Abstract
The conditions for the potential abiotic formation of organic compounds from inorganic precursors have great implications for our understanding of the origin of life on Earth and for its possible detection in other environments of the Solar System. It is known that aerosol-interfaces are effective at enhancing prebiotic chemical reactions, but the roles of salinity and pH have been poorly investigated to date. Here, we experimentally demonstrate the uniqueness of alkaline aerosols as prebiotic reactors that produce an undifferentiated accumulation of a variety of multi-carbon biomolecules resulting from high-energy processes (in our case, electrical discharges). Using simulation experiments, we demonstrate that the detection of important biomolecules in tholins increases when plausible and particular local planetary environmental conditions are simulated. A greater diversity in amino acids, carboxylic acids, N-heterocycles, and ketoacids, such as glyoxylic and pyruvic acid, was identified in tholins synthetized from reduced and neutral atmospheres in the presence of alkaline aqueous aerosols than that from the same atmospheres but using neutral or acidic aqueous aerosols.
Introduction
Tholins are complex organic materials obtained by activation, using several energy sources, of atmospheres that contain CO, CO2 or CH4 as carbon sources and N2 or NH3 as nitrogen sources. The syntheses of tholins is of high interest in the field of the prebiotic chemistry and in studies about the origin of life because in their production are simulated plausible prebiotic conditions that potentially may lead to the production of biochemically interesting organic molecules. The purpose of these simulation experiments is to obtain a better understanding of the likely environments in which life could have emerged and the first steps of a primitive biology via accumulation of multi-carbon biomolecules. Miller’s experiment1 is famous due to the production of tholins. Since this successful experiment, most of the recent Miller-type experiments simulating earth conditions used water in vapour form (e.g.2) or liquid water (e.g.3–5). A few examples reported using water in the solid state (e.g.6), and interestingly, in the last decade, the role of the aqueous aerosols in these types of experiments is being explored7–9. The possible importance of aerosols in the origin of life on the early Earth has been emphasized in recent years. Aqueous aerosols are considered to be “prebiotic microreactors”10,11 and exhibit an efficient variation in the reactivity of systems12,13. Aqueous aerosols can enhance the yield of polar organic compounds8,14,15, improve the formation of determinate organics against others15 and positively influence non-enzymatic polymerization reactions16,17.
On the other hand, pure water was employed in the majority of Miller-type experiments, and the roles of salinity and pH have been rarely studied in this type of experiment9,18–21. Because salinity and pH are important physico-chemical parameters on a planetary scale and in local environments and since the right environment (in the case that this one was unique) for the emergence of life currently remains unknown, both parameters should be taken into account in Miller-type experiments. Indeed, the formation of an aerosol depends only on a liquid water-air interface and a physical mechanism that ejects bubbles into the atmosphere, such as wind, sea waves or shock waves22,23. These water surfaces can correspond to oceans, internal seas, lakes or rives and so on, which can in turn present different compositions in terms of salinity and pH. The introduction of these parameters together with others that are more widely studied, such as the compositions of the gas mixtures, might lead to finding new clues about the puzzling trouble of the origin of life.
Therefore, in the present work, we investigate the role of the pH in the presence of aqueous aerosols in Miller-type experiments to understand what conditions are more favourable to the accumulation of organics, which may be the main characters in a plausible emergence of a primitive biology. Thus, a CH4 + N2 + H2 atmosphere or a more reducing atmosphere composed by CH4 + NH3 + H2, which can simulate the composition of volcanic gas mixtures, was activated by spark discharges in the presence of aqueous aerosols produced using several solutions with different initial pH values. The choice of these gas mixtures constraints the simulations to a particular planetary local environment, volcanoes next to any water surface. The complex mixtures obtained were studied via elemental analysis, FT-IR spectroscopy and GC-MS. Table 1 summarizes the plausible prebiotic synthetic conditions explored herein. Note that experiment 6 can seem very similar to that reported previously by Johnson et al.7. However, the conditions and the techniques for the production of aerosols are truly different, as are the prebiotic conditions simulated. Johnson et al.7 simulated the steam vapour that it is formed in volcanic eruptions and the subsequent formation of aerosols, whereas in the present case, the aerosols are formed from a pool of liquid water at r.t. that simulates any liquid water surface. The analytical techniques and the conclusions of the study by Johnson et al.7 and the work present here are also different. In the present work, a wide screening for polar organic molecules was performed via GC-MS, whereas in the case of Johnson et al.7, HPLC was used as analytical technique, and amino acids were mainly reported, although in both cases, the role of the aerosols was revealed.
Table 1.
Miller-type experiments. Synthetic conditions explored in this study.
| Experiment | Atmosphere | Salts | Initial pH | Aerosol | Ref. |
|---|---|---|---|---|---|
| 1 | CH4 + N2 + H2 | − | 7 | − | This work and8 |
| 2 | CH4 + N2 + H2 | − | 7 | + | This work and8 |
| 3 | CH4 + N2 + H2 | + | 9.8 | + | This work and9 |
| 4 | CH4 + N2 + H2 | + | 12 | + | This work and9 |
| 5 | CH4 + NH3 + H2 | − | 7 | − | This work |
| 6 | CH4 + NH3 + H2 | − | 7 | + | This work |
| 7 | CH4 + NH3 + H2 | + | 9.8 | + | This work |
| 8 | CH4 + NH3 + H2 | + | 12 | + | This work |
| 9 | CH4+N2+H2 | + | 4.8 | + | This work and9 |
| 10 | CH4+N2+H2 | + | 5.8 | + | This work and18 |
| 11 | CH4+N2+H2 | + | 7.8 | + | This work and9 |
The gas mixtures were active for 3 days in the presence of pure water (−) or saline solutions (+) as well as without (−) or with an active aerosol cycle (+). The temperature was constant throughout the reaction time, 38 °C.
Herein, as expected, a set of amino acids, carboxylic acids and N-heterocycles were identified in the hydrophilic tholins by GC-MS, but precursors of sugars were also detected. Moreover, ketoacids, such as glyoxylic acid and pyruvic acid, were identified for the first time in this type of experiment. Our analysis confirms that alkaline aerosol environments, compared to acidic or neutral environments, are particularly effective microreactors that favour the emergence of a wealth of compounds likely implicated in the first steps of a primitive biochemistry.
Materials and Methods
Simulation of alkaline reductive environments
The syntheses of the hydrophilic and hydrophobic tholins via spark discharges experiments in an atmosphere of CH4 + N2 + H2 and liquid water, aqueous aerosols or saline aqueous aerosols (experiments 1–4, Table 1) are described in references8 and9. New experiments were carried out following the methodology previously developed using a gas mixture CH4:NH3:H2 (40:30:30) purchased from Air Liquid (experiments 5–8, Table 1. For details, see the Supporting Information).
Instrumental Analyses
Gas Chromatography-Mass spectrometry (GC-MS)
GC-MS analyses in the full-scan mode were carried out on a 6850 network GC system coupled to a 5975 VL MSD with a triple-axis detector operating in electronic impact (EI) mode at 70 eV (Agilent) using an HP-5 MS column (crossbond 5% diphenyl-95% dimethyl polysiloxane, 30 m ×0.25 mm i.d. ×0.25 µm film thickness) and He as the carrier gas.
