Abstract
The growing population requires sustainable, environmentally-friendly crops. The plant growth-enhancing properties of algal extracts have suggested their use as biofertilisers. The mechanism(s) by which algal extracts affect plant growth are unknown. We examined the effects of extracts from the common green seaweed Ulva intestinalis on germination and root development in the model land plant Arabidopsis thaliana. Ulva extract concentrations above 0.1% inhibited Arabidopsis germination and root growth. Ulva extract <0.1% stimulated root growth. All concentrations of Ulva extract inhibited lateral root formation. An abscisic-acid-insensitive mutant, abi1, showed altered sensitivity to germination- and root growth-inhibition. Ethylene- and cytokinin-insensitive mutants were partly insensitive to germination-inhibition. This suggests that different mechanisms mediate each effect of Ulva extract on early Arabidopsis development and that multiple hormones contribute to germination-inhibition. Elemental analysis showed that Ulva contains high levels of Aluminium ions (Al3+). Ethylene and cytokinin have been suggested to function in Al3+-mediated root growth inhibition: our data suggest that if Ulva Al3+ levels inhibit root growth, this is via a novel mechanism. We suggest algal extracts should be used cautiously as fertilisers, as the inhibitory effects on early development may outweigh any benefits if the concentration of extract is too high.
Introduction
Plant growth, development and productivity is affected by various abiotic (physical) and biotic (biological) factors. Responses to these factors determine cropping pattern and plant distribution1. Global demand for crops is predicted to increase ~100% from 2005 to 2050, while ~795 million people worldwide were undernourished in 2014–162,3.
Current global food challenges and pressure on the food production industry are due to the exponentially growing human population and increasing soil- and water issues compounding the pressure induced by anthropogenic climate change. The frequency of abiotic environmental stresses (flooding, drought, water limitation, salinity and extreme temperatures) is increasing4 and causing crop losses worldwide5–7. More intense, frequent droughts in Africa, southern South America and southern Europe and increased flooding in temperate regions will drive future crop yield decline8–10, while soil salinity associated, for example, with drought and irrigation threatens both agriculture and natural ecosystems11–13. Intensive farming leads to unfavourable conditions for crop growth, development and survival14.
Humans have used seaweeds (macroalgae) and seaweed-based products for centuries, for food, fuel, aquaculture, cosmetics, colouring dyes and therapeutic/botanical applications14–16. The earliest written reference to using seaweed as a fertiliser is from Roman times17. Applying seaweeds/seaweed extracts in modern agriculture leads to increased seed germination rates, improved plant development (flowering, leaf quality and root system architecture), elevated defence against pathogens and pests18 and protection against nutrient deficiency and environmental stresses including salinity19, cold or drought20–23. Seaweed fertilisers have been used in agricultural programs to improve soil- and disease-management, nutritional strategies, water efficiency and drought tolerance23.
Several manufacturing practices are used to liquefy seaweed biomass21,23,24. Seaweed extracts are marketed as liquid biofertilisers or biostimulants containing a variety of plant growth-promoting components – those identified include plant growth regulators (phytohormones), minerals and trace elements, quaternary ammonium molecules (e.g. betaines and proline), polyuronides (e.g. alginates/fucoidans) and lipid-based molecules e.g. sterols23. Seaweed products are also available in soluble powder form. Depending on whether algal extract is applied as liquid fertiliser or seaweed manure to plant roots, or as a leaf spray, different plant responses to seaweeds occur14,21.
The mechanism by which seaweed fertilisers affect plant growth, development and yield is currently unknown. Crop plants treated with seaweed extracts showed similar physiological responses to those treated with plant growth-regulatory substances20. Phytohormones detected in seaweed extracts are auxins, cytokinins, gibberellins, abscisic acid and brassinosteroids25–27 but chemical components other than phytohormones, which elicit physiological responses reminiscent of plant hormones, have also been detected28. One hypothesis is that the effects of seaweed fertilisers are due either directly or indirectly to phytohormones: seaweed extracts may themselves contain beneficial phytohormones, or may contain substances that trigger land plant signaling pathways that usually respond to these signals. Which, if either, of these scenarios occurs is not clear.
Although seaweeds could potentially benefit plant growth by providing macronutrients, including nitrogen (N), phosphorus (P), ammonium (NH4+) and potassium (K), studies have consistently shown that seaweed extracts’ beneficial effects are not due to macronutrients, particularly at the concentrations used in the field20,29. Very dilute seaweed extracts (1:1000 or below) still have biological activity but the compound(s) involved are unknown: the beneficial effects may involve several plant growth-promoters working synergistically25,30–32.
Understanding at a mechanistic level how seaweed fertilisers affect land plant growth and development is important. Early plant growth and development involves germination of the seed, elongation of a primary root and subsequent branching of lateral roots (LRs) from the primary root as the seedling matures to secure anchorage and extract micro- and macronutrients from the soil33,34. Previous studies have applied a diverse range of extracts from brown, green and red seaweeds to a heterogeneous range of crop plants35,36, demonstrating conflicting effects of different concentrations of algal extract on seed germination, e.g. in tomato35,36. In terms of post-germinative growth, lower concentrations of an algal extract generally have beneficial effects on root- and shoot growth while higher concentrations have inhibitory effects36–40. Thus, algal extract concentration is critical to its effectiveness. However, because of the range of plants, seaweeds and extraction methods used, “positive” concentrations of algal extract ranged from 0.002–0.2% while inhibitory concentrations ranged from 0.1–1%.
In this paper, we establish a “standardised” laboratory-based system to determine the molecular mechanisms by which seaweeds can affect land plant productivity, using model organisms. The extensively-studied model plant Arabidopsis41, from Brassicaceae (cabbage) family, was the first plant with a sequenced genome42 and extensive mutant collections are available, including mutants in hormone signaling and perception43–47. Phytohormone biosynthetic/signalling pathways have been determined, yielding a broad understanding of plant responses to stimuli48–50. Employing Arabidopsis as a model organism has enabled translation of the understanding of plant growth and development to crops and agriculture51–53.
The green seaweed Ulva (sea lettuce; green nori) is an emerging experimentally-tractable model organism to study macroalgal development, growth, morphogenesis. Ulva is a cosmopolitan macroalgal genus, the main multicellular branch of the Chlorophyte algae, and the most abundant Ulvophyceae representative54,55. Ulvophyceae are multicellular algae with simple morphology compared to land plants. Distinctive features that make Ulva attractive as model systems are the small genome [100–300 Mb56,57] (the established model system Ulva mutabilis is currently being sequenced), symbiotic growth with bacterial epiphytes, naturally-occurring developmental mutants (in Ulva mutabilis), simple organization of the thallus (body) consisting of three differentiated cell types (blade, stem and rhizoid), laboratory cultivation58,59 and the ability to generate stable transgenic lines60–62.
The species of Ulva chosen for this study was Ulva intestinalis, an intertidal alga found worldwide, which can be lab-grown similarly to Ulva mutabilis58,63. We compared directly the growth- or inhibition parameters of different concentrations of Ulva intestinalis extract versus a control, applied to both wild-type and mutant Arabidopsis genotypes. By using two experimentally tractable organisms we have begun to understand the plant signalling pathways that can be triggered by algal extract. As Ulva genetic manipulation becomes better-established61 this raises the possibility of future engineering of improved macroalgal fertiliser properties.
Results
Concentrations of Ulva extract of 0.5% and above inhibit wild-type Arabidopsis seed germination
To investigate the effect of Ulva intestinalis extract on Arabidopsis germination, concentrations of Ulva extract ranging from 0–1.0% was tested (Fig. 1). All Ulva extract concentrations from 0.5% upwards delayed wild-type germination. The final germination percentage was reduced in 0.8% and 1.0% Ulva extract: only about half the seeds germinated in 1.0% Ulva extract after a week (Fig. 1a). Concentrations of 0.3% Ulva extract and below had no effect on seed germination and no stimulatory effect of Ulva extract on germination was observed at any concentration tested (Fig. 1a).
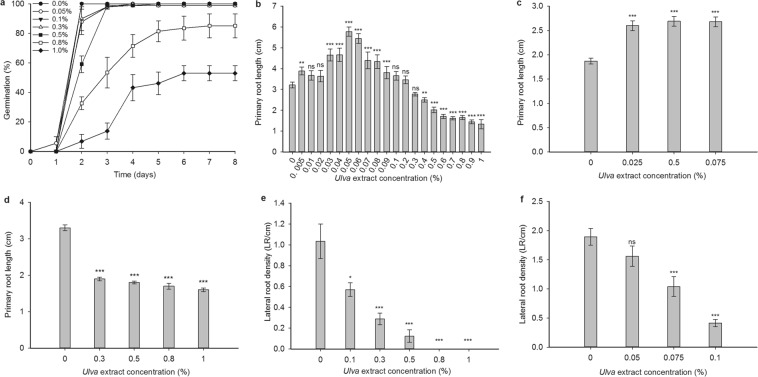
Figure 1.
