Abstract
Objective
To determine the required learning time for high-resolution anoscopy (HRA)-guided biopsy to detect histological high-risk squamous intraepithelial lesions (hHSIL) and to identify factors that impact on the training process.
Methods
All HIV-infected, screening-naïve men-who-have-sex-with-men who underwent HRA conducted by one single observer from 2010 to 2017 in a Spanish HIV-outpatient clinic were analysed.
Results
Eighty-five (14.7%) of the 581 patients included presented hHSIL. The factors associated with the capacity to detect hHSIL [adjusted odds ratio (aOR), 95% confidence interval (95%CI)] were the presence of cytological HSIL (3.04, 1.78–5.21; p < 0.001), infection with high-risk human papilloma virus (HR-HPV) (2.89, 1.38–6.05; p = 0.005), the number of biopsies taken/HRA (aOR: 1.28, 1.07–1.52; p = 0.006) and tobacco smoking (1.75; 1.12–2.73; p = 0.014). Two events independently augmented the detection rate of hHSIL: one single experienced pathologist interpreted biopsies after 409 HRA (2.80, 1.74–4.48; p = 0.035) and the anoscopist underwent an additional training after 536 HRA (2.57, 1.07–6.16; p = 0.035). A learning process could be observed throughout the whole study with stable HR-HPV prevalence.
Conclusion
The data support the growing evidence that the proposed training volume of 50–200 performances is underestimated. Extensive training of both anoscopist and pathologist is warranted and the development of tools to support the diagnostic performance may be considered.
Keywords: High-resolution anoscopy, Human papillomavirus, Anal squamous cell carcinoma, Operator experience, Learning curve, Liquid-based cytology
Highlights
-
•
HRA-guided biopsy to detect histological HSIL requires a long learning period.
-
•
581 HIV-infected men who have sex with men underwent HRA conducted by one observer.
-
•
Throughout the whole study period, a learning process was noted with stable HR-HPV.
-
•
The widely proposed training volume of 50–200 performances is probably underestimated.
-
•
Extensive training of anoscopist/pathologist, and possibly AI tools, are warranted.
1. Introduction
The incidence of anal cancer is progressively increasing, especially among patients at risk, such as HIV-infected men who have sex with men (MSM). In this specific population, incidences of up to 100 cases per 100,000 person-years (py) have been reported, as compared to 1.2 cases per 100,000 py in the overall population [1], [2], [3]. Similar to what is widely known for cervical cancer, there is extensive evidence that the development of anal cancer is preceded by high-grade squamous intraepithelial lesions (HSIL) caused by high-risk human papillomavirus (HR-HPV) genotype infection [4], [5], [6] which is frequently found in MSM [7].
High-resolution anoscopy (HRA) is currently considered the gold standard for the diagnosis of HSIL [8]. HRA consists of visualizing potentially precancerous lesions with acetic acid and Lugol's solution using a colposcope or anoscope, with subsequent confirmation by means of one or more biopsies. Although the procedure itself is very similar to comparatively simple cervical colposcopy, HRA is characterised by a long learning curve due to the anatomical topography of the anus and several factors that may obscure lesions such as folds, mucus, or haemorrhoids [9], [10]. Thus, HRA requires extensive training and observer experience in order to warrant that HSIL do not go unnoticed. While the International Anal Neoplasia Society (IANS) recommends a learning period of 50–100 HRA [10], contradictory data was obtained by other studies [11], [12], [13] and it remains unknown which factors influence the learning progression.
Therefore, this study aimed to assess the training process over time for one single observer performing HRA in HIV-infected MSM, as well as to identify parameters that may impact on the outcome.
