Abstract
Seventy-nine three-year olds and their mothers participated in a lab-based task to assess maternal hostility. Mothers also reported their behavioral regulation of their child. Seven years later, fMRI data were acquired while viewing emotional faces and completing a reward processing task. Maternal hostility predicted more negative amygdala connectivity during exposure to sad relative to neutral faces with frontal and parietal regions as well as more negative left ventral striatal connectivity during monetary gain relative to loss feedback with the right posterior orbital frontal cortex and right inferior frontal gyrus. In contrast, maternal regulation predicted enhanced cingulo-frontal connectivity during monetary gain relative to loss feedback. Results suggest parenting is associated with alterations in emotion and reward-processing circuitry 7–8 years later.
Parenting has long been recognized as an important influence on children’s psychosocial development (Baumrind, 1991; Belsky & de Haan, 2011; Bradley & Vandell, 2007; McLeod, Weisz, & Wood, 2007; Rose, Roman, Mwaba, & Ismail, 2017; Waite, Whittington, & Creswell, 2014) with this effect likely mediated by altered neural development. As such, numerous studies have examined associations between parenting behaviors or styles and the neural functioning of offspring. Understanding the links between parenting and brain development will further understanding of both normative and maladaptive neural development as well as why early childhood experiences are linked to diverse psychosocial outcomes.
The current study examined 79 three-year-olds who completed lab-based interaction tasks with a parent to assess parenting behaviors and who were then followed for seven to eight years, at which point they underwent functional magnetic resonance imaging (fMRI) during emotion- and reward-processing tasks. Examining the influence of parenting in childhood is important given that while the brain is rapidly developing in utero and during infancy, parents appear to have a particularly important effect on brain development in childhood (Belsky & de Haan, 2011; Tost, Champagne, & Meyer-Lindenberg, 2015), and in particular on connectivity between subcortical and cortical regions (Hart & Rubia, 2012; Herringa et al., 2013). This study focuses on maternal hostility and regulation of the child’s behavior, as each has been widely studied and represent core aspects of parenting that are particularly influential for children’s psychosocial development (e.g., see Baumrind, 1991; Belsky & de Haan, 2011; Bradley & Vandell, 2007; McLeod et al., 2007; Rose et al., 2017; Waite et al., 2014).
Although fMRI studies have examined associations between parenting and a range of aspects of neural functioning, the current study focuses on functional connectivity in affective processing-related circuitry, including emotional face processing and reward processing circuitry. Emotion and reward processing circuits are argued to both underpin affective processing and overlap substantially in terms of brain regions involved (see Pechtel & Pizzagalli, 2011; Tottenham & Galvan, 2016). For instance, key components of reward processing include the basal ganglia involving the ventral striatum, while the recognition and understanding of emotions recruits the amygdala, fusiform gyrus, superior temporal gyrus, and thalamus. However, both processes involve the amygdala, prefrontal cortex (PFC) regions, and the ventral striatum (Reviewed in Tottenham & Galvan, 2016), although the ventral striatum is more primarily involved in reward-based learning and prediction of outcomes (Haber, 2011; Li, Schiller, Schoenbaum, Phelps, & Daw, 2011) while the amygdala is more strongly implicated in identifying highly salient emotional stimuli (Pearce & Hall, 1980). Both, however, are implicated in associative learning (Li et al., 2011). Models of affective behavior posit that the organization between the PFC, amygdala, and ventral striatum allows for the dynamic coordination of emotional learning and responding through Pavlovian and instrumental processes that link emotion to action (Reviewed in Tottenham & Galvan, 2016). The PFC is an association area that coordinates and regulates information throughout the brain. The amygdala has strong bidirectional projections with the PFC (Barbas, Saha, Rempel-Clower, & Ghashghaei, 2003), while the ventral striatum receives unidirectional projections from the amygdala and PFC, including excitatory projections that facilitate reward learning (Stuber et al., 2011), and sends indirect projections back to the PFC (Cardinal, Parkinson, Hall, & Everitt, 2002; Casey, 2015; Cho, Ernst, & Fudge, 2013). Thus, given the importance of affective processing for a range of psychosocial outcomes, understanding how parenting influences the development of these circuits may further knowledge of the mechanisms through which parenting influences children’s psychosocial development.
Moreover, functioning in each of these circuits has important and wide-ranging implications for psychosocial functioning and underpin core aspects of psychosocial functioning in a variety of domains (Reviewed in Galvan, 2017; Tottenham & Galvan, 2016). Specifically, we examined amygdala functional connectivity during an emotional face-processing task and ventral striatum as well as anterior cingulate functional connectivity during a monetary gain versus loss task. Moreover, we sought to test the specificity of relationships between two parenting behaviors and multiple aspects of brain functional connectivity in offspring.
Parenting and emotion-processing neural circuitry
The amygdala’s role in the automatic processing of emotionally salient stimuli and production of emotional responses to stimuli is well-established (Reviewed in Callaghan & Tottenham, 2016; Sergerie, Chochol, & Armony, 2008) and appears to change with age (Somerville, Fani, & McClure-Tone, 2011). The prefrontal cortex (PFC) sends projections to the amygdala that modulate amygdala reactivity. These projections can attenuate or potentiate amygdala reactivity to emotional stimuli, thereby respectively resulting in a dampening or potentiating of automatic emotional responding (Milad, Rauch, Pitman, & Quirk, 2006). For example, at least in adults, during emotion regulation tasks, the extent of amygdala coupling with the dorsolateral PFC (dlPFC), orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), and dorsomedial PFC (dmPFC) is positively associated with post-reappraisal attenuation of negative affect (Banks, Eddy, Angstadt, Nathan, & Phan, 2007), while weak amygdala-PFC connections are correlated with emotional overarousal in adults (Motzkin, Philippi, Wolf, Baskaya, & Koenigs, 2015).
Amygdala–PFC connectivity, as well as amygdala connectivity with other brain regions (Guyer et al., 2008), appears to show particular patterns of change over development, as measured by age-related differences. Cross-sectional research in non-psychiatric samples ranging in age from early childhood to early adulthood (Gee, Gabard-Durnam, et al., 2013; Gee, Humphreys, et al., 2013; Silvers et al., 2017; Wu et al., 2016) indicates that the amygdala and PFC show positive functional connectivity (i.e., positive temporal correlation between their neuronal signals) during emotion processing in early childhood. During late childhood, these regions become increasingly unconnected (i.e. uncorrelated), and then become increasingly negatively connected (i.e., negatively correlated) with further development. This suggests that increased negative connectivity between these regions represents a normative maturational process from late childhood through early adulthood (Gee, Gabard-Durnam, et al., 2013; Gee, Humphreys, et al., 2013; Silvers et al., 2017; Wu et al., 2016). We note here that a stronger negative correlation refers to a correlation that is moving further away from zero and becoming more negative. This is interpreted as stronger but more negative connectivity between two regions as a function of levels of the predictor.
However, a limited number of studies suggest that this normative trajectory may be altered by adverse developmental experiences. Several studies following community samples have examined the associations between different forms of developmental adversity and amygdala–PFC connectivity. For instance, among older children with an average age of 12, greater early childhood stress predicted weaker amygdala-anterior cingulate connectivity while at rest (Pagliaccio et al., 2015), although they did not examine parenting per se. Among late adolescents aged 18–20 (Herringa et al., 2013), greater levels of recalled childhood parental maltreatment predicted more negative amygdala-PFC connectivity. Similarly, in a sample ranging in age from 6.5 to 17.5 (Gee, Gabard-Durnam, et al., 2013), children and adolescents with a history of early institutional care exhibited increased negative amygdala–PFC connectivity when viewing stimuli such as negatively valenced emotional faces relative to never-institutionalized children. These findings indicate that the PFC is more strongly down-regulating the amygdala’s response to emotional stimuli in children who experienced high levels of developmental adversity relative to those who experienced less. This negative correlation is similar to that seen in adults, and is consistent with theoretical models that predict a more rapid development of inverse functional connectivity between these regions in the context of developmental adversity, but an ultimate blunting of this connectivity later in development (Tottenham & Galvan, 2016). Adverse parenting may affect amygdala–PFC functional connectivity because of the absence of developmentally necessary regulation from the parent (see Tottenham, 2012). That is, over the course of development, children learn from their parents how to respond to stress and how to regulate their emotions based in part on how their parents act towards them and help (or do not help) them in times of stress. The development of amygdala–PFC connectivity may neurally mediate this effect. Maternal hostility may be related to a decreased capacity for emotional regulation as it likely exacerbates rather than assuages or teaches the child how to regulate negative emotions. Importantly, almost all prior work is cross-sectional, with some exceptions (Burghy et al., 2012; Herringa et al., 2016). Given that emotional processing and regulation have implications for risk for an array of psychopathologies as well as psychosocial functioning more generally, understanding the effects of developmental experiences of amygdala connectivity is likely highly important.