Ulva extract inhibits germination and root growth at high concentrations, promotes root growth at lower concentrations, and inhibits lateral root formation, even at concentrations that stimulate primary root growth. (a) Effect of 0–1% Ulva extract on wild-type Arabidopsis seed germination. Significant differences between treated samples and control (0%) are seen on day 3 (0.8% and 1% Ulva extract (each p = 0.038)), day 4: (0.8% (p = 0.035) and 1% (p = 0.015)), and days 5, 6, and 7 (1% (p = 0.016 on days 5 and 6; p = 0.01 on day 7)). (b) Effect of 0–1% Ulva extract on wild-type primary root growth in 14-day old seedlings. Significant differences between treatment and control (Mann-Whitney U-test) were seen with 0.005% (p = 0.006), 0.03% (p = 0.0001), 0.04% (p = 0.0005), 0.05% (p = 0.0000), 0.06% (p = 0.0000), 0.07% (p = 0.0345), 0.08% (p = 0.0009), 0.09% (p = 0.0000), 0.3% (p = 0.0053), 0.4% (p = 0.045) and 0.5%-1% (p = 0.0000). n = 10–40 seedlings per treatment. (c) Primary root growth of wild-type seedlings grown for 10 days on non-nutrient-agar is significantly stimulated by low concentrations (0.025, 0.05 and 0.075%) of Ulva extract compared to the control (p = 0.0001, p = 0.0000, p = 0.0000 respectively: t-test). n = 30–35 seedlings per treatment. (d) Effect of Ulva extract ≥0.3% on wild-type root growth after transferring 3-day old seedlings from control medium, followed by growth for 7 days. Significant inhibition compared to 0% is seen for each concentration (all p = 0.0000: t-test). n = 30–35 seedlings per treatment. (e) Effect of 0.1–1% Ulva extract on lateral root density of wild-type seedlings. Significant inhibition compared to the control (Mann-Whitney U-test) is seen at 0.3% (p = 0.0001), 0.5%, 0.8% and 1.0% (all p = 0.0000). n = 20 seedlings per treatment. (f) Effect of 0.05–0.1% Ulva extract on lateral root density of wild-type seedlings. Significant inhibition compared to the 0% control (Mann-Whitney U-test) is seen at 0.75% (p = 0.0008) and 0.1% (p = 0.0000). n = 17–20 seedlings per treatment. All panels: asterisks - significant differences compared to 0% control: *p < 0.05, **p < 0.01, ***p < 0.001. Bars - standard error of the mean.
Ulva extract stimulates Arabidopsis primary root growth at low concentrations, and inhibits root growth at higher concentrations
Having demonstrated that seed germination is inhibited by Ulva extract, we sought to discover whether the next stage of development, primary root elongation, was also affected by Ulva extract. Ulva extract significantly stimulated root growth at concentrations from 0.03–0.08% (~80% stimulation at 0.06%), while concentrations of 0.3% and above had an inhibitory effect on root growth (~68% inhibition at 2%) (Fig. 1b). The stimulatory effect of Ulva extract was similarly present when seedlings were grown on non-nutrient-containing agar (Fig. 1c).
To ascertain whether the inhibitory effect of Ulva extract concentrations ≥0.3% on root growth was simply a consequence of delayed germination (Fig. 1a), we conducted an experiment where seedlings were germinated on normal growth medium for three days before transferring to medium containing Ulva extract. Root growth was once again inhibited by Ulva extract, showing that higher concentrations of Ulva extract have an inhibitory effect on root growth, independent from any effect on germination (Fig. 1d).
Ulva extract inhibits Arabidopsis lateral root formation
Having ascertained that Ulva extract affects primary root growth, we went on to investigate the effect of Ulva extract on LR formation. Increasing concentrations of Ulva extract show a progressive inhibition in the density of LR branching from the primary root, even at concentrations that stimulate primary root growth (Fig. 1e,f).
In summary, Ulva extract inhibits germination, has a biphasic effect on primary root growth (stimulatory at low concentrations; inhibitory at higher concentrations) and inhibits LR formation. Taken together, our data are reminiscent of the effect of the plant hormone abscisic acid (ABA) on germination and early root development64–69.
The germination-inhibitory effect of Ulva extract is not apparent in an ABA-insensitive mutant
We next sought to determine whether ABA signalling could mediate the effects of Ulva extract on Arabidopsis development to uncover the mechanism by which Ulva extract inhibits germination. Arabidopsis seeds from the ABA-insensitive mutant abi170,71 were assayed for their response to Ulva extract. abi1 seeds are unresponsive to the inhibitory effect of Ulva extract and behave similarly to untreated controls under all treatments (Fig. 2a–c). This suggests that the inhibition of Arabidopsis seed germination by Ulva extract depends on a functional ABA signalling pathway in the seeds.
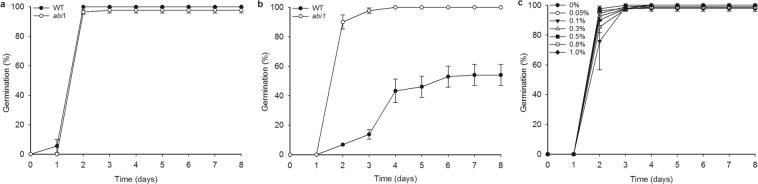
Figure 2.
The abi1 mutant is insensitive to the inhibitory effects of Ulva extract on germination. (a) Germination of WT and abi1 on control medium. There are no significant differences between genotypes on any day (Kruskal-Wallis test). (b) Germination of WT and abi1 on 1% Ulva extract. On days 2–8, wild-type is significantly (p < 0.05, Kruskal-Wallis test) different from abi1. p = 0.001 day 2, p = 0.001 day 3, p = 0.006 day 4, p = 0.005 day, p = 0.002 day 6, p = 0.001 day 7, p = 0.001 day 8. (c) Germination of abi1 seeds on increasing concentrations of Ulva extract. There are no significant differences between treatments apart from on day 2, when 0.5% and 0.8% treatments are significantly different from one another (p = 0.023). The wild type data is the same as in Fig. 1a. Bars represent standard error of the mean.
The abi1 mutant’s root growth responds normally to low concentrations of Ulva extract and is slightly insensitive to higher concentrations of Ulva extract
Since the abi1 mutant is impaired in its germination response to Ulva extract and since ABA is known to have a biphasic effect on root growth65, we tested the effect of Ulva extract on the root growth of the abi1 mutant. The abi1 mutant behaved similarly wild type plants at low concentrations (<0.1%) of Ulva extract (Fig. 3a,b). This suggests that the stimulatory effect of Ulva extract on root growth cannot be attributed to changes in ABA signalling in the plant. At higher concentrations (0.3–1%) of Ulva extract, the abi1 mutant showed some insensitivity to inhibition of root growth compared to wild-type (Fig. 3c). However, the insensitivity during root growth is slight when compared to the abi1 mutant’s complete insensitivity to the inhibitory effects of algal extract during germination. This suggests that changes in ABA signalling in Arabidopsis may partially contribute to the inhibitory effect of Ulva extract on root growth.
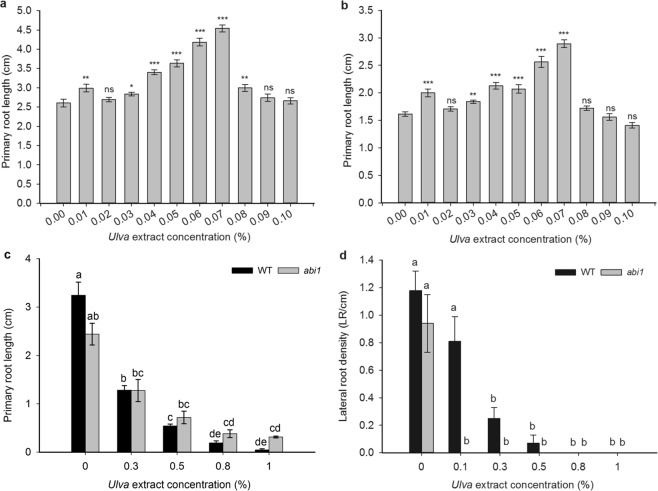
Figure 3.
The abi1 mutant is partially insensitive to the inhibitory effect of Ulva extract on root growth, responds to the stimulatory effect of Ulva extract on root growth similarly to wild-type, and is sensitive to the inhibition of lateral roots by Ulva extract. (a,b) Comparison of wild-type (a) and abi1 mutant (b) root-length responses to low concentrations (0–0.1%) of Ulva extract, measured on day 10. In wild-type, significant (p < 0.05; Mann-Whitney U-test) differences are seen at 0.01% (p = 0.0018), 0.03% (p = 0.025), 0.04% (p = 0.0000), 0.05% (0.0000), 0.06% (0.0000), 0.07% (p = 0.0000, 0.08% (p = 0.0074). In abi1 mutants, significant differences are seen at 0.01% (p = 0.0000), 0.03% (p = 0.0003), 0.04% (p = 0.0000), 0.05% (p = 0.0001), 0.06% (p = 0.0000), 0.07% (p = 0.0000), 0.1% (p = 0.0088). Asterisks: significant differences between treatments and 0% control: *p < 0.05, **p < 0.01 and ***p < 0.001. n = 26–31 seedlings for each treatment and each genotype. (c) Comparison of wild-type and abi1 mutant root-length responses to 0–1% Ulva extract, measured on day 10. Wild-type on 0% Ulva extract is significantly different to wild-type on 0.5%, 0.8% and 1% (all p < 0.001). abi1 on 0% Ulva extract is significantly different to abi1 on 0.8 and 1% Ulva extract (p < 0.001%). Ulva extract treatments decrease root length in both wild-type and abi1. Between treatments, fewer significant differences are seen with abi1 mutants than with wild-type plants, demonstrating abi1’s insensitivity to Ulva extract during root length inhibition (Kruskal-Wallis). Letters represent significant differences. n = 15–20 seedlings for each treatment and each genotype. (d) Comparison of wild-type and abi1 mutant lateral root responses to 0–1% Ulva extract calculated on day 10. Wild-type and abi1 mutants are both inhibited by Ulva extract, with wild-type being significantly inhibited by 0.3% (p = 0.001) and by 0.5%-1% Ulva extract (p < 0.001) and abi1 being significantly inhibited (p < 0.001) by 0.1–1% Ulva extract. Thus, Ulva extract treatments significantly decrease lateral root density in both wild-type and abi1 (Kruskal-Wallis). n = 30–60 seedlings for each treatment and each genotype; letters represent significant differences between genotypes and treatments. In all panels, bars represent standard error of the mean.
The abi1 mutant is sensitive to lateral root-inhibition by Ulva extract
The abi1 mutant’s LR development was inhibited by Ulva extract more strongly than wild type controls (Fig. 3d), including at 0.1% Ulva extract, which has no effect on primary root growth. This implies that the inhibition of LR development by Ulva extract is not mediated by the ABA signalling pathway and that LRs respond differently to Ulva extract compared to the primary root.
