Abstract
Melasma is a common, therapeutically challenging, and universally relapsing disorder of hyperpigmentation that is most often observed in women and individuals with Fitzpatrick Skin Types III through VI. The pathogenesis of melasma is complex and protean. Contributing factors that are often implicated in the etiopathogenesis of this condition include a genetic predisposition, intense ultraviolet radiation exposure, and hormonal influences. Therapeutic interventions for melasma include a multimodality approach incorporating photoprotection agents, topical and oral skin lighteners, and resurfacing procedures. Given our expanding knowledge of the pathogenesis of melasma, new and effective treatments are expanding our therapeutic armamentarium. This article reviews new and emerging oral and topical treatments for melasma.
Keywords: Melasma, photoprotection, lightening agents, oral treatments, topical treatments, new and emerging
Introduction
Melasma is a common acquired disorder of hyperpigmentation that is characterized by irregular light to dark brown patches on the forehead, cheeks, upper lip, and chin areas of the face. Prevalence rates vary from 1% to 50% in high-risk populations (Grimes, 1995, Grimes, 2009, Halder et al., 1983, Kim et al., 2007, Moin et al., 2006, Werlinger et al., 2007). High-risk populations include individuals with darker skin types, pregnant women, and those residing in global locations with intense ultraviolet (UV) exposure.
Patterns of distribution of melasma include a centro-facial, malar, and mandibular pattern with the centro-facial distribution as the most common. In some instances, the neck, extensor arms, and upper back are also affected. These nonfacial areas are most commonly observed in menopausal women. Recent studies have documented an increase in erythema and telangiectasias in the affected areas, which suggests a vascular component for this condition (Kim et al., 2007). Melasma occurs in all races and ethnicities, but the overwhelming majority of patients are women and individuals with Fitzpatrick skin types IV through VI, including Hispanic, Asian, and individuals of African descent (Halder et al., 1983, Kang, 2012, Kim et al., 2007, Moin et al., 2006, Rodrigues and Pandya, 2015). Men comprise the minority of patients with melasma, and account for < 20% of cases. The clinical and histologic features of melasma in men are similar to those in women as reported in the literature (Sarkar et al., 2018, Vachiramon et al., 2012). One study reported low testosterone levels in men with melasma (Sarkar et al., 2018).
Myriad quality-of-life studies report on the emotional turmoil and psychological devastation that is experienced by affected individuals (Balkrishnan et al., 2003, Ikino et al., 2015, Pawaskar et al., 2007). Patients experience significant emotional impact and often feel bothered, frustrated, embarrassed, and depressed about their skin appearance (Ikino et al., 2015).
Histologic features of melasma include an increase in the content of both epidermal and dermal melanin, but the quantity varies with the intensity of hyperpigmentation. In addition, most studies show no quantitative increase in melanocytes; however, the cells are enlarged with prominent and elongated dendrites and more abundant melanosomes. Additional features of the involved skin include solar elastosis and increased mast cells, dermal blood vessels, and expression of vascular endothelial growth factor (Grimes et al., 2005, Kang et al., 2002).
The pathogenesis of melasma is complex. Multiple pathways have been implicated in the induction of hyperpigmentation. Etiologic factors include a genetic predisposition, UV light exposure, and hormonal influences. Increased expression of stem cell factor, c-Kit, and α-melanocyte-stimulating hormone have been reported in the lesional skin (Kang et al., 2006, Lee et al., 2017). Recent studies suggest that patients with melasma express markers, which suggests increased oxidative stress (Seçkin et al., 2014). Alterations in the Wnt pathway and abnormal barrier function have also been documented (Lee, 2015). The observations of solar elastosis, increased mast cells, vascular defects, barrier dysfunction, and fatty acid abnormalities suggest that melasma may well represent a phenotype of photodamage (Passeron and Picardo, 2018).
From a historical perspective, commonly used agents for the treatment of melasma include hydroquinone, azelaic acid, kojic acid, glycolic acid, salicylic acid, and tretinoin. Of these treatments, hydroquinone remains the gold standard. Multiple studies suggest that combination formulations offer the best results (Halder et al., 1983, Kim et al., 2007, Moin et al., 2006, Werlinger et al., 2007). These triple combination formulas usually contain hydroquinone, a retinoid, and a corticosteroid in varying concentrations. The aforementioned commonly used therapies are often associated with universal relapses. Second-line treatments, such as chemical peels and lasers, are efficacious in some patients, but these approaches can be associated with acute and long-term complications, particularly in individuals with darker skin types. Given the global negative impact of melasma on the quality of life, a quest to find more efficacious treatments that offer sustained long-term remission for patients with this frustrating and therapeutically challenging disorder is ongoing.
Therapeutic interventions
The treatment of melasma should include a multimodality approach that incorporates photoprotective agents, antioxidant treatments, skin lighteners, exfoliants, and resurfacing procedures, as needed. Evidence-based studies suggest that first line therapies for melasma encompass intense photoprotection and topical lightening agents (Jutley et al., 2014, Rivas and Pandya, 2013; Sarkar et al., 2013; Sarma et al., 2017). Additionally, many studies have addressed the safety and efficacy of chemical peels and laser/light sources for pigment reduction in melasma. Such procedures are most often considered second- and third-line therapeutic approaches.
Lasers should be used with care and caution given the high rate of recurrence of melasma. In addition, laser intervention is often complicated by postinflammatory hyperpigmentation in individuals with darker skin types.
This review addresses new and emerging oral and topical agents for the treatment of melasma.
Photoprotection
Daily photoprotection is key in the management of this therapeutically challenging and universally relapsing disorder. The use of broad spectrum sunscreens is essential. Caregivers of patients with melasma often report the common occurrence of rapid and frequent relapses after intense UV exposure. Lakhdar et al. (2007) assessed the role of a broad-spectrum sunscreen in the prevention and treatment of chloasma in pregnant women during a 12-month clinical trial. Of the 185 patients who completed the study, only five new cases of chloasma (melasma) were noted, an occurrence rate of 2.7% and much lower than the 53% previously observed during pregnancy.
In addition to the deleterious impact of UV light as a trigger and relapsing factor, recent studies have implicated a role for visible light (Duteil et al., 2014, Duteil et al., 2017, Mahmoud et al., 2010). Visible blue light has been shown recently to stimulate opsin-3, which activates the melanogenesis-associated transcription factor and other melanogenic enzymes such as tyrosinase and dopachrome tautomersae (Regazzetti et al., 2018). Tyrosinase and dopachrome form a protein complex that is mainly generated in the melanocytes of dark-skinned individuals. Hence, the sustained effects of visible blue light irradiation in individuals with darker skin types are thought to induce long-lasting hyperpigmentation in individuals with skin type III and above (Regazzetti et al., 2018). Therefore, photoprotection that incorporates visible light, such as iron oxide sunscreens, is essential. There are few readily available iron oxide formulations, and such formulations optimally block UV light in the visible spectrum.
