Abstract
Aims
Carnitine and choline are major nutrient precursors for gut microbiota-dependent generation of the atherogenic metabolite, trimethylamine N-oxide (TMAO). We performed randomized-controlled dietary intervention studies to explore the impact of chronic dietary patterns on TMAO levels, metabolism and renal excretion.
Methods and results
Volunteers (N = 113) were enrolled in a randomized 2-arm (high- or low-saturated fat) crossover design study. Within each arm, three 4-week isocaloric diets (with washout period between each) were evaluated (all meals prepared in metabolic kitchen with 25% calories from protein) to examine the effects of red meat, white meat, or non-meat protein on TMAO metabolism. Trimethylamine N-oxide and other trimethylamine (TMA) related metabolites were quantified at the end of each diet period. A random subset (N = 13) of subjects also participated in heavy isotope tracer studies. Chronic red meat, but not white meat or non-meat ingestion, increased plasma and urine TMAO (each >two-fold; P < 0.0001). Red meat ingestion also significantly reduced fractional renal excretion of TMAO (P < 0.05), but conversely, increased fractional renal excretion of carnitine, and two alternative gut microbiota-generated metabolites of carnitine, γ-butyrobetaine, and crotonobetaine (P < 0.05). Oral isotope challenge revealed red meat or white meat (vs. non-meat) increased TMA and TMAO production from carnitine (P < 0.05 each) but not choline. Dietary-saturated fat failed to impact TMAO or its metabolites.
Conclusion
Chronic dietary red meat increases systemic TMAO levels through: (i) enhanced dietary precursors; (ii) increased microbial TMA/TMAO production from carnitine, but not choline; and (iii) reduced renal TMAO excretion. Discontinuation of dietary red meat reduces plasma TMAO within 4 weeks.
Keywords: Red meat, Diet, TMAO, Gut microbiota, Metabolism, Atherosclerosis
Introduction
Trimethylamine N-oxide (TMAO) is a gut microbiota-generated metabolite with mechanistic links to the pathogenesis of atherosclerotic heart disease.1–5 Elevated plasma TMAO levels are observed in subjects at risk for incident cardiovascular disease (CVD) development and adverse CVD events including heart attack, stroke, and death.1–4 A mechanistic role for TMAO in CVD pathogenesis is supported by numerous animal model studies showing that manipulation of TMAO levels modulates atherosclerosis and related processes.1,3,6–9 Notably, a clinical prognostic value of TMAO has been supported by multiple meta-analyses reaffirming that elevated circulating TMAO levels are associated with both CVD and mortality risks across multiple cohorts and continents.10–12 Thus, decreasing systemic levels of TMAO has become a rational potential therapeutic strategy for decreasing risks for the development and progression of atherosclerotic heart disease.8,13–15
Trimethylamine N-oxide is generated via a metaorganismal pathway that begins with gut microbiota-dependent formation of trimethylamine (TMA) from TMA containing nutrient precursors (Figure 1A).1–3,16 Phosphatidylcholine, the major dietary source of choline, is a major precursor for TMA generation in vegans, vegetarians, and omnivores alike and is an abundant component of human bile, and both plant and animal products. Carnitine is enriched in red meat,3 and higher total choline content is found in beef and other meats, liver, and egg yolks.17 Consequently, a diet enriched in meat, particularly red meat, has higher content of both choline and carnitine nutrient precursors for TMA and TMAO generation.1,3 Studies have also shown dose-dependent TMA/TMAO formation with egg ingestion.2,18 Beyond choline and carnitine, potential nutrient precursors for TMA production have been reported, including the carnitine related metabolites γ-butyrobetaine and crotonobetaine, and the choline oxidation product betaine.1,16,19 Some of these TMA containing compounds can participate in TMA/TMAO formation in a gut microbiota-dependent manner, and also be generated by gut microbiota metabolism of L-carnitine.1,3,16,19
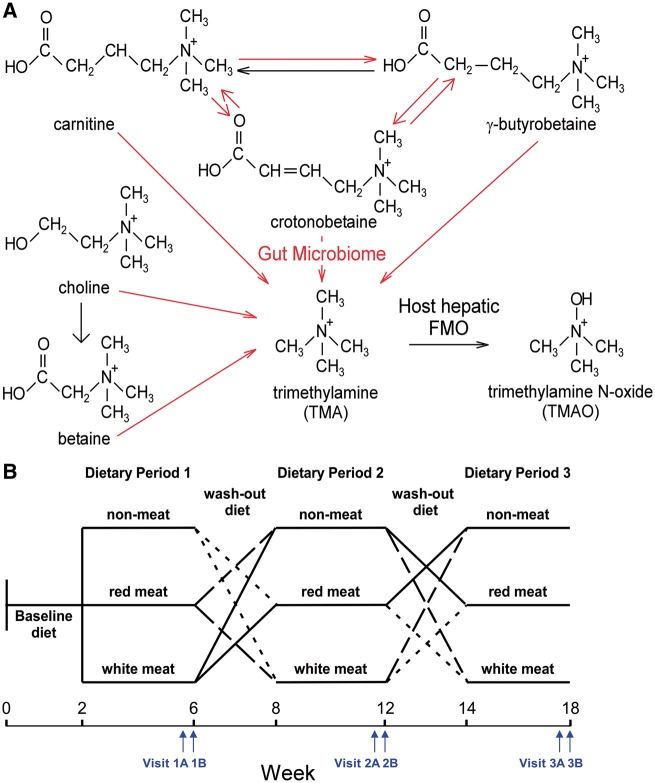
Figure 1.
Gut microbial and host pathways in trimethylamine N-oxide metabolism and study design. (A) Trimethylamine containing nutrients that can generate trimethylamine N-oxide via an initial gut microbiota-dependent step, followed by host hepatic flavin monooxygenase conversion to generate trimethylamine N-oxide. Arrows in black represent transformations performed by the host, and arrows in red represent reactions performed by gut microbes. (B) Overall study design. After consumption of a 2-week baseline diet, subjects were randomly assigned to either a high-fat or low-fat arm. Subjects in each arm underwent in cross-over design three sequential 4-week isocaloric investigational diet where protein source was derived from either red meat, white meat, or non-meat sources, with 2-week wash-out diets between each intervention dietary period, as described under Methods section. During the 4th week of each diet challenge (typically towards end), blood was collected on two separate days (Visit A and Visit B). Participants within each experimental diet arm were randomly assigned to either high- or low-saturated fat containing meal plans, as described in the text.
Few studies have systematically explored the influence of chronic dietary patterns on TMA and TMAO production, metabolism, and renal excretion. Interestingly, in small cross-sectional observational studies, plasma levels of TMAO were modestly increased in omnivores relative to vegans/vegetarians, and in recent clinical challenge studies, TMA and TMAO generation from oral carnitine was observed to be substantially reduced in vegans/vegetarians.3,19 Herein, we examined whether chronic (4 week) ingestion of an isocaloric diet containing protein derived predominantly from either red meat, white meat, or non-meat sources affects systemic levels of TMAO, many of its nutrient precursors, and both their overall metabolism and renal excretion rates in vivo.
