Abstract
Few long-term studies report late outcomes after severe traumatic brain injury. New rehabilitation techniques are needed for this heterogenous patient group. We present a dance intervention six and half years after an extreme severe TBI including excessive diffuse axonal injury, which disconnects the brain networks. Given the fact, that efficient brain function depends on the integrated operation of large-scale brain networks like default mode network (DMN), we created an intervention with multisensory and multimodal approach and goal-directed behavior. The intervention lasted four months including weekly one-hour dance lessons with the help of a physiotherapist and dance teacher. The measures included functional independence measure (FIM), repeated electroencephalogram (EEG) analysis of three subnets of DMN and clinical evaluations and observations. The results showed clear improvement after the intervention, and FIM stayed in elevated level during several years after the intervention. We present suggestion for further studies using larger patient groups.
Keywords: Neurology, Rehabilitation
1. Introduction
Few studies report outcomes in late state (over 5 years) of Traumatic Brain Injury (TBI) and there is a need to develop systematic selection of patients for design and implementation of clinical studies aiming for new therapies [1]. These new rehabilitation methods should be matched to the specific needs of individuals in order to get optimal rehabilitation interventions. There is a need to supplement randomized control trial evaluation of studies using comparative effectiveness research, in combination with high-quality observational studies. TBI population is heterogeneous and that's why observational studies would be beneficial [2]. One example of the potential promising new form of neuronal rehabilitation are music-dance-based interventions which are in emerging state [3].
Several studies have demonstrated that music- and rhythm-based interventions have positive effects in treating and rehabilitating such neurological conditions as Parkinson's disease, stroke and dementia [3, 4, 5].
In healthy brain efficient cognitive functions depend on the integrated operation of large-scale distributed brain networks [6, 7]. Higher order cognitive functions such as the capability for self-consciousness depend on the dynamic coordinated activation between different networks [8]. Perception, attention, memory, language, goal-directed behaviour and other cognitive functions are the result of interaction between distributed and overlapping brain networks [6, 7, 9]. Individuals with chronic TBI have often deficits in using higher order cognitive functions that require the coordination of multiple brain networks [10].
Default Mode Network (DMN) is active in reasonably automatic bodily actions, self-consciousness and self-related thoughts, emotions and memories [6, 7, 11, 12]. The main brain areas, which comprise DMN are posterior cingulate cortex, the ventromedial prefrontal cortex, lateral inferior parietal lobes and medial temporal structures [11, 13]. Activity in DMN has been associated with creativity [14, 15], pain reduction [16], music listening [17], musical improvisation [18], narrative thinking and autobiographical memories, as well as experiential self [7, 12], and making decisions considering self, such as future goals and events [7]. When multimodal training is combined with previously learned music and dance material and skills, memory- and motivation-related brain regions may be re-activated. Hippocampus is crucial in storing and retrieving the non-overlapping representations of the distributed cortical activities when different events are encoded [19, 20]. An important fact is that the DMN includes the hippocampus and adjacent areas [21, 22]. More specifically, many of the key neocortical regions constituting the DMN are functionally connected with the hippocampal formation and show spontaneous activity correlation with it [23].
Instead of a homogenous unit, DMN (or self-referential brain network) has also been proposed to be a heterogenous brain system which is composed of at least three spatially separable but functionally interacting subnets (or operational modules) [24, 25]. The proposed model is based on a series of electroencephalogram (EEG) -studies in normal conditions and pathological states [12, 24, 25, 26]. The revealed subnets are the anterior subnet (according to authors corresponds to Witnessing observer or “Self”), right Occipito-Parieto-Temporal subnet (corresponds to Representational-emotional agency or “Me”) and left Occipito-Parieto-temporal subnet (corresponds to Reflective agency or “I”). It has been proposed that together, these three subnets, anterior subnet and symmetrical right and left subnets, could, in large, account for the DMN [24, 25, 27]. This construct model of the DMN is called the three-dimensional construct model for the complex selfhood [24, 27] (Table 1).
Table 1.
The three-dimensional model of complex selfhood adapted from Fingelkurts and Fingelkurts [12, 25, 27] and Fingelkurts et al.24Abbreviations: DMN Default Mode Network; BA: Broadmann's area.
| DMN brain subnets of three-dimensional model of complex experiential selfhood |
|---|
| Anterior subnet |
| Witnessing observer (“Self”), which is responsible for the first-person perspective, the sense of agency and top-down attentional control. |
| Cortical areas: Left and right middle frontal gyri or Broadmann's area (BA) 8, bilateral medial areas or BA 6. |
| Right Occipito-Parieto-Temporal subnet |
| Representational-emotional agency (“Me”), which is responsible for the experience of self as a localized embodied entity (through interoceptive and exteroceptive bodily sensory processing), emotion-related thoughts, and autobiographical memories. |
| Cortical areas: Right middle temporal gyrus or BA 21, right precuneus or BA 19, right middle occipital gyrus or BA 18. |
| Left Occipito-Parieto-Temporal subnet |
| Reflective agency (“I”), is involved in experience of thinking about and reflecting upon oneself, including momentary narrative thoughts and inner speech, as well as reinterpretation of short term memory events related to self. |
| Cortical areas: Left middle temporal gyrus or BA 21, left precuneus or BA 19, left middle occipital gyrus or BA 18. |
Some researchers hypothesize that changes in DMN activity that are associated with chronic phase of TBI could be compensatory processes in the recovering brain after severe TBI [11, 28, 29]. Research suggests that DMN reorganization after severe acquired brain injury may be an important biomarker for the cognitive recovery [30]. In this context rehabilitation protocols aiming to restore functional integrity within and coordination between brain networks could be considered as very promising approaches for the persons with TBI [31].
The aim of our intervention in this case study was to assess the feasibility of intensive goal directed, personal strengths based, multimodal and multisensory dance rehabilitation intervention and to evaluate its efficacy with the rehabilitee of chronic condition (six and half years post injury) after an extremely severe TBI including excessive Diffuse Axonal Injury (DAI). The working assumption was that the process of recovery might still be accelerated even after a long and successful period of intensive multidiscipline rehabilitation. The individually tailored intervention was based on the patient's primary and current strengths, his motivation, and tasks based on his episodic memory by utilizing dance and music material, which was created by the patient himself before the accident.
2. Material and methods
2.1. Subject
The rehabilitee is a young man, aged 19 at the time of the high-energy traffic accident - g-force exceeded a hundred. Initial hit to the back of head threw the patient several meters away. Upon landing his head hit to hard material by the left side of the head. He lost his consciousness immediately and was intubated on the accident site.
