Abstract
Background
Medication adverse events are important and common yet are often not identified by clinicians. We evaluated an automated telephone surveillance system coupled with transfer to a live pharmacist to screen potentially drug-related symptoms after newly starting medications for four common primary care conditions: hypertension, diabetes, depression, and insomnia.
Methods
Cluster randomized trial with automated calls to eligible patients at 1 and 4 months after starting target drugs from intervention primary care clinics compared to propensity-matched patients from control clinics. Primary and secondary outcomes were physician documentation of any adverse effects associated with newly prescribed target medication, and whether the medication was discontinued and, if yes, whether the reason for stopping was an adverse effect.
Results
Of 4876 eligible intervention clinic patients who were contacted using automated calls, 776 (15.1%) responded and participated in the automated call. Based on positive symptom responses or request to speak to a pharmacist, 320 patients were transferred to the pharmacist and discussed 1021 potentially drug-related symptoms. Of these, 188 (18.5%) were assessed as probably and 479 (47.1%) as possibly related to the medication. Compared to a propensity-matched cohort of control clinic patients, intervention patients were significantly more likely to have adverse effects documented in the medical record by a physician (277 vs. 164 adverse effects, p < 0.0001, and 177 vs. 122 patients discontinued with documented adverse effects, p < 0.0001).
Discussion
Systematic automated telephone outreach monitoring coupled with real-time phone referral to a pharmacist identified a substantial number of previously unidentified potentially drug-related symptoms, many of which were validated as probably or possibly related to the drug by the pharmacist or their physicians. Multiple challenges were encountered using the interactive voice response (IVR) automated calling system, suggesting that other approaches may need to be considered and evaluated.
Trial Registration
Electronic supplementary material
The online version of this article (10.1007/s11606-018-4672-7) contains supplementary material, which is available to authorized users.
INTRODUCTION
Use of pharmacologic agents is ubiquitous, with more than half of the US population reporting using a prescription medication in the past year.1, 2 Medication management dominates medical encounters, with two thirds of adult ambulatory care visits resulting in a prescription or continuation of a medication, and 25% noting a new or changed prescription.1 Prior work has demonstrated that up to one in four patients prescribed a new medication experiences an adverse drug event (ADE).3, 4 The burden of outpatient ADEs on patients and health systems is substantial, resulting in more than 3.5 million physician office visits and one million US emergency room visits annually.5–8
Timely identification of symptoms related to ADEs is important to minimize harm and identify drug adverse effects more broadly. Unfortunately, many ADEs go undetected, unreported, and unaddressed.9–11 While various methods educate patients about potential ADEs (e.g., leaflets or counseling at pharmacies) and clinicians often ask about potential ADEs during follow-up encounters, proactive approaches for monitoring and detecting ADEs are rare or nonexistent in most settings.
We have deployed a variety of approaches to monitor patients for symptoms associated with starting new medications.3, 12 Given the large numbers of medications initiated in ambulatory care, manual efforts such as telephone calls should be replaced by more automated approaches to enable more efficient outreach to larger numbers of patients at risk.13 One approach is interactive voice response (IVR) technology, which enables automated telephone calls.14, 15 IVR systems use various features including voice recognition and data capture; we have previously used IVR as a tool for pharmacoepidemiologic monitoring of ADEs.16–18
In this study, we deployed IVR to call patients newly prescribed medications for hypertension, diabetes, insomnia, and depression. We developed and implemented an IVR system to contact patients approximately 1 month and 4 months after receiving a new prescription for a medication for one of these four conditions. An additional feature of this proactive surveillance was real-time transfer to a live clinical pharmacist for patients reporting potential drug-related symptoms. Here, we describe the results of a trial evaluating the impact of this approach.
METHODS
Overview
Calling for Earlier Detection of Adverse Reactions (CEDAR) was a cluster randomized controlled trial of adult primary care patients receiving care at practices affiliated with Brigham and Women’s Hospital and North Shore Physician’s Group. Using expert review and frequency of use, we identified 112 medications used for the four target conditions. Patients newly started on target medications from intervention clinics were called using a novel IVR platform for detecting patient-reported symptoms. Patients were transferred in real-time to a clinical pharmacist to assess whether the symptom was related to the new medication, filed a note in the electronic health record (EHR), and notified the appropriate clinician if symptoms required urgent follow-up. All clinics shared the same EHR, the Partners HealthCare (PHS) Longitudinal Medical Record (LMR). Clinics were randomized to intervention or control sites (or where possible, randomized by suites within clinics at each site). A cohort of propensity score-matched patients from control clinics was assembled to match patients who participated in the calls from intervention clinics. We called patients at 1 month to detect potential acute reactions and at 4 months to detect potential subacute problems. The research protocol was approved by the PHS Institutional Review Board and registered at Clinicaltrials.gov (NCT NCT02087293).
