Abstract
The default mode network (DMN) plays an important role in age-related cognitive decline. This study aims to explore the modulation effect of two mind–body interventions (Tai Chi Chuan and Baduanjin) on DMN in elderly individuals. Participants between 50 and 70 years old were recruited and randomized into a Tai Chi Chuan, Baduanjin or control group. The Wechsler Memory Scale-Chinese Revision and resting-state fMRI scans were administered at baseline and following 12 weeks of exercise. Seed-based resting-state functional connectivity (rsFC) was calculated. We found that (i) compared to the Baduanjin group, Tai Chi Chuan was significantly associated with increased rsFC between the medial prefrontal cortex (mPFC) and right putamen/caudate and (ii) compared to the control group, Tai Chi Chuan increased posterior cingulate cortex rsFC with the right putamen/caudate, while Baduanjin decreased rsFC between the mPFC and orbital prefrontal gyrus/putamen. Baseline mPFC rsFC with orbital prefrontal gyrus was negatively correlated with visual reproduction subscore. These results suggest that both Tai Chi Chuan and Baduanjin can modulate the DMN, but through different pathways. Elucidating the mechanisms underlying different mind–body interventions may shed light on the development of new methods to prevent age-related diseases as well as other disorders associated with disrupted DMN.
Keywords: Tai Chi Chuan, Baduanjin, default mode network, resting-state functional connectivity, aging, memory, mind–body exercise
Introduction
In recent years, resting-state functional connectivity (rsFC) has been widely used to investigate network changes in normal aging (Sala-Llonch et al., 2015), as well as age-related cognitive decline (Nashiro et al., 2017). One of the most well-studied brain networks associated with the aging process is the default mode network (DMN; Hafkemeijer et al., 2012).
The DMN originally referred to a set of brain regions that showed decreased neural activity during goal-oriented tasks (Fox et al., 2005; Fair et al., 2008; Andrews-Hanna et al., 2014; Raichle, 2015). It is generally accepted that the DMN includes the bilateral medial prefrontal cortex (mPFC), anterior and posterior cingulate cortices, precuneus, inferior parietal cortex, lateral temporal cortex and parahippocampal gyrus (Raichle and Snyder, 2007). Among these regions, a set of core brain regions, including the posterior cingulate cortex (PCC) and mPFC, known as ‘hubs’, plays essential roles in the DMN. Investigators have found that the PCC and anterior mPFC exhibit the highest betweenness centrality within the DMN and play an integral role in the dorsal mPFC subsystem and medial temporal lobe subsystem within the DMN (Andrews-Hanna et al., 2010). Further studies have shown that activity in the mPFC is associated with instrumental or agentic self-reflection (inward-directed), and the PCC is associated with experiential self-reflection (outward-directed; Johnson et al., 2006).
The evidence demonstrating the role of the PCC and mPFC in the processing of cognitive, emotional, pain and other self-referential modulation have increased dramatically (Kurczek et al., 2015; Martucci et al., 2015; De Pisapia et al., 2018). More recently, the literature has suggested that the DMN also plays an important and complex role in supporting cognitive function (Greicius et al., 2004; Brewer et al., 2011; Xu and Südhof, 2013). Abnormalities of the DMN have been detected in multiple age-related diseases, such as mild cognitive impairment (MCI; Gardini et al., 2015), Alzheimer’s disease (AD; Brueggen et al., 2017), depression (Ho et al., 2015) and Parkinson’s disease (Mohan et al., 2016). In addition, studies have found that stress, a risk factor for MCI and AD, is associated with alterations of the DMN (Soares et al., 2017). Taken together, these studies demonstrate the important role of the DMN in age-related cognitive impairment.
Over the past decade, a string of disappointing clinical trial results has raised concerns about our strategy of preventing the development of AD and MCI (Sperling et al., 2011). Currently, most studies are being performed on prodromal AD and MCI. At this stage, however, the progressive neurological loss and irreversible cognitive impairment may have already occurred. The failure of treatments for MCI suggests that therapy should be applied in an earlier stage of the disease (Sperling et al., 2011; Cheng et al., 2017). Thus, investigation of the early stage with no neuronal damage and still-sufficient functional compensation is crucial for the prevention of AD, MCI and other age-related dementia (Sperling et al., 2011; Jessen et al., 2014). As a result, healthy elderly adults might be the optimal stage at which to intervene with preventative therapies.
Recently, the potential of mind–body interventions in preventing age-related diseases has drawn the attention of investigators. Nevertheless, their underlying mechanisms remain unknown. Accumulating evidence suggests that the DMN may be crucial for the modulation effect of mind–body interventions. For instance, in a previous review article, (Tang et al., 2015) suggested that self-awareness is one of the key components of mind–body exercise, suggesting the involvement of the DMN. In another study, investigators found that compared with meditation-naïve controls, experienced meditators had less activation in the main nodes of the DMN (mPFC and PCC) (Brewer et al., 2011), further supporting the role of the DMN in mind–body exercise. In a more recent study, we found that Tai Chi Chuan can significantly enhance rsFC between the cognitive control network and the mPFC/anterior cingulate cortex(ACC), a key region in the DMN, in fibromyalgia patients (Kong et al., 2018).