Before the injury, he had no history of neurological or psychiatric conditions, he had degrees both in dance and music, and was highly skilled in mathematics, languages, and knowledge-based disciplines.
At admission to level I trauma centre, the Glasgow Coma Score (GCS) was 3 (out of scale 3 to 15). An isolated head trauma was found. Computed Tomography (CT) showed a left subdural haematoma with excessive oedema. 25 centimetres long fracture was found on the skull. A decompressive craniotomy and evacuation of the haematoma was performed immediately.
Control CT next day showed a large occipital epidural haematoma, which was operated. The following day CT showed a parietal contusion haematoma and subcortical temporal oedema. Intracranial pressure remained at level 15. Eight days after Magnetic Resonance Imaging (MRI) showed excessive DAI on the left hemisphere (frontal, temporal, occipital) and frontally on the right hemisphere as well as on left mesencephalon and right brain stem. He was in coma for two and half weeks and in minimally conscious state months after that. He was tracheotomised and was fed parenterally for several months.
Intensive rehabilitation started five months after the accident and lasted for six months in an inpatient rehabilitation centre. During this time his total score in the Functional Independence Measure (FIM) [32] increased from 26 to 43.
Rehabilitation procedures were continued after returning to home for five and half years, consisting of 10 to 15 hours per week in varying patterns: physiotherapy, orthopaedic manual therapy, speech and language therapy, occupational therapy, neuropsychological rehabilitation and music therapy, as well as the physiologically active substances (vitamins, nutrients, supplements and neuropeptides).
Based on the written reports of the physiotherapist, neurologist, and neuropsychologist of the rehabilitation centre, three days before the dance intervention started: The rehabilitee used a wheelchair independently inside but needed assistance with obstacles and outside in the rough terrain. He needed at least supervision and verbal guidance in many of the everyday situations. He needed guidance in planning and continuing the daily activities. He also needed guidance in extending his body in the wheel chair. He was able to work bimanually, even though the position of the left arm was not always optimal. He was able to walk inside with heavy assistance by a physiotherapist 20 meters with the walker. (Physiotherapist's report).
The function of the left upper arm was very limited and differentiated movements especially in the fingers were very rigid. (Neurologist's report).
The alertness level was better in the morning and was declining clearly during afternoons. The time of continuous working capacity was from 30 to 45 minutes. His attention regulation was noticeably difficult and variable. He had problems in sustaining attention, and it succeeded to varying degrees and was linked to the alertness level. In general, the organized functioning was noticeably slowed. (Neuropsychologist's report).
The case study and handling of the results was approved by the subject (signed written consent, subject's capability verified from court decision) and is in line with the Code of the World Medical Association (Declaration of Helsinki) and The University of Helsinki Ethical Review Board in the Humanities and Social and Behavioural Sciences.
Intervention was part of subject's normal rehabilitation process – physiotherapy – and therefore did not interfere neither with the usual medical practice, nor with the everyday rehabilitation therapies.
The evaluation of the actual intervention (dance teacher and physiotherapist), evaluation of the daily life (personal assistant/practical nurse), reports and FIM evaluations from inpatient sessions in rehabilitation centre (neurologist, neuropsychologist, nurses) and EEG measurements (BM-Science) and the use of the results was approved by the subject.
2.2. Intervention
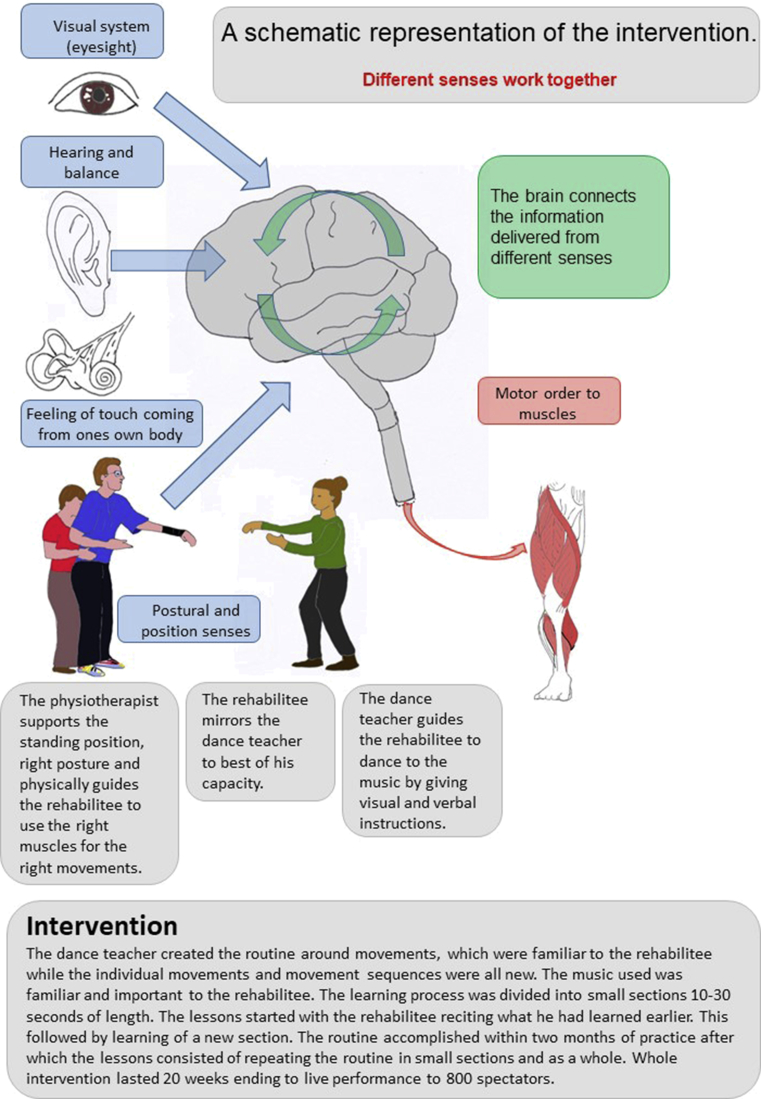
The goal directed dance rehabilitation intervention was tailored according to the needs and strengths of the rehabilitee. The highly motivating goal was to participate in to a dance competition. The 20-week intervention included a weekly 60-minute dance lesson. A professional dance teacher guided the lessons and a physiotherapist familiar with the rehabilitee provided manual instruction. Fig. 1.