Castanedo-Cazares et al. (2014) compared the effects of a UV-visible light sunscreen to a UV-only sunscreen, both with sun protection factor ≥ 50. Sixty-eight patients were included in this 8-week study (Castanedo-Cazares et al., 2014). The UV-only sunscreen contained mexoryl SX, titanium dioxide, octocrylene, Tinasorb–S, and avobenzone. The UV-visible light sunscreen contained the same regimen plus iron oxide. Both groups received hydroquinone 4% daily. Significantly greater improvement was observed in the group that was treated with the UV-visible light regimen.
In a 6-month study of 40 patients, Boukari et al. (2015) assessed the efficacy of a UV–visible light sunscreen that contained iron oxide. The control group used the same UV sunscreen that provided the same UV-B and UV-A protection without the iron oxide. A significantly greater reduction in Melasma Area Severity Index (MASI) score occurred in patients treated with the combination UV-visible light formula. Both studies document the beneficial effects of visible light protection in patients with melasma.
Polypodium leucotomos
There has been much interest recently in the use of Polypodium leucotomos (PL) as an adjunct photoprotective agent in melasma. Polypodium is a fern of the Polypodiaceae family that is unique to Central and South America. PL’s mechanisms of action include the promotion of the p53 suppressor gene expression, modulation of inflammatory cytokines, upregulation of endogenous antioxidant systems, and blockade of UV radiation-induced cyclooxygenase–2 expression (Nestor et al., 2014, Siscovick et al., 2008).
Several recent studies have documented a beneficial effect in patients with melasma (Goh et al., 2018, Martin et al., 2013). In a randomized, placebo-controlled study of 40 patients, Martin et al. (2013) assessed the efficacy of PL 240 mg twice daily versus placebo. The authors reported a statistically significant effect of PL compared with placebo. In a recent double-blind, placebo-controlled trial, Goh et al. (2018) reported the effectiveness of PL in melasma. Patients were randomized to receive either oral PL 240 mg twice daily or placebo for 12 weeks. The active and placebo groups were also treated with a broad spectrum sunscreen and hydroquinone 4% daily. The group treated with PL achieved a significantly greater reduction in MASI score at 56 and 84 days of treatment (Goh et al., 2018).
Frequently used topical agents
A variety of topical agents have been used for melasma. Historically, hydroquinone has been the gold standard. Others agents include azelaic acid, kojic acid, retinoid treatments, niacinamide, corticosteroid medications, salicylic and glycolic acid, arbutin, resveratrol, and resorcinol. These agents and their mechanisms are cited in Table 1 (Al-Niaimi and Chiang, 2017, Birk, 1985, Bissett, 2002, De Caprio, 1999, Deo et al., 2013, Glowka et al., 2018, Grimes, 1995, Grimes, 2009, Hashim et al., 2018; Huh et al., 2010; Kang, 2005; Keeling et al., 2008; Lajis et al., 2012, Menter, 2004, Monteiro et al., 2013, Navarrete-Solís et al., 2011, Niwano et al., 2018, Nordlund et al., 2006, Olejnik et al., 2018, Paine et al., 2001, Picardo and Carrera, 2007, Pires et al., 2018, Schulte et al., 2015, Tse, 2010; Videira et al., 2013; Wargniez et al., 2017, Wohlrab and Kreft, 2014). Evidence-based studies have suggested that combination products that contain hydroquinone 4%, tretinoin, and a steroid produce the best response (Jutley et al., 2014, Rivas and Pandya, 2013; Sarkar, 2013; Sarma et al., 2017). (See Table 2.)
Table 1.
Common lightening agents for melasma
| Name | Mechanism of action | Side effects | Reference |
|---|---|---|---|
| Hydroquinone | Tyrosinase inhibition | Erythema, irritation, exogenous ochronosis | De Caprio, 1999, Grimes, 2009, Nordlund et al., 2006, Tse, 2010 |
| Azelaic acid | Tyrosinase inhibition | Stinging, burning, itching, dryness | Hashim et al., 2018, Schulte et al., 2015 |
| Kojic acid | Tyrosinase inhibition | Irritation, contact dermatitis | Deo et al., 2013, Lajis et al., 2012, Monteiro et al., 2013 |
| Ascorbic acid | Inhibition of reactive oxygen species | No significant adverse event | Al-Niaimi and Chiang, 2017, Pires et al., 2018 |
| Retinoids | Downregulation of Tyrosinase | Irritant reaction, dryness, hyperpigmentation | Kang, 2005 |
| Corticosteroid treatments | Antiinflammatory and nonselective inhibition of melanogenesis | Telangiectasias, epidermal atrophy, steroid-induced acne, striae, hypopigmentation | Menter, 2004 |
| Niacinamide | Inhibition of melanasome transfer | Irritation | Bissett, 2002, Navarrete-Solís et al., 2011, Wohlrab and Kreft, 2014 |
| Licorice | Melanin dispersion, tyrosinase inhibition | No significant adverse event | Picardo and Carrera, 2007; Videira et al., 2013 |
| Undecylenoyl phenylalanine | Antagonist of α-melanocyte-stimulating hormone, β-adrenergic, stem cell receptors | No significant adverse event | Glowka et al., 2018, Olejnik et al., 2018 |
| 4-N-butylresorcinol | Tyrosinase inhibition, antioxidant, antiinflammatory | Mild erythema and itching | Huh et al., 2010; Wargniez et al., 2017 |
| Soybean | Inhibits melanosome transfer to keratinocytes | No significant adverse event | Birk, 1985, Paine et al., 2001 |
| Arbutin | Inhibition of tyrosinase | Skin irritation | Sarma et al., 2017 |
| Glucosamine | Inhibition of tyrosinase activation | Skin rash | Niwano et al., 2018 |
| Mequinol | Inhibition of tyrosinase | Skin irritation, redness, peeling | Keeling et al., 2008 |
Table 2.
