Abstract
Anurans in Peninsular India exhibit close biogeographical links with Gondwana as well as Laurasia, often explainable by the geological history of the Indian subcontinent; its breakup from Gondwanan landmasses followed by long isolation that resulted in diversification of endemic lineages, and subsequent land connections with Asia that enabled dispersal of widespread groups. Although widely distributed, the frog subfamily Microhylinae mostly comprises of geographically restricted genera found either in Southeast and East Asia or Peninsular India and Sri Lanka. Here we report a previously unknown microhylid from the Western Ghats in Peninsular India with closest relatives found over 2,000 km away in Southeast Asia. Based on integrated evidence from mitochondrial and nuclear DNA, adult and tadpole morphology, hand musculature, male advertisement call, and geographical distance, we recognize this enigmatic frog as a distinct new species and genus endemic to the Western Ghats. The discovery of Mysticellus franki gen. et sp. nov. and its close evolutionary relationship with the Southeast Asian genus Micryletta also provide insights on the biogeography of Microhylinae. Genus-level divergences within the subfamily suggest multiple Cenozoic biotic exchange events between India and Eurasia, particularly through postulated Eocene land bridges via Southeast Asia prior to accretion of the two landmasses.
Subject terms: Biodiversity, Biogeography, Phylogenetics, Taxonomy, Herpetology
Introduction
The Western Ghats is a chain of mountains that stretches over 1,600 km in southwest India. It is part of a global biodiversity hotspot with remarkable amphibian diversity and endemism1. This region is also considered as a distinct biogeographical unit, a recognition chiefly garnered due to the role of various events in the geological history of the Indian subcontinent2–13. After the initial break-up of Madagascar-Seychelles-India from Africa and Australia-Antarctica blocks (∼140–130 Mya), followed by separation of the Indian subcontinent from Madagascar (∼88 Mya) and subsequently the Seychelles (∼65 Mya), the northward-drifting Indian plate is considered to have been a “biotic ferry”14 during its course to unite with the Eurasian plate (starting ∼55 Mya)15,16. The biotic elements that moved along with the erstwhile Indian landmass during the Late Cretaceous or Palaeogene experienced long periods of isolation resulting in the origin of several endemic anuran lineages in the Western Ghats11,15. The biogeographical links of ancient frog lineages such as Nasikabatrachidae have provided crucial evidence for tracing back the evolutionary history of present day amphibians in the Western Ghats10.
While the isolation of the Indian subcontinent is regarded as a unique event, the union of the Indian plate with Eurasia is an equally intriguing juncture that provided opportunity for faunal exchanges between these landmasses4,17,18. Despite the constantly changing landscape and climatic conditions that created physical barriers for the movement of anurans19,20, several recent radiations (such as dicroglossids, microhylids and rhacophorids) acquired wider distribution patterns in India as well as South and Southeast Asia. In this context, the Northeast Indian region is often considered as a ‘gateway’21,22 bridging the Indian subcontinent with the rest of Asia, particularly before the rise of the Himalaya and upliftment of the Tibetan plateau. These present day physical features are known to have played a significant role in determining the distribution patterns of anurans in South and Southeast Asia23–25. Currently, four biogeographical regions encompassing the Indian subcontinent—Himalaya, Indo-Burma, Sundaland and Western Ghats-Sri Lanka—are recognized as global biodiversity hotspots26.
The Narrow-mouthed anuran family Microhylidae is distributed throughout the tropics and currently comprises of 653 species in 53 genera and 13 subfamilies27. Although higher-level taxonomic placement of the recognized subfamilies and their phylogenetic relationships are still disputed28–33, based on the more widely used classification by Frost et al.29 as revised by Peloso et al.33, five microhylid subfamilies are found in Asia (Asterophryinae, Chaperininae, Kalophryninae, Melanobatrachinae and Microhylinae), of which only two are known to occur in the Indian peninsula (Melanobatrachinae and Microhylinae). Melanobatrachinae is monotypic with a single known species and restricted to the southern Western Ghats of India. On the other hand, Microhylinae is widely distributed in South, Southeast and East Asia. It is a large radiation comprising of 87 species in seven genera27,34. Phylogenetically, Microhylinae is sister to Dyscophinae, and the split between the two subfamilies has been a significant point of interest for biogeographical interpretations, largely due to the congruence of geological events such as the break-up of India–Seychelles–Madagascar landmass during the Late Cretaceous12,13 with the restricted geographical distribution of members of these subfamilies to Asia and Madagascar, respectively. Phylogenetic relationships among members of the subfamily Microhylinae have been discussed in several studies12,29,31,33,35. A few have also provided insights on origin, possible routes of colonization, and patterns of distribution, however, in larger contexts of either global or selected Asian microhylids12,13,36,37. Hence, a detailed phylogenetic investigation and divergence ages within the subfamily Microhylinae are still lacking.
Our recent explorations in Peninsular India have led us to the discovery of a previously unknown microhylid frog that could not be assigned to any of the known microhylid members of this region. Evidence from multiple approaches concordantly suggests the newly discovered microhylid to be a distinct species, which also warrants recognition of a new genus within the subfamily Microhylinae. This novel evolutionary lineage is endemic to the Western Ghats, with its closest known relatives found over 2,000 km (as the crow flies) in the Indo-Burma and Sundaland hotspots. Here, we provide a formal description of Mysticellus franki gen. et sp. nov. and discuss new phylogenetic insights highlighted through this finding, along with their biogeographical implications.
Results
Amphibia Linnaeus, 1758
Anura Fischer von Waldheim, 1813
Microhylidae Günther, 1858 (1843)
Microhylinae Günther, 1858 (1843)
Mysticellus gen. nov
urn:lsid:zoobank.org:act:6145F26C-E140-4BBF-9322-550047926EA2
(Figures 1–4; Supplementary Tables S1–S4).
Figure 1.
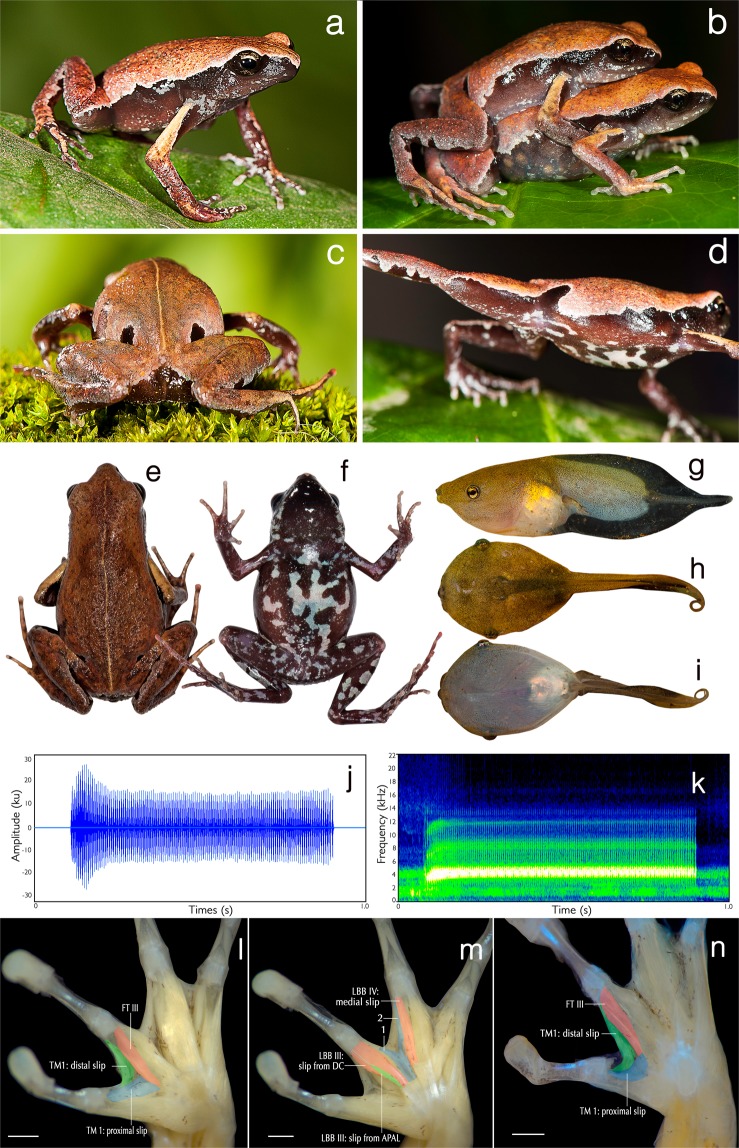
Diagnostic characteristics of Mysticellus franki gen. et sp. nov. (a–f) Adult in life. (a) Holotype (ZSI/WGRC/V/A/966, male) in dorsolateral view; (b) holotype (male) and paratype (ZSI/WGRC/V/A/971, female) in amplexus; (c) two ‘false-eye’ like spots on the back; (d) lateral markings; (e) dorsal view; (f) ventral view. (g–i) Tadpole in life. (g) Lateral view; (h) dorsal view; (i) ventral view. (j–k) Male advertisement call. (j) One second call segment showing pulsatile temporal structure; (k) spectrogram of one second call segment. (l–n) Hand musculature. (l–m) Palmar view of Mysticellus franki gen. et sp. nov. (SDBDU 2015.2870, left hand). (l) Flexor teres digiti III (FT III) passing ventrally to both slips of m. transversus metacarpus 1 (TM 1); (m) two previously unreported accessory flexor muscles on digiti III and IV (labeled as “1” and “2” respectively); (n) palmar view of Micryletta inornata (KU 328192, left hand) showing FT III dorsal to the proximal slip of TM 1 and ventral to the distal slip. Abbreviations: TM I: m. transversus metacarpus I, FT III: m. flexor teres digiti III, LBB III–IV: m. lumbricalis brevis digiti III–IV. Scale bars = 0.5 mm.