Analytical procedure
In all experiments, we collected a solution and an insoluble solid. These were separated by centrifugation and immediately freeze-dried using a standard lyophilizer; in this way, we obtained a hydrophilic tholin and a hydrophobic tholin. All samples were stored at −20 °C under a nitrogen atmosphere until they were analysed. In the case of the experiments in which saline solutions were used, prior to GC-MS analysis of the organics, the hydrophilic tholins were desalted by ion exchange chromatography [Dowex 50 W X 8–400 (H+)] using 5 N NH4OH as the first eluant (FNH3 fractions) and water as the second eluant (FH2O fractions). The fractions were lyophilized and subsequently weighed. For the identification of polar organic molecules in all freeze-dried fractions: (i) The samples were hydrolysed with 6 M HCl at 110 °C for 24 h and then freeze dried to remove water, HCl and any volatile organics; (ii) two milligrams of each hydrolysed sample in 75 µL of BSTFA with 1% TMCS [N,O-bis(trimethylsilyl)trifluoroacetamide with trimethylchlorosilane, from Thermo Scientific] was heated at 70 °C for 19 h to obtain the respective TMS derivatives; and (iii) the derivatized samples were analysed by GC-MS using the following GC oven program: 60 °C (initial temperature) with a hold time of 1.5 min, heating to 130 °C at 5 °C/min with a hold time of 11 min, heating to 180 °C at 10 °C/min with a hold time of 10 min and heating to 220 °C/min at 20 °C/min with a final hold time of 15 min. One microlitre of each sample was injected. The temperature of the injector was 275 °C, and the injections were performed in splitless mode. The detector temperature was 300 °C. The flow rate was 1.1 mL/min. As a rule, identification of the GC-MS peaks attributed to organic compounds was verified by comparison with the retention times and mass spectra of external standards (purchased from Sigma-Aldrich and Fluka).
Quantification of α-ketoacids
In the particular case of glyoxylic acid (c16) and pyruvic acid (c17), a first quantification was achieved using the multiple point external standard method, using standard solutions from 10 ppm to 100 ppm. The following procedure was used to prepare their corresponding oxime-TMS derivatives standards: (i) The needed milligrams of the α-ketoacids c16 and c17 for each standard solution were heated at 60 °C for 30 min in 500 µL of a hydroxylamine hydrochloride solution at pH 12 (20 mg of hydroxylamine hydrochloride in 1 mL of NaOH 2 N); (ii) 200 µL of HCl 6 N was added; (iii) the final mixtures were extracted with 500 µL of ethyl acetate (x2) and 500 µL diethyl ether (x1); (iv) the organic layers were combined and dried under a continuous flow of N2; (v) the samples were freeze-dried to remove residual water; and (vi) the dried residues were heated at 80 °C for 3 h in 100 µL of BSTFA + 1% TMCS. The oxime-TMS derivatives were injected and analysed by GC-MS as indicated above.
Fractionation of the hydrophilic tholins
Concentration by ultrafiltration
The fractions obtained after ion exchange chromatography using 5 N NH4OH (FNH3 fractions) were subfractionated using Nanosep® centrifugal devices (Pall, Life Sciences) to retain molecules above 3 kDa. Both the light fractions (<3 kD) and heavy fractions (<3 kDa) were analysed by polar organics as mentioned above.
Multivariate analysis
Multivariate analysis (for dependent and independent factors) was carried out using a combination of constrained and unconstrained multivariate statistical methods to account for both the total variation in the data and the variation explained by the environmental data. This statistical tool is very useful for comparing results of different experiments in which different variables have been applied. Of all the elements analysed, we retained 8 variables that had no missing values: milligrams of hydrophilic tholin (WSOM, weight of soluble organic matter), milligrams of hydrophobic tholin (WIOM, weight of insoluble organic matter), final pH, number of amino acids identified, number of carboxylic acids identified, number of N-heterocycles identified, number of polyols identified and total number of organic compounds identified. An absence-presence matrix with experimental conditions (absence-presence of NH3 in the reduced atmosphere used, salts, and aerosol) and the initial pH was generated. Detrended correspondence analysis (DCA) was performed to determine the modality of the sequence data. The analysis resulted in 0.612 segment lengths for the variable data and 1.879 segment lengths for the experimental conditions matrix. Both values were less than 3, indicating that the linear and unimodal models could be used (redundancy analysis or canonical correlation analysis). Finally, a redundancy analysis was performed because allows explain as much variance as possible and studying the influence of the different variables and experimental conditions. The significance of the first axis and that of all axes combined were tested using Monte Carlo permutation tests. DCA and RDA tests were performed using the multivariate data analysis software CANOCO 4.5 (Microcomputer Power, Ithaca, NY, USA)24. The program CANODRAW4.0 (in the CANOCO package) was used for graphical presentations.
Results
Syntheses and characterization of tholins
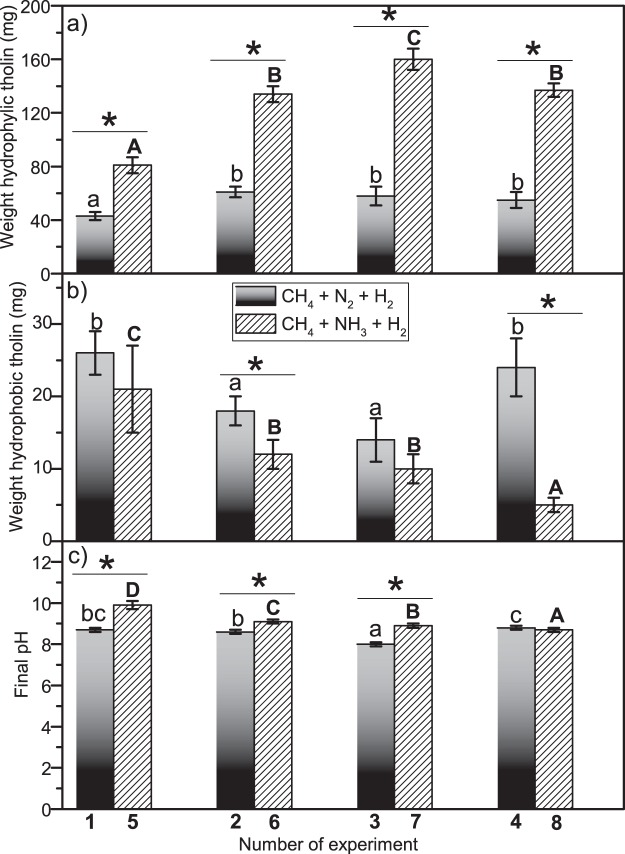
Hydrophilic tholins and hydrophobic tholins were obtained, either in experiments using an atmosphere of CH4 + N2 + H2 or an atmosphere of CH4 + NH3 + H2. Figure 1a shows the amount of hydrophilic tholin formed in each experiment. It is clear that the presence of NH3 improved the amount of soluble organic materials produced in all cases studied, and it was expected that the presence of aqueous aerosols increased the amount of soluble materials. By contrast, the presence of salts and pH variations did not have a strong influence on the total amount of soluble organic material fixed as hydrophilic tholins. In the case of the hydrophobic tholins, the effects observed were the opposite of those observed for the hydrophilic tholins. In general, the amount of hydrophobic tholins produced was less when CH4 + NH3 + H2 was used than when N2 was present in the simulations experiments, and a light influence of the initial higher pH values was observed in relationship to the weight of the hydrophobic tholins (Fig. 1b). On the other hand, in all cases, the final pH of these simulation experiments was dependent on the initial pH and salinity conditions and the presence of aqueous aerosols (Fig. 1c). In addition, the final pH depended on the atmosphere used. Higher final pH values were found for the syntheses carried out using a CH4 + NH3 + H2 atmosphere.
Figure 1.
Histograms showing the effects of the aqueous aerosols and initial pH on (a) the final weight of the hydrophilic tholins (soluble organic matter); (b) the final weight of the hydrophobic tholins (insoluble organic matter); and (c) the final pH of the crude reactions. The number shown on the x-axis corresponds to the experiment number indicated in Table 1. Statistical test: pairs test based on the confidence interval comparison (α = 0.047). *Indicates differences within groups, and letters indicate differences between groups (CH4 + N2 + H2 group = lowercase letters; CH4 + NH3 + H2 group = capital letters). The average values and the standard deviations were calculated from the results of at least five experiments.