Parenting and reward-processing circuitry
The ventral striatum (VS) is the striatal region most closely associated with reward processing (Haber, 2011). It is connected with the prefrontal, insular, and cingulate cortex in that it both sends efferent information to and receives top-down information from them (Haber, 2011). These areas, particularly the orbital and insular cortices, receive sensory information from all modalities. Additionally the VS receives dopaminergic inputs from the ventral tegmental area and substantia nigra (Haber, 2011). FMRI studies have shown that healthy adolescents show stronger VS activation to reward compared to adults (See Galvan, 2013, for a review). Adolescents also typically show heightened reward sensitivity and risk taking compared to other age groups (Galvan, 2013). However, some models contend that adolescents’ VS responsivity to reward should be blunted relative to adults as adolescents do not find modest rewards, such as those in monetary gain-loss, tasks, sufficiently appetitive (Spear, 2000). Some research has found that adolescents show reduced VS activation in response to anticipating monetary gains relative to adults, but do not differ from adults in their VS response following gains (see Geier & Luna, 2009 for a review of this topic). Much prior work has examined adolescents, whereas the current study examines a sample of older children.
VS sensitivity to reward also appears to be influenced by early developmental experiences. For instance, a history of emotional neglect by parents predicted blunted VS activation to monetary rewards in a community sample of adolescents (Hanson, Hariri, & Williamson, 2015). Moreover, adolescents with a childhood history of inhibition, a temperament trait characterized by a tendency to experience distress and to withdraw when faced with unfamiliar environments, people, or situations, which may be influenced by parenting (Fox et al., 2005), in comparison to those with no such history, showed greater striatal activation in anticipation of both monetary gain and loss (Guyer et al., 2006).
Evidence also suggests that the ACC plays an important role in reward processing across the lifespan (Lavin et al., 2013). In particular, fMRI studies in adults show activation in the ACC following losses in decision making tasks (Bush et al., 2002). More broadly, the ACC is part of a network that involves activity in the PFC during decision making (Buckley et al., 2009). It is also part of the cingulo-opercular circuit, connecting to the insula and the operculum, which provide input relative to the differences between expected and actual outcomes of a given decision. The ACC also provides outputs to coordinate dorsolateral prefrontal structures in order to organize behavioral responses (Lavin et al., 2013). Given that reward sensitivity and processing are related to risk-taking, addictive behaviors, and depression, the fronto-striatal and cingulo-opercular circuits connecting the PFC, VS, ACC, and insula also likely have important implications for understanding the neural bases of psychosocial development and functioning.
No literature of which we are aware has examined whether parenting predicts altered fronto-striatal and cingulo-opercular connectivity during reward processing. However, maternal hostility, which is a stressful experience that is threatening to the well-being of the child, may over time promote excessive attention to threats (Troller-Renfree, McDermott, Nelson, Zeanah, & Fox, 2015) and blunted attention to rewards (Starcke & Brand, 2012), which may be less salient as they do not threaten the child’s well-being. Given that the PFC sends excitatory projections to the VS and ACC that influence reward processing and facilitate reward-based learning (Haber, 2011), maternal hostility may influence the PFC to downregulate these regions’ responses to reward in order to focus attention on threats and away from rewards which may appear less salient due to chronic experiences of maternal hostility. Thus, maternal hostility may promote increasingly negative connectivity in these regions during reward processing.
Maternal regulation may also influence amygdala–PFC connectivity during emotion processing and fronto-striatal and cingulo-opercular connectivity during reward processing. Gee et al. (2014) found that children aged 4–10 showed negative amygdala-PFC connectivity when viewing images of their mother’s happy or neutral face over and above a stranger’s face. This parental effect on connectivity seemed to attenuate by adolescence. Gee et al. (2014) therefore suggest that a visual parental cue instantiates momentary increased negative connectivity in the child. Maternal regulation may therefore scaffold emotional regulation in the child through regular periods of negative connectivity, thereby promoting the normative transition from positive connectivity in childhood to negative connectivity in adolescence (argued in Callaghan & Tottenham, 2016). In the absence of parental cues, the amygdala and PFC normatively switch from positive (“non-regulatory”) to negative (“regulatory”) connectivity, with a transition point between childhood and adolescence (where connectivity hovers around zero) (Gee et al., 2013, Silvers et al., 2016). Maternal regulation in the current study may facilitate children’s regulation of their emotions in times of stress, with this effect potentially mediated by weaker (i.e., closer to zero) amygdala–PFC connectivity. This would indicate a normative pattern of development of amygdala-PFC connectivity in the current sample of 10–11 year old children (Gee et al., 2013, 2014).
Moreover, psychological autonomy support, of which appropriate behavioral regulation of the child is a core component, results in intrinsic motivation in children for reward and desired goals (Ryan & Deci, 2000). If fronto-striatal and cingulo-opercular connectivity during reward processing are neural mechanisms underpinning response to reward, maternal regulation may be related to enhanced functional connectivity in these circuits as regulation may influence prefrontal and opercular regions to upregulate the VS and ACC responses to reward.
Limitations of developmental literature predicting functional brain connectivity
Although informative, studies examining developmental predictors of brain functional connectivity have been commonly hampered by several limitations. First, the majority have been cross-sectional. Second, parenting and other forms of developmental adversity has largely been measured via self- or parent-report in child and adolescent samples (Burghy et al., 2012; Herringa et al., 2013; Herringa et al., 2016; Pagliaccio et al., 2015; Thomason et al., 2015). While informative, parent- and self-reports may be subject to errors or biases in recall due to current neural functioning, psychopathology, current mood, personality, or other factors (Hardt & Rutter, 2004). Moreover, while lab-based observations of parent-child interactions converge well with in-home observations of parent-child interactions, neither converges well with parent- or child-reports of parenting (Zaslow et al., 2006). Lab- or home-based observations of parenting have also been found in some research to be better predictors of child psychopathological outcomes than parent-reports (Zaslow et al., 2006). This suggests parent- or self-reports alone may not provide a comprehensive picture of a child’s parenting experiences. We should note there may also be limitations to lab-based observations of parenting, such as concerns about ecological validity, transient influences such as mood states, and restrictions in the range of affect and behavior elicited in the parent and child. It is therefore likely that parent-reports and lab-based observations provide unique perspectives, and so both are used in the current study. Third, the literature to date has largely examined relatively extreme forms of adversity, and examined them categorically, such as the presence or absence of physical or emotional maltreatment, poverty, or institutional rearing (for a review, see Tottenham & Galvan, 2016), although some studies in adolescents have examined adversity continuously (Burghy et al., 2012; Herringa et al., 2013; Herringa et al., 2016). More subtle and prevalent influences, such as parental behavioral regulation of the child, and hostility, each measured continuously rather than categorically, may influence a larger proportion of the population and better capture the nature of most developmental experiences. Fourth, with only two exceptions of which we are aware (Burghy et al., 2012; Herringa et al., 2016), the few longitudinal studies to date examining the influence of parenting on neural functional connectivity in these circuits have not followed children for more than a few years. Fifth, although not a limitation per se, prior studies have focused on adolescents and young adults (e.g., Burghy et al., 2012; Herringa et al., 2013; Herringa et al., 2016), rather than focusing on children, despite the presumed important role of parenting in early childhood on children’s functional brain development. Thus, examining children provides a unique test of the theorized effects of parenting in early childhood on brain functional connectivity in late childhood, and stands to further knowledge of this developmental process.
A related issue is that this literature has exclusively examined adverse or negative developmental experiences and has not examined the influence of positive parenting. This is despite evidence that positive parenting, such as high levels of warmth, care, or support, promotes a range of positive child psychological and academic outcomes (Landry, Smith, Swank, & Guttentag, 2008) as well as altered brain structure (Whittle et al., 2014; Whittle et al., 2009). This lack of research may stem from the assumption that positive parenting lies on a continuum with adverse parenting, which it is often does not (see Whittle et al., 2014 for a discussion). That is, low levels of developmental adversity (e.g., hostility) do not necessarily indicate high levels of positive parenting. The only related research to date of which we are aware suggests that positive parenting practices predict altered volume of limbic regions and thickness of various cortical regions in children and adolescents (Luby et al., 2012; Whittle et al., 2014; Whittle et al., 2009). Taken together with the literature on developmental adversity and neural functional connectivity, the field’s current knowledge of the effects of parenting on offspring functional connectivity is therefore confounded or limited by multiple important factors. The current study overcomes many of these limitations by using a 7–8 year longitudinal follow-up and observational assessments in addition to parent-reports of parenting in early childhood. To our knowledge, no study to date has used these methods, rendering the current study unique in the parenting and fMRI literature.
Overview and hypotheses.