IVR Script Development
We developed an IVR survey based on prior work17–19 which included a consent script, birthdate verification, and set of generic symptom questions followed by selected drug-specific questions (e.g., cough for ACE inhibitors). Questions required either numeric (birthday) or dichotomous YES/NO answers, with “SKIP” possible. Patients could exit the survey at any time by hanging up. The IVR script was translated into Spanish and patients were asked by the IVR system whether they preferred to participate in English or Spanish. The IVR questionnaire logic was mapped in Visio and programmed by a commercial IVR vendor (Vocantas) (Supplemental Appendix).
Patient Eligibility
Twenty-six clinic sites were identified and matched based on underlying characteristics; 13 clinic units were randomized to the intervention group. Control clinics provided patient data for chart review but no patients or providers were contacted. We included all patients over age 18 who (1) received a prescription for one of the target medications at one of the 26 clinic units; (2) evidenced no history of having been prescribed the target medication within the previous 2 years; and (3) received a prescription for more than seven doses (e.g., excluded benzodiazepine prescriptions for medical procedures or air-flight). All English- or Spanish-speaking patients were included. If an eligible patient was prescribed more than one target medication, we randomly selected only one. Patients could be enrolled/recruited for the study only once.
Randomization and Recruitment
Clinics were stratified (based on size, demographic characteristics, and network), then were randomized to intervention or control sites. Patients in intervention clinics who met eligibility criteria were sent an informational mailing describing the study and provided a phone number to call if the patient wished to opt out from receiving the call. Two weeks later (i.e., 1 month after the prescription), intervention clinic patients who had not opted out received IVR calls. Patients were called between 9 am and 7 pm, on a rotating schedule based on availability of study staff to receive transferred calls. Patients received up to 10 calls over a 2-week period, including at least one weekend day. Once reached, patients could opt out, provide verbal consent, or hang up at any point during the call. Participants were asked to verify their identity (by birthdate).
A cohort of control patients who met the same eligibility criteria was generated from control sites using propensity score matching. A logistic regression model estimated the probability that a patient would participate on the telephone interview. The model used sex, age, race, ethnicity, religion, marital status, median household income, availability of email, presence of a PCP, number of primary care visits, target drug, and diagnoses of diabetes, hypertension, depression, and insomnia as predictors. This model was then applied to the control patients to calculate a propensity score, estimating each control clinic patient’s probability of responding to the telephone survey had they been called. The propensity score of each intervention patient who responded to the telephone survey was then matched to the closest propensity score of a control patient; the pair of patients would then have an equal likelihood of responding to the survey.
Data Collection and Study Flow
Following consent by IVR, intervention participants were queried about their adherence to the target medication, and asked generic and drug-specific adverse effect questions. Any patient reporting new symptoms since starting the medication was transferred to the pharmacist who reviewed their symptoms and assessed the likelihood of a significant adverse effect, as well as triaged the patient based on their need and urgency for additional medical attention. Patients who did not report symptoms on the IVR call were also offered an opportunity to speak to the live pharmacist for any medication-related questions.
Pharmacist Protocol
The pharmacist simultaneously received the transferred call and was notified electronically via email listing symptoms reported in the IVR survey. During the call, the pharmacist inquired about adherence to the prescription and then discussed each symptom with the patient and evaluated the severity (life-threatening, serious, significant, and mild), urgency (immediate attention needed, moderate, and non-urgent), and likelihood that the symptom was drug-related (definite, probable, possible, and unlikely) using previously published modified Naranjo algorithms.20 After the call, the pharmacist documented the intervention in the EHR. For patients with life-threatening and/or urgent ADEs, the patient’s physician was contacted. The pharmacist spoke both English and Spanish and counseled patients in their preferred language.
Outcome Measures
The primary outcome was physician documentation of any adverse effects associated with the target newly prescribed medication. This was assessed by comprehensive manual chart review of 1 year of all notes following the target medication prescription by trained research assistants. Reviewers recorded all instances a physician note commented on a potential adverse event related to the target medication. Secondary outcomes included whether the study medication had been discontinued within this time and, if so, whether it was stopped due to potential adverse effects.
