Abstract
Introduction
Vitamin D deficiency is a common health problem worldwide and tends to be a risk factor for all-cause mortality. We evaluated the effect of continuous low-dose oral daily and loading dose of oral weekly and loading monthly intramuscular (IM) vitamin D3 regimens on circulating levels of total 25(OH)D and in vitamin D deficient females, and between non-obese and obese subgroups.
Material and methods
A total of 231 vitamin D deficient females were included to the study. According to treatment regimen, patients were divided into three groups: daily continuous oral, loading dose of weekly oral and monthly intramuscular. All patients in treatment groups were divided into non-obese (105) and obese (126) groups. Serum 25(OH)D and parathormone (PTH) levels were evaluated at baseline and at the third and sixth month.
Results
In obese patients oral weekly loading regimen and in non-obese patients oral daily continuous regimens were found to be more potent. Baseline PTH levels decreased when compared with the third and sixth months (p < 0.001), but between the third and sixth months it was not changed (p = 0.783).
Conclusions
Oral daily regimen in non-obese patients and loading weekly oral regimen in obese patients were more effective in achieving the target levels of 25(OH)D concentration above 30 ng/ml and provided a stable plasma vitamin D concentration over a long period of time.
Keywords: 25(OH)D3, parathormone, obese, vitamin D3 regimen
Introduction
Vitamin D deficiency and insufficiency are common health problems worldwide and tend to be risk factors for all-cause mortality. Most data show that they are related to diseases of bone, muscle strength, and other organ systems [1, 2]. Serum 25(OH)D is related to endothelial functions measured from digital pulse amplitude hyperaemic response in healthy non-smoking women [3]. Vitamin D deficiency is defined as a 25-hydroxyvitamin D [25(OH)D] level below 20 ng/ml. In its treatment, the dose of vitamin D supplementation should give plasma levels of 25(OH)D above 30 ng/ml to provide musculoskeletal health and to be protective for infectious, autoimmune, and cardiovascular diseases, and other conditions. For treatment and prevention of vitamin D deficiency, either vitamin D2 (ergocalciferol) or vitamin D3 (cholecalciferol) can be used [4]. Deficiency of vitamin D is frequently seen in 30–50% of the general population [5]. Stores of vitamin D decline with age and are affected by season, particularly in the winter [6].
It is known that there is a contrary relationship between serum circulating 25(OH)D level and body mass index (BMI) greater than 30 kg/m2; therefore, obesity may be associated with vitamin D deficiency [7]. Obese adults are at high risk of vitamin D deficiency because body fat sequesters fat-soluble vitamins. Despite the common prevalence and increasing importance of vitamin D deficiency, there is poor consensus about the treatment regimen. In the literature, there are few studies comparing the different pharmaceutical forms of vitamin D. Good studies to assess the most efficacious way of treating vitamin D deficiency and maintaining sufficiency in at-risk populations are needed.
In this current study, our aim was to evaluate the effects of administrating a continuous daily oral regimen or loading doses of weekly oral or monthly intramuscular (IM) regimens of vitamin D3 on circulating levels of total 25(OH)D for 6 months in a vitamin D deficient female population. Moreover, the efficacy of each regimen in non-obese and obese groups was compared.
Material and methods
Patients and methods
A total of 231 healthy Caucasian females between the ages of 17 and 72 years (mean: 44) with vitamin D deficiency admitted to our clinic for routine control after being diagnosed with vitamin D deficiency were randomly matched for the study. All participants were included in the study in September or October and were followed for 6 months in the same season. Patients with acute or chronic liver and/or kidney disease, malignancy, thyrotoxicosis, Paget’s disease, history of nephrolithiasis, or taking medications that might affect vitamin D metabolism, such as calcitriol anticonvulsants, glucocorticoids, and barbiturates, and patients who were taking oral calcium, vitamin D supplements, or multivitamin supplements and those that had hypercalcaemia or primary hyperparathyroidism, were excluded. Waist-to-hip ratio (WHR) was measured in all subjects.
