Abstract
Introduction
Heteroresistant vancomycin-intermediate Staphylococcus aureus (hVISA) bacteremia may result in clinical failure of vancomycin therapy, together with prolonged infection and hospitalization. This clinical problem has resulted in a search for more effective treatment options. The current study was designed to further investigate the synergistic effect of oxacillin plus vancomycin against methicillin-resistant S. aureus (MRSA) and hVISA using checkerboard and time-kill assays.
Methods
Non-duplicate S. aureus isolates including hVISA (n = 29), MRSA (n = 10) and methicillin susceptible S. aureus (MSSA, n = 11) were used for combinational testing using checkerboard and time-kill assays.
Results
Twenty-one isolates, 15 hVISA and 6 MRSA, showed synergy between oxacillin and vancomycin by checkerboard assay with fractional inhibitory concentration indices of ≤ 0.5. The addition of oxacillin to vancomycin resulted in a reduction in baseline vancomycin MIC from 1–2 to 0.06–0.5 µg/ml against MRSA and hVISA isolates. In the time-kill assay, the combination of oxacillin and vancomycin resulted in synergistic activity against hVISA (n = 23) and MRSA (n = 7) isolates. Regrowth was observed in six hVISA isolates exposed to combination in the time-kill assay, but none of them reached the original inoculum density at 24 h. All re-growth isolates showed a onefold increase in vancomycin MIC (from 1 to 2 µg/ml) and were re-confirmed as hVISA using the population-analysis profile experiment. Overall, for hVISA and MRSA, the combination of oxacillin plus vancomycin had greater antibacterial effect than each individual drug alone.
Conclusion
The present study showed the potential activity of vancomycin plus oxacillin combination against hVISA and MRSA isolates. Further, continued evaluation of this combination is warranted and may have therapeutic benefits in treating complicated MRSA infections.
Keywords: hVISA, MRSA, MSSA, Oxacillin, Vancomycin
Introduction
Staphylococcus aureus bacteremia (SAB) is a common cause of mortality and morbidity and a major burden on healthcare around the world. In patients with positive blood cultures (BC) suggestive of gram-positive cocci in clusters or with a high clinical suspicion of staphylococcal bacteremia, empirical therapy with anti-staphylococcal β-lactams such as nafcillin or cefazolin is indicated. Empirical therapy with nafcillin or cefazolin had lower 30-day mortality than second- or third-generation cephalosporins [1–3]. In settings with a methicillin-resistant S. aureus (MRSA) prevalence of > 20%, vancomycin is the usual empirical antimicrobial of choice [4]. Alternative agents include linezolid or daptomycin, but neither are superior to vancomycin in treating MRSA bacteremia [5].
A prospective study of 1994 SAB episodes found that 30-day mortality was significantly higher in patients with MRSA (30%) than MSSA (17.7%) infection [6]. Although this difference may be due to host factors, vancomycin shows slow bactericidal activity and poor tissue penetration, resulting in higher relapse rates [7–10]. In recent years, S. aureus with reduced susceptibility to vancomycin has become a significant clinical problem. Heteroresistant vancomycin-intermediate S. aureus (hVISA) is a strain of S. aureus containing a subpopulation of cells (1 in 106), that can grow within the vancomycin-intermediate susceptibility range of ≥ 4 µg/ml. hVISA bacteremia may result in clinical failure to vancomycin therapy, together with prolonged infection and hospitalization [11, 12].
This clinical problem has resulted in a search for more effective treatment options. Several in vitro and a few in vivo studies have explored the synergy between glycopeptides and various beta-lactam antibiotics against MRSA isolates with varying susceptibility to vancomycin [13–16]. These studies have described synergistic killing in most but not all tested strains. This potential synergy relies on the “seesaw effect” which demonstrates improved beta-lactam susceptibility, concomitant to reduced glycopeptide susceptibility [17]. In addition, the combination of vancomycin plus beta-lactam prevents the development of reduced vancomycin susceptibility in MRSA [18]. No published studies have documented synergism between vancomycin and daptomycin/linezolid.
Studies have also reported potential in vitro synergy between anti-staphylococcal penicillin (oxacillin or nafcillin) and vancomycin against MRSA and hVISA [19–23]. The current study was designed to further investigate the synergistic effect of oxacillin plus vancomycin against MRSA and hVISA using checkerboard and time-kill assays. These findings were compared with MSSA to establish incremental benefit.
Methods
Bacterial Strains
A total of 50 non-duplicate S. aureus isolated from the blood culture between 2016 and 2017 at a 2600-bed tertiary care hospital, Christian Medical College, Vellore, India, were included in this study. Isolates were identified and characterized using standard culture and biochemical methods [24]. Of 50 isolates, 11 were MSSA, 10 were MRSA and 29 were confirmed as hVISA, using population analysis profile-area under curve (PAP-AUC). In this study, S. aureus strains that were resistant to cefoxitin and completely susceptible to vancomycin without any heteroresistant subpopulation were specified as MRSA, while MRSA strains expressing vancomycin heteroresistance were called hVISA. The hVISA strains were subcultured and maintained in brain Heart infusion agar (BHIA) were supplemented with 1 μg/ml of vancomycin and stored at − 70 °C. The study was approved by the institutional review board (IRB. Min. No. 10643 dated April, 2017).