2. Patients and methods
2.1. Study population
This retrospective analysis of the prospective Seville Cohort of People Living with HIV at Risk for Anal Cancer (SeVIHanal Cohort, clinicaltrials.gov: NCT03713229) was conducted at a Spanish tertiary care centre in which 2500 HIV-infected patients are followed on a regular basis. From September 2010 until July 2017, all HIV-infected MSM attended at one medical office were invited to be screened for HSIL by means of HRA with biopsy. In the present study, the inclusion criteria were: patients older or equal than 18 years who for the first time underwent HRA with subsequent anal biopsy conducted by one single observer and who had no prior test for anal lesions including digital-rectal examination, HPV testing or anal liquid-based cytology (aLBC). According to the protocol followed, the same day all patients had a HR-HPV testing and an aLBC prior to HRA. Likewise, clinical and epidemiological data were prospectively entered in electronic medical records (ACyH1, Betek 43 SL, Spain).
2.2. Anoscopy/biopsy, aLBC and HPV genotyping performance and interpretation
Before starting the HSIL screening program, the observer carried out a study on the prevalence of HPV in HIV-infected MSM and received a one-day theoretical and practical training by a provider from the Callen-Lorde Centre, New York, with more than five years experience in HRA and training. Both activities were promoted by the Spanish HIV Investigation Network (RIS). HRA was performed according to the training with the support of further guiding material1. Briefly, after aLBC and HR-HPV testing, the patients were examined in the left lateral recumbent position with a lubricated/lidocaine gel treated anoscope (OCS-500, Olympus Medical Systems, Tokyo, Japan). Biopsies were taken with 3 mm Baby Tischler's forceps from areas considered abnormal by the observer after treatment with 3–5% acetic acid and Lugol's solution and subsequently analysed by a histologist blinded to the cytology/HPV results. If more than one biopsy was obtained, the highest degree of abnormality found was used as the histological diagnosis. Biopsy readings were performed using standard laboratory protocols and differentiated “normal”, low-grade anal intraepithelial neoplasia (“LSIL”) which includes condyloma and anal intraepithelial neoplasia (AIN) grade I, as well as “HSIL” which includes AIN grade II and III [14]. aLBC (ThinPrep®/ PerservCyt® solution, Hologic Inc, Barcelona, Spain) and HPV genotyping were carried out as described elsewhere [15]. Cytological HSIL was diagnosed according to the Bethesda System as encompassing moderate and severe dysplasia [16]. HPV genotypes classified as “carcinogenic” (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59) or “probably carcinogenic” (HPV 68) to humans according to the International Agency for Research on Cancer were considered HR-HPV [17].
2.3. Statistical analysis
Continuous variables were expressed as median and interquartile range (IQR) and categorical variables as the number of cases and percentages. The outcome variable was the detection of histological HSIL, as diagnosed by means of HRA with subsequent biopsy. Variables that potentially influence the outcome variable were analysed using the chi-square or the Fisher's test when categorical and the Student's t-test or the Mann–Whitney test when continuous. Two events with potential impact on the learning curve were analysed: While initially, all biopsies were interpreted by a team of three pathologists, only one single specially trained expert pathologist was responsible for all biopsy readings (Event#1) and the anoscopist underwent an additional three-day “Advanced HRA Course” organized by the IANS (San Francisco, CA) (Event#2), which consisted of an advanced training program for observers with an experience of a recommended 100–200 or more conducted HRAs. Prevalence of histological findings, aLBC and HR-HPV were calculated for the overall population and by the main stages defined for the referred events.
To compare the main slopes of the learning curve for the detection of HSIL, a linear regression model was set, including events concomitant with slopes changes. Linear binomial models were used to identify factors that independently impact on the detection of HSIL. The complementary log-log link function was selected since the outcome variable was expected to be disproportioned, including all variables with a p-value below 0.2 in the univariate analysis. Continuous variables were transformed when necessary to satisfy model assumptions. The Akaike Information Criterion (AIC) was used to assess which model best explained the detection of HSIL: the lower the AIC value, the better the fit. To calculate how many times one model is better than the other, the neperian antilogarithm was calculated according to the following formula: 1/[exp (- (AIC model 2 -AIC model 1)/2)]. Statistical analyses were performed using the SPSS v. 21.0 software (IBM, Chicago, USA) and R Statistical Software (Foundation for Statistical Computing, Vienna, Austria). P-values below 0.05 were considered significant.