Initially, patients were divided into three groups (77 patients in each). Patients within these groups were further divided into two subgroups based on BMI (calculated as weight divided by height squared, kg/m2): a non-obese group (BMI < 30 kg/m2) and an obese group (BMI ≥ 30 kg/m2). Usually, 50,000 IU once a week for 8 weeks or 6000 IU/day of vitamin D2 or D3 followed by maintenance therapy of 1500–2000 IU/day is recommended [4]. The first group was treated with an oral daily regimen by ingesting 6000 IU vitamin D3 supplementation for 8 weeks. The second group was treated with oral weekly bolus regimen by ingesting 50,000 IU of vitamin D3 once a week for 8 weeks. The third group had intramuscular (IM) injection of 200,000 IU of vitamin D3 per month for 2 months. Treatment doses of vitamin D3 were given to all patients for 2 months, followed by oral maintenance doses of 1500 IU/day for prevention of vitamin D deficiency. In obese patients, two to three times as much vitamin D is needed to achieve this same increase in serum 25(OH)D levels [2, 6]. We gave twice as many doses to obese patients. According to subgroups, 105 non-obese and 126 obese patients received three regimens of cholecalciferol randomly. All patients were evaluated by clinical and biochemical assessment for serum albumin, calcium, phosphorus, 25(OH)D, and parathormone (PTH) levels at baseline and in the third month and sixth month of the therapy. The circulating half-life of 25(OH)D is approximately 2 to 3 weeks, and it is the best indicator to monitor vitamin D status. Findings from recent studies showed that ingesting 100 IU/day of vitamin D3 increases serum 25(OH)D by less than 1 ng/ml [8, 9]. Serum calcium levels were corrected for serum albumin: measured calcium – [(4 – measured albumin) × 0.8]. The IM of 300,000 IU vitamin D3 was well tolerated among all patients and injection site reaction was not reported. The Local Ethical Committee of Ankara Numune Education and Research Hospital approved the study and all patients gave informed consent.
Laboratory analysis
The patients’ serum albumin, calcium, and phosphorus levels were measured using a photometric method; specifically, the Beckman Coulter AU5800 analyser (made by Beckman Coulter Incorporated in the United States of America [USA]) was used. The patients’ serum 25(OH)D and intact PTH levels were assessed with chemiluminescent immunoassays (CLIA); specifically, a UniCel Dxl 800 autoanalyser (also made by Beckman Coulter in the USA) was used. Serum 25(OH)D was measured with standard method, and the test sensitivity was 100% and specificity was 89%. The interassay and intra-assay precision for the test was 5.3% and 7.8%, respectively. Vitamin D deficiency was defined as a serum 25(OH)D level below 20 ng/ml, and hyperparathyroidism was defined as a PTH level over 88 pg/ml. Finally, a normal calcium range was defined as 8.5–10.5 mg/dl, while a normal phosphorus range was defined as 2.5–4.5 mg/dl.
Statistical analysis
The data was analysed using the Statistical Package for Social Sciences for Windows 20 (IBM SPSS Inc., Chicago, IL) package program. The descriptive statistics were mean ± standard deviation for normal distributions, median (minimum to maximum) for abnormal distributions, and number of cases (percentage) for nominal variables. Variations according to time were investigated with a variance analysis if the distributions were normal. If distributions were not normal, the Friedman test was used.
Because there were two groups, the significance of differences between the groups in terms of averages was investigated by t-test, and the significance of the median values was investigated by the Mann-Whitney U test. When the number of groups was more than two, the significance of differences between the groups in terms of averages was investigated by the ANOVA variance analysis test and the crucial importance in terms of median values by the Kruskal-Wallis H test. Pearson’s χ2 or Fisher’s exact tests were used to assess nominal variables. Data were compared using the two-way mixed ANOVA method when the normal distribution was found to be appropriate, depending on the type of obesity, treatment modality, and time. Finally, a Greenhouse-Geisser correction was applied when there was no assumption of globalisation.
While the relationships between continuous variables were investigated, if the distribution was normal, the Pearson correlation test was used. If a distribution was abnormal, the Spearman correlation test was used. The statistical significance level was set at a p-value less than 0.05.
Results
All subjects were female and vitamin D deficient (25(OH)D < 20 ng/ml). The characteristics of all patients and all patients mean serum 25(OH)D levels at baseline, and at the third and sixth months of each therapy are given in Table I. No statistically significant differences were detected between the groups in terms of the patients’ ages, BMIs, or WHR. Baseline level of 25(OH)D in the weekly oral group was lower than in the daily oral group (p = 0.008). At the third month, the 25(OH)D level of all groups was ≥ 30 ng/ml, and no significant difference was found between the groups. At the sixth month, the daily oral group had higher 25(OH)D levels than the weekly oral and IM groups (p < 0.001).
Table I.