Susceptibility Testing
Methicillin resistance was detected by disk diffusion testing using a 30-μg cefoxitin disk as recommended by the CLSI guidelines [25]. The MICs of oxacillin and vancomycin were determined by the CLSI-recommended broth microdilution method [26]. Oxacillin and vancomycin powders were obtained from commercial sources (Sigma, St Louis, MO, USA). Cation-adjusted Mueller–Hinton broth (CA-MHB) containing 2% NaCl was used for oxacillin MICs determination. Oxacillin and vancomycin were tested at the concentration of 0.03–512 μg/ml and 0.03–16 μg/ml, respectively. The MICs of oxacillin and vancomycin were read after incubation at 35 °C for 24 h. S. aureus ATCC 29213 and S. aureus ATCC 43300 strains were used as a quality control strains.
PCR for mec A Gene
Bacterial DNA was extracted from colonies grown overnight on blood agar using the QIAamp DNA Mini Kit and the QIAcube instrument (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. PCR was performed for the detection of mec A gene in MRSA isolates, as previously described [27]; this is considered as the gold standard method for detection of methicillin resistance in S. aureus.
Screening and Confirmation of hVISA
MRSA isolates were screened for hVISA using the glycopeptide resistance detection (GRD) E test (bioMérieux, France) and confirmed with the PAP/AUC method. The GRD E test was performed using 0.5 McFarland-adjusted inoculum swabbed onto Mueller–Hinton agar (MHA) containing 5% blood. The zone of inhibition was read at 24 and 48 h after incubation at 35 ± 2 °C [28]. The test isolate was considered positive for hVISA if the MIC of vancomycin or teicoplanin was 8 µg/ml. PAP-AUC analysis was performed as previously described [29]. The bacterial suspension was plated onto freshly prepared BHIA plates containing 0.5–8 μg/ml of vancomycin. Colonies were counted after 48 h of growth at 35 ± 2 °C. For calculation of AUC, viable counts were plotted against increasing concentration of vancomycin using the GraphPad Prism™ (v.7.0) software package. All PAP-AUC experiments were performed in duplicate. For vancomycin PAP analysis, the AUC ratio was calculated by dividing the AUC of the test strain by the AUC of the MU3 (hVISA) strain. The PAP-AUC ratio was interpreted as follows, < 0.9 as vancomycin-susceptible S. aureus (VSSA), ≥ 0.9 as hVISA phenotype, and > 1.3 as vancomycin-intermediate S. aureus (VISA). For each batch of the PAP-AUC experiment, the hVISA (MU3, ATCC 700698), VISA (MU50, ATCC 700699) and S. aureus ATCC 29213 (VSSA) were used as the reference, comparator and negative control strains, respectively.
Broth Microdilution Checkerboard Assay
Checkerboard synergy testing was performed by the microbroth dilution method, as previously described [30]. Vancomycin was tested at a concentration of 0.03–8 µg/ml. MHB containing 2% NaCl was used to perform in vitro synergy testing. In the checkerboard assay, vancomycin was combined with oxacillin in the concentration of 1–128 µg/ml for MRSA and hVISA. For MSSA, oxacillin was used at a concentration of 0.25–8 µg/ml. Microtiter plates were incubated at 37 ± 2 °C for 24 h. Growth control and sterility control were included in each test panel. The first non-turbid well in each row and column was used to calculate the fractional inhibitory concentration (FIC) index. An FIC index of ≤ 0.5 was defined as synergy, an FIC index of > 0.5 to 4.0 was defined as indifferent, and an FIC index of > 4.0 was defined as antagonistic.
Time-Kill Assay (TKA)
The time-kill assay was performed in duplicate on all PAP-confirmed hVISA, MRSA, and MSSA isolates. Time-kill assays were performed according to previously published techniques [31]. An initial bacterial inoculum of approximately 5 × 106 for each isolate was inoculated into CA-MHB containing 2% NaCl. Time-kill experiments were performed at half-MIC of oxacillin and vancomycin for the respective isolates. Each antibiotic alone, together with oxacillin in combination with vancomycin, were tested. The inoculum was diluted 1 in 100 using sterile saline. A volume of 100 µl was plated on the MHA plates at times of 0, 3, 6 and 24 h post-incubation in a shaker-incubator at 35 ± 2 °C. After 24 h of incubation (48 h for hVISA) isolates at 35 ± 2 °C, colonies were counted and results were expressed as log10CFU/ml. Each batch of testing included sterility control and growth control (without any antibiotic). Synergy was defined as ≥ 2 log10 decrease in CFU/ml for combination antibiotics in comparison to a single agent. Antagonism was defined as ≥ 2 log10 increase in CFU/ml for the combination antibiotic in comparison to the most active single agent. Bactericidal activity was defined as a ≥ 3 log10 CFU/ml reduction from baseline. Regrowth was defined as a ≥ 3 log10 decrease in CFU/ml followed by a ≥ 2 log10 increase in CFU/ml at 24 h.
Statistical Analysis
For the time-kill studies, one-way analysis of variance with Tukey’s post hoc test was used to compare changes in CFU/ml. A p value of < 0.05 was considered statistically significant. Statistical analyses were performed using Statistical Package for the Social Sciences v.16.0 (SPSS, Chicago, IL, USA).
Compliance with Ethics Guidelines
This article does not contain any studies with human participants or animals performed by any of the authors. The study was approved by an institutional review board (IRB. Min. No. 10643 dated April 2017).
