Abstract
Cancer-associated venous thromboembolism (CAT) is a common complication associated with high morbidity and mortality. In accordance with major clinical trials comparing low-molecular-weight heparin (LMWH) with a vitamin K antagonist (VKA), LMWH is currently the standard treatment for CAT, owing to its efficacy for thrombosis recurrence and improved safety profile compared to VKA. Over the past few years, direct oral anticoagulants (DOACs) have emerged as potential alternative therapies to LMWH due to their convenient route of administration and predictable pharmacokinetics, but evidence for their use in CAT is inconclusive, as only a small fraction of the study populations in these trials had CAT. Recently, two large head-to-head trials comparing DOACs to LMWH in CAT patients reported comparable efficacies of DOACs with increased bleeding risk. Occasionally, CAT treatment can be challenging due to the heterogeneity of underlying malignancies and comorbidities. Renal insufficiency and gastrointestinal defects are the main obstacles in anticoagulant selection. Careful choice of treatment candidates and proper anticoagulant strategies are critical for the treatment of CAT; hence, more studies are required to address these challenges.
Keywords: Cancer-associated Venous Thromboembolism, Low-Molecular-Weight Heparin, Direct Oral Anticoagulants
Graphical Abstract
INTRODUCTION
Since Trousseau reported migratory thrombophlebitis in a cancer patient in the 1800s, physicians have known that the incidence of thrombosis is significantly higher in patients with cancer. Cancer-associated venous thromboembolism (CAT) occurs in a number of different clinical situations. Cancer and thrombosis share diverse risk factors, and the thrombogenic potential of cancer cells themselves induces the activation of direct procoagulant properties and tissue factor-mediated coagulation.1 CAT is associated with poor outcomes and increased mortality in cancer patients and is the second leading cause of death in cancer patients.2,3,4 Frequently, both CAT and complications from its treatment significantly interrupt active cancer therapy. Therefore, proper treatment and careful follow-up are required for patients with CAT.
The incidence of CAT varies from study to study. CAT was found to account for about 20% of newly diagnosed venous thromboembolisms (VTEs) in a large population-based investigation.5 Many studies report that cancer patients have a four- to seven-fold higher relative risk of thrombosis than those without cancer.6 According to the most recent report from the Vienna Cancer and Thrombosis Study observational cohort, the incidence of CAT in cancer patients was 7.4% during the median 19 months of observation.7 In a study utilizing the Computerized Registry of Patients with Venous Thromboembolism database, 4.3% of patients who were diagnosed with an unprovoked VTE developed cancer during the 24 months of follow-up.8 A recent nationwide Korean study showed that VTE incidence is gradually increasing, which might be associated with increased awareness of VTE as well as improved survival of cancer patients.9 A recent report from a tertiary hospital in Korea actually reported that age and sex adjusted incidence of CAT has gradually increased over 10 years.10
However, there are several limitations of the use of conventional anticoagulants for the treatment of CAT, including high recurrence rates and bleeding complications.11 Injection of low-molecular-weight heparin (LMWH) showed satisfactory results in large major clinical trials and emerged as the treatment of choice12,13; however, this injection therapy, which typically lasts for more than 3 months, can be bothersome for patients. According to analyses of 2016 and 2017 US claim data, less than one-half of CAT patients received LMWH.14,15 In Korea, despite relatively low cost of LMWH, warfarin (WFR)-based treatments are still prevalent among cancer patients with thrombosis.9 In this study, only 20% of VTE patients chose LMWH treatment. Although the data did not divide the patients into CAT and non-CAT group, we can presume that a similar trend can be applied to the CAT subset. These findings suggest that patient preference can also affect actual medical practice. Furthermore, frequent interruption of anticoagulation therapy due to intervention, biopsy, or the toxicity of anticancer therapy make CAT treatment even more challenging. In these contexts, direct oral anticoagulants (DOACs), including a direct thrombin inhibitor and factor Xa inhibitors, are emerging as alternative therapies for VTE treatment. DOACs are potentially attractive for managing VTE because they can be administered orally at a fixed dose with predictable effects. In this article, we review the latest research in novel treatment options and contemplate some questions in the context of actual clinical practice.
RISK FACTOR ASSESSMENT
There have been numerous studies on the identification of CAT risk factors and high-risk patients. The following underlying patient characteristics can increase the risk of CAT development: old age, medical comorbidities, obesity, immobility, and previous VTE history.16 Risk also varies by the primary site of malignancy, histology, and disease stage.17,18,19 Pancreatic, gastric, and lung cancer are especially highly associated with CAT.20,21 Cancer treatments, including conventional chemotherapy, hormonal therapy, antiangiogenic agents, and immunomodulatory agents, can contribute to CAT development.22,23,24 Usually the incidence of CAT is highest early after cancer diagnosis, but VTE can occur at any time during the course of malignancy.
A predictive model of chemotherapy-associated VTE was developed by Khorana et al.25 using primary cancer type, hematologic profiles, and patient characteristics. Later refinements of the model introduced some biomarkers, including P-selectin and D-dimer.26,27 Although various algorithms have been proposed for risk stratification, more accurate and refined models are still needed.
ANTICOAGULATION USE FOR CAT
LMWH treatment for CAT
WFR has long been used to treat CAT, but limited by frequent drug-drug interactions (DDIs) and need for regular monitoring due to its narrow therapeutic window. Furthermore, a significant proportion of CAT cases are refractory to WFR. For these reasons, several studies sought a substitute for WFR in treatment of CAT. In 2003, the Randomized Comparison of Low-Molecular-Weight Heparin Versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) trial was the first to compare dalteparin, a LMWH, to WFR, examining 676 active cancer patients with VTE. Patients treated with LMWH showed a 55% reduction in relative risk of recurrent VTE (8.0% in dalteparin vs. 15.8% in WFR; hazard ratio [HR], 0.48; 95% confidence interval [CI], 0.30–0.77; P = 0.002) without increasing major bleeding (MB) complications (5.6% in dalteparin vs. 3.6% in WFR; P = 0.27).13 In the Comparison of Acute Treatments in Cancer Haemostasis (CATCH) trial, the largest phase III randomized trial comparing LMWH to WFR, patients treated with the tinzaparin showed a trend toward reduction in VTE recurrence (7.2% for tinzaparin vs. 10.5% for WFR; HR, 0.65; 95% CI, 0.41–1.03; P = 0.07), but the difference was not statistically significant. Tinzaparin showed a safer profile in clinically relevant non-MB (CRNMB, 10.9% vs. 15.3%; HR, 0.58; 95% CI, 0.40–0.84; P = 0.004) than WFR, but MB was similar in both groups (2.7% vs. 2.4%; P = 0.77).12 Several other studies were conducted in Europe and Canada, which consistently reported that LMWH showed comparable efficacy without increasing the risk of bleeding complications.28,29 Based on these results, current major guidelines, including those from the European Society for Medical Oncology, the National Comprehensive Care Network, the American College of Chest Physicians, the American Society of Clinical Oncology, and International Clinical Practice, consistently recommend LMWH as a first-line treatment for CAT.30,31,32,33,34 However, there are still some critical issues in the use of LMWH, specifically in that the drug requires self-injection and is not safely applicable under certain clinical situations, including severe renal impairment and thrombocytopenia.