Descriptive statistics, clinical characteristics and biochemical measurements between three regimens of vitamin D3 supplemented in participants
| Parameter | Daily oral (n = 77) | Weekly oral (n = 77) | Intramuscular (n = 77) | P-value* |
|---|---|---|---|---|
| Age [years] | 44.1 ±11.5 | 44.1 ±11.9 | 43.2 ±10.4 | 0.852 |
| Height [cm] | 160.5 ±5.9 | 159.7 ±6.3 | 161.4 ±5.2 | 0.324 |
| Weight [kg] | 73.2 ±15.9 | 77.5 ±17.1 | 74.4 ±16.3 | 0.396 |
| Waist [cm] | 73.2 ±15.9 | 91.3 ±12.8 | 89.8 ±17.9 | 0.848 |
| Hip [cm] | 109.3 ±10.5 | 110.7 ±15.9 | 112.3 ±13.4 | 0.843 |
| BMI [kg/cm2] | 27.3 ±5.8 | 28.0 ±6.5 | 26.7 ±5.5 | 0.441 |
| WHR | 0.79 ±0.06 | 0.83 ±0.09 | 0.79 ±0.09 | 0.696 |
| Baseline 25(OH)D | 9.45 ±4.10 | 7.45 ±3.38 | 7.89 ±3.69 | 0.008a, 0.081b, 1.00c |
| 3rd month 25(OH)D | 40.85 ±13.62 | 37.84 ±13.48 | 38.02 ±11.29 | 0.555a, 0.719b, 1.00c |
| 6th month 25(OH)D | 33.65 ±11.71 | 27.06 ±8.38 | 23.45 ±6.14 | < 0.001a, < 0.001b, 0.154c |
ANOVA test
BMI – body mass index, WHR – waist-to-hip ratio.
Daily oral vs. weekly oral
daily oral vs. IM
weekly oral vs. IM.
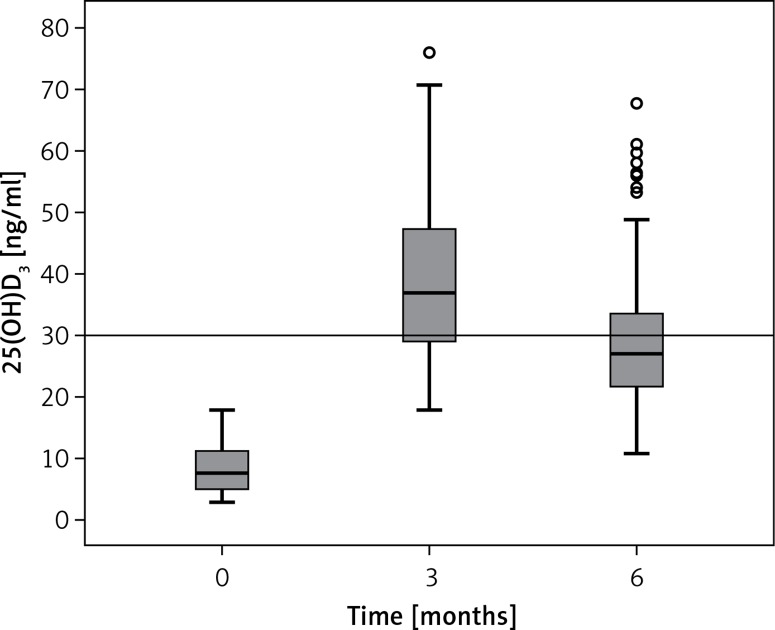
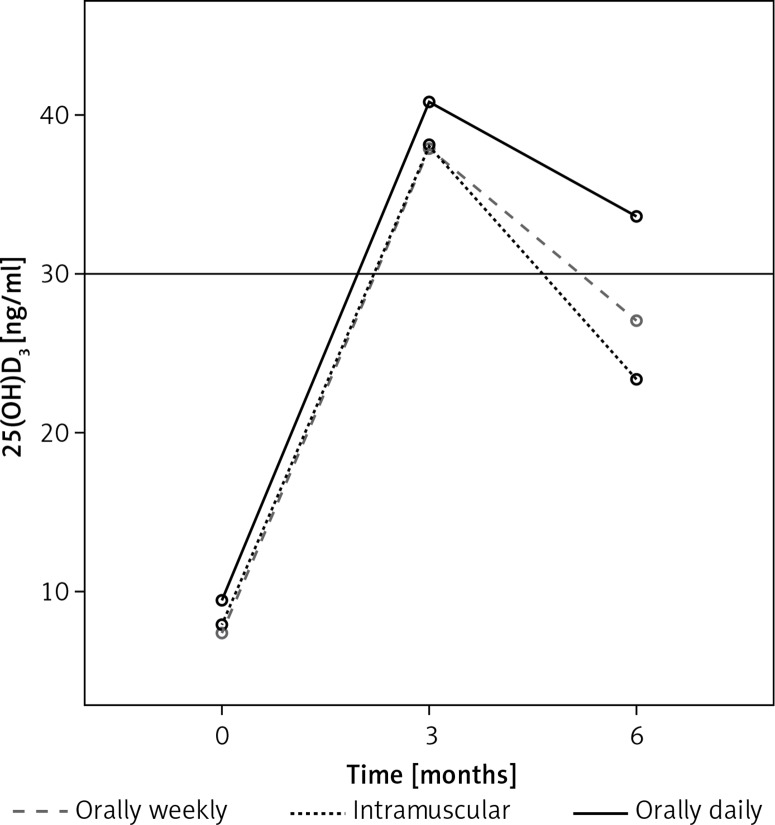
Mean serum 25(OH)D levels of patients at baseline and following vitamin D3 replacement (third and sixth months) in all treatment groups are given in Figure 1. Baseline mean 25(OH)D (ng/ml) was 7.80 (2.90–17.80), at the third month 36.88 (18.00–76.00), and at the sixth month 27.00 (10.73–67.62) (p < 0.001). Figure 2 shows that the serum 25(OH)D level increased linearly in the daily oral, weekly oral, and IM groups at the third month in all patients; however, at the sixth month of therapy 25(OH)D levels tended to decrease to < 30 ng/ml in the weekly oral and IM groups. Only the daily oral group at the sixth month, the mean serum 25(OH)D levels remained at ≥ 30 ng/ml. The oral daily regimen was found to be more effective than the oral weekly and IM regimens in the long term.
