Abstract
Organophosphorus (OP) pesticides are widely used to control pests because of their high activity. This study described a rapid and sensitive lateral flow immunochromatographic (LFIC) assay based on up-converting nanoparticles (UCNPs) for multi-residue detection of three OP pesticides. The developed assay integrated novel fluorescent material UCNPs labeled with a broad-specific monoclonal antibody. Based on the competitive platform by immobilized antigen in the test zone, the optimized UCNPs-LFIC assay enabled sensitive detection for parathion, parathion-methyl, and fenitrothion with IC50 of 3.44, 3.98, and 12.49 ng/mL (R2 ≥ 0.9776) within 40 min. The detectable ability ranged from 0.98 to 250 ng/mL. There was no cross-reactivity with fenthion, phoxim, isocarbophos, chlorpyrifos, or triazophos, even at a high concentration of 500 ng/mL. Matrix interference from various agricultural products was also studied in food sample detection. In the spiked test, recoveries of the three OP pesticides ranged from 67 to 120% and relative standard deviations were below 19.54%. These results indicated that the proposed strip assay can be an alternative screening tool for rapid detection of the three OP pesticides in food samples.
Keywords: organophosphorus pesticide, up-conversion nanoparticles, lateral flow strip, immunoassay, multi-residue detection
Introduction
Organophosphorus (OP) pesticides, a class of insecticides, are widely used in pest control for their strong effect and broad spectrum (Roberts and Aaron, 2008; Handford et al., 2015). As an inhibitor of acetylcholinesterase, OP pesticides cause the accumulation of neurotransmitters and dysfunctional neuropathy (Davies et al., 1996; Payne-sturges et al., 2009; Bouchard et al., 2011), which pose a significant threat to human health and food safety. Considering their high toxicology, many counties set strict regulations to defend against hazards from OP pesticides, as parathion and parathion-methyl were banned in the European Union, Australia and China. Unfortunately, the illegal use of them as hidden components still occurs in some areas. The potential risk of excessive residue in agriproducts is also highlighted (Liang et al., 2012). Therefore, there is an urgent need to establish highly sensitive analytical methods for large-scale monitoring of OP pesticide residue.
Many instrumental analysis methods have been established for the determination of OP pesticide residues. For example, gas chromatography (GC), high performance liquid chromatography (HPLC), and GC coupled with mass spectrometry (GC-MS) were widely used due to their high accuracy and sensitivity (Liang et al., 2008; Tao et al., 2013; Watanabe et al., 2014; Zhao et al., 2014). However, these methods are demand much in the way of equipment and professional operating technology. As an alternative, the immunoassay based on the specific combination of antigen and antibody has high sensitivity and specificity. Combining simple operation with economical material price, the immunoassay has gradually become an important detection method. However, enzyme-linked immunoassays still require laboratory operations such as multiple incubations, washing, and sample pretreatment steps, hindering application in fields and markets (Xu et al., 2011). There is high demand for developing a fast and on-site method for the determination of OP pesticides in food and environmental samples.
Lateral-flow immunochromatographic (LFIC) assay, an ideal one-step immunoassay technique, was introduced to a variety of areas for rapid detection outside the laboratory (Wang et al., 2009; Zhu et al., 2011) as a result of its great convenience. As label selection is vitally important to the assay sensitivity, colorimetric and fluorometric measurement are usually considered (Xie et al., 2014). Colloidal gold (CG) has been the most widely used label in LFIC strips because of its low-cost preparation and visual detection. However, due to the limited signal-to-noise ratio of visible lights, CG-LFIC assays may have the drawbacks of low sensitivity and a narrow detection range (Liu et al., 2012). Therefore, different fluorescence tracers are employed to LFIC assays, such as quantum dots (QDs) (Qi et al., 2016) and fluorescence microspheres (Huang et al., 2016).
Up-converting nanoparticles (UCNPs), a kind of novel fluorescence material with special luminescence properties, possess the unique optical ability to up-convert low-energy infrared radiation and emit high-energy visible light. In theory, they can circumvent the background fluorescence from biological samples. In addition, as an inorganic inert material, these particles can avoid fluorescence-quenching decay. Thus, UCNPs are beneficial for the improvement of sensitivity and accuracy with less influence from the sample matrix (Dou et al., 2013; Feng et al., 2013), and the UCNP-LFIC assays have shown great potential in the field of large-screening and point-of-care testing. According to the literature, UCNP-LFIC assays have been developed for the detection of Vibrio anguillarum (Zhao et al., 2014), foodborne pathogens (Zhao et al., 2016a), Neutrophil gelatinase-associated lipocalin (NGAL) (Lei et al., 2017), Yersinia pestis (Yan et al., 2006), and prognosis of heart failure (You et al., 2017). For the determination of small molecular compounds like pesticides, UCNP-LFIC assays require a competitive format and face a great challenge in detection. At present, only very few UCNP-LFIC assays have been established for the determination of small-molecular contaminants, such as aflatoxin B1 (Zhao et al., 2016b) and clenbuterol (Wang et al., 2016) as well as imidaclothiz (Hua et al., 2018).
In this study, we focused on preparing UCNP fluorescent probes coupled with a broad-specific monoclonal antibody (mAb) that can recognize parathion, methyl-parathion and fenitrothion simultaneously. A competitive UCNP-LFIC assay was further established for rapid quantitative determination of three OP pesticides with high sensitivity. Meanwhile, multiple detections for various agricultural matrix interference tolerance degrees were also reviewed, enabling it to screen the three OP pesticides in food samples within 40 min. To the best of our knowledge, this is the first report of using UCNP-LFIC assays for OP pesticide residue detection. It thus furthers the application of UCNP-LFIC assays in the field of food safety and quality monitoring.
Materials and Methods
Materials and Reagents
Carboxylic acid-functionalized UCP (NaYF4: Yb3+, Er3+) nanoparticles (diameter of 150 nm; excitation spectrum peak of 545 nm; emission spectrum peak of 660 nm) were obtained from Fluo Nanotech Co., Ltd (Hangzhou, China). 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC, 99%) was purchased from Sigma-Aldrich (St. Louis, MO, USA). N-Hydroxysulfosuccinimide sodium salt (sulfo-NHS, 99%), trahalose (99%), polyvinyl pyrrolidone and Tween-20 were purchased from Aladdin Industrial Corporation (Shanghai, China). 2-(N-morpholino) ethanesulfonic acid (MES) was supplied by Sangon Biotech Co., Ltd (Shanghai, China), bovine serum albumin (BSA) was obtained from Sino-American Biotechnology (Luoyang, China). N-propyl-ethylenediamine (primary secondary amine, PSA) was supplied by Agela Technologies (Tianjin, China).