Fig. 1.
A schematic representation of the intervention.
The selected dance music was very important for the rehabilitee as he had edited the music and danced to it in front of a live audience before the accident. During the intervention the dance teacher modified the choreography according to the capacity of the rehabilitee. The rehabilitee created some parts of the dance choreography and designed the costume for the competition.
In addition to the dance lessons the rehabilitee listened to the music for the choreography during his free time, did some mental exercises and watched videos which were filmed during the dance lessons. Besides this specific music-dance-focused intervention, rehabilitee's previous holistic rehabilitation continued unchanged.
After the practice period he took part in a National Dance Competition and was dancing, performing and remembering the learned dance-choreography in front of over 800-person live audience along with other competitors, of whom many were nearly professional dancers.
The competition included several hours of preparing and waiting before the actual performance. The personal assistant helped him to go on and off the stage with a wheelchair and the physiotherapist was supporting his standing posture while he was dancing. The dance teacher was present during the competition but gave no instructions for him during the performance.
2.3. Measures
The Measures included FIM, EEG, as well as observations during the intervention, observations of personal assistant (everyday life), and (external clinical) observations from the Rehabilitation Centre's neurologist and neuropsychologist, who were not aware of the intervention. Besides the observations, in the beginning of the first two lessons and during the last one, the mobility of the rehabilitee was assessed with various exercises. The rehabilitee was recorded on video both from behind (view also to the mirror) and from the left side and the mobility was compared between the first and the last lessons.
FIM is widely accepted functional outcome scale which estimates disability resulting from physical and cognitive impairments. It is used as assessment of progress during inpatient rehabilitation. The possible total score varies between 18 (lowest) to 126 (highest) 32. FIM was measured repeatedly five times before the intervention and four times after it.
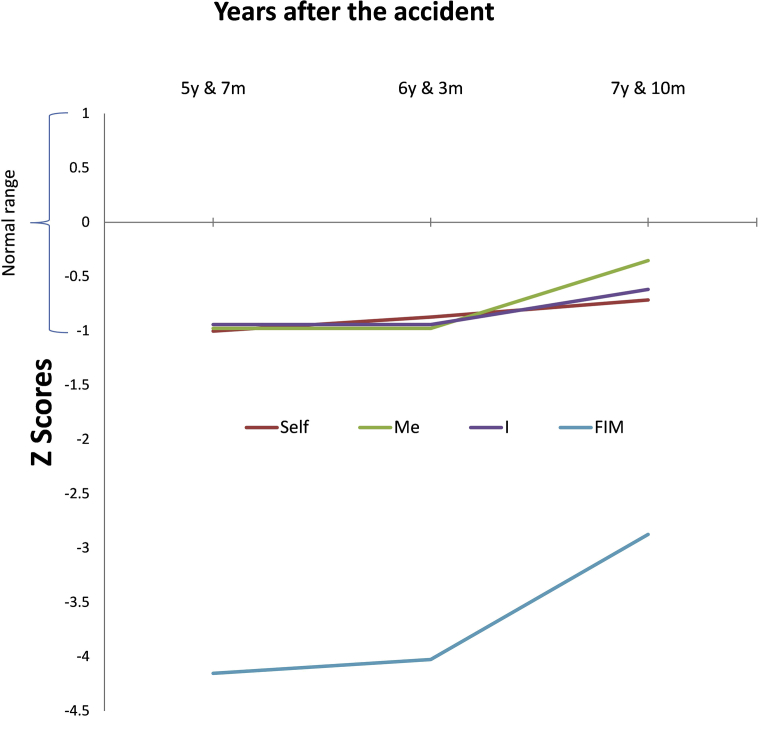
Repeated EEG measurements of the three subnets of DMN have been measured along the rehabilitee's whole rehabilitation period (from 1 year 11 months to 7 years 10 months) and are reported elsewhere [27]. For the purpose of the present case study we will present only the three last three measures of them: two before the intervention and one after it (Fig. 3). As was reported in Fingelkurts and Fingelkurts [27], operational synchrony analysis of the EEG could reliably estimate the three subnets of DMN (anterior subnet, right Occipito-Parieto-Temporal subnet and left Occipito-Parieto-Temporal subnet) that correspond to such components of selfhood as “Self”, “Me” and “I”. Details of EEG application, measurement and analysis are found in Fingelkurts and Fingelkurts [27].
Fig. 3.
Synchrony strength dynamics within three sub-nets of the brain self-referential network in the rehabilitee during three last years of observation. The Y-axis presents z-values of strength of operational synchrony for every subnet that is responsible for different aspect of complex selfhood: ‘Self’, ‘Me’ and ‘I’, as well as z-scores for FIM scales. The X-axis represents three last years after the accident. Abbreviations: FIM: Functional Independence Measure; y: year; m: month. Modified from Fingelkurts & Fingelkurts, 2017b © Clinical EEG & Neuroscience.
For each EEG-session values of the three DMN subnets were compared to population normative reference and presented as z -scores. Z-scores quantify deviation of an observed value from normative data. The deviation can be above (z > 0) or below (z < 0) the normative values in terms of standard deviation (SD). Deviation was ranged from slight (2 SD; P < .05), moderate (2,5 SD; P < .01) high (3 SD; P < .003) or very high (4 SD; P < .0001). The normative range of EEG characteristics is (-1<z < 1) [27].
3. Results
3.1. Observations and clinical examinations
According to the physiotherapist and the dance teacher rehabilitee's balance, posture, mobility and endurance got better. Procedural and episodic memory were better and he could develop mnemonics (Tables 3, 4, and 5).
Table 3.
Observational results and clinical examinations reflecting changes in DMN Right brain subnet or to the localized embodied entity from the three components of selfhood as defined in Fingelkurts and Fingelkurts [27].
| Rehabilitation Centre - Neurologist & Neuropsychologist | Intervention - Physiotherapist & Dance Teacher | Observations from real life - Personal Assistant/Practical Nurse |
|---|---|---|
| DMN Right subnet | ||
| Localized embodied entity (through interoceptive and exteroceptive bodily sensory processing) | ||
| Working bimanually more than before | Bimanual functions more frequent and self-reliant | Working with two hands in co-ordination enhanced |
| Taking into account the position of the left upper limb better than before | Understanding his bodily positions | Walking using walker, with the support of personal assistant, more frequently and longer |
| Bodily position control enhanced | ||
| Posture notably more symmetrical and no swaying | ||
| Hands raising more symmetrically and balance remaining while raising hands | ||
| Leaning further to all directions, center of gravity staying better, larger movement trajectory in the right lean | ||
| Improved mobility and control, deeper squat, ability to stay in the squat for a few seconds, no spasms appeared while straightening | ||
| Emotion-related thoughts | ||
| Performing the choreography lively in front of audience | Feeling secure to do things independently | |
| Can stay at home independently longer time | ||
| Autobiographical memories | ||
| Episodic memory improved | Recalling old dance-related events | The rehabilitee can tell what happened during important sessions during the day |
Table 4.