Results
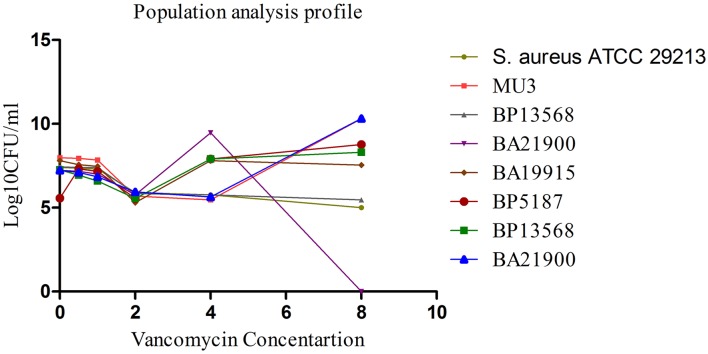
Of the tested S. aureus, MICs of oxacillin ranged between 0.5 and 1 µg/ml for MSSA and 4–128 µg/ml for hVISA and MRSA. All isolates were susceptible to vancomycin with the MIC of 1–2 µg/ml. All MRSA isolates carried the mec A gene. Twenty-nine isolates were confirmed as hVISA using the PAP-AUC method, with an AUC ratio between 0.92 and 1.33 (Fig. 1).
Fig. 1.
Population analysis profile (PAP) of vancomycin on five representative methicillin resistant Staphylococcus aureus (MRSA) isolate from bacteremia cases. PAP of vancomycin was performed to confirm the presence heterogeneous vancomycin-intermediate strains (hVISA) phenotype. MU3, hVISA as comparator control; MU50, VISA as positive control; ATCC 29213, VSSA as negative control
Twenty-one isolates, 15 hVISA and 6 MRSA, showed synergy between oxacillin and vancomycin by checkerboard assay with FIC indices of ≤ 0.5. This was not seen in any MSSA isolates (FIC 0.75–1; Table 1). The addition of oxacillin to vancomycin resulted in a reduction in baseline vancomycin MIC from 1–2 to 0.06–0.5 µg/ml against MRSA and hVISA isolates.
Table 1.
Minimum inhibitory concentration of oxacillin, vancomycin and combination activity of oxacillin plus vancomycin in time kill assay against hVISA, MRSA and MSSA isolates from bloodstream infection
| Strains | Oxacillin MIC µg/ml | Vancomycin MIC µg/ml | PAP-AUC ratio | Checker board assay | Time-kill assay ½ MIC of oxacillin + ½ MIC of vancomycin (µg/ml) at 24 h | ||
|---|---|---|---|---|---|---|---|
| ΣFIC | Activity | Combinational activity | Bactericidal activity | ||||
| hVISA_1 | 4 | 1 | 0.97 | 0.5 | Synergy | Synergy | Positive |
| hVISA_2 | 8 | 1 | 1.02 | 0.37 | Synergy | Synergy | Positive |
| hVISA_3 | 4 | 2 | 0.9 | 0.5 | Synergy | Synergy | Positive |
| hVISA_4 | 4 | 1 | 0.98 | 0.5 | Synergy | Synergy | Positive |
| hVISA_5 | 64 | 1 | 1.16 | 0.5 | Synergy | Synergy | Positive |
| hVISA_6 | 4 | 1 | 0.95 | 0.37 | Synergy | Synergy | Positive |
| hVISA_7 | 32 | 2 | 0.9 | 0.62 | Indifferent | Synergy | Positive |
| hVISA_8 | 8 | 1 | 1.03 | 0.75 | Indifferent | Synergy | Positive |
| hVISA_9 | 8 | 1 | 1.28 | 0.62 | Indifferent | Synergy | Positive |
| hVISA_10 | 8 | 1 | 0.97 | 0.75 | Indifferent | Synergy | Positive |
| hVISA_11 | 4 | 1 | 1.12 | 1.00 | Indifferent | Synergy | Positive |
| hVISA_12 | 8 | 2 | 0.9 | 1.00 | Indifferent | Synergy | Positive |
| hVISA_13 | 8 | 1 | 1.02 | 0.62 | Indifferent | Synergy | Positive |
| hVISA_14 | 16 | 1 | 1.03 | 0.75 | Indifferent | Synergy | Positive |
| hVISA_15 | 4 | 1 | 0.98 | 0.5 | Synergy | Synergy | Negative |
| hVISA_16 | 8 | 1 | 1.04 | 0.5 | Synergy | Synergy | Negative |
| hVISA_17 | 4 | 1 | 1.00 | 0.5 | Synergy | Synergy | Negative |