Statistical Analysis
Characteristics of intervention patients and their propensity-matched controls were compared using chi-square tests for categorical characteristics and t tests or Wilcoxon rank sum tests for continuous measures (Table 1). The percentages of unique patients with symptoms documented in the two arms were compared using chi-square tests. The numbers of symptoms documented in each arm were compared using Poisson regression since individual patients could have multiple events. For documented symptoms due to the target medication, with the available sample size of 776 controls and a documentation rate of 15.5%, we had 90% power if the documentation rate in the 776 intervention patients was 22% or higher. For medication discontinuation due to ADEs with available sample size of 5897 controls and discontinuation rate of 11%, we had 90% power as long as discontinuation rate in 5106 intervention patients was 13% or higher.
Table 1.
Baseline Characteristics of Intervention Participants and Matched Controls
| Intervention participants n = 776 |
Matched control patients n = 776 |
p value | |
|---|---|---|---|
| Age (mean/median (range)) | 57.2/59.0 (18–96) | 59.7/60 (19–97) | 0.0005 |
| Race/ethnicity* | < 0.0001 | ||
| White, no. (%) | 522 (67.3) | 593 (76.4) | |
| Black, no. (%) | 108 (13.9) | 63 (8.1) | |
| Hispanic, no. (%) | 112 (14.4) | 79 (10.2) | |
| Other, no. (%) | 34 (4.4) | 41 (5.3) | |
| Sex | 0.0005 | ||
| Female, no. (%) | 518 (66.6) | 581 (74.9) | |
| Clinical characteristics | |||
| Charlson Index (mean/median) | 1.5781/1.0000 | 1.4867/1.0000 | 0.6458 |
| No. of medications† (mean/median) | 10.1/8.0 | 10.2/8.0 | 0.83 |
| No. of medications at 1 year† (mean/median) | 11.5/10.0 | 11.3/9.0 | 0.71 |
| No. of medical problems† (mean/median) | 9.1/8.0 | 9.2/8.0 | 0.75 |
| No. of medical problems at 1 year†(mean/median) | 10.2/9.0 | 10.2/9.0 | 0.92 |
| Condition treated with target medication‡ | Overall target condition comparison 0.3389 | ||
| Hypertension, no. (%) | 411 (53.0) | 442 (57.0) | 0.1134 |
| Insomnia, no. (%) | 143 (18.4) | 132 (17.0) | 0.4701 |
| Depression, no. (%) | 122 (15.7) | 120 (15.5) | 0.9136 |
| Diabetes, no. (%) | 100 (12.9) | 82 (10.5) | 0.1415 |
*Race/ethnicity data based on coded fields in the EHR
†Medications and documented problems extracted from the EHR medication and problem lists at the time the target medication was prescribed and 1 year following the target prescription date
‡Treatment indication based on chart and not patient self-report
RESULTS
Recruitment
Between June 2013 and April 2015, 5143 eligible patients were identified by EHR in intervention clinics and 5985 in non-intervention control clinics. IVR calls were placed to 4876 (94.8%) eligible intervention patients, of whom 894 (17.4%) gave initial phone consent and 776 (15.1%) provided answers to at least one question in the automated survey and were considered participants. The 3982 intervention patients who did not participate included 540 (10.5%) who were never reached, 2547 (49.5%) who immediately hung up, and 895 (17.4%) who declined to participate in the call (Fig. 1).
Figure 1.
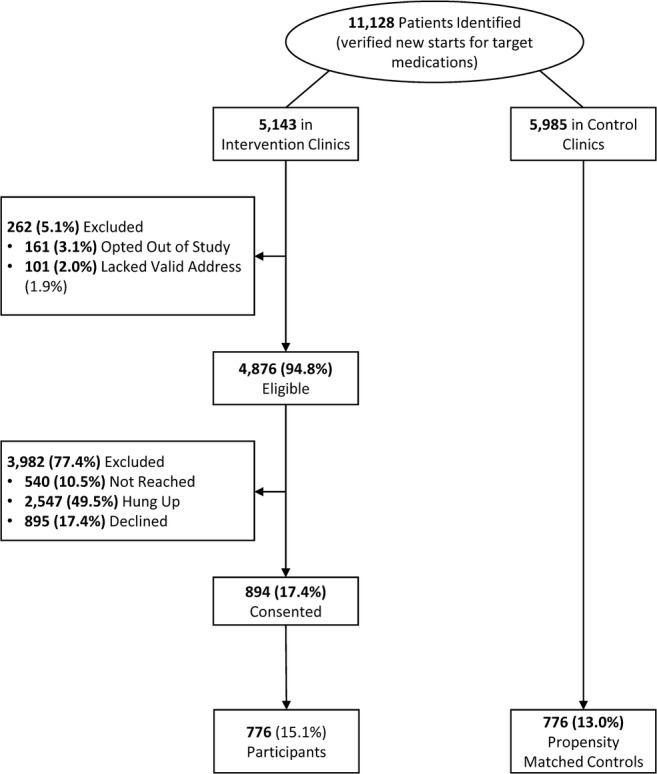
CONSORT study flow. Participants were defined as providing consent on the IVR call, completing birthdate verification, and inputting at least one question on the IVR survey.