Evidence for DOACs in early clinical trials
Since the advent of DOACs in the 2000s, they have rapidly replaced classical anticoagulants in the medical field, owing to their convenient route of administration and minimal monitoring requirements. However, the use of DOACs for CAT was limited due to a lack of evidence. Until recently, all available evidence was from subgroup analyses of cancer patients in major randomized trials.
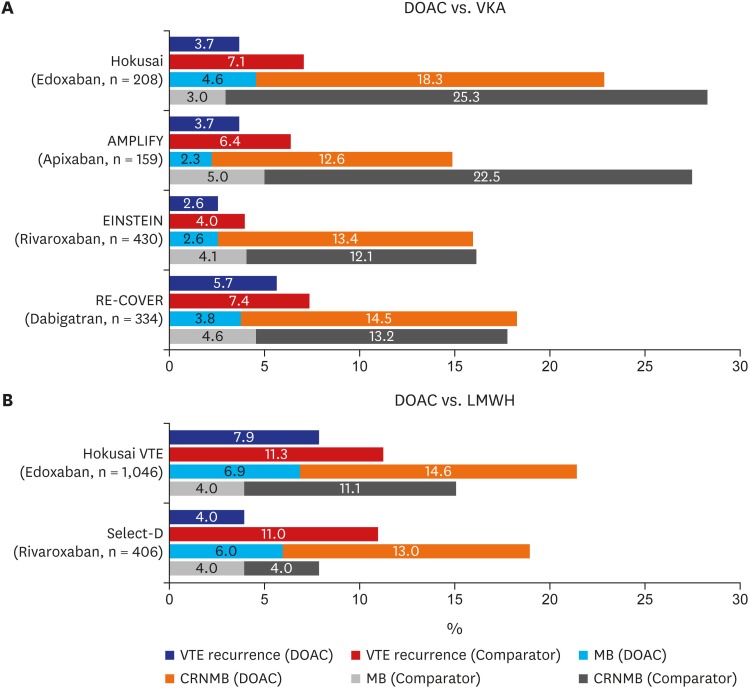
The trial of dabigatran versus warfarin in the treatment of acute venous thromboembolism (RE-COVER I) in 2009 and the subsequent RE-COVER II trial in 2014 compared the efficacy of dabigatran, a DOAC, with WFR after acute parenteral anticoagulation in VTE patients. In a pooled analysis of 4,886 patients from these two trials, there was no difference in VTE recurrence (HR, 1.09; 95% CI, 0.76–1.57) or MB (HR, 0.73; 95% CI, 0.48–1.11) between the two groups.35,36 As in the dabigatran study, studies comparing rivaroxaban (eligible patients with deep-vein thrombosis [EINSTEIN-DVT] and eligible patients with pulmonary embolism [EINSTEIN-PE]), apixaban (apixaban for the initial management of pulmonary embolism and deep-vein thrombosis as first-line therapy [AMPLIFY]), and edoxaban (Hokusai VTE) with WFR also reported that DOAC showed comparable results in terms of efficacy and complications.37,38,39,40 Based on these studies, Several meta-analyses was conducted on this issue. Vedovati et al.41 analyzed 1,132 patients with CAT from these studies and reported 3.9% of VTE recurrence in patients using DOACs, whereas 6.0% of patients developed recurrent VTE in the comparator (mainly vitamin K antagonist [VKA]) group. The DOAC group showed a lower recurrence rate but failed to show a statistically meaningful difference (odds ratio [OR], 0.63; 95% CI, 0.37–1.10; I2, 0%). MB and CRNMB showed similar trends toward nonsignificant risk reduction in DOACs (OR, 0.77; 95% CI, 0.41–1.44 for MB and OR, 0.85; 95% CI, 0.62–1.18 for CRNMB).41 The incidence of VTE recurrence and bleeding complication of cancer subpopulation in each trial are summarized in Fig. 1A. In another meta-analysis of a larger patient population, the results were generally consistent with previous study.42
Fig. 1. Comparison of VTE recurrence and bleeding (%) in major clinical trials. (A) Cancer subpopulation of DOAC vs. VKA trials and (B) DOAC vs. LMWH trials in CAT.
DOAC = direct oral anticoagulant, VKA = vitamin K antagonist, LMWH = low-molecular-weight heparin, VTE = venous thromboembolism, MB = major bleeding, CRNMB = clinically relevant non-major bleeding, AMPLIFY = apixaban for the initial management of pulmonary embolism and deep-vein thrombosis as first-line therapy, EINSTEIN = eligible patients, RE-COVER = dabigatran versus warfarin in the treatment of acute venous thromboembolism, CAT = cancer-associated venous thromboembolism.
However, there are several limitations to the adoption of these findings in real clinical practice: WFR, the main comparator of these studies, is known to be inferior to LMWH in patients with cancer. In addition, the proportion of cancer patients in these studies was very low. Even in the RE-COVER trial, which included the largest proportion of cancer patients, cancer patients only accounted for 6.5% of the entire population. Furthermore, the definition of active cancer was inconsistent among these large randomized controlled trials. For example, the RE-COVER trial defined active cancer patients as either diagnosed with or treated for active cancer within 5 years, which was a relatively broad definition compared to other studies. Consequently, in CAT patients, results are still inconclusive, despite the favorable results in subgroup analysis.