Observational results and clinical examinations reflecting changes in DMN Left brain subnet or to the thinking about and reflecting upon oneself from the three components of selfhood as defined in Fingelkurts and Fingelkurts [27].
| Rehabilitation Centre - Neurologist & Neuropsychologist | Intervention - Physiotherapist & Dance Teacher | Observations from real life - Personal Assistant/Practical Nurse |
|---|---|---|
| DMN Left subnet | ||
| Thinking about and reflecting upon oneself | ||
| Could recognize his level of alertness to a greater degree (increased self-awareness) and able to reflect that to working (self-reflection) | The awareness of his own capacity and state improved | |
| Able to notice himself the slipping of his own attentional focus | Able to regulate the activity-rest cycle by himself | |
| Uncertain of his memory capacity; although when he checks it, the memories are mostly correct | ||
| Could recognize his level of alertness to a greater degree | ||
| Momentary narrative thoughts and inner speech | ||
| Started to develop mnemonics | ||
| Reinterpretation of short-term memory, events related to self | ||
| Working memory improved | Working memory improved | |
Table 5.
Other observational results and clinical examinations.
| Rehabilitation Centre - Neurologist & Neuropsychologist | Intervention - Physiotherapist & Dance Teacher | Observations from real life - Personal Assistant/Practical Nurse |
|---|---|---|
| OTHER OBSERVATIONS | ||
| Motor function | ||
| Maximum standing from 15 to 30 minutes | Could get up to sitting position in bed | |
| Able to move from the bed to wheelchair, from wheelchair to toilet seat | ||
| Long term memory | ||
| Could memorize the whole choreography | Remembering names better and learning them faster |
According to the personal assistant (practical nurse) the rehabilitee was feeling more secure, independent and self-controlled. Executive functions got faster, alertness level and concentration were much better and he could regulate the activity-rest cycle by himself. Working and long-term memory as well as motor functions improved (Tables 2, 3, 4, and 5).
Table 2.
Observational results and clinical examinations reflecting changes in DMN Anterior brain subnet or to the first-person perspective from the three components of selfhood as defined in Fingelkurts and Fingelkurts [27].
| Rehabilitation Centre - Neurologist & Neuropsychologist | Intervention - Physiotherapist & Dance Teacher | Observations from real life - Personal Assistant/Practical Nurse |
|---|---|---|
| DMN Anterior subnet | ||
| First-person perspective | ||
| The sense of agency | ||
| Progressing self-intentionally from one phase of the action to the next phase | The motor functions notably more independent and self-controlled | |
| More self-reliant | ||
| More inclined to perform actions independently and more self-disciplinary actions | ||
| Faster speed of executive functions | ||
| Top-down attentional capacity | ||
| Attentional capacity improved | Concentration improved | |
| Stimulus dependency significantly diminished | ||
The neurological status of the rehabilitee was clinically examined and observed in the same Rehabilitation Centre before and after the applied dance -rehabilitation. According to the neuropsychologist the rehabilitees self-awareness, self-reflection, alertness level, attention and coping as well as episodic and working memory were improved (Tables 2, 3, and 4).
By the neurologist the rehabilitee acted self-intentionally, his functions were still slow but faster, stimulus-dependency was diminished and he controlled his left upper limb better and worked bimanually more (Tables 2 and 3).
3.2. Functional independence measure
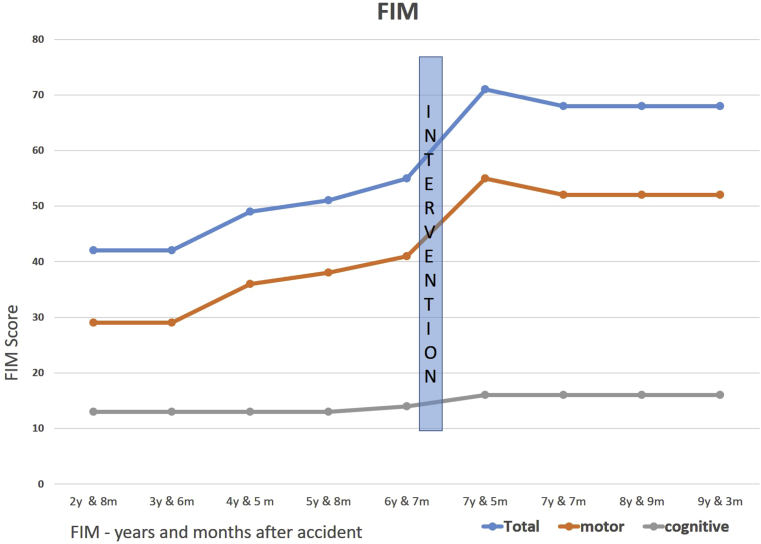
FIM had shown progressive improvement already through the six and half years after the injury. However, the steepest change was found after the dance rehabilitation intervention as it increased 29% compared to the previous measurement. Long term stabilized increase was 24%, 2 years and 3 months after intervention (Fig. 2).
Fig. 2.
Functional Independence Measure (FIM) years and months after accident.
3.3. Electroencephalogram
The recovery in three DMN subnets estimated by repeated operational synchrony analysis of the EEG was positively correlated with FIM (cited from [27]: R = 0.57; p < .05). The FIM -measures were transferred to z-scores to correlate it with the values of EEG -subnets [27]. (Fig. 3).
Please note that Figs. 2 and 3 FIM measurement points are slightly different. In Fig. 2 FIM values are in points when measurement was done and in Fig. 3 FIM Z-score values are in points when EEG measurement was done.
3.4. Combining observations, clinical examinations and DMN subnet model
The observations and clinical examinations where in line with the dynamics of DMN components [27] from the EEG analysis and from FIM measurements. These correspondences are summarised in Tables 2, 3, 4 and 5.