Nitrocellulose membranes (MDI 90, 2.0 cm × 30 cm), glass fibers, plastic adhesive backing pad, and absorbent pad were obtained from Jiening Biotech CO., Ltd (Shanghai, China). Plastic card sets fit for the reading instruments were designed by our group.
A broad-specific mAb against parathion and its coating antigen PA0304-OVA were previously produced in our laboratory (Jiao et al., 2018). Goat anti-mouse IgG was obtained from Biodragon Immunotechnologies (Beijing, China). Standards of OP pesticides, including parathion, parathion-methyl, fenitrothion, fenthion, phoxim, isocarbophos, chlorpyrifos, and triazophos, were provided by the Agro-Environmental Protection Institute, Ministry of Agriculture (Tianjin, China). All reagents were of analytical grade, used without any purification.
Apparatus
The size and surface morphology of UCNPs were characterized by transmission electron microscope (TEM, HITACHI, Japan). The F-4500 fluorescence spectrometer system (HITACHI, Japan) adapted with a 980 nm laser device (Changchun Laser Optoelectronics Technology, China) was used to determine the fluorescent spectrum. Immunoreagents were dispensed on nitrocellulose membrane by R5DDA dispense platform (Han'gan, China). Strips were prepared by a cutter (Han'gan, China). The UCNP-based LFIC (UCNP-LFIC) strips were scanned by a strip reader (Suzhou Helmen Precise Instruments, China) with 980 nm near-infrared laser excitation. An ML-902 magnetic stirring apparatus (Pujiang, Shanghai), Allegra 64R super centrifuge (Beckman, America), electric jacket and ultrasonic cleaner were also used in this study.
Preparation of UCNP-mAb Probe
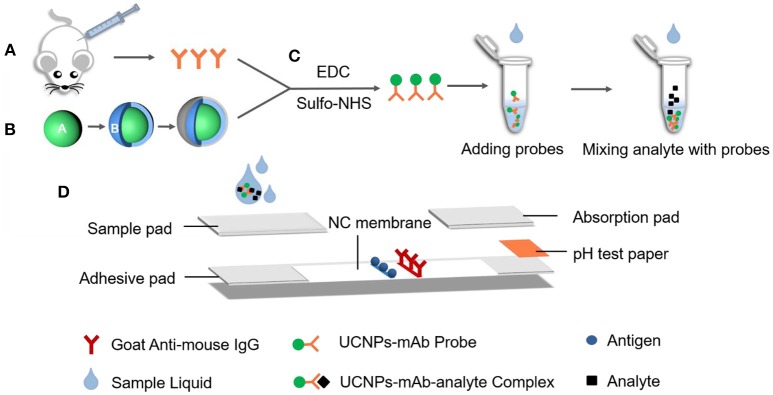
To obtain fluorescent probe, the mAb originated from BALB/c mice (Scheme 1A) was conjugated with the functional UCNPs (Scheme 1B) via EDC/sulfo-NHS mediated amidation reaction (Scheme 1C), similar to the method described in our previous work (Si et al., 2018). The modified protocol is as follows: 1 mg of carboxylic UCNPs was dissolved in 2 mL MES (0.1 mol/L, pH 5.5) solution, then activated by adding 1 mg of EDC and 1.5 mg of sulfo-NHS. After 20 min of incubation with vigorous stirring at room temperature (RT), the activated product was centrifuged and washed two times with PB (0.01 mol/L, pH 7.4), and dissolved in 1 mL of PB. Then, 1 ml of mAb at concentrations of 10, 20, 40, and 80 μg/mL were added and stirred softly in RT for 2.5 h. Afterward, 250 μL of 1% BSA (w/v) was added as a blocking buffer to avoid non-specific locus for 30 min. Finally, after being washed two times, the precipitates were resuspended in 1 mL of the preservation solution (0.03 mol/L, pH 7.2 PB buffer containing 1% BSA, 1% trehalose, and 0.1% Tween-20) for storage. The resulting UCNP-mAb probe was stored at 4°C for further use.
Scheme 1.
The fabrication process of lateral flow strips on UCNP-mAb probe. (A) MAb originated from BALB/c mice; (B) Carboxylic acid-functionalized UCNPs; (C) Bio-conjugation of functionalized UCNPs with mAb to obtain UCNPs-mAb; (D) Structure of later flow immunochromatographic strip.
Preparation of Colloidal Gold-mAb Probe
According to the procedures used in our previous work (Guo et al., 2009), CG nanoparticles were prepared with an average diameter of 20 nm and coupled with the same specific mAb.
Construction of Lateral Flow Strips
The fabrication of the lateral flow strips are as follows. Sample pad, nitrocellulose membrane, absorption pad, and pH test paper were stuck to a plastic adhesive backing pad, with 2 mm overlap between each two adjacent parts (Scheme 1D). Acting as a pump, the absorption pad was capable of liquid flow. Meanwhile, pH test paper also indicated the sample solution migrated properly through the strip. The goat anti-mouse IgG (0.3 mg/mL) and the competitive antigen of PA0304-OVA (3 mg/mL) were dispensed on the nitrocellulose membranes (0.75 μL/cm) and served as control and test lines with a 5 mm distance. Afterwards, the immobilized components were dried at 37°C for 30 min. The sample pad was pre-treated by blocking solutions, followed by drying at 37°C for 3 h. Different nitrocellulose membranes and blocking solutions were compared in this study. After overall assembly, the objective pad was cut into strips with a width of 4 mm and then stored in a sealed storage container for drying at RT for further use.
Detection Procedure of UCNP-LFIC Strip
The UCNP-mAb probe at a specific amount was mixed with 60 μL of PBS buffer containing organic solvent, shaking gently to form the homogeneous sample solution. Subsequently, the mixture was dropwise added onto the sample pad. After 15 min, the color of the pH test paper turned red, indicating successful movement of mixture liquid on the strips. The fluorescence intensity values of the T (FIT) and C (FIC) lines were recorded.