This study examined whether maternal hostility and regulation of the child’s behaviors, assessed when the child was three years old, relate to amygdala-whole brain connectivity during processing of emotional faces and VS- and ACC-whole brain connectivity during a monetary reward task seven to eight years later. This study focuses on these two parenting variables as each has been widely studied and represent core aspects of parenting that are particularly influential for children’s psychosocial development (e.g., see Bradley & Vandell, 2007; McLeod et al., 2007). Based on prior literature which suggests that maternal hostility and regulation may influence emotional and reward-processing circuitry in the developing brain (Reviewed in Tottenham & Galvan, 2016; Hanson et al., 2015), we expected high levels of maternal hostility, assessed observationally, to predict 1) increased negative amygdala connectivity (i.e., a stronger but more negative correlation) with prefrontal regions while viewing sad versus neutral faces, and 2) increased negative ventral fronto-striatal and cingulo-opercular connectivity during monetary gain versus loss feedback. We also expected high levels of maternal regulation of the child, assessed via mother-report, to predict 1) enhanced connectivity (i.e., stronger and positive) of fronto-striatal and cingulo-opercular circuits during monetary gain relative to loss, and 2) weaker (i.e., closer to zero) amygdala–PFC connectivity, reflecting typical amygdala–PFC patterns seen in children raised in positive environments (Reviwed in Tottenham & Galvan, 2016).
Methods
Participants
This study was part of a larger longitudinal study of children and their parents. Of the 559 children assessed at age 3 (M=3.44, SD=.25), 79 participants (32 Female) completed fMRI scans approximately 7–8 years later. Of those, 13 did not have usable face processing data and 10 did not have useable reward processing data because of excessive head motion. Thus, 66 had useable fMRI scans during face processing tasks while 69 had useable fMRI scans during reward processing. A total of 73 participants had useable data for one of the two tasks (M age = 10.27 years, SD = 0.92 years). Of those who had usable imaging data, 4 had missing data on mother reports of regulation. Thus, our effective sample size for the Faces task was N=62 (i.e., 66 useable scans minus 4 missing on maternal regulation) and our effective sample size for the Doors task was N = 65 (i.e., 69 useable scans minus 4 missing on maternal regulation). Participants with unusable imaging data or who had missing data on maternal regulation did not differ from those with usable data on sex (p = .09), race/ethnicity (p = .43), socioeconomic status (SES) (p = .38), the likelihood of coming from a two-parent home (p = .24), maternal hostility (p = .42), or maternal regulation (p = .98). Participants were recruited from the community utilizing commercial mailing lists, screened for any major medical conditions, and required to have at least one English-speaking biological parent. Exclusionary criteria included any developmental disabilities, metal or electronic implants, a history of head trauma, or use of medications known to affect brain functioning (e.g., antihistamines, pain killers). Participants were oversampled based on their temperamental negative emotionality, low positive emotionality, or behavioral inhibition, assessed observationally when they were three years old (see Kann, O’Rawe, Huang, Klein, & Leung, 2017). This oversampling was done as the broader goal of the study from which this sample was derived was to understand early childhood risk factors for later depressive and anxiety disorders, for which high negative emotionality, low positive emotionality, and high behavioral inhibition are risk factors (see Olino, Klein, Dyson, Rose, & Durbin, 2010, for details). Negative and positive emotionality as well as behavioral inhibition were assessed via the Laboratory Temperament Assessment Battery (Lab-TAB; Goldsmith, Reilly, Lemery, Longley, & Prescott, 1995), which involves a standardized set of tasks designed to elicit children’s bodily, vocal, and facial expressions of a range of emotions (see Olino et al., 2010). Approximately equal groups of children were selected from each of five “cells”; four cells created by crossing high negative emotionality (25 percentile and above) vs average negative emotionality (30–70th percentile) with low positive emotionality (75th percentile or below) vs average positive emotionality (30–70 percentile), and a fifth cell with children who were not in the other cells but were in the top 25% of the distribution on fear/inhibition. See Supplementary Methods S1 for further details of participant selection from the original sample. In order to confirm that results were not due to participant’s temperament group, analyses were repeated after covarying temperament group status. Kann et al. (2017) found that greater early negative emotionality predicted increased amygdala reactivity but more negative connectivity between the amygdala and the fusiform gyrus while viewing faces relative to houses.
Pubertal status was assessed with the Pubertal Development Scale (PDS) (Petersen, Crockett, Richards, & Boxer, 1988). Participants had a mean puberty score of 8.37 ± 2.36, indicating age-appropriate levels of pubertal development. One male participant did not complete the PDS. There was no significant difference in PDS scores between males (8.14 ± 2.13) and females (8.65 ± 2.67) (t < 1). Age was not significantly correlated with puberty scores (r = 0.28, p = .089), probably due to the restricted age range.
Demographic information is shown in Table 1. Children primarily came from middle- and working-class families as measured by Hollingshead’s four-factor index of social status (Hollingshead, 1975), which is based on a combination of parental education and occupational prestige. At age 3, most children lived with both biological parents and the large majority were Caucasian and non-Hispanic.
Table 1.
Demographic information
| Variable | Descriptive information |
|---|---|
| Sex | 34 male; 29 female |
| Age | 10.27 years, SD = 0.92 years |
| Race | 81.0% Caucasian |
| Socioeconomic status | M = 46.25, SD = 9.95 |
| % Living with both parents age at 3 | %85.70% |
Note: Based on sample of 63 children who provided useable faces and doors data.
Age 3 data were collected between 2004–2006 and fMRI scans were completed over the course of 2013. Informed consent was obtained from the children’s parents, and the protocol was approved by our Institutional Review Board.
Procedure
Parenting measures.
The mother’s expressions of hostility towards the child were coded while both participated in the Teaching Tasks Battery (Egeland et al., 1995) during the age 3 assessment. The teaching tasks battery is a widely-used, standardized, laboratory-based method of assessing parenting behaviors towards young children. The battery consisted of 6 standardized parent–child interaction tasks lasting a total of 25–30 minutes. The tasks, which occurred in the order listed here, were designed to elicit a variety of parent and child behaviors and consisted of book reading, naming objects with wheels, block building, matching shapes, completing a maze using an etch-a-sketch, and receiving a gift. Maternal hostility (α = .76) in each of the 6 tasks was coded on a 5-point scale ranging from “very low” to “very high” and averaged across tasks to yield a total score. Ratings of maternal hostility measure the mother’s expression of anger, frustration, annoyance, discounting, or rejection of the child. A mother scoring high on this scale clearly and openly rejects the child, blames him or her for mistakes, and otherwise makes explicit the message that she does not support the child emotionally. A score of 1, or “very low,” indicates that the mother shows no signs of anger, annoyance, frustration, or rejection. This does not necessarily indicate that she is supportive, but she does not put the child down or reject them. A score of 2, or “moderately low,” indicates that signs of hostility, such as anger, irritation, or rejection, are fleeting, but occurred on several occasions during the session, and at least one sign could be identified as clear and overt expressed or unexpressed anger towards the child. A score of 3, or “moderate,” indicates several instances of hostile or rejecting behaviors. Two or more of these events are reliably clear to observers, although the expressions are brief and do not set the tone of the mother’s interactions immediately following the episodes. Scores of 1.5 or 2.5 were for behaviors judged to be more hostile than a particular category but not sufficiently hostile to merit a one-point increase. The interrater ICC (n = 35) for maternal hostility was .83, indicating excellent interrater reliability.
Two participants were outliers on maternal hostility in that they showed particularly elevated scores (Figure 1) and introduced both skew (skew = 3.04) and kurtosis (kurtosis = 12.42) to the overall distribution. It should first be noted that these were not erroneous outliers. Rather, they were two children in the sample who experienced substantially elevated levels of maternal hostility during their lab visit. Given the pernicious nature of this variable, it is reasonable to expect a positively skewed distribution and important to retain such extreme cases.
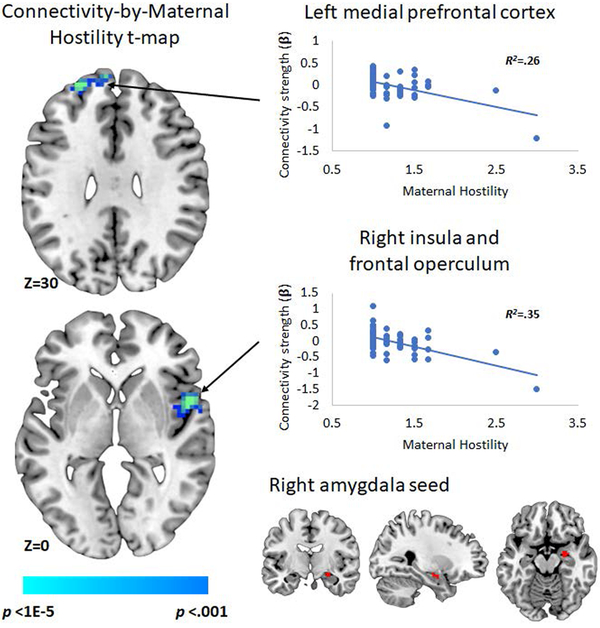
Figure 1.