Recent investigations on anticoagulation therapy for CAT
The studies mentioned above primarily compared VKA with LMWH or DOACs. Based on these results, it is expected that DOACs would have similar efficacy and safety profiles compared with LMWH. Yhim et al.43 recently conducted a prospective, open-labeled trial to investigate the efficacy of rivaroxaban in active cancer patients. The definition of active cancer in this trial was concordant with the CLOT and CATCH trials. Results implied that rivaroxaban can be used effectively and safely as a treatment for CAT. And recently, studies comparing DOAC with LMWH as treatment for CAT were successively published. First, the Hokusai VTE cancer trial reported noninferiority of edoxaban to dalteparin in CAT patients.44 This study included 1,046 patients who were diagnosed with or treated for cancer within 2 years, and 98% of the study population had active cancer at the time of the study. The primary outcome was the composite endpoint of recurrent VTE or MB. Results showed noninferiority, with an HR of 1.01 (95% CI, 0.69–1.46; P = 0.02 for noninferiority). Taking a deeper look at the endpoint, VTE recurrence did not differ between the two groups (12.8% in edoxaban vs. 13.5% in dalteparin; HR, 0.97; 95% CI, 0.70–1.36; P = 0.006), but MB was more common in the DOAC group (HR, 1.77; 95% CI, 1.03–3.04; P = 0.04). As in other studies of DOACs, the most common bleeding complication was upper gastrointestinal (GI) bleeding. In a recent report from the Select-D trial, which compared dalteparin with rivaroxaban in 406 CAT patients, cumulative VTE recurrence rates were 11% (95% CI, 7%–16%) in the dalteparin group and 4% (95% CI, 2%–9%) in the rivaroxaban group during 6 months of treatment (HR, 0.43; 95% CI, 0.19–0.99).45 MB did not differ between the two groups (4% in dalteparin vs. 6% in rivaroxaban), but CRNMB increased significantly in the rivaroxaban group (HR, 3.76; 95% CI, 1.63–8.69). In concordance with findings in other studies, most CRNMB was GI or urologic bleeding. The incidence of VTE recurrence and bleeding complication in these two studies are summarized in Fig. 1B.
Based on these recent trials, meta-analysis of these results has recently been published. DOAC showed tendency of reduced VTE recurrence by 6-months (OR, 0.65; 95% CI, 0.42–1.01; I2, 17%) with increased MB risk by 6-months (OR, 1.74; 95% CI, 1.05–2.88; I2, 0%).46 DOACs seem to be a potential alternative of LMWH for the treatment of CAT, with increased risk of CRNMB.
SPECIAL CONSIDERATIONS IN PATIENTS WITH CAT
Incidental VTE
An incidental finding of VTE during regular follow-up is common among patients with active cancer. A retrospective analysis of cancer patients receiving cisplatin-based chemotherapy at Memorial Sloan Kettering Cancer Center revealed that 43.8% of arterial or venous thromboses were detected by chance.47 Similarly, a report from Rochester University revealed that 40% of all VTE cases were incidentally diagnosed in cancer patients.48 One of the notable findings about incidental VTE is that there is no difference in terms of survival between symptomatic and incidental VTE. In both retrospective cohort and other prospective observational studies, incidental VTE also showed poor outcome as symptomatic VTE in cancer patients.49,50 Based on these findings, major guidelines currently recommend anticoagulation for incidental VTE.51
Nevertheless, some uncertainty remains in the treatment of clinically insignificant thrombosis. Some researchers have suggested that isolated subsegmental PE without DVT is not associated with increased risk of recurrent or fatal PE, even if the patient does not receive anticoagulation treatment.52 Considering that anticoagulation therapy has the potential risk of bleeding complications, the use of anticoagulants in these patients should be determined carefully, based on the patient's prognosis, symptoms, and systemic risk of thrombosis.
Renal impairment
Renal impairment is one of the most important comorbidities in hospitalized patients with VTE. Böttger et al.53 reported that 10% of patients had stage III or higher renal impairment in a study of 6,725 German VTE cohorts. It is difficult to estimate the actual incidence of renal impairment among patients with malignancy, but it is well known that the prevalence of renal impairment is higher in cancer patients than in healthy individuals, due to either the malignancy itself or treatment-related complications.54 But in major clinical trials of thromboembolism, patients with severe renal impairment were excluded.12,13,37,38,39
LMWH is partially metabolized in the kidneys, whereas unfractionated heparin is metabolized predominantly by the liver.55 Dependence on renal excretion may lead to the bioaccumulation of LMWH in patients with renal impairment, resulting in elevated bleeding risk.56 In the CLOT trial, a small proportion of patients with moderate or severe renal impairment (severe: creatinine clearance [CrCl] < 30 mL/min, 2.7%; moderate: CrCl < 60 mL/min, 21.9%) were included. In post hoc analysis, the incidence of MB in patients with renal impairment was acceptable compared with the normal renal function group.57 The CATCH trial also included some patients with renal impairment (severe, 1.8%; moderate, 13.1%). Bleeding complications among the patients with renal impairment were comparable to patients with normal renal function.58 In the prospective observation of treatment of venous thromboembolism in cancer patients with dalteparin for up to 12 months (DALTECAN), subgroup analysis of patients with a CrCl less than 50 mL/min revealed that renal impairment has no impact on bleeding risk.59 Considering these subgroup analyses from large studies, dalteparin and tinzaparin seem to be reasonable treatment options in patients with renal impairment. In general, patients with stable renal function (estimated glomerular filtration rate [GFR] > 20–30 mL/min) can use LMWH without increased bleeding risk.60
In contrast, all DOACs are excreted largely via the kidneys, so the use of DOACs in patients with renal impairment requires more clinical attention. Direct factor Xa inhibitors are known to depend on renal elimination for 20%–40% of their clearance.61 In the AMPLIFY, EINSTEIN-DVT, and EINSTEIN-PE trials investigating apixaban and rivaroxaban, renal impairment did not significantly increase bleeding risk.37,38,39 Similarly, in the Hokusai VTE trial, edoxaban also did not increase bleeding risk in patients with renal impairment.40 Overall, direct factor Xa inhibitors can also be treatment options in patients with mild-to-moderate renal impairment, as shown in these major clinical trials.
On the other hand, dabigatran, a direct thrombin inhibitor, depends 80% of their metabolism on renal excretion.62 Furthermore, it has a relatively long half-life compared to other DOACs, so special attention should be paid to its use in patients with renal impairment. In the Randomized Evaluation of Long-Term Anticoagulation Therapy trial comparing dabigatran with WFR in patients with atrial fibrillation, renal function was an important clinical factor associated with the plasma concentrations of dabigatran.63,64 The RE-COVER trial investigating dabigatran use in VTE patients also showed that renal impairment was significantly associated with bleeding complications. Based on these results, dabigatran should be avoided in patients with severe renal impairment.35,36,65
In conclusion, dalteparin, tinzaparin, and DOACs can be used in CAT patients with stable and marginal renal function, but meticulous monitoring for bleeding complications and changes in renal function is required. In addition, various formulas for the calculation of renal function can cause differences in estimated GFR and influence drug dose, especially in elderly patients.66
GI problems
It is well known that DOAC is highly associated with the occurrence of major GI bleeding in several randomized clinical trials and post-market studies.43,44,45,67 The GI tract is the most common site of MB.43,44,45 DOACs exhibit an anticoagulation effect not only by their systemic absorption through the GI tract but also by their direct local effects on intestinal mucosa and mucosal healing.68 As bleeding is a frequent problem in cancer patients with diverse etiologies, DOAC use in patients with GI malignancy should be monitored carefully.69 Additionally, as old age is consistently reported to be a major risk factor for GI bleeding, DOACs are cautiously recommended for elderly patients with a GI malignancy.