Quantitative Analysis of UCNP-LFIC Strip
By detecting a series concentration of standard analyte solution with a final concentration from 0 ng/mL (as negative for blank control) to 250 ng/mL (as positive), competitive inhibition curves of the three OP pesticides and their mixed solutions were established. The mixed standard solution was prepared by adding parathion, parathion-methyl, and fenitrothion together to the same final concentration. The ratio of FIT to FIC (FIT/ FIC) were calculated as B (positive sample) and B0 (negative control), respectively. A fitted standard curve was obtained for the inhibition rate (B0-B)/B0 × 100% against the series of concentration. The concentration causing 50% inhibition (IC50) was used to evaluate the assay sensitivity.
Optimization of UCNP-LFIC Strip
According to the maximum fluorescence, signal-noise ratio and IC50, variable factors (antigen and antibody dilution times, the concentration of organic solvent in reaction buffer and detection time) were compared based on the sensitivity performance.
Evaluation of the Assay Specificity
Eight types of OP pesticides were employed to determine the specificity of the assay. The pesticide standard solutions were diluted to a final concentration of 500 ng/mL as described above and tested individually.
Sample Pretreatment and Spiked Recovery Test
Fruit and vegetable foods including orange, cucumber, and tomato were purchased from a Walmart supermarket. West Lake water, Yuhangtang River water, and tap water were obtained in Hangzhou, China. All samples were confirmed by GC/MS to be OP pesticide-free. The samples were pretreated with a simplified QuEChERS extraction procedure. Briefly, 10 g of homogenized sample was extracted with 20 mL of acetonitrile for 10 min by vigorous shaking, 1 g of NaCl and 5 g of MgSO4 were poured and shaken by hand for 2 min. After being centrifuged at 3,500 g for 5 min, 50 mg PSA was added and supernatants were treated by nitrogen and diluted with a reaction buffer for analysis. The water sample was filtered after standing for 5 min to remove solid impurities such as gravel and sand, and a certain amount of methanol was added to the liquid, leading to a final concentration of 10% before testing.
For the recovery test, each sample was spiked with different concentrations of OP pesticides and rested for 2 h before sample preparation, followed by testing in triplicate with the UCNP-LFIC assay.
Results and Discussion
Principle of the Detection Platform
For multiple target detection, the LFIC assay usually focused on the following two types of technician paths: (1) several channels providing independent reaction areas for single-target (Zhao et al., 2016a); (2) several test lines targeting different specific UCNP-mAb probes by utilizing mono-color or multi-color fluorescent nanoparticles (Liu et al., 2017). In this study, we used a broad-specific mAb (PA-QA1-7B2) for simultaneous multi-residue detection of parathion, parathion-methyl, and fenitrothion. The broad-specific mAb was produced and their similar binding properties against three OP pesticides were proved by surface plasmon resonance-based immunosensor-based immunoassays (Jiao et al., 2018).
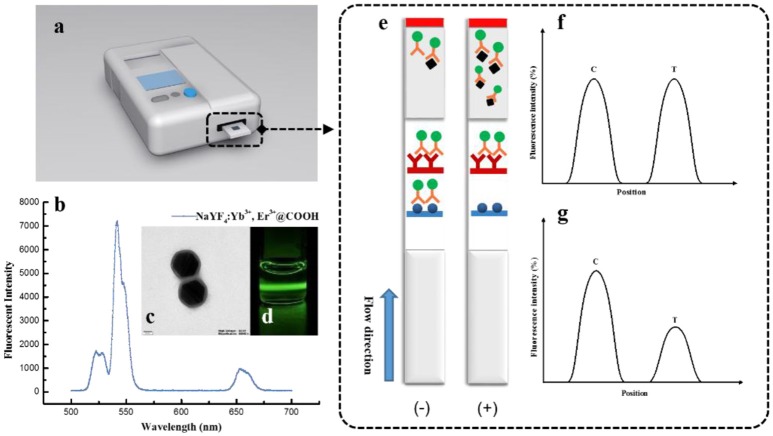
The principle of UCNP-LFIC detection is presented in Figure 1. Firstly, UCNP-mAb probes were pre-mixed with analytes to form a uniform solution. Then the sample solution flowed simultaneously through the strip by capillary force. After reaction, the fluorescent signal intensity of strips was read by the portable machine, which integrated with the NIR laser system and translated the information into an electronic data-processing computer. There was competition between the immobilized antigen and target analyte for binding to the UCNP-mAb probe. The target analytes were able to combine with the probe specifically to form the UCNP-mAb-analyte complex, and the amount of UCNP-mAb probes captured by test line decreased, indicating a positive result (+) (Figure 1G). If the target analytes were absent in the sample solution or lower than the detectable ability of the assay, all UCNP-mAb probes would react with the test line. The excess particles flowed through the membrane continuously, and then became trapped with control line. This phenomenon resulted in a negative result (–) (Figure 1F).
Figure 1.
The detection principle of UCNP-LFIC strips. (A) Developed reader for UCNP-LFIC strips; (B) Emission spectra of carboxylic acid-functionalized UCNPs; (C) TEM image of carboxylic acid-functionalized UCNPs; (D) Emission of green light under 980 nm NIR excitation; (E) Diagram of test image; (F) Negative test result; (G) Positive test result.
Development of the UCNP-LFIC Assay
The fluorescent intensity of nanoparticles is quite important in the assay. High fluorescent efficiency of single nanoparticles is beneficial for a more sensitive detection model (You et al., 2017). The dispersity and stability of UCNPs were measured via TEM and fluorescence spectrometer systems before conjugation. As a result, we observed that the size of the UCNPs was 140~150 nm, with a thickness of the SiO2 shell layer of ~10 nm (Figure 1C). With good dispersity, the UCNPs emitted green light under 980 nm NIR excitation for the doped of rare earth elements (Figures 1B,D). All characterizations indicated the UCNPs can be used for the development of fluorescent immunoassays.