Elemental analysis of Ulva intestinalis
The Ulva extract used in our experiments is a water-soluble extract, so its effects on the Arabidopsis ABA signalling pathway are most likely indirect as ABA is more soluble in organic solvents than water. We measured the concentration of a panel of 31 water-soluble ions in our Ulva intestinalis samples using ICP-MS to determine whether (i) there were substances present in the tissue that were at markedly different levels to those in a land plant standard, (ii) whether any substances were present at substantially different levels to those in our standard Arabidopsis growth medium and (iii) whether the presence of any of the substances could explain the effects of Ulva extract on Arabidopsis seedling development. Our analysis identified 16 elements present at higher levels in Ulva intestinalis extract than in the land plant control, namely Boron, Sodium, Sulphur, Lithium, Aluminium, Vanadium, Manganese, Iron, Copper, Arsenic, Strontium, Silver, Caesium, Thallium, Lead and Uranium (Table 1). Of these, 12 elements (Sodium, Lithium, Aluminium, Vanadium, Copper, Arsenic, Strontium, Silver, Caesium, Thallium, Lead and Uranium) are present in 1% Ulva intestinalis extract at higher values than in Arabidopsis growth medium (Table 1). Out of these 12 elements, only three, namely Sodium, Aluminium and Copper, are present at levels likely to have an effect on Arabidopsis development72–80. The level of Sodium in the 1% Ulva extract is 10.5 mM; Aluminium (Al3+) ions are present in 1% Ulva extract at >500 µM, and 0.05% Ulva extract at 26 µM; Copper ions (Cu2+) are present in 1% Ulva extract at 2.1 µM (Table 1). The remaining 9 elements are present at micromolar (Lithium) or nanomolar quantities, while the published literature demonstrates their effects on Arabidopsis germination and root growth only in the micromolar to millimolar81–88 range.
Table 1.
Elemental analysis of Ulva intestinalis compared to land plant (tomato) control.
| Element | Seaweed composition (mg/kg) | Tomato control composition (mg/kg) | Probability of same concentration in seaweed and tomato (Welch’s t-test) | Concentration of element in 0.05% Ulva extract - stimulatory for root growth | Concentration ofelement in 1% Ulva extract - inhibitory for germination and root growth | Concentration of element in Arabidopsis medium |
|---|---|---|---|---|---|---|
| B | 131.51 ± 5.65 | 31.18 ± 0.25 | 0.0046 | 6 µM | 121 µM | 100 µM |
| Na | 61860.69 ± 259.87 | 116.79 ± 4.96 | 0.0258 | 530 µM | 10.5 mM | 100 µM |
| Mg | 14291.19 ± 1182.33 | 10178.93 ± 71.28 | 0.1042 | 295 µM | 5.9 mM | 1.5 mM |
| P | 2511.15 ± 76.31 | 2307.47 ± 15.21 | 0.1569 | 40 µM | 800 µM | 1.25 mM |
| S | 24810.4 ± 872.48 | 9841.569 ± 110.91 | 0.0044 | 385 µM | 7.7 mM | >1.5 mM |
| K | 16466.06 ± 2383.77 | 27310.14 ± 197.01 | 0.0642 | 210 µM | 4.2 mM | >19 mM |
| Ca | 37045.93 ± 3114.25 | 46397.44 ± 256.12 | 0.1325 | 460 µM | 9.2 mM | 3 mM |
| Ti | 18.13 ± 0.36 | 18.79 ± 0.13 | 0.2782 | 190 nM | 3.8 µM | N/A |
| Li | 2.88 ± 0.19 | 0.5 ± 0.02 | 0.0097 | 150 nM | 3 µM | N/A |
| Be | 0.05 ± 0.00 | 0.01 ± 0.01 | 0.0967 | 3 nM | 60 nM | N/A |
| Al | 1397.88 ± 177.11 | 478 ± 2.71 | 0.0513 | 25.9 µM | 518 µM | N/A |
| V | 2.85 ± 0.22 | 0.74 ± 0.00 | 0.0164 | 28 nM | 560 nM | N/A |
| Cr | 2.38 ± 0.23 | 1.29 ± 0.24 | 0.1141 | 23 nM | 460 nM | N/A |
| Mn | 22.9 ± 1.23 | 228.11 ± 1.6 | 0.0003 | 210 nM | 4.2 µM | 100 µM |
| Fe | 795.33 ± 95.13 | 322.87 ± 3.18 | 0.0056 | 5 µM | 100 µM | 100 µM |
| Co | 0.34 ± 0.04 | 0.47 ± 0.00 | 0.1267 | 3 nM | 60 nM | 100 nM |
| Ni | 1.68 ± 0.13 | 1.4 ± 0.1 | 0.2871 | 154.5 nM | 290 nM | N/A |
| Cu | 13.37 ± 2.22 | 1.71 ± 0.05 | 0.0504 | 105 nM | 2.1 µM | 100 nM |
| Zn | 26.03 ± 0.87 | 25.77 ± 0.18 | 0.8336 | 200 nM | 4 µM | 30 µM |
| As | 3.07 ± 0.21 | 0.13 ± 0.00 | 0.0080 | 20.5 nM | 410 nM | N/A |
| Se | 0.13 ± 0.01 | 0.07 ± 0.00 | 0.1006 | 1 nM | 20 nM | N/A |
| Rb | 5.87 ± 0.76 | 14.09 ± 0.09 | 0.0117 | 34.5 nM | 690 nM | N/A |
| Sr | 125.03 ± 1.47 | 86.5 ± 0.29 | 0.0015 | 715 nM | 14.3 µM | N/A |
| Mo | 0.34 ± 0.04 | 0.44 ± 0.02 | 0.156 | 2 nM | 40 nM | 1 µM |
| Ag | 0.09 ± 0.00 | 0.00 ± 0.00 | 0.0001 | 415pM | 8.3 nM | N/A |
| Cd | 0.19 ± 0.01 | 1.48 ± 0.00 | p < 0.0001 | 1 nM | 20 nM | N/A |
| Cs | 0.28 ± 0.033 | 0.05 ± 0.00 | 0.0214 | 1 nM | 20 nM | N/A |
| Ba | 10.37 ± 0.91 | 64.04 ± 0.24 | 0.0002 | 38 nM | 760 nM | N/A |
| Tl | 0.09 ± 0.01 | 0.04 ± 0.00 | 0.0278 | 220pM | 4.4 nM | N/A |
| Pb | 1.79 ± 0.12 | 0.54 ± 0.00 | 0.0138 | 4.5 nM | 90 nM | N/A |
| U | 0.08 ± 0.01 | 0.03 ± 0.00 | 0.0327 | 170pM | 3.4 nM | N/A |
Elements that show a significantly higher concentration in Ulva compared to tomato are highlighted in bold. The concentration of each element in 0.05% Ulva extract (stimulates root growth) and 1% Ulva extract (inhibitory to germination and root growth) is shown, compared to the concentration of the same element in our normal Arabidopsis growth medium (0.5x MS). Elements highlighted in italics are present at higher concentrations in 1% Ulva extract than in 0.5 MS.
Auxin, ethylene, cytokinin mutants respond similarly to wild-type Arabidopsis on Ulva extract with respect to root growth
Aluminium stress on roots is mediated by a combination of ethylene (via changes in auxin transport, at higher Al3+ concentrations) and cytokinin signalling (at lower Al3+ concentrations)77. We tested mutants in auxin-, cytokinin and ethylene signalling for their root growth responses to Ulva extract (Supplemental Fig. 1). The two auxin signalling mutants used were the receptor mutant tir1-189 and the auxin-resistant signalling mutant axr1-190. The two ethylene signalling mutants used were the receptor mutant etr1-3 and the signalling mutant ein2-191. The cytokinin mutant used was the receptor mutant cre1-147. All mutants responded similarly to wild type seedlings to “inhibitory” concentrations of Ulva extract ranging from 0.1–1%, equating to approximately 50–500 µM Al3+ (Supplemental Fig. 1a). This suggests that the ethylene, auxin and cytokinin hormone signalling pathways do not participate substantially in root growth inhibition by Ulva extract. Moreover, none of the mutants were insensitive to the root growth-stimulatory effect of Ulva extract (Supplemental Fig. 1b), suggesting that these hormones do not participate in the root growth stimulation brought about by Ulva extract. Similar data was obtained when the quintuple della mutant92 was assayed for root growth stimulation and inhibition: the mutant behaved as wild-type, showing that gibberellin-DELLA signalling, which is involved in multiple plant stress- and growth-responses93 is not involved in the effects of Ulva extract on root growth (Supplemental Fig. 2).
Cytokinin- and ethylene-signalling mutants show some insensitivity to inhibition of germination by Ulva extract
As Aluminium (Al3+) ions are present in 1% Ulva extract at a root-inhibitory concentration >500 µM, and since two hormones involved in root responses to Aluminium, cytokinin and ethylene, are also regulators of seed germination94,95, we also tested the germination behaviour of cytokinin receptor mutant cre1 and the ethylene receptor mutant etr1 on Ulva extract. Both mutants’ seeds showed some insensitivity to germination-inhibition compared to wild type (Fig. 4a,b), but were not as insensitive as the abi1 mutant (Fig. 2b). Both cre1 and etr1 also showed a higher final germination percentage in comparison to WT germination on 0.8% and 1% Ulva extract over the same period of time (Fig. 1c–e). This suggests that the inhibition of Arabidopsis seed germination by Ulva extract is influenced by the cytokinin- and ethylene signalling pathways in addition to the ABA signalling pathway.
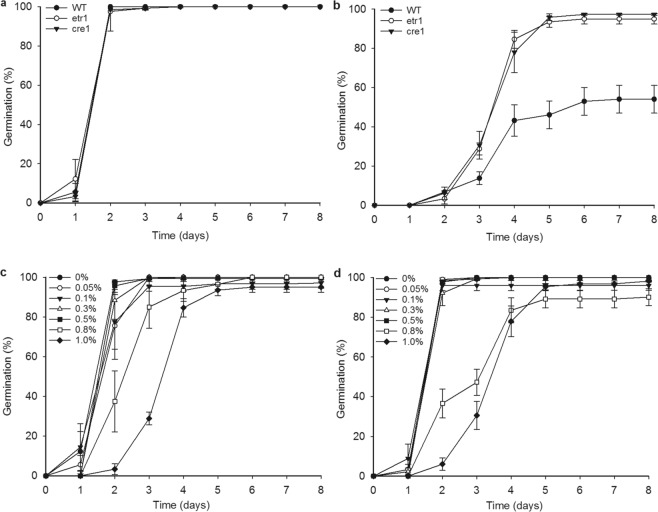
Figure 4.