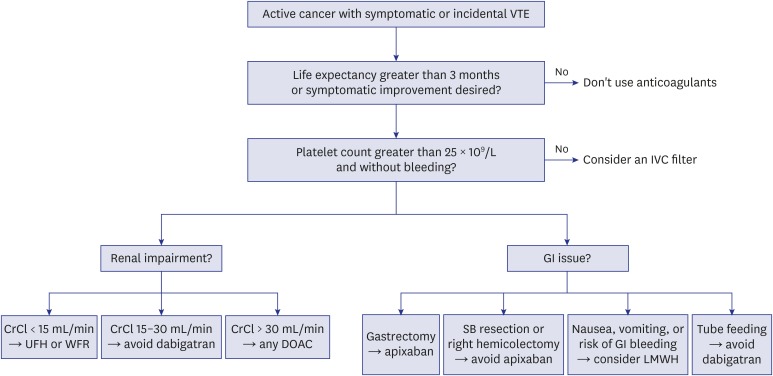
Another major consideration is the altered absorption of DOACs due to anatomical changes in patients who have undergone the surgical resection of a GI tumor. Anatomical alterations can cause changes in GI transit time, acidity, and surface area, affecting the bioavailability of DOACs.70,71 Rivaroxaban, edoxaban, and dabigatran show pH-dependent solubility and require gastric acidity for their absorption. These drugs are mainly absorbed in the distal stomach and proximal small bowel, so bioavailability is likely to decrease after gastrectomy or gastric bypass surgery.72 In such cases, apixaban can be a good alternative option, because the drug is absorbed in a pH-independent manner, and up to one-half of the drug is absorbed in the distal small bowel and ascending colon.73 For these same reasons, apixaban should be avoided in patients who have undergone small bowel resection and/or colectomy.72 Drug formulas should also be taken into account, as dabigatran should be administered in capsule form and avoided in patients with feeding tubes.74 A simplified algorithm for the selection of DOACs according to these considerations is shown in Fig. 2.
Fig. 2. DOAC selection for the treatment of CAT.
DOAC = direct oral anticoagulant, CAT = cancer-associated venous thromboembolism, VTE = venous thromboembolism, IVC = inferior vena cava, CrCl = creatinine clearance, UFH = unfractionated heparin, WFR = warfarin, SB = small bowel, GI = gastrointestinal, LMWH = low-molecular-weight heparin.
Thrombocytopenia
Thrombocytopenia is a widespread problem in patients with cancer. It can be a transient event associated with chemotherapy or a chronic problem associated with the infiltration of tumor cells into the bone marrow, immune-mediated thrombocytopenia, disseminated intravascular coagulation, or microangiopathic hemolytic anemia. Chemotherapy-induced thrombocytopenia (CIT) is one of the most prevalent causes of thrombocytopenia in cancer patients, and its incidence differs widely, according to chemotherapy regimen.75,76 In patients with CAT, thrombocytopenia increases bleeding risk but does not reduce VTE recurrence.77 For this reason, treating CAT patients with thrombocytopenia requires special attention of clinicians.78 In patients with thrombocytopenia, bleeding risk as well as risk of VTE recurrence should be considered thoroughly during treatment.
Although many studies have been published, most have been based on retrospective analyses.78,79,80,81 Currently several guidelines recommend administering full-dose anticoagulants if the platelet count exceeds 50 × 109/L.82,83 For new or recently diagnosed CAT (within 1 month), it is important not to discontinue anticoagulation therapy if possible, because the risk of recurrent thrombosis is known to be highest during the first month of treatment.13,59 In these patients, several options are acceptable if the platelet count exceeds 25 × 109/L to 30 × 109/L: reduce the LMWH dose to 50% or administer the full-dose of LMWH with platelet transfusion. And additionally, although it is not fully defined whether inferior vena cava (IVC) filter insertion is worthwhile or not, IVC filter can also be considered if DVT is present. Even with such a lack of data, current international guidelines recommend IVC filter as one of available options in thrombocytopenic patients.30,33
Debates remain regarding the treatment of CAT patients with thrombocytopenia. There are no clear answers as to which anticoagulant strategy is superior when the platelet count is between 25 × 109/L to 50 × 109/L. And currently, most of the reports on this issue only focused on CIT. Scheduling and dosing strategies for DOACs in various situations are not yet established. Additional studies on these unanswered questions are required.
DDIs
Although DDIs have decreased since mainstream CAT treatment moved from WFR to LMWH, novel treatment options still can cause DDIs in cancer patients. DOACs are metabolized mainly through the P-glycoprotein (P-gp) and cytochrome P (CYP) metabolic pathways, resulting in DDIs with other drugs that share these same mechanisms. For example, certain chemotherapeutic agents, such as adriamycin and vincristine, can reduce plasma concentrations of DOACs via CYP3A4 and P-gp induction.84 In addition to these classical cytotoxic agents, calcineurin inhibitors and tyrosine kinase inhibitors can also influence plasma levels of DOACs in P-gp- and CYP-dependent manners.85 The concurrent use of DOACs with these drugs can cause failure to achieve stable therapeutic levels, resulting in subtherapeutic effects or severe bleeding. Given that most of these drugs are used in a variety of cancers, these problems are likely to become increasingly common in the future. Unfortunately, there are currently no standardized monitoring methods or guidelines for risk assessment and dose modification. Therefore, it is reasonable to consider switching to LMWH in patients using these kinds of medications when optimal response is not achieved or when an MB event occurs with DOAC treatment.
DURATION OF ANTICOAGULATION
Decisions regarding extended anticoagulation therapy should be made very carefully. The reason of the difficulty in determining the duration of anticoagulation in CAT patients is that cancer-related VTE has a higher recurrence rate and MB risk than non-cancer VTE.11 There is insufficient evidence on the optimal duration of anticoagulation treatment in CAT patients, so therapy beyond 6 months is still controversial. According to major guidelines, the recommended period of anticoagulation therapy in CAT patients is 3–6 months, with anticoagulation therapy beyond 6 months based on the patient's active cancer status, plans for cancer treatment, and individual risk assessment for bleeding and recurrent thrombosis.16,30,31,32,33
The determination of treatment duration involves balancing the risk of thrombosis after discontinuing therapy with bleeding risk during continued therapy. In the CLOT and CATCH trials, the researchers reported 6%–7% cumulative incidence of thrombosis recurrence over 6 months in LMWH-treated patients. Subgroup analyses from DOAC trials show somewhat heterogeneous findings, ranging from 2.6%–5.8% cumulative incidence of thrombosis recurrence during treatment. On the other hand, the cumulative incidences of MB in the CLOT and CATCH trials were 6.0% and 2.5%, respectively. DOACs showed similar safety outcomes, with a cumulative incidence of MB between 2%–4%. With simple mathematical calculations, we can see that the risk of VTE recurrence after discontinuing treatment is comparable to the risk of MB when the treatment is continued.