Both Tai Chi Chuan and Baduanjin are two popular mind–body exercises (Ospina et al., 2008) that hold the potential to prevent age-related cognitive decline (Songtao, 2007; Chang et al., 2010; Kasai et al., 2010; Lam et al., 2012; Solloway et al., 2016; Sungkarat et al., 2017). Yet, the characteristics of these two interventions differ significantly, and the neural circuits through which these mind–body interventions achieve efficacy remain unclear. In this study, we investigated how Tai Chi Chuan and Baduanjin exercises modulate the rsFC of the DMN in older adults, and we explored the association with memory function changes. We hypothesized that 12 weeks of Tai Chi Chuan and Baduanjin exercise would modulate the rsFC of the DMN through different pathways due to the exercises’ differing characteristics.
Materials and methods
This study was registered at the Chinese Clinical Trial Registry Center (ChiCTR-IPR-15006131). The full details of the study are reported in previous studies (Tao et al., 2016, 2017a, 2017b, 2017c), in which we investigated rsFC changes of the hippocampus and dorsal lateral prefrontal cortex, fractional amplitude of low-frequency fluctuations and brain structure changes following Tai Chi Chuan and Baduanjin practice. In this exploratory study, we focused on the modulation effect of 12 weeks of Tai Chi Chuan and Baduanjin exercise on the rsFC of the DMN in older adults, which has not been previously published.
Healthy, right-handed subjects (50–70 years old) who had not regularly participated in physical exercise for at least 1 year and had no history of psychiatric conditions were recruited in the Gulou District, Fuzhou City, China. The minimum standard for regular physical exercise is considered to be 3 months with a frequency of 3–4 times per week and 30 min per session. Participants who had a history of stroke, any cerebrovascular/musculoskeletal system/sports injury-related contraindications or cognitive screening scores <24 on the Mini-Mental State Exam or ≥14 on the Beck Depression Inventory were excluded. To avoid the potential cross-practice between the two exercises, two cohorts of older adults from the same community were recruited independently. Participants in one cohort were randomized to the Tai Chi Chuan or control group, and those in the other cohort were randomized to the Baduanjin or control group. The two cohorts started and ended at the same time. At the end of the study, we combined the two cohorts for data analysis (Tai Chi Chuan group, Baduanjin group and control group). Please see our previous publications for more details on recruitment, intervention and data acquisition (Tao et al., 2016, 2017a, 2017b, 2017c). This study was approved by the Medical Ethics Committee at the Affiliated Rehabilitation Hospital of Fujian University of Traditional Chinese Medicine. All participants signed consent forms.
Both Tai Chi Chuan, based on Yang style 24 form (China National Sports Commission, 1983), and Baduanjin exercises, based on ‘Health Qigong––Baduanjin’ recommendations published by the General Administration of Sport in China (Health Qigong Management Center of General Administration of Sport of China, 2003), took place for 60 min per session, 5 times per week for 12 weeks. Every session included a sequence of 10 min of warm-up, 30 min of exercises, 10 min of breathing techniques and 10 min of relaxation. The control group received basic health education at the beginning of the experiment (Hughes et al., 2014), and subjects in this group were asked to maintain their original physical activity habits during the 12-week period. Two professional instructors from the Fujian University of Traditional Chinese Medicine with more than 5 years of training experience lead the Tai Chi Chuan and Baduanjin training. In addition, two other staff members supervised the training procedure to guarantee the quality of the research.
In the study, we used the Wechsler Memory Scale-Chinese Revision (WMS-CR), a Chinese version of the WMS by Gong and Wang, 1989, to assess memory function before and after the interventions. The WMS-CR includes 10 items and primarily tests long-term memory, short-term memory and transient memory. Based on the WMS-CR manual, memory quotient (MQ) were calculated as the total raw scores adjusted for age in a standardization sample.
Resting-state images were acquired at baseline and at the end of the 12-week intervention on a 3.0 T magnetic resonance scanner (MRI; General Electric Signa HDxt, USA). Resting-state fMRI data were acquired with TR = 2100 ms, TE = 30 ms, flip angle = 90 degrees, slice thickness = 3 mm, gap = 0.6 mm, acquisition matrix = 64 × 64, voxel size = 3.125 × 3.125 × 3.6 mm3, 42 axial slices, FOV = 200 × 200 mm and 160 time points. The T1-weighted images were also collected with parameters Echo time (TE) = min, flip angle = 15 degrees, slice thickness = 1 mm, field of view = 240 mm and 164 contiguous slices. Subjects were required to stay awake with their eyes closed and ears plugged during the MRI scan.
Statistical analysis
Behavioral data analysis
SPSS 18.0 software (SPSS Inc., Chicago, IL, USA) was used for the behavioral data analysis. Analysis of covariance (ANCOVA) was applied to compare changes in MQ and WMS-CR subscores across the three groups with age (years), gender and education (years) as covariates. Post hoc analysis (Bonferroni correction) was applied to explore the between-group differences.
Seed-to-voxel correlational analyses
Seed-to-voxel correlational analyses were carried out in MATLAB by applying the functional connectivity (CONN) toolbox v17.C (http://www.nitrc.org/projects/conn). The image pre-processing and first-level correlation maps were produced using methods similar to our previous study (Wang et al., 2017). For more details, please see our Supplementary Material.
