regimen.
Other prognostic factors and their association with CD163 expression and LMR
We evaluated the prognostic significance of other factors known to be associated with survival in DLBCL (Table 2). None of the factors evaluated were significantly associated with either OS or PFS for patients treated with rituximab containing regimen. Age, stage, non-GCB subtype, and MYC expression of ≥40% were significantly associated with OS, and IPI, stage, in patients treated without rituximab.
In terms of the association of known prognostic factors with either CD163 expression or low LMR, patients with advanced stage had higher number of CD163-positive cells in the microenvironment, as well as tumors with ABC subtype and high MYC expression. Patients with low LMR were more likely to present with high IPI risk score, BM involvement, or B symptoms. Due to the limited number of events in each treatment group, the independent significance of these factors was not evaluated in this study.
Discussion
In this study, we evaluated the prognostic significance of TAM and LMR in DLBCL, as well as the association of LMR and TAM, and their association with other known clinical and pathological markers of prognostic significance. We used two markers to identify TAM, CD163 and CD68, and found that the number of CD163-positive cells but not CD68-positive cells in the microenvironment was significantly associated with OS in patients treated prior to the rituximab era, but this difference was no longer evident in patients treated with rituximab. On the other hand, LMR was predictive of survival in the rituximab era, with the difference marginally lacking significance in patients treated without rituximab. There was no association between LMR and the number of CD163-positive cells. Since combination of rituximab with chemotherapy is the current standard of care for patients with DLBCL, we conclude from our study that LMR may be a better prognostic indicator than TAM in cases of DLBC.
Recent literature also suggests that the negative impact of TAMs could be dependent on the type of treatment received. Riihijarvi et al.(27) published their experience on the association of TAM assessed by CD68 mRNA and protein expression in DLBCL patients treated in the Nordic Phase II study, and compared their finding with an independent cohort of patients. They found CD68 expression at both the mRNA and protein level to be associated with favorable outcome in patients treated with rituximab containing chemo-immunotherapy, which was confirmed in independent validation cohort, but also found that the effect of CD68 expression was reversed in patients receiving chemotherapy alone without rituximab. Although the histology is different, two separate groups have shown a similar finding in patients with follicular lymphoma, where high TAM content was associated with inferior survival in the era of chemotherapy, and this negative impact could be circumvented with the incorporation of rituximab.(29, 30) Our study result is in line with these findings, suggesting an interaction of rituximab on the function of TAMs.
One major difference of the current study with the previous reports is that we assessed both CD68 and CD163 positive cells in the microenvironment, and only found CD163 to be significantly associated with survival in chemotherapy only patients, which is contrary to what have been reported.(27) There have been conflicting reports in the literature regarding the utility of CD68 and CD163 as prognostic markers in DLBCL.(22-24, 31) Different studies have used different methods of staining, scoring and cut-off values for positivity, making a direct comparison and interpretation of the studies difficult. We have therefore used cut-off values that are comparable to previous published literatures, and have confirmed the prognostic role of CD163 expression.(24, 27) Traditionally, macrophages have been classified into M1 and M2 subtype according to their phenotype, with the former exhibiting bactericidal and tumoricidal function as opposed to the protumoral phenotype of the latter.(20) CD163 is considered to be a marker relatively specific to M2 type macrophages compared to the pan-macrophage marker, CD68, and therefore, an increase in the number of CD163-positive cells would explain the increased resistance to treatment leading to decreased survival, at least in chemotherapy treated patients.(32) We note that in the current study 25 addtitional patients were analyzed for CD163 over CD68 and that the KP1 clone for CD68 was used over the PGM1 clone thought to be more specific, nevertheless, recent understanding of the tumor microenvironment suggests the polarization and classification of the different types of TAMs to be more dynamic and diverse, and a more sophisticated approach than a single marker IHC stains would be necessary to further understand the differing role and prognostic value of these cells.(20)