New oral and Topical Treatments.
| Agent | Reference | Mechanism of action | Dosing | Study outcomes | Adverse events | ||
|---|---|---|---|---|---|---|---|
| Photoprotection | Topical | Iron oxide sunscreens | Boukari et al., 2015, Castanedo-Cazares et al., 2014, Duteil et al., 2017, Lakhdar et al., 2007, Mahmoud et al., 2010, Regazzetti et al., 2018 | Blocks blue visible light | SPF ≥ 50 | UV-visible light regimen had significant improvement | None |
| Oral | Polypodium leucotomos | Goh et al., 2018, Martin et al., 2013, Nestor et al., 2014, Siscovick et al., 2008 | Promotion of p53 suppressor gene expression/modulation of inflammatory cytokines ↑ endogenous antioxidant systems blockade of UV radiation induced cyclooxygenase-2 expression |
240 mg BID | Beneficial effect Greater reduction in MASI |
Mild gastrointestinal upsets | |
| Lightening agents | Oral and topical | Tranexamic acid | Banihashemi et al., 2015; Del Rosario et al., 2018; Ebrahimi and Naeini, 2014, George, 2016, Kim et al., 2016, Lee et al., 2016, Lee et al., 2017, Na et al., 2013, Perper et al., 2017, Taraz et al., 2017, Wu et al., 2012 | Blocks conversion of plasminogen to plasmin Blocks binding of plasminogen to keratinocytes ↓ arachidonic acid release, prostaglandin synthesis and FGF ↓ melanin synthesis ↓ mast cells ↓ angiogenesis |
2-5% BID 250 mg BID |
Global studies document significant reduction in MASI score | Oral: mild gastrointestinal discomfort, hypomenorrhea, allergic skin rashes, alopecia, and mild elevations in alanine transaminase Topical: erythema, scaling, dryness Propensity to induce thromboembolic phenomena |
| Melatonin | Hamadi et al., 2009, Ryoo et al., 2001 | Potent antioxidant/free radical scavenger ↑ super oxide dismutase, glutathione reductase, glutathione peroxidase ↓ α-MSH receptors |
5% BID 3 mg daily |
Decreased MASI, malondialdehyde decreased GSH levels increased |
Not reported | ||
| GSH | Handog et al., 2016, Sonthalia et al., 2016, Watanabe et al., 2014 | decreases tyrosinase skews conversion of eumelanin to pheomelanin |
2% daily 500 mg daily |
Melanin index significantly reduced | Intravenous: Stevens-Johnson Syndrome, anaphylaxis Oral and topical: none |
||
| Topical | Cysteamine | Besouw et al., 2013, Mansouri et al., 2015 | Radio protector (via direct scavenging effects of hydroxy radicals) Tyrosinase peroxidase inhibition |
5% daily | Reduced MASI compared to placebo | None reported | |
| Pigment-correcting Serum | Makino et al., 2016 | Melanocyte activation, melanosome development, melanin synthesis, melanosome transfer keratinocyte differentiation and desquation |
Comparable efficacy to hydroquinone | Mild irritation | |||
| Methimazole | Kasraee et al., 2005, Kasraee et al., 2008 | Potent peroxidase inhibitor blocks melanin synthesis |
5% daily | No significant changes in serum TSH, free thyroxine, free triiodothymine levels | Minimal cutaneous side effects | ||
| Flutamide | Adalatkhah et al., 2011 | Blocks action of endogenous/ exogenous testosterone by binding to androgen receptor | 1% daily | MASI/colorimetry effects similar to hydroquinone 4% Patient satisfaction scores significantly high |
None | ||
BID, twice daily; FGF, fibroblast growth factor; GSH, glutathione; MASI, Melasma Area Severity Index; MSH, melanocyte-stimulating hormone; SPF, sun protection factor; TSH, thyroid-stimulating hormone; UV, ultraviolet
Hydroquinone is used globally for melasma (De Caprio, 1999, Grimes, 2009, Nordlund et al., 2006, Tse, 2010), and although used for more than 60 years, it remains our most efficacious topical agent. Hydroquinone inhibits tyrosinase, which is the rate-limiting enzyme for pigment production, and is well tolerated in most patients. The most common side effect is irritant contact dermatitis.
The majority of clinical trials that have assessed the efficacy and safety of hydroquinone have not extended beyond 6 months. Nonetheless, physicians have enormous variability in prescribing practices for hydroquinone, so there is no universal standard of treatment. In the authors’ experience, hydroquinone can be safely and effectively used beyond 6 months. However, optimal results are often best achieved using a 6-month rotational algorithm that cycles on and off hydroquinone. Other lightening agents are often essential to maintain the results achieved with hydroquinone use. Hydroquinone should not be used in pregnant or breastfeeding women due to its Category C characterization.
Hydroquinone has been banned in some countries due to concerns of ochronosis and possible cases of depigmentation. Ochronosis is characterized by hyperpigmented lichenoid, which are cavier-like papules that appear in cutaneous areas treated with hydroquinone. Ochronosis was first reported in Africa and is usually caused by the long-term use of high concentrations of hydroquinone with inadequate sun protection (Bhattar et al., 2015, Grimes, 2009). In contrast to common case reports from Africa, this condition is uncommon in the United States where most cases are secondary to the prolonged use of over-the-counter, low-concentration, cosmetic formulations of 2% hydroquinone. Cases of depigmentation caused by hydroquinone are rare.
Additional concerns with regard to the safety of hydroquinone is based on studies that suggest that benzene, which is a known leukemogenic agent, is metabolized into hydroquinone as a major by-product (Norlund et al., 2006). Benzene metabolites, including hydroquinone, phenol, or benzoquinone, do not exhibit the potency and level of the myelotoxic effect of benzene. In addition, individuals who are occupationally exposed to long-term hydroquinone have not demonstrated myelotoxic changes (De Caprio, 1999, Tse, 2010). Hydroquinone has now been used and manufactured for more than 60 years, and a review of the literature reveals no cases of skin cancer, internal malignancies, or liver damage related to the topical application of hydroquinone for skin lightening.
New oral and topical treatments
Tranexamic acid
Tranexamic acid (TA), a synthetic derivative of lysine, is a fibrinolytic agent that blocks the conversion of plasminogen to plasmin and thereby impedes the binding of plasminogen to keratinocytes (Fig. 1). Downstream effects include diminished arachidonic acid release and decreased prostaglandin and fibroblast growth factor synthesis. Prostaglandins and fibroblast growth factor both stimulate melanin synthesis. TA also decreases mast cells and angiogenesis (Kim et al., 2015). Nijo Sadako first reported the efficacy of TA in 1979 in a patient with melasma treated for chronic urticaria (George, 2016).