Figure 1.
Measurements of 25(OH)D3 levels during baseline, 3rd and 6th months in all patients
Figure 2.
Changes of mean serum 25(OH)D concentration in oral daily, oral weekly, and intramuscular groups at baseline, 3rd and 6th month of vitamin D3 treatment in all patients
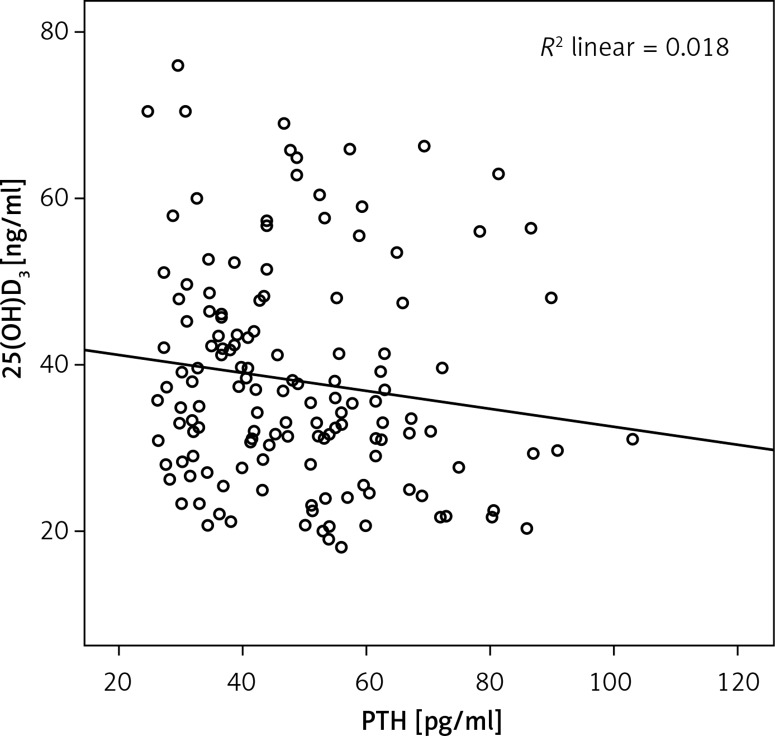
Changes in 25(OH)D, PTH, serum calcium and phosphorus levels between baseline, the third, and sixth months were compared in all patients (Table II). Baseline PTH levels decreased when compared with the third and sixth months (p < 0.001), but between the third and sixth months it remained unchanged (p = 0.783). Corrected total calcium levels were increased at baseline vs. third and sixth months (0.041 and 0.001, respectively), whereas at third vs. sixth months, it was not changed (p = 0.149). No statistically significant change was detected in serum phosphorus levels between baseline, and at the third and sixth months (p = 0.322). Because the efficacy of vitamin D treatment was assessed at the third month, the correlation between third month levels of 25(OH)D and PTH was also assessed; a weak negative correlation was found (r = –0.177, p = 0.037) (Figure 3).
Table II.
Changes of mean serum 25(OH)D, PTH, calcium, and phosphorus levels between baseline and following vitamin D3 replacement (3rd and 6th months) in all patients
| Parameter | Baseline | 3rd month | 6th month | P-value |
|---|---|---|---|---|
| 25(OH)D [ng/ml] | 7.8 (2.9–17.8) | 36.8 (18.0–76.0) | 27.0 (10.7–67.6) | < 0.001*a,b,c |
| PTH [pg/ml] | 84.0 (38.0–182.9) | 45.6 (24.7–89.9) | 50.5 (23.1–110.0) | < 0.001a,b, 0.783*c |
| Total calcium*** [mg/dl] | 9.43 ±0.44 | 9.55 ±0.42 | 9.65 ±0.41 | 0.041a, 0.001b, 0.149c** |
| Phosphorus [mg/dl] | 3.35 ±0.44 | 3.27 ±0.45 | 3.32 ±0.38 | 0.322a,b,c |
Friedman test
general linear model
corrected calcium (for albumin)
baseline vs. 3rd month
baseline vs. 6th month
3rd month vs. 6th month.
