Abstract
Objective
This study aims to determine an appropriate timeline to monitor indeterminate pulmonary nodules (IPN) in melanoma patients to confirm metastatic origin.
Materials and Methods
588 clinically non-metastatic melanoma patients underwent curative intent surgery during 3 years. Patients with baseline chest CT and at least one follow-up (FU) CT were retrospectively analyzed to assess for IPN. Patients with definitely benign nodules, metastases and non-melanoma malignancies were excluded. Change in volume from first to FU CT, initial diameter (D1) and volume (V1), distance from pleura, peripheral and perifissural location, density and clinical stage were evaluated. Nodules were volumetrically measured on CTs and were considered metastases if they increased in size between two CTs or if increase was accompanied by multiple new nodules or extrapulmonary metastases.
Results
148 patients were included. Two out of 243 baseline IPN detected in 70 patients, increased significantly in volume in 3 and 5 months and were proven metastases. During FU, 86% of 40 interval IPN detected in 28 patients, were proven metastases. Interval nodule (p < 0.0001, HR:243,CI:[57.32,1033.74]), 3-month volume change (OR:1.023,CI:[1.014,1.033]), V1 (OR:1.006,CI:[1.003,1.009]), D1 (OR:1.424,CI:[1.23,1.648]), distance from pleura (OR:1.03,CI:[1.003,1.059]), and combined stage IIC + III (OR:11.29,CI:[1.514,84.174]), were associated with increased risk for metastasis. 43%, 72% and 94% of patients with IPN were confirmed with metastases in the first FU CT at 3, 6 and 12 months respectively.
Conclusion
Baseline IPN are most likely benign, while interval IPN are high risk for metastasis. Absence of volume increase of IPN within 6 months excluded metastasis in most patients.
Abbreviations: IPN, indeterminate pulmonary nodules; FU, follow-up; GGO, ground glass opacity
Keywords: Multiple pulmonary nodules, X-ray computed tomography, Melanoma, Metastasis
1. Introduction
The management of pulmonary nodules detected in melanoma patients is a challenge for physicians as the etiology of these nodules is usually uncertain and metastatic etiology cannot be excluded. More than 80% of patients with metastatic melanoma initially show only one distant organ site involvement, most commonly the lungs [1]. Approximately 40% of cases initially show isolated pulmonary metastasis [2]. Early detection of small pulmonary metastases is of paramount importance as improved survivals of 18%–39% have been reported for pulmonary metastasectomy compared to the 5-year survival of 3%–5% for non-surgical treatment [[2], [3], [4]].
Advances in CT have increased detection of pulmonary nodules, most of which are too small to biopsy or to be characterized by PET/CT and definitive diagnosis is only established by surgery [5,6]. In this clinical context, it is common practice to recommend a 3-month FU CT for a new pulmonary nodule; however there is no agreement on how long the nodule should be monitored if it remains unchanged or if there is small increase in size on FU. Moreover, there are no guidelines regarding frequency and overall duration of FU for IPN identified on baseline CT of melanoma patients since Fleischner guidelines are applicable only for non-oncologic patients [7].
The primary outcome of our study was to explore the long-term behavior of small IPN detected either at initial staging CT or during the FU to determine an appropriate time-frame within which metastatic etiology of IPN can safely be excluded. Secondary outcome was to identify morphological and clinical predictors that would allow differentiation between benign and metastatic pulmonary nodules.
2. Materials and methods
2.1. Patients
This retrospective study included nonmetastastic consecutive patients surgically treated with curative intent for localized melanoma in our institution from September 2012 to August 2015. Research Ethics Board approved the study and consent form was waived as it was retrospective. Inclusion criteria included presence of baseline chest CT, FU CT ≥ 3 months after the baseline, and overall imaging FU for at least 12 months, unless the nodule was presumed metastatic earlier than 12 months or had resolved or improved. Patients were excluded from the study if they had definitely benign or metastatic nodules at baseline staging CT, if they had extrapulmonary metastases and/or other non-melanoma malignancies or if they received chemotherapy.
IPN were defined according to Beigelman-Aubry et al definition as “solid or subsolid nodules smaller than 10 mm in diameter with nonspiculated contours, no air bronchogram or pseudocavitation, no malignant-type calcification, and no intralesional fat or benign-type calcification” [8]. IPN detected at baseline CT were called “baseline IPN” and were followed until the last available FU CT upon completion of the study. IPN that newly developed during FU were called “interval IPN” and were also recorded. They were followed until proven metastatic or benign, or until the last available CT upon completion of the study.