In summary, in a general way, NH3 improves the production of hydrophilic tholins, prevents the formation of hydrophobic tholins and increases the final pH of the crude reactions with respect to the simulation spark experiments that used N2 as a nitrogen source. Additionally, NH3 leads to a greater number of functional groups containing nitrogen and oxygen both in hydrophilic tholins and in hydrophobic tholins. Moreover, the salinity and pH significantly change the nature of both hydrophilic and hydrophobic tholins having a greater effect than the source of nitrogen (please see the Supplementary Information for the structural characterization of tholins by elemental analysis and FT-IR spectroscopy).
GC-MS analyses of the hydrophilic tholins
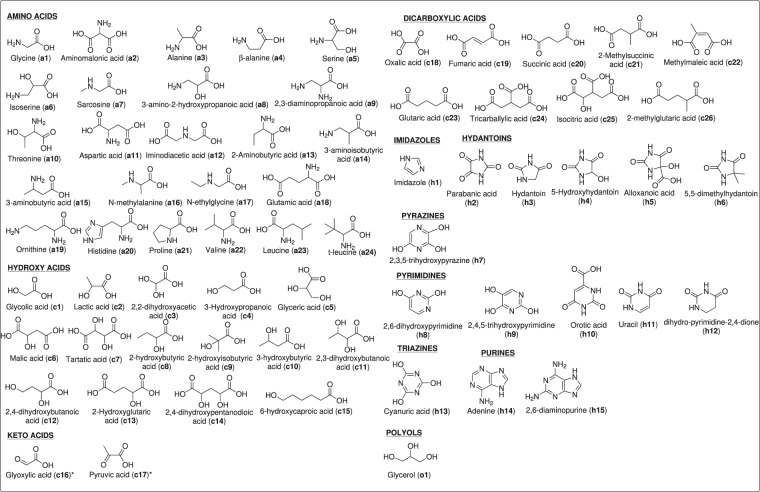
The hydrophilic tholins were analysed by GC-MS using BSTFA as a derivatization reagent to obtain the respective TMS derivatives of polar compounds, such as amino acids, carboxylic acids and several N-heterocycles. This derivatization method is not specific for each type of compound mentioned, but for comparative purposes, it provides an excellent general overview of the polar molecules present in all tholins synthesized. This analytical methodology allows discrimination among the various synthesis conditions tested to determine which are the most favourable from the point of view of the prebiotic production of polar bioorganics12. Figure 2 shows all of the analytes identified in this work.
Figure 2.
Polar organic compounds identified as their TMS derivatives by GC-MS in the present work. *Identified as oxime-TMS derivatives.
Analyses of hydrophilic tholins from pure water experiments and FNH3 fractions
In the case of the spark discharge experiments using saline solutions or alkaline aqueous aerosols, a previous step of desalting is necessary before GC-MS analyses of the hydrophilic tholins. Thus, those were desalted by an ion exchange resin, and a FNH3 fraction and a FH2O fraction were obtained for experiments 3 and 4 according to the solvents used as eluants9. The relationship FN3H/FH2O (mg of freeze-dried ammoniacal fraction/mg of freeze-dried water fraction) was 0.75 and 1.08 for experiments 3 and 4, respectively. By contrast, the amount of FH2O fraction was not significant for experiment 7, and in experiment 8, the relationship FNH3/FH2O was 76. The FNH3 fractions from experiments 3–4 and 7–8 and the bulk hydrophilic tholins from experiments 1–2 and 5–6 were acid hydrolysed and analysed by GC-MS to determine the presence of organic polar compounds (Fig. 3). The comparison between the bulk hydrophilic tholins and FNH3 fractions was made because these fractions were predominant according to weight in the CH4 + NH3 + H2 experiments, and in the case of experiment 7, the amount of the FH2O fraction obtained was so small that it did not allow GC-MS analysis. The diversity in polar organics was greater for the CH4 + N2 + H2 series (Fig. 3a,c,e) than for the CH4 + NH3 + H2 series (Fig. 3b,d,f), especially when alkaline aqueous aerosols were used at an initial pH of 12. It is interesting that some carboxylic acids that are part of the reductive tricarboxylic acid cycle (rTCA), such as maleic acid (c6), pyruvic acid (c17), fumaric acid (c19), succinic acid (c20) and isocitric acid (c25), were identified in the FNH3 fraction synthesized under those experimental conditions (Fig. 3c, exp. 4). Pyruvic acid (c17) was only identified as its oxime-TMS derivative in the CH4 + N2 + H2 series in experiment 4; by contrast, for the CH4 + NH3 + H2 series, this compound was identified under any condition when aqueous aerosols were present (Fig. 3d, experiments 6, 7 and 8). This is the first time that an α-ketoacid that forms part of the rTCA has been detected in tholins from spark discharges experiments. The formation of the oxime-TMS derivative could be explained in the same way that the oxime-TMS derivative for the glyoxylic acid (c16) from HCN polymers forms12. The quantification of pyruvic acid (c17) shows yields based on the carbon-fixed levels of 0.00015% (0.037 µmoles), 0.003% (0.07 µmoles), 0.014% (0.36 µmoles) and 0.028% (0.71 µmoles) for experiments 4, 6, 7 and 8, respectively. Note that the yields increase with the increase of the initial pH for the experiments of the CH4 + NH3 + H2 series and that the yield for the experiments at the initial pH 12 is greater when NH3 is used as a nitrogen source. The identification of tricarballylic acid (c24) (Fig. 3b,e) is also notable, and it is only identified in tholins when aqueous aerosols are used in their syntheses.
Figure 3.
Organic polar compounds identified as their TMS-derivatives by GC-MS in acid hydrolysed hydrophilic tholins synthesized using pure water (experiments 1–2 and 5–6) or in acid hydrolysed FNH3 fractions, which were obtained by desalting the hydrophilic tholins that were synthesized in the presence of alkaline aqueous aerosols at initial pH values of 9.8 or 12 (experiments 3–4 and 7–8): (a,c,d) correspond to the amino acids, carboxylic acids and N-heterocycles, respectively, found in hydrophilic tholins and FNH3 fractions from experiments using an atmosphere of CH4 + N2 + H2; (c,d,f) correspond to amino acids, carboxylic acids and N-heterocycles, respectively, found in hydrophilic tholins and FNH3 fractions from experiments using an atmosphere of CH4 + NH3 + H2. The numbers under the abscissa axis correspond to the numeration of analytes shown in Fig. 2. The coloured bars indicate the presence of a concrete analyte in the corresponding sample.
Note that although a greater diversity in the amino acids, carboxylic acids and N-heterocycles was observed in experiment 4, the yields of the compounds were greater in experiment 8 than in experiment 4 (please see Table S1 in the Supplementary Information). Thus, in experiment 4, a greater number of compounds was identified, but the yield in biomonomers was greater in experiment 8.