Study Population
Table 1 compares EHR-derived demographic characteristics and comorbidities of participants and matched controls. Compared to matched controls, participants were slightly younger (median age 59 vs. 60), less likely female (66.8% vs. 74.9%, p = 0.0005), and more racially/ethnically diverse. No significant differences were observed between intervention participants and controls with respect to Charlson comorbidity indices. We observed no significant differences in the number of active medications and problems documented in the EHR for the intervention vs. control groups, either at the time of the target medication prescription or after 1 year. The majority of the participants (53.0%) were recruited for a target medication to treat hypertension; 18.4% were recruited for an insomnia medication; 15.7% were taking a medication to treat depression; and 12.9% were prescribed an oral medication to treat diabetes.
Automated (IVR) Survey and Pharmacist Assessment
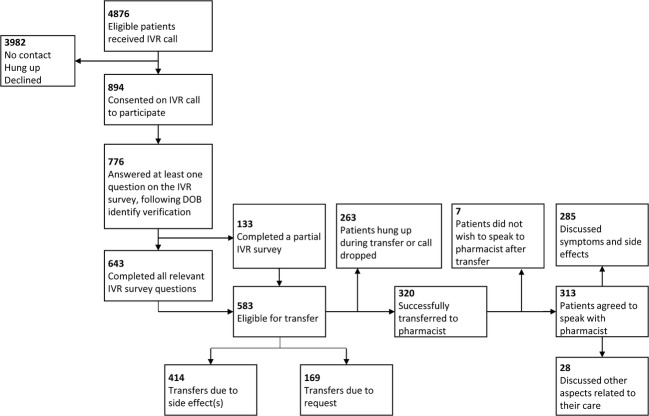
Of the 776 participants, 643 (82.9% of IVR participants) completed all relevant questions in the IVR survey; 583 (75.1%) met the requirements for automatic transfer to the pharmacist, either because they reported a potential side effect since starting the target medication (414 participants; 53.4%) or because they requested to speak to a pharmacist (169 patients; 21.8%). Three hundred twenty (41.2%) patients successfully transferred to the pharmacist, and slightly fewer (313; 40.3%) completed a phone-based encounter with the pharmacist. The remaining 263 calls eligible for transfer were either terminated or the patient declined the live encounter. The pharmacist talked about symptoms with 285 (36.7%) patients and other health-related topics with 28 (3.6%) patients (Fig. 2).
Figure 2.
Uptake of IVR and pharmacist counseling intervention.
The 776 participants reported 997 total new potentially drug-related symptoms to the IVR system; many of these were subsequently discussed with the pharmacist by the subset of patients who had their call transferred and stayed on the line. The 285 patients who spoke to the pharmacist in real-time discussed 1018 potential ADE-related symptoms. These patient-reported symptoms were related to 52 different medications. Of these, 188 (18.5%) were assessed as probably related and 479 (47.1%) possibly related to the newly initiated medication. Of the symptoms assessed as probably or possibly medication-related, 266 (39.8%) were rated as mild, 400 (59.9%) as significant, and 1 (0.15%, suicidal ideation) as life-threatening using previously validated criteria (Table 2).20, 21 Review of the EHR found that physicians explicitly referenced the pharmacist conversation/documentation in their own notes in 23 patient encounters (8.1% of those with a pharmacist note documenting patient symptoms).
Table 2.