2.2. CT protocol
All chest CT examinations - baseline and during FU - were performed using the same type of CT scanner (GE LightSpeed VCT 64 multidetector CT scanner) and the following routine clinical protocol for metastatic work-up in our department; tube voltage 120 kV, effective tube current 200 mAs with dose modulation, pitch (0.984:1), detector width 40 mm, matrix 512 × 512 and 480 mm field of view, image reconstruction with standard and lung kernel, collimation 0.625 mm, slice reconstruction thickness 2.5 mm and reconstruction interval 2 mm. All but 10 patients underwent enhanced chest CT.
2.3. Evaluation and measurement of pulmonary nodules
CT images were read in consensus by 2 radiologists: a thoracic radiologist with 15-year experience and a second year thoracic imaging fellow. Baseline and FU CTs of each patient were reviewed and nodules were evaluated on lung windows at a width of 1600 HU and a level of −700 HU. Baseline and interval IPN were volumetrically measured using automated software for volumetry of nodules (TeraRecon iNtuition, version 4.1.12). The 2 readers decided on the detection of a pulmonary nodule and marked it with a mouse click and subsequently the software automatically segmented the volume of interest of the nodule. Manual modification of segmentation was used whenever the automated procedure was not applicable, i.e.; close to bony thoracic cage or adjacent to pulmonary vessels. Semiautomated measurements including volume, maximum and minimum dimensions and area of each nodule were provided by the software and are highly reproducible for most nodules [9].
2.4. Nodule morphological characteristics
Nodules were considered metastases on FU CT if they increased in volume on 2 sequential CTs, if increase in size was accompanied by development of new pulmonary nodules and/or extrapulmonary metastases, or based on histology. Nodules were considered benign if they decreased in size or resolved on FU or if they were stable (<15% change in volume) in size for 12 months or longer. Other parameters included: distance from costal pleura, peripheral versus central location with a nodule classified as “peripheral” when the distance to costal pleura was < one third of the total distance of hilum to costal pleura, perifissural location - defined as location of the nodule adjacent to a major, minor or accessory fissure where the distance from the fissure is 0 mm, irregular versus smooth margin, and solid versus GGO density.
2.5. Nodule follow-up
A cut-off of 15% difference in volume was considered significant change for the purpose of this study and was not considered a priori progression of disease. This was taken arbitrarily lower than 20% which was the threshold for progression of disease in RECIST 1.1 criteria in order not to miss any small change that might indicate early development of metastatic disease [10]. Percent differences of volume measurements between baseline and subsequent CT (V1, V2 for baseline IPN) and between CT of first appearance and subsequent CT (V1, V2 for interval IPN) - when metastatic versus benign etiology was confirmed - were calculated.
For baseline IPN, the following two periods of time were calculated: a) time between the baseline and the last available FU CT and b) elapsed period between baseline and subsequent CT when metastatic etiology was confirmed. Similarly, for interval IPN, 2 periods of time were calculated: a) elapsed time between baseline and subsequent CT where the nodule first appeared and b) time between CT where the nodule first appeared and subsequent CT where the nodule was confirmed metastatic.
Patient demographic and clinical data was collected including age, sex, clinicopathologic stage at initial diagnosis [11]), date of baseline CT and each FU CT, volumetric and bidimensional measurements and morphological characteristics on baseline CT (for baseline IPN) and on CT of first appearance (for interval IPN).
3. Statistical analysis
Continuous variables were summarized as means with standard deviations (SD) or medians with 25–75% interquartile ranges and minimum to maximum. For independent continuous variable comparisons, the t-test or Mann Whitney U test was used, where appropriate. Categorical variables were expressed as proportions with percentages and statistical comparisons were performed by Chi-square test or Fisher’s exact test where appropriate.
Binary logistic regression analysis, and stepwise logistic regression analysis were performed to identify the predictors of metastatic etiology of the IPN including being an interval nodule (versus baseline), initial volume (V1), initial diameter (D1), %change of volume from baseline/first to FU CT [%(V2-V1)/V1], distance from pleura, solid versus GGO density, central versus peripheral, perifissural location, irregular margin and clinicopathologic stage. Receiver Operating Characteristic (ROC) curve analysis was also performed for predictors which showed significance and the optimal cut-off values based on maximizing sensitivity and specificity of the continuous variables were identified.
Time to event analysis (event was considered the confirmation of metastatic etiology of an IPN) was used to investigate time to diagnosis of metastatic etiology for baseline and interval IPN. Cox proportional hazard model was used with the sandwich estimate of variance to account for cluster effect within patients for nodules.