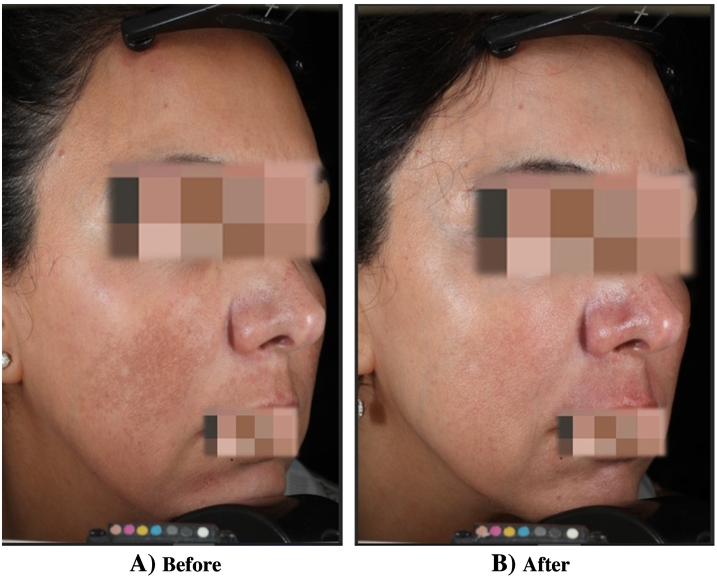
Fig. 1.
Melasma (A) before treatment and (B) after treatment with topical 3% tranexamic acid and hydroquinone. Significant improvement in areas of hyperpigmentation of the cheek and upper lip.
TA is currently used via a spectrum of delivery routes including oral, topical, intradermal, and microneedling (Taraz et al., 2017). The current oral dosing is significantly less than doses used to treat hemophilia, heavy menstrual bleeding, or other hemorrhagic conditions. Oral dosing for melasma is, on average, 250 mg twice daily compared with 3900 mg daily for bleeding diatheses (Taraz et al., 2017). Multiple studies have documented the efficacy of TA in patients with melasma (Banihashemi et al., 2015, Del Rosario et al., 2018, Ebrahimi and Naeini, 2014; Kim et al., 2017; Lee et al., 2016, Na et al., 2013, Perper et al., 2017, Wu et al., 2012).
TA has been predominantly used in Asian patients and was recently evaluated in a cohort of patients in the United States. Del Rosario et al. (2018) assessed the efficacy of TA 250 mg twice daily versus placebo in a 3-month study, followed by 3 months of sunscreen only. Thirty-nine patients completed the trial. At 3 months, there was a 49% reduction in MASI score versus 18% for the placebo control group. No serious adverse events were reported (Del Rosario et al., 2018).
The largest retrospective study of TA treatment was conducted in Singapore. The authors reviewed data from 561 patients with melasma who were treated with TA, and improvement was noted in 90% of patients. Adverse events were reported in 40 patients (7.1%), and most side effects were mild. However, one patient developed deep vein thrombosis and was later discovered to have familial protein S deficiency. There was a personal history of spontaneous miscarriage and a family history of thromboembolic issues in two siblings (Lee et al., 2016).
General concerns linger with regard to the safety profile given TA’s propensity to induce thromboembolic phenomena. Hence, TA is contraindicated in patients with clotting disorders or a history of thromboembolism (Kim et al., 2017). Major side effects in melasma clinical trials are rarely reported. Other adverse events related to TA use include mild gastrointestinal discomfort, hypomenorrhea, allergic skin rashes, alopecia, and mild elevations in alanine transaminase levels. Oral TA should be prescribed with care and caution. A detailed history should be taken for each patient to exclude individuals at risk for untoward complications.
Several studies have addressed the efficacy and safety of topical TA (Banihashemi et al., 2015, Ebrahimi and Naeini, 2014, Kim et al., 2016). In a 12-week, split-face, prospective trial, Banihashemi et al. (2015) compared the efficacy of a liposomal 5% TA formulation to hydroquinone 4%. Both treatments showed significant clinical improvement without significant differences between the two groups, which suggests comparable efficacy.
In a separate split-face study, a TA 3% suspension was applied to one side of the face and a suspension with hydroquinone 2%, dexamethasone 0.01%, and vitamin C to the opposite side. Both formulations showed significant improvement, which suggests similar topical efficacy for hydroquinone-based formulations (Ebrahimi and Naeini, 2014). Topical side effects included erythema, scaling, irritation, and dryness (Banihashemi et al., 2015, Ebrahimi and Naeini, 2014, Kim et al., 2016).
Melatonin
The hormone melatonin, which is secreted by the pineal gland, is a potent antioxidant and free-radical scavenger that stimulates several antioxidant enzymes, including superoxide dismutase, glutathione reductase, and glutathione peroxidase. Melatonin also inhibits α-melanocyte-stimulating hormone receptors. Oral and topical melatonin were evaluated in a series of 36 patients with melasma and 10 healthy control participants. Patients were randomized to several groups, including topical melatonin only, topical melatonin plus screen, and a combination of topical and oral melatonin and hydroquinone 4% for 90 days. At 90 days, all patients with melasma demonstrated a significant reduction in MASI score. In addition, malondialdehyde, which is a measure of oxidative stress, decreased and glutathione (GSH) levels increased and suggest significant improvement in oxidative stress. These seminal observations also suggest that additional studies to assess the efficacy and safety of melatonin in patients with melasma are warranted (Hamadi et al., 2009l Ryoo et al., 2001).
Glutathione
GSH is one of the most powerful endogenous antioxidants produced by cells in the human body and is a tripeptide of glutamate, cysteine, and glycine. Mechanisms that induce lightening of the skin include inhibition of tyrosinase and the ability to skew production of eumelanin to pheomelanin. There has been enormous recent publicity with regard to the use of intravenous GSH for general skin lightening (Sonthalia et al., 2016). Multiple articles have been published in the lay press on the use of GSH for a variety of diseases including melasma. Intravenous use of GSH has been associated with severe life-threatening reactions including Stevens–Johnson syndrome and anaphylaxis.
Several studies have evaluated oral and topical GSH for general skin lightening. In an 8-week study, Handog et al. (2016) treated 30 healthy Filipino women with a 500 mg buccal glutathione lozenge. The melanin index showed a significant reduction, and global assessments reported moderate lightening in 90% of subjects (Handog et al., 2016). A topical glutathione 2% suspension was assessed in a randomized, double-blind, split-face, 10-week study, in which GSH was applied to one side and a placebo to the opposite side. The melanin index was significantly reduced in the GSH-treated side (Watanabe et al., 2014). Oral and topical GSH are well tolerated with no significant adverse events. The results of these studies suggest the need to test oral and topical GSH for melasma.
New topical agents
Cysteamine
Cysteamine hydrochloride (ß–mercaptoethylanine hydrochloride) is naturally produced in the human body and is a degradation product of the amino acid L-cysteine. Cysteamine is also a radio protector that protects cells from the mutagenic and other lethal effects of ionizing radiation via its direct scavenging effects on hydroxy radicals (Besouw et al., 2013).
Recently, several studies have documented the efficacy of cysteamine in patients with melasma. In a randomized, double-blind trial of 40 patients, a significant improvement in melasma lesions was observed compared with patients who were treated with placebo. Mansouri et al. (2015) assessed the efficacy of 5% cysteamine in 50 patients with melasma. Cysteamine induced significant reductions in MASI scores at 16 weeks compared with placebo.
