See the article by John et al, pp. 264–273.
Glioblastomas (GBMs) have for decades been characterized in detail by their intratumor heterogeneity (cellular heterogeneity within tumors) as well as their intertumor heterogeneity (heterogeneity between GBM patients). The heterogeneity within and between patients represents a key challenge for the development of future new effective therapies. A rigorous pathological diagnosis is therefore important in order to design new treatment alternatives in both a primary and a recurrent setting. At present, neuropathological analysis, taking into consideration the latest World Health Organization classification guidelines, represents the gold standard to delineate different tumor types. Also, recent evidence suggests that an additional level of accuracy can be obtained at the molecular level using DNA methylation-based profiling, which may show applicability in routine diagnostic settings.1 Transcriptional complexity in GBM has also been shown through the Ivy Glioblastoma Atlas Project (Ivy GAP), which represents a resource database describing the spatial anatomic and genetic bases of GBMs at the cellular and molecular levels.2 To some extent longitudinal clinical information and MRI time course data are also available within the Ivy GAP project.
During the last decades there have been continuous development and improvement in neuroimaging techniques based on the development of new imaging hardware and data analysis tools, including machine learning, where data from MRI and PET have continued to improve based on increased resolution, contrast enhancement, and the development of new tracers. A future challenge will be to determine to what extent advanced molecular imaging data within tumor-specific subregions can predict the molecular cellular heterogeneity at the genomic level. Imaging genomic mapping may therefore serve as a future tool for identifying glioma subtypes.3
In a recent article published in Neuro-Oncology,4 the authors used a combined MRI/PET approach to define GBM-specific subregions associated with structural and metabolic abnormalities. Using MRI (contrast enhanced T1, T2/fluid attenuated inversion recovery MR apparent diffusion coefficient maps from diffusion-weighted imaging) and amino acid PET (alpha-[11C]-methyl-L-tryptophan [AMT-PET]), 115 subregions were classified in 30 GBM patients. Comparative imaging analyses of the subregions revealed a high AMT uptake within the contrast enhancing tumor mass that extended into non-enhancing areas with low apparent diffusion coefficient, suggesting a high cellularity in these areas.
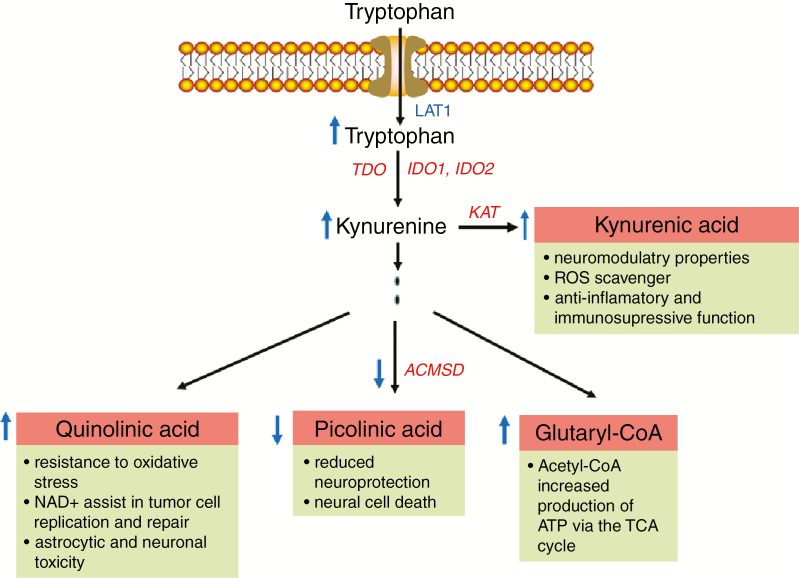
Amino acid PET has for years been used as a diagnostic tool to assess tumor burden and progression. It is well known that GBMs have a high uptake of amino acid PET tracers. In the context of AMT-PET, AMT is transported into the tumors by the amino acid receptor LAT1.5 Compared with tryptophan, however, AMT is not incorporated into proteins based on an added methyl group to the molecule. Yet it can undergo enzymatic conversion by the enzyme indoleamine 2,3-dioxygenase (IDO).6 The metabolization of tryptophan in tumors occurs via the kynurenine immunomodulatory pathway—which plays an important role in immune tolerance7—as well as by the production of the NAD+ precursor quinolinic acid, which has been shown to confer glioma resistance to oxidative stress and neuronal toxicity. In addition, the enzyme ACMSD (α-amino-β-carboxymuconate-ε-semialdehyde decarboxylase) may be reduced in gliomas leading to less neuroprotection by the well-known neuroprotective agent picolinic acid.8 An increase in tryptophan uptake should therefore in theory favor tumor growth at many different levels (Fig. 1).