Beyond prognostic value, this study also provides interesting insight into the role of macrophages in the tumor microenvironment of DLBCL, especially in association with the use of rituximab. One of the mechanisms of action of rituximab in the treatment of B-cell lymphoma is through antibody-dependent cellular cytotoxicity (ADCC), and therefore, in the era of rituximab use, increase of macrophages in the tumor microenvironment may have been a marker of enhanced ADCC, leading to improved survival.(33) In vitro study has shown that macrophages differentiated with M-CSF stimulation (M2 phenotype) exhibited greater phagocytic activity against rituximab opsonized cells compared to GM-CSF induced, M1 macrophages.(34) This phenomena might explain the difference seen in the current study that macrophages delineated by the more M2 macrophage specific marker CD163 showed stronger signal compared to CD68 delineated macrophages. Other possibilities include the use of rituximab stimulating the change in the polarization of macrophages into a tumoricidal, M1-type phenotype to promote their anti-tumoral function. Targeting of macrophages with colony-stimulating factor 1 receptor (CSF1R) inhibitors or monoclonal antibodies have gained interest and tested in the clinical setting against various solid tumors and Hodgkin lymphoma, where high TAM content has been associated with poor prognosis.(35, 36) Putting together our current result as well as prior findings, this type of approach may not be as appealing in DLBCL compared to solid tumors. However, our data suggests that through further understanding of the mechanism of improved survival of patients with the addition of rituximab to the treatment scheme and its association with macrophage content, a treatment modality targeting the change in the macrophage polarity might be a more appealing approach to improve prognosis of patients with DLBCL
In this study, we have also looked in detail the prognostic role of LMR in DLBCL and its association with macrophage signature in the tissue. Although the association of low LMR with prognosis did not reach statistical significance, we believe this was limited due to the relatively small sample size in the non-rituximab containing patient group, and overall, our result suggests that LMR has its prognostic relevance regardless of the incorporation of rituximab in the treatment regimen. While the origin of TAMs were initially considered to be peripheral blood monocytes, recent findings suggest at least two other origins of TAM; the yolk-sac and the fetal liver, which both give rise to tissue-resident macrophages.(37) The difference in the effect of rituximab use seen between tissue CD163-positive cells versus LMR, which reflects the peripheral blood monocytes, might be explained by this multiple origin of TAM, which is reflected on the lack of association between LMR and number of CD163-positive cells in the tumor.
Due to the retrospective as well as a single institute nature of this study, our result is limited in exploring the effect of different combination chemotherapy with or without rituximab that may have affected the role of macrophage in treatment effect. However, since the majority of patients received CHOP-based chemotherapy, or treatment modality that includes the use of same drug in a different dosing schedule,(38) we believe that this effect is minimal. Another limitation to the current study is the limited sample size, limiting the assessment of independent association of macrophage/monocyte associated markers in addition to currently known prognostic features. Especially, we have identified that the number of CD163-positive cells in the tissue is associated with non-GCB phenotype and high MYC expression that are known markers of more aggressive disease phenotype.(9, 39, 40) Therefore, the increase of CD163-positive cells may reflect the rapid tumor growth and resulting cellular turnover, and hence may not have been an independent prognostic marker. Nevertheless, the data on TAM in DLBCL is controversial with discordant results and not ready for real time assessment with questions remaining on mechanisms (41). In conclusion we have confirmed the prognostic role of TAMs assessed by CD163-positive cells in the tumor microenvironment in patients with DLBCL, when treated with chemotherapy alone but not with rituximab combined chemo-immunotherapy. We have also identified that LMR remains to be a significant prognostic factor in the era of rituximab use. Our study adds to the literature in the current understanding in the role of TAMs as a prognostic marker in DLBCL. The scientific rationale behind the differing role of these markers as well as the difference in response to rituximab use needs further exploration and validation in prospective clinical studies.
Acknowledgments
We would like to thank Ms Irina Linkov for her support in performing the immunohistochemistry stains. EM received fellowship support from Yoshida Scholarship Foundation.
Footnotes
This work was in part presented at ASH December 2012 in Atlanta, GA and AACR April 2015 in Philadelphia, PA
Disclosure/Conflict of Interest
The authors have no relevant conflict of interest to declare.