The DALTECAN study was a prospective, observational study of VTE recurrence and bleeding complications at regular time points during 12 months of extended anticoagulation.59 The incidence of recurrent VTE was highest within the first month of treatment (5.7%), stabilized to 3.4% during the second to sixth months, and rose to 4.0% thereafter. MB showed a similar pattern, with a cumulative incidence of 3.6% within the first month of treatment, rising to 4.6% during the second to sixth months, and dropping to 4.2% until the completion of treatment.59 The Cancer-Duration of Anticoagulation Based on Compression UltraSonography study also examined the rate of VTE recurrence.86 After classifying patients according to the presence of remnant VTE after 6 months of treatment with LMWH, the researchers randomized the patients with remnant VTE into an extended therapy group and an observation group. The incidence of recurrent VTE was significantly higher in patients with remnant VTE. Although extended therapy did not decrease the risk of recurrent VTE in this study (HR, 1.37; 95% CI, 0.7–2.5; P = 0.311), the results suggested that recurrence rates differ widely, depending on certain risk factors, and that extended therapy might be required for this group of patients.86 The Tinzaparin in Cancer-Associated Thrombosis Beyond Six Months study, conducted in Spain, investigated VTE recurrence rates and related bleeding complications during extended tinzaparin treatment.87 The rate of VTE recurrence with extended tinzaparin treatment was 4.5% (95% CI, 2.2–7.8) from 1 to 6 months and 1.1% (95% CI, 0.1–3.9) from 7 to 12 months. This study showed the safety of extended anticoagulation therapy, in line with previous studies. Based on the results of these studies, it can be assumed that extended anticoagulation treatment in high-risk patients is relatively safe in terms of VTE recurrence and bleeding.
To summarize, it is important to individualize the duration of anticoagulation treatment after an evaluation of each patient's current status, risk factors for recurrence and bleeding, and patient preference. Currently, there is insufficient evidence to establish reliable guidelines for anticoagulation treatment duration that are applicable to all CAT patients; thus, additional studies are needed.
SUMMARY
Management of CAT is more challenging than that of cancer-naïve VTE. Not only is CAT itself difficult to treat, but various complex medical situations should also be taken into consideration, and careful monitoring for complications is critical. Understanding both the individual needs of each cancer patient and proper drug selection are cornerstones of this therapy. More research is required to address the remaining challenges of CAT management.
Key message
1. In CAT, DOAC can be a reasonable option in terms of thrombosis recurrence and bleeding complications.
2. Anticoagulation should be based on patients' underlying disease, major organ function, concurrent drugs, and compliance.
Footnotes
Funding: This study was supported by a grant from Seoul National University Bundang Hospital Research Fund (grant No.04-2012-003).
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Bang SM.
- Investigation: Kim SA.
- Writing - original draft: Kim SA.
- Writing - review & editing: Yhim HY, Bang SM.
References
- 1.Sørensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343(25):1846–1850. doi: 10.1056/NEJM200012213432504. [DOI] [PubMed] [Google Scholar]
- 2.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5(3):632–634. doi: 10.1111/j.1538-7836.2007.02374.x. [DOI] [PubMed] [Google Scholar]
- 3.Khorana AA. Venous thromboembolism and prognosis in cancer. Thromb Res. 2010;125(6):490–493. doi: 10.1016/j.thromres.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoon SY, Lee MY, Yoon J, Kim HJ, Kim KH, Kim SH, et al. The incidence of venous thromboembolism is not lowin Korean patients with advanced pancreatic cancer. Blood Res. 2018;53(3):227–232. doi: 10.5045/br.2018.53.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heit JA, O'Fallon WM, Petterson TM, Lohse CM, Silverstein MD, Mohr DN, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med. 2002;162(11):1245–1248. doi: 10.1001/archinte.162.11.1245. [DOI] [PubMed] [Google Scholar]
- 6.Noble S, Pasi J. Epidemiology and pathophysiology of cancer-associated thrombosis. Br J Cancer. 2010;102(Suppl 1):S2–S9. doi: 10.1038/sj.bjc.6605599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riedl J, Kaider A, Reitter EM, Marosi C, Jäger U, Schwarzinger I, et al. Association of mean platelet volume with risk of venous thromboembolism and mortality in patients with cancer. Results from the Vienna Cancer and Thrombosis Study (CATS) Thromb Haemost. 2014;111(4):670–678. doi: 10.1160/TH13-07-0603. [DOI] [PubMed] [Google Scholar]
- 8.Jara-Palomares L, Otero R, Jimenez D, Praena-Fernandez JM, Font C, Falga C, et al. Validation of a prognostic score for hidden cancer in unprovoked venous thromboembolism. PLoS One. 2018;13(3):e0194673. doi: 10.1371/journal.pone.0194673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong J, Lee JH, Yhim HY, Choi WI, Bang SM, Lee H, et al. Incidence of venous thromboembolism in Korea from 2009 to 2013. PLoS One. 2018;13(1):e0191897. doi: 10.1371/journal.pone.0191897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park TY, Jung JW, Choi JC, Shin JW, Kim JY, Choi BW, et al. Epidemiological trend of pulmonary thromboembolism at a tertiary hospital in Korea. Korean J Intern Med. 2017;32(6):1037–1044. doi: 10.3904/kjim.2016.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prandoni P, Lensing AW, Piccioli A, Bernardi E, Simioni P, Girolami B, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100(10):3484–3488. doi: 10.1182/blood-2002-01-0108. [DOI] [PubMed] [Google Scholar]
- 12.Lee AY, Kamphuisen PW, Meyer G, Bauersachs R, Janas MS, Jarner MF, et al. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314(7):677–686. doi: 10.1001/jama.2015.9243. [DOI] [PubMed] [Google Scholar]