Methods
Trial participants and study designs
Healthy adult participants (N = 113, all omnivores, 44 males and 69 females, with normal renal function; age: minimum 21 years, median 45 years, and maximum 65 years; body mass index: minimum 18.2, median 25.3, and maximum 35.3) were recruited for a study initially designed to test the effects of red meat, white meat, or non-meat protein sources on lipoprotein particles in the context of either high- or low-saturated fat intake (‘Dietary Protein Sources and Atherogenic Dyslipidemia’, ClinicalTrials.gov Identifier: NCT01427855, described elsewhere20). Before recruitment began, an additional study was designed to be carried out within this protocol to examine the impact of dietary protein and saturated fat on TMAO metabolism amongst all subjects. All study protocols were approved by the Institutional Review Boards of Children’s Hospital and Research Center of Oakland and the Cleveland Clinic. All participants gave written informed consent.
This dietary intervention study had a randomized three-period crossover design. After consuming a 2-week baseline (run-in) diet designed to reflect a typical American diet (carbohydrate 49%, protein 14%, and fat 37%; Supplementary material online, Table S1, for details regarding nutrient and TMA precursor content of baseline and experimental diets), the volunteers were assigned to three experimental diets (red meat, white meat, or non-meat) in random order for 4 weeks, each separated by a 2–7 weeks washout period, during which they were instructed to consume their habitual diet (Figure 1B). All diets were isocaloric and prepared in the metabolic kitchen of the Bionutrition Unit, University of California San Francisco-based Clinical and Translational Science Institute. Fasting plasma and urine samples were collected on two separate days in the last week of each dietary intervention (Visit A and Visit B, respectively, Figure 1B). Unless noted, results presented represent analyses of plasma and urine recovered from Visit B (longest duration on dietary intervention). Within a random subset (N = 13), heavy isotope tracer studies were performed using oral d6(N,N-dimethyl)-choline and d3(N-methyl)-carnitine challenges during the last week of each experimental diet. A description of the isotope label challenge protocol is provided in the Supplementary material online.
Metabolite quantification by mass spectrometry
All plasma and urine metabolites were quantified by stable isotope dilution high-performance liquid chromatography (HPLC) with on-line tandem mass spectrometry.16,19 Full descriptions of methods are provided in the Supplementary material online.
Statistical analysis
Scatter plots are shown with group means and 95% confidence intervals. Box–whisker plots are also shown, with boxes representing interquartile range, line inside the box indicating median, and whiskers indicating 5th and 95th percentile levels. Non-parametric repeated measures analysis of variance (ANOVA) (Friedman) test, followed by post hoc Wilcoxon matched pairs test, were used to compare analytes and fractional renal excretion levels among different diets. The Mann–Whitney U test or t-test was performed to compare non-paired samples. Spearman rank correlations were used to test associations for non-normally distributed data. Analyses were performed using R 3.4.1.21 A P-value of <0.05 was considered significant.
Results
A chronic diet enriched in red meat substantially increases plasma and urine trimethylamine N-oxide levels, and may be reversed within 1 month
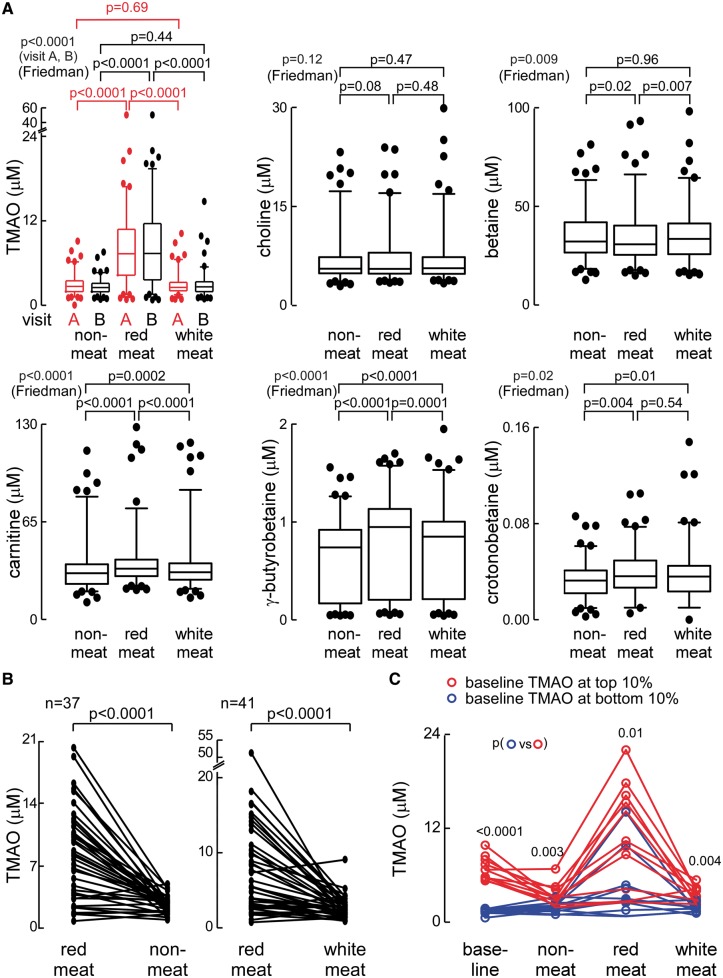
The metaorganismal pathways and metabolites monitored in the present studies are shown in Figure 1A; and overall study design of the dietary interventions are illustrated in Figure 1B. The impact of a chronic (4 weeks) diet in which the primary protein source was derived from either red meat, white meat, or non-meat sources on plasma and urine concentrations of TMAO and its various choline and carnitine nutrient precursors and related metabolites are shown in Figures 2 and 3. After 1 month of the red meat diet, an increase in plasma TMAO levels was observed in the majority of subjects. On average, plasma TMAO levels increased approximately three-fold (P < 0.0001) during the red meat diet, compared with the white meat or non-meat diets, with some subjects showing over a 10-fold increase (Figure 2A). A similar significant increase in urine levels of TMAO were noted following chronic ingestion of the red meat containing diet (P < 0.0001; Figure 3). Day-to-day variations in plasma TMAO levels could adversely impact the ability to use TMAO as a monitor of diet intervention. We, therefore, measured plasma TMAO from blood collected on two separate days in the last week of each 4-week diet intervention period (Visit A and Visit B; Figure 1B). Notably, essentially identical results were observed when analysing samples from different days in subjects. Specifically, in both cases, 1 month consumption of the red meat rich isocaloric diet led to significant and comparable increase in plasma TMAO levels (Figure 2A; P = 0.14 for comparison of Visit A vs. Visit B, yet P < 0.0001 for comparison of red meat vs. either non-meat or white meat diets at either Visit A or Visit B). Day-to-day (Visit A vs. Visit B) plasma TMAO levels across all diet arms were highly correlated (r = 0.75; P < 0.001), showing a coefficient of variance of 0.41, higher than that reported for total cholesterol, triglyceride, high-density lipoprotein cholesterol, or low-density lipoprotein cholesterol.22 However, day-to-day variability in TMAO levels were greatest with low levels of the marker (e.g. bottom two quartiles), well below clinically significant cut-offs. For example, in the bottom half of the normal range (median level ∼3.5 μM), TMAO fluctuated substantially (inter-day coefficient of variance 0.43), yet at higher levels (e.g. >6.2 μM, the top quartile2,10) where TMAO shows greater association with incident CVD events, the inter-day coefficient of variance observed was 0.30.