2.4. Ethics approval and consent to participate
The study was designed and performed according to the Helsinki declaration and was approved by the Ethics Committee of the Virgen del Rocío University Hospital (Seville, Spain; Ref. 03/2006). All patients gave their written informed consent both to be included in the database, as well as to the use of their anonymised data, photographies or videoscopies for scientific research and HRA training programs, thereby ensuring the protection of personal data in accordance with the Spanish Personal Data Protection Organic Law15/199 enacted on December 13, 1999.
3. Results
3.1. Characteristics of the study population
A total of 581 HIV-infected MSM who firstly underwent HRA were included in the analysis, of whom 405 subjects had an undetectable plasma HIV RNA from a median (IQR) time of 17 (8−37) months. The median (IQR) CD4+ T-cell count was 630 (483−800) cells/μL. Further characteristics of the study population are shown in Table 1.
Table 1.
Characteristics of the study population (n = 581) at the moment of high resolution anoscopy performance.
| Characteristic | Value |
|---|---|
| Age, yearsa | 38 (31–47) |
| Educational level, n (%) | |
| None/primary | 121 (20.8) |
| Secondary | 211 (36.3) |
| University | 249 (42.9) |
| Tobacco use, n (%) | |
| Current | 186 (32) |
| Past | 56 (9.6) |
| Never | 339 (58.3) |
| Undetectable plasma HIV RNA, n (%) | 405 (69.7) |
| CD4+ T-cell nadir, cells/μLa | 290 (187–404) |
| C4+/CD8+ ratioa | 0.68 (0.45–0.91) |
| CDC category C, n (%) | 61 (10.5) |
| Gonorrhoea and/or chlamydia, n (%)b | 40 (12.6) |
median (interquartile range).
available in 370 patients.
3.2. Results of anal mucosa biopsy, aLBC and HPV genotyping
The histological results in the overall population were as follows: normal, 235 (40.4%); LSIL (AIN grade I and condyloma), 192 (33.1%); AIN grade II, 59 (10.2%) and AIN grade III, 26 (4.5%), while 69 (11.9%) samples were not valid. Thus, a total of 85/581 HRA (14.6%) had a histological HSIL diagnosis. The HSIL prevalences before Event#1, between Event#1 and Event#2 and after Event#2 were 38/409 HRA (9%), 29/127 HRA (23%) and 18/45 HRA (39%), respectively, p < 0.001. The corresponding prevalences for non-valid biopsies were 12.2%, 12.6% and 6.7%, respectively [p (before Event#1 versus after Event #2) < 0.001]. HPV genotyping was available in 560 (96.4%) patients, of which 428 (76.4%) individuals showed a HR-HPV. Histologic HSIL was observed in 70 (16.4%) of those with HR-HPV versus 8 (6.1%) subjects without HR-HPV, p = 0.003. Fifty (9%) out of 557 patients with a valid aLBC showed cytological HSIL. Of those with cytological HSIL, 18 (36%) patients also showed histological HSIL versus 61 (12%) patients without HSIL in aLBC, p < 0.001. The corresponding figures for smokers versus non-smokers were 34 (18.3%) versus 51 (12.9%), p = 0.102, and where one, two, three or four biopsy samples were taken per HRA, HSIL was detected in 71 (12.8%), 9 (50%), 3 (60%) and 2 (100%) individuals, p < 0.001. Median (IQR) age was 37 (29−46) years and 39 (32−47) years in patients with and without HSIL, p = 0.148.