Pigment-correcting serum
Pigment-correcting serum was recently reformulated to address the multiple pathways involved in the induction of hyperpigmentation. These pathways include melanocyte activation, melanosome development, melanin synthesis, melanosome transfer, and keratinocyte differentiation and desquamation. Pigment-correcting serum contains TA, tetrapeptides, plankton extracts, niacinamide, phenylethyl resorcinol, and undecylenol phenylalanine. In a randomized, double-blind comparison trial of 43 patients, pigment-correcting serum was compared with hydroquinone 4% and showed overall comparable efficacy to hydroquinone in patients with melasma and postinflammatory hyperpigmentation (Makino et al., 2016).
Methimazole
Methimazole is an oral anti-thyroid medication used to treat patients with hyperthyroidism and has been shown to cause depigmentation when applied topically. Methimazole has been used in patients with melasma and postinflammatory hyperpigmentation and causes significant skin lightening. Methimazole is a potent peroxidase inhibitor that blocks melanin synthesis. Absorption studies of topically applied methimazole 5% revealed minimal detectable serum levels and no abnormalities in thyroid function tests (Kasraee et al., 2005, Kasraee et al., 2008). Methimazole 5% was applied daily in 20 patients with epidermal melasma and did not induce any significant changes in serum thyroid-stimulating hormone, free thyroxine, and free triiodothyronine levels. Methimazole was well tolerated with minimal cutaneous side effects. However, Kasraee et al. (2008) recommend that methimazole only be applied to areas affected by melasma and should not be used as a general cosmetic lightening agent.
Flutamide
A recent paper evaluated the efficacy of topical flutamide in patients with melasma. Flutamide is a nonsteroidal antiandrogen that blocks the action of endogenous and exogenous testosterone by binding to the androgen receptor. Seventy-four women were enrolled in a parallel, randomized, 16-week trial that compared once daily flutamide 1% with hydroquinone 4%. Skin hyperpigmentation improved, and the results of MASI and colorimetry scores revealed similar efficacy for flutamide and hydroquinone 4%. However, patient satisfaction scores were significantly higher in the flutamide group (Adalatkhah and Sadeghi-Bazargani, 2015).
Conclusions
Melasma remains a chronic, therapeutically challenging, and universally relapsing condition. This psychologically devastating disorder should be treated with a multimodality approach that incorporates photoprotective agents, antioxidant treatments, skin lighteners, exfoliants, and resurfacing procedures in severe cases. A myriad of new oral, topical, and combination therapies for melasma have been introduced to expand our repertoire of therapies, and warrant additional trials to substantiate their efficacy and safety.
References
- Adalatkhah H., Pourfarzi F., Sadeghi-Bazargani H. Flutamide versus a cyproterone acetate-ethinyl estradiol combination in moderate acne: a pilot randomized clinical trial. Clin Cosmet Investig Dermatol. 2011;4:117. doi: 10.2147/CCID.S20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adalatkhah H., Sadeghi-Bazargani H. The first clinical experience on efficacy of topical flutamide on melasma compared with topical hydroquinone: A randomized clinical trial. Drug design, development and therapy. Drug Des Devel Ther. 2015;9:4219–4225. doi: 10.2147/DDDT.S80713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Niaimi F., Chiang N.Y.Z. Topical vitamin C and the skin: Mechanisms of action and clinical applications. J Clin Aesthet Dermatol. 2017;10(7):14–17. [PMC free article] [PubMed] [Google Scholar]
- Balkrishnan R., McMichael A.J., Camacho F.T., Saltzberg F., Housman T.S., Grummer S. Development and validation of a health-related quality of life instrument for women with melasma. Br J Dermatol. 2003;149(3):572–577. doi: 10.1046/j.1365-2133.2003.05419.x. [DOI] [PubMed] [Google Scholar]
- Banihashemi M., Zabolinejad N., Jaafari M.R., Salehi M., Jabari A. Comparison of therapeutic effects of liposomal tranexamic acid and conventional hydroquinone on melasma. J Cosmet Dermatol. 2015;14(3):174–177. doi: 10.1111/jocd.12152. [DOI] [PubMed] [Google Scholar]
- Besouw M., Masereeuw R., van den Heuvel L., Levtchenko E. Cysteamine: An old drug with new potential. Drug Discov Today. 2013;18(15-16):785–792. doi: 10.1016/j.drudis.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Bhattar P.A., Zawar V.P., Godse K.V., Patil S.P., Nadkarni N.J., Gautam M.M. Exogenous ochronosis. Indian J Dermatol. 2015;60(6):537–543. doi: 10.4103/0019-5154.169122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk Y. The Bowman-Birk inhibitor. Trypsin- and chymotrypsin-inhibitor from soybeans. Int J Pept Protein Res. 1985;25(2):113–131. doi: 10.1111/j.1399-3011.1985.tb02155.x. [DOI] [PubMed] [Google Scholar]
- Bissett D. Topical niacinamide and barrier enhancement. Cutis. 2002;70:8–12. [PubMed] [Google Scholar]
- Boukari F., Jourdan E., Fontas E., Montaudié H., Castela E., Lacour J.P. Prevention of melasma relapses with sunscreen combining protection against UV and short wavelengths of visible light: A prospective randomized comparative trial. J Am Acad Dermatol. 2015;72(1):189–190. doi: 10.1016/j.jaad.2014.08.023. e1. [DOI] [PubMed] [Google Scholar]
- Castanedo-Cazares J.P., Hernandez-Blanco D., Carlos-Ortega B., Fuentes-Ahumada C., Torres-Álvarez B. Visible-light photoprotection in melasma. Photodermatol Photoimmunol Photomed. 2014;30(1):35–42. doi: 10.1111/phpp.12086. [DOI] [PubMed] [Google Scholar]
- De Caprio A.P. The toxicology of hydroquinone-relevance to occupational and environmental exposure. Crit Rev Toxicol. 1999;29:283–330. doi: 10.1080/10408449991349221. [DOI] [PubMed] [Google Scholar]