| hVISA_18 | 64 | 2 | 0.93 | 0.5 | Synergy | Synergy | Negative |
| hVISA_19 | 4 | 1 | 0.92 | 0.06 | Synergy | Synergy | Negative |
| hVISA_20 | 64 | 1 | 1.1 | 0.5 | Synergy | Synergy | Negative |
| hVISA_21 | 4 | 1 | 0.92 | 0.07 | Synergy | Synergy | Negative |
| hVISA_22 | 4 | 2 | 1.00 | 0.5 | Synergy | Synergy | Negative |
| hVISA_23 | 4 | 1 | 1.01 | 0.37 | Synergy | Indifferent | Negative |
| hVISA_24 | 8 | 1 | 1.52 | 0.75 | Indifferent | Synergy | Regrowth |
| hVISA_25 | 4 | 1 | 0.91 | 0.5 | Indifferent | Indifferent | Regrowth |
| hVISA_26 | 8 | 1 | 1.33 | 0.56 | Indifferent | Indifferent | Regrowth |
| hVISA_27 | 64 | 1 | 1.01 | 1.00 | Indifferent | Indifferent | Regrowth |
| hVISA_28 | 4 | 1 | 1.14 | 0.75 | Indifferent | Indifferent | Regrowth |
| hVISA_29 | 8 | 1 | 1.23 | 0.62 | Indifferent | Indifferent | Regrowth |
| MRSA_1 | 128 | 1 | 0.28 | 0.5 | Synergy | Synergy | Positive |
| MRSA_2 | 64 | 1 | 0.39 | 0.5 | Synergy | Synergy | Positive |
| MRSA_3 | 4 | 1 | 0.37 | 0.75 | Indifferent | Synergy | Positive |
| MRSA_4 | 8 | 1 | 0.48 | 0.62 | Indifferent | Synergy | Positive |
| MRSA_5 | 8 | 1 | 0.55 | 0.62 | Indifferent | Synergy | Positive |
| MRSA_6 | 64 | 1 | 0.37 | 0.5 | Synergy | Indifferent | Positive |
| MRSA_7 | 4 | 1 | 0.47 | 0.5 | Synergy | Synergy | Negative |
| MRSA_8 | 8 | 1 | 0.32 | 0.5 | Synergy | Synergy | Negative |
| MRSA_9 | 8 | 1 | 0.35 | 0.5 | Synergy | Indifferent | Negative |
| MRSA_10 | 4 | 1 | 0.72 | 0.62 | Indifferent | Indifferent | Negative |
| MSSA_1 | 0.5 | 1 | ND | 0.75 | Indifferent | Indifferent | Positive |
| MSSA_2 | 1 | 1 | ND | 0.75 | Indifferent | Indifferent | Positive |
| MSSA_3 | 1 | 0.5 | ND | 1.00 | Indifferent | Indifferent | Positive |
| MSSA_4 | 0.5 | 1 | ND | 0.70 | Indifferent | Indifferent | Positive |
| MSSA_5 | 0.5 | 1 | ND | 0.74 | Indifferent | Indifferent | Positive |
| MSSA_6 | 1 | 0.5 | ND | 1.007 | Indifferent | Indifferent | Negative |
| MSSA_7 | 0.5 | 1 | ND | 1.007 | Indifferent | Indifferent | Positive |
| MSSA_8 | 1 | 0.25 | ND | 0.75 | Indifferent | Indifferent | Positive |
| MSSA_9 | 0.5 | 0.12 | ND | 0.75 | Indifferent | Indifferent | Positive |
| MSSA_10 | 1 | 0.5 | ND | 1.007 | Indifferent | Indifferent | Negative |
| MSSA_11 | 1 | 0.5 | ND | 1.007 | Indifferent | Indifferent | Positive |
ND not done
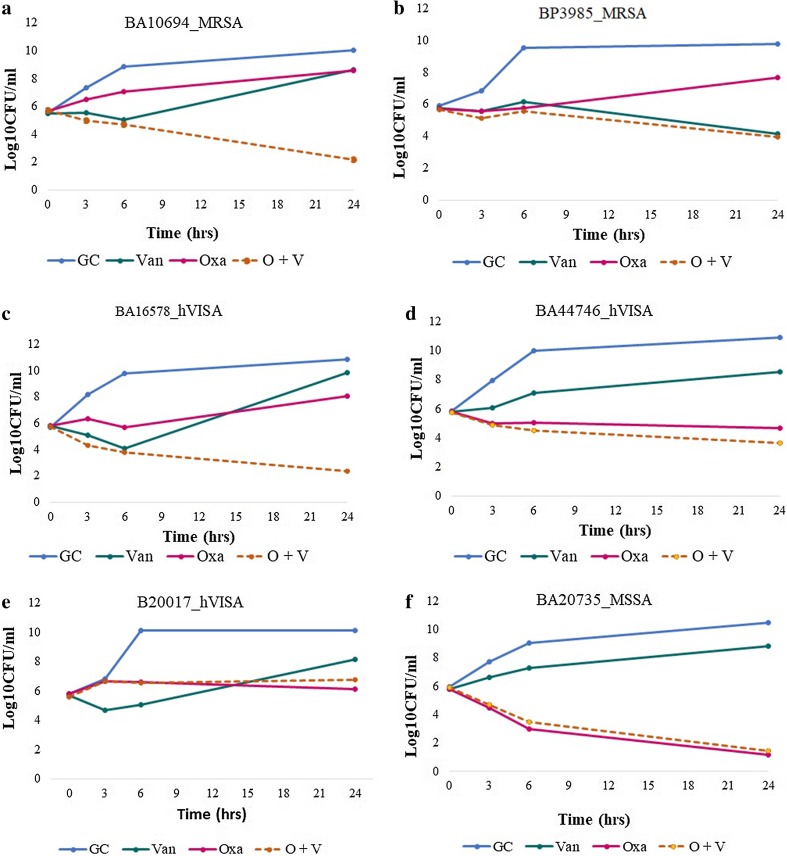
In time-kill assays, the combination of oxacillin and vancomycin resulted in synergistic activity against hVISA (n = 23) and MRSA (n = 7) isolates (Table 1). Time-kill curves of the oxacillin–vancomycin combination, oxacillin, and vancomycin alone against representative hVISA, MRSA and MSSA are shown in Fig. 2. Although a higher rate of synergy was observed against hVISA, marked heterogeneity in bactericidal activity was observed.
Fig. 2.