Effects of age 3 maternal hostility on functional connectivity of the left and right amygdala while viewing sad relative to neutral faces. Note: N = 61. Effects on connectivity of left amygdala significant at peak p < .001 with cluster-wise FWEc. Labels refer to entire cluster, including but not limited to those brain regions visible in the slice. Scatterplots based on effects in peak voxel in each cluster (See Table 2). R2 values represent the effect of the labelled predictor when entered alone in the regression model.
However, in order to examine robustness of results after normalizing data, where results showed an effect of maternal hostility on functional connectivity, ancillary confirmatory analyses were conducted both after log-transforming maternal hostility as well as winsorizing the two outliers to within the 95th percentile. Transformation removed outliers, removed skewness (skew = .11), and substantially reduced kurtosis, although the data remained mildly kurtotic (kurtosis = −2.01).
Maternal regulation of the child’s behaviors at age 3 was assessed via the regulation subscale of the Parenting Styles and Dimensions Questionnaire (PSDQ; Robinson, Mandleco, Olsen, & Hart, 2001), a widely used parent-report measure of parenting styles. Regulation refers to appropriate rule and limit setting for the child along with appropriate, rational explanations for those rules and limits. It comprises 5 items. Sample items include “Gives child reasons why rules should be obeyed” and “Explains to child how we feel about the child’s good and bad behavior.” Parents are asked “for each item, rate how often you exhibit this behavior with your child.” Response options range from 1 (“Never”) to 3 (“About half of the time”) to 5 (“Always”). The PSDQ has shown good internal consistency, agreement with informants, and convergent validity with child psychosocial functioning (see Olivari et al., 2013 for a review; Robinson et al., 2001). Maternal regulation was normally distributed (skew = −.60, kurtosis = .69). In the current sample, internal consistency for regulation was .79.
fMRI Emotional Faces task.
Each participant performed three iterations of four kinds of task blocks that lasted 16 seconds each in one of two pseudo-random orders. The task blocks were interleaved with fixation blocks of 14 seconds each. The four task conditions were defined by the type of stimuli presented in each: neutral faces, sad faces, happy faces, and houses. At the beginning of each block, there was a one second warning period, in which the fixation cross changed in color from black to blue cuing the starting of the block, followed by four trials, two match trials and two non-match trials. Each trial began with a fixation cross that lasted 600 ms followed by the simultaneous presentation of two images for 3000 ms. Participants were asked to make a button press to indicate whether or not the two images were identical or different. The task lasted a total of 6.2 minutes for each subject. Within the entire task, 186 volumes were collected.
fMRI Monetary Reward task.
An event-related design for the monetary reward task was used. On each trial, participants viewed two doors on the screen, and were asked to choose a door. One door led to feedback of monetary gain, and the other loss, with a 50% chance of a win or loss after each choice. Participants completed a total of 60 trials (30 win, 30 loss). There were two pseudorandom sequences of win/loss trials that lasted 16 seconds each. At the beginning of each trial, participants were presented with a fixation point lasting 2 seconds. The two doors were then presented for 2 seconds for the participants to make their choice of the left or right door with a button press. After a fixation period of 800 ms, feedback (i.e., “You Win” versus “You Lose”) was presented for 1.2 seconds. The interstimulus interval was jittered (M = 2 s, range = 0 – 14 s). The average trial duration was 8 seconds. The task lasted a total of 8.2 minutes for each subject. Within this task, there were two runs and each consisted of 130 volumes.
Scanning procedure.
On the day of scanning, children first participated in mock scanning to acclimate them to the experimental environment utilizing an MRI simulator (Model number: PST-100355 from Psychological Software Tools, USA). During the mock scanning session, children were given feedback regarding their head motion utilizing the Flock of Birds motion tracking system (Ascension Technology Corporation, USA) and MoTrack motion tracking software (Psychology Software Tools, USA). During both the mock and the actual scanning, foam pads were placed around the participant’s head to reduce head movement.
Data Acquisition, Preprocessing, and Analysis.
All images were acquired utilizing a Siemens Trio 3 T scanner. For each child, the scanning session began with the acquisition of T1-weighted high resolution structural images using the following MPRAGE sequence: slices = 176, slice thickness = 1 mm, TR = 2400 ms, TE = 3.16 ms, flip angle = 8°, matrix size = 256 × 256, FOV = 256 × 256, resolution = 1 × 1 × 1 mm3. After that, a set of inplane T2-weighted structural images were collected in the axial oblique plane, parallel to the AC-PC plane with the following parameters: slices = 37, slice thickness = 3.5mm, TR = 6450 ms, TE = 88 ms, flip angle = 120°, matrix size = 256 × 256, FOV = 256 × 256, resolution = 1 × 1 × 3.5 mm3. Slice orientation used T2*-weighted axial images, acquired using the following EPI sequence: 37 interleaved axial slices, slice thickness = 3.5 mm, TR = 2000 ms, TE = 30 ms, flip angle = 900, matrix size = 64 × 64, FOV = 224×224 mm, resolution = 3.5 × 3.5 × 3.5 mm3.
All images were processed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm8/). Motion outliers were defined as volumes with a scan-to-scan motion greater than 0.5 mm in either the x, y or z plane, or 0.01 radians in either the roll, pitch or yaw rotation, using the Artifact Detection Tools (ART) (http://www.nitrc.org/projects/artifact_detect/). On average 17% of scans were identified as outliers per run (ranging from 5% to 31% across subjects). Runs were excluded from analysis if more than 1/3 of volumes were outliers. Prior to analysis, images were corrected for slice timing, realigned to the middle scan, and from this realignment 6 parameter rigid body transformations were calculated. Structural images were coregistered with the mean functional image, segmented, and then normalized to a standard space using the MNI template. Functional images were then normalized using the same parameters as the structural normalization. Finally, the functional images were smoothed using a 6mm FWHM Gaussian kernel.
Univariate analysis was conducted using the General Linear Model (GLM). Each stimulus condition was modeled in the GLM as a regressor using a boxcar function convolved with the canonical hemodynamic response function. The 6 motion parameters and volumes with high motion (Composite Motion >= 0.5 mm, Mean Global Signal (z) >= 3.0) were entered into the model as covariates of no interest. For the faces task, beta values were estimated for each voxel for each of the two conditions of interest (i.e., neutral, sad), and these estimated parameters were used to generate the contrasts of interest. For the monetary reward task, the same procedure was followed, but the conditions were monetary gain feedback versus loss feedback. The respective sad versus neutral faces and the gain versus loss contrast images from the Emotional Faces and the Monetary Reward tasks were then entered in second-level one-sample t-test models.
Seed regions for emotional face processing were functionally defined following a small volume search of the left and right amygdala and the sample’s average peak voxel reactivity in each. Seed regions for reward processing were functionally defined based on the sample’s average peak voxel reactivity in left and right VS and medial (bilateral) ACC during processing of monetary gains versus loss. Spheres with a 6mm radius centered on those peak voxel coordinates were created and used as seed regions for each respective task. These seeds were then constrained within the boundaries of the regions of interest as defined by the AAL atlas. Psychophysiological Interaction (O’Reilly, Woolrich, Behrens, Smith, & Johansen-Berg, 2012) analyses were conducted to examine task-dependent connectivity of the amygdala with the whole brain during the processing of sad versus neutral faces and of the VS and ACC with the whole brain during the processing of monetary gain versus loss as a function of early parenting experiences. That is, PPI analyses tested the extent to which the connectivity of the amygdala, VS, or ACC with other brain regions during one task condition relative to another correlated with early parenting experiences.
All functional connectivity whole-brain maps at the group level were thresholded using a cluster-defining threshold (CDT; i.e., voxel-wise threshold) of p<.001 and a family-wise error cluster (FWEc) correction of p<.05, as implemented in SPM. Using this correction method based on Gaussian random field theory (RFT), the FWE corrected significance of each cluster is obtained and can be further corrected (e.g., with a Bonferroni correction) for the multiple parenting variables and PPI seeds tested. We thus performed a Bonferroni correction based on two parenting variables and five seed regions, therefore correcting for 10 comparisons in total. This resulted in a p-threshold of .005 (.05/10 = .005). If an effect did not survive this level of correction, we also report whether it survived correction for two separate parenting predictors, which yields a p-threshold of .025 (.05/2 = .025).