We used two core subregions of the DMN as seeds: the PCC (Tal peak coordinate: −2, −36, 37, sphere with 6 mm radius, Figure 1A_1) and mPFC (Tal peak coordinate: 1, 54, 21, sphere with 6 mm radius, Figure 1B_1). The coordinates of the two seeds were derived from a previous study by (Fox et al., 2005). Age was included in the analysis as a covariate of non-interest. Group analysis was applied with a random effects model. To explore the association between clinical outcomes and rsFC, we also performed multiple regression analyses using the PCC and mPFC as seeds in all subjects and WMS-CR MQ and visual reproduction subscore at baseline. A threshold of voxel-wise P < 0.005 uncorrected and cluster-level P < 0.05 false discovery rate corrected were used for the seed-to-voxel analyses.
Fig. 1.
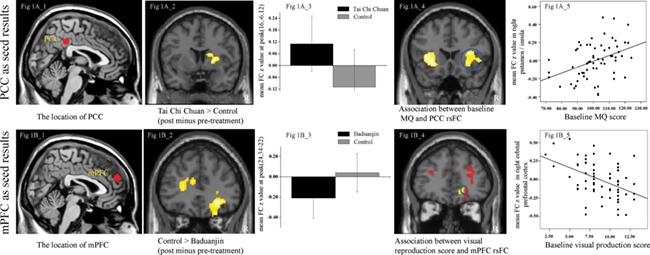
Results of DMN rsFC. Figure 1A_1, location of seed of PCC; Figure 1A_2 and 1A_3, significant rsFC changes between the PCC and right putamen/caudate in the Tai Chi Chuan group compared to the control group; Figure 1A_4, multiple regression analyses showed significant positive correlation between PCC and bilateral anterior insula/putamen/operculum rsFC and baseline MQ score across all subjects adjusted for age; Figure 1A_5, scatter plots indicate the correlation between MQ and mean Z-values in the right putamen/insula cluster (the blue circles of Figure 1A_4) adjusted for age. Figure 1B_1, location of the seed of mPFC; Figure 1B_2 and 1B_3, significant rsFC changes in the Baduanjin group compared to the control group using the mPFC as a seed; Figure 1B_4, the overlapping (the blue circles: right orbital prefrontal gyrus) brain region between significant rsFC changes in the control group compared to the Baduanjin group using the mPFC as a seed (red) and multiple regression analyses showing significant negative correlations between mPFC rsFC and visual reproduction subscores (yellow); Figure 1B_5, scatter plots indicate the correlation between visual reproduction subscores and mean Z-values in the right orbital prefrontal cluster (the blue circles in the Figure 1B_4) adjusted for age; R, right.
Results
There were no significant differences in the demographic data at baseline across the three groups (see our Supplementary Material and Supplementary Table S1 for details). ANCOVA analysis revealed a significant difference in MQ among the three groups before and after treatment (F = 25.94, P < 0.001). Post hoc analysis (Bonferroni correction) showed that compared with the control group, MQ scores significantly increased in the Tai Chi Chuan and Baduanjin groups (Tai Chi Chuan, P < 0.001; Baduanjin, P < 0.001). No significant difference was found between the Tai Chi Chuan and Baduanjin groups (P = 0.238). ANCOVA analysis on WMS-CR subscores showed that both Tai Chi Chuan and Baduanjin significantly increased visual reproduction subscores compared to the control group. Baduanjin also produced greater improvements in mental control, recognition, touch and comprehension memory subscores compared to the control group and improvements in touch subscores compared to the Tai Chi Chuan group (Bonferroni correction, P < 0.0063; Supplementary Table S2).
PCC rsFC results
We found an increased rsFC between the PCC and right putamen/caudate after 12 weeks of Tai Chi Chuan practice compared to the control group (Table 1; Figure 1A_2 and 1A_3). There was no other significant group difference above the threshold we set.
Table 1.
Regions showing significant functional connectivity changes with the DMN (R, right; L, left; ACC, anterior cingulate cortex)
| Seed | Cluster size | Brain regions | Z-value | MNI coordinate (mm) | |||
|---|---|---|---|---|---|---|---|
| PCC | Tai Chi Chuan > Control | 518 | R putamen/caudate | 4.13 | 16 | −6 | 12 |
| Control > Tai Chi Chuan | No regions above the threshold | ||||||
| Baduanjin > Control | No regions above the threshold | ||||||
| Control > Baduanjin | No regions above the threshold | ||||||
| Tai Chi Chuan > Baduanjin | No regions above the threshold | ||||||
| Baduanjin > Tai Chi Chuan | No regions above the threshold | ||||||
| mPFC | Tai Chi Chuan > Control | 649 | R temporal pole/middle & inferior temporal gyrus | 4.18 | 60 | −6 | −28 |
| Control > Tai Chi Chuan | No regions above the threshold | ||||||
| Baduanjin > Control | No regions above the threshold | ||||||
| Control > Baduanjin | 1865 | R orbital prefrontal gyrus | 4.36 | 24 | 34 | −22 | |
| R medial frontal gyrus | 3.51 | 28 | 48 | 16 | |||
| R putamen | 3.61 | 22 | 4 | −2 | |||
| R anterior insula | 3.23 | 42 | −2 | 10 | |||
| 753 | L ACC | 3.46 | −8 | 30 | 20 | ||
| L anterior insula | 3.06 | −34 | 20 | 6 | |||
| R caudate | 3.00 | 8 | 16 | 6 | |||
| 539 | R supramarginal gyrus | 3.52 | 44 | −32 | 50 | ||
| Tai Chi Chuan > Baduanjin | 808 | R putamen | 3.83 | 22 | 4 | −2 | |
| R caudate | 3.22 | 14 | 20 | −4 | |||
| Baduanjin > Tai Chi Chuan | No regions above the threshold | ||||||
Since both the Tai Chi Chuan and Baduanjin groups produced greater improvements in MQ and visual reproduction subscores compared to the control group, we also applied a multiple regression analysis using the two measurements separately across all subjects at baseline to explore the association between the measurements and PCC rsFC. We found a significant positive association between the baseline MQ and the PCC rsFC at the bilateral anterior insula/putamen/operculum (Table 2; Figure 1A_4 and 1A_5) and a negative association between the baseline MQ and the PCC rsFC at the right middle and superior temporal gyrus/fusiform gyrus, bilateral posterior rostral medial prefrontal gyrus, right orbital prefrontal gyrus and bilateral precuneus/left posterior cingulate gyrus. No significant association was observed between the baseline visual reproduction subscores and the PCC rsFC at baseline.