All analyses were two-tailed, and p values less than or equal to 0.05 were considered significant. The statistical analyses described above were performed using SAS version 9.4 for windows (SAS Institute, Cary, North Carolina, USA) and IBM SPSS statistical software (IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp).
4. Results
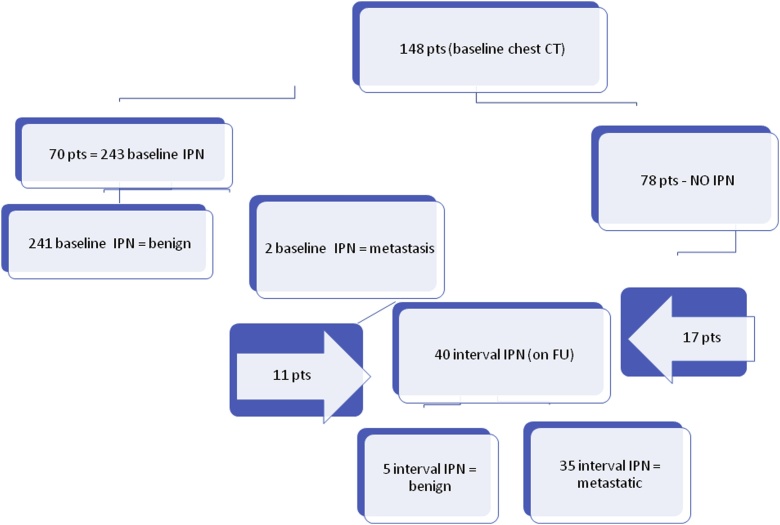
588 patients underwent primary surgery for melanoma between September 2012 and August 2015. 148 patients had baseline chest CT and FU CT at ≥12 months and were included in the study. On baseline CT, 70 patients (group A) had at least 1 IPN and the remaining 78 patients (group B) had no nodules detected (Fig. 1).
Fig. 1.
Flowchart showing the detection of IPN nodules at baseline chest CT (baseline IPN*) and during follow-up (interval IPN). *IPN: indeterminate pulmonary nodule.
4.1. IPN at baseline chest CT
A total of 243 IPN were identified on baseline CT (baseline IPN) (Fig. 1). They were followed for a median of 17 months (range: 12–42) with a median of 3 subsequent chest CTs (range: 2–5). Only 2 out of 243 nodules were proven metastatic and demonstrated 405% and 7326% increase in volume on 5 and 4-month FU CT respectively. The remaining nodules either remained stable (48) demonstrating <15% change in volume, decreased in size (83) or completely resolved (17) (Fig. 2). 94 nodules initially demonstrated 16% median volume increase in the first FU and either resolved or decreased in size in the second FU (Table 1).
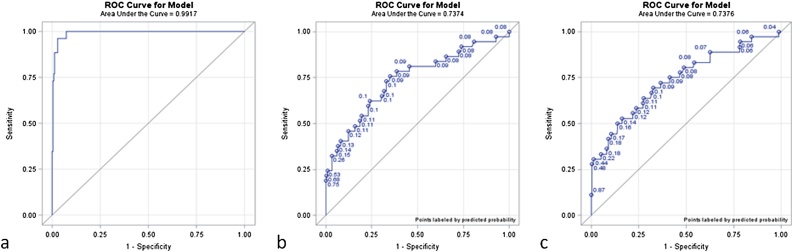
Fig. 2.
ROC curves of the regression analysis for significant independent univariable parameters: a) % increase V2**-V1*/V1, b) initial V1, c) initial D1***. *V1: volume of nodule at initial CT, **V2: volume of nodule at first follow-up CT, ***D1: diameter of nodule at initial CT.
Table 1.
Follow-up of 243 baseline IPN (group A).
| Metastasis (%) | 2 / 243 (1) |
|---|---|
| Stable (%) | 48 / 243 (19) |
| Decreased in volume > 15% (%) | 83 / 243 (34) |
| Initially increased and later decreased or resolved (%) | 94 / 243 (39) |
| Resolved (%) | 17 / 243 (7) |
| Median time to resolution - months (range) | 7 (3–21) |
4.2. IPN newly developed during FU
During FU of both groups (A and B), overall 28 patients developed 40 new IPN. Of the 40 interval nodules, 35 nodules in 25 patients (8 patients -group A, 17 patients - group B) proved metastatic (Fig. 1). Patients’ demographics with interval IPN nodules are shown in Table 2.