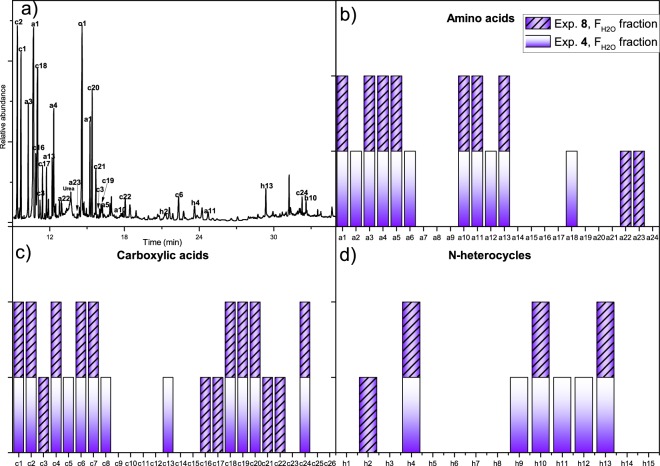
Analyses of the FH2O fractions
The FH2O fraction from experiment 8 was analysed and compared with the previous results from experiment 4 (Fig. 4). It is interesting for the further development of the “glyoxylic acid scenario” proposed by Eschenmoser25 that glyoxylic acid (c16) was identified as its oxime-TMS derivative in this fraction from experiment 8. The yield of glyoxylic acid (c16) was 0.078% (2.98 µmol) based on the initial carbon input into the system. Additionally, pyruvic acid (c17) was detected with a yield for this fraction of 0.016% (0.41 µmoles). Together with the amount found in the FNH3 fraction, this result indicates a total amount of pyruvic acid from experiment 8 of 1.12 µmoles. It is also notable that glycerol (o1) (Fig. 4a) has a yield of 0.238% (6.23 µmoles). To our knowledge, this is the first time that a sugar derivative has been found in this type of prebiotic simulation.
Figure 4.
Organic polar compounds identified as their TMS derivatives by GC-MS in acid hydrolysed FH2O fractions, which were obtained by desalting hydrophilic tholins synthesized in the presence of alkaline aqueous aerosols at an initial pH = 12 (experiments 4 and 8): (a) Representative GC-MS chromatogram of FH2O fraction from experiment 8; (b–d) correspond to amino acids, carboxylic acids and N-heterocycles, respectively, found in FH2O fractions from experiments 4 and 8. The numbers shown on the peaks of the GC-MS chromatogram and under the abscissa axis correspond with the numeration of analytes shown in Fig. 2. The coloured bars indicate the presence of a concrete analyte in the corresponding sample.
The results shown in Fig. 4 indicate that the diversities of biomonomers and related compounds in experiments 4 and 8 are similar. This result is apparently not in agreement with the results discussed above. Thus, this point will be discussed later.
Analyses of non-hydrolysed samples and the subfractionations of the FNH3 fractions
For comparative purposes with the GC-MS analysis data discussed above and to develop a more exhaustive understanding of the system, non-hydrolysed samples were also studied. Moreover, the FNH3 fractions from experiments 7 and 8 were subfractionated by ultrafiltration using centrifugal filter devices with a cut-off of 3 kDa, collected in both cases from a light fraction (<3 kDa) and a heavy fraction (>3 kDa). These heavy fractions were 6% and 9% in weight of the total amount of hydrophilic tholins from experiments 7 and 8, respectively. Either the light fractions or heavy fractions were acid hydrolysed and analysed by GC-MS. Figure S5 (please see the Supporting Information) shows the qualitative data analysis of the samples indicated in this section.
As a result, as expected, the diversity of amino acids and carboxylic acids is increased when the samples are hydrolysed, while the diversity in hydantoins is greater in the non-hydrolysed samples because this type of compound is partially or fully ring-opened under heating in an acid medium to lead to amino acids (Fig. S5a, S5b and S5c). On the other hand, in general, the number of organic compounds identified when FNH3 fractions 7 and 8 are subfractionated using ultrafiltration is greater than of the corresponding non-subfractionated samples (please compare Fig. 3b,d,f with Fig. S5d, S5e,S5f). Subfractionation allows the identification of compounds previously not found in the bulk FNH3 fractions.
At this point, it is difficult to compare the analytical results from the different experiments. On the one hand, one needs to take into account the experimental conditions of synthesis, and on the other hand, it is also important to note how the samples are handled because the number of organics identified depends on both aspects. To provide a global view, a statistical multivariate analysis was carried out, considering all of the organics identified in each sample and the experimental synthetic conditions.
Multivariate analysis
The RDA technique generates an ordination diagram in which axes are created by a combination of variables26. The eigenvalues for each axis generated by the RDA indicate how much of the variation observed in species data can be explained by that axis. In this case, 91% of the correlation between experimental conditions, experiments and variables was explained by two axes (p-value = 0.03) (Fig. 5). For comparative purposes in this statistical study, data from experiments 1–8 were included together with data from experiments 9–11 (Table 1), which had an acidic initial pH or a slightly alkaline initial pH9,18. The first axis shows a positive correlation between the final pH and number of amino acids. The rest of the variables and experimental conditions showed a negative correlation with this axis, where the initial pH showed the highest negative correlation. The triplot showed a positive correlation between experiments performed with salts and aerosols and the total number of organic compounds identified, number of carboxylic acids and number of amino acids. The high pH experiments showed a positive correlation with the number of polyols, number of N-heterocycles and milligrams of hydrophilic tholin. Finally, the NH3-based atmosphere showed a positive correlation with the number of polyols, milligrams of hydrophilic tholin, milligrams of hydrophobic tholin and high final pH. The experiments were plotted on different areas of the diagram depending on their experimental characteristics. The combination of the first and second axes allowed discrimination among the different experiments. For example, experiments 7 and 8 were located in the negative part of the first axis and positive part of the second axis, because they were performed in the presence of salts, aerosols and NH3. Experiments 1, 5 and 6 were plotted in the positive part of the fist axis and second axis because they were performed in the absence of salts. Experiments 2, 9 and 10 were performed in the presence of aerosols and absence of NH3 and plotted in the positive part of the first axis and negative part of the second axis. Finally, experiments 3, 4 and 11 were situated in the negative part of the first and second axes because they were performed in the presence of salts and aerosols. On the other hand, in general, the distribution of the experiments in the upper part or bottom part of the diagram is also related to the presence of NH3.
Figure 5.
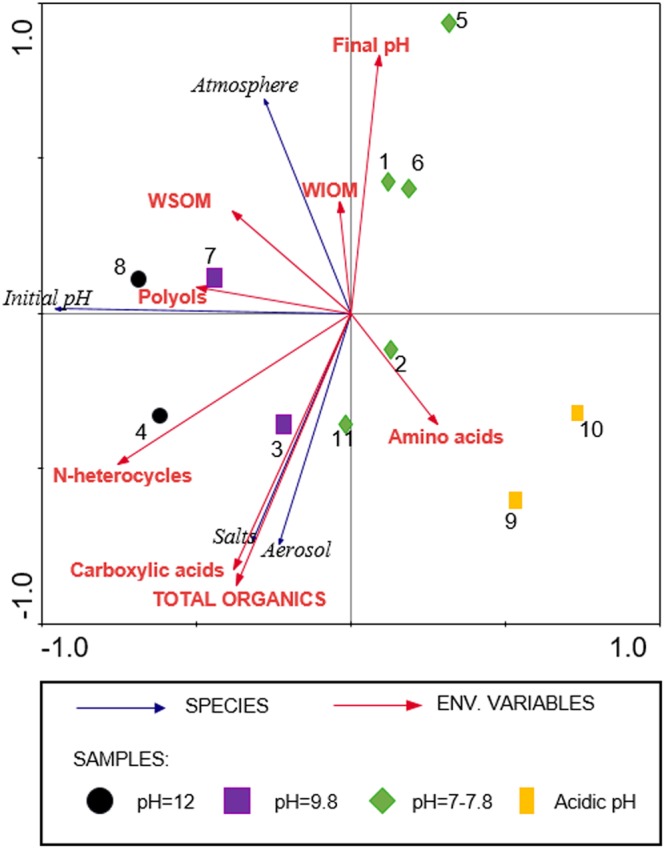
RDA triplot correspondence analysis relating variables and experimental conditions. Experimental conditions are shown by blue arrows. Variables used in the analysis are shown by red arrows. Experiments are indicated by numbers and different symbols depending on the initial pH. The synthetic conditions of experiments 1–8 are shown in Table 1. The initial pH of experiments 9, 10 and 11 was 4, 5.8 and 7.8, respectively, and an atmosphere of CH4 + N2 + H2 (40:30:30), saline aqueous aerosols and spark discharges were used in the same way as experiments 3 and 4 (Table 1).