Table 2.
Regions showing significant correlations with the MQ and visual reproduction subscores at baseline across all subjects (R, right; L, left)
| Seed | Contrast | Cluster size | Brain regions | Z-value | MNI coordinates (mm) | |||
|---|---|---|---|---|---|---|---|---|
| PCC | MQ | positive | 681 | L anterior insula/putamen/operculum | 3.98 | −28 | 22 | −4 |
| 478 | R anterior insula/putamen/operculum | 3.65 | 30 | 26 | 6 | |||
| negative | 628 | R middle & superior temporal gyrus/fusiform gyrus | 4.28 | 60 | 0 | −20 | ||
| 926 | Bilateral posterior rostral medial prefrontal gyrus | 4.14 | 0 | 62 | 28 | |||
| 868 | R orbital prefrontal gyrus | 4.00 | 12 | 62 | −12 | |||
| 1237 | Bilateral precuneus | 3.79 | 4 | −52 | 14 | |||
| L posterior cingulate gyrus | 3.60 | −4 | −38 | 36 | ||||
| Visual reproduction | positive | No regions above the threshold | ||||||
| negative | No regions above the threshold | |||||||
| mPFC | MQ | positive | No regions above the threshold | |||||
| negative | 556 | R angular gyrus | 3.78 | 38 | −68 | 38 | ||
| Visual reproduction | positive | 1746 | L inferior & medial frontal gyrus | 3.69 | −54 | 10 | 12 | |
| L anterior insula | 3.08 | −28 | 24 | −2 | ||||
| 831 | R medial & inferior frontal gyrus | 3.72 | 50 | 30 | 26 | |||
| R anterior insula | 3.34 | 36 | 22 | 2 | ||||
| 501 | L supramarginal gyrus/parietal operculum | 3.10 | −60 | −48 | 18 | |||
| negative | 519 | R angular gyrus | 3.37 | 48 | −58 | 32 | ||
| 421 | R orbital prefrontal gyrus | 3.80 | 12 | 62 | −2 | |||
| 809 | Bilateral precuneus/posterior cingulate gyrus | 3.54 | 12 | −50 | 24 | |||
We also explored the association between the pre- and post-treatment behavioral measurements and corresponding PCC rsFC changes (MQ and visual reproduction subscores) in the Tai Chi Chuan and Baduanjin groups separately and found no significant association at the threshold we set. When we applied a less conservative threshold of voxel-wise P < 0.005 uncorrected with 20 continuous voxels, there was still no significant finding.
mPFC rsFC results
We found an increased rsFC between the mPFC and right temporal pole/middle and inferior temporal gyrus in the Tai Chi Chuan group compared with the control group (Table 1). We also found a significantly decreased rsFC between the mPFC and right orbital prefrontal gyrus, right medial frontal gyrus, right putamen, right anterior insula, left ACC,left anterior isula, right caudate and right supramarginal gyrus in the Baduanjin group compared to the control group (Table 1, Figure 1B_1 and 1B_2). The direct comparison between the two mind–body interventions revealed that Tai Chi Chuan was significantly associated with increased rsFC between the mPFC and right putamen/caudate compared with the Baduanjin group.
A multiple regression analysis revealed a negative correlation (Table 2) between (i) the baseline MQ and mPFC rsFC with the right angular gyrus and (ii) the baseline visual reproduction subscore and mPFC rsFC with the right angular, right orbital prefrontal gyrus and bilateral precuneus/posterior cingulate gyrus. A positive association between the visual reproduction subscore and mPFC rsFC with left inferior and medial frontal gyrus, left anterior insula, right medial and inferior frontal gyrus, right anterior insula, and left supramarginal gyrus/parietal operculum was also observed.
Interestingly, we found that the association analysis (between the baseline visual production and mPFC rsFC) and the mPFC rsFC pre- and post-treatment differences between the Baduanjin and control groups overlapped at the right orbital prefrontal gyrus (Figure 1B_4 and 1B_5).
We also explored the association between the pre- and post-treatment behavioral measurements and mPFC rsFC changes in Tai Chi and Baduanjin groups separately and found no significant association at the threshold we set. However, at a lower threshold (a threshold of voxel-wise P < 0.005 uncorrected with 20 continous voxels), we found a positive association between the change in MQ/visual reproduction and the change in rsFC of the right temporal pole mPFC after Tai Chi training using multiple whole-brain regression analyses adjusted for age (visual reproduction regression: cluster, 42; peak T-value, 3.45; and MNI coordinate, 46, 10, −44; MQ regression: cluster, 302; peak T-value, 4.86; and MNI coordinate, 48, 10, −44).