Table 2.
Demographics of patients with interval IPN nodules.
| IPN proven benign | IPN proven metastases | |
|---|---|---|
| Patients (n) | 73 | 27 |
| Female | 39/73 | 4/27 |
| Age, median (range) | 62 (24–85) | 56 (31–87) |
| Stage I (%) | 4 | 0 |
| Stage IIA (%) | 4 | 0 |
| Stage IIB (%) | 10 | 4 |
| Stage IIC (%) | 8 | 11 |
| Stage III (%) | 74 | 85 |
Table 3 compares characteristics between metastatic and non-metastatic nodules. The median change in volume (%[V2-V1]/V1) in benign IPN was decrease by 7%. Benign IPN that initially increased in size had a median volume increase of 16%. On the contrary metastatic nodules had a volume increase on FU CT, within a median 3-month interval time, was 326% (p < 0.0001) (Table 3).
Table 3.
Univariate comparison of characteristics between metastatic and non-metastatic nodules.
| IPN proven benign (n = 246) |
IPN proven metastases (n = 37) |
P | |||||
|---|---|---|---|---|---|---|---|
| Initial V1, median (25%-75%), (min-max) mm3 | 24.3 | (13.4–50.3) | (4.29–568) | 67.4 | (39.5–272) | (4.14–22,000) | <0.001* |
| Initial D1, median (25%-75%), (min-max) mm | 4.17 | (3.48–5.34) | (1–13.6) | 6.2 | (4.37–10.95) | (2.1–42) | <0.001* |
| %Change V2-V1/V1, median (25%-75%), (min-max) % | −7 | (−31.73–10.73) | (−100–499) | 326 | (118.67–646.37) | (40–7326) | <0.001* |
| %Increase V2-V1/V1, median (25%-75%), (min-max) % | 16 | (8.61–30.1) | (1–499) | 326 | (118.67–646.37) | (40–7326) | <0.001* |
| Distance from pleura, median(25%-75%), (min-max) mm | 2 | (0–10) | (0–56) | 7 | (3–17) | (0–38) | 0.006* |
| Solid, n (%) | 221 | (90) | 37 | (100) | 0.054♦ | ||
| GGO, n (%) | 25 | (10) | 0 | (0) | 0.054♦ | ||
| Central, n (%) | 28 | (11) | 5 | (14) | 1.000♦ | ||
| Peripheral, n (%) | 218 | (89) | 30 | (81) | 0.102♦ | ||
| Perifissural, n (%) | 41 | (17) | 4 | (11.1) | 0.475♦ | ||
| Irregular margin, n (%) | 4 | (2) | 1 | (3) | |||
Mann-Whitney test for continuous variables.
Fisher exact test for categorical variables.
The median period between baseline CT and first appearance of metastatic interval IPN was 11 months (range: 2–29 months). The median elapsed time between first appearance of interval IPN and FU CT when metastasis was confirmed was 3 months.
The 5 benign interval IPN in 3 patients, resolved or decreased in a median 3-month interval time without chemotherapy. There was an 8-month median period between baseline CT and first appearance of interval IPN.
4.3. Independent predictors of metastasis
We compared the imaging and clinical characteristics between metastatic and benign IPN. The analyses were done independently for each variable. A multivariable model was not possible due to multicollinearity between variables and model instability due to complete separation. The strongest univariable predictor was the percent change of volume which explained 71% (Nagelkerke R2) of variance of IPN (OR:1.02). Initial volume (V1) (OR 1.006), initial diameter (D1) (OR:1.42) and distance from costal pleura (OR:1.03) were statistically significantly higher in metastatic compared to benign IPN. Metastatic IPN were significantly associated with stage IIC&III at diagnosis (OR:11.29) compared to lower stages (Table 4). Solid density was more common in metastatic IPN but this was not statistically significant (Table 3).
Table 4.