Ethylene and cytokinin signalling mutants are slightly insensitive to the inhibition of germination by Ulva extract. (a) Wild type seed, etr1 mutant seed and cre1 mutant seed all germinate similarly on standard growth medium. No significant differences between genotypes are seen on any day. (b) etr1 and cre1 mutant seed germinate faster than wild type in the presence of 1% Ulva extract. Significant (p < 0.05%; Kruskal-Wallis) differences are seen between wild-type and cre1 on day 5 (p = 0.007) and on days 6–8 (each p = 0.02). Significant differences between wild-type and etr1 are soon on days 4 (p = 0.028), 5 (p = 0.009), 6 (p = 0.004), 7 and 8 (each p = 0.002). (c) Germination of cre1 mutant seed on varying concentrations of Ulva extract. On day 2, there is a significant (p < 0.05%; Kruskal-Wallis) difference between the 0% control and both 0.8% (p = 0.035) and 1% (p = 0.015). (d) Germination of etr1 mutant seed on varying concentrations of Ulva extract. On day 2, 1% Ulva extract is significantly different (p < 0.05; Kruskal-Wallis) from the control (p = 0.041). On day 3, 1% Ulva extract is significantly different from control (p = 0.025). Wild type data in (a,b) is the same as in Fig. 2.
Discussion
Ulva extract at concentrations of 0.5–1% inhibits wild-type Arabidopsis seed germination. Ulva extract ≥0.3% reduces wild-type Arabidopsis primary root growth and the extract inhibits wild type LR formation even at concentrations below 0.1%, suggesting that LRs are more sensitive than the primary root to the inhibitory agent(s) in the Ulva extract. This effect of Ulva extract resembles the effect of ABA, which is a negative regulator of germination64, shows a biphasic effect on primary root growth65–67, and inhibits LR growth at concentrations that stimulate primary root growth but do not affect germination68.
Our results concur with other studies where seaweed extract at high concentrations inhibited seed germination and seedling growth. Reduced germination occurred in pepper seeds primed with brown seaweed (Ascophyllum) extract at 1:250 (0.4%) and at higher concentrations (10%) of Maxicrop (commercial seaweed extract) solution compared to control seeds96. A higher concentration (1.0%) of water-extracts from the brown seaweeds Caulerpa sertularioides, Padina gymnospora and Sargassum liebmannii reduced tomato germination and seedling development36. Aqueous extracts (2–10%) from Sargassum johnstonii led to similar effects on tomato97. Concentrations of kelp waste extracts (KWE) from 10–100% inhibited germination of pakchoi (Brassica chinensis L.). This was attributed to high levels of NaCl98, which are absent from our Ulva extract. Arnon and Johnson99 reported detrimental effects on early tomato development as a result of higher pH in the growth medium. In our experiments, the pH was adjusted to be the same for all concentrations of Ulva extract so the effects are not due to altered pH.
Ulva extract has a growth-stimulating effect on wild type Arabidopsis primary root elongation specifically at concentrations between 0.025–0.08%. This is in accordance with data from other species, suggesting that Arabidopsis is a good model for studying the effects of seaweed extracts.
Seaweed extract may improve water and nutrient uptake efficiency by root systems100 leading to enhanced plant growth and vigour. Commercial extracts from the brown seaweed Ecklonia maxima stimulated tomato root growth at low concentrations (1:600; 0.17%) while higher concentrations (1:100; 1%) strongly inhibited root growth35. Root growth enhancement was seen in Arabidopsis plants treated with aquaeous Ascophyllum nodosum extracts (0.1gL−1; 0.01%), whereas plant height and number of leaves were affected positively at 1gL−1 (0.1%)101. Lower concentrations (0.2%) of extracts of both Ulva lactuca (green seaweed) and P. gymnospora (brown) were more effective at enhancing tomato seed germination36. We observed no boost in Arabidopsis germination with Ulva intestinalis extract under our growth conditions where we vernalise seeds to break dormancy before an assay, so this may explain the discrepancy between the experiments.
Kelp waste extract (KWE) at 2% stimulated pakchoi seed germination98. Pakchoi seedling growth (plumule length, radicle length, fresh weight and dry weight) was improved by treatment with 2–5% KWE98. This data is in-line with our observed root growth stimulation at low concentrations. The KWE was prepared differently (cell wall digestion and centrifugation) to our Ulva intestinalis extract, which may explain why higher concentrations of KWE than Ulva extract give stimulatory effects.
The stimulatory effect of KWE on pakchoi may be attributed to the combined effects of soluble sugars, amino acids and mineral elements98. Sugars are immediate substrates for intermediary metabolism and effective signaling molecules: thus accessibility of sugars influences plant growth and development102. The growth-enhancing potential of algal extract correlates with the presence of diverse polysaccharides, including unusual/complex polysaccharides not present in land plants21,103. However, a role for macro- and microelements, vitamins and phytohormones is also suggested20,27,32,104–106.
Our Arabidopsis mutant analysis demonstrates that germination-inhibition by Ulva extract is dependent on activation of the Arabidopsis ABA signaling pathway, with cytokinin- and ethylene-signaling also playing a role. Since our Ulva extracts are water-based, it is unlikely that they contain high quantities of plant hormones, which are largely soluble in organic solvents, even though Ulva107 and other seaweeds108 are known to produce ABA. This suggests that a substance(s) in Ulva extract activates endogenous plant hormone signaling to inhibit germination. Ulva extract-mediated inhibition of primary root growth is partly blocked in an ABA-insensitive mutant, while cytokinin-, auxin- ethylene- and gibberellin signaling mutants all respond similarly to wild type with respect to root growth. This implies that although ABA signaling plays a role in primary root growth inhibition by Ulva extract, additional pathways also contribute. Lateral root development is inhibited via a different mechanism to primary root growth, as the ABA-insensitive abi1 mutant’s LR development is inhibited by Ulva extract to a greater extent than wild-type (Fig. 3d).
Our elemental analysis of Ulva tissue suggests that the most likely cation contributing to the inhibitory effects of Ulva extract is Al3+, which is present in quantities known to inhibit Arabidopsis primary root growth76,77. Even 5 µM Al3+ can slow root growth76 while 500 µM Al3+ can reduce root growth by around 80%77. Thus, the elevated Al3+ levels in the Ulva extract could be contributing to the inhibition in root growth that we see at concentrations of Ulva extract ≥0.3%.
Al3+ may not be the only inhibitory substance present: previous research has demonstrated a role for auxin, ethylene and cytokinin in root responses to Al3+ stress77 and this is not apparent from our mutant root assays. Conversely, there may be other hormones involved in seed- and root responses to Al3+ stress: the effects of Al3+ on germination and lateral root development in Arabidopsis has not previously been studied. The toxic effect of Al3+ in the Ulva extract may be partially countered by the relatively high levels of Mg2+ also present in the extract (In 1% Ulva extract, 4x that present in Arabidopsis growth medium - Table 184).
Al3+ stress has a range of physiological effects that could affect root growth and development. Al3+ stress alters membrane potentials, which affects transport of ions, including Ca2+, across membranes. This can result in changes in cytoplasmic Ca2+ homeostasis, which controls cell signaling, metabolism and cell-growth processes including root development109. Al3+ stress induces changes in the expression and activity of the plasma membrane H+-ATPase that controls cytosolic pH and membrane potentials110.
Copper levels of 1.6 µM have previously been described as rhizotoxic81 and 20–25 µM Cu2+ inhibits root elongation in several studies78. Higher levels of copper (500µM-2mM) inhibit seed germination79,80. Thus, it is unlikely that the elevated copper levels in the Ulva extract are causing germination inhibition, but they could be partly contributing to the inhibition in root growth that we see in ≥0.3% Ulva extract.
Seaweeds contain high levels of particular cations: macroelements (Na, P, K, Ca) and microelements (Fe, B, Mn, Ca, Mo, Zn, Co) that have critical roles in plant development and growth111,112. In many vegetable crops, the accumulation of sodium ions restrains embryo or seedling development, leading to reduced germination, uneven morphogenesis and loss of crop production e.g.113. Our data suggests that the only macroelement present at higher concentrations in Ulva extract than in plant tissues (or indeed plant growth medium) is Na+, but Na+ is not present at high enough concentrations to explain the inhibition of germination, root growth and lateral root development that we see. Arabidopsis germination is inhibited only by concentrations of salt above 150 mM72. Thus, the level of germination-inhibition with Ulva extract at ≥0.3% is not attributable to the levels of Sodium in the extract. Arabidopsis root growth is inhibited by concentrations of 25 mM Na+ and above73. Thus, the inhibition of root growth seen in our experiments is unlikely to be attributable wholly to salt stress. This conclusion is in accordance with the fact that the abi1 mutant is not wholly insensitive to the root growth inhibition (Fig. 3c) since salt stress responses are mediated by ABA signalling74,75.
Ulva species tolerate low salinity despite being marine algae. Our Ulva sampling site is where a river meets the sea: the salinity of the seawater is low (F. Ghaderiardakani, unpublished). A reduction in germination rate and growth of tomato attributable to salt (and perhaps reduced imbibition of water by seeds) was suggested upon applying brown seaweed (Caulerpa sertularioides and Sargassum liebmannii) liquid extracts, but not with U. lactuca and P. gymnospora with a lower salt concentration36.
Some seaweed extracts alleviate salt stress: the survival of Kentucky bluegrass (Poa pratensis L. cv. Plush) treated with a proprietary seaweed extract (38Lha−1) increased significantly, under various levels of salinity, with improved growth and promotion of rooting of the grass at a soil salinity of 0.15Sm−1 19. Application of seaweed extract activated a mechanism reducing the accumulation of Na+ in plants; grass treated with seaweed extract had less sodium in the shoot tissue114,115.