Figure 3.
Correlation* analysis of the 3rd month of 25(OH)D and PTH levels. There was a weak negative correlation between the 3rd month of 25(OH)D and PTH levels (r = –0.177; p = 0.037)
*Spearman’s correlation test.
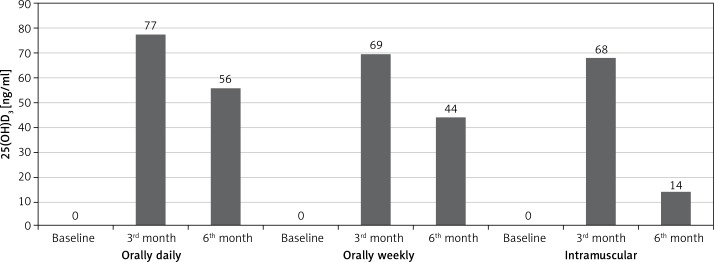
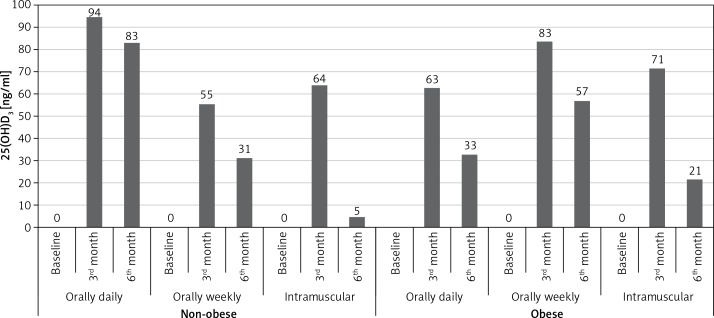
We compared percentages of all patients who had vitamin D levels higher than 30 mg/dl according to treatment regimens (Figure 4). At the third month, the percentage of patients who received daily oral regimen was higher (n = 77%). Similarly, at the sixth month, serum 25(OH)D tended to remain at > 30 ng/ml in the daily oral group more than in the weekly oral and IM groups (n = 56% vs. 44% and 14%, respectively; p < 0.001 for both). Percentages of obese and non-obese subgroups were compared according to treatment regimens (Figure 5). In non-obese patients, the daily oral regimen was found to be more successful than the weekly oral and IM regimens for keeping 25(OH)D ≥ 30 at third and sixth months ((94% vs. 55% and 64%, respectively; p < 0.001 for both) and (83% vs. 31% and 5%, respectively; p < 0.001 for both)). On the other hand, in obese patients, weekly oral therapy was found to be successful, compared to the daily oral and IM regimens at the third and sixth months ((83% vs. 63% and 71%, respectively; p < 0.001 for both) and (57% vs. 33% and 21%, respectively; p < 0.001 for both)).
Figure 4.
The percentages of patients receiving three regimens of vitamin D3, who achieved > 30 ng/ml, among all patients (25(OH)D, n = (%))
Figure 5.
The percentages of patients who received three regimens of vitamin D3, who achieved > 30 ng/ml, between non-obese and obese patients (25(OH)D > 30 (%))
Discussion
It is estimated that most people worldwide are vitamin D deficient. A study from Turkey showed that vitamin D insufficiency has 80% prevalence in both the reproductive-aged and the elderly female populations [10]. It was shown that a regimen of vitamin D three times a year, once a week, or once a day can be effective in maintaining serum 25(OH)D levels in both children and adults. Two to three times as much vitamin D, however, is required to achieve this same increase in serum 25(OH)D levels in patients who are obese [4].
Vitamin D3 is the most commonly used treatment choice for vitamin D insufficient or deficient patients. Previous studies compared effects of vitamin D2 and D3 preparations on total serum 25(OH)D by using different supplementation regimens in humans. They found that vitamin D3 was more potent than vitamin D2 [11–14]. Ingestion of 4000 IU cholecalciferol daily resulted in 97.5% of the population having a 25(OH)D level of 32 ng/ml or greater [15]. Therefore, we used vitamin D3 preparations for our patients. Although many controlled trial data are available for comparing vitamin D2 and D3, limited data are available for comparing vitamin D3 supplementation regimens. Our study aimed to fill this gap in experience. We investigated the efficacy of three regimens of vitamin D3 for treatment and maintenance therapy of vitamin D deficiency in female populations. Also, we compared the effective treatment between obese and non-obese subgroups.