Documented Symptoms and Drug Discontinuations, Intervention vs. Control
| Intervention participants n = 776 |
Control patients n = 776 |
p value | |
|---|---|---|---|
| Total symptoms collected by IVR system | 997 | NA | |
| Total symptoms discussed by pharmacist | 1018 | NA | |
| Related to target medication—probable | 188 (18.5%) | NA | |
| Related to target medication—possible | 479 (47.1%) | NA | |
| Related to target medication—unlikely | 351 (34.5%) | NA | |
| Severity of pharmacist-confirmed possible and probable symptoms (N = 668) | |||
| Mild | 266 (39.8%) | NA | |
| Significant | 400 (59.9%) | NA | |
| Life-threatening | 1 (0.15%) | NA | |
| Total symptoms documented by MD in notes | 277 | 164 | < 0.0001 |
| Number of symptoms per 100 patients | 36 | 21 | < 0.0001 |
| Number of patients with symptoms documented by MD | 177 | 122 | < 0.0001 |
| Total unique symptoms documented (including IVR documented and MD notes) | 1303 | 164 | < 0.0001 |
| Total unique patients with symptoms documented (including IVR documented) | 448 | 120 | < 0.0001 |
| Total unique symptoms documented by MD or RPh as probably or possibly related to the drug | 753 | 164 | < 0.0001 |
| Total unique patients with symptoms documented by MD or RPh as probably or possibly related to the drug | 425 | 120 | < 0.0001 |
| Drug discontinuations (CPOE d/c code reason) |
N = 5106* No. (%) |
N = 5897† No. (%) |
|
| Discontinuations 1 year from prescription | 1694 (33.2) | 1977 (33.5) | 0.70 |
| Discontinuations due to adverse event‡ | 254 (15.0) | 217 (11.0) | 0.0003 |
*Missing data on target medication discontinuations for 37 patients
†Missing data on target medication discontinuations for 88 patients
‡Discontinuation due to an adverse event as a coded justification in the EHR
Documentation of Symptoms and Drug Discontinuations
For our primary measure comparing physician documentation of patient-reported drug-related symptoms, we compared IVR participants with a propensity-matched cohort of control patients and found significant increases in documentation of symptoms, number of symptoms documented, and number of patients with symptoms documented in the intervention group. Detailed review of these clinicians’ notes identified a total of 277 symptoms that they specifically documented as potentially related to the index medication, compared to 164 in the propensity-matched control patients (p < 0.0001, Table 2). We observed a greater number of unique symptoms documented and unique patients with symptoms documented in the intervention group (p < 0.0001 for each category).
Analyzing the results by overall intention to treat (entire cohort assigned to intervention vs. control) using our EMR-based discontinuation rates with structured reasons, there was no significant difference in medications discontinued; however, there was a highly significant increased proportion discontinued due to adverse events in the intervention group (15% vs. 11%, p = 0.0003) (Table 2).
Table 3 summarizes frequencies of symptoms that patients reported on the IVR calls. It shows the frequencies of symptoms by each of the four conditions being treated and reveals that patients being treated with medications for depression and insomnia reported more symptoms overall.
Table 3.
Patient-Reported Symptoms on IVR
| Condition treated by target medication for IVR participants | ||||
|
Hypertension n = 335 No. (%) |
Diabetes n = 76 No. (%) |
Depression n = 144 No. (%) |
Insomnia n = 85 No. (%) |
|
| Broad symptom screening | ||||
| Skin rash | 12 (3.6) | 3 (3.9) | 7 (4.9) | 1 (1.2) |
| Memory or confusion | 16 (4.8) | 7 (9.2) | 15 (10.4) | 7 (8.2) |
| Stomach or intestinal problems | 16 (4.8) | 14 (18.4) | 19 (13.2) | 2 (2.4) |
| Problems with sexual function | 15 (4.5) | 6 (7.9) | 16 (11.1) | 5 (5.9) |
| Frequent headaches | 25 (7.5) | 8 (10.5) | 25 (17.4) | 8 (9.4) |
| Dizziness or problems with balance | 35 (10.4) | 5 (6.6) | 26 (18.1) | 8 (9.4) |
| Muscle aches | 41 (12.2) | 12 (15.8) | 23 (16.0) | 10 (11.8) |
| Weight changes | 61 (18.2) | 14 (18.4) | 39 (27.1) | 13 (15.3) |
| Drug-specific symptom screening | ||||
| ACE inhibitors | N = 72 | |||
| New cough | 19 (26.4%) | |||
| Swelling of the face, lips, tongue | 4 (5.6%) | |||
| Beta/calcium channel blockers | N = 132 | |||
| New shortness of breath | 14 (10.6) | |||
| Swelling of the legs | 11 (8.3) | |||
| No. of symptoms reported on broad symptom screen | Burden of symptoms reported by survey completers | |||
|
Hypertension N = 264 No. (%) |
Diabetes N = 58 No. (%) |
Depression N = 117 No. (%) |
Insomnia N = 60 No. (%) |
|
| 0 | 157 (59.5) | 24 (41.4) | 43 (36.8) | 35 (58.3) |
| 1 | 51 (19.3) | 14 (24.1) | 29 (24.8) | 14 (23.3) |
| 2 | 25 (9.5) | 10 (17.2) | 22 (18.8) | 5 (8.3) |
| 3 | 18 (6.8) | 7 (12.1) | 12 (10.3) | 2 (3.3) |
| 4 | 4 (1.5) | 3 (5.2) | 7 (6.0) | 1 (1.7) |