The microelements B and Fe are present at higher concentrations in Ulva tissue than in our land plant control, but at levels that are very similar to that found in our Arabidopsis growth medium, so cannot be contributing to the observed stimulatory or inhibitory effects. The content of minerals in Ulva intestinalis is in-line with values for Ulva spp. reported previously, e.g. Ulva lactuca36 and Ulva reticulata40,111.
Using seaweed extracts as biofertilisers due to their direct or indirect stimulatory impacts on plant metabolism has been suggested as one of their key beneficial applications23. Taken together, our results and others’ suggest that for plants to benefit optimally from algal extracts, only a small quantity should be used or could be mixed with commercially available fertilisers for a synergistic effect on crop yield and a reduction in quantities and costs of chemical fertilisers applied116.
Our data demonstrates that Ulva extract can inhibit Arabidopsis seed germination, early root growth and lateral root development, even at concentrations below 1%, by activating endogenous plant hormone signaling pathways. Could this in itself be useful? One of the top priorities in organic agriculture is the eradication of weeds from the production area117. Concerns about improvements in agriculture focus on diminishing weeds’ adverse effects on the environment and improving the sustainable development of agricultural systems. New approaches are required to integrate biological and ecological processes into food production and minimize the use of practices that lead to the environmental harm118. Considering the observed biological inhibitory effects resulting from the action of seaweed extracts on crops’ germination and early development particularly at high concentration, it might be worthwhile to employ seaweed extracts as organic herbicides. The evidence at hand establishes that there are benefits to be obtained from utilizing macroalgal products in agricultural systems. Further translational studies are required to define the appropriate algal sources for commercial biostimulants (considering inherently different algal extracts and also the availability of seaweed biomass in a particular area), their application form and frequency, the timing of applications in relation to plant development and the optimal dosages needed to maximise both agricultural productivity and economic advantages.
In conclusion, water-soluble algal extracts from Ulva intestinalis were effective at stimulating the primary root growth of Arabidopsis thaliana only when applied at low concentrations. High concentrations of Ulva extract inhibit germination and root development, perhaps in part due to Al3+ toxicity, with endogenous plant ABA signalling playing a role in this inhibition. The effects of algal extracts on Arabidopsis development are likely mediated by a complex interplay of hormones. Future work targeting candidate genes in Ulva62 may uncover how Ulva extracts exerts their effects on plant hormone signalling. Although if used sparingly, seaweed extracts are potential candidates to produce effective biostimulants, they may be just as beneficial as organic herbicides by targeting plants’ ABA signalling mechanisms. Cross-disciplinary research could help farmers to benefit optimally from the use of algal extracts in the future, particularly for cost-effective organic farming and an environmentally-friendly approach for sustainable agriculture.
Methods
Collection and Identification of Algal Samples
Vegetative and fertile U. intestinalis blades were collected from the intertidal zone at low tide, three times between March 2015 and April 2016, from the coastal area of Llantwit Major beach, South Wales, UK (51°40′N; 3°48′W). Excess water and epiphytic species were removed at the site by blotting the sample’s surface before storage on ice for transport back to the laboratory. Epiphyte-free samples were subjected to a molecular identification using plastid-encoded rbcL (large unit ribulose bisphosphate carboxylase) and tufA (plastid elongation factor) markers because identification solely by morphological characteristics is not reliable63.
Preparation of water-soluble Ulva Extract
Ulva samples were washed with tap water to remove surface salt, shade dried for 10 days, oven-dried for 48 h at 60 °C, then ground to a fine powder using a coffee grinder (Crofton, China) to less than 0.50 mm. 10 g of this milled material was added to 100 mL of distilled water with constant stirring for 15 min followed heating for 45 minutes at 60 °C in water bath40. The contents were filtered through two layers of muslin cloth. This Ulva extract was designated as 10% stock solution and added to 0.5x Murashige and Skoog (MS) Arabidopsis growth medium (Sigma M0404) to make up specific concentrations and autoclaved. 1% Ulva extract stock was subjected to pH measurement and elemental analysis. All measurements were performed in triplicate.
Digestion of plant material for elemental analysis
Ulva samples were digested using a microwave system, comprising a Multiwave 3000 platform with a 48-vessel MF50 rotor (Anton Paar GmbH, Graz, Austria); digestion vessels comprised perfluoroalkoxy liner material and polyethylethylketone pressure jackets (Anton Paar GmbH). Dried samples (~0.2 g) were digested in 2 mL 70% Trace Analysis Grade HNO3, 1 mL Milli-Q water (18.2 MΩ cm; Fisher Scientific UK Ltd, Loughborough, UK), and 1 mL H2O2 with microwave settings as follows: power = 1400 W, temp = 140 C, pressure = 20 Bar, time = 45 minutes. Two operational blanks and two certified reference material of leaf (Tomato SRM 1573a, NIST, Gaithersburg, MD, USA) were included in each digestion run. Following digestion, each tube was made up to a final volume of 15 mL by adding 11 mL of Milli-Q water and transferred to a universal tube and stored at room temperature.
Elemental analysis
Sample digestates were diluted 1-in-10 using Milli-Q water prior to elemental analysis. The concentrations of 28 elements were obtained using inductively coupled plasma-mass spectrometry (ICP-MS; Thermo Fisher Scientific iCAPQ, Thermo Fisher Scientific, Bremen, Germany); Ag, Al, As, B, Ba, Ca, Cd, Cr, Co, Cs, Cu, Fe, K, Mg, Mn, Mo, Na, Ni, P, Pb, Rb, S, Se, Sr, Ti, U, V, Zn. Operational modes included: (i) a helium collision-cell (He-cell) with kinetic energy discrimination to remove polyatomic interferences, (ii) standard mode (STD) in which the collision cell was evacuated, and (iii) a hydrogen collision-cell (H2-cell). Samples were introduced from an autosampler incorporating an ASXpress™ rapid uptake module (Cetac ASX-520, Teledyne Technologies Inc., Omaha, NE, USA) through a PEEK nebulizer (Burgener Mira Mist, Mississauga, Burgener Research Inc., Canada). Internal standards were introduced to the sample stream on a separate line via the ASXpress unit and included Sc (20 µgL−1), Rh (10 µgL−1), Ge (10 µgL−1) and Ir (5 µgL−1) in 2% trace analysis grade HNO3 (Fisher Scientific UK Ltd). External multi-element calibration standards (Claritas-PPT grade CLMS-2; SPEX Certiprep Inc., Metuchen, NJ, USA) included Ag, Al, As, B, Ba, Cd, Ca, Co, Cr, Cs, Cu, Fe, K, Mg, Mn, Mo, Na, Ni, P, Pb, Rb, S, Se, Sr, Ti (semi-quant), U, V and Zn, in the range 0–100 µgL−1 (0, 20, 40, 100 µgL−1). A bespoke external multi-element calibration solution (PlasmaCAL, SCP Science, Courtaboeuf, France) was used to create Ca, K, Mg and Na standards in the range 0–30 mgL−1. Boron, P and S calibration utilized in-house standard solutions (KH2PO4, K2SO4 and H3BO3). In-sample switching was used to measure B and P in STD mode, Se in H2-cell mode and all other elements in He-cell mode. Sample processing was undertaken using Qtegra™ software (Thermo Fisher Scientific) with external cross-calibration between pulse-counting and analogue detector modes when required119. Differences between seaweed and tomato control were analysed using a Welch’s t-test.
Germination Bioassay
Arabidopsis thaliana wild-type Col-0 and mutant lines abi1-1, tir1-1, axr1-3, cre1-12, etr1-3, and ein3-1 were obtained from the Nottingham Arabidopsis Stock Centre (Loughborough, UK). Arabidopsis seeds were sterilised in 20% ParozoneTM bleach on a turning wheel for 10 minutes and subsequently washed 2-3 times in sterile water. Seeds were vernalized at 4 °C for 48 h and placed on 1% agar, containing 0.5x MS and Ulva extract. Plates were transferred to the growth room for 7–10 days and incubated at 22 ± 2 °C with a 16-h-light photoperiod and light intensity of 120 µmolm−2 s−1. Germination was observed daily as in120. A seed was scored as germinated when its radicle had emerged from within the seed coat. Germination percentage (GP) was calculated as follows: GP = (the number of germinated seeds/total number of seeds) × 100). Data from three independent biological repeats (n = 30–90 seeds per genotype and treatment) were combined. To identify significant differences between treatments and genotypes, Kruskal-Wallis one-way ANOVA on ranks followed by Dunn’s post-hoc tests were performed using SigmaPlot 13 software (Systat Software, San Jose, CA).
Root Bioassay
Experiments were conducted using 10 cm square agar plates. 20 seeds were placed individually on the agar following a line across the top of the plate. The plates were sealed with Micropore tape (3M), taped together and incubated vertically in standard growth conditions as in120.
From day 7 to 14 the seedlings were photographed and primary root (PR) lengths were measured with ImageJ open-source software (http://rsb.info.nih.gov/ij). For some assays, the number of visible emerged lateral roots (LR) on each primary root was also counted and the lateral root density was calculated by dividing the number of LRs present by the length of that root. To identify significant differences between treatments and controls in wild-type plants, data were first checked to confirm normality, then appropriate two-tailed t-tests (normal data) or Mann-Whitney U-tests (non-normal data) were performed in Excel using an Excel template from Gianmarco Alberti’s lab (xoomer.alice.it/Exceltemplates.pdf), comparing the results of each Ulva extract concentration to the control (without Ulva extract). To identify significant differences between treatments and genotypes, Kruskal-Wallis one-way ANOVA on ranks followed by a Dunn’s post-hoc test were performed using SigmaPlot 13 software (Systat Software, San Jose, CA). All experiments were repeated a minimum of two and a maximum of four times with similar trends observed in each biological repeat.
Supplementary information
Acknowledgements
This work was funded by an Islamic Development Bank PhD scholarship to F.G. and University of Birmingham funds for J.C. to host E.C., D.K.D. and K.T. in the lab.