All analyses included both maternal regulation and hostility as simultaneous predictors to examine their independent effects adjusting for each other. All analyses were repeated after covarying age during the scan as well as sex and socioeconomic status. None were related to functional connectivity of any seed with any brain region during any task, and results reported below remained virtually identical with age, sex, or socioeconomic status in the models. Results are therefore reported without these covariates in the models. One-sample t-tests are reported for all results, which is the standard approach to reporting the effects of a covariate on seed-based functional connectivity. Based on prior literature (Burghy et al., 2012; Herringa et al., 2013; Herringa et al., 2016), we expected medium to large effect sizes. Power analyses indicated a sample of 68 participants would provide 85% power to detect medium to large effects (R2 = .20) at p < .01. Finally, no correlation was found between any of the parenting measures and mean frame-wise displacement on any of the tasks.
Results
Bivariate associations between parenting variables.
Mean maternal hostility scores were 1.19 (SD = .32, Range = 1–3) while mean maternal regulation scores were 20.61 (SD = 3.00, range = 13–25). Maternal hostility was not significantly associated with maternal regulation (r = −.14, p = .24, N = 73).
Reactivity to sad versus neutral faces.
At the group level, direct contrast of sad versus neutral faces revealed significant activation in the right inferior frontal gyrus (IFG) pars triangularis, left middle and right inferior temporal gyrus, bilateral inferior and superior parietal lobes, right angular gyrus, left inferior occipital cortex, left fusiform gyrus, right middle occipital cortex (see supplementary Figure S2 and Table S1).
Reactivity to monetary gain versus loss feedback.
A direct contrast of monetary gain versus loss revealed significant activation in the bilateral VS and ACC, as well as various frontal, parietal, temporal, occipital and cerebellar regions (see supplementary Figure S3 and Table S2).
PPI – Effects of early childhood parenting on amygdala-whole brain connectivity during emotional face processing.
With the left amygdala as a seed (Figure 1A), increased maternal hostility predicted more negative connectivity with the right insula and operculum. With the right amygdala as a seed region (Figure 1B), when viewing sad relative to neutral faces, increased maternal hostility predicted more negative connectivity with the right insula and operculum and bilateral mPFC (i.e. medial superior frontal gyrus) (Table 2). There were no significant effects of maternal regulation on connectivity of either the left or right amygdala.
Table 2.
Effects of maternal hostility on connectivity with the right and left amygdala while viewing sad faces relative to neutral faces (N = 61).
| Region | MNI Coordinates (X, Y, Z) |
Cluster size |
Peak-level t (df = 59) |
Cluster- level R2 |
Cluster-level p (FWEc) |
|---|---|---|---|---|---|
|
Seed: Left Amygdala (X, Y, Z = 21, −3, −18) |
|||||
| Right insula and operculum | 54, 6, −3 | 63 | −4.73 | .36 | .005* |
|
Seed: Right Amygdala
(X, Y, Z = −21 −6, −18) |
|||||
| Right insula and operculum | 51, 6, 0 | 79 | −5.44 | .36 | .001* |
| Left medial superior frontal gyrus | −24, 54, 30 | 66 | −5.11 | .26 | .004* |
Note: R2 values represent the effect of maternal hostility over and above that of maternal regulation.
Survives correction for 10 comparisons (2 parenting variables X 5 PPI seeds); Coordinates and statistics are reported for the peak voxel in each cluster.
PPI - Effects of early childhood parenting on VS- and ACC-whole brain connectivity during monetary reward processing.
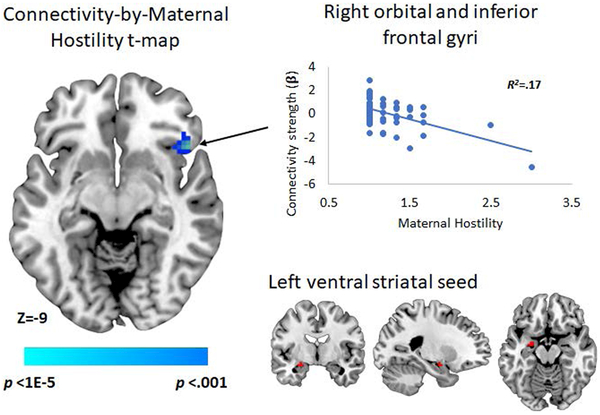
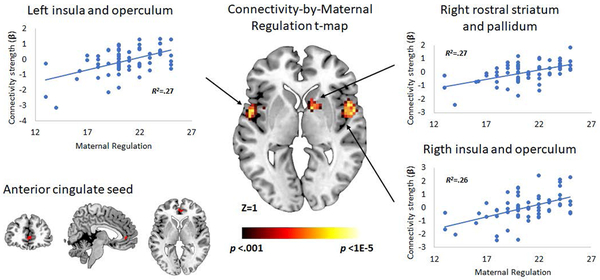
With the left VS as a seed region, greater maternal hostility predicted more negative connectivity with the right posterior OFC and IFG (Table 3; Figure 2A, top). With the ACC as a seed region (Figure 2B), greater maternal regulation predicted more positive connectivity with bilateral insular and opercular regions as well as the right anterior striatum/pallidum (Table 3). There were no significant effects of maternal hostility on connectivity of the right VS or the ACC, or of maternal regulation on connectivity of the left or right VS.
Table 3.
Effects of maternal hostility and regulation on connectivity of the left ventral striatum (VS) and the anterior cingulate cortex (ACC) while viewing monetary gain versus loss (N=65).
| Region | MNI Coordinates (X, Y, Z) |
Cluster size |
Peak-level t
(df =62) |
Cluster- level R2 |
Cluster-level p (FWEc) |
|---|---|---|---|---|---|
|
Seed: ACC (X, Y, Z = 3, 47, 0) Predictor: Maternal regulation |
|||||
| Right insula and operculum | 54, 0, 3 | 84 | 4.69 | .29 | .001* |
| Left insula and operculum | −45, 3, −6 | 125 | 4.45 | .27 | <.001* |
| Right anterior striatum/pallidum | 12, 6, 0 | 40 | 4.31 | .25 | .041 |
|
Seed: Left VS (X, Y, Z = −12, 9, −3) Predictor: Maternal hostility |
|||||
| Right orbital and inferior frontal gyri | 48, 21, −9 | 79 | −4.25 | .14 | .002* |
Note: R2 values represent the effect of the labelled predictor (i.e., maternal hostility or maternal regulation) over and above that of the other predictor (i.e., maternal regulation or maternal hostility).
Survives correction for 10 comparisons (2 parenting variables X 5 PPI seeds); Coordinates and statistics are reported for the peak voxel in each cluster.
Figure 2.
Effects of age 3 maternal hostility on functional connectivity of the left VS (top) and of age 3 maternal regulation on functional connectivity of the ACC during monetary gain relative to loss (bottom). Note: N = 65. All effects significant at peak p < .001 with cluster-wise FWEc. Labels refer to entire cluster, including but not limited to those brain regions visible on the brain sections. Scatterplots based on effects in peak voxel in each cluster (See Table 3). R2 values represent the effect of the labelled predictor when entered alone in the regression model.
Ancillary confirmatory analyses
In order to confirm the robustness of results after either log-transforming maternal hostility or winsorizing the two outliers on this variable, parameter estimates of connectivity averaged across the voxels within each cluster were extracted and Pearson’s correlations were computed between these connectivity estimates and transformed or winsorized maternal hostility. We also applied a Bonferroni correction based on 10 comparisons (2 parenting variables multiplied by 5 seeds). As such, our p-value threshold was reduced to .005 (.05/10 = .005). Both maternal regulation and hostility were simultaneously included as predictors in each model.
All effects of maternal hostility on amygdala connectivity during the faces task and of left ventral striatal connectivity during the monetary loss-gain task survived correction for 10 comparisons. Higher levels of maternal hostility continued to predict more negative connectivity of the left amygdala with the right insula/operculum (Log Maternal Hostility: β = −.49, t(59) = −4.19, p < .001; Winsorized Maternal Hostility: β = −.56, t(59) = −5.10, p < .001). Higher levels of maternal hostility also continued to predict more negative connectivity of the right amygdala and the right insular/operculum (Log Maternal Hostility: β = −.45, t(59) = −3.75, p < .001; Winsorized Maternal Hostility: β = −.48, t(59) = −4.07, p =. 002) as well as the mPFC (Log Maternal Hostility: β = −.44, t(59) = −3.65, p = .001; Winsorized Maternal Hostility: β = −.34, t(59) = −2.74, p = .008). Greater maternal hostility also continued to predict more negative connectivity of the left ventral striatum with the right posterior OFC and IFG, which were both contained in one cluster (Log Maternal Hostility: β = −.38, t(62) = −3.22, p = .002; Winsorized Maternal Hostility: β = −.39, t(62) = −3.31, p < .001).