Discussion
In this study, we investigated the modulation effect of Tai Chi Chuan and Baduanjin on the DMN using the PCC and mPFC as seeds. We found that compared to the control group, (i) Tai Chi Chuan increased rsFC between the PCC and right putamen/caudate, while Baduanjin decreased rsFC between the mPFC and right orbital prefrontal gyrus, right putamen and left anterior cingulate gyrus; compared to the Baduanjin group, Tai Chi Chuan produced greater mPFC rsFC at the right putamen/caudate; (ii) both the Tai Chi Chuan and Baduanjin groups demonstrated increased MQ and visual reproduction subscores of the WMS-CR. Baseline rsFC between the mPFC and right orbital prefrontal gyrus was negatively correlated with visual reproduction subscores, and the strength of this rsFC was decreased in the Baduanjin group after 12 weeks of exercise compared to the control group.
A recent study reported that the DMN is associated with a wide range of cognitive tasks, particularly prospective memory (Raichle, 2015). Vatansever et al., 2016 reported that the whole-brain connectivity of the DMN’s core hubs (i.e. the PCC and mPFC) changes across three levels of n-back tasks, suggesting the involvement of the DMN in working memory. The PCC and mPFC are two core subdivisions of the DMN (Koshino et al., 2014). The PCC plays a crucial role in memory function and has strong interconnections with other memory-related structures, such as the parahippocampal formation in subserving episodic memory retrieval (Torta and Cauda, 2011; Shapira-Lichter et al., 2013). A previous study suggests that age-related memory decline in older adults is associated with decreased connectivity between the PCC and parietal regions (Bernard et al., 2015). In addition, the mPFC also plays an important role in the retrieval of long-term memories and the consolidation of a wide range of memories (Euston et al., 2012). Lesion studies highlight the critical roles of the mPFC in memory integration, which involves combining information acquired from different events (Koscik and Tranel, 2012).
In the present study, we found altered rsFC in brain regions with PCC and mPFC after mind–body exercises (Tai Chi Chuan and Baduanjin). A review conducted by (Tang et al., 2015) suggested that different types and forms of meditation, such as Tai Chi and Qigong, include the three most important components (self-awareness, attention control and emotion regulation) to constitute the self-regulation process. A study by Saunders et al., (2007) showed that for first year medical students, mind–body skills course can promote their self-awareness and reflection.
Tai Chi Chuan is a complex exercise and requires the practitioners to perform spiral movements of the extremities with delicate control. Furthermore, bodyweight shifting, body rotation and single-leg standing in different postures are practiced repeatedly (Lan et al., 2002; Innes et al., 2016). We found increased rsFC between the putamen/caudate and PCC in the Tai Chi Chuan group compared to the control group. The putamen and caudate were also positively correlated with changes in MQ scores, which represent the total subscores of WMS-CR adjusted for age (Butters et al., 1995). This result is consistent with a previous study in which the authors found that mindfulness exercises could modulate brain functions by enhancing intrinsic activities of the putamen and caudate (Tang et al., 2015). Our findings are also in line with another study that found that MQ scores are positively associated with the average degree, global efficiency and local efficiency of the DMN in young, healthy subjects (Tao et al., 2015).
Both the putamen and caudate are involved in movement control and regulation. In addition, these two regions play a key role in memory processing. The literature suggests that the putamen is involved in maintaining the memory of stimuli across a delay (Janes et al., 2015) and during the working memory encoding phase (Cairo et al., 2004). A previous study found that D2 dopamine receptor levels in the caudate were positively associated with episodic memory and that high memory performance was characterized by increased functional connectivity between the caudate and hippocampus (Nyberg et al., 2016).
In addition, both animal (Olson et al., 2007) and human (Ng et al., 2014) studies have demonstrated the indispensable role of the medial temporal pole in memory processing. Our present study showed an increased rsFC between the mPFC and temporal pole after 12 weeks of Tai Chi Chuan exercise, implying that Tai Chi Chuan may prevent memory decline by strengthening the connection between the DMN and memory-related networks, such as the putamen/caudate and temporal pole.
Baduanjin, another mind–body exercise, consists of eight simple movements and focuses on increasing flexibility and strengthening muscles and tendons. Previous studies suggest that stretching may impact the plasticity of connective tissue, which may produce anti-inflammatory effects (Berrueta et al., 2016). We found that baseline rsFC between the mPFC and orbital prefrontal gyrus was negatively correlated with visual reproduction subscores, and the strength of this rsFC was decreased in the Baduanjin group after 12 weeks of exercise compared to the control group. The visual reproduction test is a non-verbal visuospatial memory task in the WMS-CR that examines the spatial immediate memory and delayed recall function and is consistently and strongly associated with age-related memory decline (McCarty et al., 1982; Haaland et al., 2003). Frey and Petrides, 2002 found that the orbitofrontal cortex is a critical frontal region for memory information processes. (McCormick et al., 2014) found altered visuospatial memory capacity associated with different parts of the DMN in patients with unilateral medial temporal lobe epilepsy. Taken together, our results suggest that Baduanjin may prevent memory decline by decreasing the rsFC between the mPFC and orbital prefrontal gyrus.