Figure 4.
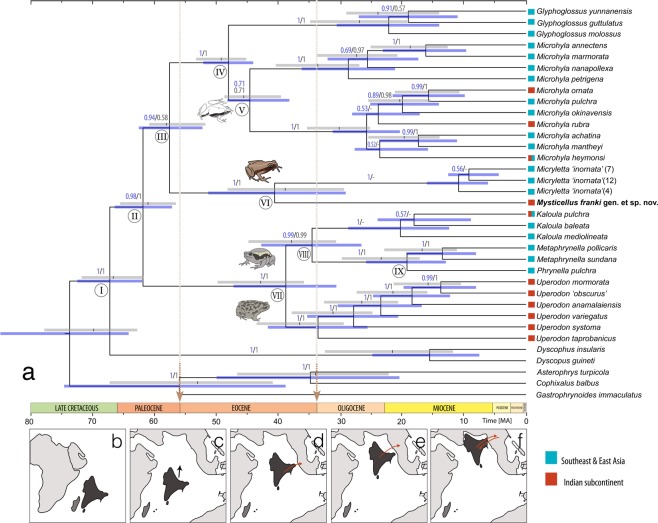
Estimated divergence ages in the subfamily Microhylinae and coinciding dispersal events. (a) Time-calibrated phylogeny from BEAST analysis using the Best-fit models of evolution. Node numbers are referenced in Table 1; node support values > 0.50 are provided above the branches (analysis A followed by B); 95% HPD intervals of the inferred age estimates are represented by blue (analysis A) and grey (analysis B) bars; geographical distribution of species are indicated alongside taxon labels. (b–f) Cenozoic position of the Indian subcontinent and postulated biotic exchange events between India and Eurasia. (b) Separated India and Madagascar landmasses (K/Pg boundary); (c) isolation of the northward drifting Indian subcontinent (Paleocene); (d) first Eocene land bridge between India and Southeast Asia through Sumatra; (e) second Eocene land bridge between India and mainland Southeast Asia through Myanmar-Malay Peninsula; (f) final accretion of India and Eurasia (Oligocene/Miocene). Suggestive paleomaps are based on Bossuyt and Milinkovitch8, Klaus et al.52 and Grismer et al.53.
Type species
Mysticellus franki sp. nov.
Etymology
The genus name, Mysticellus, is a masculine noun derived from the Latin mysticus (meaning mysterious) + ellus (a diminuitive), highlighting the ability of this small frog to remain out of sight despite its occurrence in wayside areas surrounding human settlements.
Suggested common name
Mysterious Narrow-mouthed Frog.
Diagnosis
The new genus Mysticellus differs from other Microhylinae genera by the combination of following characters: small adult snout-vent size (male SVL 23.0–27.5 mm, N = 5; female SVL 27.0–28.9 mm, N = 2), slender body; snout longer than eye length, male SL 2.8–3.0 mm, 2.9 ± 0.1 mm, N = 5, female SL 3.0–3.2 mm, 3.1 ± 0.1 mm, N = 2 vs. male EL 2.4–2.6 mm, 2.5 ± 0.1 mm, N = 5; female EL 2.5–2.7 mm, 2.6 ± 0.1 mm, N = 2; absence of maxillary and vomerine teeth; finger and toe tips rounded, with small discs; presence of well-developed subarticular tubercles on all fingers and toes, rounded, alternating with additional smaller tubercles; prominent inner metatarsal tubercle and a small outer metatarsal tubercle on foot; webbing between fingers absent; rudimentary webbing between toes; lateral surfaces from tip of the snout up to the groin prominently dark blackish-brown; two prominent dark blackish-brown ‘false-eye’ like spots on either side of the groin extending just above the hind legs; a thin mid-dorsal line invariably extending from tip of the snout up to the vent; ventral surfaces of throat, belly, arms and legs dark brown with a violet tinge and various sized greyish-white blotches and speckles. This new genus can also be distinguished from other members of the subfamily by its hand musculature in having the m. flexor teres digiti III ventral to both slips of the m. transversus metacarpus 138; and the dorsal surface with two previously unreported flexor muscles on digits III and IV, one lateral to the m. lumbricalis brevis digiti III, and the other medial to the medial slip of the m. lumbricalis brevis digiti IV (Fig. 1).
Morphological comparison
The new genus Mysticellus differs from Glyphoglossus Günther, 1869 by its slender body (vs. robust), absence of maxillary and vomerine teeth (vs. present), horizontal pupil (vs. vertical), and well-developed tubercles on the hand (vs. not enlarged); differs from Kaloula Gray, 1831 by its smaller adult snout-vent size, SVL < 30 mm (vs. SVL > 30 mm), slender body (vs. robust), absence of ridge on posterior sides of choanae (vs. present), supratympanic fold absent (vs. present), finger and toe tips slightly enlarged (vs. enlarged with prominent discs), and inner metatarsal tubercle not enlarged (vs. enlarged and spatulate); differs from Metaphrynella Parker, 1934 by absence of ridge on posterior sides of choanae (vs. present), finger and toe tips slightly enlarged (vs. enlarged with prominent discs), webbing absent between fingers (vs. present), terrestrial habitat (vs. predominantly arboreal), and behavior of breeding around temporary water puddles (vs. tree holes); differs from Microhyla Tschudi, 1838 by its body relatively linear in shape (vs. triangular), lateral surfaces of the body prominently dark blackish-brown from tip of the snout up to the lower abdomen, tapering close to the groin and extending towards the dorsal surface just above the hind legs in the form of two prominent blackish-brown ‘false-eye’ like spots on either side (vs. dark colouration absent or discontinuous and ‘false-eye’ like spots absent), and prominent subarticular tubercles alternating with additional smaller tubercles (vs. absence of additional smaller tubercles); differs from Micryletta Dubois, 1987 by its tympanum externally indistinct (vs. distinct), and lateral surfaces of the body prominently dark blackish-brown from tip of the snout up to the lower abdomen, tapering close to the groin and extending towards the dorsal surface just above the hind legs in the form of two prominent blackish-brown ‘false-eye’ like spots on either side (vs. dark colouration absent or discontinuous and ‘false-eye’ like spots absent); differs from Phrynella Boulenger, 1887 by its finger and toe tips slightly enlarged (vs. enlarged with prominent discs), metatarsal tubercles separate (vs. united), and rudimentary webbing between toes (vs. nearly fully webbed); and differs from Uperodon Duméril and Bibron, 1841 by its slender body (vs. robust and globular), absence of ridge on posterior sides of choanae (vs. present), prominent subarticular tubercles alternating with additional smaller tubercles (vs. absence of additional smaller tubercles), lateral surfaces of the body prominently dark blackish-brown from tip of the snout up to the lower abdomen, tapering close to the groin and extending towards the dorsal surface just above the hind legs in the form of two prominent blackish-brown ‘false-eye’ like spots on either side (vs. absent), finger and toe tips slightly enlarged (vs. enlarged with prominent discs except in U. systoma and U. globulosus), inner metatarsal tubercle not enlarged (vs. enlarged or spatulate), and rudimentary webbing between toes (vs. prominent webbing, except in U. systoma).