Logistic regression analysis on each variable (independently).
| B | SE | Wald | p | Odds ratio | 95% C.I. |
Nagelkerke R2 | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Initial V1, (mm3) | 0.00584 | 0.00143 | 16.65 | <0.0001 | 1.006 | 1.003 | 1.009 | 22.59 |
| Initial D1 | 0.3534 | 0.0745 | 22.51 | <0.0001 | 1.424 | 1.23 | 1.648 | 22.70 |
| % Change V2-V1/V1 | 0.0230 | 0.004 | 24.32 | <0.0001 | 1.023 | 1.014 | 1.033 | 70.76 |
| Distance from pleura | 0.0298 | 0.0139 | 4.5883 | 0.0322 | 1.03 | 1.003 | 1.059 | 2.75 |
| Stage IIC&III | 1.212 | 0.5125 | 5.5925 | 0.018 | 11.29 | 1.514 | 84.174 | 7.8 |
4.4. Optimal cut-off values distinguishing metastatic IPN
ROC analysis identified a percent volume change (%[V2-V1]/V1) cut-off value of 51% (95%CI: 36.76–85.47) with sensitivity 96.2% and specificity 95.9% to distinguish metastatic from benign nodules (AUC: 0.9917).
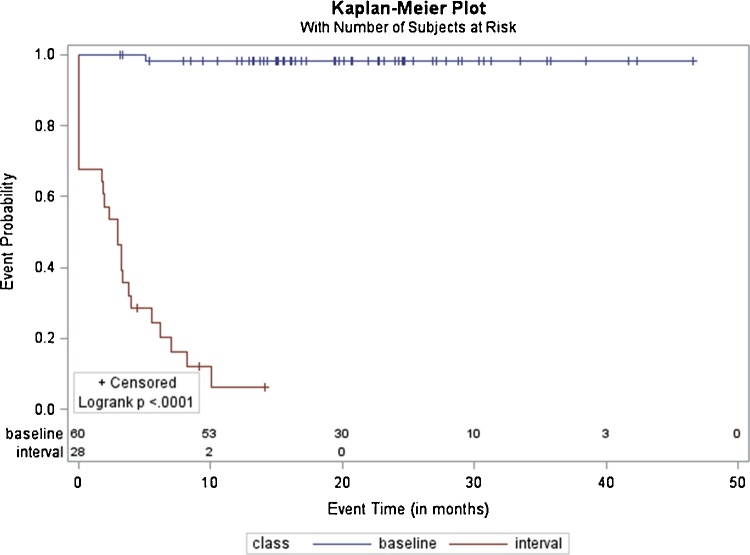
4.5. Time to event analysis
Time to event analysis (event was considered the confirmation of metastasis) showed that patients with baseline nodules only, would rarely prove to have metastases (Fig. 3). Development of an interval nodule is the best predictor of metastasis (p < 0.0001, HR: 243, 95%CI:[57.32, 1033.74]).
Fig. 3.
Kaplan-Meier plot shows time to event analysis for patients with an IPN nodule found at baseline chest CT (blue graph), or with an IPN nodule found at baseline and or during follow up (red graph) to be confirmed having pulmonary metastatic disease (event). Y axis shows the probability of not being confirmed with pulmonary metastatic disease and x axis shows the time to event (in months). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
43% of patients with both baseline and/or interval nodules were confirmed with metastases in 3 months, 72% in 6 months and 94% in 12 months (Fig. 3).
5. Discussion
Our study shows that change in volume on 3-month CT, initial volume, diameter, stage, and distance from pleura are independent predictors of metastatic etiology of IPN in melanoma. Baseline IPN are most likely benign, while IPN detected during FU have a high risk of being metastatic. 43% and 72% of patients with baseline or interval metastatic IPN were confirmed at 3 and 6 months respectively.
The larger the initial volume and diameter, the more likely for an IPN is to be metastatic. Size was a reliable predictor of malignancy in lung cancer screening studies where the risk of cancer in nodules <6 mm was considerably less than 1% even in high-risk patients [12,13]. In a study about colorectal cancer, size of IPN was not found to be a significant factor for development of metastases [14]. The authors reported that this might have been associated with the increased slice thickness used. In another study investigating the significance of pulmonary nodules <4 mm in oncologic patients, 28% of patients at initial staging were found to have metastases [15]. However, in that study, patients with a variety of primary malignancies, disease stage and receiving chemotherapy were included.
In our study, although most of the benign IPN decreased in volume during FU, some IPN increased and then subsequently decreased/resolved. This can be explained by the evolving nature of inflammation or infection. The change in volume on 3-month FU was found to be a predictor of metastasis of IPN in melanoma. Volumetric measurement has been found to be more accurate than uni- or bidimensional measurements of nodules and to depict more subtle changes between studies [16,17]. This is reasonable knowing that a nodule measuring 5 mm in diameter (65 mm3) will have doubled in volume when it measures 6.3 mm [18]. Moreover it has been reported that volumetry was underestimated manually by 24.1% for nodules of any density compared with 7.6% semi-automatically, as performed in our study [19].