Our first key finding was that all three regimens were found to be successful in the treatment. However, regarding long-term efficacy, we recommend giving a daily oral regimen for non-obese and general female populations. With a decreasing rate, parenteral high-dose vitamin D regimens are still used throughout the world. In the literature about using an injection regimen of high-dose vitamin D, some cautions are noted regarding toxicity and adherence. Hashemipour et al. indicated late and long-lasting effects of intramuscular (IM) vitamin D3. They showed that injection of vitamin D doses higher than 300,000 IU may be associated with 25(OH)D concentrations that exceed the upper limits of normal and may cause hypervitaminosis D [16]. Acute vitamin D toxicity may cause symptoms of hypercalcaemia or hypercalciuria. A study for the treatment of vitamin D deficiency with oral or IM ergocalciferol established a greater variability and low effectivity in 25(OH)D concentration with IM injections compared with oral administration [17]. Hackman et al. compared the efficacy and safety of oral continuous low-dose versus short-term high-dose vitamin D in the vitamin D deficient population. Participants received high-dose (50,000 IU) daily vitamin D3 for 10 days or continuous low-dose (3000 IU) daily vitamin D3 for 30 days, followed by 1000 IU daily for 60 days. They found similar increases in serum 25(OH)D in both groups [18]. In a study, it was reported that annual IM injection of 600,000 IU vitamin D3 was an effective and safe treatment for vitamin D deficiency in 50 Australians [19]. In these studies, the course of vitamin D level has not been stated in the long term after the treatment dose was discontinued or tapered.
A previous study compared two different dosing regimens of cholecalciferol in elderly women with vitamin D deficiency at the sixth month of the follow-up period. Patients received 300,000 IU every three months, (intermittent D3 group) or 1000 IU/day (daily D3 group). The intermittent D3 group was found to be more effective than the daily D3 group for correcting vitamin D deficiency. The intermittent D3 group received 3.3 times more dose of cholecalciferol than the daily D3 group, but the mean absolute increase of 25(OH)D was not 3.3 times as high [20]. The response of therapy in the high-dose group is not similar to our study. In a recent study targeting the therapeutic range of vitamin D in elderly patients with hip fracture, patients received either placebo or a loading dose of 50,000 or 100,000 IU vitamin D2 with a daily dose of 1000 IU vitamin D3 over a period of 90 days. They showed that an additional loading dose of 50,000 IU had better results than 100,000 IU (75% vs. 44%) [21]. Contrary to our study, some studies comparing daily to weekly or monthly regimens have reported suboptimal vitamin D levels at the end of the study despite supplementation. Ish-Shalom et al. [22] found that only half of the patients with initial 25(OH)D below 50 nmol/l, and 80% of those with 25(OH)D 50 nmol/l and above, achieved the therapeutic target (75 nmol/l) after 8 weeks of 1500 IU vitamin D3 daily. In nursing homes where residents received 600 IU vitamin D3 daily, fewer than 40% achieved vitamin D levels of 75 nmol/l after 4 months [23]. We think that vitamin D absorption defects or causes affect the vitamin D metabolism and must be carefully evaluated for the success of the treatment.
A study that compared pharmacological doses of oral and IM cholecalciferol regimens in the treatment of vitamin D deficiency determined that both regimens were effective, safe, and practical. They revealed the superiority of an oral route, similarly to our results [24]. We believe that prolonged or inadvertent intake of high loading doses of weekly or monthly treatment regimens may result in vitamin D toxicity. Thus, taking a daily vitamin D replacement is a safe method, and our study found it to be a more effective treatment and maintenance regimen in females.
However, one issue that needs to be explained is the prevention method that should be taken in order to avoid recurrence of hypovitaminosis D after the treatment is discontinued or tapered. For this reason, we tried to determine which treatment regimen was more successful in order to avoid the recurrence of the disease. We found that in the studied population continuous oral daily treatment was more effective than weekly oral loading and monthly IM doses for the treatment in achieving the target level, and this provides a stable plasma D vitamin concentration over a long period of time.