| 5 | 9 (3.4) | 0 | 3 (2.6) | 1 (1.7) |
| 6 | 0 | 0 | 1 (0.9) | 2 (3.3) |
DISCUSSION
Patients starting medications frequently experience drug-related symptoms that may be unreported or overlooked. We deployed an IVR pharmacosurveillance system coupled with pharmacist transfer and uncovered a significant number of patient-reported symptoms, many of which were later validated as likely drug-related by pharmacist assessment and/or documentation in physician notes. Compared to a matched control group, there was a 50% increase in potential ADE symptoms documented by the PCP in patients who participated in the intervention compared to matched controls and when we also include symptoms reported to the IVR, a more than threefold increase (753 vs. 164) in total overall potential adverse symptoms documented. Despite the fact that only one of eight patients in the overall intervention group participated in the call, we nonetheless were able to observe a significant difference in the proportion of patients having their drugs discontinued by their physician with the physician-coded discontinuation reason listed as an adverse event.
Reported symptoms varied by drug and target condition. Patients treated for insomnia and depression reported more symptoms on the IVR than patients newly started on drugs for diabetes or hypertension. These differences may be due either to patient- or medication-related factors. While patients reported a large number of non-specific symptoms on the IVR “since starting the (new) medication,” the high rates also likely reflect an inherent bias that patients experiencing problems might be more likely to interact with our IVR and pharmacist outreach. Nonetheless, after careful review by our study pharmacist, nearly half met the criteria for being probably or possibly likely related to the newly started drugs.
It is important to note that many drug-related symptoms do not necessarily warrant discontinuation of the medication. A drug treatment’s net risk and benefits must be weighed through shared decision-making between patient and prescriber, discussing various treatment options and alternatives (e.g., non-drug options, adjunctive medications, dosing alterations). Awareness of ADEs by the ordering clinician however facilitates such discussions and may also improve medication adherence. While heightening patients’ awareness of possible adverse events might lead to greater non-adherence, our study pharmacist identified a number patients who were already not taking their medications as prescribed due to perceived symptoms, often unreported or unknown to their physicians.
Effectively and efficiently detecting ADEs is clinically and logistically challenging.22, 23 Prior studies have shown that manual phone-based outreach can identify symptoms, and automated calling systems such as IVR can facilitate large-scale outreach3, 17, 24, 25 and, based on an economic analysis of this effort, can be done at reasonable cost.19 However, we encountered a number of challenges in implementing our IVR system that may limit broad application. In the past, patients typically had landline telephones and calls reached them at home at times they might be available to answer survey questions. Currently, many patients have mobile phones and have wearied of “robo-calls” from advertisers, politicians, and other surveys.26 As we documented in our suboptimal response rates and high percentage of patients who immediately hung up, the ability to reach patients using this technology has limitations on response rates. Because our calls came at unexpected and often inconvenient times, and patients might not have trusted that they were legitimately integrated into their healthcare, they may have had reservations about interacting with the system and providing confidential medical information.
Setting up and deploying an IVR system in a complex healthcare organization were also challenging. Although we created an algorithm to identify newly started medications, limitations in our system’s coded records, new patient referrals, and complexities of our medication ordering process resulted in ongoing prescriptions initially being inaccurately labeled as new starts, requiring manual review to remove these ineligible cases. Creating the IVR script required extensive efforts and iterative testing with a professional vendor to ensure the logic and content of the call obtained accurate answers and was easy to use and interpret. Finally, the study’s measurement was subject to limitations related to the fact that charts contained the pharmacist’s notes; thus, reviewers could not be fully blinded to intervention vs. control patients (although we applied strict predefined criteria to the review solely of the physician notes), as well as any potential measured and unmeasured differences confounding our propensity-matched selected controls.