Our results showed that baseline IPN in melanoma are frequent, seen in 47% of cases. Similarly, in a study for renal cell carcinoma, IPN were seen in 38% of patients [20]. However, in a study for colorectal cancer, the incidence was as low as 4% [14]. In lung cancer screening studies, although the individuals were not oncologic, IPN ranged from 8 to 51% in different studies [[21], [22], [23]] rendering the detection of IPN is a huge clinical burden.
Although baseline IPNs were frequently detected in our study, these were almost always proven benign on FU. Only 2/243 nodules proved metastatic on FU CT and this was confirmed on 5 and 4-month FU. Similarly in another study about colorectal cancer, only 1/100 patients had an IPN on preoperative CT that proved metastatic. Such low risk suggested that IPN should not cause further preoperative work-up or FU aside from routine regimens [24]. In a study about biopsied lung nodules in melanoma, 32% of the nodules detected at initial diagnosis were metastases, however, patients with extrapulmonary metastases and non-melanoma cancers were also included increasing the possibility of biopsied nodules being malignant [25]. Munden et al. reported that 28% of oncologic patients with IPN measuring ≤4 mm showed increased size on FU and were considered metastases [15]. However patients with different primary malignancies were included and clinical stage was not reported. In our study, stage IV patients or under chemotherapy were excluded. In a study based on data from Nelson trial for screening lung cancer, incidental solid IPNs on baseline study were mostly stable (89.8%), while resolved nodules were 10.2%. Only 3.1% of stable nodules proved malignant [26].
Our study showed that interval IPN are high risk for being metastatic. 85% of the interval nodules were proven metastatic. No other study specifically investigated the difference in the incidence of metastasis between baseline and interval IPN. 43% of patients with metastatic nodules were confirmed at 3 months, 72% in 6 months and 94% in 12 months. However FU CT was not consistently done at 3 months and in many cases it might have been delayed up to 6 months or even longer for various reasons including cancellations, delays or missed appointments. These are limitations inherent to the retrospective nature of the study. One might speculate that if all nodules were reassessed precisely at 3-month time interval, all of them might have proven metastatic earlier. Munden et al reported that 15% of oncologic patients who had pulmonary metastases showed increase of nodule size in 3 months and 25% in 6 months. This may partly be explained by the large variety of primary malignancies included in that study, which might have different aggressiveness and propensity to metastasize to the lungs compared to the homogeneous population with melanoma in our study [15].
In oncologic patients, there are no specific guidelines for the FU of IPNs on baseline staging or FU CT. Most radiologists depend on their own experience and on institutional guidelines for management. A FU CT chest after 3 months is the most common recommendation when IPN is detected in oncologic patients [27]. Our study has shown that 3-month FU is critical in confirming significant increase in size of metastatic nodules and if there is borderline increase in size then another FU in 6 months will confirm metastatic etiology in the majority of the nodules. A cut-off value of 51% percent increase in volume from CT of the first appearance of the nodule until subsequent FU CT, was found to be significant indicator of metastatic etiology. On the contrary if a nodule is stable for 6 months it is highly unlikely that this is metastatic.
Distance from pleura differentiated metastatic from benign IPN. Nodules closer to the pleura and fissures were most likely benign and consistent with intraparenchymal nodes. In another study more intraparenchymally located IPN were more likely metastases [24]. IPNs that are solid, peripheral, subpleural or polygonal with smooth margins are usually benign - representing mostly intrapulmonary nodes [28]. In our study although many perifissural nodules were benign, perifissural location was not a predictor of metastasis. However, 11% of metastatic interval IPN were perifissural with smooth margins. Therefore, perifissural location does not exclude metastasis and short-term FU is strongly recommended.
Combination of stage IIC and III was found to be an independent predictor of metastatic etiology [11]. Similarly in a study about colorectal cancer, tumor stage correlated with progression of IPN to metastasis [14].
Limitations of the study include its retrospective nature, the inability to precisely define the time of nodule increase or resolution, the large variability of interval periods between baseline and FU CTs and the lack of histologic confirmation. Finally another limitation could be considered that some of the interval pulmonary nodules might not have been recorded if the patients with baseline IPNs were excluded due to follow up shorter than 12 months.
In conclusion, we have shown that baseline IPN are more likely benign, while interval IPN are high risk for metastases. FU in 3 months and if need be in 6 months will confirm the metastatic etiology in most cases. If a nodule is stable or regressed in 6 months in a patient not receiving treatment, it is highly unlikely that it will start growing after 6 months and prove metastatic. Future research should aim at establishing guidelines with specific recommendations regarding frequency and duration of monitoring of IPN in patients with different primary malignancies.