The correlations found with the initial pH are interesting. Experiments with an initial high pH plotted in the negative part of the first axis, and experiments performed at low pH plotted in the positive part of this axis, while the neutral initial pH values plotted the experiments in the central part of the graph. On the other hand, the experiments performed under extreme conditions (lowest pH and upper pH) presented a high correlation with the total number of organic compounds identified, especially if they were performed in the presence of aerosols and salts (experiments 4, 8 and 9). Between these samples, the special conditions of 4 (absence of NH3) provided more N-heterocycles, conditions of 8 (presence of NH3 and higher pH) provided more milligrams of hydrophilic tholin and conditions of 9 (lowest pH and absence of NH3) provided the highest number of amino acids.
In any case, independent of the presence of NH3, a greater diversity of organic compounds was related to the presence of aerosols, salts and alkaline pH values, as shown in Fig. 5. In addition, some advantages of alkaline pH values compared to acidic pH values for the emergence of life are that amino acids can be converted into peptides under alkaline conditions26 and alkaline pH values favour the polymerization and synthesis of possible protobiopolymers9. Although it is beyond the scope of the present paper, it is worth mentioning that the FNH3 fractions have a macromolecular nature with an electrophoretic mobility that is not present in the FH2O fractions9 and that the presence of NH3 and alkaline pH values increases the production of these fractions and therefore the likely production of protobiopolymers. On the other hand, we propose that experiments with gas mixtures based on CH4, electric discharges, aqueous aerosols and alkaline pH values would lead to the production of sugars because the activation of CH4 with electric discharges leads to the formation of formaldehyde (e.g.27), aqueous aerosols increase the formation of hydroxylated compounds8, and the alkaline pH favours analogous reactions to result in the formose reaction (see e.g.28). In addition, it has been recently demonstrated that aerosols and alkaline pH might favour the emergence of a possible protometabolism when cyanide is used as the carbon source12,15, and it is well known that HCN is formed by the activation of CH4 and NH3/N2 by spark discharges27.
Discussion
The chemical mixtures obtained in Miller-type experiments pose notable analytical challenges due to the heterogeneity of the samples and the large diversity of the obtained organic compounds. In this work, we focus on comparing several experimental conditions with respect to three main classes of polar organic compounds: carboxylic acids, N-heterocycles and amino acids. We also consider their possible implications for the main hypotheses about the origin life: i) the autotrophic origin, which can be related to the findings of some particular carboxylic acids, and ii) the heterotrophic origin, which can be related to the accumulation of the main components of nucleic acids and proteins.
Carboxylic acids
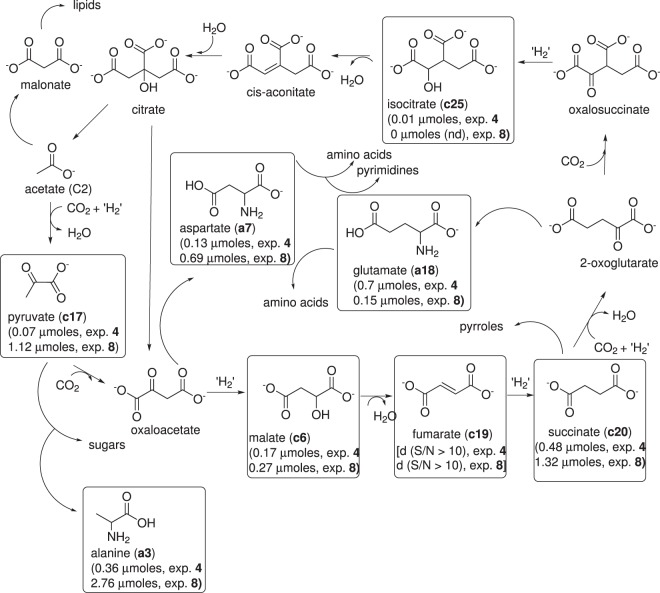
The identification and formation of carboxylic acids under possible prebiotic conditions is a key component of the autotrophic hypothesis about the origin of life and the emergence of a primitive metabolism29. This hypothesis is mainly based on the reductive fixation of inorganic environmental carbon. In this context, the rTCA cycle has been proposed to be a plausible mechanism for carbon fixation and energy storage at the time of the emergence of life29. This cycle is the central axis of the universal metabolism. The combination of the rTCA cycle and its exit products operates as a factory for the synthesis of the main classes of biomolecules. This cycle is shown in Fig. 6. The key components identified via GC-MS in hydrophilic tholins from experiments 4 and 8 are marked in boxes. In general, quantitative/semiquantitative analyses of the carboxylic acids implicated in the rTCA cycles (Fig. 6) indicate that the yields for these compounds are increased when the plausible prebiotic conditions of experiment 8 are used. The rTCA cycle is effectively a mechanism of carbon fixation that can be started at any point along the cycle. Nonenzymatic chemical pathways for some steps of the rTCA cycle, similar to the initial input of the species involved, remain a challenging problem for the viability of the proposed prebiotic cycle. However, recently, some important advances have been made in this field30–32. In addition to those, herein, it is shown that alkaline reduced environments, especially in the presence of NH3, could be favourable scenarios for the emergence of some components of an ancient metabolism. However, the viability and likely emergence of the rTCA cycle in a non-enzymatic scenario has been evaluated33. In this sense, Zubarev and colleagues presented several examples of other auto-catalytic cycles in an rTCA supernetwork using a reactivity model34. These auto-catalytic cycles involve molecules with the highest closeness centrality in the rTCA network, and both sequences are based on glyoxylate (Fig. S6, Supporting Information). The glyoxylic acids (c16) and other compounds related to these cycles were found in the hydrophilic tholin from experiment 8 (Fig. S6, Supporting Information) in highly alkaline environments. Thus, some components of these auto-catalytic cycles could be available in a significant manner in alkaline environments. In addition, a protometabolic analogue of the rTCA cycle involving two linked cycles has recently been experimentally demonstrated35. In this approach, to understand the origin of life, glyoxylate acts as a carbon source, and pyruvate is used as one main reagent. On the other hand, in the abovementioned “glyoxylate scenario”, glyoxylate and its formal dimer, dihydroxyfumarate, are suggested to be the key starting materials of the chemical constitution of a possible metabolism, serving as a source of the main biomonomers, such as sugars, amino acids, pyrimidines and the constituents of the rTCA cycle25. To the best of our knowledge, few works describe plausible prebiotic syntheses of glyoxylate12,36,37. Thus, the alkaline conditions using aqueous aerosols described here can be additional prebiotic sources for this important keto acid due to the relatively high yields obtained.
Figure 6.
Reductive tricarboxylic acid cycle (rTCAC) (adapted from Guzman and Martin). In boxes the components of this cycle identified in hydrophilic tholins from experiments 4 and 8, which used alkaline aqueous aerosols are marked. The µmoles for the carboxylic acids were calculated using the multiple point external standard method, and µmoles of amino acids were semi-quantitatively calculated from the peak of glycine, considering the related areas of the peaks versus the area of the peak of glycine. For the quantification of glycine, a multiple point external standard method was used.