The intervention was well tolerated and subject was highly committed to it, as reported by the physiotherapist and the dance teacher. There were no adverse or unanticipated negative effects during or after the intervention.
4. Discussion
Our aim was to assess the feasibility of goal-directed multimodal and multisensory dance rehabilitation in the chronic state of extreme severe TBI, and to follow the outcome during several years. The results showed accelerated improvement in the total FIM score which raised 29 % during the intervention (from 55 to 71 points). This increase was stable still 2 years 3 months after the intervention, the total FIM score being 24 % higher than before the intervention. EEG –analysis of the three-dimensional DMN brain network [27] revealed, that all three subnets recovered as compared to the time before and after the intervention. The three subnets of the DMN network had been shown to be positively correlated with FIM (R = 0.57; p < .05) already in an earlier study [27]. The clearest improvement was seen in the right Occipito-Parieto-Temporal DMN -subnet [27]. In this intervention both embodied actions, memory and emotional based elements were strongly presented, and that could explain this result. Observations and clinical evaluations revealed several positive outcomes in self-related functions as well as balance, posture, mobility, and endurance within the rehabilitee after the intervention. All observational and clinical examination results, FIM and DMN changes were in line. Importantly, the intervention took place in the chronic phase of extreme severe traumatic brain injury. Moreover, FIM stayed in an elevated level during several years after the intervention.
As a future direction, also other brain networks besides Default Mode Network could be studied in this context of rehabilitation. Salience Network (SN) is active when sudden attention and higher order cognitive control is needed and it seems to work in counter-synchrony with the DMN: When the activity of SN increases, the activity in DMN decreases [6]. Brain areas associated with SN are mostly the rostral anterior cingulate cortex/presupplementary motor area and anterior insulae [11, 33, 34].
Default Mode Network, Dorsal Attention Network (DAN) and Fronto Parieto Control Network (FPCN), which is active during tasks which require executive functions, are supposed to work in synchrony to provide internally and externally focused goal-directed cognition and other higher order cognitive functions [7, 10, 35]. It is of special importance, that large-scale brain systems like default and executive control networks, which can show antagonistic relation, seem to work co-operatively during creative cognition and artistic performance. Complex cognitive processes, especially goal-directed, self-generated thought, seem to be supported by this dynamic process [36].
TBI may disrupt normal interaction between the SN and the DMN [37], thus causing cognitive and motor functioning disruption [6, 37, 38, 39], as well as leading to deficits in self-control and self-monitoring [40]. Structural and functional disconnection within the DMN is linked to impairments of sustained attention and generally degraded self-awareness [6, 40, 41], whereas the damage within the SN is linked to failure of DMN control and impairments of attention and executive function [37, 42, 43], motor network dysfunction [44], spatial neglect [45], working memory disturbances [46], and impaired memory retrieval [47].
According to earlier studies it seems that multimodal rehabilitation with dance and music-related activity affects brain plasticity. It has been reported that music-related activities influence brain plasticity both in white and grey matter level, cause structural and functional plastic changes in hippocampus, and structural changes in the brain sensorimotor network [48, 49, 50, 51]. Functional changes have been observed also in Salience Network after long-term musical training [52]. It has been documented that music and dance training affects Default Mode Network by enlarging the volume and interactions of the key brain structures involved in DMN which are responsible for visual imagery, episodic memory, bodily self and generally for self-regulation and self-enhancement and goal directed behaviour [18, 53, 54, 55, 56]. Dancing with music is multisensory and multimodal activity, which requires coordination of motor, cognitive, imaginative and emotional brain networks and includes action observational and simulation networks, as well as self-reflecting [57, 58, 59, 60, 61, 62, 63, 64, 65]. When people are dancing or playing music, they often react to rhythm with spatiotemporal and affective coordination [67]. The sensory and motor systems in the brain seem to have a connection while perceiving rhythm [68], and musical rhythm has been shown to affect several brain networks e.g. Fronto-Parieto-Cerebellar motor system and corticospinal excitability [69, 70, 71]
Capitalizing on the evidence of the relation between DMN and other brain networks [7, 10, 36], and considering the findings that dance and music could modify them [48, 49, 50, 51], our view is that dance- and music-based interventions may have extra effect on the brain networks coordination and hence accelerate the rehabilitation outcome in a chronic state of TBI. Activity in DMN has been associated with making decisions considering one's future goals [7], and goal-directed behaviour seems to be a result of interaction between DMN and other large-scale brain networks [6, 7, 9]. DMN -integration can be an important biomarker of the recovering brain networks after severe acquired brain injury [30].
4.1. Suggestions for further studies
-
i.
By providing emotionally-charged, memory-retrieving (pre-accident learned) multisensory information (dancing and music) to the multimodal brain regions through remaining/intact pathways with repeated rehearsal, it may be possible to restore/strengthen the connections within and between different brain areas more effectively and possibly stabilize the putative balance between DMN, SN and other brain networks activity.
-
ii.
The memory system might be activated during rehabilitation by accessing several pre-injury learned components into the process.
-
iii.
In the beginning of the training period the brain functions and activity outcomes are likely to be slow and in the need of conscious control. However, after a certain amount of training, when the brain networks are hypothetically restored or reconnected, cognitive control, attention and movements are likely to become more automatic and therefore faster than in the beginning of the training period.
Further studies should use objective methodologies such as Magnetic Resonance Imaging (MRI), Diffuse Tensor Imaging (DTI) and advanced functional connectivity EEG analysis [12], to evaluate the structural and functional recovery of brain networks and their connections (such as DMN, SN and FPCN) as a result of dance rehabilitation intervention as in this way it may be possible to create personally tailored rehabilitation programs for subjects suffering TBI. This kind of new therapy might have substantial effects to individuals in late state of TBI and their families, and for the society owing to the economic burden. As in all studies, possible contraindications should also be considered.
While designing future research protocols it should be taken care that rehabilitation is not too intense due the possible negative changes in plasticity. It should be considered at which stage of rehabilitation process this kind of intense rehabilitation protocol could be started.
4.2. Limitations
Despite the promising results (changes in the rehabilitee), the fact that it was only one patient suggests that evaluation of the results should be done with caution before providing indications for clinicians and should be considered preliminary. To confirm the results presented in this report, future studies with a larger group of patients are warranted.
Traditional methods used in scientific studies, specifically standardization of subjects and methods, cannot be used in evaluation of individualized rehabilitation methods. It's something, which should be taken care, while designing future research protocols.