We also found that Tai Chi Chuan produced increased rsFC between the mPFC and right putamen/caudate in the Tai Chi Chuan group compared with the Baduanjin group, suggesting that although both Tai Chi Chuan and Baduanjin can improve memory performance, the two may do so through different neural mechanisms. We speculate that these differences may be due to the different characteristics of the two mind–body interventions. Compared with Baduanjin, Tai Chi Chuan involves much more complicated postures and movement learning. Both the putamen and caudate are key regions in the basal ganglia (Olson et al., 2007), which is responsible for controlling motor learning (Nyberg et al., 2016), specifying amplitudes of movement (Olson et al., 2007), movement sequences (Ng et al., 2014) and complex movements requiring sequential rhythm (Haaland et al., 2003). We thus believe that the increased rsFC between the mPFC and putamen in the Tai Chi Chuan group may reflect the increased complexity of Tai Chi Chuan compared to Baduanjin exercise.
There are several limitations in the present study. First, 12 weeks of exercise is a relatively short duration. Therefore, the long-term effects of Tai Chi Chuan and Baduanjin need to be studied further. In addition, both Tai Chi Chuan and Baduanjin are mind–body interventions (Chen et al., 2017), thus we cannot separate the role of physical activity from the mental component in memory improvement and rsFC changes. Further studies should compare the effects of physical exercise vs meditation on memory improvement. Finally, the sample size in our study is relatively small, and future studies with a larger sample size are needed to replicate our findings.
Conclusion
We found that Tai Chi Chuan and Baduanjin significantly modulate the rsFC of the DMN through different pathways. Elucidating how various mind–body interventions can modulate the DMN may shed light on the development of new interventions to prevent age-related disorders such as MCI and AD, as well as other diseases associated with a disrupted DMN.
Funding
This work was supported by the Special Scientific Research Fund of Public Welfare Profession of China (grant no. 201307004), Ministry of Science and Technology and Ministry of Finance of the People’s Republic of China and the National Rehabilitation Research Center of Traditional Chinese Medicine, Fujian Rehabilitation Industrial Institution and Fujian Rehabilitation Tech Co-innovation Center (grant no. X2012007-Collaboration).
Author contributions
L.C., experimental design; J.K., analysis and manuscript preparation; J.L. and J.T., experimental design, data analysis and manuscript preparation; M.Y. and M.L., data collection and data analysis; W.L. and J.H., data collection; X.X., Y.T. and W.G., manuscript preparation/revision. All authors contributed to drafting the manuscript and have read and approved the final manuscript.
Conflict of interest.
J.K. has a disclosure to report (holding equity in a start-up company, MNT, and a pending patent to develop a new brain stimulation device), but declares no conflict of interest. All other authors declare no competing interests.
Supplementary Material
Acknowledgements
We thank two professional instructors from the Fujian University of Traditional Chinese Medicine, Hongmei Yi and Tingjin Duan, for their training in Tai Chi Chuan and Baduanjin exercise.
References
- Andrews-Hanna J.R., Reidler J.S., Sepulcre J., et al. (2010). Functional-anatomic fractionation of the brain’s default network. Neuron, 65, 550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J.R., Smallwood J., Spreng R.N. (2014). The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Annals of the New York Academy of Sciences, 1316, 29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard C., Dilharreguy B., Helmer C., et al. (2015). PCC characteristics at rest in 10-year memory decliners. Neurobiology of Aging, 36, 2812–2820. [DOI] [PubMed] [Google Scholar]
- Berrueta L., Muskaj I., Olenich S., et al. (2016). Stretching impacts inflammation resolution in connective tissue. Journal of Cellular Physiology, 231, 1621–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer J.A., Worhunsky P.D., Gray J.R., et al. (2011). Meditation experience is associated with differences in default mode network activity and connectivity. Proceedings of the National Academy of Sciences of the United States of America, 108, 20254–20259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggen K., Fiala C., Berger C., et al. (2017). Early changes in alpha band power and DMN BOLD activity in Alzheimer’s disease: a simultaneous resting state EEG–fMRI study. Frontiers in Aging Neuroscience, 9, 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butters N., Delis D.C., Lucas J.A. (1995). Clinical assessment of memory disorders in amnesia and dementia. Annual Review of Psychology, 46, 493–523. [DOI] [PubMed] [Google Scholar]
- Cairo T.A., Liddle P.F., Woodward T.S., et al. (2004). The influence of working memory load on phase specific patterns of cortical activity. Cognitive Brain Research, 21, 377–387. [DOI] [PubMed] [Google Scholar]
- Chang Y.-K., Nien Y.-H., Tsai C.-L., et al. (2010). Physical activity and cognition in older adults: the potential of Tai Chi Chuan. Journal of Aging and Physical Activity, 18, 451–472. [DOI] [PubMed] [Google Scholar]
- Chen T., Yue G.H., Tian Y., et al. (2017). Baduanjin mind–body intervention improves the executive control function. Frontiers in Psychology, 7, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y.W., Chen T.F., Chiu M.J. (2017). From mild cognitive impairment to subjective cognitive decline: conceptual and methodological evolution. Neuropsychiatric Disease and Treatment, 13, 491–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- China National Sports Commission (1983). Simplified Taijiquan, Beijing: China Sports Editorial Board. [Google Scholar]