Author Contributions
F.G. and J.C.C. conceived the study and designed experiments. All authors performed experiments and analysed data: F.G. - F.G. - Fig. 1a,d,e, Fig. 2, Fig. 3a,b,c,d; Fig. 4; E.C. - Fig. 1a,c, Supplemental Fig. 1; Fig. 4; D.K.D. – Fig. 1e, Fig. 3c,d, Supplemental Fig. 1; K.T. – Fig. 1a; Fig. 2; N.S.G. - Table 1, J.C.C. – Supplemental Fig. 2. F.G. supervised E.C., D.K.D., K.T. in the lab; J.C.C. supervised F.G., E.D., D.K.D., K.T. F.G., N.S.G. and J.C.C. wrote the paper.
Data Availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ellen Collas, Deborah Kohn Damiano and Katherine Tagg contributed equally.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-38093-2.
References
- 1.Duque, A. S. et al. In Abiotic Stress-Plant responses and applications in agriculture. (InTech, 2013).
- 2.Tilman D, Balzer C, Hill J, Befort BL. Global food demand and the sustainable intensification of agriculture. Proceedings of the National Academy of Sciences. 2011;108:20260–20264. doi: 10.1073/pnas.1116437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGuire S. FAO, IFAD, and WFP. The State of Food Insecurity in the World 2015: Meeting the 2015 International Hunger Targets: Taking Stock of Uneven Progress. Rome: FAO, 2015. Advances in Nutrition: An International Review Journal. 2015;6:623–624. doi: 10.3945/an.115.009936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atkinson NJ, Urwin PE. The interaction of plant biotic and abiotic stresses: from genes to the field. Journal of Experimental Botany. 2012;63:3523–3543. doi: 10.1093/jxb/ers100. [DOI] [PubMed] [Google Scholar]
- 5.Challinor AJ, Simelton ES, Fraser ED, Hemming D, Collins M. Increased crop failure due to climate change: assessing adaptation options using models and socio-economic data for wheat in China. Environmental Research Letters. 2010;5:034012. doi: 10.1088/1748-9326/5/3/034012. [DOI] [Google Scholar]
- 6.Barriopedro D, Fischer EM, Luterbacher J, Trigo RM, García-Herrera R. The hot summer of 2010: redrawing the temperature record map of Europe. Science. 2011;332:220–224. doi: 10.1126/science.1201224. [DOI] [PubMed] [Google Scholar]
- 7.Cramer GR, Urano K, Delrot S, Pezzotti M, Shinozaki K. Effects of abiotic stress on plants: a systems biology perspective. BMC Plant Biology. 2011;11:163. doi: 10.1186/1471-2229-11-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schellnhuber, H. J. et al. Turn down the heat: why a 4 C warmer world must be avoided. (World Bank, 2012).
- 9.Shepherd, A. et al. The geography of poverty, disaster and climate extremes in 2030 (2013).
- 10.Kit, O. Turn down the heat: confronting the new climate normal (2014).
- 11.Sowers J, Vengosh A, Weinthal E. Climate change, water resources, and the politics of adaptation in the Middle East and North Africa. Climatic Change. 2011;104:599–627. doi: 10.1007/s10584-010-9835-4. [DOI] [Google Scholar]
- 12.Shahbaz M, Ashraf M. Improving salinity tolerance in cereals. Critical Reviews in Plant Sciences. 2013;32:237–249. doi: 10.1080/07352689.2013.758544. [DOI] [Google Scholar]
- 13.Vengosh A, Jackson RB, Warner N, Darrah TH, Kondash A. A critical review of the risks to water resources from unconventional shale gas development and hydraulic fracturing in the United States. Environmental Science & Technology. 2014;48:8334–8348. doi: 10.1021/es405118y. [DOI] [PubMed] [Google Scholar]
- 14.Battacharyya D, Babgohari MZ, Rathor P, Prithiviraj B. Seaweed extracts as biostimulants in horticulture. Scientia Horticulturae. 2015;196:39–48. doi: 10.1016/j.scienta.2015.09.012. [DOI] [Google Scholar]
- 15.Kelly, M. & Dworjanyn, S. The potential of marine biomass for anaerobic biogas production: a feasibility study with recommendations for further research. Scotland: Scottish Association for Marine Science (2008).
- 16.Notoya, M. In, Seaweeds and their role in globally changing environments. 217–228 (Springer, 2010).
- 17.Henderson, J. The Roman Book of Gardening. (Psychology Press, 2004).
- 18.Zhang X, Ervin E, Schmidt R. Physiological effects of liquid applications of a seaweed extract and a humic acid on creeping bentgrass. Journal of the American Society for Horticultural Science. 2003;128:492–496. doi: 10.21273/JASHS.128.4.0492. [DOI] [Google Scholar]
- 19.Nabati D, Schmidt R, Parrish D. Alleviation of salinity stress in Kentucky bluegrass by plant growth regulators and iron. Crop Science. 1994;34:198–202. doi: 10.2135/cropsci1994.0011183X003400010035x. [DOI] [Google Scholar]
- 20.Khan W, et al. Seaweed extracts as biostimulants of plant growth and development. Journal of Plant Growth Regulation. 2009;28:386–399. doi: 10.1007/s00344-009-9103-x. [DOI] [Google Scholar]
- 21.Craigie JS. Seaweed extract stimuli in plant science and agriculture. Journal of Applied Phycology. 2011;23:371–393. doi: 10.1007/s10811-010-9560-4. [DOI] [Google Scholar]
- 22.Du Jardin, P. The Science of Plant Biostimulants–A bibliographic analysis, Ad hoc study report. (European Commission, 2012).
- 23.Arioli T, Mattner SW, Winberg PC. Applications of seaweed extracts in Australian agriculture: past, present and future. Journal of Applied Phycology. 2015;27:2007–2015. doi: 10.1007/s10811-015-0574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milton, R. Improvements in or relating to horticultural and agricultural fertilizers. British Patent664989 (1952).
- 25.Crouch IJ, Van Staden J. Commercial seaweed products as biostimulants in horticulture. Journal of Home & Consumer Horticulture. 1993;1:19–76. doi: 10.1300/J280v01n01_03. [DOI] [Google Scholar]
- 26.Hussain A, Boney A. Hydrophilic growth inhibitors from Laminaria and Ascophyllum. New Phytologist. 1973;72:403–410. doi: 10.1111/j.1469-8137.1973.tb02048.x. [DOI] [Google Scholar]
- 27.Stirk W, et al. Changes in cytokinin and auxin concentrations in seaweed concentrates when stored at an elevated temperature. Journal of Applied Phycology. 2004;16:31–39. doi: 10.1023/B:JAPH.0000019057.45363.f5. [DOI] [Google Scholar]
- 28.Wally OS, et al. Regulation of phytohormone biosynthesis and accumulation in Arabidopsis following treatment with commercial extract from the marine macroalga Ascophyllum nodosum. Journal of Plant Growth Regulation. 2013;32:324–339. doi: 10.1007/s00344-012-9301-9. [DOI] [Google Scholar]
- 29.Blunden G, Jenkins T, Liu Y-W. Enhanced leaf chlorophyll levels in plants treated with seaweed extract. Journal of Applied Phycology. 1996;8:535–543. doi: 10.1007/BF02186333. [DOI] [Google Scholar]
- 30.Fornes F, Sanchez-Perales M, Guardiola J. Effect of a seaweed extract on the productivity of’de Nules’ clementine mandarin and navelina orange. Botanica Marina. 2002;45:486–489. doi: 10.1515/BOT.2002.051. [DOI] [Google Scholar]
- 31.Vernieri P, Borghesi E, Ferrante A, Magnani G. Application of biostimulants in floating system for improving rocket quality. Journal of Food Agriculture and Environment. 2005;3:86. [Google Scholar]
- 32.Tay SA, Macleod JK, Palni LMS, Letham DS. Detection of cytokinins in a seaweed extract. Phytochemistry. 1985;24:2611–2614. doi: 10.1016/S0031-9422(00)80679-2. [DOI] [Google Scholar]
- 33.Lynch J. Root architecture and plant productivity. Plant Physiology. 1995;109:7–13. doi: 10.1104/pp.109.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang L, Ramireddy E, Schmülling T. Lateral root formation and growth of Arabidopsis is redundantly regulated by cytokinin metabolism and signalling genes. Journal of Experimental Botany. 2013;64:5021–5032. doi: 10.1093/jxb/ert291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finnie J, Van Staden J. Effect of seaweed concentrate and applied hormones on in vitro cultured tomato roots. Journal of Plant Physiology. 1985;120:215–222. doi: 10.1016/S0176-1617(85)80108-5. [DOI] [Google Scholar]
- 36.Hernández-Herrera RM, Santacruz-Ruvalcaba F, Ruiz-López MA, Norrie J, Hernández- Carmona G. Effect of liquid seaweed extracts on growth of tomato seedlings (Solanum lycopersicum L.) Journal of Applied Phycology. 2014;26:619–628. doi: 10.1007/s10811-013-0078-4. [DOI] [Google Scholar]
- 37.Rao GN, Chatterjee R. Effect of seaweed liquid fertilizer from Gracilaria textorii and Hypnea musciformis on seed germination and productivity of some vegetable crops. Universal Journal of Plant Science. 2014;2:115–120. [Google Scholar]
- 38.Kalaivanan C, Venkatesalu V. Utilization of seaweed Sargassum myriocystum extracts as a stimulant of seedlings of Vigna mungo (L.) Hepper. Spanish Journal of Agricultural Research. 2012;10:466–470. doi: 10.5424/sjar/2012102-507-10. [DOI] [Google Scholar]
- 39.Kumar, N. A., Vanlalzarzova, B., Sridhar, S. & Baluswami, M. Effect of liquid seaweed fertilizer of Sargassum wightii Grev. on the growth and biochemical content of green gram (Vigna radiata (L.) R. Wilczek). Recent Research in Science and Technology 4 (2012).