Generally, crude UCNPs are a kind of inorganic material without active groups to combine with biomolecules. The surface modifications provided the ability to covalently conjugate with antibodies (Liang et al., 2017). In this work, the commercial UCNPs modified with carboxylic groups were coupled with mAb under the EDC/sulfo-NHS-mediated reaction. It should be noted that the quality of the UCNP-mAb probes is a key factor. The dispersion and size performance of the probes is directly relevant to the selection of nitrocellulose membranes. Differences in migration speed thus significantly affect the fluorescent intensity and the assay's sensitivity. Thus, the selection and optimization of an appropriate coupling system is crucial. The above conditions were optimized in this study (illustrated in part of Preparation of UCNPs-mAb probe). The freshly-prepared complex generally dispersed well in the preservation solution.
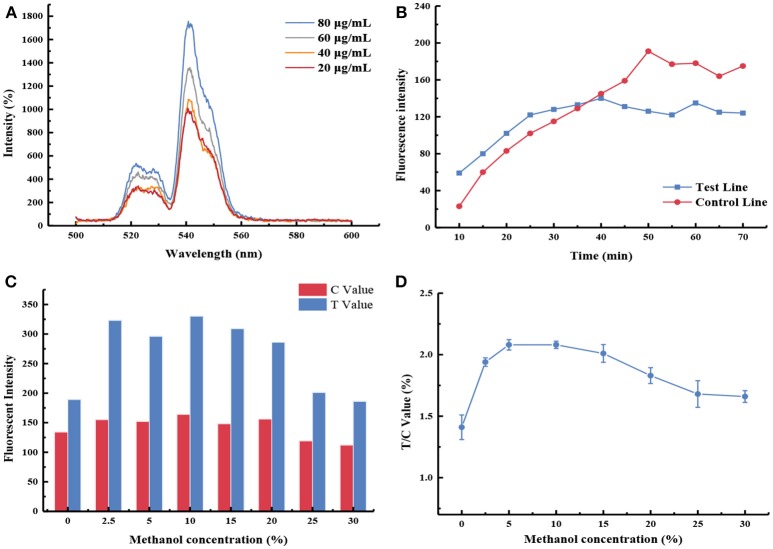
After systematic optimization (Figure 2), 80 μg/mL of mAb was conjugated with UCNPs, and PA0304-OVA (3 mg/mL) and goat anti-mouse IgG (0.3 mg/mL) were immobilized on the test and control zones, respectively. PBS (0.01 M, pH 7.4) with a final methanol content of 10% was selected as the reaction buffer. Meanwhile, it was proved that 40 min was sufficient for strip reading (more details are available in the Supplementary Material).
Figure 2.
Optimization of UCNP-LF assay. (A) The fluorescence intensity emitted by different antibody labeling amounts; (B) Immunological kinetics analysis of probes on the strip; (C) The fluorescence intensity values of the test line (T Value) and control line (C Value) were recorded by using buffers containing different methanol concentrations; (D) Influence of methanol concentration in the FIT/ FIC value.
Evaluation of the UCNP-LFIC Assay
In a previous study, the produced mAb targeting parathion was identified through an indirect competitive chemiluminescence enzyme-linked immunoassay (CLEIA). It showed broad specificity to three OP pesticides, including parathion, methyl-parathion and fenitrothion, with IC50 values of 5.57, 2.30, and 2.62 ng/mL, respectively (Zou et al., 2017). The CLEIA indicated the mAb may have better affinity to two other analog compounds in this detection format.
In this work, we also selected parathion as a priority to test. Serial dilutions ranging from 0.39 to 500 ng/mL in the extraction solution were prepared and tested in quintuplicate, respectively.
Afterward, the standard curves for each and the mixed solution of the three OP pesticides were established (Figure 3). The indirect competitive UCNP-LFIC assay displayed good linear fitting (R2 ≥ 0.9776) within the analyte concentration ranging from 0.98/1.95 to 250 ng/mL (Table 1). According to China's food safety national standard allowance, the minimum concentration of linear detection range was lower than the maximum residue limit (MRL) value. The IC50 values of parathion, parathion-methyl and fenitrothion were 3.44, 3.98, 12.49 ng/mL, respectively. By comparison, there is quite different in the sensitivity order between CLEIA (homogeneous reaction mode) and the UCNP-LFIC assay (paper-based flow reaction mode) using the same mAb and competitive antigen. It indicates that the selection of the reaction mode has a significant influence on assay sensitivity. This conclusion was also demonstrated in the SPR-based immunoassay (Jiao et al., 2018).
Figure 3.
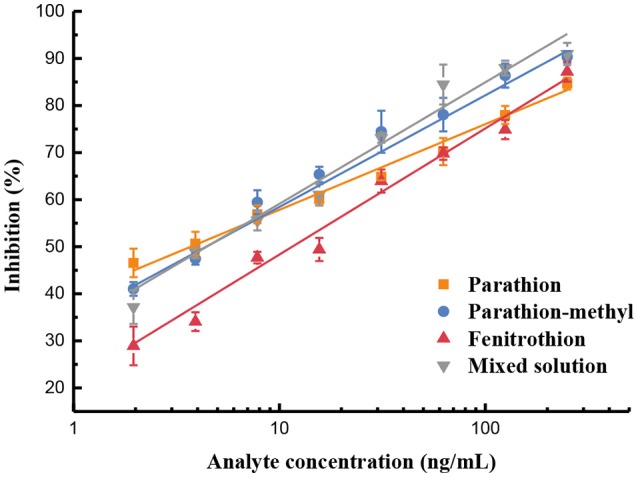
Sensitivity evaluation based on UCNP-LFIC of parathion, parathion-methyl, fenitrothion, and mixed solution.
Table 1.
Linear regression equations based on UCNP-LFIC of OP pesticides (n = 5).
| Pesticide | Standard Curve | R2 | IC50 (ng/mL) | Linear range (ng/mL) | MRLa (mg/kg) |
|---|---|---|---|---|---|
| Parathion | y = 7.575 ln(x) + 40.64 | 0.9907 | 3.44 | 0.98~250 | 0.01 |
| Parathion-methyl | y = 10.381 ln(x) + 35.67 | 0.9853 | 3.98 | 1.95~250 | 0.01 |
| Fenitrothion | y = 11.702 ln(x) + 20.45 | 0.9851 | 12.49 | 0.98~250 | 0.1 |
| Mixed solution | y = 11.517 ln(x) + 31.74 | 0.9776 | 4.88 | 1.95~250 | – |
MRL value refers to the maximum residue limit allowed in the national food safety standard method GB 2763-2016 of China.