- Del Rosario E., Florez-Pollack S., Zapata L., Jr., Hernandez K., Tovar-Garza A., Rodrigues M. Randomized, placebo-controlled, double-blind study of oral tranexamic acid in the treatment of moderate to severe melasma. J Am Acad Dermatol. 2018;78(2):363–369. doi: 10.1016/j.jaad.2017.09.053. [DOI] [PubMed] [Google Scholar]
- Deo K.S., Dash K.N., Sharma Y.K., Virmani N.C., Oberai C. Kojic acid vis-a-vis its combinations with hydroquinone and betamethasone valerate in melasma: A randomized, single blind, comparative study of efficacy and safety. Indian J Dermatol. 2013;58(4):281–285. doi: 10.4103/0019-5154.113940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi B., Naeini F.F. Topical tranexamic acid as a promising treatment for melasma. J Res Med Sci. 2014;19(8):753–757. [PMC free article] [PubMed] [Google Scholar]
- Duteil L., Cardot-Leccia N., Queille-Roussel C., Maubert Y., Harmelin Y., Boukari F. Differences in visible light-induced pigmentation according to wavelengths: A clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014;27(5):822–826. doi: 10.1111/pcmr.12273. [DOI] [PubMed] [Google Scholar]
- Duteil L., Esdaile J., Maubert Y., Cathelineau A.C., Bouloc A., Queille-Roussel C. A method to assess the protective efficacy of sunscreens against visible light-induced pigmentation. Photodermatol Photoimmunol Photomed. 2017;33(5):260–266. doi: 10.1111/phpp.12325. [DOI] [PubMed] [Google Scholar]
- George A. Tranexamic acid: An emerging depigmenting agent. Pigment Int. 2016;3(2):66–71. [Google Scholar]
- Glowka A., Olejnik A., Nowak I. The release studies of undecylenoyl phenylalanine through cellulose based membranes. Saudi Pharm J. 2018;26:709–718. doi: 10.1016/j.jsps.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh C.L., Chuah S.Y., Tien S., Thng G., Vitale M.A., Delgado-Rubin A. Double-blind, placebo-controlled trial to evaluate the effectiveness of Polypodium leucotomos extract in the treatment of melasma in Asian skin: A pilot study. J Clin Aesthet Dermatol. 2018;11(3):14–19. [PMC free article] [PubMed] [Google Scholar]
- Grimes P.E. Melasma: Etiologic and therapeutic considerations. Arch Dermatol. 1995;131(12):1453–1457. doi: 10.1001/archderm.131.12.1453. [DOI] [PubMed] [Google Scholar]
- Grimes P.E. Management of hyperpigmentation in darker racial ethnic groups. Semin Cutan Med Surg. 2009;28(2):77–85. doi: 10.1016/j.sder.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Grimes P.E., Yamada N., Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27(2):96–101. doi: 10.1097/01.dad.0000154419.18653.2e. [DOI] [PubMed] [Google Scholar]
- Halder R.M., Grimes P.E., McLaurin C.I., Kress M.A., Kenney J.A., Jr. Incidence of common dermatoes in a predominantly black dermatologic practice. Cutis. 1983;32(4):388–390. [PubMed] [Google Scholar]
- Hamadi S.A., Mohammed M.M., Aljaf A.N., Abdulrazak A. The role of topical and oral melatonin in management of melasma patients. J Arab Univ Basic Appl Sci. 2009;8:30–42. [Google Scholar]
- Handog E.B., Datuin M.S., Singzon I.A. An open-label, single-arm trial of the safety and efficacy of a novel preparation of glutathione as a skin-lightening agent in Filipino women. Int J Dermatol. 2016;55(2):153–157. doi: 10.1111/ijd.12999. [DOI] [PubMed] [Google Scholar]
- Hashim P.W., Chen T., Harper J.C., Kircik L.H. The efficacy and safety of azelaic acid 15% foam in the treatment of facial acne vulgaris. J Drugs Dermatol. 2018;17(6):641–645. [PubMed] [Google Scholar]
- Huh S.Y., Shin J.W., Na J.I., Huh C.H., Youn S.W., Park K.C. Efficacy and safety of liposome‐encapsulated 4‐n‐butylresorcinol 0.1% cream for the treatment of melasma: A randomized controlled split‐face trial. J Dermatol. 2010;37(4):311–315. doi: 10.1111/j.1346-8138.2010.00787.x. [DOI] [PubMed] [Google Scholar]
- Ikino J.K., Nunes D.H., Da Silva V.P.M., Fröde T.S., Sens M.M. Melasma and assessment of the quality of life in Brazilian women. An Bras Dermatol. 2015;90(2):196–200. doi: 10.1590/abd1806-4841.20152771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutley G.S., Rajaratnam R., Halpern J., Salim A., Emmett C. Systematic review of randomized controlled trials on interventions for melasma. J Am Acad Dermatol. 2014;70(2):369–373. doi: 10.1016/j.jaad.2013.07.044. [DOI] [PubMed] [Google Scholar]
- Kang H.Y. Melasma and aspects of pigmentary disorders in Asians. Ann Dermatol Venereol. 2012;139:S92–S95. doi: 10.1016/S0151-9638(12)70126-6. [DOI] [PubMed] [Google Scholar]
- Kang H.Y., Hwang J.S., Lee J.Y., Ahn J.H., Kim J.Y., Lee E.S. The dermal stem and c-Kit are overexpressed in melasma. Br J Dermatol. 2006;154(6):1094–1099. doi: 10.1111/j.1365-2133.2006.07179.x. [DOI] [PubMed] [Google Scholar]
- Kang S. The mechanism of action of topical retinoids. Cutis. 2005;75:10–13. [PubMed] [Google Scholar]
- Kang W.H., Yoon K.H., Lee E.S., Kim J., Lee K.B., Yim H. Melasma: Histopathological characteristics in 56 Korean patients. Br J Dermatol. 2002;146(2):228–237. doi: 10.1046/j.0007-0963.2001.04556.x. [DOI] [PubMed] [Google Scholar]
- Kasraee B., Handjani F., Parhizgar A., Omrani G.R., Fallahi M.R., Amini M. Topical methimazole as a new treatment for postinflammatory hyperpigmentation: Report of the first case. Dermatology. 2005;211(4):360–362. doi: 10.1159/000088509. [DOI] [PubMed] [Google Scholar]
- Kasraee B., Safaee Ardekani G.H., Parhizgar A., Handjani F., Omrani G.R., Samani M. Safety of topical methimazole for the treatment of melasma: Transdermal absorption, the effect on thyroid function and cutaneous adverse effects. Skin Pharmacol Physiol. 2008;21(6):300–305. doi: 10.1159/000148222. [DOI] [PubMed] [Google Scholar]