Figure 2.
Differences in plasma trimethylamine N-oxide and other trimethylamine containing compounds following a 4-week exposure to different diets. (A) Box–whisker plots of trimethylamine N-oxide, choline, betaine, carnitine, γ-butyrobetaine, and crotonobetaine levels after 4 week consumption of the red meat, white meat, or non-meat diets. (B) Changes in plasma trimethylamine N-oxide in subjects at completion of the 4-week red meat investigational diet period, upon switching to either the 4-week non-meat or white meat investigational diets. (C) After completion of the 2-week run-in baseline diet, subjects with the top (red) and bottom (blue) 10 percentile levels of trimethylamine N-oxide were identified, and their trimethylamine N-oxide levels plotted following completion of the indicated 4-week interventional dietary period. Except where indicated, all metabolite levels were from blood drawn on Visit B.
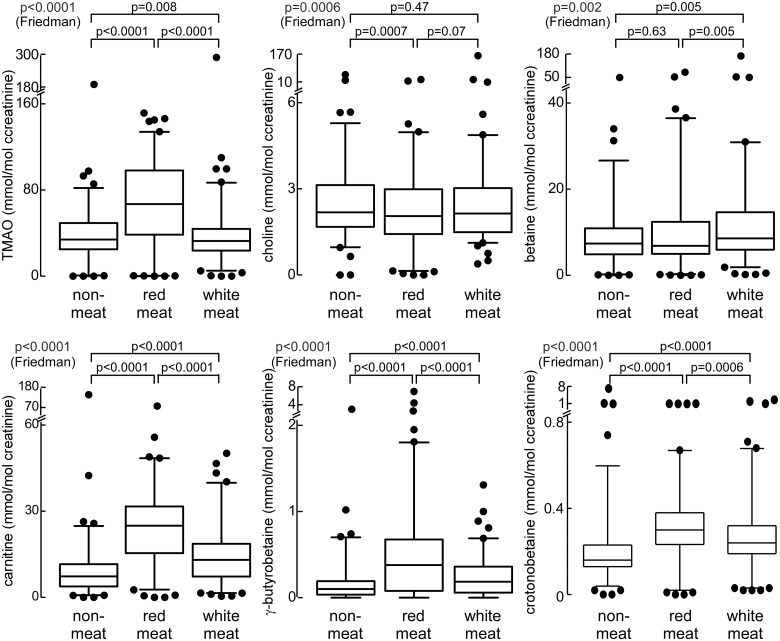
Figure 3.
Differences in urine trimethylamine N-oxide and other trimethylamine containing compounds following a 4-week exposure to different diets.
Interestingly, very modest but statistically significant reductions were noted in plasma betaine and urine choline concentrations following chronic consumption of the red meat diet compared with the non-meat or white meat diets (Figures 2A and 3). Two additional carnitine related metabolites generated by gut microbiota, γ-butyrobetaine, and crotonobetaine,16,19 both showed significant increases in both plasma (P < 0.0001 and P = 0.004, respectively) and urine (P < 0.0001 each) concentrations following 4 weeks of the red meat diet, though the relative magnitude of the increase was smaller than that for TMAO (Figures 2A and 3). In analyses examining the relationship between plasma and urine levels of TMAO, plasma TMAO levels were highly correlated with concurrently collected spot urine TMAO concentration (r = 0.41, P < 0.0001; N = 113) or urine TMAO/creatinine (r = 0.55, P < 0.0001; N = 113), and 24 h urine TMAO collections (r = 0.74, P < 0.001; N = 13).
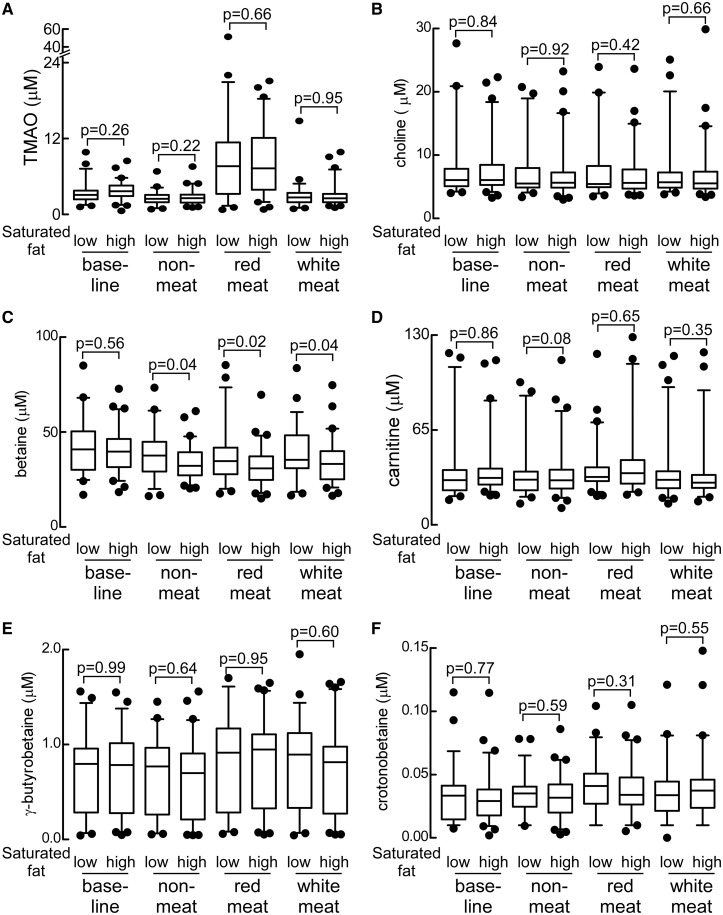
When the impact of order of the diets was examined on plasma and urine levels, no statistically significant difference in the observed effects of diet on the monitored metabolites was observed. Thus, following a participant’s completion of the red meat diet, TMAO levels were at their highest (compared with other diet arms). Moreover, when subjects discontinued the red meat diet and moved to either the white meat or non-meat diet, a marked reduction in fasting plasma TMAO level was observed (Figure 2B). Plasma levels of each of the monitored metabolites following 4 weeks of low- vs. high-saturated fat diets failed to show any significant differences (Figure 4A). Thus, increased level of dietary-saturated fat had no effect on plasma concentrations of TMAO. Similarly, no changes in plasma concentrations of other TMA containing compounds (choline, carnitine, γ-butyrobetaine, and crotonobetaine; all P > 0.05, Figure 4B and D–F) were noted with high- vs. low-saturated fat except for a modest reduction in betaine levels with increased saturated fat (P < 0.05; Figure 4C). Consequently, for all remaining study results, data shown are only for the primary randomization among red meat, white meat, and non-meat diet interventions.
Figure 4.