Time-kill curves of oxacillin (1/2 MIC) in combination with vancomycin (1/2 MIC), vancomycin, oxacillin alone and vancomycin alone. MRSA showing a synergy and b indifferent with oxacillin with vancomycin combination; hVISA displaying c synergy, d indifferent, e regrowth with this combination, d MSSA exhibiting indifferent effect with this combination in time-kill assays
The combination of oxacillin plus vancomycin demonstrated bactericidal activity in 14 of 29 hVISA isolates and 6 of 10 MRSA isolates at 24 h. Regrowth was observed in six hVISA isolates exposed to combination in time-kill assays, but none of them reached the original inoculum density at 24 h. All re-growth isolates showed a onefold increase in vancomycin MIC (from 1 to 2 µg/ml) and were re-confirmed as hVISA using the PAP-AUC method. Overall, for hVISA and MRSA, the combination of oxacillin plus vancomycin had greater antibacterial effect than each individual drug alone.
Table 2 documents the mean change in log10 CFU/ml of bacterial inoculum treated with oxacillin, vancomycin, and the oxacillin–vancomycin combination against hVISA, MRSA, and MSSA at 24 h. The combination of oxacillin plus vancomycin was superior at inhibiting hVISA and MRSA than vancomycin alone (p < 0.01). The combinations resulted in a 1.4-fold reduction in hVISA and a 0.7-fold reduction in MRSA than with vancomycin alone.
Table 2.
Mean log10 colony-forming unit (CFU)/ml of hetero-resistant vancomycin intermediate S. aureus (hVISA), methicillin resistant S. aureus (MRSA) and methicillin susceptible S. aureus (MSSA) isolates treated with oxacillin alone, vancomycin alone and oxacillin plus vancomycin combination in time-kill assays
| Organism | Growth control log10 CFU/ml ± SD |
Time kill assay aMean log10 CFU/ml ± SD |
||
|---|---|---|---|---|
| Oxacillin | Vancomycin | bOxacillin at ½ X MIC + vancomycin ½ X MIC | ||
| hVISA (n = 29) | 8.3 ± 0.3 | 6.5 ± 0.7 | 6.4 ± 0.5 | 5.0 ± 0.7 |
| MRSA (n = 10) | 8.1 ± 0.1 | 5.1 ± 0.3 | 4.6 ± 0.3 | 3.9 ± 0.4 |
| MSSA (n = 5) | 8.4 ± 0.3 | 3.7 ± 0.3 | 6.9 ± 0.2 | 3.6 ± 1.0 |
aAll data are presented as mean ± standard deviation (SD)
b1/2 MICs of oxacillin and vancomycin are derived from hVISAMIC of individual isolates as listed in Table 1
In time-kill studies, the combination of oxacillin plus vancomycin showed indifferent activity against all MSSA isolates. Oxacillin was more effective than combination therapy at inhibiting MSSA (p < 0.01). No antagonism was seen in any of the tested isolates with either checkerboard or time-kill assays.
Discussion
Clinical guidelines recommend at least 14 days of antibiotic therapy to treat for treating uncomplicated S. aureus bacteremia [32]. Empiric therapy for S. aureus bacteremia often includes beta-lactam with additional vancomycin until susceptibility of the isolate are known [33].
MRSA is inherently resistant to all β-lactams except ceftaroline fosamil and ceftobiprole. Several studies have established that beta-lactams and vancomycin show synergy against MRSA with varying vancomycin susceptibility (MIC, ≤ 2 to ≥ 4 µg/ml). [13–16, 19–23, 34]. Recently, Tran et al. have reported that vancomycin in combination with various beta-lactams including nafcillin, cefazolin, cefepime and ceftaroline resulted in a 4- to 16-fold reduction in baseline vancomycin MIC values [15]. Further, a marked “seesaw effect” was demonstrated in MRSA isolates with increased susceptibility to ceftaroline associated with decreased glycopeptide and daptomycin susceptibility [17]. An in vitro study has shown that sub-MIC concentrations of oxacillin plus vancomycin prevents the selection of vancomycin heteroresistance [19]. However, strong evidence to support this hypothesis has not been established through in vivo studies.
Sieradzki et al. have reported that increased glycopeptide MICs were associated with reduced β-lactam resistance in MRSA isolates [35, 36]. Notably, the addition of oxacillin resulted in a fourfold reduction (32 to 2 µg/ml) of vancomycin MIC in VRSA isolates [37]. An in vitro pharmacokinetic/pharmacodynamic study demonstrated that nafcillin in combination with vancomycin resulted in more rapid killing of MRSA (6.3 h) than vancomycin alone (72 h) [23].
Although, studies have attempted to assess the activity of nafcillin/oxacillin and vancomycin, the mechanism behind this synergistic effect is unclear. Vancomycin and beta-lactam have different targets. Vancomycin binds with the d-ala-d-ala peptide and beta-lactam suicide-inhibits transpeptidase [21, 36]. Specifically, beta-lactam antibiotics bind to PBPs other than PBP2’ in hVISA and vancomycin-intermediate S. aureus (VISA).