- De Pisapia N., Barchiesi G., Jovicich J., et al. (2018). The role of medial prefrontal cortex in processing emotional self-referential information: a combined TMS/fMRI study. Brain Imaging and Behavior, 1–12. [DOI] [PubMed] [Google Scholar]
- Euston D.R., Gruber A.J., McNaughton B.L. (2012). The role of medial prefrontal cortex in memory and decision making. Neuron, 76, 1057–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Cohen A.L., Dosenbach N.U.F., et al. (2008). The maturing architecture of the brain’s default network. Proceedings of the National Academy of Sciences of the United States of America, 105, 4028–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., et al. (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America, 102, 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S., Petrides M. (2002). Orbitofrontal cortex and memory formation. Neuron, 36, 171–176. [DOI] [PubMed] [Google Scholar]
- Gardini S., Venneri A., Sambataro F., et al. (2015). Increased functional connectivity in the default mode network in mild cognitive impairment: a maladaptive compensatory mechanism associated with poor semantic memory performance. Journal of Alzheimer’s Disease, 45, 457–470. [DOI] [PubMed] [Google Scholar]
- Gong Y., Wang D.J. (1989). Handbook of Wechsler Memory Scale––Revised, Changsha, China: Bulletin. [Google Scholar]
- Greicius M.D., Srivastava G., Reiss A.L., et al. (2004). Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proceedings of the National Academy of Sciences of the United States of America, 101, 4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaland K.Y., Price L., Larue A. (2003). What does the WMS-III tell us about memory changes with normal aging? Journal of the International Neuropsychological Society, 9, 89–96. [DOI] [PubMed] [Google Scholar]
- Hafkemeijer A., van der Grond J., Rombouts S.A. (2012). Imaging the default mode network in aging and dementia. Biochimica et Biophysica Acta, 1822, 431–441. [DOI] [PubMed] [Google Scholar]
- Health Qigong Management Center of General Administration of Sport of China (2003). Health Qigong––Baduanjin, Beijing: People’s Sports Publishing House of China. [Google Scholar]
- Ho T.C., Connolly C.G., Henje Blom E., et al. (2015). Emotion-dependent functional connectivity of the default mode network in adolescent depression. Biological Psychiatry, 78, 635–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes T.F., Flatt J.D., Fu B., et al. (2014). Interactive video gaming compared with health education in older adults with mild cognitive impairment: a feasibility study. International Journal of Geriatric Psychiatry, 29, 890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innes K.E., Selfe T.K., Khalsa D.S., et al. (2016). Effects of meditation versus music listening on perceived stress, mood, sleep, and quality of life in adults with early memory loss: a pilot randomized controlled trial. Journal of Alzheimer’s Disease, 52, 1277–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes A.C., Ross R.S., Farmer S., et al. (2015). Memory retrieval of smoking-related images induce greater insula activation as revealed by an fMRI-based delayed matching to sample task. Addiction Biology, 20, 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F., Amariglio R.E., van Boxtel M., et al. (2014). A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s & Dementia, 10, 844–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M.K., Raye C.L., Mitchell K.J., et al. (2006). Dissociating medial frontal and posterior cingulate activity during self-reflection. Social Cognitive and Affective Neuroscience, 1, 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai J.Y.T., Busse A.L., Magaldi R.M., et al. (2010). Effects of Tai Chi Chuan on cognition of elderly women with mild cognitive impairment. Einstein (São Paulo), 8, 40–45. [DOI] [PubMed] [Google Scholar]
- Kong J., Wolcott E., Wang Z., et al. (2018). Altered resting state functional connectivity of the cognitive control network in fibromyalgia and the modulation effect of mind–body intervention. Brain Imaging and Behavior, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koscik T.R., Tranel D. (2012). The human ventromedial prefrontal cortex is critical for transitive inference. Journal of Cognitive Neuroscience, 24, 1191–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshino H., Minamoto T., Yaoi K., et al. (2014). Coactivation of the default mode network regions and working memory network regions during task preparation. Scientific Reports, 4, 5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurczek J., Wechsler E., Ahuja S., et al. (2015). Differential contributions of hippocampus and medial prefrontal cortex to self-projection and self-referential processing. Neuropsychologia, 73, 116–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam L.C.W., Chau R.C.M., Wong B.M.L., et al. (2012). A 1-year randomized controlled trial comparing mind body exercise (Tai Chi) with stretching and toning exercise on cognitive function in older Chinese adults at risk of cognitive decline. Journal of the American Medical Directors Association, 13, 568–e15. [DOI] [PubMed] [Google Scholar]
- Lan C., Lai J.-S., Chen S.-Y. (2002). Tai Chi Chuan: an ancient wisdom on exercise and health promotion. Sports Medicine (Auckland, N.Z.), 32, 217–224. [DOI] [PubMed] [Google Scholar]
- Martucci K.T., Shirer W.R., Bagarinao E., et al. (2015). The posterior medial cortex in urologic chronic pelvic pain syndrome: detachment from default mode network––a resting-state study from the MAPP Research Network. Pain, 156, 1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty S.M., Siegler I.C., Logue P.E. (1982). Cross-sectional and longitudinal patterns of three Wechsler memory scale subtests. Journal of Gerontology, 37, 169–175. [DOI] [PubMed] [Google Scholar]
