Abstract
Background
Early stage melanoma survivors are typically otherwise healthy adults with a median age of 56.5 years for women at the time of diagnosis. Women have a projected lifespan of 20 to 30 additional years during which they should be able to enjoy and benefit from leisure outdoor physical activities while limiting their risk of a second melanoma from unprotected sun exposure.
Objective
This study evaluated the physical activity of melanoma survivors before their diagnosis of melanoma as well as 2 to 3 months and 12 months after surgical resection.
Methods
Participants in this observational study were early stage melanoma survivors (Stage 0-1A) who were surgically treated for melanoma within the last 6 months. Participants completed three online surveys (Leisure Time Exercise Questionnaire) that estimated their physical activity 2 to 3 months prior to the diagnosis with melanoma as well as 2 to 3 months and 12 months after surgery.
Results
All 75 participants were non-Hispanic white, and 38 of 75 participants (50.6%) were women. The median age of this urban/suburban Midwest population was 52 years. Prior to their diagnosis, all melanoma survivors were active, and 55% of women reported vigorous leisure physical activity. Two to three months after surgery, 11 of 38 women (30%) were inactive and 31% were inactive at 12 months. At 12 months after surgery, inactivity was positively correlated with older age (61-80 + years; F1050 = 15.38; p < .001) and being a woman (0.05; F1050 = 11.02; p < .01).
Conclusion
Dermatologists are in a position to promote a healthy lifestyle for melanoma survivors, especially older women who can be expected to live many more years and may restrict leisure outdoor physical activity to comply with sun protection recommendations. When considering the overall health of older, female melanoma survivors, dermatologists may recommend they walk with a friend at any time of the day while wearing a hat, protective clothing, and sunscreen on exposed skin.
Keywords: melanoma, physical activity, inactivity, healthy lifestyle
Introduction
From 2005 to 2014, melanoma incidence among women increased significantly across all age groups: Age 45 to 54 years (average annual percent change of 1%), 55 to 65 years (2.3%), 65 to 74 years (2.7%), 75 to 84 years (2.9%), and > 85 years (3.5 %; Holman et al., 2018). In part due to indoor tanning by women, the annual number of new melanoma cases are expected to rise from 70,000 between 2007 and 2011 to 116,000 between 2016 and 2031 (American Cancer Society, 2016a, Coelho and Hearing, 2010, Cust et al., 2011). Currently among the more than 1.2 million melanoma survivors in the United States, 46% are 40 to 60 years old and 84% have disease that is localized to the skin (Whiteman et al., 2016). Since 80% are expected to be early stage melanoma survivors, the overall number of survivors will rapidly increase and remain elevated (Holman et al., 2018). Early stage melanoma survivors are typically otherwise healthy adults with a median age at the time of diagnosis of 56.5 years for women and 62.6 for men (American Cancer Society, 2016b). These women have a projected lifespan of 20 to 30 years during which they should be able to enjoy and benefit from outdoor leisure physical activities while limiting their risk of a second melanoma from unprotected sun exposure (Najita et al., 2016).
Dermatologists perform the resection of early stage melanomas and most melanoma survivors have continuing care with dermatologists who recommend sun protection to reduce the incidence of a second melanoma (Green et al., 2010). Sun protection recommendations often suggest shifting outdoor activities to nonpeak hours of intense sun before 10 a.m. and after 4 p.m. The patient may interpret this as a prohibition and stay out of the sun. An overly rigid focus on sun avoidance may result in unhealthy reductions in physical activity, especially in older adults (King et al., 2013). Since approximately 59% of melanoma survivors are overweight (Coups and Osteroff, 2005), an assessment of physical activity among early stage melanoma survivors who are cared for by dermatologists is indicated. This study evaluates the physical activity of mobile, early stage melanoma survivors before their diagnosis of melanoma as well as 2 to 3 months and 12 months after surgical resection of the melanoma.
Methods
Participants in this observational study were early stage melanoma survivors (Stage 0-1A) who were treated for melanoma within the last 6 months and enrolled in a randomized clinical trial that provided education on skin self-examination (Robinson et al., 2016). Eligibility criteria included being over 18 years old, not mobility impaired, and no comorbid diseases that limited the ability to walk the distance of two blocks. Due to concerns about mobility, melanoma survivors with lesions on the lower leg or foot were excluded from the study. Participants were required to have access to a computer and received no compensation for the online completion of the Leisure Time Exercise Questionnaire (Table 1; Godin and Shephard, 1985). Participants completed three separate surveys that estimated their physical activity 2 to 3 months prior to their diagnosis with melanoma as well as 2 to 3 months after surgical resection of the melanoma and 12 months after surgery. The Institutional Review Board of Northwestern University approved the study.