Controlling for temperament group
When including temperament group as the only predictor of functional connectivity, there were no significant main effects of group on functional connectivity. We then computed models examining our parenting predictors while including temperament group as a covariate. In nearly all models, there remained no significant effect of temperament at a voxel-wise threshold of p < .001 with cluster correction threshold of FWEc p < .05, and most of the main effects of parenting on functional connectivity remained virtually unchanged. However, our finding of maternal hostility predicting decreased connectivity between left amygdala and right insula/operculum during processing of sad versus neutral faces no longer survived FWEc (p=.235), although it survived voxel-wise threshold of p<.001 (k=22; Table S3). Similarly, our finding of maternal regulation predicting increased connectivity between ACC and right anterior striatum/pallidum during monetary loss versus gain no longer survived FWEc (p=.286), but it survived voxel-wise threshold of p<.001 (k=20; Table S4). In addition, when examining right VS functional connectivity during monetary loss versus gain while including temperament group in the model, a significant effect of maternal hostility emerged, although no significant main effect of temperament group was detected. Specifically, maternal hostility predicted decreased connectivity of the right VS during monetary gain versus loss with right posterior OFC and IFG, right temporal gyri, and right inferior parietal lobe (Table S4).
Discussion
This study examined the associations, over seven to eight years, between parenting experiences, assessed in early childhood at age 3 years, and late childhood neural functional connectivity during affective processing tasks including emotional faces and monetary gain versus loss, between the ages of 10 and 11 years, as assessed via fMRI. This is, to our knowledge, the first study to prospectively examine the relationships between parenting in early childhood on neural development over such a long time period (with two exceptions, Burghy et al., 2012; Herringa et al., 2016), and to overcome limitations of much prior work by using both lab-based observations of parenting as well as parent-reports. This is also the first study to simultaneously examine the association of both positive and aversive parenting in early childhood with multiple aspects of late childhood neural functional connectivity. Specifically, greater maternal hostility was associated with increased negative connectivity of the amygdala with frontal, temporal, parietal and insular regions, including the mPFC, during processing of sad faces, as well as increased negative VS connectivity with the ventrolateral PFC (i.e. the IFG) during processing of reward. In addition, elevated maternal regulation was related to more positive ACC connectivity with the bilateral insula during processing of reward.
Research into developmental predictors of neural functional connectivity during emotional and reward processing has largely relied on adults’ retrospective reports of developmental experiences or has examined cross-sectional associations between parental reports and children’s and adolescents’ neural function in community samples (with some exceptions, e.g., Burghy et al., 2012; Herringa et al., 2016). The current study’s incorporation of both direct observations and maternal reports of parenting, as well as our finding that these relationships persist seven to eight years later, extend our knowledge of the relationship between parenting and late childhood neural functional connectivity. Moreover, in contrast to most prior research (Gee et al., 2013; Herringa et al., 2013; Wolf & Herringa, 2016), the current study sampled a normative range of early experiences (although for an exception, see Graham, Pfeifer, Fisher, Carpenter, & Fair, 2015). Results suggest that relatively subtle forms of adverse parenting, such as maternal hostility and regulation, relate to brain functional connectivity over childhood. Given we did not examine more extreme forms of adversity, it is unclear whether effects found in the current study would be comparable to the effects of more extreme adversity found in other studies. Prior studies have also not examined the influence of positive, rather than aversive or negative parenting. These more subtle forms of parenting, both negative and positive, affect a greater proportion of the population than do more extreme experiences, making their influence on neural development particularly important to understand.
Parenting and emotional processing neural circuitry
Only maternal hostility predicted increased negative amygdala connectivity with frontal, parietal, and insular regions. The literature, which is largely cross-sectional (with some exceptions, Burghy et al., 2012; Herringa et al., 2016), indicates that early adversity, such as early childhood stress (Pagliaccio et al., 2015), childhood maltreatment (Herringa et al., 2013; Thomason et al., 2015), and institutional rearing (Gee, Gabard-Durnam, et al., 2013) is associated with the development of increasingly negative amygdala-frontal connectivity in community samples of children and adolescents. The current study supports this possibility and demonstrates that associations of maternal hostility, assessed in three-year-olds, with emotion processing circuitry may be visible at least 7–8 years later. Furthermore, these results support the contention that relatively subtle forms of adverse early-life caregiving are associated with altered capacity for emotional regulation in the developing brain (See Tottenham, 2012), although we did not examine emotional regulation in the current study. In addition, alterations in connectivity between the amygdala and insular/opercular regions may indicate that maternal hostility adversely impacts individuals’ capacity for cognitive control or regulation in the context of negative or aversive stimuli (Chang, Yarkoni, Khaw, & Sanfey, 2013).
That the amygdala shows increasingly negative connectivity at high levels of maternal hostility is consistent with models of normative development that suggest these regions should be less strongly connected in late compared to early childhood (Silvers et al., 2017; Wu et al., 2016; See Tottenham, 2012) but that childhood stress results in adult-like patterns of amygdala-connectivity (Reviewed in Callaghan & Tottenham, 2016; Tottenham, 2012; Tottenham & Galvan, 2016). Results therefore provide novel evidence that relatively subtle adverse parenting in early childhood is associated with accelerated development of increased negative coupling between the amygdala and various brain regions implicated in emotional processing as much as seven to eight years later.
Parenting and reward processing neural circuitry.
Prior research suggests that developmental adversity promotes hyporeactivity of the VS to reward (Reviewed in Tottenham & Galvan, 2016). However, no studies of which we are aware have examined the effects of developmental experiences on functional connectivity between regions during reward processing tasks, although Fareri et al. (2017), found that children and adolescents with a history of institutional care early in life but who were adopted at varying ages into foster homes showed increased VS-mPFC connectivity while at rest relative to children raised by their biological parents. Maternal hostility was associated with more negative connectivity of the left VS with the right IFG and posterior OFC. Maternal hostility in childhood may therefore be associated with a more limited capacity of the developing brain’s frontal regions to regulate, via various aspects of executive functioning and cognitive control (Jiang et al., 2015), the VS response to reward. These findings are consistent with theories (Auerbach, Admon, & Pizzagalli, 2014) which posit that early life stress may confer vulnerability to later maladaptive psychosocial functioning because of its effects on reward processing neural circuitry. Greater maternal regulation was also associated with more positive connectivity of the ACC with the bilateral insula/operculum. Part of the cingulo-opercular circuit, the ACC receives inputs from the insula relative to the differences between expected and actual outcomes of a given decision and provides outputs to coordinate dorsolateral prefrontal structures in order to organize behavioral responses (Haber, 2011; Shackman et al., 2011). As such, maternal regulation may enhance the capacity of this circuit to detect and respond to salient or rewarding outcomes and decide how to respond.
Implications
While results suggest long-term relationships between parenting and brain functional connectivity, they may also suggest some specificity in the relationships of different parenting behaviors on different aspects of neural function, although these findings should be interpreted with caution until they are replicated. Only maternal hostility predicted altered amygdala and VS connectivity. Given that hostility, as measured in the current study, is an aversive parenting behavior, it is possible that only aversive or particularly salient developmental experiences, although still within the normative range of developmental experiences, influence amygdala connectivity during emotional processing and VS connectivity during reward processing (Reviewed in Tottenham & Galvan, 2016). Finally, parental regulation is characterized by appropriate rule and limit setting while still permitting exploration and independence (Robinson et al., 2001). Psychological autonomy support, of which appropriate regulation is a core component, results in intrinsic motivation in children for reward and desired goals, which is in turn related to increased goal-oriented behaviors (Ryan & Deci, 2000). As such, enhanced ACC-insula (i.e., cingulo-opercular) functional connectivity may represent a neural mechanism underpinning motivational states for reward which is developmentally influenced by parental regulation.
Results may also be consistent with broader models of parental socialization of children. Grusek and Davidov (2010) proposed that there are specific domains of socialization of children by parents that may have unique effects on specific domains of children’s psychosocial development. For example, Grusek and Davidov (2010) propose that ‘protection’ is an important domain of parenting, the primary goal of which is to alleviate the child’s distress. Elevated maternal hostility in the current study may signal not just a lack of protection but an active threat to the child’s well-being from their primary protector during this developmental phase. Maternal hostility may also convey to the child that their parent will not be there in times of need. Grusek and Davidov (2010) suggest that children who perceive a lack of protection from their parents will lack the ability to self-regulate their own emotional distress; this suggestion may be consistent with our finding of that maternal hostility relates to increased negative connectivity of the amygdala and prefrontal regions during emotional processing. However, they also note that domains of socialization and child outcomes are interconnected. As such, maternal hostility may also be relevant to Grusek and Davidov’s (2010) control domain, which involves the parent controlling the child’s actions to promote what the parent believes are socially acceptable behaviours. However, hostile parents may threaten the child or withdraw rewards to control their child. This may explain the current finding that elevated maternal hostility relates to increased negative ventral striatal connectivity during reward processing, as hostility may represent an inappropriate form of control. In contrast, behavioral regulation of the child is likely an adaptive form of control of the child. Grusek and Davidov (2010) suggest that children who are appropriately controlled likely show greater anticipation of rewards and positive outcomes, a possibility consistent with our finding that high levels of maternal regulation relate to enhanced connectivity of neural reward processing circuitry.