Impact of high-saturated fat on plasma trimethylamine N-oxide and other trimethylamine-related metabolites. Comparison of plasma levels of the indicated metabolites in subjects on the low-saturated fat vs. high-saturated fat diet arms, stratified by dietary protein source (A-F). Values reported for base-line diet are those for participants later randomized to low vs. high saturated fat in their first intervention (protein source) diet. P-values were calculated by unpaired t-test.
It was interesting to note that following the initial 2 weeks defined run-in (baseline) diet, if a subject had a higher TMAO level at baseline, their TMAO level tended to be higher following other diet arms (compared with those with low TMAO at baseline, Figure 2C). In addition, subjects with high TMAO levels following the baseline diet were also more susceptible to larger elevations in TMAO during consumption of the red meat containing diet (Figure 2C). Also evident in Figure 2C is that some subjects had higher TMAO level on the baseline run-in diet compared with the non-meat diet. Interestingly, examination of the carnitine content of the diets (Supplementary material online, Table S1) reveals the baseline diet had 2 × the carnitine level (56 mg) of the non-meat (high-saturated fat) diet (22 mg). In additional analyses, we examined the relationships between plasma levels of TMAO and the various precursor nutrients and related metabolites amongst all 113 subjects. Similar relationships were observed for the correlations amongst the choline and carnitine derived metabolites within each of the diet interventions (see Supplementary material online, Table S2). Notably, plasma levels of TMAO were most strongly correlated to choline and carnitine (and choline and carnitine with one another), particularly following 4 weeks of the red meat and white meat diets.
Chronic dietary exposure to red meat diet differentially impacts fractional renal excretion of trimethylamine N-oxide vs. carnitine and its metabolites
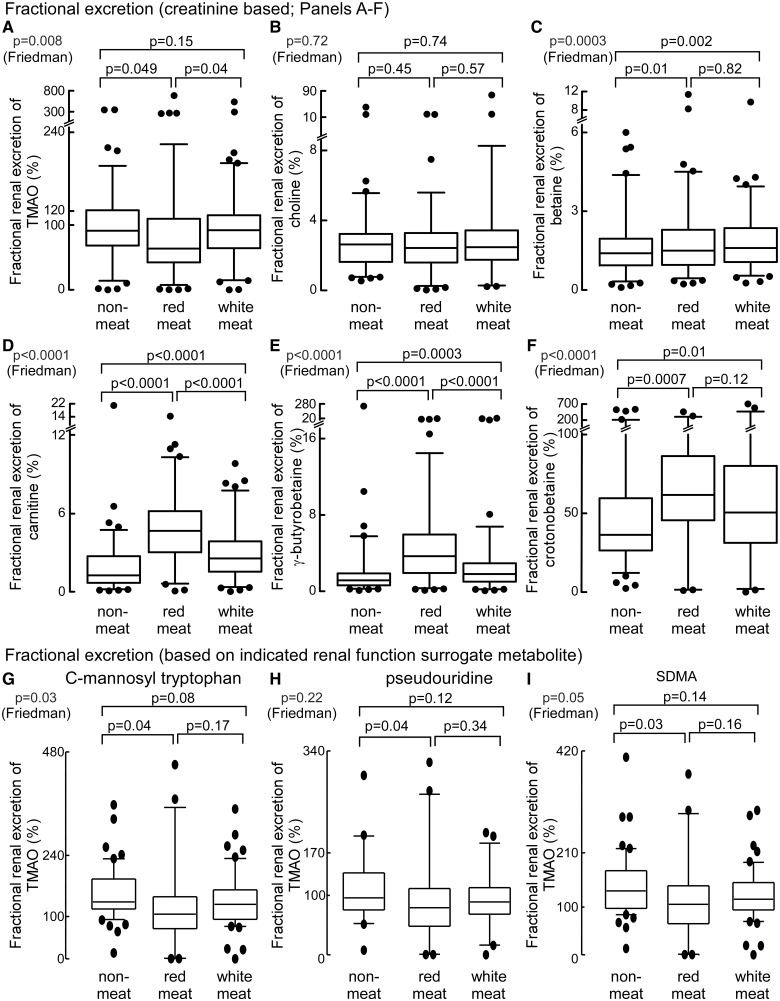
The kidney is an important organ to maintain homoeostasis of plasma metabolites,23 yet little is known about factors impacting renal excretion of TMAO or its related metabolites. We, therefore, examined the impact of diet on fractional renal excretion of TMAO and each of the other choline and carnitine-related metabolites monitored. The fractional excretion of a compound is simply a measure of the renal clearance of that compound divided by the glomerular filtration rate (i.e. the ‘fraction’ excreted), and was calculated using metabolite concentrations recovered from paired plasma and urine samples collected at the same time, as described under Methods section. First, compared to the other metabolites, the fractional excretion of TMAO was found to be both higher, and to demonstrate a remarkably broader range of values extending from nearly zero to over 700%, with a sizable proportion of subjects (almost half) showing >100%, particularly following the white meat and non-meat diet interventions. Thus, within a cohort of healthy volunteer subjects with normal renal function, the fractional renal clearance of TMAO demonstrates a surprisingly broad dynamic range. Perhaps even more surprising, the distinct chronic dietary exposures differentially impacted fractional renal excretion of the various metabolites. Following chronic ingestion of the red meat diet, the fractional excretion rate of TMAO was significantly (P < 0.05) reduced compared with white meat or non-meat diets (Figure 5A). In contrast, the fractional renal excretion of carnitine, γ-butyrobetaine, and crotonobetaine increased significantly (P < 0.0001, P < 0.0001, and P = 0.0007, respectively) on the red meat diet in comparison to the non-meat diet (Figure 5D–F). In contrast, no significant change in renal clearance of choline and only modest changes with betaine were observed among the three diets (Figure 5C). Lastly, in contrast to TMAO, the fractional renal excretion of choline, betaine, carnitine, and γ-butyrobetaine were significantly less than 100%, indicating that after filtering through the kidneys, most of these metabolites are reabsorbed, but less so following chronic dietary red meat exposure (Figure 5B–E).
Figure 5.
Impact of diet on fractional renal excretion of trimethylamine N-oxide and both choline and carnitine related metabolites (N = 113 subjects) based on paired plasma and urine levels of concurrently measured creatinine (A–F). Fractional renal excretion was also calculated by replacing plasma and urine creatinine concentrations with paired plasma and urine concentrations of alternative metabolites (concurrently measured in the same samples) whose concentrations are highly correlated with measures of renal function (C-mannosyl-tryptophan, pseudouridine, or symmetric dimethylarginine) (G–I). Fractional renal excretion of the indicated metabolites were determined following 4-weeks of the indicated diet. SDMA, symmetric dimethylarginine.