Table 1.
Self-reported survey of leisure time physical activity
| Times per week | Average duration each time you exercised (min) | |
|---|---|---|
| 1) Strenuous exercise (Heart beats rapidly) (e.g., running, jogging, hockey, football, soccer, squash, basketball, cross country skiing, judo, roller skating, vigorous swimming, vigorous long distance bicycling) |
________ |
________ |
| 2) Moderate exercise (not exhausting) (e.g., fast walking, baseball, tennis, easy bicycling, volleyball, badminton, easy swimming, alpine skiing, popular and folk dancing) |
________ |
________ |
| 3) Mild exercise (minimal effort) (e.g., yoga, archery, fishing from river bank, bowling, horseshoes, golf, snowmobiling, easy walking) |
________ |
________ |
Measures
Demographic information was obtained about age, sex, residence (i.e., urban, suburban, and rural), access to parks and walking/biking trails, education, income, and stage of melanoma confirmed with the medical record. The three-item Leisure Time Exercise Questionnaire (Godin and Shephard, 1985) asked participants to recall their average weekly leisure time for physical activity (not including work or household activities). The instructions stated: “When considering the number of times per week, please only include those times that you were physically active for more than 15 minutes” (Table 1). Inactivity was defined as < 60 min of vigorous exercise or < 150 min of moderate-or-mild exercise per week (Denmark-Wahnefried et al., 2015).
The survey conducted 12 months after surgery invited participants to enter free text to describe their three most commonly performed, weekly leisure time physical activities that were performed for more than 15 minutes. Participants were instructed to not include work or household activities. Also, the survey that was conducted 12 months after surgery identified participants who were inactive, and invited them to respond to the following items: a) Do you expect to change the amount of leisure time physical activity in the next 6 months? Yes/No; b) If yes, what change do you expect to make? Become more or less active; c) If you expect to become more active, then please tell us what you may find helpful? (free text); d) If no, then please tell us why you do not believe you will change your leisure physical activity. (free text).
Statistical analysis
Descriptive statistics for physical activity and strategies to obtain physical activity were calculated for the total sample and by age category and sex. To test the differences in three levels of physical activity (i.e., vigorous, moderate-mild, and inactive) multiplied by three (pre-diagnosis, 2-3 months after surgical treatment, and 12 months after surgery), mixed measures analysis of variance tests were tested on physical activity by age category and sex. All analyses were conducted using SPSS software (v23.0; SPSS Inc., Chicago, IL).
Results
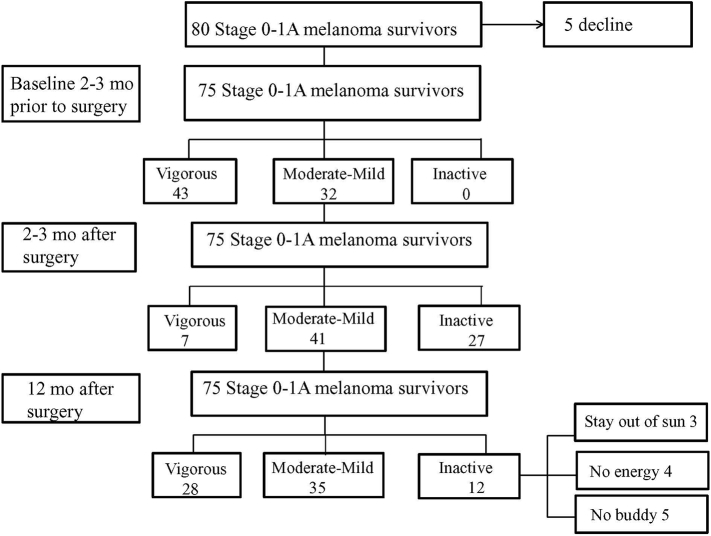
Participation in the survey was offered to 80 randomly selected melanoma survivors with Stage 0 to 1A disease among the 100 participants enrolled in the skin self-examination research who met eligibility criteria (Fig. 1). Five participants declined to participate because they did not want to take online surveys. The median age of the population was 52 years (range, 27-82 years), and there was no significant difference in age between men and women. All subjects were non-Hispanic white and 38 of 75 participants (50.6%) were women. Over half of the study participants had a college degree or higher and an income of US $51,000 or higher. The participants lived in urban (47%) and suburban (53%) locations, all had access to parks, and 84% had access to walking/biking trails.
Fig. 1.