Contents
The new genus currently contains a single species Mysticellus franki sp. nov.
Mysticellus franki sp. nov
urn:lsid:zoobank.org:act:B1FBD56B-B6B3-412A-8988-E527A082C4C5
(Figures 1–4; Supplementary Figs S1–S6; Supplementary Tables S1–S4).
Etymology
The species name, franki, is a Latin genitive honoring evolutionary biologist Prof Franky Bossuyt (Vrije Universiteit Brussel), recognizing his role in global amphibian research and education, and particularly for his contribution to the study of Indian amphibians.
Suggested common name
Franky’s Narrow-mouthed Frog.
Holotype
ZSI/WGRC/V/A/966, an adult male, SVL 23.0 mm, from Suganthagiri (11°32′19″ N 76°3′14″ E, 852 m asl), Wayanad district, Kerala state, India, collected by Sonali Garg and SD Biju on 05 June 2015.
Paratypes
ZSI/WGRC/V/A/967–970, four adult males, and ZSI/WGRC/V/A/971–972, two adult females, collected along with the holotype.
Description of holotype (measurements in mm)
Small adult male (SVL 23.0), rather slender; head wider than long (HW 7.4, HL 6.3), flat above; snout nearly truncate in dorsal and ventral view, acute in lateral view, snout length (SL 2.9) longer than horizontal diameter of eye (EL 2.4); loreal region acute, rounded canthus rostralis; interorbital space flat, twice (IUE 3.2) as wide as upper eyelid (UEW 1.6) and internarial distance (IN 1.6); nostril oval, closer to snout (NS 0.7) than eye (EN 1.3); pupil oval; vomerine ridge absent; tongue small, not emarginated, spatulate, bearing no median lingual process. Arms long, forearm length (FAL 5.5) shorter than hand length (HAL 7.0); relative length of fingers I < IV < II < III (FLI 2.5, FLII 3.1, FLIII 4.8, FLIV 2.7); finger tips rounded, slightly enlarged with small discs, fingers without dermal fringes, webbing absent between fingers; subarticular tubercles prominent, rounded, all present, alternating with additional smaller tubercles; three prominent palmar tubercles, middle tubercle rounded (PTL 0.5), outer tubercle oval (OPTL 0.7), inner tubercle rounded (IPTL 0.4); supernumerary tubercles present. Hind limbs long, thigh (THL 10.3) slightly shorter than shank (SHL 10.5) and shorter than foot (FOL 11.7); distance from the base of tarsus to the tip of toe IV (TFOL 17.0); toes long, relative length of toes I < V < II < III < IV; toe tips rounded, slightly enlarged with small discs, toes with weakly developed dermal fringes, rudimentary webbing between toes; subarticular tubercles prominent, oval, all present, alternating with additional smaller tubercles; inner metatarsal tubercle prominent (IMTL 0.8), oval; outer metatarsal tubercle small (OMTL 0.4), rounded; supernumerary tubercles present on toes (Fig. 1a–f; Supplementary Fig. S1).
Skin of snout and upper eyelids shagreened with scattered granular projections; anterior and posterior parts of back, and upper and lower parts of flank shagreened with more prominent granular projections compared to the snout region; dorsal surfaces of forelimb, thigh and shank shagreened with granular projections; skin surrounding the anal region prominently granular. Ventral surface of throat finely granular; chest, belly and limbs smooth with scattered granular projections (Fig. 1a–f; Supplementary Fig. S1).
Colour of holotype in life
Dorsum brick red to reddish-brown; a thin mid-dorsal line extending from the tip of snout up to the vent; lateral surfaces prominently dark blackish-brown from tip of the snout up to the lower abdomen, tapering close to the groin and extending towards the dorsal surface just above the hind legs in the form of two prominent blackish-brown ‘false-eye’ like spots on either side; dorsal surface of forearm light brown and hand (including fingers) reddish-brown; dorsal surface of hind limbs (including toes) brick red to reddish-brown with faint greyish-brown transverse bands; posterior parts of thigh light brown, shank and tarsus reddish-brown. Ventral surfaces of throat, belly, fore- and hind limbs dark brown with a violet tinge and various sized greyish-white blotches and speckles (Fig. 1a–f).
Diagnosis
As for Mysticellus gen. nov.
Ecology and Behavior
A large number of animals usually aggregate around temporary water collection sites about two to three days after the first monsoon showers. Individuals were collected from grass adjacent to water puddles in a small wayside quarry (about 25 m2 area). The specific site was located close to a secondary forest. Breeding activities were observed only for four to five days, after which the animals disappeared and no individuals could be located despite repeated visits. Tadpoles (stage 34) were observed at the same site towards the end of July (Fig. 1g–i). Calling males exhibited a peculiar behavior of raising the hind part of their body displaying a pair of black ‘false-eye’ like spots. On some occasions, similar behavior was observed when individuals were disturbed, suggesting that the ‘false-eye’ spots may be serving a defensive role against predators (Fig. 1c).
Vocalization
Males of Mysticellus franki sp. nov. produce a single type of call with pulsatile temporal structure. Calls have uniform intervals and are not delivered in groups. Characteristics of a single male call (ZSI/WGRC/V/A/966) are as follows: call duration, 1771.4 ms; call rise time, 91.4 ms; call fall time, 1681.9 ms; number of pulses, 141; pulse rate, 80.2 pulses per second; call spectrum with a single broad peak and mean dominant frequency of 3.7 kHz (Fig. 1j–k; Supplementary Fig. S5).
Note
For colour description of holotype in preservation, secondary sexual characters, variations, hand musculature, and description of tadpole, see additional taxonomic description in the electronic supplementary material.
Distribution
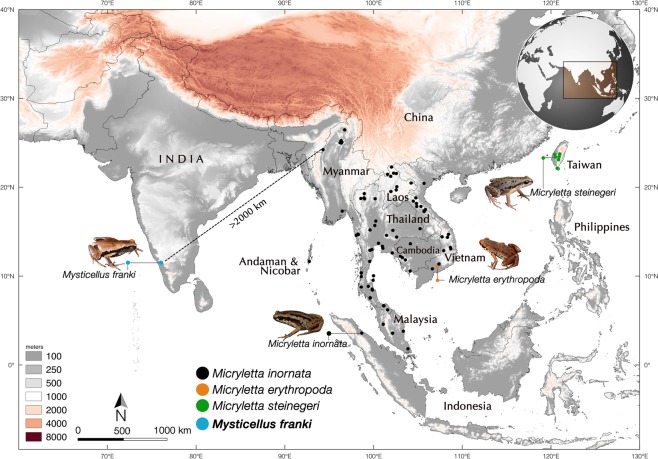
Mysticellus franki gen. et. sp. nov. is presently known only from the type locality in southern Western Ghats region of Kerala, Peninsular India (Fig. 2).
Figure 2.
Distribution of Mysticellus franki gen. et sp. nov. and its closest generic relative Micryletta. Map prepared using QGIS 2.6.1 (http://www.qgis.org). Image credits: Micryletta inornata (M.A.M.M. Akil), Micryletta erythropoda (J. Rowley) and Micryletta steinegeri (N.A. Poyarkov Jr.).