Regardless of the in vitro methodology, our study agrees with previous studies in demonstrating synergistic activity against MRSA and hVISA isolates [14–18]. The present study clearly demonstrates that the inhibitory activity of vancomycin is enhanced by the addition of oxacillin. Consistent with previous studies [13–16, 19–23, 34, 38], we found that vancomycin had the least bactericidal activity against MRSA than all other antibiotics. Cell wall thickening in hVISA/VISA leads to clogging of vancomycin and prevents binding at the target site [12]. Alteration in the cell wall structure is induced by binding of beta-lactam to PBPs other than PBP2′ which promotes the binding of vancomycin to the d-ala-d-ala subunit [18]. In addition, an inverse relationship between vancomycin and oxacillin MIC was reported in an in vitro study [36]. This finding suggests that the “seesaw effect” may contribute to the enhanced synergistic activity of oxacillin plus vancomycin against hVISA, in comparison to single agent vancomycin alone. Neither antagonism nor synergism was observed with the vancomycin–oxacillin combination against MSSA isolates. This was similar to the findings of Joukhadar et al. [39]. It is well established that beta-lactam is superior to vancomycin in treating MSSA infections [40–42].
Dilworth et al. have studied the microbiological impact of adding an anti-staphylococcal β-lactams to vancomycin and reported increased microbiological eradication with this combination [43]. Similarly, McConeghy et al. have reported that the combination of vancomycin with beta-lactam could improve the outcome of S. aureus infection [33]. Furthermore, a multicenter randomized controlled trial (ACTRN12610000940077) found that the duration of MRSA bacteremia was shortened from 3 days in patients receiving vancomycin alone to 1.94 days in patients receiving the combination of vancomycin and flucloxacillin. However, no significant difference in 90-day mortality was noted [44]. Collectively, this suggests that combination therapy has a role in the treatment of MRSA bacteremia (Table 3).
Table 3.
In vitro and in vivo evidence for increased antibacterial activity of anti-staphylococcal penicillin/beta-lactam with vancomycin
| Study | hVISA/VISA | MRSA | MSSA |
|---|---|---|---|
| In vitro | In nearly all studies, consistent synergistic bacterial killing was reported in most but not all strains tested. Synergy was proportional to vancomycin MIC, and increasing degree of synergy was seen with increasing vancomycin MIC | Consistently reported synergistic bacterial killing in most but not all strains tested | Least effect against MSSA. Neither synergy nor antagonism were evident in any strain, using both fixed dose concentration and dynamic model stimulating clinical dosing |
| In vivo | No data | Higher microbiological eradication, improved outcome and shortens the duration of MRSA bacteremia [33, 43, 44] | β-lactam is superior to vancomycin in treating MSSA infection [40–42] |
There are certain limitations to the present study as the data from presented here represent in vitro synergistic activity. However, additional in vivo studies are required to support these findings. Further, this may not be translated into clinical benefit for patients. Thus, additional clinical studies are warranted to establish the superiority of this combination to vancomycin alone in treating severe MRSA infections.
Conclusion
This present study confirms previous findings that vancomycin and oxacillin are synergistic against MRSA including hVISA strains. However, oxacillin alone was more potent against MSSA than combination therapy. A randomized controlled trial is warranted to establish whether combination therapy should be recommended as standard therapy to reduce morbidity and mortality among recurrent or difficult-to-treat hVISA/MRSA infections.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Yamuna Devi Bakthavatchalam, Ravikar Ralph, Balaji Veeraraghavan, Priyanka Babu and Elakkiya Munusamy have nothing to disclose.
Compliance with Ethics Guidelines
This article does not contain any studies with human participants or animals performed by any of the authors The study was approved by an institutional review board (IRB. Min. No. 10643 dated April 2017).
Data Availability
All data generated or analyzed during this study are included in this published article.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced digital features
To view enhanced digital features for this article go to 10.6084/m9.figshare.7315073.
References
- 1.Paul M, Zemer-Wassercug N, Talker O, Lishtzinsky Y. Are all beta-lactams similarly effective in the treatment of methicillin-sensitive Staphylococcus aureus bacteraemia? Clin Microbiol Infect Dis. 2011;17(10):1581–1586. doi: 10.1111/j.1469-0691.2010.03425.x. [DOI] [PubMed] [Google Scholar]
- 2.Lee S, Choe PG, Song K-H, Park S-W. Is cefazolin inferior to nafcillin for treatment of methicillin-susceptible Staphylococcus aureus bacteremia? Antimicrob Agents Chemother. 2011;55(11):5122–5126. doi: 10.1128/AAC.00485-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li J, Echevarria KL, Traugott KA. β-Lactam therapy for methicillin-susceptible Staphylococcus aureus bacteremia: a comparative review of cefazolin versus antistaphylococcal penicillins. Pharmacotherapy. 2016 doi: 10.1002/phar.1892. [DOI] [PubMed] [Google Scholar]
- 4.Solomkin JS, Bjornson HS, Cainzos M, Dellinger EP. A consensus statement on empiric therapy for suspected gram-positive infections in surgical patients. Am J Surg. 2004;187(1):134–145. doi: 10.1016/j.amjsurg.2003.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Holmes NE, Tong SYC, Davis JS, van Hal SJ. Treatment of methicillin-resistant Staphylococcus aureus: vancomycin and beyond. Semin Respir Crit Care Med. 2015;36(1):17–30. doi: 10.1055/s-0034-1397040. [DOI] [PubMed] [Google Scholar]