The information of the rehabilitee was collected from real-life clinical examinations and rehabilitative observations and not as a result of a designed research protocol. For example, mobility measurements, evaluation of the movement patterns and their documentation was unsystematic. Accordingly, the results should be considered as descriptive.
4.3. Conclusion
The presented observations and measures show that there has been a considerable acceleration in the rehabilitation process in a chronic condition of an extremely severe traumatic brain injury after the goal-directed applied dance rehabilitation intervention and that the results have been stable for several years after the intervention. Rehabilitation interventions specifically targeted to restoring the integrity of DMN network and connectivity with other networks could offer a promising future rehabilitation method, and more studies with larger patient groups are needed to confirm our view.
Declarations
Author contribution statement
Marjo Kullberg-Turtiainen: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Kaisa Vuorela, Lilli Huttula: Performed the experiments.
Petri Turtiainen: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Sanna Koskinen: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors want to thank Dr. Andrew Fingelkurts and Dr. Alexander Fingelkurts for very insightful and substantial discussions related to this study.
References
- 1.Wilson L., Stewart W., Dams-O’Connor K., Diaz-Arrastia R., Horton L., Menon D.K., Polinder S. Traumatic brain injury 4. The chronic and evolving neurological consequences of traumatic brain injury. Lancet Neurol. 2017;16:813–825. doi: 10.1016/S1474-4422(17)30279-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maas A.I., Menon D.K., Adelson P.D. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16:987–1048. doi: 10.1016/S1474-4422(17)30371-X. [DOI] [PubMed] [Google Scholar]
- 3.Sihvonen A.J., Särkämö T., Leo V., Tervaniemi M., Altenmüller E., Soinila S. Music-based interventions in neurological rehabilitation. Review. Lancet Neurol. 2017;16:648–660. doi: 10.1016/S1474-4422(17)30168-0. [DOI] [PubMed] [Google Scholar]
- 4.Bradt J., Magee W.L., Dileo C., Wheeler B.L., Mcgilloway E. Music therapy for acquired brain injury. Cochrane Database Syst. Rev. 2010;7:1–42. doi: 10.1002/14651858.CD006787.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Magee W.L., Clark I., Tamplin J., Bradt J. Music interventions for acquired brain injury. Cochrane Database Syst. Rev. 2017;1:CD006787. doi: 10.1002/14651858.CD006787.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharp D.J., Scott G., Leech R. Network dysfunction after traumatic brain injury. Nat. Rev. Neurol. 2014;10:156–166. doi: 10.1038/nrneurol.2014.15. [DOI] [PubMed] [Google Scholar]
- 7.Andrews-Hanna J.R., Smallwood J., Spreng R.N. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann. N. Y. Acad. Sci. 2014;1316:29–52. doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fingelkurts An A., Fingelkurts Al A., Neves C.F.H. Phenomenological architecture of a mind and Operational Architectonics of the brain: the unified metastable continuum. New Math. Nat. Comput. 2009;5:221–244. [Google Scholar]
- 9.Sepulcre J. Functional streams and cortical integration in the human brain. Neuroscientist. 2014;20:499–508. doi: 10.1177/1073858414531657. [DOI] [PubMed] [Google Scholar]
- 10.Han K., Chapman S.B., Krawczyk D.C., Barch D.M., Verfaellie M., Rao S.M. Disrupted intrinsic connectivity among default, dorsal attention, and frontoparietal control networks in individuals with chronic traumatic brain injury. J. Int. Neuropsychol. Soc. 2016;22:263–279. doi: 10.1017/S1355617715001393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ham T.E., Sharp D.J. How can investigation of network function inform rehabilitation after traumatic brain injury? Curr. Opin. Neurol. 2012;25:662–669. doi: 10.1097/WCO.0b013e328359488f. [DOI] [PubMed] [Google Scholar]
- 12.Fingelkurts An A., Fingelkurts Al A. Persistent operational synchrony within brain default-mode network and self-processing operations in healthy subjects. Brain Cogn. 2011;75:79–90. doi: 10.1016/j.bandc.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. A default mode of brain function. Proc. Natl. Acad. Sci. U. S. A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu J., Yang H., Zhang X., He H., Luo C., Yao D. The brain functional state of music creation: an fMRI study of composers. Sci. Rep. 2015;5:1–8. doi: 10.1038/srep12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beaty R.E., Benedek M., Wilkins R.W., Jauk E., Fink A., Silvia P.J., Hodges D.A., Koschutnig K., Neubauer A.C. Creativity and the default network: a functional connectivity analysis of the creative brain at rest. Neuropsychologia. 2014;64:92–98. doi: 10.1016/j.neuropsychologia.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garza-Villarreal E.A., Jiang Z., Vuust P., Alcauter S., Vase L., Pasaye E.H., Cavazos-Rodriguez R., Brattico E., Jensen T.S., Barrios F.A. Music reduces pain and increases resting state fMRI BOLD signal amplitude in the left angular gyrus in fibromyalgia patients. Front. Psychol. 2015;6:1–11. doi: 10.3389/fpsyg.2015.01051. Article 1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kay B.P., Meng X., DiFrancesco M.W., Holland S.K., Szaflarski J.P. Moderating effects of music on resting state networks. Brain Res. 2012;1447:53–64. doi: 10.1016/j.brainres.2012.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Limb C.J., Braun A.R. Neural substrates of spontaneous musical performance: an fMRI study of jazz improvisation. PLoS One. 2008;3:1–9. doi: 10.1371/journal.pone.0001679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norman K.A., O’Reilly R.C. Modeling hippocampal and neocortical contributions to recognition memory: a complementary-learning-systems approach. Psychol. Rev. 2003;110:611–646. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- 20.Rugg M.D., Wilding E.L. Retrieval processing and episodic memory. Trends Cognit. Sci. 2000;4:108–115. doi: 10.1016/s1364-6613(00)01445-5. [DOI] [PubMed] [Google Scholar]