3.3. Predictive factors for the detection of HSIL
In an univariate analyses, the following factors were associated with a p < 0.2 with the detection of HSIL: the presence of HSIL in aLBC (crude OR: 3.48, 95%CI: 2.05–5.91 p < 0.001), the presence of HR-HPV (crude OR: 2.86, 95%CI: 1.37–5.94, p = 0.005), the number of biopsies taken per HRA (crude OR: 1.43, 95%CI: 1.26–1.63, p < 0.001), age (crude OR: 0.84, 95%CI: 0.67–1.04, p = 0.108), accumulated number of HRA (crude OR: 1.51, 95%CI: 1.21–1.89, p < 0.001) and accumulated number of HRA by events: Event#1 vs no-event (crude OR: 2.50, 95%CI: 1.56–4.04, p < 0.001) and Event#2 vs no-event (crude OR: 4.97, 95%CI: 2.79–8.85, p < 0.001). Regarding the demographic or HIV-related factors associated with presence of histological HSIL, only tobacco smoking showed a positive trend (crude OR: 1.46, 95%CI: 0.95–2.26, p = 0.088). There were no differences with a statistical significance of p < 0.2 for CD4+ T-cell count nadir or at time of intervention, CD4+/CD8+ ratio, presence of or time of undetectable plasma HIV RNA, presence of gonorrhoea/chlamydia, nor CDC category C.
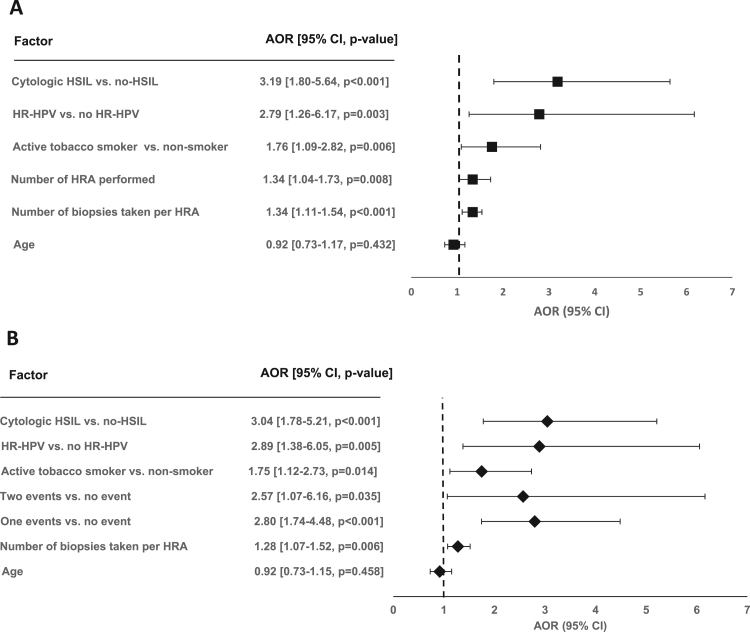
In the binomial regression models, the highest AOR were associated with the presence of HSIL in aLBC, the presence of HR-HPV, as well as the accumulated number of HRA (Model 1) and Event#1 and Event#2 (Model 2) (Fig. 1). Based on the AIC fit index, Model 2, including concomitant events with slope changes showed a 90.02-fold better fit than Model 1.
Fig. 1.
Binominal linear models to identify independent predictors for the detection of high-grade squamous intraepithelial lesions (HSIL) in high resolution anoscopies (HRA) with subsequent biopsy considering the number of HRA performed (Model 1, Fig. 2A) and the events that showed an impact on slope steepness of the learning curve (Model 2, Fig. 2B) in HIV-infected men who have sex with men seen between 2010 and 2017 in an HIV outpatient clinic of a tertiary care centre in Seville, Spain. AOR: adjusted odds ratio; CI: confidence interval; HR-HPV: high-risk human papillomavirus; Event #1: One single expert pathologist responsible for biopsy interpretation; Event #2: Observer participates at additional one week expert training. Akaike Information Criteria were 389,398 for Model 1 and 380.526 for Model 2, respectively.