- 13.Lee AY, Levine MN, Baker RI, Bowden C, Kakkar AK, Prins M, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146–153. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- 14.Khorana AA, Yannicelli D, McCrae KR, Milentijevic D, Crivera C, Nelson WW, et al. Evaluation of US prescription patterns: are treatment guidelines for cancer-associated venous thromboembolism being followed? Thromb Res. 2016;145:51–53. doi: 10.1016/j.thromres.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Khorana AA, McCrae KR, Milentijevic D, Fortier J, Nelson WW, Laliberté F, et al. Current practice patterns and patient persistence with anticoagulant treatments for cancer-associated thrombosis. Res Pract Thromb Haemost. 2017;1(1):14–22. doi: 10.1002/rth2.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyman GH, Khorana AA, Kuderer NM, Lee AY, Arcelus JI, Balaban EP, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31(17):2189–2204. doi: 10.1200/JCO.2013.49.1118. [DOI] [PubMed] [Google Scholar]
- 17.Yhim HY, Jang MJ, Bang SM, Kim KH, Kim YK, Nam SH, et al. Incidence of venous thromboembolism following major surgery in Korea: from the Health Insurance Review and Assessment Service database. J Thromb Haemost. 2014;12(7):1035–1043. doi: 10.1111/jth.12611. [DOI] [PubMed] [Google Scholar]
- 18.Lee KW, Bang SM, Kim S, Lee HJ, Shin DY, Koh Y, et al. The incidence, risk factors and prognostic implications of venous thromboembolism in patients with gastric cancer. J Thromb Haemost. 2010;8(3):540–547. doi: 10.1111/j.1538-7836.2009.03731.x. [DOI] [PubMed] [Google Scholar]
- 19.Choi S, Lee KW, Bang SM, Kim S, Lee JO, Kim YJ, et al. Different characteristics and prognostic impact of deep-vein thrombosis/pulmonary embolism and intraabdominal venous thrombosis in colorectal cancer patients. Thromb Haemost. 2011;106(6):1084–1094. doi: 10.1160/TH11-07-0505. [DOI] [PubMed] [Google Scholar]
- 20.Walker AJ, Card TR, West J, Crooks C, Grainge MJ. Incidence of venous thromboembolism in patients with cancer - a cohort study using linked United Kingdom databases. Eur J Cancer. 2013;49(6):1404–1413. doi: 10.1016/j.ejca.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 21.Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166(4):458–464. doi: 10.1001/archinte.166.4.458. [DOI] [PubMed] [Google Scholar]
- 22.Alcalay A, Wun T, Khatri V, Chew HK, Harvey D, Zhou H, et al. Venous thromboembolism in patients with colorectal cancer: incidence and effect on survival. J Clin Oncol. 2006;24(7):1112–1118. doi: 10.1200/JCO.2005.04.2150. [DOI] [PubMed] [Google Scholar]
- 23.Connolly GC, Dalal M, Lin J, Khorana AA. Incidence and predictors of venous thromboembolism (VTE) among ambulatory patients with lung cancer. Lung Cancer. 2012;78(3):253–258. doi: 10.1016/j.lungcan.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Kröger K, Weiland D, Ose C, Neumann N, Weiss S, Hirsch C, et al. Risk factors for venous thromboembolic events in cancer patients. Ann Oncol. 2006;17(2):297–303. doi: 10.1093/annonc/mdj068. [DOI] [PubMed] [Google Scholar]
- 25.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111(10):4902–4907. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parker A, Peterson E, Lee AYY, de Wit C, Carrier M, Polley G, et al. Risk stratification for the development of venous thromboembolism in hospitalized patients with cancer. J Thromb Haemost. 2018;16(7):1321–1326. doi: 10.1111/jth.14139. [DOI] [PubMed] [Google Scholar]
- 27.Ay C, Dunkler D, Marosi C, Chiriac AL, Vormittag R, Simanek R, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116(24):5377–5382. doi: 10.1182/blood-2010-02-270116. [DOI] [PubMed] [Google Scholar]
- 28.Meyer G, Marjanovic Z, Valcke J, Lorcerie B, Gruel Y, Solal-Celigny P, et al. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: a randomized controlled study. Arch Intern Med. 2002;162(15):1729–1735. doi: 10.1001/archinte.162.15.1729. [DOI] [PubMed] [Google Scholar]
- 29.Hull RD, Pineo GF, Brant RF, Mah AF, Burke N, Dear R, et al. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med. 2006;119(12):1062–1072. doi: 10.1016/j.amjmed.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 30.Mandalà M, Falanga A, Roila F ESMO Guidelines Working Group. Management of venous thromboembolism (VTE) in cancer patients: ESMO clinical practice guidelines. Ann Oncol. 2011;22(Suppl 6):vi85–vi92. doi: 10.1093/annonc/mdr392. [DOI] [PubMed] [Google Scholar]
- 31.National Comprehensive Cancer Network (NCCN) NCCN clinical practice guidelines in oncology, cancer-associated venous thromboembolic disease. [Updated 2018]. [Accessed March 22, 2018]. https://www.nccn.org/professionals/physician_gls/pdf/vte.pdf.
- 32.Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2) Suppl:e419S–e496S. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farge D, Bounameaux H, Brenner B, Cajfinger F, Debourdeau P, Khorana AA, et al. International clinical practice guidelines including guidance for direct oral anticoagulants in the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2016;17(10):e452–e466. doi: 10.1016/S1470-2045(16)30369-2. [DOI] [PubMed] [Google Scholar]
- 34.Lyman GH, Bohlke K, Khorana AA, Kuderer NM, Lee AY, Arcelus JI, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American society of clinical oncology clinical practice guideline update 2014. J Clin Oncol. 2015;33(6):654–656. doi: 10.1200/JCO.2014.59.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361(24):2342–2352. doi: 10.1056/NEJMoa0906598. [DOI] [PubMed] [Google Scholar]
- 36.Schulman S, Kakkar AK, Goldhaber SZ, Schellong S, Eriksson H, Mismetti P, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. 2014;129(7):764–772. doi: 10.1161/CIRCULATIONAHA.113.004450. [DOI] [PubMed] [Google Scholar]
- 37.EINSTEIN Investigators. Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499–2510. doi: 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- 38.EINSTEIN-PE Investigators. Büller HR, Prins MH, Lensin AW, Decousus H, Jacobson BF, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366(14):1287–1297. doi: 10.1056/NEJMoa1113572. [DOI] [PubMed] [Google Scholar]
- 39.Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799–808. doi: 10.1056/NEJMoa1302507. [DOI] [PubMed] [Google Scholar]