- 21.Greicius M.D., Srivastava G., Reiss A.L., Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buckner R., Andrews-Hanna, Schacter D.L. The brain’s default network anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 23.Vincent J.L., Snyder A.Z., Fox M.D., Shannon B.J., Andrews J.R., Raichle M.E., Buckner R.L. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J. Neurophysiol. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- 24.Fingelkurts A.A., Fingelkurts A.A., Kallio-Tamminen T. Trait lasting alteration of the brain default mode network in experienced meditators and the experiential selfhood. Self Ident. 2016;15:381–393. [Google Scholar]
- 25.Fingelkurts A.A., Fingelkurts A.A. Three-dimensional components of selfhood in treatment-naive patients with major depressive disorder: a resting-state qEEG imaging study. Neuropsychologia. 2017;99:30–36. doi: 10.1016/j.neuropsychologia.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 26.Fingelkurts A.A., Fingelkurts A.A., Bagnato S., Boccagni C., Galardi G. The chief role of frontal operational module of the brain default mode network in the potential recovery of consciousness from the vegetative state: a preliminary comparison of three case reports. Open Neuroimaging J. 2016;10:41–51. doi: 10.2174/1874440001610010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fingelkurts A.A., Fingelkurts A.A. Longitudinal dynamics of 3-dimensional components of Selfhood after severe traumatic brain injury: a qEEG case study. Clin. EEG Neurosci. 2017;48:327–337. doi: 10.1177/1550059417696180. [DOI] [PubMed] [Google Scholar]
- 28.Sharp D.J., Beckmann C.F., Greenwood R., Kinnunen K.M., Bonnelle V., De Boissezon X., Powell J.H., Counsell S.J., Patel M.C., Leech R. Default mode network functional and structural connectivity after traumatic brain injury. Brain. 2011;134:2233–2247. doi: 10.1093/brain/awr175. [DOI] [PubMed] [Google Scholar]
- 29.Hillary F.G., Slocomb J., Hills E.C., Fitzpatrick N.M., Medaglia J.D., Wang J., Good D.C., Wylie G.R. Changes in resting connectivity during recovery from severe traumatic brain injury. Int. J. Psychophysiol. 2011;82:115–123. doi: 10.1016/j.ijpsycho.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 30.Caravasso F.C., de Pasquale F., Ciurli P., Catani S., Formisano R., Sabatini U. The default mode network connectivity predicts cognitive recovery in severe acquired brain injured patients: a longitudinal study. J. Neurotrauma. 2016;33:1247–1262. doi: 10.1089/neu.2015.4003. [DOI] [PubMed] [Google Scholar]
- 31.Bagnato S., Boccagni C., Sant'Angelo A., Fingelkurts Al A., Fingelkurts, An A., Giuseppe G. Emerging from an unresponsive wakefulness syndrome: brain plasticity has to cross a threshold level. Neurosci. Biobehav. Rev. 2013;37:2721–2736. doi: 10.1016/j.neubiorev.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Shukla D., Devi I.B., Agrawal A. Outcome measures for traumatic brain injury. Clin. Neurol. Neurosurg. 2011;113:435–441. doi: 10.1016/j.clineuro.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 33.Ham T.E., de Boissezon X., Leff A., Beckmann C., Hughes E., Kinnunen K.M., Leech R., Sharp D.J. Distinct frontal networks are involved in adapting to internally and externally signaled errors. Cerebr. Cortex. 2013;23:703–713. doi: 10.1093/cercor/bhs056. [DOI] [PubMed] [Google Scholar]
- 34.Seeley W.V., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vincent J.L., Kahn I., Snyder A.Z., Raichle M.E., Buckner R.L. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J. Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beaty R.E., Benedek M., Silvia P.J., Schacter D.L. Creative cognition and brain network dynamics. Trends Cognit. Sci. 2016;20:87–95. doi: 10.1016/j.tics.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonnelle V., Ham T.E., Leech R., Kinnunen K.M., Mehta M.A., Greenwood R.J., Sharp D.J. Salience network integrity predicts default mode network function after traumatic brain injury. Proc. Natl. Acad. Sci. U. S. A. 2012;109:469095. doi: 10.1073/pnas.1113455109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kinnunen K.M., Greenwood R., Powell J., Leech R., Hawkins P.C., Bonnelle V., Patel M.C., Counsell S.J., Sharp D.J. White matter damage and cognitive impairment after traumatic brain injury. Brain. 2011;134:449–463. doi: 10.1093/brain/awq347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fagerholm E.D., Hellyer P.J., Scott G., Leech R., Sharp D.J. Disconnection of network hubs and cognitive impairment after traumatic brain injury. Brain. 2015;138:1696–1709. doi: 10.1093/brain/awv075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fingelkurts An A., Fingelkurts Al A., Bagnato S., Boccagni C., Galardi G. DMN operational synchrony relates to self-consciousness: evidence from patients in vegetative and minimally conscious states. Open Neuroimaging J. 2012;6:55–68. doi: 10.2174/1874440001206010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonnelle V., Leech R., Kinnunen K.M., Ham T.E., Beckmann C.F., De Boissezon X., Greenwood R.J., Sharp D.J. Default mode network connectivity predicts sustained attention deficits after traumatic brain injury. J. Neurosci. 2011;31:13442–13451. doi: 10.1523/JNEUROSCI.1163-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spitz G., Maller J.J., O'Sullivan R., Ponsford J.L. White matter integrity following traumatic brain injury: the association with severity of injury and cognitive functioning. Brain Topogr. 2013;26:648–660. doi: 10.1007/s10548-013-0283-0. [DOI] [PubMed] [Google Scholar]
- 43.Caeyenberghs K., Leemans A., Leunissen I., Gooijers J., Michiels K., Sunaert S., Swinnen S. Altered structural networks and executive deficits in traumatic brain injury patients. Brain Struct. Funct. 2014;219:193–209. doi: 10.1007/s00429-012-0494-2. [DOI] [PubMed] [Google Scholar]
- 44.Kasahara M., Menon D.K., Salmond C.H., Outtrim J.G., Tavares J.V.T., Carpenter T.A., Pickard J.D., Sahakian B.J., Stamatakis E.A. Altered functional connectivity in the motor network after traumatic brain injury. Neurology. 2010;75:168–176. doi: 10.1212/WNL.0b013e3181e7ca58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corbetta M., Shulman G.L., Hyman S.E., Jessell T.M., Shatz C.J., Stevens C.F., Zoghbi H.Y. Spatial neglect and attention networks. Ann. Rew. Neurosci. 2011;34:569–599. doi: 10.1146/annurev-neuro-061010-113731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kasahara M., Menon D.K., Salmond C.H., Outtrim J.G., Tavares J.V.T., Carpenter T.A., Pickard J.D., Sahakian B.J., Stamatakis E.A. Traumatic brain injury alters the functional brain network mediating working memory. Brain Inj. 2011;25:1170–1187. doi: 10.3109/02699052.2011.608210. [DOI] [PubMed] [Google Scholar]