Comparison Between the UCNP-LFIC and CG-LFIC Assays
Label selection is a vital factor in improving the sensitivity of LFIC. Two LFIC (CG-LFIC and UCNP-LFIC) assays were established based on the competitive format after systemic optimization. The CG-LFIC assay was established and optimized in accordance with the procedures reported in our lab (Guo et al., 2009). Selection of crucial working conditions was similar to the UCNP-LFIC assay, including the reaction buffer, the concentration of coating materials and methanol, and the amount of mAb labeled with CG nanoparticles.
By adopting a series concentration of mixed standard solution, the sensitivity of strips was determined by the naked eye. The color of the test line in CG-LFIC assay remained even at an extreme high concentration of 1,000 ng/mL. It was too hard to meet the detection need when CG was applied as a label. Meanwhile, the same line nearly disappeared at a concentration of 15.6 ng/mL in the UCNP-LFIC assay (Figure 4). The results indicate that the UCNPs are more suitable than traditional CG as a label in this study. The variation is probably due to the properties of distinct labeling materials and different coupling methods. Furthermore, UCNPs have unique optical properties in size, color, and fluorescence intensity under different processes of synthesis and modification. The versatile performance thus enhances the potential for trace detection.
Figure 4.
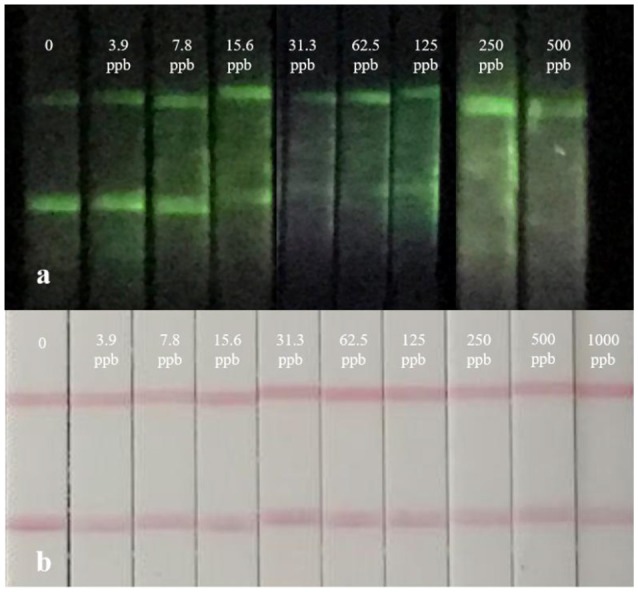
Real detection figure of UCNP-LFIC assay and GC-LFIC assay of mixed solution. (A) UCNP-LIFC assay. (B) GC-LFIC assay.
Selectivity of UCNP-LFIC Assay
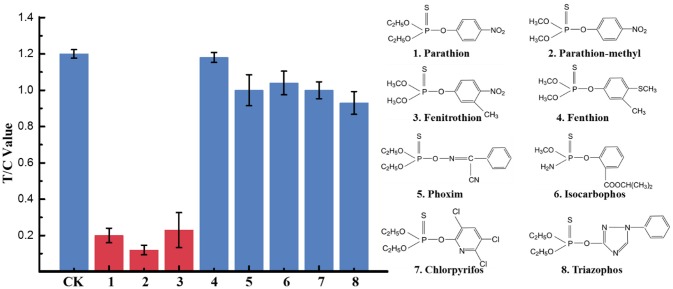
To determine the specificity of the immunoassay, eight OP pesticides including parathion, parathion-methyl, fenitrothion, fenthion, phoxim, isocarbophos, chlopyrifos, and triazophos were investigated. Each analyte diluted to a standard solution with a final concentration of 500 ng/mL was tested by the developed UCNP-LFIC assay. The FIT/FIC values of blank control and annalyte standard solutions were determined based on the corresponding test and control lines. In contrast, the inhibition rates by parathion, parathion-methyl, and fenitrothion were significantly higher than those from the other five compounds (Figure 5), suggesting the assay selectivity was consistent with the mAb specificity characterized in our previous work (Zou et al., 2017; Jiao et al., 2018).
Figure 5.
Selectivity evaluation of UCNP-LFIC. Eight OPs including parathion, parathion-methyl, fenitrothion, fenthion, phoxim, isocarbophos, chlopyrifos, and triazophos with a final concentration of 500 ng/mL.
Matrix Interference in Real Samples
Immunoassay performance can be interfered with by the complicated matrix compositions in the samples, such as protein, lipid, pigment, and fiber. These components might cause antibody denaturation, non-specific absorption, and then affect the sensitivity.
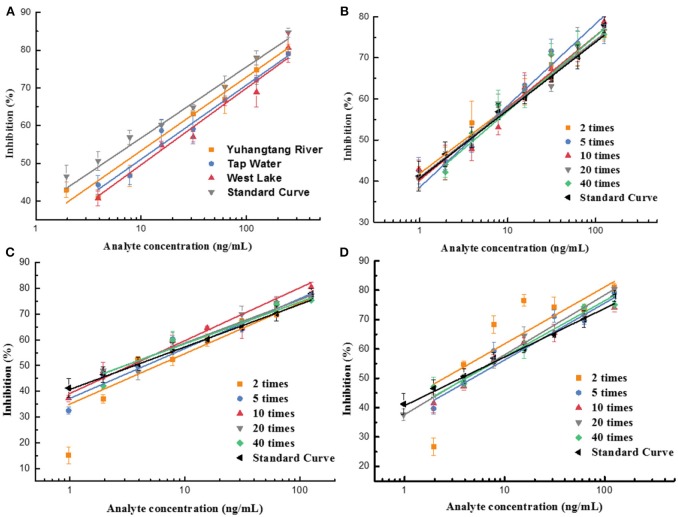
In this study, the impact of the matrix in different vegetables, fruits, and water on the UCNP-LFIC assay's sensitivity was evaluated. To eliminate the matrix interference and guarantee the credibility of the method, sample dilution was conducted prior to detection. According to the pretreatment method, food sample extracts were diluted to 2, 5, 10, 20, and 40 times with PBS buffer containing 10% methanol. Then, a series of sample buffers with different matrix concentrations was used to prepare standard solutions. As shown in Table 2 and Figure 6, the matrix effect values of cucumber and tomato matrices were within 80–120% under 2 times' dilution. The results indicated the matrix interference could be ignored in the above dilution times (Matuszewski et al., 2003), whereas the orange matrix should be diluted 5 times, and there was no extra dilution for water.