- 40.Selvam GG, Sivakumar K. Effect of foliar spray from seaweed liquid fertilizer of Ulva reticulata (Forsk.) on Vigna mungo L. and their elemental composition using SEM–energy dispersive spectroscopic analysis. Asian Pacific Journal of Reproduction. 2013;2:119–125. doi: 10.1016/S2305-0500(13)60131-1. [DOI] [Google Scholar]
- 41.Koornneef M, Meinke D. The development of Arabidopsis as a model plant. The Plant Journal. 2010;61:909–921. doi: 10.1111/j.1365-313X.2009.04086.x. [DOI] [PubMed] [Google Scholar]
- 42.The Arabidopsis Genome Initiative Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature. 2000;408:796. doi: 10.1038/35048692. [DOI] [PubMed] [Google Scholar]
- 43.Azpiroz-Leehan R, Feldmann KA. T-DNA insertion mutagenesis in Arabidopsis: going back and forth. Trends in Genetics. 1997;13:152–156. doi: 10.1016/S0168-9525(97)01094-9. [DOI] [PubMed] [Google Scholar]
- 44.Tissier AF, et al. Multiple independent defective suppressor-mutator transposon insertions in Arabidopsis: a tool for functional genomics. The Plant Cell. 1999;11:1841–1852. doi: 10.1105/tpc.11.10.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parinov S, Sundaresan V. Functional genomics in Arabidopsis: large-scale insertional mutagenesis complements the genome sequencing project. Current Opinion in Biotechnology. 2000;11:157–161. doi: 10.1016/S0958-1669(00)00075-6. [DOI] [PubMed] [Google Scholar]
- 46.Sussman MR, Amasino RM, Young JC, Krysan PJ, Austin-Phillips S. The Arabidopsis knockout facility at the University of Wisconsin–Madison. Plant Physiology. 2000;124:1465–1467. doi: 10.1104/pp.124.4.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alonso JM, et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science. 2003;301:653–657. doi: 10.1126/science.1086391. [DOI] [PubMed] [Google Scholar]
- 48.Hasegawa PM, Bressan RA, Zhu J-K, Bohnert HJ. Plant cellular and molecular responses to high salinity. Annual Review of Plant Biology. 2000;51:463–499. doi: 10.1146/annurev.arplant.51.1.463. [DOI] [PubMed] [Google Scholar]
- 49.Qin F, Shinozaki K, Yamaguchi-Shinozaki K. Achievements and challenges in understanding plant abiotic stress responses and tolerance. Plant and Cell Physiology. 2011;52:1569–1582. doi: 10.1093/pcp/pcr106. [DOI] [PubMed] [Google Scholar]
- 50.Wani SH, Kumar V, Shriram V, Sah SK. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. The Crop. Journal. 2016;4:162–176. [Google Scholar]
- 51.Hayashi M, Nishimura M. Arabidopsis thaliana—a model organism to study plant peroxisomes. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 2006;1763:1382–1391. doi: 10.1016/j.bbamcr.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 52.Hochholdinger F, Zimmermann R. Conserved and diverse mechanisms in root development. Current Opinion in Plant Biology. 2008;11:70–74. doi: 10.1016/j.pbi.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 53.Sah, S. K., Reddy, K. R. & Li, J. Abscisic acid and abiotic stress tolerance in crop plants. Frontiers in Plant Science7 (2016). [DOI] [PMC free article] [PubMed]
- 54.Hayden HS, et al. Linnaeus was right all along: Ulva and Enteromorpha are not distinct genera. European Journal of Phycology. 2003;38:277–294. doi: 10.1080/1364253031000136321. [DOI] [Google Scholar]
- 55.Guiry MD, et al. AlgaeBase: An On-line Resource for Algae. Cryptogamie, Algologie. 2014;35:105–115. doi: 10.7872/crya.v35.iss2.2014.105. [DOI] [Google Scholar]
- 56.Le Gall Y, Brown S, Marie D, Mejjad M, Kloareg B. Quantification of nuclear DNA and GC content in marine macroalgae by flow cytometry of isolated nuclei. Protoplasma. 1993;173:123–132. doi: 10.1007/BF01379001. [DOI] [Google Scholar]
- 57.Kapraun D. Karyology and cytophotometric estimation of nuclear DNA variation in seven species of Ulvales (Chlorophyta). Japanese. Journal of Phycology. 1992;40:13–24. [Google Scholar]
- 58.Spoerner M, Wichard T, Bachhuber T, Stratmann J, Oertel W. Growth and thallus morphogenesis of Ulva mutabilis (Chlorophyta) depends on a combination of two bacterial species excreting regulatory factors. Journal of Phycology. 2012;48:1433–1447. doi: 10.1111/j.1529-8817.2012.01231.x. [DOI] [PubMed] [Google Scholar]
- 59.Vesty EF, et al. The decision to germinate is regulated by divergent molecular networks in spores and seeds. New Phytologist. 2016;211:952–966. doi: 10.1111/nph.14018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fjeld, A. & Lovlie, A. Genetics of multicellular marine algae. Botanical Monographs (1976).
- 61.Oertel W, Wichard T, Weissgerber A. Transformation of Ulva mutabilis (Chlorophyta) by vector plasmids integrating into the genome. Journal of Phycology. 2015;51:963–979. doi: 10.1111/jpy.12336. [DOI] [PubMed] [Google Scholar]
- 62.Wichard, T. et al. The green seaweed Ulva: a model system to study morphogenesis. Frontiers in Plant Science6 (2015). [DOI] [PMC free article] [PubMed]
- 63.Ghaderiardakani F, Coates JC, Wichard T. Bacteria-induced morphogenesis of Ulva intestinalis and Ulva mutabilis (Chlorophyta): a contribution to the lottery theory. FEMS Microbiology Ecology. 2017;93:fix094. doi: 10.1093/femsec/fix094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li X-J, et al. Abscisic acid pretreatment enhances salt tolerance of rice seedlings: proteomic evidence. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics. 2010;1804:929–940. doi: 10.1016/j.bbapap.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 65.Li X, Chen L, Forde BG, Davies WJ. The Biphasic Root Growth Response to Abscisic Acid in Arabidopsis Involves Interaction with Ethylene and Auxin Signalling Pathways. Frontiers in Plant Science. 2017;8:1493. doi: 10.3389/fpls.2017.01493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Watts S, Rodriguez J, Evans SE, Davies W. Root and shoot growth of plants treated with abscisic acid. Annals of Botany. 1981;47:595–602. doi: 10.1093/oxfordjournals.aob.a086056. [DOI] [Google Scholar]
- 67.Xu W, et al. Abscisic acid accumulation modulates auxin transport in the root tip to enhance proton secretion for maintaining root growth under moderate water stress. New Phytologist. 2013;197:139–150. doi: 10.1111/nph.12004. [DOI] [PubMed] [Google Scholar]
- 68.De Smet I, et al. An abscisic acid‐sensitive checkpoint in lateral root development of Arabidopsis. The Plant Journal. 2003;33:543–555. doi: 10.1046/j.1365-313X.2003.01652.x. [DOI] [PubMed] [Google Scholar]
- 69.De Smet I, Vanneste S, Inzé D, Beeckman T. Lateral root initiation or the birth of a new meristem. Plant Molecular Biology. 2006;60:871–887. doi: 10.1007/s11103-005-4547-2. [DOI] [PubMed] [Google Scholar]
- 70.Meyer K, Leube MP, Grill E. A protein phosphatase 2C involved in ABA signal transduction in Arabidopsis thaliana. Science. 1994;264:1452–1454. doi: 10.1126/science.8197457. [DOI] [PubMed] [Google Scholar]
- 71.Leung J, et al. Arabidopsis ABA response gene ABI1: features of a calcium-modulated protein phosphatase. Science-AAAS. 1994;264:1448–1451. doi: 10.1126/science.7910981. [DOI] [PubMed] [Google Scholar]
- 72.Gao X, Ren F, Lu Y-T. The Arabidopsis mutant stg1 identifies a function for TBP-associated factor 10 in plant osmotic stress adaptation. Plant and Cell Physiology. 2006;47:1285–1294. doi: 10.1093/pcp/pcj099. [DOI] [PubMed] [Google Scholar]
- 73.Beisson F, Li Y, Bonaventure G, Pollard M, Ohlrogge JB. The acyltransferase GPAT5 is required for the synthesis of suberin in seed coat and root of Arabidopsis. The Plant Cell. 2007;19:351–368. doi: 10.1105/tpc.106.048033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shi H, Lee B-h, Wu S-J, Zhu J-K. Overexpression of a plasma membrane Na+/H+ antiporter gene improves salt tolerance in Arabidopsis thaliana. Nature Biotechnology. 2003;21:81–85. doi: 10.1038/nbt766. [DOI] [PubMed] [Google Scholar]
- 75.West G, Inzé D, Beemster GT. Cell cycle modulation in the response of the primary root of Arabidopsis to salt stress. Plant Physiology. 2004;135:1050–1058. doi: 10.1104/pp.104.040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang, Z. B. et al. Synergistic action of auxin and cytokinin mediates aluminum‐induced root growth inhibition in Arabidopsis. EMBO Reports, e201643806 (2017). [DOI] [PMC free article] [PubMed]
- 77.Sun P, Tian Q-Y, Chen J, Zhang W-H. Aluminium-induced inhibition of root elongation in Arabidopsis is mediated by ethylene and auxin. Journal of Experimental Botany. 2009;61:347–356. doi: 10.1093/jxb/erp306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yuan H-M, Xu H-H, Liu W-C, Lu Y-T. Copper regulates primary root elongation through PIN1-mediated auxin redistribution. Plant and Cell Physiology. 2013;54:766–778. doi: 10.1093/pcp/pct030. [DOI] [PubMed] [Google Scholar]
- 79.Gill T, Dogra V, Kumar S, Ahuja PS, Sreenivasulu Y. Protein dynamics during seed germination under copper stress in Arabidopsis over-expressing Potentilla superoxide dismutase. Journal of Plant Research. 2012;125:165–172. doi: 10.1007/s10265-011-0421-2. [DOI] [PubMed] [Google Scholar]
- 80.Andrés‐Colás N, et al. The Arabidopsis heavy metal P‐type ATPase HMA5 interacts with metallochaperones and functions in copper detoxification of roots. The Plant Journal. 2006;45:225–236. doi: 10.1111/j.1365-313X.2005.02601.x. [DOI] [PubMed] [Google Scholar]