References
- 1.Xie Y, Pittaluga S, Jaffe ES. The Histological Classification of Diffuse Large B-cell Lymphomas. Seminars in Hematology. 2015;52:57–66. doi: 10.1053/j.seminhematol.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swerdlow SH International Agency for Research on Cancer., World Health Organization. WHO classification of tumours of haematopoietic and lymphoid tissues. 4. International Agency for Research on Cancer; Lyon, France: 2008. p. 439. [Google Scholar]
- 3.Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of a Standard Regimen (CHOP) with Three Intensive Chemotherapy Regimens for Advanced Non-Hodgkin’s Lymphoma. New England Journal of Medicine. 1993;328:1002–6. doi: 10.1056/NEJM199304083281404. [DOI] [PubMed] [Google Scholar]
- 4.Pfreundschuh M, Trumper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. The Lancet Oncology. 2006;7:379–91. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 5.Pfreundschuh M, Schubert J, Ziepert M, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60) The Lancet Oncology. 2008;9:105–16. doi: 10.1016/S1470-2045(08)70002-0. [DOI] [PubMed] [Google Scholar]
- 6.Feugier P, Van Hoof A, Sebban C, et al. Long-Term Results of the R-CHOP Study in the Treatment of Elderly Patients With Diffuse Large B-Cell Lymphoma: A Study by the Groupe d’Etude des Lymphomes de l’Adulte. Journal of Clinical Oncology. 2005;23:4117–26. doi: 10.1200/JCO.2005.09.131. [DOI] [PubMed] [Google Scholar]
- 7.The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329:987–94. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 8.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–11. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 9.Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:1937–47. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 10.Wilson WH, Young RM, Schmitz R, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nature medicine. 2015;21:922–6. doi: 10.1038/nm.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–82. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 12.Choi WW, Weisenburger DD, Greiner TC, et al. A new immunostain algorithm classifies diffuse large B-cell lymphoma into molecular subtypes with high accuracy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:5494–502. doi: 10.1158/1078-0432.CCR-09-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutierrez-Garcia G, Cardesa-Salzmann T, Climent F, et al. Gene-expression profiling and not immunophenotypic algorithms predicts prognosis in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Blood. 2011;117:4836–43. doi: 10.1182/blood-2010-12-322362. [DOI] [PubMed] [Google Scholar]
- 14.Read JA, Koff JL, Nastoupil LJ, et al. Evaluating cell-of-origin subtype methods for predicting diffuse large B-cell lymphoma survival: a meta-analysis of gene expression profiling and immunohistochemistry algorithms. Clinical lymphoma, myeloma & leukemia. 2014;14:460–7.e2. doi: 10.1016/j.clml.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lenz G, Wright G, Dave SS, et al. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med. 2008;359:2313–23. doi: 10.1056/NEJMoa0802885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linderoth J, Eden P, Ehinger M, et al. Genes associated with the tumour microenvironment are differentially expressed in cured versus primary chemotherapy-refractory diffuse large B-cell lymphoma. British journal of haematology. 2008;141:423–32. doi: 10.1111/j.1365-2141.2008.07037.x. [DOI] [PubMed] [Google Scholar]
- 17.Klemm F, Joyce JA. Microenvironmental regulation of therapeutic response in cancer. Trends in Cell Biology. 2015;25:198–213. doi: 10.1016/j.tcb.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer cell. 2012;21:309–22. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 19.Steidl C, Lee T, Shah SP, et al. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med. 2010;362:875–85. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ostuni R, Kratochvill F, Murray PJ, Natoli G. Macrophages and cancer: from mechanisms to therapeutic implications. Trends Immunol. 2015;36:229–39. doi: 10.1016/j.it.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Kridel R, Xerri L, Gelas-Dore B, et al. The Prognostic Impact of CD163-Positive Macrophages in Follicular Lymphoma: A Study from the BC Cancer Agency and the Lymphoma Study Association. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21:3428–35. doi: 10.1158/1078-0432.CCR-14-3253. [DOI] [PubMed] [Google Scholar]
- 22.Hasselblom S, Hansson U, Sigurdardottir M, et al. Expression of CD68+ tumor-associated macrophages in patients with diffuse large B-cell lymphoma and its relation to prognosis. Pathol Int. 2008;58:529–32. doi: 10.1111/j.1440-1827.2008.02268.x. [DOI] [PubMed] [Google Scholar]
- 23.Wada N, Zaki MA, Hori Y, et al. Tumour-associated macrophages in diffuse large B-cell lymphoma: a study of the Osaka Lymphoma Study Group. Histopathology. 2012;60:313–9. doi: 10.1111/j.1365-2559.2011.04096.x. [DOI] [PubMed] [Google Scholar]