Table 2.
Matrix effect of food sample.
| Sample | Dilution | Standard curve | R2 | IC50 (ng/mL) | Matrix effect |
|---|---|---|---|---|---|
| Cucumber | 2 | y = 7.2632 ln(x) + 41.578 | 0.9840 | 3.19 | 95.88% |
| 5 | y = 8.003 ln(x) + 40.649 | 0.9765 | 3.22 | 105.65% | |
| 10 | y = 7.5116 ln(x) + 40.655 | 0.9710 | 3.47 | 99.16% | |
| 20 | y = 7.5349 ln(x) + 40.367 | 0.9790 | 3.59 | 99.47% | |
| 40 | y = 8.0456 ln(x) + 39.71 | 0.9604 | 3.59 | 106.21% | |
| Tomato | 2 | y = 8.5744 ln(x) + 35.617 | 0.9524 | 5.35 | 113.19% |
| 5 | y = 8.4773 ln(x) + 37.712 | 0.9528 | 4.26 | 111.91% | |
| 10 | y = 8.2276 ln(x) + 40.291 | 0.9698 | 3.25 | 108.61% | |
| 20 | y = 7.992 ln(x) + 40.146 | 0.9679 | 3.24 | 105.50% | |
| 40 | y = 7.9008 ln(x) + 39.713 | 0.9564 | 3.68 | 104.30% | |
| Orange | 2 | y = 10.64 ln(x) + 35.738 | 0.7138 | 3.82 | 140.46% |
| 5 | y = 8.855 ln(x) + 36.932 | 0.9483 | 4.37 | 116.90% | |
| 10 | y = 7.9104 ln(x) + 37.85 | 0.9731 | 4.65 | 104.43% | |
| 20 | y = 8.5783 ln(x) + 38.273 | 0.9880 | 3.92 | 113.24% | |
| 40 | y = 7.5503 ln(x) + 40.195 | 0.9705 | 3.66 | 99.67% |
Figure 6.
Fitted standard curve results of mixed OPs in different dilution times of real sample matrix. (A) Water. (B) Cucumber. (C) Tomato. (D) Orange.
Detection of Spiked Sample
In order to evaluate the accuracy of the UCNP-LFIC assay, we performed spiked recovery tests within the detection linear range of the assay. Various samples spiked with different concentrations of parathion and the mixed solutions of three OP pesticides (2.5, 5, and 10 ng/mL) were analyzed in triplicates. Based on the standard curve, the pesticide amount and recovery rate were calculated. At the same time, the precision and repeatability among batches were also evaluated by relative standard deviation (RSD). As listed in Table 3, the recovery rates ranged from 67 to 120% with RSD ≤ 19.54%, which generally satisfied the requirement of trace detection.
Table 3.
Recovery rate determination of parathion and mixed solution of three OP pesticides by UCNP-LFIC strips.
| Sample | Spiked level (ng/g or ng/mL) | Parathion | Mixed solution | ||||
|---|---|---|---|---|---|---|---|
|
Detected amount (ng/g or ng/mL) |
Recovery rate (%) |
RSD (%) (n = 6) |
Detected amount (ng/g or ng/mL) |
Recovery rate (%) |
RSD (%) (n = 6) |
||
| Cucumber | 5 | 5.78 | 116 | 2.20 | 3.60 | 72 | 3.53 |
| 10 | 9.50 | 95 | 12.96 | 6.70 | 67 | 12.55 | |
| 20 | 19.48 | 97 | 7.49 | 21.50 | 108 | 8.39 | |
| Tomato | 5 | 5.14 | 103 | 17.42 | 4.40 | 88 | 12.90 |
| 10 | 9.04 | 90 | 15.77 | 7.28 | 73 | 4.71 | |
| 20 | 19.50 | 98 | 7.29 | 18.46 | 92 | 18.31 | |
| Orange | 12.5 | 10.35 | 83 | 1.95 | 9.60 | 77 | 14.28 |
| 25 | 28.90 | 116 | 27.90 | 26.25 | 105 | 19.54 | |
| 50 | 51.75 | 104 | 10.11 | 55.25 | 111 | 7.97 | |
| Yuhangtang River | 2.5 | 2.05 | 82 | 14.20 | 1.97 | 79 | 9.36 |
| 5 | 5.13 | 103 | 2.14 | 6.01 | 120 | 10.53 | |
| 10 | 9.48 | 95 | 10.32 | 10.22 | 102 | 11.98 | |
| Tap Water | 2.5 | 1.87 | 75 | 12.42 | 2.81 | 112 | 4.31 |
| 5 | 4.98 | 100 | 10.35 | 5.23 | 105 | 10.23 | |
| 10 | 11.29 | 113 | 11.94 | 9.21 | 92 | 15.23 | |
| West Lake | 2.5 | 2.34 | 94 | 8.47 | 2.9 | 116 | 6.51 |
| 5 | 5.02 | 100 | 10.62 | 4.56 | 91 | 9.12 | |
| 10 | 11.52 | 115 | 5.24 | 9.92 | 99 | 14.56 | |
However, the UCNP-LFIC assay was not able to distinguish parathion, parathion-methyl, and fenitrothion, instead only indicating the mixture levels. In any case, it can work as a screening tool for large-scale multi-residue detection of the three OP pesticides and thus contribute to a reduction of the sample amount for further instrumental analysis.
Conclusion
In this study, we successfully established a UCNP-based competitive LFIC strip for rapid multi-residue detection of three OP pesticides with good sensitivity in food samples. The UCNP-LFIC immunoassay showed a good linear fit (R2 ≥ 0.9776) in the range of 0.98/1.95–250 ng/mL, and high sensitivity for parathion, parathion-methyl, and fenitrothion (IC50 ranging from 3.44 to 12.49 ng/mL). Meanwhile, it improved the determination of multiple OP pesticide residues from qualitative to semi-quantitative detection within 40 min. This is the first report on using a UCNP-LFIC assay for OP pesticide residue detection. The assay revealed the broad prospects of UCNPs for the detection of pesticides and other small contaminant molecules.