Phylogenetic relationship
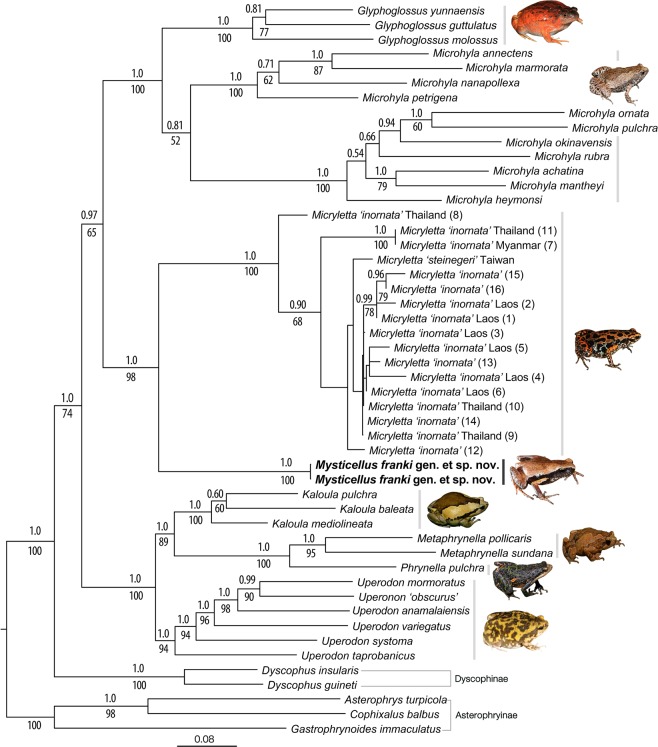
The ML and Bayesian phylogenies showed similar topologies and relationships were congruent with previous studies. Microhylinae was recovered as the sister group of Dyscophinae, with three members of Asterophryinae forming the outgroup clade. Within Microhylinae, all the known genera were recovered as well-supported clades (BPP > 0.95, BS > 80), except Microhyla for which monophyly was weakly supported (Fig. 3). Our phylogenetic analyses concordantly recovered Mysticellus franki gen. et sp. nov. (Western Ghats) as a distinct and well-supported sister lineage to genus Micryletta (Indo-Burma + Sundaland). The new lineage was also well differentiated in independent analyses of nuclear and mitochondrial gene datasets. Pairwise distances for the mitochondrial 16S rRNA gene sequences showed considerable genetic differentiation (minimum 8.2%) between the new species and members of its closest generic relative Micryletta. This was also concordant with genetic divergences observed among various known genera in the subfamily Microhylinae (Fig. 3; Supplementary Fig. S6; Supplementary Tables S1–S3). Hence, based on presented evidence, Mysticellus gen. nov. is phylogenetically defined as the most inclusive clade comprising Mysticellus franki gen. et sp. nov., but not members of the genus Micryletta.
Figure 3.
Maximum likelihood phylogram showing the phylogenetic position of Mysticellus franki gen. et sp. nov. in the subfamily Microhylinae. Bayesian Posterior Probabilities (BPP) and RAxML Bootstrap support values (BS) are shown above and below the branches, respectively. Image credits: Glyphoglossus (B. Tapley), Metaphrynella (A. Figueroa), Micryletta (N.A. Poyarkov Jr.) and Phrynella (D. Bickford).
Divergence time
Based on dating estimations, Mysticellus gen. nov. diverged from its sister lineage Micryletta ~39.7 (40.6–38.8) Mya during the Eocene (Fig. 4; Table 1). These age estimates based on two different sets of substitution models were within the 95% confidence interval (CI) values derived from both the analyses. Considering the timings of continental drift during the Cenozoic, the split between these two genera is likely to have occurred as the Indian landmass established close proximity with mainland Southeast Asia through Myanmar-Malay Peninsula during Middle/Late Eocene (Fig. 4e). As evident from age estimates observed in subfamily Microhylinae, most genus-level clades were established during the Eocene, suggesting land bridge connections between India and Eurasia well before the final accretion of these landmasses (Fig. 4d–e). On the other hand, the Oligocene epoch largely witnessed diversification within genus-level endemic radiations, possibly due to isolation. Subsequently, upon India’s final collision and accretion with Eurasia during the Miocene, diversification and exchanges only at the species-level are likely to have been prevalent between India and the rest of Asia (Fig. 4f).
Table 1.
Estimated divergence ages of major microhyline lineages.
| Node | Divergence event | Estimated age ± SD (95% HPD confidence interval) | ||
|---|---|---|---|---|
| Best-fit models (Analysis A) | HKY model (Analysis B) | Average age | ||
| I | Split of Microhylinae from Dyscophinae | 67.3 (62.1–72.6) | 66.6 (61.6–71.6) | 67.0 (K-Pg boundary) |
| II^ | Spilt between (Glyphoglossus + Microhyla + Micryletta + Mysticellus) and (Kaloula + Metaphrynella + Phrynella + Uperodon) | 61.9 (57.2–66.5) | 61.0 (56.6–65.5) | 61.5 (Paleocene) |
| III^ | Spilt between (Glyphoglossus + Microhyla) and (Micryletta + Mysticellus) | 57.6 (52.3–62.6) | 58.0 (51.7–60.9) | 57.8 (Paleocene/Eocene) |
| IV* | Spilt between Glyphoglossus and Microhyla | 48.1 (44.1–52.2) | 49.2 (45.1–53.2) | 48.7 (Eocene) |
| V* | Spilt between two major Microhyla clades | 44.6 (38.2–48.3) | 45.5 (39.6–48.7) | 45.1 (Eocene) |
| VI ** | Split between Micryletta and Mysticellus | 40.6 (29.1–51.4) | 38.8 (29.5–48.1) | 39.7 (Eocene) |
| VII** | Split between Uperodon and (Kaloula + Metaphrynella + Phrynella) | 38.8 (30.6–47.3) | 42.7 (35.8–49.8) | 40.8 (Eocene/Oligocene) |
| VIII^ | Split between Kaloula and (Metaphrynella + Phrynella) | 34.6 (26.6–42.8) | 37.8 (30.8–44.8) | 36.2 (Eocene/Oligocene) |
| IX^ | Split between Metaphrynella and Phrynella | 19.2 (12.9–25.8) | 23.3 (17.1–29.9) | 21.3 (Oligocene/Miocene) |
Ages are in Million years; HPD = highest posterior density. Best-fit models are provided in Supplementary Table S4. *Denotes first postulated Miocene exchange; **Denotes second postulated Miocene exchange; ^Denotes diversification events associated with periods of isolation.
Discussion
Phylogenetic and geographical relationship of the new genus
The discovery of a novel microhylid genus from the Western Ghats reemphasizes the importance of this region as a reservoir of several evolutionarily significant anuran lineages. It also indicates that extensive explorations can still result in identification of unknown taxa, which is necessary before a comprehensive understanding of existing diversity, their systematic relationships and patterns of biogeographical distribution can be achieved. The new frog Mysticellus franki gen. et sp. nov. is most closely related to the Southeast Asian genus Micryletta, which comprises of three recognized species and is largely endemic to Southeast Asia (namely Cambodia, Indonesia, Laos, Malaysia, Myanmar, Thailand, and Vietnam) and China, including Taiwan27. Even though molecular data have supported monophyly of Micryletta, the phylogenetic position of this clade has remained confusing13,29,31–33,35–37. In our phylogenetic analyses (Fig. 3), Micryletta shows a sister relationship with Mysticellus gen. nov. Together, these two genera form a clade that is sister to the group containing Microhyla + Glyphoglossus, which is largely consistent with the findings of Van der Meijden et al.36 and Peloso et al.33. Based on the current taxonomic arrangements in the family, we excluded Chaperina from our Microhylinae dataset33. However upon inclusion, Chaperina was found sister to the group containing Microhyla + Glyphoglossus in agreement with Kurabayashi et al.13 and Pyron and Wiens32, and the Micryletta + Mysticellus gen. nov. clade consistently occupied a basal position to these groups. Previous studies have also reported the presence of genus Micryletta in Andaman and Nicobar islands and Manipur in Northeast India39,40. However, based on close geographical proximity, the populations from Andaman and Nicobar islands are likely to be similar to Micryletta inornata originally described from the Indonesian island of Sumatra, whereas those from Manipur could be related to members in the adjacent regions of mainland Southeast Asia. Further molecular and morphological confirmation will be necessary to ascertain the identity of these records. Yet, despite the ambiguity in species-level identification of several currently known Micryletta populations, their distribution pattern suggests that the genus is largely restricted to the Indo-Burma and Sundaland biodiversity hotspots, with its range extending up to East Asia. On the other hand, its sister group Mysticellus franki gen. et sp. nov. is a distinct lineage found in Peninsular India.