- 40.Hokusai-VTE Investigators. Büller HR, Décousus H, Grosso MA, Mercuri M, Middeldorp S, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369(15):1406–1415. doi: 10.1056/NEJMoa1306638. [DOI] [PubMed] [Google Scholar]
- 41.Vedovati MC, Germini F, Agnelli G, Becattini C. Direct oral anticoagulants in patients with VTE and cancer: a systematic review and meta-analysis. Chest. 2015;147(2):475–483. doi: 10.1378/chest.14-0402. [DOI] [PubMed] [Google Scholar]
- 42.van Es N, Coppens M, Schulman S, Middeldorp S, Büller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124(12):1968–1975. doi: 10.1182/blood-2014-04-571232. [DOI] [PubMed] [Google Scholar]
- 43.Yhim HY, Choi WI, Kim SH, Nam SH, Kim KH, Mun YC, et al. Long-term rivaroxaban for the treatment of acute venous thromboembolism in patients with active cancer in a prospective multicenter trial. Korean J Intern Med. 2018 doi: 10.3904/kjim.2018.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raskob GE, van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia D, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378(7):615–624. doi: 10.1056/NEJMoa1711948. [DOI] [PubMed] [Google Scholar]
- 45.Young AM, Marshall A, Thirlwall J, Chapman O, Lokare A, Hill C, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D) J Clin Oncol. 2018;36(20):2017–2023. doi: 10.1200/JCO.2018.78.8034. [DOI] [PubMed] [Google Scholar]
- 46.Li A, Garcia DA, Lyman GH, Carrier M. Direct oral anticoagulant (DOAC) versus low-molecular-weight heparin (LMWH) for treatment of cancer associated thrombosis (CAT): a systematic review and meta-analysis. Thromb Res. 2019;173:158–163. doi: 10.1016/j.thromres.2018.02.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moore RA, Adel N, Riedel E, Bhutani M, Feldman DR, Tabbara NE, et al. High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: a large retrospective analysis. J Clin Oncol. 2011;29(25):3466–3473. doi: 10.1200/JCO.2011.35.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Menapace LA, Peterson DR, Berry A, Sousou T, Khorana AA. Symptomatic and incidental thromboembolism are both associated with mortality in pancreatic cancer. Thromb Haemost. 2011;106(2):371–378. doi: 10.1160/TH10-12-0789. [DOI] [PubMed] [Google Scholar]
- 49.den Exter PL, Hooijer J, Dekkers OM, Huisman MV. Risk of recurrent venous thromboembolism and mortality in patients with cancer incidentally diagnosed with pulmonary embolism: a comparison with symptomatic patients. J Clin Oncol. 2011;29(17):2405–2409. doi: 10.1200/JCO.2010.34.0984. [DOI] [PubMed] [Google Scholar]
- 50.Font C, Farrús B, Vidal L, Caralt TM, Visa L, Mellado B, et al. Incidental versus symptomatic venous thrombosis in cancer: a prospective observational study of 340 consecutive patients. Ann Oncol. 2011;22(9):2101–2106. doi: 10.1093/annonc/mdq720. [DOI] [PubMed] [Google Scholar]
- 51.Di Nisio M, Lee AY, Carrier M, Liebman HA, Khorana AA Subcommittee on Haemostasis and Malignancy. Diagnosis and treatment of incidental venous thromboembolism in cancer patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2015;13(5):880–883. doi: 10.1111/jth.12883. [DOI] [PubMed] [Google Scholar]
- 52.Di Nisio M, Carrier M. Incidental venous thromboembolism: is anticoagulation indicated? Hematology Am Soc Hematol Educ Program. 2017;2017(1):121–127. doi: 10.1182/asheducation-2017.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Böttger B, Wehling M, Bauersachs RM, Amann S, Schuchert A, Reinhold C, et al. Prevalence of renal insufficiency in hospitalised patients with venous thromboembolic events: a retrospective analysis based on 6,725 VTE patients. Thromb Res. 2014;134(5):1014–1019. doi: 10.1016/j.thromres.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 54.Givens ML, Wethern J. Renal complications in oncologic patients. Emerg Med Clin North Am. 2009;27(2):283–291. doi: 10.1016/j.emc.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 55.Hirsh J, Warkentin TE, Shaughnessy SG, Anand SS, Halperin JL, Raschke R, et al. Heparin and low-molecular-weight heparin: mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119(1) Suppl:64S–94S. doi: 10.1378/chest.119.1_suppl.64s. [DOI] [PubMed] [Google Scholar]
- 56.Scotté F, Rey JB, Launay-Vacher V. Thrombosis, cancer and renal insufficiency: low molecular weight heparin at the crossroads. Support Care Cancer. 2012;20(12):3033–3042. doi: 10.1007/s00520-012-1590-9. [DOI] [PubMed] [Google Scholar]
- 57.Woodruff S, Feugère G, Abreu P, Heissler J, Ruiz MT, Jen F. A post hoc analysis of dalteparin versus oral anticoagulant (VKA) therapy for the prevention of recurrent venous thromboembolism (rVTE) in patients with cancer and renal impairment. J Thromb Thrombolysis. 2016;42(4):494–504. doi: 10.1007/s11239-016-1386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bauersachs R, Lee AYY, Kamphuisen PW, Meyer G, Janas MS, Jarner MF, et al. Renal impairment, recurrent venous thromboembolism and bleeding in cancer patients with acute venous thromboembolism-analysis of the CATCH study. Thromb Haemost. 2018;118(5):914–921. doi: 10.1055/s-0038-1641150. [DOI] [PubMed] [Google Scholar]
- 59.Francis CW, Kessler CM, Goldhaber SZ, Kovacs MJ, Monreal M, Huisman MV, et al. Treatment of venous thromboembolism in cancer patients with dalteparin for up to 12 months: the DALTECAN study. J Thromb Haemost. 2015;13(6):1028–1035. doi: 10.1111/jth.12923. [DOI] [PubMed] [Google Scholar]
- 60.Hughes S, Szeki I, Nash MJ, Thachil J. Anticoagulation in chronic kidney disease patients-the practical aspects. Clin Kidney J. 2014;7(5):442–449. doi: 10.1093/ckj/sfu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mavrakanas T, Bounameaux H. The potential role of new oral anticoagulants in the prevention and treatment of thromboembolism. Pharmacol Ther. 2011;130(1):46–58. doi: 10.1016/j.pharmthera.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 62.Stangier J, Rathgen K, Stähle H, Gansser D, Roth W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol. 2007;64(3):292–303. doi: 10.1111/j.1365-2125.2007.02899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 64.Liesenfeld KH, Lehr T, Dansirikul C, Reilly PA, Connolly SJ, Ezekowitz MD, et al. Population pharmacokinetic analysis of the oral thrombin inhibitor dabigatran etexilate in patients with non-valvular atrial fibrillation from the RE-LY trial. J Thromb Haemost. 2011;9(11):2168–2175. doi: 10.1111/j.1538-7836.2011.04498.x. [DOI] [PubMed] [Google Scholar]