However, the technical process for UCNP-LFIC strips should be further improved. Although the fresh synthesized UCNP-mAb probes have homogeneous dispersibility, the stock solution of UCNP-mAb probes was aggregated to form flocculent precipitates after 2 days' storage. Careful optimization of the probes' preparation method and the preservation buffer may solve this problem to some extent. In view of high requirements in the analytical field, it is still necessary to improve UCNP-LFIC sensitivity for trace detection. The main approaches include the synthesis of new UCNPs with high luminous intensity, the modification of UCNPs' surface with more active sites, and the development of a UCNP detector apparatus with enhanced optical performance.
Ethics Statement
This study on mice was carried out in accordance with the recommendations of the animal welfare committee of Zhejiang University in China.
Author Contributions
YG and YihL designed the study and guided the experiments. RZ carried out experiments, analyzed data, and wrote the manuscript draft. YC, TZ, and FS assisted with experiments. YinL and YZ assisted with data analysis. MZ, XY, XQ, and GZ helped to design the study and supply the facility. YG and YihL revised the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was financially supported by the National Key R&D Program of China (2017YFF0210200 & 2016YFD0201302) and the National Natural Science Foundation of China (31401768).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2019.00018/full#supplementary-material
References
- Bouchard M. F., Chevrier J., Harley K. G., Kogut K., Vedar M., Calderon M., et al. (2011). Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children. Environ. Health Perspect. 119, 1189–1195. 10.1289/ehp.1003185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H. G., Richter R. J., Keifer M., Broomfield C. A., Sowalla J., Furlong C. E. (1996). The effect of the human serum paraoxonase polymorphism is reversed with diazoxon, soman and sarin. Nat. Genet. 14, 334–336. 10.1038/ng1196-334 [DOI] [PubMed] [Google Scholar]
- Dou Q. Q., Idris N. M., Zhang Y. (2013). Sandwich-structured upconversion nanoparticles with tunable color for multiplexed cell labeling. Biomaterials 34, 1722–1731. 10.1016/j.biomaterials.2012.11.011 [DOI] [PubMed] [Google Scholar]
- Feng W., Han C. M., Li F. Y. (2013). Upconversion-nanophosphor-based functional nanocomposites. Adv. Mater. 25, 5287–5303. 10.1002/adma.201301946 [DOI] [PubMed] [Google Scholar]
- Guo Y. R., Liu S. Y., Gui W. J., Zhu G. N. (2009). Gold immunochromatographic assay for simultaneous detection of carbofuran and triazophos in water samples. Anal. Biochem. 389, 32–39. 10.1016/j.ab.2009.03.020 [DOI] [PubMed] [Google Scholar]
- Handford C. E., Elliott C. T., Campbell K. (2015). A review of the global pesticide legislation and the scale of challenge in reaching the global harmonization of food safety standards. Integr. Environ. Assess. Manag. 11, 525–536. 10.1002/ieam.1635 [DOI] [PubMed] [Google Scholar]
- Hua X. D., You H. J., Yang J. C., Shi H. Y., Wang M. H. (2018). Immunochromatographic assay for detection of imidaclothiz based on upconversion fluorescence labeling. Chinese J. Anal. Chem. 46, 413–421. 10.11895/j.issn.0253-3820.171149 [DOI] [Google Scholar]
- Huang X., Aguilar Z. P., Xu H., Lai W., Xiong Y. (2016). Membrane-based lateral flow immunochromatographic strip with nanoparticles as reporters for detection: a review. Biosens. Bioelectron. 75, 166–180. 10.1016/j.bios.2015.08.032 [DOI] [PubMed] [Google Scholar]
- Jiao S. S., Liu P. Y., Liu Y., Zou R. B., Zhao Y., Liu Y. H., et al. (2018). Binding properties of broad-specific monoclonal antibodies against three organophosphorus pesticides by a direct surface plasmon resonance immunosensor. Anal. Bioanal. Chem. 410, 7263–7273. 10.1007/s00216-018-1337-7 [DOI] [PubMed] [Google Scholar]
- Lei L. J., Zhu J., Xia G. Q., Feng H., Zhang H. M., Han Y. W. (2017). A rapid and user-friendly assay to detect the Neutrophil gelatinase-associated lipocalin (NGAL) using up-converting nanoparticles. Talanta 162, 339–344. 10.1016/j.talanta.2016.10.009 [DOI] [PubMed] [Google Scholar]
- Liang Y., Liu X. J., Liu Y., Yu X. Y., Fan M. T. (2008). Synthesis of three haptens for the class-specific immunoassay of O,O-dimethyl organophosphorus pesticides and effect of hapten heterology on immunoassay sensitivity. Anal. Chim. Acta 615, 174–183. 10.1016/j.aca.2008.03.OSO [DOI] [PubMed] [Google Scholar]
- Liang Y., Wang W., Shen Y., Liu Y., Liu X. J. (2012). Effects of home preparation on organophosphorus pesticide residues in raw cucumber. Food Chem. 133, 636–640. 10.1016/j.foodchem.2012.01.016 [DOI] [Google Scholar]
- Liang Z. Q., Wang X. C., Zhu W., Zhang P. P., Yang Y. X., Sun C. Y., et al. (2017). Upconversion nanocrystals mediated lateral-flow nanoplatform for in vitro detection. ACS Appl. Mater. Interfaces 9, 3497–3504. 10.1021/acsami.6b14906 [DOI] [PubMed] [Google Scholar]
- Liu B. B., Gong H., Wang Y. L., Zhang X. S., Li P., Qiu Y. L. (2017). A gold immunochromatographic assay for simultaneous detection of parathion and triazophos in agricultural products. Anal. Methods 10, 422–428. 10.1039/c7ay02481a [DOI] [Google Scholar]
- Liu D. B., Chen W. W., Wei J. H., Li X. B., Wang Z., Jiang X. Y. (2012). A highly sensitive, dual-readout assay based on gold nanoparticles for organophosphorus and carbamate pesticides. Anal. Chem. 84, 4185–4191. 10.1021/ac300545p [DOI] [PubMed] [Google Scholar]