3.4. Learning curve
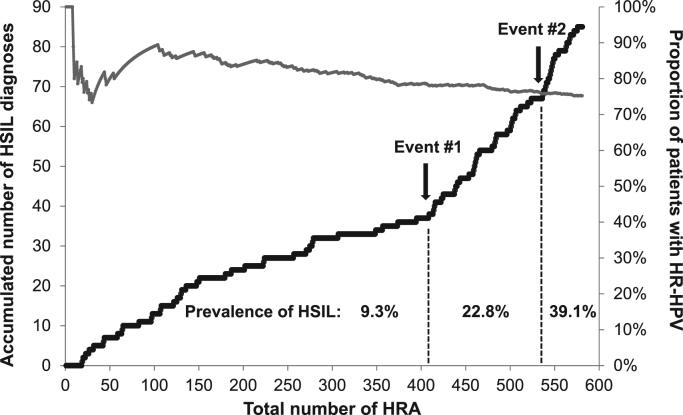
The accumulated numbers of HSIL detected with increasing number of total HRA conducted, as well as the prevalence of HR-HPV over the study time, are depicted in Fig. 2. There were three mains slopes for the detection of HSIL. The second slope was observed after 409 HRAs and the third slope after 536 HRAs. Both slopes were steeper compared to the first one, with differences of 5.40 and 22.11 in histological HSIL (p < 0.001), respectively. The emergence of both slopes could be attributed to Event#1 and Event#2. The slope of the concomitant HR-HPV prevalence curve was negative (-0.0003) and liner (r2 = 0.97) after a considerable number (100 HRA) to calculate the prevalence of HR-HPV at every HRA conducted was reached.
Fig. 2.
Accumulated high-grade anal squamous neoplasias (HSIL) according to the total number of high resolution anoscopies (HRA) with subsequent biopsy (black line) and the prevalence of high-risk human papillomavirus (HR-HPV) (grey line) in 581 HIV-infected men who have sex with men seen between 2010 and 2017 in an HIV outpatient clinic of a tertiary care centre in Seville, Spain. Event #1: One single expert pathologist responsible for biopsy interpretation; Event #2: Observer participates at additional one week expert training. The slope of the HR-HPV curve from the hundredth HRA onwards was −0.0003 (r2 = 0.97).
4. Discussion
The present study confirms a long learning curve for HRA and, furthermore, the data suggest that the amount of training recommended by different guidelines may not be sufficient [8]. To our knowledge, this is the first study to determine factors that impact on the learning process and underlines the importance of both training and experience not only of the anoscopist but also of the pathologist, which should be taken into account when performing HRA.
Up to the end of the study where almost 600 HRA had been performed, a learning process could be observed as indicated by the lack of a plateau on the learning curve that would represent the moment of the detection of a constant number of HSIL. Data on observer experience in HRA are scarce and include small and/or heterogeneous populations. Furthermore, in these studies a concomitant cytology outcome is mainly used to validate HRA in spite of the considerably poor diagnostic values of aLBC, which may result in misleading interpretations [8]. In this context, a study conducted in 384 MSM suggests a training period of 200 HRA as sufficient to consider that the observer obtained sufficient experience [11]. It is to note though that, even after that point, a learning phase could be observed visually in the learning curve but, unfortunately, statistical analysis was not done in the study. Likewise, data from another study that measured the diagnostic performance of HRA by the concordance between cytological and histological diagnosis suggested that the results can improve when training is continued to 800 interventions [13]. Finally, our findings are in accordance to a recent study conducted by Hillmann et al. suggesting that a minimum of 500 procedures are needed for an anoscopist to reach maximum achievable diagnostic accuracy [12]. In contrast to that study, in the present study only patients without prior data acquisition were included in order to avoid biases. All this suggests that the already considerably high training period of 50–200 procedures as suggested by expert panels [8], [9], [10] may still not be sufficient. To solve this problem, the development of tools to shorten the learning process and facilitate the identification of sites were biopsies should be taken, such as the use of computer algorithms to process HRA based on artificial intelligence, are warranted.