N-Heterocycles
The hypothesis of an RNA world, wherein RNA plays both the role of an informational carrier and the role of a catalyst, is one of the most popular early life hypotheses. In contrast, the emergence of RNA from potentially natural pathways is still open to debate, and it is postulated that RNA could be a product of a multistep evolution process, as proposed in38. In this line of thinking, the development of non-natural base pairs for incorporation into DNA and RNA has been the focus of several research groups for a number of years, e.g.39. Recent studies investigating RNA as an early genetic code found that cyanuric acid (h13) and triaminopyrimidine self-assemble in water to create aggregates resembling contemporary nucleic acid base pairs. Triaminopyrimidine forms nucleosides with ribose, and upon heating, the cyanuric acid (h13) mixture forms gene-length polymers that have been termed proto-RNA40. This polymerization suggests that cyanuric acid (h13) and related cyclic compounds may have played a role in the initiation of life on Earth. To test this hypothesis, prebiotic production of cyanuric acid (h13) must be demonstrated. Here, prebiotic synthesis of cyanuric acid (h13) is demonstrated, indicating that its production is possible using alkaline aqueous aerosols, a reductive atmosphere and spark discharges. This synthetic method increases the scarce number of possible prebiotic syntheses previously reported: from the hydrolysis of HCN polymers12; from CO, H2, and an NH3 mixture41; and from urea subjected to freeze-thaw cycles and spark discharges under CH4/N2/H2 or argon6. In the last case, the formation pathway of cyanuric acid (h13) is based on the fact that biuret (NH2CONHCONH2) is a condensation product of two urea molecules and is a precursor of cyanuric acid (h13). The use of spark discharges for the formation of cyanuric acid (h13) suggests that free radicals are involved in the processes42. In the present case, the production of cyanuric acid (h13) would follow the same pathway because urea was formed in all of the experiments analysed (1–8), and iminoacetic acid (a12), the oxidase form of the biuret, was also identified. Moreover, it is well known that aqueous aerosols stabilize and favour the production of free radicals, e.g18. On the other hand, ribonucleotides and RNA are also thought to be preceded by simpler progenitors comprising the nucleotides containing C3 and C4 units based on glycerol and tetrose sugar as information carriers. Flexible nucleic acid (FNA)43, glycerol nucleic acid (GNA)44, and isoGNA45, based on a simple 3-carbon glycerol, were thus considered. In this manner, the identification of glycerol (o1) in the hydrophilic tholins from experiment 8 is also very interesting because this is the first time that this compound has been found under simulating spark discharge conditions and because the prebiotic syntheses of these biological compounds have rarely been described46–48. In addition, beyond its role as a constituent of a pre-RNA world, glycerol (o1) is well known to be a key building block of lipids, which are central components of all cellular membranes. Additionally, acyclic nucleic acid based on glyceric acid (c5) has been proposed49.
Amino acids
The identification and quantification/semi-quantification of glycine (a1), alanine (a3), and aspartic (a11) and glutamic acids (a18), in addition to those of glycolic (c1), lactic (c2), malic (c6) and 2-hydroxyglutaric (c13) acids, are in good agreement with the findings of Parker and colleagues based on analyses of several Miller-type reaction mixtures via ultrahigh-performance liquid chromatography and triple quadrupole mass spectrometry50. These results indicate that the production of depsipeptides as a plausible prior step to the prebiotic formation of polypeptides51 could be initiated using glycine (a1), alanine (a3), and glycolic (c1) and lactic (c2) acids due to the presence of major compounds in the mixture reactions, along with smaller quantities of aspartic (a11), glutamic (a18), malic (c6) and 2-hydroxyglutaric (c13) acids. Our detection limits using GC-MS are greater than those reported by Parker et al50. when using UHPLC-QqQMS. Additionally, our yields are also minor, probably due to the work-up and derivatization of the samples since in the work of Parker et al., the samples are directly injected in solution onto the chromatography column. In any case, the ratio between the different amino acids and hydroxy acids is similar, and the production of these compounds is favoured in an alkaline medium, as in the case of Parker et al.50. On the other hand, in relationship with the abiotic production of peptides, hydantoins have been suggested to be a precursor for the emergence of prebiotic peptides and amino acids. Moreover, it has been hypothesized that primitive microorganisms on Earth may be able to use hydantoins as C or N sources. The findings presented here demonstrate that hydantoins can be prebiotically synthesized using reduced gas mixtures activated by spark discharges at middle temperatures. These synthetic conditions extend the scope of experiments that lead to the abiotic synthesis of hydantoins, which was previously always demonstrated in icy environments6,37,52.
Therefore, the analyses of the tholins here described show that some components implicated in an autotrophic origin of life and those implicated in a heterotrophic origin can be synthetized simultaneously under particular environmental conditions: alkaline aerosols, reduced gas mixtures and an external energy source. The existence of alkaline lakes on the modern Earth, and their plausible existence on the early Earth, increases the likelihood of auspicious alkaline environments that are capable of generating bioorganics. Finally, alkaline aerosols and a reduced atmosphere may have been possible on ancient Mars due to the past volcanic activity of this planet and the recently demonstrated existence of ancient alkaline lakes, such as that located at Gale Crater, where the Curiosity Rover is operating53. In addition, although not discussed in this work, a protocell can also be defined as a large ordered structure enclosed by a membrane that performs some life activities, such as growth and division. These last characteristics can be found in atmospheric aerosols54. Thus, plausible alkaline environmental conditions and the analytical findings checked for the tholins synthetized under such conditions lead to proposing a vision about the origin of life such as that previously suggested by Eschemnoser via “an iconoclastic attitude among genetic, metabolism, and compartmentalization towards one of openness to horizontal transfer of ideas and insights” in the field of the prebiotic chemistry55.
Conclusions
It has been speculated that a non-enzymatic version of the rTCA cycle may have been central to the origin of life. Our experiments demonstrate both the formation of key elements of the rTCA cycle and of some sugar precursors. In particular, our experiments demonstrate that salinity and pH significantly change the nature of both hydrophilic and hydrophobic tholins. This is the first time that an α-ketoacid that forms part of the rTCA cycle has been detected in tholins from spark discharge experiments. Quantification of the pyruvic acid in tholins shows yields based on a carbon fixation of up to 0.044%. In general, the yields increase with the increase of the initial pH for the experiments of the CH4 + NH3 + H2 series, and the yield for the experiments at an initial pH 12 is greater with NH3. Glyoxylic acid was found with a yield of 0.078%. It is also notable that glycerol was identified to have a yield of 0.238%. This is the first time that a sugar derivative has been found in this type of prebiotic simulation. Indeed, contrasting our analysis with the control experiments demonstrates that the presence of aerosols, salts and alkaline pH values leads to a greater diversity of organic compounds. The volcanic scenario proposed here not only allows for the simultaneous emergence of metabolic, informational and structural components of a plausible primitive biochemistry but also the availability of containers for them. Our experiments confirm that volcanic environments next to alkaline pools of water would be ideal prebiotic niches for the accumulation of organics with biological interest.
Electronic supplementary material
Acknowledgements
The authors used the research facilities of the Centro de Astrobiología (CAB) and were supported by the Instituto Nacional de Técnica Aeroespacial “Esteban Terradas” (INTA) and by the Spanish MINECO project ESP2014-55811-C2-2-P. M.R.M.-Y. was supported by a research training grant from INTA. C.M. and P.E. were fellows of “Plan de Formación” from INTA. M.-P.Z. and E.G.-T. acknowledge the Spanish MINECO projects CGL2014-55230-R and CGL2015-69758-P. Additionally, we are grateful to M.T. Fernández for the recording of the FT-IR spectra.
Author Contributions
M.R.-B. conceived the project and designed the experiments. C.M., M.R.M.-Y. and P.E. performed the experiments. E.G.-T. realized the multivariate analysis. M.R.-B. and M.-P.Z. wrote the main manuscript text with inputs from C.M., M.R.M.-Y. and P.E.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-36579-7.