- Keeling J., Cardona L., Benitez A., Epstein R., Rendon M. Mequinol 2%/tretinoin 0.01% topical solution for the treatment of melasma in men: a case series and review of the literature. Cutis. 2008;81(2):179–183. [PubMed] [Google Scholar]
- Kim E.H., Kim Y.C., Lee E.S., Kang H.Y. The vascular characteristics of melasma. J Dermatol Sci. 2007;46(2):111–116. doi: 10.1016/j.jdermsci.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Kim M.S., Bang S.H., Kim J.H., Shin H.J., Choi J.H., Chang S.E. Tranexamic acid diminishes laser-induced melanogenesis. Ann Dermatol. 2015;27(3):250–256. doi: 10.5021/ad.2015.27.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.J., Moon S.H., Cho S.H., Lee J.D., Sung Kim H. Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm Venereol. 2017;97(6-7):776–781. doi: 10.2340/00015555-2668. [DOI] [PubMed] [Google Scholar]
- Kim S.J., Park J.Y., Shibata T., Fujiwara R., Kang H.Y. Efficacy and possible mechanisms of topical tranexamic acid in melasma. Clin Exp Dermatol. 2016;41(5):480–485. doi: 10.1111/ced.12835. [DOI] [PubMed] [Google Scholar]
- Lajis A.F., Hamid M., Ariff A.B. Depigmenting effect of Kojic acid esters in hyperpigmented B16F1 melanoma cells. J Biomed Biotechnol. 2012;2012:95245. doi: 10.1155/2012/952452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhdar H., Zouhair K., Khadir K., Essari A., Richard A., Seite S. Evaluation of the effectiveness of a broad-spectrum sunscreen in the prevention of chloasma in pregnant women. J Eur Acad Dermatol Venereol. 2007;21(6):738–742. doi: 10.1111/j.1468-3083.2007.02185.x. [DOI] [PubMed] [Google Scholar]
- Lee A.Y. Recent progress in melasma pathogenesis. Pigment Cell Melanoma Res. 2015;28(6):648–660. doi: 10.1111/pcmr.12404. [DOI] [PubMed] [Google Scholar]
- Lee B.W., Schwartz R.A., Janniger C.K. Melasma. G Ital Dermatol Venereol. 2017;152(1):36–45. doi: 10.23736/S0392-0488.16.05425-0. [DOI] [PubMed] [Google Scholar]
- Lee H.C., Thng T.G., Goh C.L. Oral tranexamic acid (TA) in the treatment of melasma: A retrospective analysis. J Am Acad Dermatol. 2016;75(2):385–392. doi: 10.1016/j.jaad.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Mahmoud B.H., Ruvolo E., Hexsel C.L., Liu Y., Owen M.R., Kollias N. Impact of long-wavelength UVA and visible light on melanocompetent skin. J Invest Dermatol. 2010;130(8):2092–2097. doi: 10.1038/jid.2010.95. [DOI] [PubMed] [Google Scholar]
- Makino E.T., Kadoya K., Sigler M.L., Hino P.D., Mehta R.C. Development and clinical assessment of a comprehensive product for pigmentation control in multiple ethnic populations. J Drugs Dermatol. 2016;15(12):1562–1570. [PubMed] [Google Scholar]
- Mansouri P., Farshi S., Hashemi Z., Kasraee B. Evaluation of the efficacy of cysteamine 5% cream in the treatment of epidermal melasma: A randomized double-blind placebo-controlled trial. Br J Dermatol. 2015;173(1):209–217. doi: 10.1111/bjd.13424. [DOI] [PubMed] [Google Scholar]
- Martin L.K., Caperton C., Woolery-Lloyd H., Avashia N. A randomized double-blind placebo controlled study evaluating the effectiveness and tolerability of oral Polypodium leucotomos in patients with melasma. JAMA Dermatol. 2013;149(8):981–983. doi: 10.1001/jamadermatol.2013.4294. [DOI] [PubMed] [Google Scholar]
- Menter A. Rationale for the use of topical corticosteroids in melasma. J Drugs Dermatol. 2004;3(2):169–174. [PubMed] [Google Scholar]
- Moin A., Jabery Z., Fallah N. Prevalence and awareness of melasma during pregnancy. Int J Dermatol. 2006;45(3):285–288. doi: 10.1111/j.1365-4632.2004.02470.x. [DOI] [PubMed] [Google Scholar]
- Monteiro R.C., Kishore B.N., Bhat R.M., Sukumar D., Martis J., Ganesh H.K. A comparative study of the efficacy of 4% hydroquinone vs 0.75% Kojic acid cream in the treatment of facial melasma. Indian J Dermatol. 2013;58(2):157. doi: 10.4103/0019-5154.108070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na J.I., Choi S.Y., Yang S.H., Choi H.R., Kang H.Y., Park K.C. Effect of tranexamic acid on melasma: A clinical trial with histological evaluation. J Eur Acad Dermatol Venereol. 2013;27(8):1035–1039. doi: 10.1111/j.1468-3083.2012.04464.x. [DOI] [PubMed] [Google Scholar]
- Navarrete-Solís J., Castanedo-Cázares J.P., Torres-Álvarez B., Oros-Ovalle C., Fuentes-Ahumada C., Gonzalez F.J. A double-blind, randomized clinical trial of niacinamide 4% versus hydroquinone 4% in the treatment of melasma. Dermatol Res Pract. 2011;2011:379173. doi: 10.1155/2011/379173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor M.S., Bucay V.W., Callender V.D., Cohen J.L., Sadick N., Waldorf H. Polypodium leucotomos as an adjunct treatment of pigmentary disorders. J Clin Aesthet Dermatol. 2014;7(3):13–17. [PMC free article] [PubMed] [Google Scholar]
- Niwano T., Terazawa S., Sato Y., Kato T., Nakajima H., Imokawa G. Glucosamine abrogates the stem cell factor + endothelin-1-induced stimulation of melanogenesis via a deficiency in MITF expression due to the proteolytic degradation of CREB in human melanocytes. Arch Dermatol Res. 2018;310:625–637. doi: 10.1007/s00403-018-1850-8. [DOI] [PubMed] [Google Scholar]
- Nordlund J.J., Grimes P.E., Ortonne J.P. The safety of hydroquinone. J Eur Acad Dermatol Venereol. 2006;20:781–787. doi: 10.1111/j.1468-3083.2006.01670.x. [DOI] [PubMed] [Google Scholar]
- Olejnik A., Glowka A., Nowak I. Release studies of undecylenoyl phenylalanine from topical formulations. Saudi Pharm J. 2018;26:709–718. doi: 10.1016/j.jsps.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine C., Sharlow E., Liebel F., Eisinger M., Shapiro S., Seiberg M. An alternative approach to depigmentation by soybean extracts via inhibition of the PAR-2 pathway. J Invest Dermatol. 2001;116(4):587–595. doi: 10.1046/j.1523-1747.2001.01291.x. [DOI] [PubMed] [Google Scholar]