These results may also contribute to our understanding of the mechanisms underlying the well-established relationship between parenting and later risk for psychopathology in offspring. Deficits in emotional regulation and reward processing are transdiagnostically related to a wide variety of mental illnesses including depression, anxiety, bipolar disorder, schizophrenia, substance abuse, and eating disorders (Barch, 2008; Pechtel & Pizzagalli, 2011). If replicated, such evidence would help elucidate the phenomenon of multifinality (Cicchetti & Rogosch, 1996), which refers to the fact that multiple outcomes (i.e., mental illnesses) can share the same origins (i.e., adverse parenting). Examining the effects of more specific parenting behaviors, as opposed to examining adverse parenting as a unitary construct, may help to explain why adverse parenting when examined as a unitary construct predicts so many diverse outcomes (i.e., multifinality). That is, specific parenting behaviors may relate to specific aspects of neural functioning, which may in turn relate to different psychosocial outcomes. However, we should note that these implications are speculative and go well beyond the data in the current study. As such, they should be interpreted cautiously and explored in further research specifically designed to address these issues.
Limitations
Despite several notable strengths, including observational in addition to parent-report measures of parenting behaviors, a 7–8 year longitudinal period, and the examination of multiple aspects of parenting and neural functional connectivity, some limitations should be noted. Perhaps most importantly, parenting, but not neural functional connectivity, was assessed at age 3, while neural functioning, but not parenting, was assessed at age 10–11. Thus, it is possible that our effects are the result of unmeasured cross-sectional associations between parenting and child brain functioning in early childhood or that both are influenced by some third, unmeasured factor (Rothbart, 2007). However, disentangling this relationship would require repeated assessments of both parenting and functional connectivity over a 7–8 year period.
Another limitation is that the range of maternal hostility scores was relatively restricted. While it is scored on a five-point scale, the highest score was 3.0. Thus, it is unclear how maternal hostility would relate to brain functioning if we had observed a full range of maternal hostility. Related to this, while results remained significant after log transforming maternal hostility or winsorizing the two outliers, effects of maternal hostility on brain functioning are also likely partially driven by these two outliers.
Additionally, our sample size was modest, and so may or may not generalize to a broader population and analyses may be underpowered to detect effects or examine if results would vary by gender. Future research should also examine the relative contributions of maternal and paternal parenting behaviors to offspring brain development. Finally, our emotion processing analyses compared sad relative to neutral faces, in contrast to the bulk of prior literature which has examined fearful or angry relative to neutral faces. While results confirm that sad faces elicit alterations in amygdala connectivity with various brain regions relative to neutral faces that is related to early childhood parenting experiences, it is unclear whether or how results would extend to fearful or angry faces.
Conclusion
This study provided novel evidence that parenting behaviors, assessed via lab-based observations or parental report, when children were 3 years old, predicted a range of individual differences in brain functional connectivity in those children when they were 10–11 years old. Specifically, maternal hostility was associated with more negative connectivity of the amygdala with frontal and insular regions, and of the VS with the IFG. Maternal regulation was uniquely associated with more positive connectivity of the ACC with the insula. Results highlight an important link between parenting experiences and children’s functional brain development and indicate that these effects persist for at least 7–8 years. Such advances in our understanding of the effects of parenting on the developing brain will likely be necessary in order to develop a fuller picture of the development of individual differences in brain functioning, and its effects on psychosocial functioning.
Supplementary Material
Acknowledgements
We gratefully acknowledge the assistance of Drs. Joan Luby and Deanna Barch in planning the fMRI portion of the current study. We also gratefully acknowledge the help of Laura Klein and Dr. Anna Allmann for their help in data collection.
Acknowledgements: Supported by NIMH grant RO1 MH45757 (Klein), as well as a post-doctoral fellowship from the Social Sciences and Humanities Research Council of Canada (Kopala-Sibley).
References
- Auerbach RP, Admon R, & Pizzagalli DA (2014). Adolescent depression: stress and reward dysfunction. Harv Rev Psychiatry, 22(3), 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, & Phan KL (2007). Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci, 2(4), 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, Saha S, Rempel-Clower N, & Ghashghaei T (2003). Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC Neurosci, 4, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM (2008). Emotion, motivation, and reward processing in schizophrenia spectrum disorders: what we know and where we need to go. Schizophr Bull, 34(5), 816–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumrind D (1991). The influence of parenting style on adolescent competence and substance use. The Journal of Early Adolescence, 11(1), 56–95. [Google Scholar]
- Belsky J, & de Haan M (2011). Annual Research Review: Parenting and children’s brain development: the end of the beginning. J Child Psychol Psychiatry, 52(4), 409–428. [DOI] [PubMed] [Google Scholar]
- Bradley RH, & Vandell DL (2007). Child care and the well-being of children. Arch Pediatr Adolesc Med, 161(7), 669–676. [DOI] [PubMed] [Google Scholar]
- Buckley MJ, Mansouri FA, Hoda H, Mahboubi M, Browning PG, Kwok SC, et al. (2009). Dissociable components of rule-guided behavior depend on distinct medial and prefrontal regions. Science, 325(5936), 52–58. [DOI] [PubMed] [Google Scholar]
- Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, et al. (2012). Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nat Neurosci, 15(12), 1736–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, et al. (2002). Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci U S A, 99(1), 523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BL, & Tottenham N (2016). The Neuro-Environmental Loop of Plasticity: A Cross-Species Analysis of Parental Effects on Emotion Circuitry Development Following Typical and Adverse Caregiving. Neuropsychopharmacology, 41(1), 163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, & Everitt BJ (2002). Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev, 26(3), 321–352. [DOI] [PubMed] [Google Scholar]
- Casey BJ (2015). Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annu Rev Psychol, 66, 295–319. [DOI] [PubMed] [Google Scholar]
- Chang LJ, Yarkoni T, Khaw MW, & Sanfey AG (2013). Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb Cortex, 23(3), 739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YT, Ernst M, & Fudge JL (2013). Cortico-amygdala-striatal circuits are organized as hierarchical subsystems through the primate amygdala. J Neurosci, 33(35), 14017–14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, & Rogosch FA (1996). Equifinality and multifinality in developmental psychopathology. Development and Psychopathology, 8, 597–600. [Google Scholar]
- Egeland B, Weinfield N, Hiester M, Lawrence C, Pierce S, Chippendale K, et al. (1995). Teaching tasks administration and scoring manual. Minneapolis, MN: University of Minnesota. [Google Scholar]
- Fareri DS, Gabard-Durnam L, Goff B, Flannery J, Gee DG, Lumian DS, et al. (2017). Altered ventral striatal-medial prefrontal cortex resting-state connectivity mediates adolescent social problems after early institutional care. Dev Psychopathol, 29(5), 1865–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, & Raichle ME (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A, 102(27), 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A (2013). The teenage brain: Sensitivity to rewards. Current Directions in Psychological Science, 22(2), 88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A (2017). Adolescence, brain maturation and mental health. Nat Neurosci, 20(4), 503–504. [DOI] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam L, Telzer EH, Humphreys KL, Goff B, Shapiro M, et al. (2014). Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychol Sci, 25(11), 2067–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, et al. (2013). Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci U S A, 110(39), 15638–15643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, et al. (2013). A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J Neurosci, 33(10), 4584–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier C, & Luna B (2009). The maturation of incentive processing and cognitive control. Pharmacol Biochem Behav, 93(3), 212–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith HH, Reilly J, Lemery KS, Longley S, & Prescott A (1995). Laboratory Temperament Assessment Battery: Preschool version Unpublished Manuscript. Department of Psychology, University of Wisconsin, Madison, WI. [Google Scholar]
- Graham AM, Pfeifer JH, Fisher PA, Carpenter S, & Fair DA (2015). Early life stress is associated with default system integrity and emotionality during infancy. J Child Psychol Psychiatry, 56(11), 1212–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler AD, et al. (2008). A developmental examination of amygdala response to facial expressions. J Cogn Neurosci, 20(9), 1565–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Nelson EE, Perez-Edgar K, Hardin MG, Roberson-Nay R, Monk CS, et al. (2006). Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. J Neurosci, 26(24), 6399–6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN (2011). Neuroanatomy of Reward: A View from the Ventral Striatum In Gottfried JA (Ed.), Neurobiology of Sensation and Reward. Boca Raton (FL). [PubMed] [Google Scholar]