CONSORT Flow Diagram
CONSORT indicates Consolidated
Standards of Reporting Clinical Trials
Prior to their diagnosis, all melanoma survivors were active and 21 of 38 women (55%) reported vigorous activity with 60 minutes or more per week (Table 2). Two to three months after surgery, 11 of 38 women (30%) were inactive. Most failed to meet the American Cancer Society guideline recommendation for adults of 150 minutes of exercise per week (Denmark-Wahnefried et al., 2015). Inactivity increased significantly in both men and women and among those ages 41 to 60 years and 61 to 80 + years (F1050 = 9.07; p < .05). Twelve months after surgical treatment, 17.33% of participants were inactive in comparison with 36% at 2 to 3 months after surgery. Analyses showed a significant interaction of time with age and sex, and failure to resume prediagnostic levels of leisure physical activity at 12 months after surgery was positively correlated with older age (61-80 + years; F1050 = 15.38; p < .001) and being a woman (0.05; F1050 = 11.02; p < .01).
Table 2.
Leisure time physical activity of the population of Stage 0-1A melanoma survivors (n = 75)
| Physical activity sustained > 15 min | Sex |
Age (yrs) |
||||
|---|---|---|---|---|---|---|
| Men | Women | 27-40 | 41-60 | 61-80 + | ||
| 2-3 months before diagnosis | mean min/wk (standard deviation) | n = 37 n (%) |
n = 38 n (%) |
n = 16 n (%) |
n = 31 n (%) |
n = 28 n (%) |
| Vigorous | 92 (18) | 22 (59) | 21 (55) | 15 (94) | 24 (77) | 4 (14) |
| Moderate-mild | 120 (48) | 15 (41) | 17 (45) | 1 (6) | 7 (23) | 24 (86) |
| Inactive < 60 min | 0 | 0 | 0 | 0 | 0 | |
| 2-3 months after surgery | ||||||
| Vigorous | 20 (5) | 6 (16)⁎ | 1 (2)⁎ | 5 (32)⁎ | 2 (6)⁎ | - |
| Moderate-mild | 101 (56) | 15 (41) | 26 (68)⁎ | 10 (62)⁎ | 18 (59)⁎ | 13 (64)⁎ |
| Inactive < 60 min | 16 (43)⁎ | 11 (30)⁎ | 1 (6) | 11 (35)⁎ | 15 (36)⁎ | |
| 12 months after surgery | ||||||
| Vigorous | 60 (12) | 19 (52) | 9 (24)† | 13 (75)† | 15 (50)† | - |
| Moderate-mild | 105 (61) | 18 (48) | 17 (45) | 4 (25)† | 12 (40)† | 19 (68)† |
| Inactive < 60 min | - | 12 (31)† | - | 3 (10)† | 9 (32)† | |
Significant differences from prediagnosis to 2 to 3 months after surgery; p < .05 for all age categories and both sexes for vigorous activity and inactivity, and women in the mild-moderate activity category.
Significant differences from prediagnosis to 12 months after surgery; p < .05 for vigorous exercise in all age categories, for moderate-mild in ages 27-40 years and 61-80 + years, and inactive for ages 41-60 years and 61-80 + years. Women had significant differences in vigorous activity and inactivity.
Preferred forms of exercise varied by age and sex. Women ages 61 to 80 years preferred to exercise in water with a class (49%), Zumba dance class (31%), and walks with a friend (20%). Men ages 61 to 80 years played golf (49%), went to the gym to work out (27%), and walked their dog (24%). The sex difference in preferred activities persisted among those ages 41 to 60 years with women preferring yoga class (63%), running (29%), and swimming (7%) and men playing golf (52%), going to the gym (35%), and biking (13%). Among those ages 27 to 40 years, there was no sex difference in the preferred activities of running (40%), biking (37%), and playing outdoor sports (e.g., tennis and golf; 23%).
All 12 inactive women indicated that they did not expect to change their leisure physical activity and offered the following free text responses: Stay out of the sun (n = 3), no energy (too tired/weak; n = 4), and no buddy (no one to remind me/do leisure physical activities with me; (n = 5).