- 6.Turnidge JD, Kotsanas D, Munckhof W, Roberts S. Staphylococcus aureus bacteraemia: a major cause of mortality in Australia and New Zealand. Med J Aust. 2006;184(8):384–388. doi: 10.5694/j.1326-5377.2009.tb02841.x. [DOI] [PubMed] [Google Scholar]
- 7.Pletz MW, Burkhardt O, Welte T. Nosocomial methicillin-resistant Staphylococcus aureus (MRSA) pneumonia: linezolid or vancomycin?—Comparison of pharmacology and clinical efficacy. Eur J Med Res. 2010;15(12):507–513. doi: 10.1186/2047-783X-15-12-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kollef MH. Limitations of vancomycin in the management of resistant staphylococcal infections. Clin Infect Dis. 2007;45(Suppl 3):S191–S195. doi: 10.1086/519470. [DOI] [PubMed] [Google Scholar]
- 9.Welsh KJ, Skrobarcek KA, Abbott AN, Lewis CT. Predictors of relapse of methicillin-resistant Staphylococcus aureus bacteremia after treatment with vancomycin. J Clin Microbiol. 2011;49(10):3669–3672. doi: 10.1128/JCM.05287-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakoulas G, Moise-Broder PA, Schentag J, Forrest A. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol. 2004;42(6):2398–2402. doi: 10.1128/JCM.42.6.2398-2402.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casapao AM, Leonard SN, Davis SL, Lodise TP. Clinical outcomes in patients with heterogeneous vancomycin-intermediate Staphylococcus aureus (hVISA) bloodstream infection. Antimicrob Agents Chemother. 2013;57(9):4252–4259. doi: 10.1128/AAC.00380-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang S, Sun X, Chang W, Dai Y. Systematic review and meta-analysis of the epidemiology of vancomycin-intermediate and heterogeneous vancomycin-intermediate Staphylococcus aureus isolates. PLoS ONE. 2015;10(8):e0136082. doi: 10.1371/journal.pone.0136082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis JS, Van Hal S, Tong SYC. Combination antibiotic treatment of serious methicillin-resistant Staphylococcus aureus infections. Semin Respir Crit Care Med. 2015;36(1):3–16. doi: 10.1055/s-0034-1396906. [DOI] [PubMed] [Google Scholar]
- 14.Sy CL, Huang TS, Chen CS, Chen YS, Tsai HC, Wann SR, Wu KS, Chen JK, Lee SS, Liu YC. Synergy of β-lactams with vancomycin against methicillin-resistant Staphylococcus aureus: correlation of disk diffusion and checkerboard methods. J Clin Microbiol. 2016;54(3):565–568. doi: 10.1128/JCM.01779-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao CH, Chen SY, Huang YT, Tsai HY, Hsueh PR. Comparison of in vitro synergy of various β-lactams with vancomycin against methicillin-resistant Staphylococcus aureus. J Infect. 2017;74(3):324–325. doi: 10.1016/j.jinf.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 16.Tran KN, Rybak MJ. β-Lactam combinations with vancomycin show synergistic activity against vancomycin-susceptible Staphylococcus aureus, vancomycin-intermediate S. aureus (VISA), and heterogeneous VISA. Antimicrob Agents Chemother. 2018;62(6):e00157-18. doi: 10.1128/AAC.00157-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barber KE, Ireland CE, Bukavyn N, et al. Observation of “seesaw effect” with vancomycin, teicoplanin, daptomycin and ceftaroline in 150 unique MRSA strains. Infect Dis Ther. 2014;3:35–43. doi: 10.1007/s40121-014-0023-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng X, Berti AD, McCrone S, Roch M, Rosato AE, Rose WE, Chen B. Combination antibiotic exposure selectively alters the development of vancomycin intermediate resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 2018;62(2):e02100-17. doi: 10.1128/AAC.02100-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Domaracki BE, Evans AM, Venezia RA. Vancomycin and oxacillin synergy for methicillin-resistant staphylococci. Antimicrob Agents Chemother. 2000;44(5):1394–1396. doi: 10.1128/AAC.44.5.1394-1396.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Werth BJ, Vidaillac C, Murray KP, Newton KL. Novel combinations of vancomycin plus ceftaroline or oxacillin against methicillin-resistant vancomycin-intermediate Staphylococcus aureus (VISA) and heterogeneous VISA. Antimicrob Agents Chemother. 2013;57(5):2376–2379. doi: 10.1128/AAC.02354-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dilworth TJ, Sliwinski J, Ryan K, Dodd M. Evaluation of vancomycin in combination with piperacillin-tazobactam or oxacillin against clinical methicillin-resistant Staphylococcus aureus isolates and vancomycin-intermediate S. aureus isolates in vitro. Antimicrob Agents Chemother. 2014;58(2):1028–1033. doi: 10.1128/AAC.01888-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagihara M, Wiskirchen DE, Kuti JL, Nicolau DP. In vitro pharmacodynamics of vancomycin and cefazolin alone and in combination against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2012;56(1):202–207. doi: 10.1128/AAC.05473-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leonard SN. Synergy between vancomycin and nafcillin against Staphylococcus aureus in an in vitro pharmacokinetic/pharmacodynamic model. PLoS ONE. 2012;7(7):e42103. doi: 10.1371/journal.pone.0042103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winn WC, Koneman EW. Koneman’s color atlas and textbook of diagnostic microbiology. Philadelphia: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 25.CLSI . Performance standards for antimicrobial susceptibility testing. CLSI approved standard M100-S19. Wayne: Clinical and Laboratory Standards Institute; 2016. [Google Scholar]
- 26.Clinical and Laboratory Standards Institute (CLSI). Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically; approved standard-10th edition. CLSI document M07-A10. CLSI, 940 West Valley Road, Suite 1400, Wayne, PA; 2015.