In addition to the constant learning process due to acquisition of experience, two events significantly marked an increase in the diagnostic performance, one was based on the observer performing HRA, but the other was not. This is an important finding since it points out that, on the one hand, adequate teaching of the observer is crucial and still effective after a considerable training period. In fact, a further training within an advanced program (Event #2) was independently associated with higher performance. It is to note that this program addressed anoscopists with an experience in the range of the minimum recommended training period as mentioned above, thus further underlining the probable underestimation of 50–100 HRA to be sufficient for reaching expert status. Also, the proportion of invalid biopsies decreased almost 50% to a number representative for expert anoscopists. On the other hand, the data show the importance of factors associated with learning process and that may not be appreciated as they are less obvious, such as the training and skills of the pathologist. Both events were observed independently from other known factors associated with HSIL such as the presence of HSIL in cytology and HR-HPV and, to a lower extent, smoking [18]. To our knowledge, the pathologist's experience is not considered in the currently available studies on the learning process for HRA but should be taken into account in the future.
This study has several limitations. First, since HRA is currently considered the gold standard [8], there was no optimum measure to confirm the HRA results. However, there is a very high association between HR-HPV genotypes and anal cancer [18] and the proportion of patients with HR-HPV did not only remain stable when a reasonable number of patients was reached, there was also even a trend of a descending prevalence of HR-HPV over time. Therefore, it can be assumed that the proportion of patients with HGAIN was similar throughout the study period and if there was a change it would have been in a way that at the end of the study the prevalence would have been lower. This would make it even more difficult to detect HSIL, however, the learning curve clearly steepened towards the end, indicating more HSIL detection per number of HRA. Second, data of only one observer was analysed. Still, as mentioned above, the results of the present study are similar to what was described earlier and it is therefore likely that the results represent an adequate approximation of the general population. Finally, following the advanced training, the concentration of acetic acid used in HRA was enhanced from 3% to 5% in order to improve visibility of HSIL. However, while this may contribute to a higher yield of detected HSIL, the crucial point is the interpretation of what is visualized, depending on the observer's skills. Therefore, the use of a different concentration merely represents part of the training as it is linked with its interpretation. The considerable decrease in the proportion of invalid biopsies further confirms the learning effect of the additional training.
In conclusion, the findings described herein support the growing evidence that the widely proposed training volume is underestimated. Extensive training of both anoscopist and pathologist is warranted and the development of observer-independent visualization tools based on artificial intelligence algorithms to support the diagnostic performance may be considered.
Declarations of interest
None.
Funding sources
This work was partially funded by the Plan Nacional R+D+I and Red de Investigación en SIDA, Spain [grant number RD16/0025/0020-ISCIII-FEDER]. K.N. is the recipient of a Miguel Servet research grant [grant number CPII18/00033] from the Instituto de Salud Carlos III, Spain.
Footnotes
A review on HRA technique that was used for guidance.