A second key finding in this study was which of the vitamin D treatment and maintenance regimens was more effective for obese patients. The reasons for the increased risk of vitamin D deficiency in the obese population is unclear, but it has been suggested that they mght avoid exposure to solar ultraviolet radiation, which is required for the production of vitamin D3 on the skin [25]. Furthermore, in obesity the metabolic clearance of vitamin D may rise, possibly with greater uptake by adipose tissue [26]. With the effect of body weight on the dose response curve, larger doses of vitamin D may be required in the treatment of vitamin D insufficiency in obese patients, depending on possible decreased bioavailability of 25(OH)D from cutaneous and dietary sources because of its deposition in body fat compartments [6]. Usually obese patients need two to three times as much vitamin D to achieve an increase in serum 25(OH)D levels [27, 28]. Therefore, in our study, two times as much vitamin D replacement was given to obese patients. We observed the obese group respond to oral weekly loading dose better than the non-obese group. In addition, while taking maintenance therapy, at the sixth month the percentages of patients who received weekly oral loading regimen were greatet than the other two treatment regimens. For this reason, we think that weekly oral regimen is more appropriate for patients who are obese.
The third key point is PTH level, which tends to decrease after treatment. Deficiency of vitamin D causes the reduction of intestinal calcium and phosphorus absorption resulting in an increase in PTH levels. Data indicate that when the level of 25(OH)D reached 30 ng/ml or less, there was a significant decrease in intestinal calcium absorption that was associated with increased parathyroid hormone [2]. The relationship of PTH and 25(OH)D is not curvilinear, and there is no absolute threshold level of 25(OH)D at which PTH levels rise [29, 30]. In our study, after vitamin D3 treatment the PTH level was decreased, but between the third and sixth months it remained unchanged. A negative and significant correlation was found between 25(OH)D and PTH levels at the third month of therapy.
There are some limitations to our study. The first is that the study was done only with the female population. If male patients were included in the study, the study population would be more appropriate to real life data. The second limitation is that since there was no control group in this study, placebo was not used. If a placebo was used, a better comparison could be made. Other limitations included the fact that urinary calcium excretion levels were not measured in patients if it looked the hypercalciuria effect of the treatment could be better followed.
In conclusion, in non-obese patients, a daily continuous oral vitamin D3 regimen and in obese patients a weekly loading oral vitamin D3 regimen were each effective in maintaining vitamin D levels above 30 ng/ml long term. Prescription and exposure to higher doses can lead to vitamin D intoxication, which can be harmful even in vitamin D deficient subjects. According to the results of this study, administration of three regimens of vitamin D3 can achieve the target levels of 25(OH)D concentration. We established the superiority of a continuous oral daily regimen among all patients in general. We found an oral daily regimen was more effective in non-obese patients, and a weekly oral bolus in obese patients was effective in achieving the target levels and providing a stable plasma D vitamin concentration over a long period of time. Further investigations with long follow-up periods are needed to find the appropriate treatment approach.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Neale RE, Webb PM, van der Pols JC. Vitamin 18 D for prevention of chronic disease: the need for continued research. Intern Med J. 2008;38:813–5. doi: 10.1111/j.1445-5994.2008.01813.x. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D deficiency. N Engl j Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Ertek S, Akgül E, Cicero AF, et al. 25-Hydroxy vitamin D levels and endothelial vasodilator function in normotensive women. Arch Med Sci. 2012;8:47–52. doi: 10.5114/aoms.2012.27280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metabol. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 5.Lee JH, O’Keefe JH, Bell D, Hensrud DD, Holick MF. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol. 2008;52:1949–56. doi: 10.1016/j.jacc.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 6.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67:373–8. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 7.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–3. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 8.Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204–10. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]