The reduction in the fractional excretion rate of TMAO (Figure 5A) observed following a month of the red meat diet suggests that the kidney becomes less efficient at eliminating TMAO relative to creatinine, yet in contrast, the increase in fractional excretion observed for carnitine, γ-butyrobetaine, and crotonobetaine, indicates red meat diet has a divergent effect—enhancement in their efficiency of renal excretion (Figure 5A). We are unaware of other examples of such diet-induced changes in renal excretion. We also noted that following the red meat diet, subjects showed a modest but significant (P = 0.006; Supplementary material online, Table S3) increase in serum creatinine (still within the normal range), while urine creatinine concentrations were increased (within normal range). While we could not find any recommended dietary restrictions before measurement of fractional renal excretion, we thought it reasonable to confirm our results using a method independent of creatinine. To do so, we sought to identify alternative analytes in plasma that serve as robust measures of renal function, which we could then utilize (paired plasma and urine analyses) in place of creatinine in the fractional renal excretion calculations (Methods section). In a recent untargeted metabolomics study examining nearly 500 analytes to identify those that most closely correlate with renal function, C-mannosyl tryptophan and pseudouridine were identified as excellent renal functional surrogates.24 Further, we too had performed unpublished untargeted metabolomics analyses and identified symmetric dimethylarginine (SDMA) as being highly correlated with renal function (r > 0.80; P < 0.0001) in both men and women alike (data not shown), and alternative published studies have similarly suggested SDMA serves as an excellent endogenous marker of renal function.25 We, therefore, developed stable isotope dilution liquid chromatography (LC)/mass spectrometry (MS)/MS methods for quantification of these three surrogate indicators of renal function, and then quantified their concentrations within the same paired plasma and urine samples used for the creatinine-based fractional excretion measurements. Results from these analyses are shown in Figure 5G–I and Supplementary material online, Tables S3–S5. Notably, in contrast to creatinine, none of the surrogate indicators of renal function showed significant changes in plasma or urine levels based on diet, and all were highly correlated with creatinine (Supplementary material online, Tables S3 and S4). Remarkably, fractional excretion calculations for TMAO using each of the three alternative renal function metabolite surrogates (C-mannosyl tryptophan, pseudouridine, and SDMA) showed similar results to that observed using creatinine (i.e. red meat diet-induced significant reduction in fractional excretion rate of TMAO, yet conversely, significant increases in the fractional excretion rates for carnitine, γ-butyrobetaine, and crotonobetaine; Figure 5D–F and Supplementary material online, Table S5).
Isotope tracer studies reveal enhanced microbial production of trimethylamine and trimethylamine N-oxide from carnitine but not choline in subjects following a chronic red meat rich diet
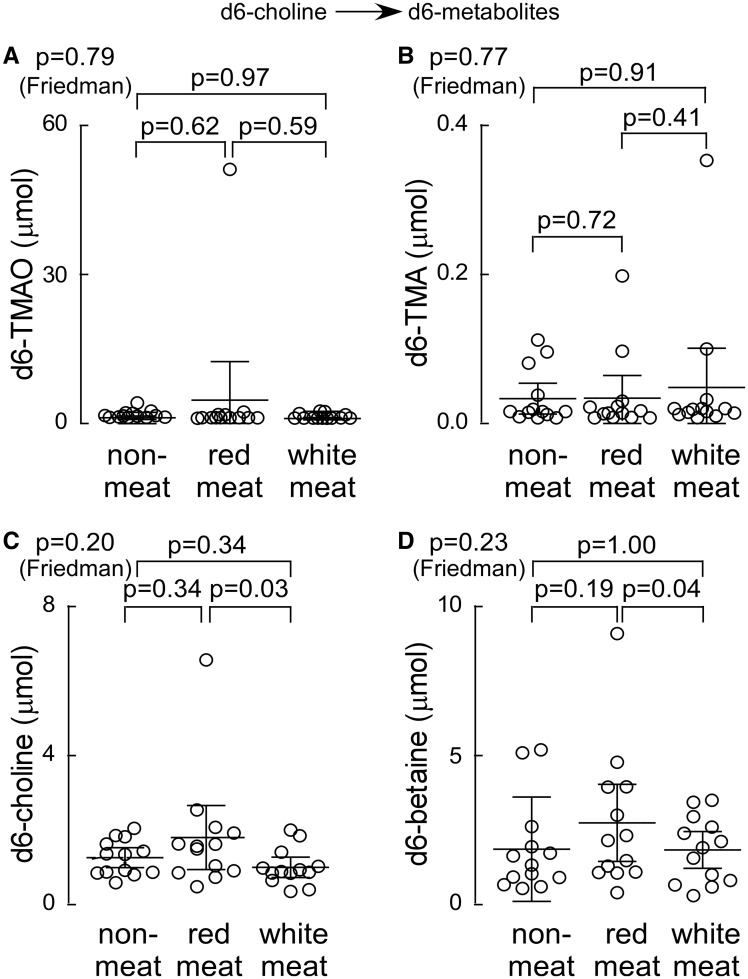
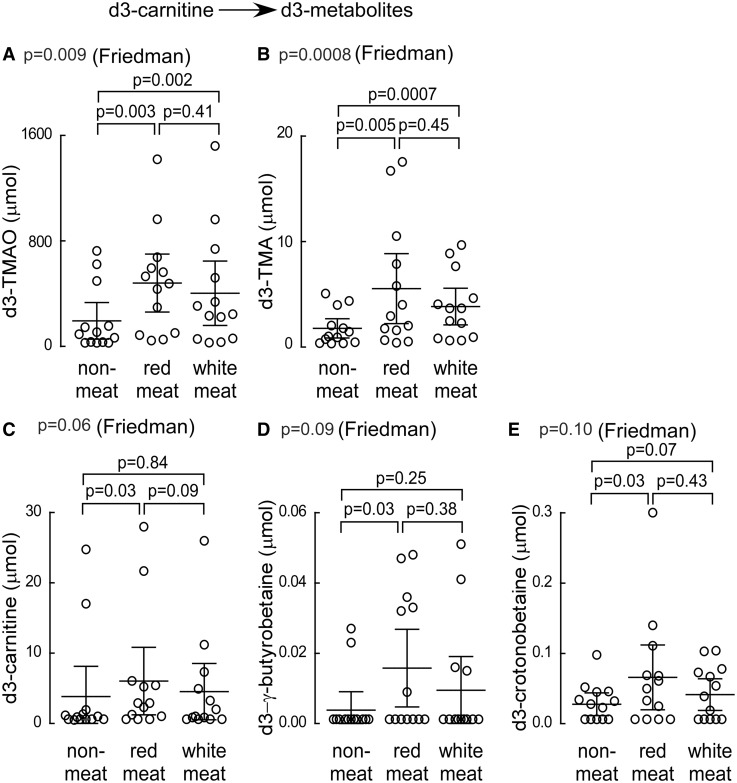
In additional studies, we examined 24 h production levels of TMA and TMAO by the nutrient precursors, choline, and carnitine, in a random subset of consented subjects (N = 13) following chronic dietary exposure to each diet arm. During the last week of each experimental diet, subjects ingested synthetic d6-choline and d3-carnitine in capsule form, and both d6- (derived from choline; Figure 6A–D) and d3- (derived from carnitine; Figure 7A–E) labelled isotopologues of TMA, TMAO, and the appropriate choline and carnitine derived metabolites were examined. Remarkably, 24 h urine choline-derived d6-TMA and d6-TMAO showed no significant differences within subjects following the month long exposures to each of the distinct diets (Figure 6A and B). Similarly, 24 h urinary total d6-choline and d6-betaine recovered following each diet intervention showed no significant differences, except for a modest decrease following 4 weeks of white meat diet vs. red meat (Figure 6C and D). In contrast, chronic exposure to the different diets substantially affected 24 h production of the d3-carnitine—derived metabolites (Figure 7A–E). For example, the 24 h urine d3-TMA and d3-TMAO recovered following ingestion of d3-carnitine was significantly (several-fold) higher in subjects following either the red meat or white meat diets compared to the non-meat diet (Figure 7A and B). Similarly, the 24 h urine d3-carnitine, d3-γ-butyrobetaine, and d3-crotonobetaine were all significantly increased in subjects following the red meat diet (Figure 7C–E).