References
- 1.D’Souza G., Wiley D.J., Li X., Chmiel J.S., Margolick J.B., Cranston R.D. Incidence and epidemiology of anal cancer in the multicenter AIDS cohort study (MACS) J. Acquir. Immune Defic. Syndr. 2008;48:491–499. doi: 10.1097/QAI.0b013e31817aebfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piketty C., Selinger-Leneman H., Bouvier A.M., Belot A., Mary-Krause M., Duvivier C. Incidence of HIV-related anal cancer remains increased despite long-term combined antiretroviral treatment: results from the french hospital database on HIV. J. Clin. Oncol. 2012;30:4360–4366. doi: 10.1200/JCO.2012.44.5486. [DOI] [PubMed] [Google Scholar]
- 3.Shiels M.S., Pfeiffer R.M., Chaturvedi A.K., Kreimer A.R., Engels E.A. Impact of the HIV epidemic on the incidence rates of anal cancer in the United States. J. Natl. Cancer Inst. 2012;104:1591–1598. doi: 10.1093/jnci/djs371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouvard V., Baan R., Straif K., Grosse Y., Secretan B., Ghissassi F El. A review of human carcinogens—Part B: biological agents. Lancet Oncol. 2009;10:321–322. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 5.de Sanjosé S., Brotons M., Pavón M.A. The natural history of human papillomavirus infection. Best Pract. Res. Clin. Obstet. Gynaecol. 2018;47:2–13. doi: 10.1016/j.bpobgyn.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Lin C., Franceschi S., Clifford G.M. Human papillomavirus types from infection to cancer in the anus, according to sex and HIV status: a systematic review and meta-analysis. Lancet Infect. Dis. 2017;18:198–206. doi: 10.1016/S1473-3099(17)30653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torres M., González C., Del Romero J., Viciana P., Ocampo A., RodrÍguez-Fortunez P. Anal human papillomavirus genotype distribution in hiv-infected men who have sex with men by geographical origin, age, and cytological status in a spanish cohort. J. Clin. Microbiol. 2013;51:3512–3520. doi: 10.1128/JCM.01405-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leeds I.L., Fang S.H. Anal cancer and intraepithelial neoplasia screening: a review. World J. Gastrointest. Surg. 2016;8:41. doi: 10.4240/wjgs.v8.i1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palefsky J.M. Practising high-resolution anoscopy. Sex. Health. 2012;9:580–586. doi: 10.1071/SH12045. [DOI] [PubMed] [Google Scholar]
- 10.Hillman R.J., Cuming T., Darragh T., Nathan M., Berry-Lawthorn M., Goldstone S. 2016 IANS international guidelines for practice standards in the detection of anal cancer precursors. J. Low. Genit. Tract Dis. 2016;20:283–291. doi: 10.1097/LGT.0000000000000256. [DOI] [PubMed] [Google Scholar]
- 11.Richel O., Prins J.M., de Vries H.J. Screening for anal cancer precursors: what is the learning curve for high-resolution anoscopy? AIDS. 2014;28:1376–1377. doi: 10.1097/QAD.0000000000000227. [DOI] [PubMed] [Google Scholar]
- 12.Hillman R.J., Gunathilake M.P.W., Jin F., Tong W., Field A., Carr A. Ability to detect high-grade squamous anal intraepithelial lesions at high resolution anoscopy improves over time. Sex. Health. 2016;13:177–181. doi: 10.1071/SH15170. [DOI] [PubMed] [Google Scholar]
- 13.Mathews C., Caperna J., Cachay E.R., Cosman B. Early impact and performance characteristics of an established anal dysplasia screening program: program evaluation considerations. Open Aids. J. 2007;1:11–20. doi: 10.2174/1874613600701010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darragh T.M. The LAST project and the diagnostic bottom line. Cytopathology. 2015;26:343–345. doi: 10.1111/cyt.12299. [DOI] [PubMed] [Google Scholar]
- 15.González C., Torres M., Benito A., Del Romero J., Rodríguez C., Fontillón M. Anal squamous intraepithelial lesions are frequent among young HIV-infected men who have sex with men followed up at the Spanish AIDS research network cohort (CoRIS-HPV) Int. J. Cancer. 2013;133:1164–1172. doi: 10.1002/ijc.28102. [DOI] [PubMed] [Google Scholar]
- 16.Solomon D., Davey D., Kurman R., Moriarty A., O’Connor D., Prey M. The 2001 bethesda system: terminology for reporting results of cervical cytology. JAMA. 2002;287:2114–2119. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 17.IARC, Agents Classified by the IARC Monographs, IARC. Monogr, 2012, pp. 1–25.
- 18.Daling J., Sherman K., Hislop T., Maden C., Mandelson M., Beckmann A. Cigarette smoking and the risk of anogenital cancer. Am. J. Epidemiol. 1992;135:180–189. doi: 10.1093/oxfordjournals.aje.a116270. [DOI] [PubMed] [Google Scholar]