- 27.Ghaznavi-Rad E, Nor Shamsudin M, Sekawi Z, van Belkum A. A simplified multiplex PCR assay for fast and easy discrimination of globally distributed staphylococcal cassette chromosome mec types in meticillin-resistant Staphylococcus aureus. J Med Microbiol. 2010;59(Pt 10):1135–1139. doi: 10.1099/jmm.0.021956-0. [DOI] [PubMed] [Google Scholar]
- 28.Leonard SN, Rossi KL, Newton KL, Rybak MJ. Evaluation of the Etest GRD for the detection of Staphylococcus aureus with reduced susceptibility to glycopeptides. J Antimicrob Chemother. 2009;63(3):489–492. doi: 10.1093/jac/dkn520. [DOI] [PubMed] [Google Scholar]
- 29.Wootton M, Howe RA, Hillman R, Walsh TR. A modified population analysis profile (PAP) method to detect hetero-resistance to vancomycin in Staphylococcus aureus in a UK hospital. J Antimicrob Chemother. 2001;47(4):399–403. doi: 10.1093/jac/47.4.399. [DOI] [PubMed] [Google Scholar]
- 30.Odds FC. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother. 2003;52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 31.Isenberg H. Time-kill assay. In: Garcia LS, Isenberg HD, editors. Clinical microbiology procedures handbook. Washington, DC: ASM; 2010. pp. 5.10.2.1–5.10.2.12. [Google Scholar]
- 32.Liu C, Bayer A, Cosgrove SE, Daum RS. Clinical practice guidelines by the infectious diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 33.McConeghy KW, Bleasdale SC, Rodvold KA. The empirical combination of vancomycin and a β-lactam for Staphylococcal bacteremia. Clin Infect Dis. 2013;57(12):1760–1765. doi: 10.1093/cid/cit560. [DOI] [PubMed] [Google Scholar]
- 34.Climo MW, Patron RL, Archer GL. Combinations of vancomycin and beta-lactams are synergistic against staphylococci with reduced susceptibilities to vancomycin. Antimicrob Agents Chemother. 1999;43(7):1747–1753. doi: 10.1128/AAC.43.7.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sieradzki K, Wu SW, Tomasz A. Inactivation of the methicillin resistance gene mecA in vancomycin-resistant Staphylococcus aureus. Microb Drug Resist. 1999;5(4):253–257. doi: 10.1089/mdr.1999.5.253. [DOI] [PubMed] [Google Scholar]
- 36.Sieradzki K, Tomasz A. Suppression of beta-lactam antibiotic resistance in a methicillin-resistant Staphylococcus aureus through synergic action of early cell wall inhibitors and some other antibiotics. J Antimicrob Chemother. 1997;39(Suppl A):47–51. doi: 10.1093/jac/39.suppl_1.47. [DOI] [PubMed] [Google Scholar]
- 37.Tabuchi F, Matsumoto Y, Ishii M, Tatsuno K. Synergistic effects of vancomycin and β-lactams against vancomycin highly resistant Staphylococcus aureus. J Antibiot (Tokyo) 2017;70(6):771–774. doi: 10.1038/ja.2017.7. [DOI] [PubMed] [Google Scholar]
- 38.Rose WE, Knier RM, Hutson PR. Pharmacodynamic effect of clinical vancomycin exposures on cell wall thickness in heterogeneous vancomycin-intermediate Staphylococcus aureus. J Antimicrob Chemother. 2010;65(10):2149–2154. doi: 10.1093/jac/dkq292. [DOI] [PubMed] [Google Scholar]
- 39.Joukhadar C, Pillai S, Wennersten C, Moellering RC. Lack of bactericidal antagonism or synergism in vitro between oxacillin and vancomycin against methicillin-susceptible strains of Staphylococcus aureus. Antimicrob Agents Chemother. 2010;54(2):773–777. doi: 10.1128/AAC.00348-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong D, Wong T, Romney M, Leung V. Comparative effectiveness of β-lactam versus vancomycin empiric therapy in patients with methicillin-susceptible Staphylococcus aureus (MSSA) bacteremia. Ann Clin Microbiol Antimicrob. 2016;15:27. doi: 10.1186/s12941-016-0143-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDanel JS, Perencevich EN, Diekema DJ, Herwaldt LA. Comparative effectiveness of beta-lactams versus vancomycin for treatment of methicillin-susceptible Staphylococcus aureus bloodstream infections among 122 hospitals. Clin Infect Dis. 2015;61(3):361–367. doi: 10.1093/cid/civ308. [DOI] [PubMed] [Google Scholar]
- 42.Lodise TP, McKinnon PS, Levine DP, Rybak MJ. Impact of empirical-therapy selection on outcomes of intravenous drug users with infective endocarditis caused by methicillin-susceptible Staphylococcus aureus. Antimicrob Agents Chemother. 2007;51(10):3731–3733. doi: 10.1128/AAC.00101-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dilworth TJ, Ibrahim O, Hall P, Sliwinski J. β-Lactams enhance vancomycin activity against methicillin-resistant Staphylococcus aureus bacteremia compared to vancomycin alone. Antimicrob Agents Chemother. 2014;58(1):102–109. doi: 10.1128/AAC.01204-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davis JS, Sud A, O’Sullivan MVN, Robinson JO. Combination of vancomycin and β-lactam therapy for methicillin-resistant Staphylococcus aureus bacteremia: a pilot multicenter randomized controlled trial. Clin Infect Dis. 2016;62(2):173–180. doi: 10.1093/cid/civ808. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.