Figure 6.
Urine analyses (24 h) following ingestion of d6-choline isotope tracer. During the last week of each interventional dietary period (red meat, white meat, or non-meat), subjects (N=13) underwent an oral d6-choline challenge and 24 h urine output of the indicated d6-labelled metabolites (A-D) was collected and analyzed as described (Methods section).
Figure 7.
Urine analyses (24 h) following ingestion of d3-carnitine isotope tracer. During the last week of each interventional dietary period (red meat, white meat, or non-meat), subjects (N=13) underwent an oral d3-carnitine challenge and 24 h urine output of the indicated d3-labelled metabolites (A-E) was collected and analyzed as described (Methods section).
Discussion
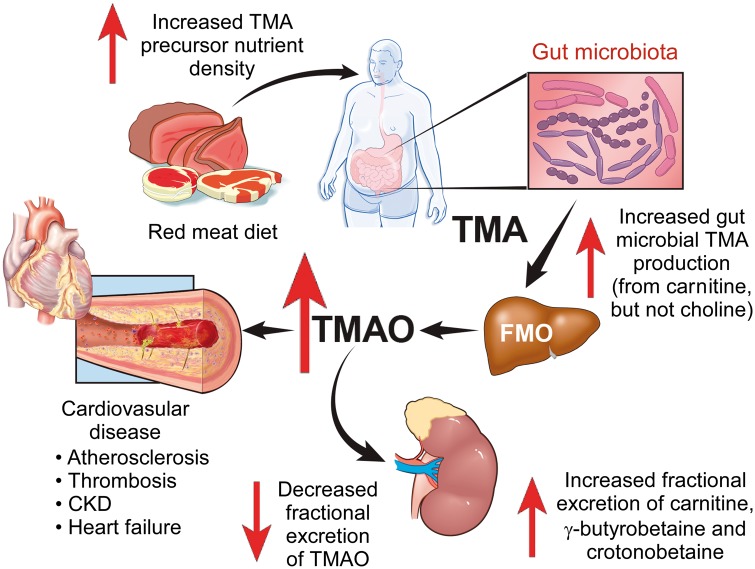
A major component of preventive efforts for CVD risk reduction, both at the population and individual targeted level, is the adoption of healthy lifestyles, including dietary recommendations.26 Based upon the many epidemiological and nutrition studies suggesting a positive association between red meat ingestion and CVD risks,27–33 most dietary recommendations advise limiting red meat ingestion.26 However, some recent meta-analyses suggest the association between red meat ingestion and adverse changes to traditional CVD risk factors is not as strong,28 despite the relatively consistent association with hard endpoints like heart attack, stroke, mortality, and CVD development.29–33 Such findings arguably suggest that factors alternative to traditional CVD risk factors exist to help explain the association observed between red meat ingestion and heightened CVD risks. One potential candidate for this is the metaorganismal TMAO pathway.1,14,15 Despite the many striking associations between elevated TMAO levels and incident CVD risks,1–4,10–12 and the numerous animal model studies supporting a mechanistic contribution of TMAO to adverse CVD-related phenotypes,1,3–9,13 the direct contribution of TMAO to CVD pathogenesis in humans is unclear, and the impact of dietary interventions on TMAO have not been extensively investigated. The present study demonstrates several important points regarding chronic dietary patterns with respect to protein source, and their impact on TMAO levels and its associated metaorganismal metabolism. Foremost is the unambiguous demonstration that a diet in which the major protein source is derived from red meat, compared with either white meat or non-meat sources (keeping total calories constant), results in substantial increases in fasting plasma and urine TMAO levels (Take home figure). Moreover, adherence to a red meat diet raises systemic TMAO levels by three different mechanisms: (i) enhanced nutrient density of dietary TMA precursors; (ii) increased microbial TMA/TMAO production from carnitine, but not choline; and (iii) reduced renal TMAO excretion. Interestingly, discontinuation of dietary red meat reduced plasma TMAO within 4 weeks. Also notable was that the kidneys appear to dynamically regulate fractional excretion of TMAO over a remarkably broad range amongst subjects with normal renal function, suggesting a potential functional role. It was somewhat unanticipated that there was no increase in the conversion of isotope labelled choline into TMA and TMAO in subjects following chronic exposure to the red meat diet. While the total choline content of the red meat diet provided approximately 15% and 28% more compared with the average daily total choline content of the white meat and non-meat diets, respectively (Supplementary material online, Table S1), this increase was substantially less than the 3.8-fold and 7.9-fold higher carnitine content present in the red meat diet compared with the white meat and non-meat diets, respectively (Supplementary material online, Table S1).
Take home figure.
Summary scheme: effect of a red meat containing diet on the metaorganismal trimethylamine N-oxide pathway.