Despite these limitations, our automated system was able to collect a substantial number of previously unidentified drug-related symptoms from more than 15% of a large cohort of patients. The IVR scripts we prepared can serve as ready-made templates for others seeking to do similar surveillance. Newer technologies such as text messaging, email communication, or patient portals could build on this work, overcoming some of the IVR hurdles we encountered and facilitating more convenient outreach.23, 26, 27 Regardless of what methods ultimately prove most efficient, this and other studies demonstrate that many ADEs are present and are often overlooked, yet could be detected with more systematic, proactive, and timely surveillance.
Electronic Supplementary Material
(PDF 76 kb)
Acknowledgments
The team would like to acknowledge Japneet Kwatra, SM for her research assistance.
Funding
This research was supported by the Agency for Healthcare Research and Quality (AHRQ), H.I.T Center for Education and Research on Therapeutics (CERT) grant number 1U19HS021094-01, part of AHRQ’s CERT funding mechanism.
Compliance with Ethical Standards
The research protocol was approved by the PHS Institutional Review Board and registered at Clinicaltrials.gov (NCT NCT02087293).
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of AHRQ.
Conflict of Interest
Dr. Schiff previously received grant compensation to evaluate the Medaware software. Dr. Bates consults for EarlySense, which makes patient safety monitoring systems. He receives cash compensation from CDI (Negev), Ltd., which is a not-for-profit incubator for health IT startups. He receives equity from ValeraHealth which makes software to help patients with chronic diseases. He receives equity from Clew which makes software to support clinical decision-making in intensive care. He receives equity from MDClone which takes clinical data and produces deidentified versions of it. Dr. Schiff’s and Dr. Bates’ financial interests have been reviewed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their institutional policies.
The remaining authors declare no potential conflicts of interest.
REFERENCES
- 1.CDC. National Center for Health Statistics: Therapeutic Drug Use. 2015; http://www.cdc.gov/nchs/fastats/drug-use-therapeutic.htm. Accessed 12/5/2016.
- 2.Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the united states from 1999-2012. JAMA. 2015;314(17):1818–1830. doi: 10.1001/jama.2015.13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gandhi TK, Weingart SN, Borus J, et al. Adverse drug events in ambulatory care. N Engl J Med. 2003;348(16):1556–1564. doi: 10.1056/NEJMsa020703. [DOI] [PubMed] [Google Scholar]
- 4.Taché SV, Sönnichsen A, Ashcroft DM. Prevalence of adverse drug events in ambulatory care: a systematic review. Ann Pharmacother. 2011;45(7–8):977–989. doi: 10.1345/aph.1P627. [DOI] [PubMed] [Google Scholar]
- 5.Budnitz DS, Shehab N, Kegler SR, Richards CL. Medication use leading to emergency department visits for adverse drug events in older adults. Ann Intern Med. 2007;147(11):755–765. doi: 10.7326/0003-4819-147-11-200712040-00006. [DOI] [PubMed] [Google Scholar]
- 6.Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365(21):2002–2012. doi: 10.1056/NEJMsa1103053. [DOI] [PubMed] [Google Scholar]
- 7.Shehab N, Lovegrove MC, Geller AI, Rose KO, Weidle NJ, Budnitz DS. US emergency department visits for outpatient adverse drug events, 2013-2014. JAMA. 2016;316(20):2115–2125. doi: 10.1001/jama.2016.16201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrato EH. The Case for Funding Our National Drug Safety Surveillance System. In: LWW; 2017. [DOI] [PubMed]
- 9.Classen DC, Resar R, Griffin F, et al. ‘Global trigger tool’shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff. 2011;30(4):581–589. doi: 10.1377/hlthaff.2011.0190. [DOI] [PubMed] [Google Scholar]
- 10.Bourgeois FT, Shannon MW, Valim C, Mandl KD. Adverse drug events in the outpatient setting: an 11-year national analysis. Pharmacoepidemiol Drug Saf. 2010;19(9):901–910. doi: 10.1002/pds.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weissman JS, Schneider EC, Weingart SN, et al. Comparing patient-reported hospital adverse events with medical record review: do patients know something that hospitals do not? Ann Intern Med. 2008;149(2):100–108. doi: 10.7326/0003-4819-149-2-200807150-00006. [DOI] [PubMed] [Google Scholar]