Fig. 1.
The tryptophan-kynurenine pathway and major functional consequences in the context of GBM growth. It should be emphasized that this pathway may regulate, through inflammatory reactions, also other pro-oncogenic factors such as transforming growth factor ß and signal transducer and activator of transcription signaling (not described here).9
Putting this knowledge in the context of the findings by John et al,4 it is somewhat surprising that the authors show that high AMT uptake in the MRI contrast enhancing regions is associated with a better prognosis regardless of other prognostic variables. In this context, however, it should be emphasized that although there is an abundant mechanistic insight into the tryptophan-kynurenine metabolic pathway, we still do not completely understand how this pathway regulates tumor growth and progression at various tumor sites within a given tumor.
Based on the data provided by the authors, it is also questionable, given the relatively low number of patients analyzed (n = 30), that true conclusions can be made that will warrant routine clinical implementation of the findings presented by the authors. From the article, it is also somewhat difficult to foresee how the MRI techniques described can be repeated by others: There is only a very brief description of the MR protocols used. For instance, an explanation of the reasons for choosing these MR sequences, including choice of b-values, is not clear, as well as a discussion on to what extent the MR results presented in this article are scanner specific or not. Also, the reason for the choice of time windows for reconstruction of the dynamic PET data is not clear, nor to what extent such parameters would have an influence on the results presented.
In order to get a comprehensive functional overview of the data presented, it will be important to stereotactically select tumor specimens from non-contrast enhancing as well as high contrast enhancing AMT regions. The aim here should be to delineate in detail how the kynurenine metabolic pathway is regulated in non-contrast and contrast enhancing regions with a high AMT-PET uptake. In the clinical setting, there has been a strong focus on the development of IDO1 inhibitors where some have entered or are in the process of entering clinical trials, such as Epacadostat, Indoximod, and PF-06840003, which inhibit the oxidation of tryptophan into kynurenine. A focus here is to assess how such inhibitors can be used in combination with immunotherapy. It may be of interest also in the future to see how IDO1 targeting will affect the AMT-PET signatures described by the authors.
References
- 1. Capper D, Jones DTW, Sill M, et al. DNA methylation-based classification of central nervous system tumours. Nature. 2018;555(7697):469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Puchalski RB, Shah N, Miller J, et al. An anatomic transcriptional atlas of human glioblastoma. Science. 2018;360(6389):660–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Smits M, van den Bent MJ. Imaging correlates of adult glioma genotypes. Radiology. 2017;284(2):316–331. [DOI] [PubMed] [Google Scholar]
- 4. John F, Bosnyák E, Robinette NL, et al. Multimodal imaging-defined subregions in newly-diagnosed glioblastoma: impact on overall survival. Neuro Oncol. 2019;21(2), 264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boado RJ, Li JY, Nagaya M, Zhang C, Pardridge WM. Selective expression of the large neutral amino acid transporter at the blood-brain barrier. Proc Natl Acad Sci U S A. 1999;96(21):12079–12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Diksic M, Nagahiro S, Sourkes TL, Yamamoto YL. A new method to measure brain serotonin synthesis in vivo. I. Theory and basic data for a biological model. J Cereb Blood Flow Metab. 1990;10(1):1–12. [DOI] [PubMed] [Google Scholar]
- 7. Uyttenhove C, Pilotte L, Théate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9(10):1269–1274. [DOI] [PubMed] [Google Scholar]
- 8. Adams S, Teo C, McDonald KL, et al. Involvement of the kynurenine pathway in human glioma pathophysiology. PLoS One. 2014;9(11):e112945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wirthgen E, Hoeflich A, Rebl A, Günther J. Kynurenic acid: the janus-faced role of an immunomodulatory tryptophan metabolite and its link to pathological conditions. Front Immunol. 2017;8:1957. [DOI] [PMC free article] [PubMed] [Google Scholar]