Colonization of subfamily Microhylinae
The Southeast Asian affinity of the new frog also provides interesting biogeographical insights. The family Microhylidae originated on Gondwanaland and acquired wide distribution on most continents including Africa, Asia, Australia, and North and South America12,23,36,41. Colonization and diversification of Microhylidae remains a complex subject that requires understanding of all possible dispersal routes, vicariance events, as well as major geological and climatological changes ever since the Early Cretaceous, as various landmasses of Gondwanan origin drifted towards their present day continental positions12,13,36. The subfamily Microhylinae, however, originated during the Late Cretaceous and is restricted to South, Southeast and East Asia8,12,13,17,36. Ancestors of Microhylinae (Asia) split from Dyscophinae (Madagascar), an event that coincides with the separation between Madagascar and Indian subcontinent, subsequent to which the latter moved northwards (Fig. 4b,c)8,12,36. The long isolation of the Indian subcontinent during the Late Cretaceous (Fig. 4c) led to the origin and diversification of several microhylid lineages, many of which probably remained restricted to the southern peninsular region by the Deccan traps that erupted around the Cretaceous-Palaeogene (K/Pg) boundary8,11,12, and later dispersed to Asia through various terrestrial connections.
In general, the dispersal route that formed following the collision of India with Asia has often been considered instrumental in faunal exchange between these landmasses4,5,7,9,42–45, particularly for colonization of anuran groups of Late Cretaceous Gondwanan origin from India to Asia8,11–13,25. Although the same may be regarded as the most parsimonious explanation for colonization of Microhylinae into Asia, a single dispersal event is less likely to have resulted in the origin of multiple microhyline lineages with Indo-Southeast Asian affinity since the peninsular elements remained isolated by the massive Deccan traps for a long duration after the K/Pg boundary8,11. If microhylids are believed to have been more widespread on the Indian subcontinent prior to the Deccan Traps, especially in the northern regions, even then their colonization solely through the Himalaya-Tibetan Plateau-Northeast Indian route would have been difficult during Oligocene–Miocene because of the enormous geological processes (subduction vs. orogeny), topographical changes (connection vs. isolation) and climatic fluctuations (favorable vs. unfavorable) that resulted after India’s collision4,6,8,19,24,25,46,47. These massive changes had important consequences to the movement of amphibians due to their limited dispersal abilities24,48.
In an alternate scenario, as the Indian subcontinent drifted closer towards Asia during the Palaeogene, it is postulated to have made brief land connections with insular Southeast Asia and mainland Southeast Asia, opening dispersal opportunities early during the Eocene49–51. Our discovery of a new microhylid genus from the Western Ghats with closest links in Southeast Asia provides evidence for at least one or more dispersal events between India and Southeast Asia (Figs 3 and 4), well before the Tibetan-Himalayan route became available (Fig. 4d–f). Most microhylines are restricted either to Peninsular India and Sri Lanka, or Indo-Burma and Sundaland, suggestive of early land connections between these regions followed by isolation, as India drifted closer towards Eurasia. The most recent common ancestor (MRCA) of Glyphoglossus + Microhyla (~48.7 Mya) and the MRCA of two large radiations of Microhyla seem to have utilized Early Eocene land bridges (~45.1 Mya) to colonize Southeast Asia through direct land connection with Sundaic regions such as Sumatra (believed to have been a single landmass). Whereas, MRCAs of microhyline radiations such as Uperodon + (Kaloula + Metaphrynella + Phrynella) (~40.8 Mya) and Micryletta + Mysticellus (~39.7 Mya) possibly dispersed through a second land bridge with Myanmar–Malay Peninsula later during the Eocene25,52,53 (Fig. 4; Table 1). Since the exact path and timing of landmass movement remains debatable49,51,54, such connections could have either been multiple events intermitted with brief isolation periods53, or the Indian subcontinent could have followed a counter clockwise trajectory along the western boundary of Southeast Asia (Sumatra–Myanmar–Malay Peninsula) forming more prolonged connections [e.g., ref.52 and references therein]. The subsequent period starting around Oligocene is largely represented by diversification within the genus-level endemic radiations (Fig. 4), suggesting periods of geographical isolation apart from the continued separation of Indian Peninsula by the Deccan traps, until India’s hard collision with Asia around Miocene. Several widespread microhylid species, particularly those with distributions north of the Deccan traps (such as Microhyla spp), could have then utilized the newly created dispersal opportunities to move into Asia. At the same time, Southeast Asian taxa in groups such as Microhyla, Micryletta and Kaloula probably also recolonized parts of Northeast India through these connections (Fig. 4). Such exchanges between India and Southeast Asia would have continued after the closure of Tethys sea, until the upliftment of Himalaya and Tibetan plateau, and embayment in Assam and Myanmar regions during the Miocene8. The latter geotectonic features once again restricted faunal exchange between the Indian subcontinent and neighboring regions24,46, probably accounting for the present day species-level endemism patterns observed in several Indo-Asian microhyline groups.
Multiple faunal exchanges between India and Asia
Phylogenetic evidence from the study of Microhylinae is suggestive of multiple dispersal events between India and Asia through postulated land bridges right from Eocene up to Miocene. While the more widely discussed single Miocene exchange after India’s final accretion with Asia explains the affinities between Indian and Southeast Asian faunal elements, it does not provide a convincing justification for early genus-level divergences and the parallel occurrence of endemism as well as widespread distribution patterns observed in Microhylinae. Hence, a trichotomy of (1) Eocene land bridges between India and Southeast Asia prior to the final Indo-Asia accretion, also termed as the “Eocene exchange hypothesis”53, (2) prolonged isolation of Indian Peninsula by the Deccan traps (since the K/Pg boundary) along with intermitted periods of isolation during Eocene–Oligocene, and (3) limited Miocene exchange between northern Indian regions (presently comprising of Himalaya–Tibetan plateau and Northeast India) and Asia due to complex geological and paleoclimatological events associated with Indo-Asia collision—explains early colonization of certain faunal groups from India to Southeast Asia, as well as the observed patterns of regional endemism and widespread distributions. Future research would benefit from wider taxon sampling across faunal groups with Indo-Southeast Asian affinities, and a better understanding of the exact timings and positions of land bridges, which remain debatable till date.
Methods
Field surveys and sampling
Field surveys were conducted during the monsoon season in the months of June and July (2015). Sampled individuals were photographed and euthanized in aqueous solution of Tricaine methanesulfonate (MS-222). Adults were fixed in 4% formalin and preserved in 70% alcohol. Tadpoles were preserved in 10% neutral-buffered formalin. Prior to preservation, tissue samples from the tadpole (tail muscle) and adults (thigh muscle) were taken in absolute alcohol for molecular studies and stored at −20 degree in Systematics Lab, Department of Environmental Studies, University of Delhi (SDBDU). Male advertisement calls were recorded using a Marantz PMD620 solid-state digital recorder (44.1 kHz sampling rate, 16-bit resolution) with a handheld Sennheiser ME 66 unidirectional microphone. Air temperature (dry bulb and wet bulb) at the calling site was recorded to the nearest 0.1 °C. Type specimens are deposited in Zoological Survey of India–Western Ghats Regional Centre (ZSI-WGRC), Kozhikode and referred specimens are available in the SDBDU collection. The study was conducted with permission and following the guidelines by the concerned authorities in the State Forest Department, Government of Kerala (Permit No. WL10-25421/2014).
DNA extraction, PCR and sequencing
Total genomic DNA was extracted from adult and tadpole tissue samples using Qiagen DNeasy tissue kit, following the manufacturer’s protocols. Based on availability of GenBank data, the following gene fragments were PCR-amplified using previously published primers: two mitochondrial (mt) genes—ribosomal subunit 16 S rRNA (16 S, ∼560 bp, primer set 16Sar and 16Sbr)55 and cytochrome oxidase I (CO1, ∼650 bp, primer set Chmf4 and Chmr4)56, and four nuclear (nu) genes—brain-derived neurotrophic factor (BDNF, ∼700 bp, primer set BDNF.Amp.F1 and BDNF.Amp.R1)36, histone H3 (His3, ∼330 bp, primer set H3F and H3R)57, seven in absentia homolog 1 (SIA1, ∼399 bp, primer set SIA1 and SIA2)58 and tyrosinase (Tyr, ∼530 bp, primer set TyrC and TyrG)15. Sequencing was performed on both strands using the BigDye terminator cycle sequencing kit on ABI 3730 automated DNA sequencer (Applied Biosystems). Nucleotide sequences were checked and assembled in ChromasPro v1.34 (Technelysium Pty Ltd.), and deposited in the Genbank under accession numbers MK285340–MK285351.