References
- 1.Miller SL. A Production of Amino Acids Under Possible Primitive Earth Conditions. Science. 1953;17:528–529. doi: 10.1126/science.117.3046.528. [DOI] [PubMed] [Google Scholar]
- 2.Utsumi Y, Hattori T. Synthesis of ammonium and organic compounds from N2, H2O vapour, and CO2 gas mixture by synchrontron radiation induced photochemical reactions at atmospheric pressure and at room temperature. Review of Scientific Instruments. 2002;73:1387–1389. doi: 10.1063/1.1423625. [DOI] [Google Scholar]
- 3.Takahashi J, et al. Photochemical abiotic synthesis of amino-acid precursors from simulated planetary atmospheres by vacuum ultraviolet light. J. App. Phys. 2005;98:024097. [Google Scholar]
- 4.Plankensteiner K, Reiner H, Schranz B, Rode BM. Prebiotic formation of amino acids in a neutral atmosphere by electric discharge. Angew. Chem. Int. Ed. 2004;43:1886–1895. doi: 10.1002/anie.200353135. [DOI] [PubMed] [Google Scholar]
- 5.Takano Y, Ohashi A, Kaneko T, Kobayashi K. Abiotic synthesis of high-molecular-weight organics from an inorganic gas mixture of carbon monoxide, ammonia, and water by 3 MeV proton irradiation. Appl. Phys. Lett. 2003;84:1410–1412. doi: 10.1063/1.1646757. [DOI] [Google Scholar]
- 6.Menor-Salván C, Ruiz-Bermejo M, Guzmán MI, Osuna-Esteban S, Veintemillas-Verdaguer S. Synthesis of pyrimidines and triazines in ice: implications for the prebiotic chemistry of nucleobases. Chem. Eur. J. 2009;15:4411–4418. doi: 10.1002/chem.200802656. [DOI] [PubMed] [Google Scholar]
- 7.Johnson AP, et al. The Miller volcanic spark discharge experiment. Science. 2008;322:404. doi: 10.1126/science.1161527. [DOI] [PubMed] [Google Scholar]
- 8.Ruiz-Bermejo M, Menor-Salván C, Osuna-Esteban S, Veintemillas-Verdaguer S. rebiotic Microreactors: A Synthesis of Purines and Dihydroxy Compounds in Aqueous Aerosol. Origin Life Evol Biosph. 2007;37:123–142. doi: 10.1007/s11084-006-9026-5. [DOI] [PubMed] [Google Scholar]
- 9.Ruiz-Bermejo M, Rivas AL, Palacín A, Menor-Salván C, Osuna-Esteban S. Prebiotic synthesis of protobiopolymers under alkaline ocean conditions. Orig. Life Evol. Biosph. 2011;41:331–345. doi: 10.1007/s11084-010-9232-z. [DOI] [PubMed] [Google Scholar]
- 10.Donaldson DJ, Tervahattu H, Tuck AF, Vaida V. Organic aerosols and the origin of life: an hypothesis. Orig. Life Evol. Biosph. 2004;34:57–67. doi: 10.1023/B:ORIG.0000009828.40846.b3. [DOI] [PubMed] [Google Scholar]
- 11.Dobson CM, Ellison GB, Tuck AF, Vaida V. Atmospheric aerosols as prebiotic chemical reactors. Proc. Nat. Acad. Sci. USA. 2000;97:11864–11868. doi: 10.1073/pnas.200366897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marín-Yaseli MR, González-Toril E, Mompeán C, Ruiz-Bermejo M. The role of the aqueos aerosols in the “Glyoxylate Scenario”: An experimental approach. Chem. Eu. J. 2016;22:12785–12799. doi: 10.1002/chem.201602195. [DOI] [PubMed] [Google Scholar]
- 13.Ruiz-Bermejo M, Susana Osuna-Esteban S, Zorzano M-P. Role of ferrocyanides in the prebiotic synthesis of α-amino acids. Origin. Life Evol. Biosph. 2013;43:191–206. doi: 10.1007/s11084-013-9336-3. [DOI] [PubMed] [Google Scholar]
- 14.Griffith EC, Vaida V. J. Ionization state of L‐Phenylalanine at the Air−Water Interface. J. Am. Chem. Soc. 2013;135:710–716. doi: 10.1021/ja308089n. [DOI] [PubMed] [Google Scholar]
- 15.Marín-Yaseli MR, Mompéan C, Ruiz-Bermejo M. A prebiotic synthesis of pterins. Chem. Eur. J. 2015;21:13531–13534. doi: 10.1002/chem.201502345. [DOI] [PubMed] [Google Scholar]
- 16.Griffith EC, Tuck AF, Vaida V. Ocean–atmosphere interactions in the emergence of complexity in simple chemical systems. Acc. Chem. Res. 2012;45:2106–2113. doi: 10.1021/ar300027q. [DOI] [PubMed] [Google Scholar]
- 17.Griffith E. C. & Vaida V. In situ observation of peptide bond formation at the water-air interface. Proc. Nat. Acad. Sci. USA, 10.1073/pnas.1210029109 (2012). [DOI] [PMC free article] [PubMed]
- 18.Ruiz-Bermejo M, Menor-Salván C, Osuna-Esteban S, Veintemillas-Verdaguer S. The effects of ferrous and other ions on the abiotic formation of biomolecules using aqueous aerosols and spark discharges. Origin Life Evol Biosph. 2007;37:507–521. doi: 10.1007/s11084-007-9107-0. [DOI] [PubMed] [Google Scholar]
- 19.Plankensteiner K, Reiner H, Schranz B, Rode BM. Amino acids on the rampant primordial Earth: electric discharges and the hot salty ocean. Molecular Diversity. 2006;10:3–7. doi: 10.1007/s11030-006-7009-0. [DOI] [PubMed] [Google Scholar]
- 20.Manolache S, Romanescu C, Cobialeac G, Simonescu CI. Some terpenes and related derivatives synthesized in cold plasma systems. Molecules. 1997;2:158–164. doi: 10.3390/21100158. [DOI] [Google Scholar]
- 21.Kobayashi K, Tsuchiya M, Oshima T, Yanagawa H. Abiotic synthesis of amino acids and imidazole by proton irradiation of simulated primitive Earth atmospheres. Orig. Life Evol. Biosph. 1990;20:99–109. doi: 10.1007/BF01808270. [DOI] [Google Scholar]
- 22.Lerman L. The bubble-aerosol-droplet cycle: a prebiotic geochemical reactor (that must have been) Orig. Life Evol. Biosph. 1996;26:369–370. doi: 10.1007/BF02459814. [DOI] [Google Scholar]
- 23.Ellison GB, Tuck AF, Vaida V. Atmospheric processing of organic aerosols. J. Geophys. Res. 1999;104:11633–11641. doi: 10.1029/1999JD900073. [DOI] [Google Scholar]
- 24.Eschenmoser A. The search for the chemistry of life’s origin. Tetrahedron. 2007;63:12821–12844. doi: 10.1016/j.tet.2007.10.012. [DOI] [Google Scholar]
- 25.ter Braak, C. J. F. & Šmilauer P. CANOCO reference manual and CanoDraw for Window’s user’s guide: software for canonical community ordination (version 4.5). Ithaca: Microcomputer Power. 500 p (2002).