Discussion
This study demonstrated a decrease in leisure physical activity after treatment for early stage melanoma that was positively correlated at 12 months after surgery with older age (61-80 + years) and being a woman. In general, women tend to be more aware of their risk of developing skin cancer and are more proactive with regard to sun protection than men. Although melanoma survivors may be aware of the harms of sun exposure and have already suffered the consequences of prior unprotected sun exposure, women may have been more likely to act on counseling by their dermatologist and avoid strong sunlight by limiting outdoor leisure physical activity. Because few adults practice all recommended behaviors, at-risk adults are provided with a choice of multiple sun protection behaviors (Buller et al., 2011). Dermatologists may simplify the recommendations for melanoma survivors by placing emphasis on a single behavior, especially if a single behavior reduces the patient’s resistance to perform another behavior. For example, the recommendation may be to stay in the shade, which makes wearing a hat less important and avoids patients’ objections to hats. Since the use of sunscreen often prolongs time in the sun and wearing less protective clothing (Passantino et al., 2013), which can increase the risk of sunburn (Robinson, 1992, Harris and Alberts, 2004), dermatologists may say to stay out of the sun.
The majority of Americans identify their physician as the primary source of advice concerning health information (Hesse et al., 2005); therefore, it is important for dermatologists to consider the overall health of melanoma survivors, especially older women who may limit their outdoor leisure physical activity to stay out of the sun. Sunny days encourage outdoor physical activity, which is beneficial to health in terms of obesity, diabetes, coronary disease, and bone health (a particular concern for older women). Dermatologists need to strike a balance among the three types of prevention strategies: Protection with sunscreen, avoidance by staying in the shade or indoors during peak hours of sun intensity and covering up with clothing, and the wellbeing of the patient.
Twelve months after surgery, inactivity was reported by 31% of this urban/suburban population of Stage 0-1A melanoma survivors and all were women. Melanoma survivors who enrolled in this study had no functional impairment and most had access to parks and walking/biking trails. The mobility of the participants and access to safe environments to perform leisure indoor and outdoor physical activity contributed to the < 68% inactivity reported by melanoma survivors in the 2000 National Health Interview Survey (Coups and Osteroff, 2005). Although the number of inactive women in this study was small, their qualitative responses indicated that older women prefer low impact exercise in classes that are usually indoors; however, their preference for walking with a friend may provide guidance to the dermatologist to recommend walking at any time of the day while wearing a hat, protective clothing, and sunscreen on exposed skin.
The National Health Interview Survey also found that 59% of melanoma survivors were overweight (Coups and Osteroff, 2005). Obesity and physical inactivity are at least as prevalent among cancer survivors as in the general population, and have been shown to increase after diagnosis for patients with breast cancer (Irwin et al., 2003). Since weight and height information was not obtained in this study, obesity was not assessed.
This study is not without limitations. The melanoma survivors in this study were of higher socioeconomic status than the general population of the United States; however, melanoma survivors are generally of higher socioeconomic status than the general population (Irwin et al., 2003, Jiang et al., 2015). The population was composed of Midwestern urban and suburban residents; therefore, the findings may not be generalizable to other locations. Furthermore, the data are self-reported, subject to potential bias (e.g., recall bias and socially desirable responses), and did not include assessments of weight and height. Future research should examine obesity among early stage melanoma survivors.
Conclusions
Dermatologists are in a position to promote a healthy lifestyle for melanoma survivors, especially older women who can be expected to live many more years and restrict leisure outdoor physical activity to comply with sun protection recommendations. Improving the overall wellbeing and quality of life of early stage melanoma survivors needs to emphasize the use of sun protection and physical activity throughout life to reduce obesity and maintain bone health.
Acknowledgement
Racheal Reavy, PhD, of the Biobehavioral Health and Prevention Research Center at The Pennsylvania State University in University Park, PA performed the statistical analysis.
Footnotes
Funding sources: This work was supported by grant number R01 CA154908 to June K. Robinson, MD from the National Cancer Institute.
Conflicts of interest: None.