Declarations of interest
None.
Contributor Information
Magdy Soliman, Email: mezzelarab@hotmail.com.
Teresa Petrella, Email: teresa.petrella@sunnybrook.ca.
Pascal Tyrrell, Email: pascal.tyrrell@utoronto.ca.
Frances Wright, Email: Frances.Wright@sunnybrook.ca.
Nicole J. Look Hong, Email: Nicole.LookHong@sunnybrook.ca.
Hua Lu, Email: henryhua.lu@mail.utoronto.ca.
Petros Zezos, Email: pzezos@nosm.ca.
Laura Jimenez-Juan, Email: laura.jimenezjuan@sunnybrook.ca.
Anastasia Oikonomou, Email: anastasia.oikonomou@sunnybrook.ca.
References
- 1.Younes R., Abrao F.C., Gross J. Pulmonary metastasectomy for malignant melonoma: prognostic factors for long-term survival. Melanoma Res. 2013;23:307–311. doi: 10.1097/CMR.0b013e3283632cbe. [DOI] [PubMed] [Google Scholar]
- 2.Petersen R.P., Hanish S.I., Haney J.C. Improved survival with pulmonary metastasectomy: an analysis of 1720 patients with pulmonary metastatic melanoma. J. Thorac. Cardiovasc. Surg. 2007;133(2007):104–110. doi: 10.1016/j.jtcvs.2006.08.065. [DOI] [PubMed] [Google Scholar]
- 3.Mrazek A.A., Chao C. Surviving cutaneous melanoma: a clinical review of follow-up practices, surveillance, and management of recurrence. Surg. Clin. North Am. 2014;94:989–1002. doi: 10.1016/j.suc.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coit D.G., Thompson J.A., Algazi A. Melanoma, version 2.2016, NCCN clinical practice guidelines in oncology. J. Compr. Canc. Netw. 2016;14:450–473. doi: 10.6004/jnccn.2016.0051. [DOI] [PubMed] [Google Scholar]
- 5.Mosmann M.P., Borba M.A., de Macedo F.P., Liguori Ade A., Villarim Neto A., de Lima K.C. Solitary pulmonary nodule and (18)F-FDG PET/CT. Part 2: accuracy, cost-effectiveness, and current recommendations. Radiol. Bras. 2016;49:104–111. doi: 10.1590/0100-3984.2014.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winokur R.S., Pua B.B., Sullivan B.W., Madoff D.C. Percutaneous lung biopsy: technique, efficacy, and complications. Semin. Intervent. Radiol. 2013;30:121–127. doi: 10.1055/s-0033-1342952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacMahon H., Naidich D.P., Goo J.M. Guidelines for management of incidental pulmonary nodules detected on CT Images: from the Fleischner Society 2017. Radiology. 2017;284:228–243. doi: 10.1148/radiol.2017161659. [DOI] [PubMed] [Google Scholar]
- 8.Beigelman-Aubry C., Hill C., Grenier P.A. Management of an incidentally discovered pulmonary nodule. Eur. Radiol. 2007;17:449–466. doi: 10.1007/s00330-006-0399-7. [DOI] [PubMed] [Google Scholar]
- 9.Massion P.P., Walker R.C. Indeterminate pulmonary nodules: risk for having or for developing lung cancer? Cancer Prev. Res. (Phila) 2014;7:1173–1178. doi: 10.1158/1940-6207.CAPR-14-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisenhauer E.A., Therasse P., Bogaerts J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Balch C.M., Gershenwald J.E., Soong S.J. Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horeweg N., van Rosmalen J., Heuvelmans M.A. Lung cancer probability in patients with CT-detected pulmonary nodules: a prespecified analysis of data from the NELSON trial of low-dose CT screening. Lancet Oncol. 2014;15:1332–1341. doi: 10.1016/S1470-2045(14)70389-4. [DOI] [PubMed] [Google Scholar]
- 13.McWilliams A., Tammemagi M.C., Mayo J.R. Probability of cancer in pulmonary nodules detected on first screening CT. N. Engl. J. Med. 2013;369:910–919. doi: 10.1056/NEJMoa1214726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quyn A.J., Matthews A., Daniel T., Amin A.I., Yalamarthi S. The clinical significance of radiologically detected indeterminate pulmonary nodules in colorectal cancer. Colorectal Dis. 2012;14:828–831. doi: 10.1111/j.1463-1318.2011.02722.x. [DOI] [PubMed] [Google Scholar]