Perhaps the most surprising finding of the present studies was the observation that the fractional renal excretion of TMAO and the carnitine family of nutrient precursors and metabolites (carnitine, γ-butyrobetaine, and crotonobetaine) were all dynamically (and differentially) regulated by the preceding dietary exposure. These findings appear to be robust, as they were observed using multiple different indices of renal function beyond creatinine (C-mannosyl tryptophan, pseudouridine, and SDMA). Thus, after 1 month of the red meat diet, fractional renal excretion of TMAO was significantly reduced, which would contribute to the observed rise in plasma TMAO levels. In contrast, carnitine and both of the other carnitine derived gut microbial metabolites, γ-butyrobetaine and crotonobetaine, all showed marked (2–5 fold) elevation in fractional renal excretion following the red meat diet. The mechanism(s) contributing to the observed altered fractional renal excretion of these metabolites is unknown. Indeed, it is unclear what metabolites and host receptors/sensor(s) contribute to the observed changes in fractional renal excretion based upon dietary protein source. One can speculate that dynamic regulation of TMAO excretion, for example, might be linked to renal tissue oncotic pressure and osmoregulation, given that TMAO has been shown to play a role in osmoregulation in some animal species.34
The present studies have clear potential clinical relevance. Numerous studies have revealed a dose-dependent relationship between circulating levels of TMAO and incident adverse CVD risks, as reviewed in several recent meta-analyses.10–12 In one meta-analysis involving >25 000 subjects cumulatively with a mean follow-up duration amongst all studies examined of 4.3 ± 1.5 years, the relative risk for all-cause mortality was calculated to increase by 7.6% per each 10 μmol/L increment of TMAO.10–12 In the present study, subjects experienced on average an absolute change in median TMAO level of 5.9 μM and 5.7 μM during the red meat diet arm (compared with non-meat and white meat diets, respectively), corresponding to approximately two portions of red meat per day. According to the meta-analysis [and assuming changes observed following 1 month of diet can be extended to the mean length of follow-up (4.3 year) in the meta-analysis], the increases in plasma TMAO observed with the red meat diet would correspond to an approximate 4.5% increase in relative risk of mortality compared with the non-meat diet, and a 4.3% difference in mortality compared with white meat diet. Further, use of TMAO levels from the independent alternative blood draw in the last week of diet intervention for each subject gave comparable results, with projected red meat diet associated increases in relative risk for all-cause mortality of 4.6% or 4.7%, compared with non-meat or white meat diets, respectively. Moreover, recent human observational and interventional studies show that TMAO levels can increase substantially (>10 μM) with chronic supplementation.4,5 Further, TMAO has been shown to directly interact with platelets (human and murine), increasing their responsiveness, and promoting a prothrombotic phenotype.4,5 Such observations may account for the heightened risk for thrombotic events like heart attack and stroke observed amongst subjects with elevated TMAO levels.4,10–12 The present studies thus suggest that a diet rich in red meat would be associated with heightened TMAO levels, and potential heightened thrombotic event risk. Indeed, numerous epidemiological studies show a dose-dependent heightened risk of thrombotic events, CVD, and mortality risks with red meat consumption.29–33 However, the contribution of TMAO to heightened cardiovascular risks from a chronic diet rich in red meat is unclear. Importantly, the present studies reveal that switching from a diet rich in red meat to either a white meat or non-meat protein source (yet maintaining same calories, and proportion of protein in the diet) can substantially reduce TMAO levels within several weeks. To our knowledge, the present studies are the first to directly explore the relationship of dietary protein source to plasma TMAO levels with defined isocaloric randomized diets, and the time needed to reduce TMAO levels with dietary changes. They thus reveal that beyond quantity, the quality of diet composition (with respect to protein source, but not saturated fat content) impacts overall TMAO metabolism and excretion.
Few studies have examined the influence of controlled diets on TMAO levels. Reduced urine TMAO levels were recently reported in an observational study among subjects following a Mediterranean diet in a post hoc cross-sectional analysis comparing omnivores vs. vegetarians or vegans35; moreover, a trend towards reduced urinary TMAO levels was observed among subjects with increased reported compliance with the Mediterranean diet (based on the PREDIMED trial compliance tool).35 The Mediterranean diet in the PREDIMED trial, which showed a 30% reduction in CVD risk, was characterized by avoidance of red meat as a protein source.36,37 The present results add substantially to our understanding of diet and TMAO metabolism, and raise intriguing questions about the selection of dietary protein source in subjects at heightened CVD risks with increased TMAO levels. For example, elevated TMAO levels are frequently observed in patients with impaired renal function in whom increased incidence of CVD is not accounted for by traditional risk factors. Given the growing body of evidence suggesting a mechanistic link between TMAO and CVD pathogenesis, there is substantial interest in development of TMAO reducing therapeutic interventions. The present studies begin to provide some evidence-based results regarding dietary manipulations that can effectively reduce TMA/TMAO levels.
Limitations
There are several limitations to this study. Blood collection time after meal was not controlled, which may lead to fluctuation of plasma metabolite levels. Only two visits of blood and urine for each diet arm were collected, which may not reflect the total intra-individual variability in subjects. Fractional excretion calculations utilized creatinine plasma and urine concentrations, which varied based on diet, though we also quantified three separate alternative metabolites identified to serve as surrogate markers of renal function, and observed qualitatively comparable results.
Supplementary Material
Acknowledgements
The authors thank Cewin Chao, MS, RD, MBA, Monique Schloetter, RD, Laurie Herraiz, RD, and the staff of the Bionutrition Unit of the UCSF CTSI for design of the dietary protocols and meal preparation, Megan Bennett for co-ordination of recruitment and clinic activities, Barbara Sutherland PhD, and Alison Brown for monitoring the dietary intervention and compliance, and Sarah King, PhD for management of samples in Dr Krauss’ laboratory.
Funding
This work was supported by the National Institutes of Health and the Office of Dietary Supplements [R01 HL103866 to S.L.H., R01 HL126827 and R01 DK106000 to W.H.W.T. and S.L.H., RO1 HL106003 to R.M.K. and N.B., R01 HL130819 to Z.W. S.L.H. is also partially supported by an award from the Leducq Foundation.] and The University of California, San Francisco (UCSF) Clinical and Translational Science Unit.
Conflict of interest: Z.W., B.S.L., and S.L.H. are named as co-inventors on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics, and have the right to receive royalty payment for inventions or discoveries related to cardiovascular diagnostics or therapeutics from Cleveland Heart Lab, Quest Diagnostics, and Proctor & Gamble. S.L.H. reports having been paid as a consultant from Proctor & Gamble, and having received research funds from Proctor & Gamble and Roche. All other authors report that they have no relationships relevant to the contents of this article to disclose.