- 47.Levine B., Cabeza R., McIntosh A.R., Black S.E., Grady C.L., Stuss D.T. Functional reorganisation of memory after traumatic brain injury: a study with H2150 positron emission tomography. J. Neurol. Neurosurg. Psychiatr. 2002;73:173–181. doi: 10.1136/jnnp.73.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scholz J., Klein M.C., Behrens T.E.J., Johansen-Berg H. Training induces changes in white–matter architecture. Nat. Neurosci. 2009;12:1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fauvel B., Groussard M., Chételat G., Fouquet M., Landeau B., Eustache F., Desgranges B., Platel H. Morphological brain plasticity induced by musical expertise is accompanied by modulation of functional connectivity at rest. Neuroimage. 2014;90:179–188. doi: 10.1016/j.neuroimage.2013.12.065. [DOI] [PubMed] [Google Scholar]
- 50.Hüfner K., Binetti C., Hamilton D., Stephan T., Flanagin V.I., Linn J., Labudda K., Markowitsch H., Glausauer S., Jahn K., Strupp M., Brandt T. Structural and functional plasticity of the hippocampal formation in professional dancers and slackliners. Hippocampus. 2011;21:855–865. doi: 10.1002/hipo.20801. [DOI] [PubMed] [Google Scholar]
- 51.Hänggi J., Koeneke S., Bezzola L., Jäncke L. Structural neuroplasticity in the sensorimotor network of professional female ballet dancers. Hum. Brain Mapp. 2010;31:1196–1206. doi: 10.1002/hbm.20928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luo C., Tu S., Peng Y., Gao S., Li J., Dong L., Li G., Lai Y., Li H., Yao D. Long-term effects of musical training and functional plasticity in salience system. Neural Plast. 2014;2014:1–13. doi: 10.1155/2014/180138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sato K., Kirino E., Tanaka S.A. Voxel-based morphometry study of the brain of university students majoring in music and nonmusic disciplines. Behav. Neurol. 2015;2015 doi: 10.1155/2015/274919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beaty R.E. The neuroscience of musical improvisation. Neurosci. Biobehav. Rev. 2015;51:108–117. doi: 10.1016/j.neubiorev.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 55.Ermutlu N., Yücesir I., Eskikurt G., Temel T., İşoğlu-Alkaç Ü. Brain electrical activities of dancers and fast ball sports athletes are different. Cogn. Neurodyn. 2015;9:257–263. doi: 10.1007/s11571-014-9320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elvers P. Songs for the ego: theorizing musical self-enhancement. Front. Psychol. 2016;7:1–11. doi: 10.3389/fpsyg.2016.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Homann K.B. Embodied concepts of neurobiology in dance/movement therapy practice. Am. J. Dance Ther. 2010;32:80–99. [Google Scholar]
- 58.Thomas J.P., Shiffrar M. I can see you better if I can hear you coming: action-consistent sounds facilitate the visual detection of human gait. J. Vis. 2010;10:1–11. doi: 10.1167/10.12.14. [DOI] [PubMed] [Google Scholar]
- 59.Calvo-Merino B., Grezes J., Glaser D.E., Passingham R.E., Haggard P. Seeing or doing? Influence of visual and motor familiarity in action observation. Curr. Biol. 2006;16:1905–1910. doi: 10.1016/j.cub.2006.07.065. [DOI] [PubMed] [Google Scholar]
- 60.Calvo-Merino B., Glaser D.E., Grèzes J., Passingham R., Haggard P. Action observation and acquired motor skills: an fMRI study with expert dancers. Cerebr. Cortex. 2005;15:1243–1249. doi: 10.1093/cercor/bhi007. [DOI] [PubMed] [Google Scholar]
- 61.Cross E.S., Kraemer D.J.M., Hamilton A.F.C., Kelley W.M., Grafton S.T. Sensitivity of the action observation network to physical and observational learning. Cerebr. Cortex. 2009;19:315–326. doi: 10.1093/cercor/bhn083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fink A., Graif B., Neubauer A.C. Brain correlates underlying creative thinking: EEG alpha activity in professional vs. novice dancers. Neuroimage. 2009;46:854–862. doi: 10.1016/j.neuroimage.2009.02.036. [DOI] [PubMed] [Google Scholar]
- 63.Bläsing B., Calvo-Merino B., Cross E.S., Jola C., Honisch J., Stevens C.J. Neurocognitive control in dance perception and performance. Acta Psychol. 2012;139:300–308. doi: 10.1016/j.actpsy.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 64.Munzert J., Zentgraf K., Stark R., Vaitl D. Neural activation in cognitive motor processes: comparing motor imagery and observation of gymnastic movements. Exp. Brain Res. 2008;188:437–444. doi: 10.1007/s00221-008-1376-y. [DOI] [PubMed] [Google Scholar]
- 65.Jola C., Abedian-Amiri A., Kuppuswamy A., Pollick F.E., Grosbras M.-H. Motor simulation without motor expertise: enhanced corticospinal excitability in visually experienced dance spectators. PLoS One. 2012;7:1–12. doi: 10.1371/journal.pone.0033343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Phillips-Silver J., Keller P.E. Searching for roots of entrainment and joint action in early musical interactions. Front. Hum. Neurosci. 2012;6:1–11. doi: 10.3389/fnhum.2012.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Repp B.R., Su Y.-H. Sensorimotor synchronization: a review of recent research (2006–2012) Psychon. Bull. Rev. 2013;20:403–452. doi: 10.3758/s13423-012-0371-2. [DOI] [PubMed] [Google Scholar]
- 69.Konoike N., Kotozaki Y., Miyachi S., Miyauchi C.M., Yomogida Y., Akimoto Y., Kuraoka K., Sugiura M., Kawashima, Nakamura K. Rhythm information represented in the fronto-parieto-cerebellar motor system. Neuroimage. 2012;63:328–338. doi: 10.1016/j.neuroimage.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 70.Thaut M.H. Neural basis of rhythmic timing- Networks in the human brain. Ann. N. Y. Acad. Sci. 2003;999:364–373. doi: 10.1196/annals.1284.044. [DOI] [PubMed] [Google Scholar]
- 71.Michaelis K., Wiener M., Thompson J.C. Passive listening to preferred motor tempo modulates corticospinal excitability. Front. Hum. Neurosci. 2014;8:1–26. doi: 10.3389/fnhum.2014.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]