See the article by Poore et al, pp. 252–263.
Pediatric low-grade gliomas (pLGGs) are the most common brain tumor of childhood and are associated with significant morbidity. When present in favorable locations, pLGG can be cured with surgical resection alone. However, those tumors present in unresectable locations or those which recur will require additional therapy, with children commonly receiving several different therapies throughout the course of their childhood. While children with these tumors have excellent overall survival,1 current standards of care, which most commonly comprise combination chemotherapy regimens, have clear limitations. Across a variety of prospective studies investigating standard chemotherapy regimens, only 35–45% of children will be progression free 5 years after treatment.2 Therefore, the majority of children with pLGG will progress following these regimens and require further therapy. While recent genomic profiling efforts of pLGGs have shed tremendous insights into the genetic drivers of pLGGs, determining how to incorporate these with current standards of care remains a significant challenge.
In this issue of Neuro-Oncology, Poore et al investigate a novel combination treatment regime introducing a small molecular inhibitor, everolimus, to a traditional chemotherapy (carboplatin) backbone.3 The authors demonstrate the synergistic benefit of this combinatorial approach in pLGG models both in vitro and in vivo. They also demonstrate a biologic basis of this effect through the depletion of glutathione.
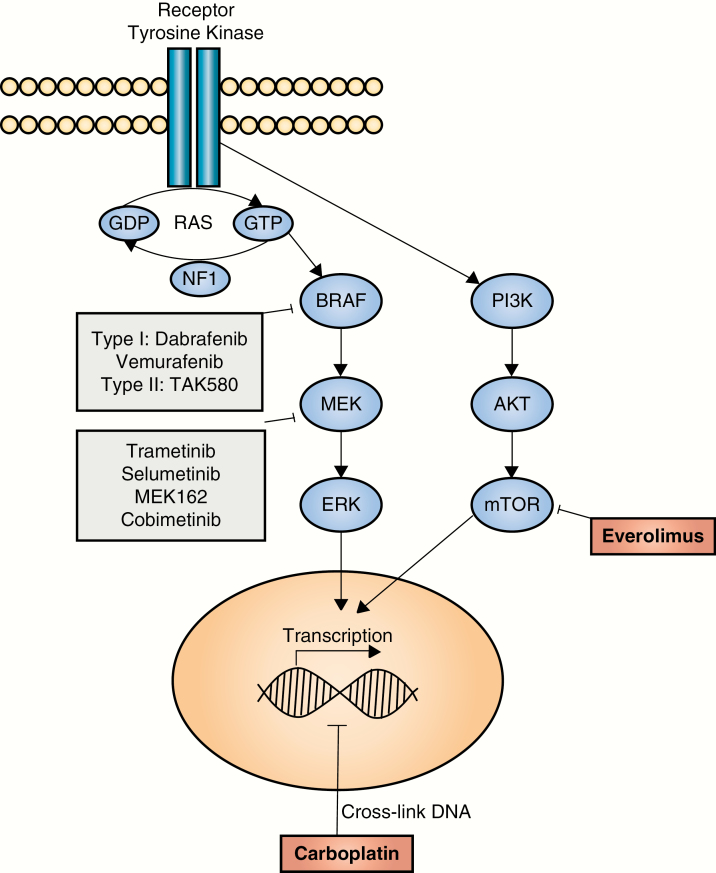
The authors’ choice of everolimus stems from the recognition that the majority of pLGGs are associated with molecular alterations that activate the mitogen-activated protein kinase (MAPK) pathway.4–6 The most frequent of these involve the BRAF gene and include the KIAA1549:BRAF fusion duplication and the BRAF V600E mutation. With this knowledge, treatment strategies have shifted toward molecular targeted approaches aimed at interrupting MAPK hyperactivation. Mammalian target of rapamycin (mTOR) is a downstream element of the phosphatidylinositol 3-kinase (PI3K) pathway, and can also be activated by MAPK signaling. Everolimus, a potent and selective mTOR inhibitor, was the first small-molecule inhibitor trialed in children with progressive/recurrent low-grade gliomas. These trials demonstrated efficacy (tumor stability or response) and the drug was well tolerated.7 Newer agents have sought to inhibit either BRAF or MEK directly (Fig. 1). There are several MEK inhibitors under evaluation which have been used in patients with both KIAA1549:BRAF fusion duplications and BRAF V600E mutations, including selumetinib, trametinib, MEK162, and cobimetinib. Selumetinib is the agent with the most mature data, with a completed phase II showing partial response in one third of all patients and 66% ± 11% progression-free survival at 2 years.8 The other 3 agents are part of ongoing phase I/II clinical trials with results forthcoming. Clinical trial experience with BRAF V600E inhibitors is likewise growing. Two type I BRAF inhibitors included in ongoing pediatric phase II trials are dabrafenib and vemurafenib, which have encouraging preliminary results in pLGG with BRAF V600E mutations.9 However, when used to treat KIAA1549:BRAF fusion positive tumors, type I inhibitors can result in paradoxical activation of the MAPK pathway with tumor growth. Type II BRAF inhibitors are not associated with such paradoxical activation in preclinical studies,10 and are currently undergoing phase I and II clinical testing. While targeted therapy shows promising preliminary results, the durability of response is unknown. Additionally, it is not known how these agents may behave in combination—whether in combination with other targeted therapies or with traditional chemotherapy agents.