Footnotes
See page 595 for the editorial comment on this article (doi: 10.1093/eurheartj/ehy905)
References
- 1. Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL.. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL.. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 2013;368:1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL.. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013;19:576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, Sartor RB, McIntyre TM, Silverstein RL, Tang WHW, DiDonato JA, Brown JM, Lusis AJ, Hazen SL.. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 2016;165:111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhu W, Wang Z, Tang WHW, Hazen SL.. Gut microbe-generated trimethylamine N-oxide from dietary choline is prothrombotic in subjects. Circulation 2017;135:1671–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bennett BJ, de Aguiar Vallim TQ, Wang Z, Shih DM, Meng Y, Gregory J, Allayee H, Lee R, Graham M, Crooke R, Edwards PA, Hazen SL, Lusis AJ.. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab 2013;17:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shih DM, Wang Z, Lee R, Meng Y, Che N, Charugundla S, Qi H, Wu J, Pan C, Brown JM, Vallim T, Bennett BJ, Graham M, Hazen SL, Lusis AJ.. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J Lipid Res 2015;56:22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, Gu X, Huang Y, Zamanian-Daryoush M, Culley MK, DiDonato AJ, Fu X, Hazen JE, Krajcik D, DiDonato JA, Lusis AJ, Hazen SL.. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell 2015;163:1585–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Warrier M, Shih DM, Burrows AC, Ferguson D, Gromovsky AD, Brown AL, Marshall S, McDaniel A, Schugar RC, Wang Z, Sacks J, Rong X, Vallim TA, Chou J, Ivanova PT, Myers DS, Brown HA, Lee RG, Crooke RM, Graham MJ, Liu X, Parini P, Tontonoz P, Lusis AJ, Hazen SL, Temel RE, Brown JM.. The TMAO-generating enzyme flavin monooxygenase 3 is a central regulator of cholesterol balance. Cell Rep 2015;10:326–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schiattarella GG, Sannino A, Toscano E, Giugliano G, Gargiulo G, Franzone A, Trimarco B, Esposito G, Perrino C.. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis. Eur Heart J 2017;38:2948–2956. [DOI] [PubMed] [Google Scholar]
- 11. Qi J, You T, Li J, Pan T, Xiang L, Han Y, Zhu L.. Circulating trimethylamine N-oxide and the risk of cardiovascular diseases: a systematic review and meta-analysis of 11 prospective cohort studies. J Cell Mol Med 2018;22:185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heianza Y, Ma W, Manson JE, Rexrode KM, Qi L.. Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: a systematic review and meta-analysis of prospective studies. J Am Heart Assoc 2017;6 pii: e004947. 1–12. doi: 10.1161/JAHA.116.004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roberts AB, Gu X, Buffa JA, Hurd AG, Wang Z, Zhu W, Gupta N, Skye SM, Cody DB, Levison BS, Barrington WT, Russell MW, Reed JM, Duzan A, Lang JM, Fu X, Li L, Myers AJ, Rachakonda S, DiDonato JA, Brown JM, Gogonea V, Lusis AJ, Garcia-Garcia JC, Hazen SL.. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med 2018;24:1407–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brown JM, Hazen SL.. Microbial modulation of cardiovascular disease. Nat Rev Microbiol 2018;16:171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brown JM, Hazen SL.. Targeting of microbe-derived metabolites to improve human health: the next frontier for drug discovery. J Biol Chem 2017;292:8560–8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koeth RA, Levison BS, Culley MK, Buffa JA, Wang Z, Gregory JC, Org E, Wu Y, Li L, Smith JD, Tang WH, DiDonato JA, Lusis AJ, Hazen SL.. γ-butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab 2014;20:799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Conlon MA, Bird AR.. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2014;7:17–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miller CA, Corbin KD, da Costa KA, Zhang S, Zhao X, Galanko JA, Blevins T, Bennett BJ, O'Connor A, Zeisel SH.. Effect of egg ingestion on trimethylamine-N-oxide production in humans: a randomized, controlled, dose-response study. Am J Clin Nutr 2014;100:778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koeth RA, Lam-Galvez BR, Kirsop J, Wang Z, Levison BS, Gu X, Copeland MF, Bartlett D, Cody DB, Dai HJ, Culley MK, Li XS, Fu X, Wu Y, Li L, DiDonato JA, Tang WHW, Garcia-Garcia JC, Hazen SL.. L-Carnitine in omnivorous diets induces an atherogenic gut microbial pathway in humans. J Clin Invest 2018; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bergeron N, Williams PT, Lamendella R, Faghihnia N, Grube A, Li X, Wang Z, Knight R, Jansson JK, Hazen SL, Krauss RM.. Diets high in resistant starch increase plasma levels of trimethylamine-N-oxide, a gut microbiome metabolite associated with CVD risk. Br J Nutr 2016;116:2020–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Core TR. A Language and Environment for Statistical Computing v.3.4.1. R. Vienna, Austria: R Foundation for Statistical Computing; 2017.
- 22. Bookstein L, Gidding SS, Donovan M, Smith FA.. Day-to-day variability of serum cholesterol, triglyceride, and high-density lipoprotein cholesterol levels. Impact on the assessment of risk according to the National Cholesterol Education Program guidelines. Arch Intern Med 1990;150:1653–1657. [PubMed] [Google Scholar]
- 23. Thurau KW. Autoregulation of renal blood flow and glomerular filtration rate, including data on tubular and peritubular capillary pressures and vessel wall tension. Circ Res 1964;15(Suppl):132–141. [PubMed] [Google Scholar]
- 24. Sekula P, Goek ON, Quaye L, Barrios C, Levey AS, Romisch-Margl W, Menni C, Yet I, Gieger C, Inker LA, Adamski J, Gronwald W, Illig T, Dettmer K, Krumsiek J, Oefner PJ, Valdes AM, Meisinger C, Coresh J, Spector TD, Mohney RP, Suhre K, Kastenmuller G, Kottgen A.. A metabolome-wide association study of kidney function and disease in the general population. J Am Soc Nephrol 2016;27:1175–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kielstein JT, Salpeter SR, Bode-Boeger SM, Cooke JP, Fliser D.. Symmetric dimethylarginine (SDMA) as endogenous marker of renal function–a meta-analysis. Nephrol Dial Transplant 2006;21:2446–2451. [DOI] [PubMed] [Google Scholar]
- 26. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corra U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Lochen ML, Lollgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, Binno S; ESC Scientific Document Group. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abete I, Romaguera D, Vieira AR, Lopez de Munain A, Norat T.. Association between total, processed, red and white meat consumption and all-cause, CVD and IHD mortality: a meta-analysis of cohort studies. Br J Nutr 2014;112:762–775. [DOI] [PubMed] [Google Scholar]
- 28. O'Connor LE, Kim JE, Campbell WW.. Total red meat intake of >/=0.5 servings/d does not negatively influence cardiovascular disease risk factors: a systemically searched meta-analysis of randomized controlled trials. Am J Clin Nutr 2017;105:57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schwingshackl L, Schwedhelm C, Hoffmann G, Lampousi AM, Knuppel S, Iqbal K, Bechthold A, Schlesinger S, Boeing H.. Food groups and risk of all-cause mortality: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr 2017;105:1462–1473. [DOI] [PubMed] [Google Scholar]
- 30. Haring B, Wang W, Fretts A, Shimbo D, Lee ET, Howard BV, Roman MJ, Devereux RB.. Red meat consumption and cardiovascular target organ damage (from the Strong Heart Study). J Hypertens 2017;35:1794–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Micha R, Michas G, Lajous M, Mozaffarian D.. Processing of meats and cardiovascular risk: time to focus on preservatives. BMC Med 2013;11:136.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim K, Hyeon J, Lee SA, Kwon SO, Lee H, Keum N, Lee JK, Park SM.. Role of Total, Red, Processed, and white meat consumption in stroke incidence and mortality: a systematic review and meta-analysis of prospective cohort studies. J Am Heart Assoc 2017;6: pii:e005983 1–16. doi:10.1161/JAHA.117.005983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wolk A. Potential health hazards of eating red meat. J Intern Med 2017;281:106–122. [DOI] [PubMed] [Google Scholar]
- 34. Yancey PH. Compatible and counteracting solutes: protecting cells from the Dead Sea to the deep sea. Sci Prog 2004;87:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, Serrazanetti DI, Di Cagno R, Ferrocino I, Lazzi C, Turroni S, Cocolin L, Brigidi P, Neviani E, Gobbetti M, O'Toole PW, Ercolini D.. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016;65:1812–1821. [DOI] [PubMed] [Google Scholar]
- 36. Estruch R, Ros E, Salas-Salvadó J, Covas M-I, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pintó X, Basora J, Muñoz MA, Sorlí JV, Martínez JA, Martínez-González MA; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–1290. [DOI] [PubMed] [Google Scholar]
- 37. Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pintó X, Basora J, Muñoz MA, Sorlí JV, Martínez JA, Fitó M, Gea A, Hernán MA, Martínez-González MA; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med 2018;378:e34.. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.