- Passeron T., Picardo M. Melasma, a photoaging disorder. Pigment Cell Melanoma Res. 2018;31(4):461–465. doi: 10.1111/pcmr.12684. [DOI] [PubMed] [Google Scholar]
- Pawaskar M.D., Parikh P., Markowski T., McMichael A.J., Feldman S.R., Balkrishnan R. Melasma and its impact on health-related quality of life in Hispanic women. J Dermatolog Treat. 2007;18(1):5–9. doi: 10.1080/09546630601028778. [DOI] [PubMed] [Google Scholar]
- Perper M., Eber A.E., Fayne R., Verne S.H., Magno R.J., Cervantes J. Tranexamic acid in the treatment of melasma: A review of the literature. Am J Clin Dermatol. 2017;18(3):373–381. doi: 10.1007/s40257-017-0263-3. [DOI] [PubMed] [Google Scholar]
- Picardo M., Carrera M. New and experimental treatments of cloasma and other hypermelanoses. Dermatol Clin. 2007;25(3):353–362. doi: 10.1016/j.det.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Pires A., Marques R., Encarnação J., Abrantes A., Marques I.A., Laranjo Ascorbic acid chemosensitizes colorectal cancer cells and synergistically inhibits tumor growth. Front Physiol. 2018;9:911. doi: 10.3389/fphys.2018.00911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regazzetti C., Sormani L., Debayle D., Bernerd F., Tulic M.K., De Donatis G.M. Melanocytes sense blue light and regulate pigmentation through the Opsin3. J Invest Dermatol. 2018;138(1):171–178. doi: 10.1016/j.jid.2017.07.833. [DOI] [PubMed] [Google Scholar]
- Rivas S., Pandya A. Treatment of melasma with topical agents, peels and lasers: An evidence-based review. Am J Clin Dermatol. 2013;14(5):359–376. doi: 10.1007/s40257-013-0038-4. [DOI] [PubMed] [Google Scholar]
- Rodrigues M., Pandya A. Melasma: Clinical diagnosis and management options. Australas J Dermatol. 2015;56(3):151–163. doi: 10.1111/ajd.12290. [DOI] [PubMed] [Google Scholar]
- Ryoo Y.W., Suh S.I., Mun K.C., Kim B.C., Lee K.S. The effects of the melatonin on ultraviolet-B irradiation cultured dermal fibroblasts. J Dermatol Sci. 2001;27(3):162–169. doi: 10.1016/s0923-1811(01)00133-5. [DOI] [PubMed] [Google Scholar]
- Sarkar R. Chemical peels for melasma. Pigment Cell Melanoma Res. 2013;26(3):451. [Google Scholar]
- Sarkar R., Ailawadi P., Garg S. Melasma in men: A review of clinical, etiological, and management issues. J Clin Aesthet Dermatol. 2018;11(2):53–59. [PMC free article] [PubMed] [Google Scholar]
- Sarma N., Chakraborty S., Poojary S.A., Rathi S., Kumaran S., Nirmal B. Evidence-based review, grade of recommendation, and suggested treatment recommendations for melasma. Indian Dermatol Online J. 2017;8(6):406–442. doi: 10.4103/idoj.IDOJ_187_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte B.C., Wu W., Rosen T. Azelaic acid: Evidence-based update on mechanism of action and clinical application. J Drugs Dermatol. 2015;14(9):964–968. [PubMed] [Google Scholar]
- Seçkin H.Y., Kalkan G., Baş Y., Akbaş A., Önder Y., Özyurt H. Oxidative stress status in patients with melasma. Cutan Ocul Toxicol. 2014;33(3):212–217. doi: 10.3109/15569527.2013.834496. [DOI] [PubMed] [Google Scholar]
- Siscovick J.R., Zapolanski T., Magro C., Carrington K., Prograis S., Nussbaum M. Polypodium leucotomos inhibits ultraviolet B radiation-induced immunosuppression. Photodermatol Photoimmunol Photomed. 2008;24(3):134–141. doi: 10.1111/j.1600-0781.2008.00352.x. [DOI] [PubMed] [Google Scholar]
- Sonthalia S., Daulatabad D., Sarkar R. Glutathione as a skin whitening agent: Fact, myths, evidence and controversies. Indian J Dermatol Venereol Leprol. 2016;82(3):262–272. doi: 10.4103/0378-6323.179088. [DOI] [PubMed] [Google Scholar]
- Taraz M., Niknam S., Ehsani A.H. Tranexamic acid in treatment of melasma: A comprehensive review of clinical studies. Dermatol Ther. 2017:30(3). doi: 10.1111/dth.12465. [DOI] [PubMed] [Google Scholar]
- Tse T. Hydroquinone for skin lightening: Safety profile, duration of use and when should we stop? J Dermatolog Treat. 2010;21(5):272–275. doi: 10.3109/09546630903341945. [DOI] [PubMed] [Google Scholar]
- Vachiramon V., Suchonwanit P., Thadanipon K. Melasma in men. J Cosmet Dermatol. 2012;11:151–157. doi: 10.1111/j.1473-2165.2012.00613.x. [DOI] [PubMed] [Google Scholar]
- Videira I.F., Moura D.F., Magina S. Mechanisms regulating melanogenesis. An. Bras. Dermatol. 2013;88:76–83. doi: 10.1590/S0365-05962013000100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wargniez W., Jungman E., Wilkinson S., Seyler N., Grégoire S. Inter-laboratory skin distribution study of 4-n-butyl resorcinol: The importance of liquid chromatography/mass spectrometry (HPLC-MS/MS) bioanalytical validation. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1060:416–423. doi: 10.1016/j.jchromb.2017.05.026. [DOI] [PubMed] [Google Scholar]
- Watanabe F., Hashizume E., Chan G.P., Kamimura A. Skin-whitening and skin-condition-improving effects of topical oxidized glutathione: A double-blind and placebo-controlled clinical trial in healthy women. Clin Cosmet Investig Dermatol. 2014;7:267–274. doi: 10.2147/CCID.S68424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werlinger K.D., Guevara I.L., González C.M., Rincón E.T., Caetano R., Haley R.W. Prevalence of self-diagnosed melasma among premenopausal Latino women in Dallas and Fort Worth. Tex Arch Dermatol. 2007;143(3):424–425. doi: 10.1001/archderm.143.3.424. [DOI] [PubMed] [Google Scholar]
- Wohlrab J., Kreft D. Niacinamide - Mechanisms of action and its topical use in dermatology. Skin Pharmacol Physiol. 2014;27(6):311–315. doi: 10.1159/000359974. [DOI] [PubMed] [Google Scholar]
- Wu S., Shi H., Wu H., Yan S., Guo J., Sun Y. Treatment of melasma with oral administration of tranexamic acid. Aesthet Plast Surg. 2012;36(4):964–970. doi: 10.1007/s00266-012-9899-9. [DOI] [PubMed] [Google Scholar]