Fig. 1.
Pediatric low-grade gliomas exhibit frequent genetic alterations that activate the mitogen-activated protein kinase pathway (depicted). Small-molecule inhibitors that have been trialed (or are currently being trialed) in early-phase pediatric clinical trials are shown. The combination of carboplatin and everolimus (shown in red) makes use of distinct mechanisms of action.
A key clinical challenge is how to incorporate these small-molecule inhibitors with combination treatments. Combining these targeted therapies is not without risk. The long-term toxicity profiles of individual agents are still to be determined and the risks associated with common pathway inhibition and potential for overlapping toxicity have not been defined in gliomas. Combination therapies for targeted inhibitors also pose practical challenges regarding timing of clinical development (for example, phase I vs phase II agents) and the ability to design combination trials with small molecule inhibitors developed by different pharmaceutical companies. Thus, the approach used by Poore et al in this issue of Neuro-Oncology is particularly compelling.3 In using a traditional carboplatin backbone, they capitalize on the well-known safety profile of this long-used agent. Carboplatin’s mechanism of action differs significantly from their targeted agent of choice, thus minimizing a risk of overlapping toxicity. Additionally, everolimus is a targeted agent which has well-documented antineoplastic properties across cancers; has safely been combined with traditional chemotherapy in other cancers; and was the first targeted inhibitor to undergo clinical trials for children with pLGG and showed clinical effect. The authors demonstrated that these drugs synergized in multiple in vitro models harboring characteristic pLGG molecular alterations.
Additional challenges remain. We do not yet know the ideal timing of combination chemotherapy/small-molecule inhibitors—at diagnosis to improve response and progression-free survival, or at relapse/recurrence given improved effect over single-agent therapy. Additionally, one wonders where the newer BRAF and MEK inhibitors will fall. What is the durability of single-agent response? When, if ever, should they be used in combination? What is the role of traditional chemotherapy in combination with these newer agents? These questions remain to be answered in the next generation of clinical trials for children with both newly diagnosed and recurrent pLGG.
Conflict of interest statement
P.B. receives grant funding from Novartis Institutes for Biomedical Research for an unrelated project.
References
- 1. Bandopadhayay P, Bergthold G, London WB, et al. Long-term outcome of 4,040 children diagnosed with pediatric low-grade gliomas: an analysis of the Surveillance Epidemiology and End Results (SEER) database. Pediatr Blood Cancer. 2014;61(7):1173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jones DTW, Kieran MW, Bouffet E, et al. Pediatric low-grade gliomas: next biologically driven steps. Neuro Oncol. 2018;20(2):160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Poore B, Yuan M, Arnold A, et al. mTORC1 inhibition in pediatric low-grade glioma depletes glutathione and therapeutically synergizes with carboplatin. Neuro Oncol. 2018. doi: 10.1093/neuonc/noy150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jones DT, Hutter B, Jäger N, et al. Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat Genet. 2013;45(8):927–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang J, Wu G, Miller CP, et al. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet. 2013;45(6):602–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bandopadhayay P, Ramkissoon LA, Jain P, et al. MYB-QKI rearrangements in angiocentric glioma drive tumorigenicity through a tripartite mechanism. Nat Genet. 2016;48(3):273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kieran MW, Yao X, Macy M, et al. Final results of a prospective multi-institutional phase II study of everolimus (RAD001), an mTOR inhibitor, in pediatric patients with recurrent or progressive low-grade glioma. A POETIC consortium trial. Neuro Oncol. 2014;16(Suppl 3):iii27. [Google Scholar]
- 8. Fangusaro J, Onar-Thomas A, Young-Poussaint T, et al. A phase II prospective study of selumetinib in children with recurrent or refractory low-grade glioma (LGG): A Pediatric Brain Tumor consortium (PBTC) study. J Clin Oncol; 2017;35(Suppl 15):10504––10504.. [Google Scholar]
- 9. Kieran MW, Bouffet E, Tabori U, et al. The First Study of Dabrafenib in Pediatric Patients with BRAF V600-Mutant Relapsed or Refractory Low-Grade Gliomas. Abstract Book of the 41st ESMO Congress; 2016:abstract LBA19_MR. [Google Scholar]
- 10. Sun Y, Alberta JA, Pilarz C, et al. A brain-penetrant RAF dimer antagonist for the noncanonical BRAF oncoprotein of pediatric low-grade astrocytomas. Neuro Oncol. 2017;19(6):774–785. [DOI] [PMC free article] [PubMed] [Google Scholar]