- 24.Nam SJ, Go H, Paik JH, et al. An increase of M2 macrophages predicts poor prognosis in patients with diffuse large B-cell lymphoma treated with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone. Leukemia & lymphoma. 2014;55:2466–76. doi: 10.3109/10428194.2013.879713. [DOI] [PubMed] [Google Scholar]
- 25.Porrata LF, Ristow K, Habermann TM, et al. Absolute monocyte/lymphocyte count prognostic score is independent of immunohistochemically determined cell of origin in predicting survival in diffuse large B-cell lymphoma. Leukemia & lymphoma. 2012;53:2159–65. doi: 10.3109/10428194.2012.690605. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe R, Tomita N, Itabashi M, et al. Peripheral blood absolute lymphocyte/monocyte ratio as a useful prognostic factor in diffuse large B-cell lymphoma in the rituximab era. European journal of haematology. 2014;92:204–10. doi: 10.1111/ejh.12221. [DOI] [PubMed] [Google Scholar]
- 27.Riihijarvi S, Fiskvik I, Taskinen M, et al. Prognostic influence of macrophages in patients with diffuse large B-cell lymphoma: a correlative study from a Nordic phase II trial. Haematologica. 2015;100:238–45. doi: 10.3324/haematol.2014.113472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin B, Chen C, Qian Y, Feng J. Prognostic role of peripheral blood lymphocyte/monocyte ratio at diagnosis in diffuse large B-cell lymphoma: a meta-analysis. Leukemia & lymphoma. 2015:1–6. doi: 10.3109/10428194.2015.1014367. [DOI] [PubMed] [Google Scholar]
- 29.Canioni D, Salles G, Mounier N, et al. High numbers of tumor-associated macrophages have an adverse prognostic value that can be circumvented by rituximab in patients with follicular lymphoma enrolled onto the GELA-GOELAMS FL-2000 trial. J Clin Oncol. 2008;26:440–6. doi: 10.1200/JCO.2007.12.8298. [DOI] [PubMed] [Google Scholar]
- 30.Taskinen M, Karjalainen-Lindsberg M-L, Nyman H, Eerola L-M, Leppä S. A High Tumor-Associated Macrophage Content Predicts Favorable Outcome in Follicular Lymphoma Patients Treated with Rituximab and Cyclophosphamide-Doxorubicin-Vincristine-Prednisone. Clinical Cancer Research. 2007;13:5784–9. doi: 10.1158/1078-0432.CCR-07-0778. [DOI] [PubMed] [Google Scholar]
- 31.Marchesi F, Cirillo M, Bianchi A, et al. High density of CD68+/CD163+ tumour-associated macrophages (M2-TAM) at diagnosis is significantly correlated to unfavorable prognostic factors and to poor clinical outcomes in patients with diffuse large B-cell lymphoma. Hematological oncology. 2014 doi: 10.1002/hon.2142. [DOI] [PubMed] [Google Scholar]
- 32.Zaki MA, Wada N, Ikeda J, et al. Prognostic implication of types of tumor-associated macrophages in Hodgkin lymphoma. Virchows Arch. 2011;459:361–6. doi: 10.1007/s00428-011-1140-8. [DOI] [PubMed] [Google Scholar]
- 33.Glennie MJ, French RR, Cragg MS, Taylor RP. Mechanisms of killing by anti-CD20 monoclonal antibodies. Molecular immunology. 2007;44:3823–37. doi: 10.1016/j.molimm.2007.06.151. [DOI] [PubMed] [Google Scholar]
- 34.Leidi M, Gotti E, Bologna L, et al. M2 macrophages phagocytose rituximab-opsonized leukemic targets more efficiently than m1 cells in vitro. Journal of immunology. 2009;182:4415–22. doi: 10.4049/jimmunol.0713732. [DOI] [PubMed] [Google Scholar]
- 35.Ries CH, Hoves S, Cannarile MA, Ruttinger D. CSF-1/CSF-1R targeting agents in clinical development for cancer therapy. Current opinion in pharmacology. 2015;23:45–51. doi: 10.1016/j.coph.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 36.Moskowitz C, Younes A, de Vos S, et al. CSF1R inhibition by PLX3397 in patients with relapsed or refractory Hodgkin lymphoma: results from a phase 2 single agent clinical trial. Blood. 2012;120:1638. [Google Scholar]
- 37.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Portlock CS, Qin J, Schaindlin P, et al. The NHL-15 protocol for aggressive non-Hodgkin’s lymphomas: a sequential dose-dense, dose-intense regimen of doxorubicin, vincristine and high-dose cyclophosphamide. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2004;15:1495–503. doi: 10.1093/annonc/mdh390. [DOI] [PubMed] [Google Scholar]
- 39.Cook JR, Goldman B, Tubbs RR, et al. Clinical significance of MYC expression and/or “high-grade” morphology in non-Burkitt, diffuse aggressive B-cell lymphomas: a SWOG S9704 correlative study. The American journal of surgical pathology. 2014;38:494–501. doi: 10.1097/PAS.0000000000000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valera A, Lopez-Guillermo A, Cardesa-Salzmann T, et al. MYC protein expression and genetic alterations have prognostic impact in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Haematologica. 2013;98:1554–62. doi: 10.3324/haematol.2013.086173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kridel R, Steidl C, Gascoyne RD. Tumor-associated macrophages in diffuse large B-cell lymphoma. Haematologica. 2015 Feb;100(2):143–145. doi: 10.3324/haematol.2015.124008. [DOI] [PMC free article] [PubMed] [Google Scholar]