- Hanson JL, Hariri AR, & Williamson DE (2015). Blunted Ventral Striatum Development in Adolescence Reflects Emotional Neglect and Predicts Depressive Symptoms. Biol Psychiatry, 78(9), 598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt J, & Rutter M (2004). Validity of adult retrospective reports of adverse childhood experiences: review of the evidence. J Child Psychol Psychiatry, 45(2), 260–273. [DOI] [PubMed] [Google Scholar]
- Hart H, & Rubia K (2012). Neuroimaging of child abuse: a critical review. Front Hum Neurosci, 6, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa RJ, Birn RM, Ruttle PL, Burghy CA, Stodola DE, Davidson RJ, et al. (2013). Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc Natl Acad Sci U S A, 110(47), 19119–19124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa RJ, Burghy CA, Stodola DE, Fox ME, Davidson RJ, & Essex MJ (2016). Enhanced prefrontal-amygdala connectivity following childhood adversity as a protective mechanism against internalizing in adolescence. Biol Psychiatry Cogn Neurosci Neuroimaging, 1(4), 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB (1975). Four Factor Index of Social Status. Yale University. [Google Scholar]
- Jiang L, Xu T, He Y, Hou XH, Wang J, Cao XY, et al. (2015). Toward neurobiological characterization of functional homogeneity in the human cortex: regional variation, morphological association and functional covariance network organization. Brain Struct Funct, 220(5), 2485–2507. [DOI] [PubMed] [Google Scholar]
- Kann SJ, O’Rawe JF, Huang AS, Klein DN, & Leung HC (2017). Preschool negative emotionality predicts activity and connectivity of the fusiform face area and amygdala in later childhood. Soc Cogn Affect Neurosci, 12(9), 1511–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry SH, Smith KE, Swank PR, & Guttentag C (2008). A responsive parenting intervention: the optimal timing across early childhood for impacting maternal behaviors and child outcomes. Dev Psychol, 44(5), 1335–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin C, Melis C, Mikulan E, Gelormini C, Huepe D, & Ibanez A (2013). The anterior cingulate cortex: an integrative hub for human socially-driven interactions. Front Neurosci, 7, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Schiller D, Schoenbaum G, Phelps EA, & Daw ND (2011). Differential roles of human striatum and amygdala in associative learning. Nat Neurosci, 14(10), 1250–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, Barch DM, Belden A, Gaffrey MS, Tillman R, Babb C, et al. (2012). Maternal support in early childhood predicts larger hippocampal volumes at school age. Proc Natl Acad Sci U S A, 109(8), 2854–2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod BD, Weisz JR, & Wood JJ (2007). Examining the association between parenting and childhood depression: a meta-analysis. Clin Psychol Rev, 27(8), 986–1003. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL, Pitman RK, & Quirk GJ (2006). Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol, 73(1), 61–71. [DOI] [PubMed] [Google Scholar]
- Motzkin JC, Philippi CL, Wolf RC, Baskaya MK, & Koenigs M (2015). Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biol Psychiatry, 77(3), 276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly JX, Woolrich MW, Behrens TE, Smith SM, & Johansen-Berg H (2012). Tools of the trade: psychophysiological interactions and functional connectivity. Soc Cogn Affect Neurosci, 7(5), 604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olino TM, Klein DN, Dyson MW, Rose SA, & Durbin CE (2010). Temperamental emotionality in preschool-aged children and depressive disorders in parents: associations in a large community sample. J Abnorm Psychol, 119(3), 468–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivari MG, Tagliabue S, & Confalonieri E (2013). Parenting Style and Dimensions Questionnaire: A review of reliability and validity. Marriage & Family Review, 49(6), 465–490. [Google Scholar]
- Pagliaccio D, Luby JL, Bogdan R, Agrawal A, Gaffrey MS, Belden AC, et al. (2015). Amygdala functional connectivity, HPA axis genetic variation, and life stress in children and relations to anxiety and emotion regulation. J Abnorm Psychol, 124(4), 817–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce JM, & Hall G (1980). A model for Pavlovian learning: Variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychological Review, 87(6), 532–552. [PubMed] [Google Scholar]
- Pechtel P, & Pizzagalli DA (2011). Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology (Berl), 214(1), 55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, & Boxer A (1988). A self-report measure of pubertal status: Reliability, validity, and initial norms. J Youth Adolesc, 17(2), 117–133. [DOI] [PubMed] [Google Scholar]
- Philip NS, Kuras YI, Valentine TR, Sweet LH, Tyrka AR, Price LH, et al. (2013). Regional homogeneity and resting state functional connectivity: associations with exposure to early life stress. Psychiatry Res, 214(3), 247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CC, Mandleco B, Olsen SF, & Hart CH (2001). The parenting styles and dimensions questionnaire (PSDQ). In Perlmutter BF, Touliatos J & Holden GW (Eds.), Handbook of Family Measurement Techniques (Vol. 3, pp. 319–321). Thousand Oaks, CA: SAGE Publications, Inc. [Google Scholar]
- Rose J, Roman N, Mwaba K, & Ismail K (2017). The relationship between parenting and internalizing behaviours of children: a systematic review. Early Child Development and Care, 1–19. [Google Scholar]
- Rothbart MK (2007). Temperament, development, and personality. Current directions in psychological science, 16(4), 207–212. [Google Scholar]
- Ryan RM, & Deci EL (2000). Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am Psychol, 55(1), 68–78. [DOI] [PubMed] [Google Scholar]
- Sergerie K, Chochol C, & Armony JL (2008). The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev, 32(4), 811–830. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, & Davidson RJ (2011). The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci, 12(3), 154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JA, Insel C, Powers A, Franz P, Helion C, Martin RE, ... & Ochsner KN (2016). vlPFC–vmPFC–amygdala interactions underlie age-related differences in cognitive regulation of emotion. Cerebral Cortex, 27(7), 3502–3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Fani N, & McClure-Tone EB (2011). Behavioral and neural representation of emotional facial expressions across the lifespan. Dev Neuropsychol, 36(4), 408–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starcke K, & Brand M (2012). Decision making under stress: a selective review. Neurosci Biobehav Rev, 36(4), 1228–1248. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, et al. (2011). Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature, 475(7356), 377–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Marusak HA, Tocco MA, Vila AM, McGarragle O, & Rosenberg DR (2015). Altered amygdala connectivity in urban youth exposed to trauma. Soc Cogn Affect Neurosci, 10(11), 1460–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tost H, Champagne FA, & Meyer-Lindenberg A (2015). Environmental influence in the brain, human welfare and mental health. Nat Neurosci, 18(10), 1421–1431. [DOI] [PubMed] [Google Scholar]
- Tottenham N (2012). Human amygdala development in the absence of species-expected caregiving. Dev Psychobiol, 54(6), 598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, & Galvan A (2016). Stress and the adolescent brain: Amygdala-prefrontal cortex circuitry and ventral striatum as developmental targets. Neurosci Biobehav Rev, 70, 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troller-Renfree S, McDermott JM, Nelson CA, Zeanah CH, & Fox NA (2015). The effects of early foster care intervention on attention biases in previously institutionalized children in Romania. Dev Sci, 18(5), 713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Werff SJ, Pannekoek JN, Veer IM, van Tol MJ, Aleman A, Veltman DJ, et al. (2013). Resting-state functional connectivity in adults with childhood emotional maltreatment. Psychol Med, 43(9), 1825–1836. [DOI] [PubMed] [Google Scholar]
- Waite P, Whittington L, & Creswell C (2014). Parent-Child Interactions and Adolescent Anxiety: A Systematic Review. Psychopathology Review, 1(1), 51–76. [Google Scholar]
- Whittle S, Simmons JG, Dennison M, Vijayakumar N, Schwartz O, Yap MBH, et al. (2014). Positive parenting predicts the development of adolescent brain structure: A longitudinal study. Developmental Cognitive Neuroscience, 8, 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S, Yap MB, Yucel M, Sheeber L, Simmons JG, Pantelis C, et al. (2009). Maternal responses to adolescent positive affect are associated with adolescents’ reward neuroanatomy. Soc Cogn Affect Neurosci, 4(3), 247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf RC, & Herringa RJ (2016). Prefrontal-Amygdala Dysregulation to Threat in Pediatric Posttraumatic Stress Disorder. Neuropsychopharmacology, 41(3), 822–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Kujawa A, Lu LH, Fitzgerald DA, Klumpp H, Fitzgerald KD, ... & Phan KL (2016). Age‐related changes in amygdala–frontal connectivity during emotional face processing from childhood into young adulthood. Human Brain Mapping, 37(5), 1684–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaslow MJ, Weinfield NS, Gallagher M, Hair EC, Ogawa JR, Egeland B, et al. (2006). Longitudinal prediction of child outcomes from differing measures of parenting in a low-income sample. Dev Psychol, 42(1), 27–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.