- 26.Huber C, Wächtershäuser G. Peptides by activation of amino acids with CO on (Ni, Fe)Ssurfaces: implications for the origin of life. Science. 1998;281:670–672. doi: 10.1126/science.281.5377.670. [DOI] [PubMed] [Google Scholar]
- 27.Stribling R, Miller SL. Energy yields for hydrogen cyanide abd formaldehyde syntheses: The HCN and amino acid concentrations in the primitive ocean. Origins of Life. 1987;17:261–273. doi: 10.1007/BF02386466. [DOI] [PubMed] [Google Scholar]
- 28.Loewenthal E, Eschenmoser A. Chemistry of Potentially Prebiological Natural Products. Chem. Soc. Rev. 1992;21:1–16. doi: 10.1039/cs9922100001. [DOI] [Google Scholar]
- 29.Morowitz HJ, Kostelnik JD, Yang J, Cody GD. The origin of intermediary metabolism. Proc. Natl. Acad. Sci. USA. 2000;97:7704–7708. doi: 10.1073/pnas.110153997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keller MA, Kampjut D, Harrison SA, Ralser M. Sulfate radicals enable a non-enzymatic Krebs cycle precursor. Nature Ecol. Evol. 2017;1:0083. doi: 10.1038/s41559-017-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muchowska KB, et al. Metals promote sequences of the reverse Krebs cycle. Nature Ecology and Evolution. 2017;1:1716–1721. doi: 10.1038/s41559-017-0311-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varma SJ, Muchowska KB, Chatelain P, Moran J. Native iron reduces CO2 to intermediates and end-products of the acetyl-CoA pathway. Nature Ecology and Evolution. 2017;2:1019–1024. doi: 10.1038/s41559-018-0542-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meringer M, Cleaves HJ. Computational exploration of the chemical structure space of possible reverse tricarboxylic acid cycle constituents. Scientific Reports. 2017;7:17540. doi: 10.1038/s41598-017-17345-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zubarev DY, Rappoport D, Aspuru-Guzik A. Uncertainty of prebiotic scenarios: The case of the non-enzymatic reverse tricarboxylic acid cycle. Scientific Reports. 2015;5:8009. doi: 10.1038/srep08009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Springsteen G, Yerabolu JR, Nelson J, Rhea CJ, Krishnamurthy R. Linked cycles of oxidative decarboxylation of glyoxylate as protometabolic analogs of the citric acid cycle. Nature Comm. 2018;9:9. doi: 10.1038/s41467-017-02591-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohammed FS, et al. A Plausible Prebiotic Origin of Glyoxylate: Nonenzymatic Transamination Reactions of Glycine with Formaldehyde. Synlett. 2017;28:93–97. [Google Scholar]
- 37.Menor-Salvan C, Marin-Yaseli MR. A New Route for the Prebiotic Synthesis of Nucleobases and Hydantoins in Water/Ice Solutions Involving the Photochemistry of Acetylene. Chem.-Eur. J. 2013;19:6488–6497. doi: 10.1002/chem.201204313. [DOI] [PubMed] [Google Scholar]
- 38.Yu H, Zhang S, Chaput JC. Darwinian evolution of an alternative genetic system provides support for TNA as an RNA progenitor. Nat. Chem. 2012;4:183–187. doi: 10.1038/nchem.1241. [DOI] [PubMed] [Google Scholar]
- 39.Krueger AT, Kool ET. Redesigning the architecture of the base pair: toward biochemical and biological function of new genetic sets. Chem. Biol. 2009;16:242–248. doi: 10.1016/j.chembiol.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hud N. Our Odyssey to Find a Plausible Prebiotic Path to RNA: The First Twenty Years. Synlett. 2017;28:36–55. doi: 10.1055/s-0036-1588646. [DOI] [Google Scholar]
- 41.Hayatsu R, Studier MH, Oda A, Fuse K, Anders E. Organic matter in the solar system II. Nitrogen compounds. Geochim. Cosmochim. Acta. 1968;32:175–190. doi: 10.1016/S0016-7037(68)80003-1. [DOI] [Google Scholar]
- 42.Jeilani YA, Orlando TM, Pope A, Pirim C, Nguyen MT. Prebiotic synthesis of triazines from urea: a theoretical study of free radical routes to melamine, ammeline, ammelide and cyanuric acid. RSC Adv. 2014;4:32375–32382. doi: 10.1039/C4RA03717K. [DOI] [Google Scholar]
- 43.Schneider KC, Benner SAJ. Oligonucleotides containing flexible nucleoside analogs. J. Am. Chem. Soc. 1990;112:453–455. doi: 10.1021/ja00157a073. [DOI] [Google Scholar]
- 44.Zhang L, Peritz A, Meggers EA. A Simple Glycol Nucleic Acid. J. Am. Chem. Soc. 2005;127:4174–4175. doi: 10.1021/ja042564z. [DOI] [PubMed] [Google Scholar]
- 45.Karri P, Punna V, Kim K, Krishnamurthy R. Base-pairing properties of a structural isomer of glycerol nucleic acid. Angew. Chem., Int. Ed. 2013;52:5840–5844. doi: 10.1002/anie.201300795. [DOI] [PubMed] [Google Scholar]
- 46.Cooper G, et al. Carbonaceous meteorites as a source of sugar-related organic compounds for the early Earth. Nature. 2001;414:879–883. doi: 10.1038/414879a. [DOI] [PubMed] [Google Scholar]
- 47.Nuevo M, Bredehoft JH, Meierhenrich UJ, d’Hendecourt L, Thiemann WHP. Urea, Glycolic Acid, and Glycerol in an Organic Residue Produced by Ultraviolet Irradiation of Interstellar/Pre-Cometary Ice Analogs. Astrobiology. 2010;10:245–256. doi: 10.1089/ast.2009.0358. [DOI] [PubMed] [Google Scholar]
- 48.Kaiser RI, Maity S, Jones BM. Synthesis of Prebiotic Glycerol in Interstellar Ices. Angew. Chem., Int. Ed. 2015;54:195–200. doi: 10.1002/anie.201408729. [DOI] [PubMed] [Google Scholar]
- 49.Hernandez-Rodriguez M, Xie J, Osornio YM, Krishnamurthy R. Mapping the Landscape of Potentially Primordial Informational Oligomers: (3′- > 2′)-D-Phosphoglyceric Acid Linked Acyclic Oligonucleotides Tagged with 2,4-Disubstituted 5-Aminopyrimidines as Recognition Elements. Chem.-Asian J. 2011;6:1252–1262. doi: 10.1002/asia.201000828. [DOI] [PubMed] [Google Scholar]
- 50.Parker ET, Cleaves HJ, Bada JL, Fernández FM. Quantification of α-hydroxy acids in complex mixtures via liquid chromatography/tandem mass spectrometry. Rapid. Commun. Mass. Spectrom. 2016;30:2043–2051. doi: 10.1002/rcm.7684. [DOI] [PubMed] [Google Scholar]
- 51.Forsythe JG, et al. Ester-Mediated Amide Bond Formation Driven by Wet-Dry Cycles: A Possible Path to Polypeptides on the Prebiotic Earth. Angew. Chem., Int. Ed. 2015;54:9871–9875. doi: 10.1002/anie.201503792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Marcellus P, Bertrand M, Nuevo M, Westall F, d’Hendecourt LL. Prebiotic Significance of Extraterrestrial Ice Photochemistry: Detection of Hydantoin in Organic Residues. Astrobiology. 2011;11:847–854. doi: 10.1089/ast.2011.0677. [DOI] [PubMed] [Google Scholar]
- 53.Hurowitz JA, et al. Redox stratification of an ancient lake in Gale crater, Mars. Science. 2017;356:eaah6849. doi: 10.1126/science.aah6849. [DOI] [PubMed] [Google Scholar]
- 54.Donaldson DJ, Tuck AF, Vaida V. Spontaneous fission of atmospheric aerosol particles. Phys. Chem. Chem. Phys. 2001;3:5270–5273. doi: 10.1039/b105215m. [DOI] [Google Scholar]
- 55.Eschenmoser A. On a Hypothetical Generational Relationship between HCN and Constituents of the Reductive Citric Acid Cycle. Origin. Life Evol. Biosph. 2007;43:554–573. doi: 10.1002/cbdv.200790050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.