References
- American Cancer Society Cancer treatment and survivorship statistics [Internet] 2016. http://www.cancer.org/research/cancerfactsstatistics/survivor-facts-figures [cited 2017 January 3]. Available from:
- American Cancer Society . American Cancer Society; Atlanta, GA: 2016. Cancer treatment and survivorship facts and figures 2016-2017. [Google Scholar]
- Buller D.B., Cokkinides V., Hall H.I., Hartman A.M., Saraiya M., Miller E. Prevalence of sunburn, sun protection, and indoor tanning behaviors among Americans: Review from national surveys and case studies of 3 states. J Am Acad Dermatol. 2011;65(5):S114. doi: 10.1016/j.jaad.2011.05.033. e111–4. [DOI] [PubMed] [Google Scholar]
- Coelho S.G., Hearing V.J. UVA tanning is involved in the increased incidence of skin cancers in fair-skinned young women. Pigment Cell Mel Res. 2010;23(1):57–63. doi: 10.1111/j.1755-148X.2009.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coups E.J., Osteroff J.S. A population-based estimate of the prevalence of behavioral risk factors among adult cancer survivors and noncancer controls. Prev Med. 2005;40(6):702–712. doi: 10.1016/j.ypmed.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Cust A.E., Armstrong B.K., Goumas C., Jenkins M.A., Schmid H., Hopper J.L. Sunbed use during adolescence and early adulthood is associated with increased risk of early-onset melanoma. Int J Cancer. 2011;128(10):2425–2435. doi: 10.1002/ijc.25576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denmark-Wahnefried W., Rogers L.Q., Alfano C.M., Thomson C.A., Courneya K.S., Meyerhardt J.A. Practical clinical interventions for diet, physical activity, and weight control in cancer survivors. CA Cancer J Clin. 2015;65:167–189. doi: 10.3322/caac.21265. [DOI] [PubMed] [Google Scholar]
- Godin G., Shephard R.J. A simple method to assess exercise behavior in the community. Can J Appl Spt Sci. 1985;10(3):141–146. [PubMed] [Google Scholar]
- Green A.C., Williams G.M., Logan V., Strutton G.M. Reduced melanoma after regular sunscreen use: Randomized trial follow-up. J Clin Oncol. 2010;29:257–263. doi: 10.1200/JCO.2010.28.7078. [DOI] [PubMed] [Google Scholar]
- Harris R.B., Alberts D.S. Strategies for skin cancer prevention. Int J Dermatol. 2004;43(4):243–251. doi: 10.1111/j.1365-4632.2004.01966.x. [DOI] [PubMed] [Google Scholar]
- Hesse B.W., Nelson D.E., Kreps G.L., Croyle R.T., Arora N.K., Rimer B.K. Trust and sources of health information: the impact of the Internet and its implications for health care providers: Findings from the first Health Information National Trends Survey. Arch Intern Med. 2005;165:2618–2624. doi: 10.1001/archinte.165.22.2618. [DOI] [PubMed] [Google Scholar]
- Holman D.M., Freeman M.B., Shoemaker M.L. Trends in melanoma incidence among non-Hispanic Whites in the United States, 2005 to 2014. JAMA Dermatol. 2018;154(3):361–362. doi: 10.1001/jamadermatol.2017.5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin M.L., Crumley D., McTiernan A., Bernstein L., Baumgartner R., Gilliland F.D. Physical activity levels before and after a diagnosis of breast carcinoma: The Health, Eating, Activity, and Lifestyle (HEAL) study. Cancer. 2003;97(7):1746–1757. doi: 10.1002/cncr.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang A.J., Rambhatla P.V., Eide M.J. Socioeconomic and lifestyle factors and melanoma: A systematic review. Br J Dermatol. 2015;172:885–915. doi: 10.1111/bjd.13500. [DOI] [PubMed] [Google Scholar]
- King A.C., Hekler E.B., Grieco L.A., Winter S.J., Sheats J.L., Buman M.P. Harnessing different motivational frames via mobile phones to promote daily physical activity and reduce sedentary behavior in aging adults. PLoS One. 2013;8(4) doi: 10.1371/journal.pone.0062613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najita J.S., Sweeter S.M., Geller A.C., Gershenwald J.E., Zelen M., Lee S.J. Sex differences in age at primary melanoma diagnosis in a population-based analysis (US Surveillance, Epidemiology, and End Results, 2005-2011) J Investig Dermatol. 2016;136(7):1894–1897. doi: 10.1016/j.jid.2016.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passantino L., Costa M., Matsui M. Skin cancer prevention strategies. In: Vereecken P., editor. Highlights in Skin Cancer [Internet] 2013. https://www.intechopen.com/books/highlights-in280skin-cancer/skin-cancer-prevention-strategies [cited 2017 January 3]. Available from: [Google Scholar]
- Robinson J.K. Compensation strategies in sun protection behaviors by a population with nonmelanoma skin cancer. Prev Med. 1992;21(6):754–765. doi: 10.1016/0091-7435(92)90082-s. [DOI] [PubMed] [Google Scholar]
- Robinson J.K., Wayne J.D., Martini M.C., Hultgren B.A., Mallett K.A., Turrisi R. Early detection of new melanomas by patients with melanoma and their partners using a structured skin self-examination skills training intervention: A randomized clinical trial. JAMA Dermatol. 2016;152(9):979–985. doi: 10.1001/jamadermatol.2016.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman D.C., Green A.C., Olsen C.M. The growing burden of invasive melanoma: projections of incidence rates and numbers of new cases in six susceptible populations through 2013. J Investig Dermatol. 2016;136:1161–1171. doi: 10.1016/j.jid.2016.01.035. [DOI] [PubMed] [Google Scholar]