Phylogenetic analyses
DNA sequences for 43 taxa representing all the recognized genera in subfamily Microhylinae and five outgroup taxa (Asterophryinae and Dyscophinae) were retrieved from the GenBank (Supplementary Table S1). In addition, newly generated sequences of the unknown microhylid were included in the study. Sequences were aligned using MEGA 6.059. For non-coding DNA, ambiguous portions were identified manually and excluded from the analyses. A combined mitochondrial and nuclear DNA dataset of 3,088 basepairs was assembled and phylogenetic estimations were made based on Maximum Likelihood (ML) and Bayesian analyses. The data matrix was partitioned by genes and appropriate likelihood models were estimated independently using Modeltest 3.460 that yielded GTR + G + I as the best-fitting model for each gene. We used RAxML 7.3.061 as implemented in raxmlGUI 1.162 to perform ML searches with GTRGAMMA model recommended in RAxML61. The rapid bootstrap algorithm63 was executed with 1000 replicates and a thorough ML search based on 200 independent runs (20 percent of bootstrap replicates). Bootstrap support (BS) was computed on the resulting majority-rule consensus tree. Bayesian analysis was performed in MrBayes 3.1.264 using GTR + G + I model across all gene partitions. Two parallel runs of four Markov Chain Monte Carlo (MCMC) chains were executed for 10 million generations with sampling at every 1000th generation. Convergence of the parallel runs was determined by split frequency of <0.01 standard deviations and potential scale reduction factors of ∼1.0. Stationarity of the likelihood scores and effective sample sizes (ESS) for all parameters were checked in Tracer v1.665. Trees were summarized with a burn-in value of 10 percent and Bayesian Posterior Probabilities (BPP) were used to estimate clade credibility. Additionally, ML trees were generated for each nuclear and mitochondrial gene in order to independently assess distinctness of the new lineage. Uncorrected intra- and interspecific pairwise genetic distances were computed for the 16S mitochondrial gene sequences using PAUP* 4.0b1066. Pairwise identity was graphically visualized using the Sequence Demarcation Tool v1.267.
Dating estimates
For estimation of divergence times in the subfamily Microhylinae, an uncorrelated lognormal relaxed-clock analysis was performed in the software package BEAST v2.4.468. The data set was partitioned by genes and best-fitting substitution models (Supplementary Table S4) were determined in PartitionFinder 2.1.169 using the ‘greedy’ search option and ‘mrbayes’ models under Bayesian information criterion. The Birth-death model was used for tree priors as it takes into account two parameters, i.e. lineage birth as well as extinction, while estimating phylogenetic diversification70. All other parameters were left as default. For dating of nodes, three calibration points were used following Van Bocxlaer et al.12 with normally distributed priors: (1) the split between Dyscophinae and Microhylinae representing the most recent common ancestor (MRCA) of Microhylinae was calibrated at a mean age of 68 Mya and standard deviation of 3.5 Mya, (2) the MRCA of Kaloula and Microhyla was calibrated at a mean age of 62 Mya and standard deviation of 3.5 Mya, and (3) the MRCA of Glyphoglossus and Microhyla was calibrated at a mean age of 48 Mya and standard deviation of 2.5 Mya. Four independent runs with MCMC chain length of 50 million each were executed in BEAST v2.4.468 and trees were sampled at every 500th generation. Convergence of the runs was assessed in Tracer v1.665 and the initial 10 percent trees were discarded as burn-in. The results were combined in LogCombiner68 and ESS values of over 200 were largely achieved for all parameters, except posterior (ESS = 126). In order to avoid overparameterization, an additional analysis of 100 million generations with 10 percent burn-in was performed using only the HKY model for each locus, which yielded ESS values over 1000 for all parameters.
Adult morphology
Sex and maturity of specimens were determined either by the presence of secondary sexual characters (nuptial pads and vocal sacs in males) or by examining the gonads through a small ventral incision. Only adult specimens were used for morphometric studies. Measurements were taken to the nearest 0.1 mm by using a digital slide-caliper or a binocular microscope with a micrometer ocular. Measurements and associated terminologies follow Biju et al.71,72; for abbreviations see electronic supplementary material. All measurements provided in the taxonomy section are in millimeters. Webbing formulae and the degree of webbing is described following Biju et al.73. Measurements and photographs were taken for the right side of the specimens, except when a character was damaged, in which case they were taken for the left side.
Tadpole morphology
The tadpoles were morphologically examined and staged according to Gosner74. Descriptions are based on stage 34 tadpoles (N = 3). Measurements were taken to the nearest 0.01 mm using a stereomicroscope with a micrometer ocular or a digital slide-caliper. Measurements and larval terminologies were adapted from McDiarmid and Altig75. Oral morphology and margins of papillae were visualized using blue ink.
Hand musculature
Palmar musculature was examined under stereomicroscope after dissection and removal of superficial layers (see electronic supplementary material). Dissection procedures, character sampling, terminologies and abbreviations follow Burton38,76 and Blotto et al.77.
Acoustic analysis
Temporal and spectral properties were measured for a single call using Raven Pro 1.478. Dominant frequency was measured after averaging spectrum over the entire call. Terminologies and graphical representation of the analysed call properties follow Bee et al.79.
Nomenclatural acts
This article is published in an electronic journal with an ISSN (2045–2322), and has been archived in PubMed Central. Taxonomic nomenclature published in this article conforms to the requirements of the amended International Code of Zoological Nomenclature (ICZN), and hence is available under ICZN. This publication and the nomenclatural acts it contains have been registered in ZooBank, the proposed online registration system for the ICZN. The ZooBank LSID (Life Science Identifier) for this publication can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix ‘http://zoobank.org/’. The LSID for this publication is urn:lsid:zoobank.org:pub:522D72B4-87B3-4A9B-B533-EA794695755D; LSID for Mysticellus gen. nov. is urn:lsid:zoobank.org:act:6145F26C-E140-4BBF-9322-550047926EA2; LSID for Mysticellus franki sp. nov. is urn:lsid:zoobank.org:act:B1FBD56B-B6B3-412A-8988-E527A082C4C5.
Supplementary information
Acknowledgements
We are grateful to Boris L. Blotto, Robin Suyesh and Gayani Senevirathne for help in the study of hand musculature, vocalization, and larval morphology, respectively. Philippe J. R. Kok provided valuable advice on dating analysis. We also thank Guntupalli V. R. Prasad, Rohan Pethiyagoda and Darrel R. Frost for comments and advice. Jeff Streicher (NHM, London) provided museum support and specimen loan. This study was in part funded by the following grants to S.D.B.: Critical Ecosystem Partnership Fund, Conservation International, USA (Project 55918/ 2009); University of Delhi Research and Development Grant 2015–16; and DST Purse Grant Phase II (2015–16), Department of Science and Technology, Government of India. S.G. received research fellowships from University of Delhi Teaching Assistantship Scheme (2010/56562) and Council for Scientific and Industrial Research (CSIR No. 9/45(1381)/2015-EMR-I), and financial support from Amphibian Evolution Lab (http://www.amphibia.be) for a study visit to Vrije Universiteit Brussel. The State Forest Department of Kerala provided the field study permit (No. WL10-25421/2014).
Author Contributions
S.G. and S.D.B. collected and analyzed the data, conceived and wrote the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/28/2019
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-38133-x.
References
- 1.Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GA, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403(6772):853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- 2.Hora SL. Satpura hypothesis of the distribution of Malayan fauna and flora of peninsular India. Proc. Nat. Inst. Sci. India. 1949;15:309–314. [Google Scholar]
- 3.Croizat, L. The biogeography of India: a note on some of its fundamentals. Proc. Symp. Recent Advances in Trop. Ecol. (eds Misra, R. & Gopal, B.) 545–590 (Inter. Soc. for Trop. Ecol., Varanasi, India, 1968).