- 15.Munden R.F., Erasmus J.J., Wahba H., Fineberg N.S. Follow-up of small (4 mm or less) incidentally detected nodules by computed tomography in oncology patients: a retrospective review. J. Thorac. Oncol. 2010;5:1958–1962. doi: 10.1097/JTO.0b013e3181f2636e. [DOI] [PubMed] [Google Scholar]
- 16.Marten K., Auer F., Schmidt S., Kohl G., Rummeny E.J., Engelke C. Inadequacy of manual measurements compared to automated CT volumetry in assessment of treatment response of pulmonary metastases using RECIST criteria. Eur. Radiol. 2006;16:781–790. doi: 10.1007/s00330-005-0036-x. [DOI] [PubMed] [Google Scholar]
- 17.Revel M.P., Bissery A., Bienvenu M., Aycard L., Lefort C., Frija G. Are two-dimensional CT measurements of small noncalcified pulmonary nodules reliable? Radiology. 2004;231:453–458. doi: 10.1148/radiol.2312030167. [DOI] [PubMed] [Google Scholar]
- 18.Gavrielides M.A., Kinnard L.M., Myers K.J., Petrick N. Noncalcified lung nodules: volumetric assessment with thoracic CT. Radiology. 2009;251:26–37. doi: 10.1148/radiol.2511071897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie X., Zhao Y., Snijder R.A. Sensitivity and accuracy of volumetry of pulmonary nodules on low-dose 16- and 64-row multi-detector CT: an anthropomorphic phantom study. Eur. Radiol. 2013;23:139–147. doi: 10.1007/s00330-012-2570-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu R., Horick N., McGovern F.J. Prognostic significance of indeterminate lung nodules in renal cell carcinoma. Urol. Oncol. 2014;32:355–361. doi: 10.1016/j.urolonc.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Ruparel M., Quaife S.L., Navani N., Wardle J., Janes S.M., Baldwin D.R. Pulmonary nodules and CT screening: the past, present and future. Thorax. 2016;71:367–375. doi: 10.1136/thoraxjnl-2015-208107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toghiani A., Adibi A., Taghavi A. Significance of pulmonary nodules in multi-detector computed tomography scan of noncancerous patients. J. Res. Med. Sci. 2015;20:460–464. doi: 10.4103/1735-1995.163967. PMID:26487874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henschke C.I., Yankelevitz D.F., Naidich D.P. CT screening for lung cancer: suspiciousness of nodules according to size on baseline scans. Radiology. 2004;231:164–168. doi: 10.1148/radiol.2311030634. [DOI] [PubMed] [Google Scholar]
- 24.Nordholm-Carstensen A., Wille-Jørgensen P.A., Jorgensen L.N., Harling H. Indeterminate pulmonary nodules at colorectal cancer staging: a systematic review of predictive parameters for malignancy. Ann. Surg. Oncol. 2013;20:4022–4030. doi: 10.1245/s10434-013-3062-y. [DOI] [PubMed] [Google Scholar]
- 25.Smyth E.C., Hsu M., Panageas K.S., Chapman P.B. Histology and outcomes of newly detected lung lesions in melanoma patients. Ann. Oncol. 2012;23:577–582. doi: 10.1093/annonc/mdr364. Epub 2011 Aug 4. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Y.R., Heuvelmans M.A., Dorrius M.D. Features of resolving and nonresolving indeterminate pulmonary nodules at follow-up CT: the NELSON study. Radiology. 2014;270:872–879. doi: 10.1148/radiol.13130332. Epub 2013 Nov 18. [DOI] [PubMed] [Google Scholar]
- 27.Occhipinti M., Heidinger B.H., Pfannenberg C., Munden R.F., Eisenberg R.L., Bankier A.A. Managing incidental lung nodules in patients with a history of oncologic disease: a survey of thoracic radiologists. J. Thorac. Imaging. 2017;32:115–120. doi: 10.1097/RTI.0000000000000231. [DOI] [PubMed] [Google Scholar]
- 28.Takashima S., Sone S., Li F. Small solitary pulmonary nodules (< or =1 cm) detected at population-based CT screening for lung cancer: Reliable high-resolution CT features of benign lesions. AJR Am. J. Roentgenol. 2003;180:955–964. doi: 10.2214/ajr.180.4.1800955. PMID: 12646435. [DOI] [PubMed] [Google Scholar]