- 12.Berner ES, Ray MN, Panjamapirom A, et al. Exploration of an automated approach for receiving patient feedback after outpatient acute care visits. J Gen Intern Med. 2014;29(8):1105–1112. doi: 10.1007/s11606-014-2783-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berner ES, Burkhardt JH, Panjamapirom A, Ray MN. Cost implications of human and automated follow-up in ambulatory care. Am J Manag Care. 2014;20(11 Spec No. 17):SP531–540. [PubMed]
- 14.Willig JH, Krawitz M, Panjamapirom A, et al. Closing the feedback loop: an interactive voice response system to provide follow-up and feedback in primary care settings. J Med Syst. 2013;37(2):9905. doi: 10.1007/s10916-012-9905-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Auger C, Forster AJ, Oake N, Tamblyn R. Usability of a computerised drug monitoring programme to detect adverse drug events and non-compliance in outpatient ambulatory care. BMJ Qual Saf. 2013;22(4):306–316. doi: 10.1136/bmjqs-2012-001492. [DOI] [PubMed] [Google Scholar]
- 16.Houser SH, Ray MN, Maisiak R, et al. Telephone follow-up in primary care: can interactive voice response calls work? Stud Health Technol Inform. 2013;192:112–116. [PMC free article] [PubMed] [Google Scholar]
- 17.Haas JS, Iyer A, Orav EJ, Schiff GD, Bates DW. Participation in an ambulatory e-pharmacovigilance system. Pharmacoepidemiol Drug Saf. 2010;19(9):961–969. doi: 10.1002/pds.2006. [DOI] [PubMed] [Google Scholar]
- 18.Haas JS, Amato M, Marinacci L, Orav EJ, Schiff GD, Bates DW. Do package inserts reflect symptoms experienced in practice?: assessment using an automated phone pharmacovigilance system with varenicline and zolpidem in a primary care setting. Drug Saf. 2012;35(8):623–628. doi: 10.1007/BF03261959. [DOI] [PubMed] [Google Scholar]
- 19.Mahida S, Seoane E, Klinger E, et al. Cost of reporting possible adverse Drug reactions in medical Outpatients using a Telephonic interactive Voice response system. Value Health. 2015;18(3):A257. doi: 10.1016/j.jval.2015.03.1498. [DOI] [Google Scholar]
- 20.Rehan HS, Chopra D, Kakkar AK. Physician’s guide to pharmacovigilance: Terminology and causality assessment. Eur J Intern Med. 2009;20(1):3–8. doi: 10.1016/j.ejim.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 21.Farcas A, Bojita M. Adverse drug reactions in clinical practice: a causality assessment of a case of drug-induced pancreatitis. J Gastrointestin Liver Dis. 2009;18(3):353–358. [PubMed] [Google Scholar]
- 22.Bates DW, Evans RS, Murff H, Stetson PD, Pizziferri L, Hripcsak G. Detecting adverse events using information technology. J Am Med Inform Assoc. 2003;10(2):115–128. doi: 10.1197/jamia.M1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forster AJ, Erlanger TE, Jennings A, et al. Effectiveness of a computerized drug-monitoring program to detect and prevent adverse drug events and medication non-adherence in outpatient ambulatory care: study protocol of a randomized controlled trial. Trials. 2015;16(1):1. doi: 10.1186/1745-6215-16-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forster AJ, Auger C. Using information technology to improve the monitoring of outpatient prescribing. JAMA Intern Med. 2013;173(5):382–384. doi: 10.1001/jamainternmed.2013.2002. [DOI] [PubMed] [Google Scholar]
- 25.Falconi M, Johnston S, Hogg W. A scoping review to explore the suitability of interactive voice response to conduct automated performance measurement of the patient’s experience in primary care. Prim Health Care Res Dev. 2016;17(3):209–225. doi: 10.1017/S1463423615000407. [DOI] [PubMed] [Google Scholar]
- 26.Boulos MNK, Wheeler S, Tavares C, Jones R. How smartphones are changing the face of mobile and participatory healthcare: an overview, with example from eCAALYX. Biomed Eng Online. 2011;10(1):1. doi: 10.1186/1475-925X-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Link MW, Murphy J, Schober MF, et al. Mobile technologies for conducting, augmenting and potentially replacing surveys: report of the AAPOR task force on emerging technologies in public opinion research. Deerfield: AAPOR; 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 76 kb)