- 4.Mani M. S. Monographiae Biologicae. Dordrecht: Springer Netherlands; 1974. Biogeography of the Himalaya; pp. 664–681. [Google Scholar]
- 5.Briggs JC. The historic biogeography of India: isolation or contact? Syst. Zool. 1989;38(4):322–332. [PubMed] [Google Scholar]
- 6.Briggs JC. The biogeographic and tectonic history of India. J. Biogeogr. 2003;30(3):381–388. [Google Scholar]
- 7.Prasad GVR, Sahni A. First Cretaceous mammal from India. Nature. 1988;332(6165):638–640. [Google Scholar]
- 8.Bossuyt F, Milinkovitch MC. Amphibians as indicators of early tertiary “out-of-India” dispersal of vertebrates. Science. 2001;292(5514):93–95. doi: 10.1126/science.1058875. [DOI] [PubMed] [Google Scholar]
- 9.Gower DJ, et al. A molecular phylogeny of ichthyophiid caecilians (Amphibia: Gymnophiona: Ichthyophiidae): out of India or out of South East Asia? Proc. R. Soc. Lond. B. 2002;269(1500):1563–1569. doi: 10.1098/rspb.2002.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biju SD, Bossuyt F. New frog family from India reveals an ancient biogeographical link with the Seychelles. Nature. 2003;425(6959):711–714. doi: 10.1038/nature02019. [DOI] [PubMed] [Google Scholar]
- 11.Roelants K, Jiang J, Bossuyt F. Endemic ranid (Amphibia: Anura) genera in southern mountain ranges of the Indian subcontinent represent ancient frog lineages: evidence from molecular data. Mol. Phylogenet. Evol. 2004;31(2):730–740. doi: 10.1016/j.ympev.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Van Bocxlaer I, Roelants K, Biju SD, Nagaraju J, Bossuyt F. Late Cretaceous vicariance in Gondwanan amphibians. PLoS ONE. 2006;1(1):e74. doi: 10.1371/journal.pone.0000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurabayashi A, et al. From Antarctica or Asia? New colonization scenario for Australian-New Guinean narrow mouth toads suggested from the findings on a mysterious genus Gastrophrynoides. BMC Evol. Biol. 2012;11(1):175. doi: 10.1186/1471-2148-11-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hedges SB. Biogeography: The coelacanth of frogs. Nature. 2003;425(6959):669–670. doi: 10.1038/425669a. [DOI] [PubMed] [Google Scholar]
- 15.Bossuyt F, Milinkovitch MC. Convergent adaptive radiations in Madagascan and Asian ranid frogs reveal covariation between larval and adult traits. Proc. Natl. Acad. Sci. USA. 2000;97(12):6585–6590. doi: 10.1073/pnas.97.12.6585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scotese, C. R. Atlas of Earth History Vol. 1, Paleogeography (Paleomap Project, Arlington, Texas, 2001).
- 17.Duellman, W. E. & Trueb, L. Biology of Amphibians (McGraw Hill, 1986).
- 18.Klaus S, Morley RJ, Plath M, Zhang YP, Li JT. Biotic interchange between the Indian subcontinent and mainland Asia through time. Nat. Commun. 2016;7:12132. doi: 10.1038/ncomms12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhisheng A, Kutzbach JE, Prell WL, Porter SC. Evolution of Asian monsoons and phased uplift of the Himalaya–Tibetan plateau since Late Miocene times. Nature. 2001;411(6833):62–66. doi: 10.1038/35075035. [DOI] [PubMed] [Google Scholar]
- 20.Harris N. The elevation history of the Tibetan Plateau and its implications for the Asian monsoon. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006;241(1):4–15. [Google Scholar]
- 21.Mani, M. S. Biogeography in India (Surya Publications, Dehra Dun, Uttaranchal, India, 1995).
- 22.Kamei RG, et al. Discovery of a new family of amphibians from northeast India with ancient links toAfrica. Proc. R. Soc. Lond., B, Biol. Sci. 2012;279(1737):2396–2401. doi: 10.1098/rspb.2012.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bossuyt F, Brown RM, Hillis DM, Cannatella DC, Milinkovitch MC. Phylogeny and biogeography of a cosmopolitan frog radiation: Late Cretaceous diversification resulted in Continental-Scale Endemism in the Family Ranidae. Syst. Biol. 2006;55(4):579–594. doi: 10.1080/10635150600812551. [DOI] [PubMed] [Google Scholar]
- 24.Che J, et al. Spiny frogs (Paini) illuminate the history of the Himalayan region and Southeast Asia. Proc. Natl. Acad. Sci. USA. 2010;107(31):13765–13770. doi: 10.1073/pnas.1008415107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li JT, et al. Diversification of rhacophorid frogs provides evidence for accelerated faunal exchange between India and Eurasia during the Oligocene. Proc. Natl. Acad. Sci. USA. 2013;110(9):3441–3446. doi: 10.1073/pnas.1300881110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mittermeier, R. A. et al. Hotspots revisited: Earth’s biologically richest and most endangered terrestrial ecoregions (CEMEX Books on Nature Series, Mexico City, 2004).
- 27.Frost, D. R. Amphibian Species of the World: an Online Reference, Version 6.0 web application, American Museum of Natural History, New York, U.S.A., http://research.amnh.org/herpetology/amphibia/index.html Accessed: 01 June 2018 (2018).
- 28.Dubois A. Notes sur la classification des ranidae (Amphibiens, Anoures) Bull. Soc. Linn. Lyon. 1992;61(10):305–352. [Google Scholar]
- 29.Frost DR, et al. The amphibian tree of life. Bull. Am. Mus. Nat. Hist. 2006;297:1–370. [Google Scholar]
- 30.Bossuyt, F. & Roelants, K. Frogs and toads (Anura). In The Timetree of Life (eds Hedges, S. B. & Kumar, S.) 357–364 (Oxford University Press, 2009).
- 31.Matsui M, et al. Systematic relationships of Oriental tiny frogs of the family Microhylidae (Amphibia, Anura) as revealed by mtDNA genealogy. Mol. Phylogenet. Evol. 2011;61(1):167–176. doi: 10.1016/j.ympev.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 32.Pyron RA, Wiens JJ. A large-scale phylogeny of Amphibia including over 2800 species, and a revised classification of extant frogs, salamanders, and caecilians. Mol. Phylogenet. Evol. 2011;61(2):543–583. doi: 10.1016/j.ympev.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Peloso PL, et al. The impact of anchored phylogenomics and taxon sampling on phylogenetic inference in narrow‐mouthed frogs (Anura, Microhylidae) Cladistics. 2016;32(2):113–140. doi: 10.1111/cla.12118. [DOI] [PubMed] [Google Scholar]
- 34.AmphibiaWeb, Information on amphibian biology and conservation web application, Berkeley, California, U.S.A, http://amphibiaweb.org/ Accessed: 01 June 2018 (2018)
- 35.de Sá RO, et al. Molecular phylogeny of microhylid frogs (Anura: Microhylidae) with emphasis on relationships among New World genera. BMC Evol. Biol. 2012;12:241. doi: 10.1186/1471-2148-12-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Der Meijden A, et al. Nuclear gene phylogeny of narrow-mouthed toads (Family: Microhylidae) and a discussion of competing hypotheses concerning their biogeographical origins. Mol. Phylogenet. Evol. 2007;44(3):1017–1030. doi: 10.1016/j.ympev.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 37.Blackburn DC, et al. An adaptive radiation of frogs in a Southeast Asian island archipelago. Evolution. 2013;67(9):2631–2646. doi: 10.1111/evo.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burton TC. Pointing the way: the distribution and evolution of some characters of the finger muscles of frogs. Am. Mus. Novit. 1998;3229:1–13. [Google Scholar]
- 39.Chanda, S. K. Handbook—Indian Amphibians (Zoological Survey of India, Kolkata, 2002).
- 40.Mathew, R. & Sen, N. Pictorial Guide to Amphibians of North East India (Zoological Survey of India, Kolkata, 2010).