- Matuszewski B. K., Constanzer M. L., Chavez-Eng C. M. (2003). Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal. Chem. 75, 3019–3030. 10.1021/ac020361s [DOI] [PubMed] [Google Scholar]
- Payne-sturges D., Cohen J., Castorina R., Axelrad D. A., Woodruff T. J. (2009). Evaluating cumulative organophosphorus pesticide body burden of children: a national case study. Environ. Sci. Technol. 43, 7924–7930. 10.1021/es900713s [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X. P., Huang Y. Y., Lin Z. S., Xu L., Yu H. (2016). Dual-quantum-dots-labeled lateral flow strip rapidly quantifies procalcitonin and C-reactive protein. Nanoscale Res. Lett. 11:167. 10.1186/s11671-016-1383-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. M., Aaron C. K. (2008). Managing acute organophosphorus pesticide poisoning. Br. Med. J. 334, 629–634. 10.1136/bmj.39134.566979.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si F. F., Zou R. B, Jiao S. S., Qiao X. S., Guo Y. R., Zhu G. N. (2018). Inner filter effect-based homogeneous immunoassay for rapid detection of imidacloprid residue in environmental and food samples. Ecotoxicol. Environ. Saf. 148, 862–868. 10.1016/j.ecoenv.2017.11.062 [DOI] [Google Scholar]
- Tao X. Q., Chen M., Jiang H. Y., Shen J. Z., Wang Z. H., Wang X., et al. (2013). Chemiluminescence competitive indirect enzyme immunoassay for 20 fluoroquinolone residues in fish and shrimp based on a single-chain variable fragment. Anal. Bioanal. Chem. 405, 7477–7484. 10.1007/s00216-013-7174-9 [DOI] [PubMed] [Google Scholar]
- Wang P. L., Wang R. G., Zhang W., Su X. O., Luo H. F. (2016). Novel fabrication of immunochromatographic assay based on up conversion phosphors for sensitive detection of clenbuterol. Biosens. Bioelectron. 77, 866–870. 10.1016/j.bios.2015.10.053 [DOI] [PubMed] [Google Scholar]
- Wang S., Zhang C., Mang Y. (2009). lateral flow colloidal gold-based immunoassay for pesticide. Methods Mol. Biol. 504, 237–252. 10.1007/978-1-60327-569-9_15 [DOI] [PubMed] [Google Scholar]
- Watanabe E., Kobara Y., Baba K., Eun H. (2014). Aqueous acetonitrile extraction for pesticide residue analysis in agricultural products with HPLC–DAD. Food Chem. 154, 7–12. 10.1016/j.foodchem.2013.12.075 [DOI] [PubMed] [Google Scholar]
- Xie Q. Y., Wu Y. H., Xiong Q. R., Xu H. Y., Xiong Y. H., Liu K., et al. (2014). Advantages of fluorescent microspheres compared with colloidal gold as a label in immunochromatographic lateral flow assays. Biosens. Bioelectron. 54, 262–265. 10.1016/j.bios.2013.11.002 [DOI] [PubMed] [Google Scholar]
- Xu Z. L., Wang Q., Lei H. T., Eremin S. A., Shen Y. D., Wang H., et al. (2011). A simple, rapid and high-throughput fluorescence polarization immunoassay for simultaneous detection of organophosphorus pesticides in vegetable and environmental water samples. Anal. Chim. Acta 708, 123–129. 10.1016/j.aca.2011.09.040 [DOI] [PubMed] [Google Scholar]
- Yan Z. Q., Zhou L., Zhao Y. K., Wang J., Huang L. H., Hu K. X., et al. (2006). Rapid quantitative detection of Yersinia pestis, by lateral-flow immunoassay and up-converting phosphor technology-based biosensor. Sensors Actuators B Chem. 119, 656–663. 10.1016/j.snb.2006.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You M. L., Lin M., Gong Y., Wang S. R., Li A., Ji L. Y., et al. (2017). Household fluorescent lateral flow strip platform for sensitive and quantitative prognosis of heart failure using dual-color upconversion nanoparticles. ACS Nano 11, 6261–6270. 10.1021/acsnano.7b02466 [DOI] [PubMed] [Google Scholar]
- Zhao P., Wu Y. Y., Zhu Y. H., Yang X. L., Jiang X., Xiao J. F., et al. (2014). Upconversion fluorescent strip sensor for rapid determination of Vibrio anguillarum. Nanoscale 6, 3804–3809. 10.1039/c3nr06549a [DOI] [PubMed] [Google Scholar]
- Zhao X., Kong W., Wei J., Yang M. (2014). Gas chromatography with flame photometric detection of 31 organophosphorus pesticide residues in Alpinia oxyphylla dried fruits. Food Chem. 162, 270–276. 10.1016/j.foodchem.2014.04.060 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Liu X., Wang X. C., Sun C. Y., Wang X. R., Zhang P. P., et al. (2016b). Development and evaluation of an up-converting phosphor technology-based lateral flow assay for rapid and quantitative detection of aflatoxin B1 in crops. Talanta 161, 297–303. 10.1016/j.talanta.2016.08.058 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Wang H. R., Zhang P. P., Sun C. Y., Wang X. C., Wang X. R., et al. (2016a). Rapid multiplex detection of 10 foodborne pathogens with an up-converting phosphor technology-based 10-channel lateral flow assay. Sci. Report 6:21342. 10.1038/srep21342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J. M., Zou N. L., Zhu D. N., Wang J., Jin Q. H., Zhao J. L., et al. (2011). Simultaneous detection of high-sensitivity cardiac troponin I and myoglobin by modified sandwich lateral flow immunoassay: proof of principle. Clin. Chem. 57, 1732–1738. 10.1373/clinchem.2011.171694 [DOI] [PubMed] [Google Scholar]
- Zou R. B., Liu Y., Wang S. J., Zhang Y., Guo Y. R., Zhu G. N. (2017). Development and evaluation of chemiluminescence enzyme-linked immunoassay for residue detection of three organophosphorus pesticides. Chinese J. Pesticide Sci. 19, 37–45. 10.16801/j.issn.1008-7303.2017.0006 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.