- McCormick C., Protzner A.B., Barnett A.J., et al. (2014). Linking DMN connectivity to episodic memory capacity: what can we learn from patients with medial temporal lobe damage? NeuroImage: Clinical, 5, 188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan A., Roberto A.J., Mohan A., et al. (2016). The significance of the default mode network (DMN) in neurological and neuropsychiatric disorders: a review. Yale Journal of Biology and Medicine, 89, 49–57. [PMC free article] [PubMed] [Google Scholar]
- Nashiro K., Sakaki M., Braskie M.N., et al. (2017). Resting-state networks associated with cognitive processing show more age-related decline than those associated with emotional processing. Neurobiology of Aging, 54, 152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng C.-W., Plakke B., Poremba A. (2014). Neural correlates of auditory recognition memory in the primate dorsal temporal pole. Journal of Neurophysiology, 111, 455–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L., Karalija N., Salami A., et al. (2016). Dopamine D2 receptor availability is linked to hippocampal–caudate functional connectivity and episodic memory. Proceedings of the National Academy of Sciences of the United States of America, 113, 7918–7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson I.R., Plotzker A., Ezzyat Y. (2007). The enigmatic temporal pole: a review of findings on social and emotional processing. Brain, 130, 1718–1731. [DOI] [PubMed] [Google Scholar]
- Ospina M., Bond K., Karkhaneh M., et al. (2008). Meditation practices for health: state of the research. Evidence Report/Technology Assessment, 155. [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E. (2015). The brain’s default mode network. Annual Review of Neuroscience, 38, 433–447. [DOI] [PubMed] [Google Scholar]
- Raichle M.E., Snyder A.Z. (2007). A default mode of brain function: a brief history of an evolving idea. NeuroImage, 37, 1083–1090. [DOI] [PubMed] [Google Scholar]
- Sala-Llonch R., Bartrés-Faz D., Junqué C. (2015). Reorganization of brain networks in aging: a review of functional connectivity studies. Frontiers in Psychology, 6, 663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders P.A., Tractenberg R.E., Chaterji R., et al. (2007). Promoting self-awareness and reflection through an experiential mind–body skills course for first year medical students. Medical Teacher, 29, 778–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira-Lichter I., Oren N., Jacob Y., et al. (2013). Portraying the unique contribution of the default mode network to internally driven mnemonic processes. Proceedings of the National Academy of Sciences of the United States of America, 110, 4950–4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares J.M., Marques P., Magalhães R., et al. (2017). The association between stress and mood across the adult lifespan on default mode network. Brain Structure and Function, 222, 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solloway M.R., Taylor S.L., Shekelle P.G., et al. (2016). An evidence map of the effect of Tai Chi on health outcomes. Systematic Reviews, 5, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songtao W. (2007). Effect of Baduanjin on physiological age of intelligence for old people. Journal of Clinical Rehabilitative Tissue Engineering Research, 11, 7910–7913. [Google Scholar]
- Sperling R.A., Jack C.R., Aisen P.S. (2011). Testing the right target and right drug at the right stage. Science Translational Medicine, 111, 111cm33–111cm33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sungkarat S., Boripuntakul S., Chattipakorn N., et al. (2017). Effects of Tai Chi on cognition and fall risk in older adults with mild cognitive impairment: a randomized controlled trial. Journal of the American Geriatrics Society, 65, 721–727. [DOI] [PubMed] [Google Scholar]
- Tang Y.Y., Hölzel B.K., Posner M.I. (2015). The neuroscience of mindfulness meditation. Nature Reviews Neuroscience, 16, 213–225. [DOI] [PubMed] [Google Scholar]
- Tao J., Chen X., Egorova N., et al. (2017a). Tai Chi Chuan and Baduanjin practice modulates functional connectivity of the cognitive control network in older adults. Scientific Reports, 7, 41581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J., Liu J., Liu W., et al. (2017b). Tai Chi Chuan and Baduanjin increase grey matter volume in older adults: a brain imaging study. Journal of Alzheimer’s Disease, 60, 389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J., Xiangli C., Liu J., et al. (2017c). Tai Chi Chuan and Baduanjin mind–body training changes resting-state low-frequency fluctuations in the frontal lobe of older adults: a resting-state fMRI study. Frontiers in Human Neuroscience, 11, 514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J., Liu J., Egorova N., et al. (2016). Increased hippocampus-medial prefrontal cortex resting-state functional connectivity and memory function after Tai Chi Chuan practice in elder adults. Frontiers in Aging Neuroscience, 8, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y., Liu B., Zhang X., et al. (2015). The structural connectivity pattern of the default mode network and its association with memory and anxiety. Frontiers in Neuroanatomy, 9, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torta D.M., Cauda F. (2011). Different functions in the cingulate cortex, a meta-analytic connectivity modeling study. NeuroImage, 56, 2157–2172. [DOI] [PubMed] [Google Scholar]
- Vatansever D., Manktelow A.E., Sahakian B.J., et al. (2016). Angular default mode network connectivity across working memory load. Human Brain Mapping, 52, 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Wang X., Liu J., et al. (2017). Acupuncture treatment modulates the corticostriatal reward circuitry in major depressive disorder. Journal of Psychiatric Research, 84, 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W., Südhof T.C. (2013). A neural circuit for memory specificity and generalization. Science, 339, 1290–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