- 9.Holick MF, Biancuzzo RM, Chen TC, et al. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab. 2008;93:677–81. doi: 10.1210/jc.2007-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arasil T, Uysal AR, Idil A, et al. Vitamin D status of women living in Ankara. Turk J Endocrinol Metabolism. 2010:39–44. [Google Scholar]
- 11.Romagnoli E, Mascia M, Cipriani C, et al. Short and long-term variations in serum calciotropic hormones after a single very large dose of ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3) in the elderly. J Clin Endocrinol Metabolism. 2008;93:3015–20. doi: 10.1210/jc.2008-0350. [DOI] [PubMed] [Google Scholar]
- 12.Binkley N, Gemar D, Engelke J, et al. Evaluation of ergocalciferol or cholecalciferol dosing, 1,600 IU daily or 50,000 IU monthly in older adults. J Clin Endocrinol Metabolism. 2011;96:981–8. doi: 10.1210/jc.2010-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heaney R, Recker R, Grote J, Horst R, Armas L. Vitamin D3 is more potent than vitamin D2 in humans. J Clin Endocrinol Metabolism. 2010;96:447–52. doi: 10.1210/jc.2010-2230. [DOI] [PubMed] [Google Scholar]
- 14.Trang HM, Cole DE, Rubin LA, Pierratos A, Siu S, Vieth R. Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nutr. 1998;68:854–8. doi: 10.1093/ajcn/68.4.854. [DOI] [PubMed] [Google Scholar]
- 15.Heaney RP. Barriers to optimizing vitamin D3 intake for the elderly. J Nutr. 2006;136:1123–5. doi: 10.1093/jn/136.4.1123. [DOI] [PubMed] [Google Scholar]
- 16.Hashemipour S, Sarukhani MR, Asefzadeh S. Effect of different doses of parenteral vitamin D3 on serum 25 (OH)D concentrations. Daru. 2009;17(Suppl 1):26–30. [Google Scholar]
- 17.Stephens WP, Klimiuk PS, Berry JL, Mawer EB. Annual high-dose vitamin D prophylaxis in Asian immigrants. Lancet. 1981;2:1199–202. doi: 10.1016/s0140-6736(81)91439-2. [DOI] [PubMed] [Google Scholar]
- 18.Hackman KL, Gagnon C, Briscoe RK, Lam S, Anpalahan M, Ebeling PR. Efficacy and safety of oral continuous low-dose versus short-term high-dose vitamin D: a prospective randomised trial conducted in a clinical setting. Med J Aust. 2010;192:686–9. doi: 10.5694/j.1326-5377.2010.tb03702.x. [DOI] [PubMed] [Google Scholar]
- 19.Diamond TH, Ho KW, Rohl PG, Meerkin M. Annual intramuscular injection of a megadose of cholecalciferol for treatment of vitamin D deficiency: efficacy and safety data. Med J Aust. 2005;183:10–2. doi: 10.5694/j.1326-5377.2005.tb06879.x. [DOI] [PubMed] [Google Scholar]
- 20.Giusti A, Barone A, Pioli G, et al. Heterogeneity in serum 25-hydroxy-vitamin D response to cholecalciferol in elderly women with secondary hyperparathyroidism and vitamin D deficiency. J Am Geriatr Soc. 2010;58:1489–95. doi: 10.1111/j.1532-5415.2010.02970.x. [DOI] [PubMed] [Google Scholar]
- 21.Papaioannou A, Kennedy CC, Giangregorio L, et al. A randomized controlled trial of vitamin D dosing strategies after acute hip fracture: no advantage of loading doses over daily supplementation. BMC Musculoskel Disorders. 2011;12:135. doi: 10.1186/1471-2474-12-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ish-Shalom S, Segal E, Salganik T, Raz B, Bromberg IL, Vieth R. Comparison of daily, weekly, and monthly vitamin D3 in ethanol dosing protocols for two months in elderly hip fracture patients. J Clin Endocrinol Metab. 2008;93:3430–5. doi: 10.1210/jc.2008-0241. [DOI] [PubMed] [Google Scholar]
- 23.Chel V, Wijnhoven HA, Smit JH, Ooms M, Lips P. Efficacy of different doses and time intervals of oral vitamin D supplementation with or without calcium in elderly nursing home residents. Osteoporos Int. 2008;19:663–71. doi: 10.1007/s00198-007-0465-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zabihiyeganeh M, Jahed A, Nojomi M. Treatment of hypovitaminosis D with pharmacologic doses of cholecalciferol, oral vs intramuscular; 1, an open labeled RCT. Clin Endocrinol. 2013;78:210–6. doi: 10.1111/j.1365-2265.2012.04518.x. [DOI] [PubMed] [Google Scholar]
- 25.Compston JE, Vedi S, Ledger JE, Webb A, Gazet JC, Pilkington TRE. Vitamin D status and bone histomorphometry in gross obesity. Am J Clin Nutr. 1981;34:2359–63. doi: 10.1093/ajcn/34.11.2359. [DOI] [PubMed] [Google Scholar]
- 26.Liel Y, Ulmer E, Shary J, Hollis BW, Bell NH. Low circulating vitamin D in obesity. Calcif Tissue Int. 1988;43:199–201. doi: 10.1007/BF02555135. [DOI] [PubMed] [Google Scholar]
- 27.Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004. Am J Clin Nutrition. 2008;88:1519–27. doi: 10.3945/ajcn.2008.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hintzpeter B, Scheidt-Nave C, Müller MJ, Schenk L, Mensink GB. Higher prevalence of vitamin D deficiency is associated with immigrant background among children and adolescents in Germany. J Nutrition. 2008;138:1482–90. doi: 10.1093/jn/138.8.1482. [DOI] [PubMed] [Google Scholar]
- 29.Heaney RP. The vitamin D requirement in health and disease. J Steroid Biochem Mol Biol. 2005;97:13–9. doi: 10.1016/j.jsbmb.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 30.Steingrimsdottir L, Gunnarsson O, Indridason OS, Franzson L, Sigurdsson G. Relationship between serum parathyroid hormone levels, vitamin D sufficiency, and calcium intake. JAMA. 2005;294:2336–41. doi: 10.1001/jama.294.18.2336. [DOI] [PubMed] [Google Scholar]