- 81.Zhao C-R, et al. Comparative transcriptomic characterization of aluminum, sodium chloride, cadmium and copper rhizotoxicities in Arabidopsis thaliana. BMC Plant Biology. 2009;9:32. doi: 10.1186/1471-2229-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miwa K, Takano J, Fujiwara T. Improvement of seed yields under boron‐limiting conditions through overexpression of BOR1, a boron transporter for xylem loading, in Arabidopsis thaliana. The Plant Journal. 2006;46:1084–1091. doi: 10.1111/j.1365-313X.2006.02763.x. [DOI] [PubMed] [Google Scholar]
- 83.Singh BT, Wort D. Effect of vanadium on growth, chemical composition, and metabolic processes of mature sugar beet (Beta vulgaris L.) plants. Plant Physiology. 1969;44:1321–1327. doi: 10.1104/pp.44.9.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Deng W, et al. Overexpression of an Arabidopsis magnesium transport gene, AtMGT1, in Nicotiana benthamiana confers Al tolerance. Journal of Experimental Botany. 2006;57:4235–4243. doi: 10.1093/jxb/erl201. [DOI] [PubMed] [Google Scholar]
- 85.Sundaram S, Wu S, Ma LQ, Rathinasabapathi B. Expression of a Pteris vittata glutaredoxin PvGRX5 in transgenic Arabidopsis thaliana increases plant arsenic tolerance and decreases arsenic accumulation in the leaves. Plant, Cell & Environment. 2009;32:851–858. doi: 10.1111/j.1365-3040.2009.01963.x. [DOI] [PubMed] [Google Scholar]
- 86.Vanhoudt N, et al. Unraveling uranium induced oxidative stress related responses in Arabidopsis thaliana seedlings. Part I: responses in the roots. Journal of Environmental Radioactivity. 2011;102:630–637. doi: 10.1016/j.jenvrad.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 87.Shi H, Xiong L, Stevenson B, Lu T, Zhu J-K. The Arabidopsis salt overly sensitive 4 mutants uncover a critical role for vitamin B6 in plant salt tolerance. The Plant Cell. 2002;14:575–588. doi: 10.1105/tpc.010417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Strader LC, Beisner ER, Bartel B. Silver ions increase auxin efflux independently of effects on ethylene response. The Plant Cell. 2009;21:3585–3590. doi: 10.1105/tpc.108.065185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ruegger M, et al. The TIR1 protein of Arabidopsis functions in auxin response and is related to human SKP2 and yeast Grr1p. Genes & Development. 1998;12:198–207. doi: 10.1101/gad.12.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lincoln C, Britton JH, Estelle M. Growth and development of the axr1 mutants of Arabidopsis. The Plant Cell. 1990;2:1071–1080. doi: 10.1105/tpc.2.11.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guzman P, Ecker JR. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. The Plant Cell. 1990;2:513–523. doi: 10.1105/tpc.2.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Belfield EJ, et al. Genome-wide analysis of mutations in mutant lineages selected following fast-neutron irradiation mutagenesis of Arabidopsis thaliana. Genome Research. 2012;22:1306–1315. doi: 10.1101/gr.131474.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Colebrook EH, Thomas SG, Phillips AL, Hedden P. The role of gibberellin signalling in plant responses to abiotic stress. Journal of Experimental Biology. 2014;217:67–75. doi: 10.1242/jeb.089938. [DOI] [PubMed] [Google Scholar]
- 94.Riefler M, Novak O, Strnad M, Schmülling T. Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. The Plant Cell. 2006;18:40–54. doi: 10.1105/tpc.105.037796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Linkies A, Leubner-Metzger G. Beyond gibberellins and abscisic acid: how ethylene and jasmonates control seed germination. Plant Cell Reports. 2012;31:253–270. doi: 10.1007/s00299-011-1180-1. [DOI] [PubMed] [Google Scholar]
- 96.Sivritepe N. Organic priming with seaweed extract (Ascophyllum nodosum) affects viability of pepper seeds. Asian Journal of Chemistry. 2008;20:5689. [Google Scholar]
- 97.Kumari R, Kaur I, Bhatnagar A. Effect of aqueous extract of Sargassum johnstonii Setchell & Gardner on growth, yield and quality of Lycopersicon esculentum Mill. Journal of Applied Phycology. 2011;23:623–633. doi: 10.1007/s10811-011-9651-x. [DOI] [Google Scholar]
- 98.Zheng, S., Jiang, J., He, M., Zou, S. & Wang, C. Effect of Kelp Waste Extracts on the Growth and Development of Pakchoi (Brassica chinensis L.). Scientific Reports6 (2016). [DOI] [PMC free article] [PubMed]
- 99.Arnon DI, Johnson CM. Influence of hydrogen ion concentration on the growth of higher plants under controlled conditions. Plant Physiology. 1942;17:525. doi: 10.1104/pp.17.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Crouch I, Beckett R, Van Staden J. Effect of seaweed concentrate on the growth and mineral nutrition of nutrient-stressed lettuce. Journal of Applied Phycology. 1990;2:269–272. doi: 10.1007/BF02179784. [DOI] [Google Scholar]
- 101.Rayorath P, et al. Rapid bioassays to evaluate the plant growth promoting activity of Ascophyllum nodosum (L.) Le Jol. using a model plant, Arabidopsis thaliana (L.) Heynh. Journal of Applied Phycology. 2008;20:423–429. doi: 10.1007/s10811-007-9280-6. [DOI] [Google Scholar]
- 102.Smeekens S, Ma J, Hanson J, Rolland F. Sugar signals and molecular networks controlling plant growth. Current Opinion in Plant Biology. 2010;13:273–278. doi: 10.1016/j.pbi.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 103.Blunden G, Cripps A, Gordon S, Mason T, Turner C. The characterisation and quantitative estimation of betaines in commercial seaweed extracts. Botanica Marina. 1986;29:155–160. doi: 10.1515/botm.1986.29.2.155. [DOI] [Google Scholar]
- 104.Tay S, Palni L, MacLeod J. Identification of cytokinin glucosides in a seaweed extract. Journal of Plant Growth Regulation. 1987;5:133–138. doi: 10.1007/BF02087181. [DOI] [Google Scholar]
- 105.Crouch I, Van Staden J. Effect of seaweed concentrate on the establishment and yield of greenhouse tomato plants. Journal of Applied Phycology. 1992;4:291–296. doi: 10.1007/BF02185785. [DOI] [Google Scholar]
- 106.Stirk W, Van Staden J. Isolation and identification of cytokinins in a new commercial seaweed product made from Fucus serratus L. Journal of Applied Phycology. 1997;9:327–330. doi: 10.1023/A:1007910110045. [DOI] [Google Scholar]
- 107.Tietz A, Ruttkowski U, Kohler R, Kasprik W. Further investigations on the occurrence and the effects of abscisic acid in algae. Biochemie und Physiologie der Pflanzen. 1989;184:259–266. doi: 10.1016/S0015-3796(89)80011-3. [DOI] [Google Scholar]
- 108.Moore, K. K. Using Seaweed Compost To Grow Bedding Plants. Bio Cycle45 (2004).
- 109.Panda SK, Baluška F, Matsumoto H. Aluminum stress signaling in plants. Plant Signaling & Behavior. 2009;4:592–597. doi: 10.4161/psb.4.7.8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang J, et al. The Role of the Plasma Membrane H+-ATPase in Plant Responses to Aluminum Toxicity. Frontiers in Plant Science. 2017;8:1757. doi: 10.3389/fpls.2017.01757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hong DD, Hien HM, Son PN. Seaweeds from Vietnam used for functional food, medicine and biofertilizer. Journal of Applied Phycology. 2007;19:817–826. doi: 10.1007/s10811-007-9228-x. [DOI] [Google Scholar]
- 112.Rayirath P, et al. Lipophilic components of the brown seaweed, Ascophyllum nodosum, enhance freezing tolerance in Arabidopsis thaliana. Planta. 2009;230:135–147. doi: 10.1007/s00425-009-0920-8. [DOI] [PubMed] [Google Scholar]
- 113.Almodares A, Hadi M, Dosti B. Effects of salt stress on germination percentage and seedling growth in sweet sorghum cultivars. Journal of Biological Sciences. 2007;7:1492–1495. doi: 10.3923/jbs.2007.1492.1495. [DOI] [Google Scholar]
- 114.Yan, J. Influence of plant growth regulators on turfgrass polar lipid composition, tolerance to drought and salinity stresses, and nutrient efficiency, (PhD Thesis, Virginia Tech, 1993).
- 115.Latef AAHA, Srivastava AK, Saber H, Alwaleed EA, Tran L-SP. Sargassum muticum and Jania rubens regulate amino acid metabolism to improve growth and alleviate salinity in chickpea. Scientific Reports. 2017;7:10537. doi: 10.1038/s41598-017-07692-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sridhar, S. & Rengasamy, R. Significance of seaweed liquid fertilizers for minimizing chemical fertilizers and improving yield of Arachis hypogaea under field trial. Recent Research in Science and Technology2 (2010).
- 117.Walz, E. Final results of the third biennial national organic farmers’ survey (1999).
- 118.Pretty J. Agricultural sustainability: concepts, principles and evidence. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2008;363:447–465. doi: 10.1098/rstb.2007.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Thomas C, et al. Root morphology and seed and leaf ionomic traits in a Brassica napus L. diversity panel show wide phenotypic variation and are characteristic of crop habit. BMC Plant Biology. 2016;16:214. doi: 10.1186/s12870-016-0902-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Moody LA, et al. An ancient and conserved function for Armadillo‐related proteins in the control of spore and seed germination by abscisic acid. New Phytologist. 2016;211:940–951. doi: 10.1111/nph.13938. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.