- 41.San Mauro D, Vences M, Alcobendas M, Zardoya R, Meyer A. Initial diversification of living amphibians predated the breakup of Pangaea. Am. Nat. 2005;165(5):590–599. doi: 10.1086/429523. [DOI] [PubMed] [Google Scholar]
- 42.Macey JR, et al. Evaluating trans-tethys migration: an example using acrodont lizard phylogenetics. Syst Biol. 2000;49(2):233–256. doi: 10.1093/sysbio/49.2.233. [DOI] [PubMed] [Google Scholar]
- 43.Conti E, Eriksson T, Schö Nenberger J, Sytsma KJ, Baum DA. Early Tertiary out-of-India dispersal of Crypteroniaceae: evidence from phylogeny and molecular dating. Evolution. 2002;56(10):1931–1941. doi: 10.1554/0014-3820(2002)056[1931:ETOOID]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 44.Wilkinson MA, Sheps J, Oommen OV, Cohen BL. Phylogenetic relationships of Indian caecilians (Amphibia: Gymnophiona) inferred from mitochondrial rRNA gene sequences. Mol Phylogenet Evol. 2002;23(3):401–407. doi: 10.1016/s1055-7903(02)00031-3. [DOI] [PubMed] [Google Scholar]
- 45.Karanth KP. Out-of-India: Gondwanan origin of some tropical Asian biota. Curr Sci. 2006;90(6):789–792. [Google Scholar]
- 46.Zhang P, et al. Phylogeny, evolution, and biogeography of Asiatic Salamanders (Hynobiidae) Proc. Natl. Acad. Sci. USA. 2006;103(19):7360–7365. doi: 10.1073/pnas.0602325103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morley, R. J. Cretaceous and Tertiary climate change and the past distribution of megathermal rainforests. In Tropical rainforest responses to climatic change (eds Bush, M. B. & Flenley, J. R.) 1–34 (Springer, Berlin, Heidelberg, 2011).
- 48.Vences M, et al. Multiple overseas dispersal in amphibians. Proc. R. Soc. Lond. B. 2003;270(1532):2435–2442. doi: 10.1098/rspb.2003.2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Acton, G. D. Apparent polar wander of India since the Cretaceous with implications for regional tectonics and true polar wander. In The Indian Subcontinent and Gondwana: a Palaeomagnetic and Rock Magnetic Perspective (eds Radhakrishna, T. & Piper, J. D. A.). 44, 129–175 (Mem. Geol. Soc. India, 1999).
- 50.Aitchison JC, Ali JR, Davis AM. When and where did India and Asia collide? J. Geophys. Res. 2007;112:B05423. [Google Scholar]
- 51.Ali JR, Aitchison JC. Gondwana toAsia: Plate tectonics, paleogeography and the biological connectivity of the Indian sub-continent from the Middle Jurassic through latest Eocene (166-35 Ma) Earth Sci. Rev. 2008;88(3-4):145–166. [Google Scholar]
- 52.Klaus S, Schubart CD, Streit B, Pfenninger M. When Indian crabs were not yet Asian—biogeographic evidence for Eocene proximity of India and Southeast Asia. BMC Evol. Biol. 2010;10:287. doi: 10.1186/1471-2148-10-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grismer JL, et al. The Eurasian invasion: phylogenomic data reveal multiple Southeast Asian origins for Indian Dragon Lizards. BMC Evol. Biol. 2016;16(1):43. doi: 10.1186/s12862-016-0611-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schettino A, Scotese CR. Apparent polar wander paths for the major continents (200 Ma to the present day): a palaeomagnetic reference frame for global plate tectonic reconstructions. Geophys. J. Int. 2005;163(2):727–759. [Google Scholar]
- 55.Simon C, et al. Evolution, weighting and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 1994;87(6):651–701. [Google Scholar]
- 56.Che J, et al. Universal COI primers for DNA barcoding amphibians. Mol. Ecol. Resour. 2012;12(2):247–258. doi: 10.1111/j.1755-0998.2011.03090.x. [DOI] [PubMed] [Google Scholar]
- 57.Colgan DJ, et al. Histone H3 and U2 sn-RNA DNA sequences and arthropod molecular evolution. Aust. J. Zool. 1999;46(5):419–437. [Google Scholar]
- 58.Bonacum J, et al. New nuclear and mitochondrial primers for systematics and comparative genomics in Drosophilidae. Drosoph. Inf. Serv. 2001;84:201–204. [Google Scholar]
- 59.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Posada D, Crandall KA. Modeltest: testing the model of DNA substitution. Bioinformatics. 1998;14(9):817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- 61.Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22(21):2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- 62.Silvestro D, Michalak I. raxmlGUI: a graphical front-end for RAxML. Org. Divers. Evol. 2012;12(4):335–337. [Google Scholar]
- 63.Stamatakis A, Hoover P, Rougemont J, Renner S. A rapid bootstrap algorithm for the RAxML Web Servers. Syst. Biol. 2008;57(5):758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- 64.Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19(12):1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 65.Rambaut, A., Suchard, M. A., Xie, D. & Drummond, A. J. Tracer v1.6, Available from, http://tree.bio.ed.ac.uk/software/tracer/ (2014).
- 66.Swofford, D. L. PAUP*: Phylogenetic Analysis Using Parsimony (*and other methods). Version 4.0b10. Sinauer Association Inc., Sunderland, Massachusetts (2002).
- 67.Muhire BM, Varsani A, Martin DP. SDT: a virus classification tool based on pairwise sequence alignment and identity calculation. PLoS ONE. 2014;9:e108277. doi: 10.1371/journal.pone.0108277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bouckaert R, et al. BEAST 2: a software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2014;10(4):e1003537. doi: 10.1371/journal.pcbi.1003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lanfear R, Frandsen PB, Wright AM, Senfeld T, Calcott B. PartitionFinder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2016;34(3):772–773. doi: 10.1093/molbev/msw260. [DOI] [PubMed] [Google Scholar]
- 70.Drummond, A. J. & Bouckaert, R. R. Bayesian Evolutionary Analysis With BEAST. (Cambridge University Press, 2015).
- 71.Biju SD, Garg S, Gururaja KV, Shouche Y, Walujkar SA. DNA barcoding reveals unprecedented diversity in Dancing Frogs of India (Micrixalidae, Micrixalus): a taxonomic revision with description of 14 new species. Ceylon J. Sci. Biol. Sci. 2014;43(1):37–123. [Google Scholar]
- 72.Biju SD, et al. Frankixalus, a new rhacophorid genus of tree hole breeding frogs with oophagous tadpoles. PLoS ONE. 2016;11(1):e0145727. doi: 10.1371/journal.pone.0145727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Biju SD, et al. DNA barcoding, phylogeny and systematics of Golden-backed frogs (Hylarana, Ranidae) of the Western Ghats-Sri Lanka biodiversity hotspot, with the description of seven new species. Contrib. Zool. 2014;83(4):269–335. [Google Scholar]
- 74.Gosner KL. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica. 1960;16(3):183–190. [Google Scholar]
- 75.McDiarmid, R. W. & Altig, R. Tadpoles: The Biology of Anuran Larvae. Chicago: The University of Chicago Press (1999).
- 76.Burton TC. Variation in the hand and superficial throat musculature of neotropical leptodactylid frogs. Herpetologica. 1998;54(1):53–72. [Google Scholar]
- 77.Blotto BL, Pereyra MO, Faivovich J, Dias PHDS, Grant T. Concentrated evolutionary novelties in the foot musculature of Odontophrynidae (Anura: Neobatrachia), with comments on adaptations for burrowing. Zootaxa. 2017;4258(5):425–442. doi: 10.11646/zootaxa.4258.5.2. [DOI] [PubMed] [Google Scholar]
- 78.Charif, R. A., Waack, A. M. & Strickman, L. M. Raven Pro 1.4 User’s Manual. Cornell Lab of Ornithology, Ithaca, 367 pp (2010).
- 79.Bee MA, Suyesh R, Biju SD. Vocal behavior of the Ponmudi Bush Frog (Raorchestes graminirupes): repertoire and individual variation. Herpetologica. 2013;69(1):22–35. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.