- 65.Goldhaber SZ, Schulman S, Eriksson H, Feuring M, Fraessdorf M, Kreuzer J, et al. Dabigatran versus warfarin for acute venous thromboembolism in elderly or impaired renal function patients: pooled analysis of RE-COVER and RE-COVER II. Thromb Haemost. 2017;117(11):2045–2052. doi: 10.1160/TH17-03-0176. [DOI] [PubMed] [Google Scholar]
- 66.Lutz J, Jurk K, Schinzel H. Direct oral anticoagulants in patients with chronic kidney disease: patient selection and special considerations. Int J Nephrol Renovasc Dis. 2017;10:135–143. doi: 10.2147/IJNRD.S105771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Desai JC, Chatterjee P, Friedman K, Aisenberg J. Incidence and clinical presentation of gastrointestinal bleeding in atrial fibrillation patients taking direct oral anticoagulants. Am J Gastroenterol Suppl. 2016;3(1):13–21. [Google Scholar]
- 68.Desai J, Kolb JM, Weitz JI, Aisenberg J. Gastrointestinal bleeding with the new oral anticoagulants--defining the issues and the management strategies. Thromb Haemost. 2013;110(2):205–212. doi: 10.1160/TH13-02-0150. [DOI] [PubMed] [Google Scholar]
- 69.Yarris JP, Warden CR. Gastrointestinal bleeding in the cancer patient. Emerg Med Clin North Am. 2009;27(3):363–379. doi: 10.1016/j.emc.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 70.Degen LP, Beglinger C. Postoperative gastrointestinal physiology following operations on the stomach. Pancreatology. 2001;1(Suppl)(1):9–13. [Google Scholar]
- 71.Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11(1):41–50. doi: 10.1111/j.1467-789X.2009.00614.x. [DOI] [PubMed] [Google Scholar]
- 72.Hakeam HA, Al-Sanea N. Effect of major gastrointestinal tract surgery on the absorption and efficacy of direct acting oral anticoagulants (DOACs) J Thromb Thrombolysis. 2017;43(3):343–351. doi: 10.1007/s11239-016-1465-x. [DOI] [PubMed] [Google Scholar]
- 73.Song Y, Wang X, Perlstein I, Wang J, Badawy S, Frost C, et al. Relative bioavailability of apixaban solution or crushed tablet formulations administered by mouth or nasogastric tube in healthy subjects. Clin Ther. 2015;37(8):1703–1712. doi: 10.1016/j.clinthera.2015.05.497. [DOI] [PubMed] [Google Scholar]
- 74.Boehringer Ingelheim Pharmaceuticals. Pradaxa (dabigatran) Prescribing Information. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals; 2018. [Google Scholar]
- 75.Ten Berg MJ, van den Bemt PM, Shantakumar S, Bennett D, Voest EE, Huisman A, et al. Thrombocytopenia in adult cancer patients receiving cytotoxic chemotherapy: results from a retrospective hospital-based cohort study. Drug Saf. 2011;34(12):1151–1160. doi: 10.2165/11594310-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 76.Wu Y, Aravind S, Ranganathan G, Martin A, Nalysnyk L. Anemia and thrombocytopenia in patients undergoing chemotherapy for solid tumors: a descriptive study of a large outpatient oncology practice database, 2000–2007. Clin Ther. 2009;31(Pt 2):2416–2432. doi: 10.1016/j.clinthera.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 77.Samuelson Bannow BR, Lee AYY, Khorana AA, Zwicker JI, Noble S, Ay C, et al. Management of anticoagulation for cancer-associated thrombosis in patients with thrombocytopenia: a systematic review. Res Pract Thromb Haemost. 2018;2(4):664–669. doi: 10.1002/rth2.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kopolovic I, Lee AY, Wu C. Management and outcomes of cancer-associated venous thromboembolism in patients with concomitant thrombocytopenia: a retrospective cohort study. Ann Hematol. 2015;94(2):329–336. doi: 10.1007/s00277-014-2198-6. [DOI] [PubMed] [Google Scholar]
- 79.Nazari H, Shirazi A, Shams-Esfandabadi N, Afzali A, Ahmadi E. The effect of amniotic membrane stem cells as donor nucleus on gene expression in reconstructed bovine oocytes. Int J Dev Biol. 2016;60(4-6):95–102. doi: 10.1387/ijdb.160010hn. [DOI] [PubMed] [Google Scholar]
- 80.Oliver N, Short B, Thein M, Duong VH, Tidwell ML, Sausville EA, et al. Treatment of catheter-related deep vein thrombosis in patients with acute leukemia with anticoagulation. Leuk Lymphoma. 2015;56(7):2082–2086. doi: 10.3109/10428194.2014.982640. [DOI] [PubMed] [Google Scholar]
- 81.Houghton DE, Key NS, Zakai NA, Laux JP, Shea TC, Moll S. Analysis of anticoagulation strategies for venous thromboembolism during severe thrombocytopenia in patients with hematologic malignancies: a retrospective cohort. Leuk Lymphoma. 2017;58(11):2573–2581. doi: 10.1080/10428194.2017.1306644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Samuelson Bannow BT, Lee A, Khorana AA, Zwicker JI, Noble S, Ay C, et al. Management of cancer-associated thrombosis in patients with thrombocytopenia: guidance from the SSC of the ISTH. J Thromb Haemost. 2018;16(6):1246–1249. doi: 10.1111/jth.14015. [DOI] [PubMed] [Google Scholar]
- 83.Mantha S, Miao Y, Wills J, Parameswaran R, Soff GA. Enoxaparin dose reduction for thrombocytopenia in patients with cancer: a quality assessment study. J Thromb Thrombolysis. 2017;43(4):514–518. doi: 10.1007/s11239-017-1478-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee AY, Peterson EA. Treatment of cancer-associated thrombosis. Blood. 2013;122(14):2310–2317. doi: 10.1182/blood-2013-04-460162. [DOI] [PubMed] [Google Scholar]
- 85.Short NJ, Connors JM. New oral anticoagulants and the cancer patient. Oncologist. 2014;19(1):82–93. doi: 10.1634/theoncologist.2013-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Napolitano M, Saccullo G, Malato A, Sprini D, Ageno W, Imberti D, et al. Optimal duration of low molecular weight heparin for the treatment of cancer-related deep vein thrombosis: the Cancer-DACUS study. J Clin Oncol. 2014;32(32):3607–3612. doi: 10.1200/JCO.2013.51.7433. [DOI] [PubMed] [Google Scholar]
- 87.Jara-Palomares L, Solier-Lopez A, Elias-Hernandez T, Asensio-Cruz M, Blasco-Esquivias I, Marin-Barrera L, et al. Tinzaparin in cancer associated thrombosis beyond 6 months: TiCAT study. Thromb Res. 2017;157:90–96. doi: 10.1016/j.thromres.2017.07.004. [DOI] [PubMed] [Google Scholar]